Abstract
Background
Cancers from lung and esophagus are the leading causes of cancer-related deaths in China and share many similarities in terms of histological type, risk factors and genetic variants. Recent genome-wide association studies (GWAS) in Chinese esophageal cancer patients have demonstrated six high-risk candidate single nucleotide polymorphisms (SNPs). Thus, the present study aimed to determine the risk of these SNPs predisposing to lung cancer in Chinese population.
Methods
A total of 1170 lung cancer patients and 1530 normal subjects were enrolled in this study from high-incidence areas for esophageal cancer in Henan, northern China. Five milliliters of blood were collected from all subjects for genotyping. Genotyping of 20 high-risk SNP loci identified from genome-wide association studies (GWAS) on esophageal, lung and gastric cancers was performed using TaqMan allelic discrimination assays. Polymorphisms were examined for deviation from Hardy-Weinberg equilibrium (HWE) using Х2 test. Bonferroni correction was performed to correct the statistical significance of 20 SNPs with the risk of lung cancer. The Pearson’s Х2 test was used to compare the distributions of gender, TNM stage, histopathological type, smoking and family history by lung susceptibility genotypes. Kaplan-Meier and Cox regression analyses were carried out to evaluate the associations between genetic variants and overall survival.
Results
Four of the 20 SNPs identified as high-risk SNPs in Chinese esophageal cancer showed increased risk for Chinese lung cancer, which included rs3769823 (OR = 1.26; 95% CI = 1.107–1.509; P = 0.02), rs10931936 (OR = 1.283; 95% CI = 1.100–1.495; P = 0.04), rs2244438 (OR = 1.294; 95% CI = 1.098–1.525; P = 0.04) and rs13016963 (OR = 1.268; 95% CI = 1.089–1.447; P = 0.04). All these SNPs were located at 2q33 region harboringgenes of CASP8, ALS2CR12 and TRAK2. However, none of these susceptibility SNPs was observed to be significantly associated with gender, TNM stage, histopathological type, smoking, family history and overall survival.
Conclusions
The present study identified four high-risk SNPs at 2q33 locus for Chinese lung cancer and demonstrated the shared susceptibility loci at 2q33 region for Chinese lung and esophageal cancers.
Introduction
Cancers from lung and esophagus are the most common cancers and remain the leading causes of cancer-related deaths in China. For both cancers, squamous cell carcinoma is the predominant histopathological type and cigarette smoking and alcohol consumption are the main risk factors [1–5]. The concurrent occurrence of malignant lesions from lung and esophagus is not uncommon. Lung is the most common site for secondary primary malignancies in patients with esophageal cancer [4]. Accumulating evidence indicates that lung and esophageal cancers share many common genetic variants, such as deletion of CASP and CYP2A6 gene [3, 6], SOX2 expression [2], IFGR-IGFBP axis [7] and WDHD1 [8], especially in an Asian population.
Recently, a couple of genome-wide association studies (GWAS) demonstrated that a group of common genetic variants increase the risk to tumors from esophagus, lung and stomach [9–21]. Notably, the SNPs among these genetic variants have not been well characterized in lung cancer from Chinese Han population. Based on the published GWAS data from lung cancer, esophageal squamous cell carcinoma (ESCC) and gastric adenocarcinoma (GA), a group of 20 SNPs of interest were selected to determine their relevance on the susceptibility and prognosis in 1170 patients with lung cancer and 1530 healthy control subjects from northern China.
Methods
Ethics statement
The study was approved by the Ethics Committee of The First Affiliated Hospital of Zhengzhou University, China and conducted according to Declaration of Helsinki principles. Written informed consent was obtained from all participants.
Patients and follow-up
For genotyping, 1170 lung cancer patients were enrolled in this study from January 1, 2011 to December 31, 2012, including 811 males with a mean age of 60±12 years (mean±SD) and 359 females with a mean age of 58±13 years. All patients were Chinese Han descent and were from multiple hospitals across Henan province, northern China, one of the high-incidence areas for both ESCC and GA. Detailed clinical information including histological type, lymph node metastasis, pathological stage, family history, cigarette smoking and treatment methods were retrieved from medical records. All patients were followed up until the end of 2014 with a median of 3.5 years. Survival status was available for all the patients genotyped in this study. In addition, 1530 healthy control subjects of Chinese Han descent were from the same regions of above lung cancer patients. 5ml peripheral venous blood was extracted from all the subjects enrolled in this study, blood DNA from all the subjects were retrospectively genotyped.
Family history was self-reported and defined as any first-degree relatives (parents or siblings) who had been diagnosed with lung cancer reported by the subjects. Positive smoking history was defined as more than 15 pack-years. Never smokers were individuals who reported smoking less than 100 cigarettes in their lifetime [22]. TNM stages were defined according to the seventh edition of the American Joint Committee on Cancer staging system, published in 2009 [23].
DNA extraction and SNP genotyping
DNA was extracted from buffy coat of 1170 patients diagnosed with lung cancer and 1530 healthy controls using Puregene Kit (Qiagen, Valencia, CA, USA). All DNA samples were genotyped for the 20 susceptibility SNPs at 17 risk loci derived from lung cancer, ESCC or GA using TaqMan allelic discrimination assays (Applied Biosystems, Foster City, CA, USA). The reaction consisted of 2μl of 10 ng/μl DNA, 0.5μl of 2ⅹTaqman Universal PCR Master Mix, No AmpErase UNG (Bio-Rad, Hercules, CA, USA). The fluorescent intensities of each sample were measured before and after PCR using an Applied Biosystems 7500 Real-Time PCR System (Applied Biosystems). Data obtained was analyzed and genotypes assigned using 7300 System SDS Software, version 1.4 (Applied Biosystems). Because of less quantity and poor quality of DNA, 1005 effective genotypes with lung cancer and 1473 effective genotypes with healthy control were obtained in our study finally.
Statistical analyses
All polymorphisms were examined for deviation from Hardy-Weinberg equilibrium (HWE) using Х2 test. Bonferroni correction was performed to correct the statistical significance of 20 SNPs with the risk of lung cancer. Clinical parameters were examined for associations with individual SNPs using Fisher’s Exact Test. Kaplan-Meier and Cox regression analyses were carried out to evaluate the associations between genetic variants and overall survival. The Pearson’s Х2 test was used to estimate the associations of gender, grade, tumor type, cigarette smoking and family history with lung susceptibility genotypes of selected SNPs.
Results
People characteristics
The information, including age and gender of the subjects involved in the present study, were summarized in Table 1. The mean ages for male and female lung cancer patients and healthy control were 60±12, 58±13 and 48±14, 49±12 years, respectively. The majority of the cases were males (811/1170, 69.32%). Cox regression analyses did not reveal association between risk loci and gender, age.
Table 1. Demographic characteristics of patients and healthy controls.
| Lung cancer | Healthy Controls | |
|---|---|---|
| Age(Mean±SE) | ||
| male | 60±12 | 48±14 |
| female | 58±13 | 49±12 |
| Sex(n) | ||
| male | 811 | 728 |
| female | 359 | 802 |
| Total | 1170 | 1530 |
Lung cancer susceptibility genotypes
The distribution of genotypes in this patient cohort was shown in Table 2. Twenty SNPs in 17 genes (Tables 2 and 3) were analyzed for association with the risk of lung cancer. Finally, five SNPs (rs3769823, rs10931936, rs2244438, rs13016963, rs7578456) were significant association with the increased risk of lung cancer in the high-incidence area of ESCC in northern China with p value of 0.001, 0.002, 0.002, 0.002 and 0.007. However, after Bonferroni correction multiple comparisons, of the original five significant SNPs, one SNP (rs7578456) was identified without significant p value (0.14). The other four SNPs (rs3769823, rs2244438, rs10931936, and rs13016963) showed significant associations with the increased risk of lung cancer with p value of 0.02, 0.04, 0.04 and 0.04, respectively. The 95% CIs were shown in Table 2. High linkage disequilibrium SNPs, which map to a region including CASP8, ALS2CR12 and TRAK2, identified as high-risk SNPs in Chinese esophageal cancer, showed significant associations with the increased risk of lung cancer.
Table 2. Association of twenty genetic variants and risk of lung cancer.
| Risk genotypes | F_ A | F_ U | P | Bonferroni correction P |
OR | 95%CI |
|---|---|---|---|---|---|---|
| rs3769823 rs10931936 rs2244438 rs13016963 rs7578456 rs36600 rs13042395 rs1926203 rs9841504 rs4254535 rs8034191 rs1530057 rs2352028 rs4975616 rs2808630 rs748404 rs753955 rs2909430 rs13361707 rs78378222 |
0.2923 0.2999 0.2470 0.3051 0.2727 0.1882 0.2883 0.1476 0.1413 0.2530 0.0494 0.0845 0.2011 0.1470 0.1332 0.0805 0.3548 0.0499 0.4926 0.1774 |
0.2422 0.2504 0.2023 0.2571 0.2319 0.2127 0.2634 0.1655 0.1246 0.2739 0.0417 0.0749 0.1885 0.1385 0.1538 0.0861 0.3464 0.0481 0.4921 0.0862 |
0.0010 0.0020 0.0020 0.0020 0.0070 0.0777 0.1086 0.1579 0.1590 0.1705 0.2873 0.3128 0.3602 0.4832 0.5105 0.5635 0.6139 0.8091 0.9797 0.5200 |
0.02 0.04 0.04 0.04 0.14 1.554 2.172 3.158 3.18 3.41 5.746 6.256 7.204 9.664 10.21 11.27 12.278 16.182 19.594 10.4 |
1.2920 1.2830 1.2940 1.2680 1.2420 0.8585 1.1330 0.8731 1.1560 0.8977 1.1950 1.1390 1.0840 1.0720 0.8442 0.9302 1.0370 1.0400 1.0020 0.8500 |
1.107–1.509 1.100–1.495 1.098–1.525 1.089–1.447 1.061–1.045 0.724–1.017 0.973–1.319 0.723–1.054 0.945–1.414 0.769–1.408 0.860–1.661 0.885–1.446 0.912–1.287 0.882–1.303 0.511–1.369 0.728–1.189 0.899–1.197 0.759–1.425 0.874–1.148 0.460–1.530 |
F_ A = Variant frequency of cancer loci; F_ U = Normal control variant frequency; OR = odds ratio.
Table 3. Distribution of SNPs genotyping in case (patients with esophageal, lung or gastric cancer) and controls (normal healthy subjects).
| SNPs | Gene | Allele | MAF(n) | P | OR | 95%CI | Reference number | Cancer type | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Cases | Controls | |||||||||
| rs36600 | MTMR3 | T/C | 0.117(1978) | 0.086(2540) | 0.0000 | 1.38 | 1.21 | 1.58 | [14] | LC |
| Rs3769823 | CASP8 | T/C | 0.29(2961) | 0.27(3400) | 0.0001 | 1.29 | 1.18 | 1.38 | [9] | EC |
| Rs2244438 | TRAK2 | G/A | 0.25(2961) | 0.23(3400) | 0.000 | 1.35 | 1.26 | 1.49 | [9] | EC |
| rs748404 | TGM5 | C/T | 0.825(1447) | 0.797(36256) | 0.0005 | 1.08 | 1.33 | 1.20 | [16] | LC |
| rs9841504 | ZBTB20 | G/C | 0.09(4294) | 0.14(5882) | 0.000 | 0.58 | 0.47 | 0.72 | [20] | GA |
| rs2909430 | TP53 | G/A | 0.17(443) | 0.10(547) | 0.000 | 1.94 | 1.06 | 3.57 | [18] | LC |
| rs78378222 | TP53 | A/C | 0.21(1014) | 0.19(1839) | 0.0001 | 0.85 | 0.46 | 1.53 | [19] | LC |
| rs10931936 | CASP8 | T/C | 0.27(1974) | 0.31(2617) | 0.0000 | 1.29 | 1.19 | 1.40 | [3] | EC |
| rs4254535 | GKN2/GKN1 | C/T | 0.67(1452) | 0.68(36203) | 0.0000 | 0.97 | 0.89 | 1.05 | [16] | LC |
| rs13042395 | C20orf54 | T/C | 0.47(380) | 0.48(380) | 0.0000 | 0.98 | 0.72 | 1.33 | [20] | EC |
| rs8034191 | AGPHD1 | C/T | 0.21(467) | 0.16(388) | 0.0036 | 1.61 | 1.17 | 2.21 | [15,17] | LC |
| rs7578456 | TRAK2 | A/G | 0.24(1974) | 0.28(2617) | 0.0000 | 1.27 | 0.17 | 1.38 | [11] | EC |
| rs2352028 | GPC5 | T/C | 0.32(295) | 0.35(598) | 0.0000 | 0.89 | 0.66 | 1.20 | [13] | LC |
| rs13016963 | ALS2CR12 | A/G | 0.27(2961) | 0.31(3400) | 0.0000 | 1.29 | 1.19 | 1.40 | [9] | EC |
| rs4975616 | TERT/CLPTM1 | G/A | 0.42(239) | 0.48(553) | 0.001 | 0.56 | 0.40 | 0.80 | [16] | LC |
| rs1530057 | RBMS3 | A/C | 0.05(1429) | 0.05(36224) | 0.0000 | 0.01 | 0.83 | 1.23 | [16] | LC |
| rs1926203 | ACTA2 | T/G | 0.67(1466) | 0.67(36273) | 0.0000 | 0.99 | 0.90 | 1.08 | [16] | LC |
| rs13361707 | PRKAA1 | T/C | 0.57(4294) | 0.48(5882) | 0.0000 | 1.42 | 1.24 | 1.62 | [20] | GA |
| rs2808630 | CRPP1/CRP | C/T | 0.40(96) | 0.41(124) | 0.0000 | 1.04 | 0.74 | 1.36 | [16] | LC |
| rs753955 | MIPEP/TNFRSF | C/T | 0.33(2331) | 0.28(3065) | 0.000 | 1.24 | 1.14 | 1.35 | [14] | LC |
Relationships between lung cancer susceptibility SNPs and overall survival
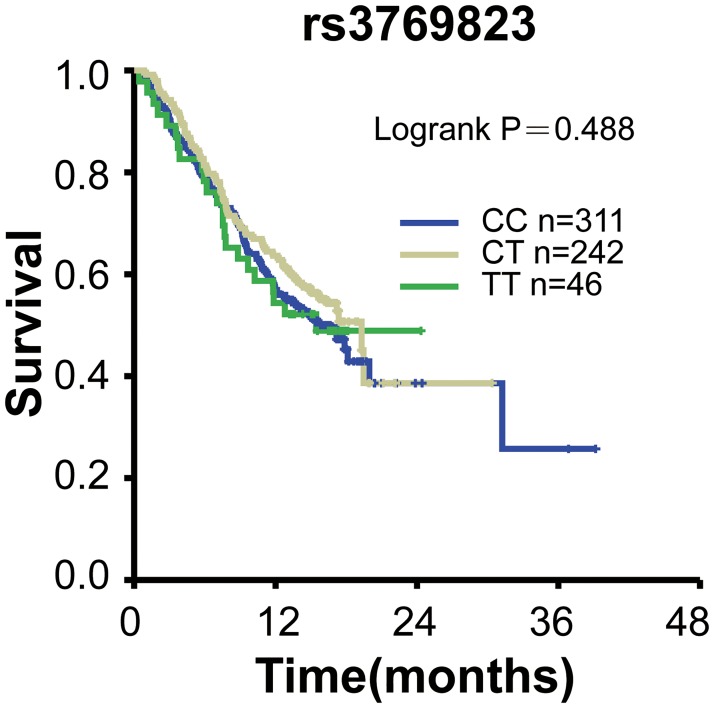
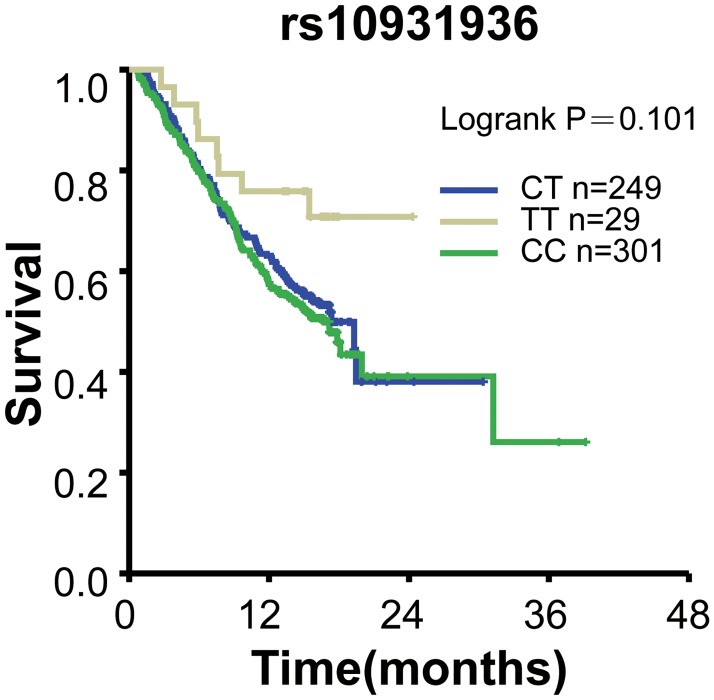
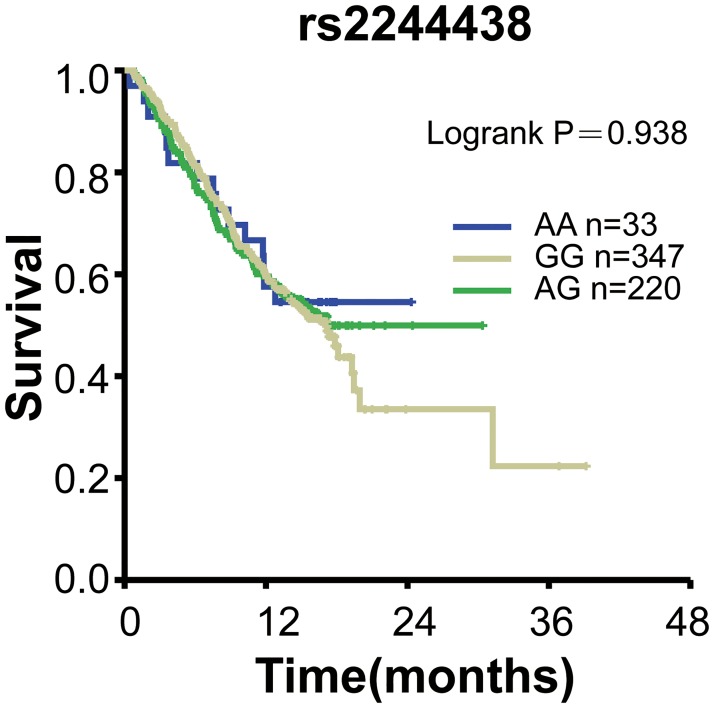
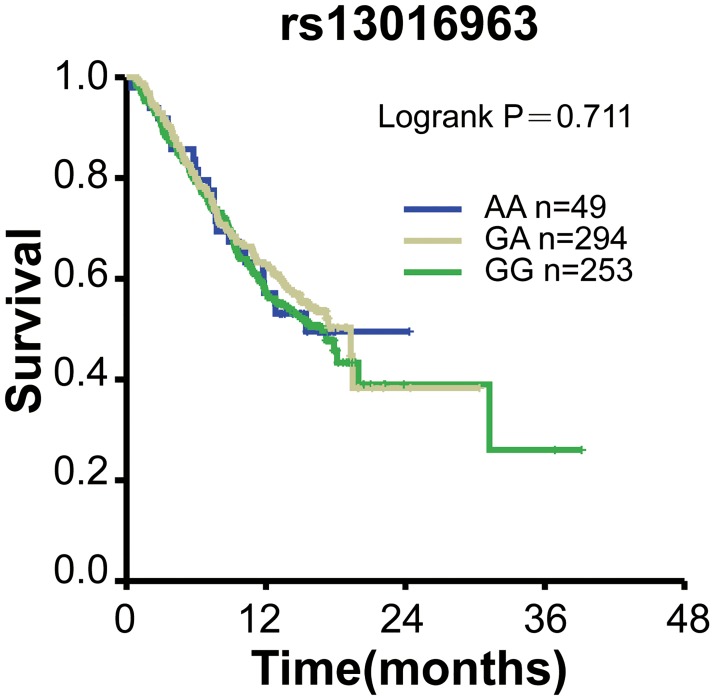
To test whether these ESCC and lung cancer susceptibility SNPs variants correlate with the overall survival of lung cancer patients, we performed Kaplan-Meier survival analyses. Our results showed that no significant differences between overall survival (OS) and these SNPs were observed in patients (P>0.05; Table 4). 599 patients were genotyped for rs3769823, 579 for rs10931936, 600 for rs2244438, 596 for 13016963, respectively. Kaplan-Meier curve of OS for rs3769823 genotype was shown in Fig 1. Log-rank tests showed no significant difference between OS and rs10931936 genotype (log-rank P = 0.488). Fig 2 showed Kaplan-Meier curve of OS for rs10931936 genotype. OS of the TT genotype group increased in comparison with CT and AG genotype groups but without statistical significance. Kaplan-Meier curves of OS for rs2244438, rs13016963 genotypes were shown in Figs 3 and 4, respectively, and Log-rank analysis showed no significant differences between OS and these genotypes (log-rank P = 0.101, P = 0.938, P = 0.711, respectively).
Table 4. Association between overall survival and 4 risk genotypes of lung cancer.
| SNP and genotype | Risk allele | Hazard ratio | 95%CI | P | Case number | Total | |
|---|---|---|---|---|---|---|---|
| Yes/No | Total | ||||||
| Rs3769823 | |||||||
| C/C | T | 1.656 | 1.425–1.887 | 0.488 | 160/151 | 311 | |
| T/T | 1.286 | 1.064–1.509 | 23/23 | 46 | 599 | ||
| T/C | 1.517 | 1.340–1.694 | 111/131 | 242 | |||
| Rs10931936 | |||||||
| C/C | T | 1.672 | 1.438–1.907 | 0.101 | 153/148 | 301 | |
| T/T | 1.624 | 1.383–1.866 | 8/21 | 29 | 579 | ||
| T/C | 1.499 | 0.324–1.673 | 117/132 | 249 | |||
| Rs2244438 | |||||||
| A/A | A | 1.367 | 1.108–1.626 | 0.938 | 15/18 | 33 | |
| A/G | 1.576 | 1.439–1.713 | 104/116 | 220 | 600 | ||
| G/G | 1.609 | 1.383–1.835 | 176/171 | 347 | |||
| Rs1301663 | |||||||
| A/A | A | 1.373 | 1.118–1.535 | 0.711 | 24/25 | 49 | |
| A/G | 1.501 | 1.326–1.676 | 118/13 | 253 | 596 | ||
| G/G | 1.667 | 1.432–1.902 | 150/144 | 294 | |||
Fig 1. Kaplan-Meier survival curves with regards to rs3769823 genotypes.
Fig 2. Kaplan-Meier survival curves with regards to rs10931936 genotypes.
OS of the TT genotype group was increased compared with CT and AG genotype groups before expansion of abbreviation region.
Fig 3. Kaplan-Meier survival curves with regards to rs2244438 genotypes.
Fig 4. Kaplan-Meier survival curves with regards to rs13016963 genotypes.
Associations between the four risk genotypes of lung cancer and clinical characteristics
Table 5 summarizes the baseline characteristics of patients in this study. Among 1170 lung cancer patients, the main histopathological types were adenocarcinoma and squamous cell carcinoma. The majority of male patients of lung cancer were smokers. We found that rs10931936 CT genotype was borderline significantly associated with an increase risk in male subgroup (P = 0.086; Table 5). No statistically significant differences were found between four genotypes and gender, tumor types, grades, smoking and family history (P> 0.05, Table 5) when genotype and the allele distribution of polymorphisms were compared. The uninformative cases were due to lost of connection or low genome QC level.
Table 5. Association of four risk genotypes of lung cancer and clinical characteristics.
| Characteristics | Rs22244438 | Rs3769823 | Rs10931936 | Rs13016963 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | GT | N (%) | P value | Total | GT | N (%) | P value | Total | GT | N (%) | P value | Total | GT | N (%) | P value | |
| Gender | ||||||||||||||||
| Male | 674 | AA | 41 (6) | 674 | CC | 325 (48) | 674 | CT | 296 (44) | 674 | AA | 64 (9) | ||||
| AG | 263 (39) | TT | 61 (9) | CC | 316 (47) | GG | 313 (47) | |||||||||
| GG | 370 (55) | TC | 288 (43) | TT | 62 (9) | GA | 295 (43) | |||||||||
| Female | 305 | AA | 18 (6) | 305 | CC | 167 (55) | 305 | CT | 113 (37) | 305 | AA | 27 (9) | ||||
| AG | 104 (34) | TT | 24 (8) | CC | 166 (54) | GG | 161 (53) | |||||||||
| GG | 183 (60) | 0.311 | TC | 114 (37) | 0.173 | TT | 26 (9) | 0.086 | GA | 115 (38) | 0.158 | |||||
| HPTP | ||||||||||||||||
| LAC | 287 | AA | 19 (7) | 287 | CC | 145 (50) | 273 | CT | 115 (42) | 286 | AA | 30 (10) | ||||
| AG | 110 (38) | TT | 27 (9) | CC | 143 (52) | GG | 139 (49) | |||||||||
| GG | 158 (55) | TC | 115 (41) | TT | 15 (6) | GA | 117 (41) | |||||||||
| LSC | 201 | AA | 11 (6) | 200 | CC | 100 (51) | 188 | CT | 80 (42) | 199 | AA | 19 (9) | ||||
| AG | 77 (38) | TT | 18 (9) | CC | 100 (53) | GG | 98 (49) | |||||||||
| GG | 113 (56) | TC | 82 (40) | TT | 8 (5) | GA | 82 (42) | |||||||||
| LSCC | 153 | AA | 11 (7) | 153 | CC | 82 (54) | 153 | CT | 59 (41) | 152 | AA | 19 (9) | ||||
| AG | 49 (32) | TT | 13 (9) | CC | 80 (55) | GG | 79 (52) | |||||||||
| GG | 93 (61) | TC | 58 (37) | TT | 6 (4) | GA | 59 (39) | |||||||||
| Other | 47 | AA | 6 (13) | 47 | CC | 22 (47) | 47 | CT | 19 (46) | 47 | AA | 10 (21) | ||||
| AG | 19 (40) | TT | 10 (21) | CC | 18 (44) | GG | 19 (40) | |||||||||
| GG | 22 (47) | 0.447 | TC | 15 (32) | 0.237 | TT | 4 (10) | 0.269 | GA | 18 (39) | 0.592 | |||||
| TNM Stages | ||||||||||||||||
| I | 19 | AA | 0 (0) | 19 | CC | 11 (58) | 19 | CT | 7 (37) | 19 | AA | 1 (5) | ||||
| AG | 9 (47) | TT | 1 (5) | CC | 11 (58) | GG | 10 (53 | |||||||||
| GG | 10 (53) | TC | 7 (37) | TT | 1 (5) | GA | 8 (42) | |||||||||
| II | 24 | AA | 3 (13) | 24 | CC | 8 (33) | 22 | CT | 12 (55) | 24 | AA | 4 (17) | ||||
| AG | 10 (41) | TT | 4 (17) | CC | 8 (36) | GG | 8 (33) | |||||||||
| GG | 11 (46) | TC | 12 (50) | TT | 2 (9) | GA | 12 (50) | |||||||||
| III | 60 | AA | 4 (7) | 60 | CC | 28 (47) | 57 | CT | 27 (47) | 59 | AA | 6 (10) | ||||
| AG | 24 (40) | TT | 7 (12) | CC | 27 (47) | GG | 26 (44) | |||||||||
| GG | 32 (53) | TC | 25 (41) | TT | 3 (6) | GA | 27 (46) | |||||||||
| IV | 67 | AA | 5 (7) | 67 | CC | 31 (46) | 60 | CT | 26 (43) | 60 | AA | 10 (15) | ||||
| AG | 23 (34) | TT | 9 (13) | CC | 31 (52) | GG | 31 (46) | |||||||||
| GG | 39 (58) | 0.565 | TC | 27 (41) | 0.785 | TT | 3 (5) | 0.678 | GA | 26 (39) | 0.761 | |||||
| Smoking | ||||||||||||||||
| Yes | 523 | AA | 31 (7) | 522 | CC | 255 (49) | 494 | CT | 227 (46) | 484 | AA | 220 (46) | ||||
| AG | 203 (38) | TT | 47 (9) | CC | 247 (50) | GG | 249 (51) | |||||||||
| GG | 289 (55) | TC | 220 (42) | TT | 20 (4) | GA | 15 (3) | |||||||||
| No | 389 | AA | 22 (6) | 389 | CC | 205 (53) | 374 | CT | 156 (42) | 370 | AA | 149 (42) | ||||
| AG | 143 (37) | TT | 28 (7) | CC | 202 (54) | GG | 207 (54) | |||||||||
| GG | 224 (57) | 0.782 | TC | 156 (40) | 0.444 | TT | 16 (4) | 0.459 | GA | 14 (4) | 0.467 | |||||
| Family history | ||||||||||||||||
| Yes | 165 | AA | 7 (4) | 165 | CC | 85 (52) | 157 | CT | 69 (44) | 165 | AA | 12 (7) | ||||
| AG | 55 (33) | TT | 10 (6) | CC | 83 (53) | GG | 82 (50) | |||||||||
| GG | 103 (63) | TC | 70 (42) | TT | 5 (3) | GA | 71 (43) | |||||||||
| No | 745 | AA | 46 (6) | 744 | CC | 369 (50) | 708 | CT | 314 (44) | 741 | AA | 71 (10) | ||||
| AG | 294 (40) | TT | 68 (9) | CC | 361 (51) | GG | 354 (48) | |||||||||
| GG | 405 (54) | 0.152 | TC | 307 (41) | 0.414 | TT | 33 (5) | 0.75 | GA | 316 (42) | 0.64 | |||||
HPTP = histopathological types; LAC = lung adenocarcinoma; LSC = lung squamous carcinoma; LSCC = lung small cell carcinoma; GT = genotype; Total = number of subjects examined
Other: other malignant tumor types of lung, hamartoma(3), carcinoma sareomatodes(1), undifferentiated carcinoma(3), adenosquamous carcinoma(14), mixed carcinoma(5), mucoepidermoid carcinoma(1), carcinoid tumor(2), tumor type undiagnosable(18)
Discussion
In this study, we investigated the association between lung cancer and these functional polymorphisms at 2q33 predisposing to the risk of ESCC. The patients with lung cancer we studied came from high-incidence regions of ESCC, where exhibits a considerable high-incidence of lung cancer as well. Of all 20 SNPs, 6 SNPs were risk loci of ESCC, 12 SNPs were susceptibility loci of LC and only 2 SNPs were associated with GC. Interestingly, 4 of the 6 ESCC susceptibility SNPs showed significant association with LC in high-incidence areas of ESCC in northern China. All the ESCC patients associated with the six SNPs were Chinese people, and majority of them were from high-incidence area of ESCC in China. The fact may reveal lung cancer and ESCC may share many similar genetic variants. However, none of the 12 LC susceptibility SNPs showed significant association with LC in this study. Only 3 LC SNPs were from Chinese, other 9 of them were from foreigners. The result may indicate that there are significant regional and ethnic differences in susceptibility to lung cancer.
Our data revealed that four functional polymorphisms at 2q33 (rs13016963, rs3769823, rs10931936, rs2244438) out of 20 SNPs were associated with the risk of lung cancer (P< 0.01) and ESCC of Chinese Han as well. However, we did not find evidence of significant associations between overall survival of lung cancer and any of the four SNPs at 2q33.
Unlike highly penetrant germline mutations causing ‘hereditary’ cancers, such as CDKN2A mutations in melanoma and BRCA1 mutations in breast cancer, lung cancer and ESCC belong to ‘sporadic’ (‘non-hereditary’) malignant phenotype but a number of recent studies of heritability assessment suggests that these phenotypes (include twelve cancer types) of sporadic tumors share common genetic variants [24]. In other words, the genetic variants in cancer susceptibility play important roles in several common cancers and more loci of remaining polygenic components should be discovered continuously. The effects of genes and common environmental factors on heritability should not simply be distinguished [24]. In fact, more novel cancer susceptibility loci identified by GWAS and contribution of all genetic variants to malignant disease should be simultaneously taken into consideration instead of just few known loci [9, 25, 26].
Numerous studies investigated whether variation in the CASP8 [27, 28] gene region alters cancer risk, including cancers of lung [29], breast [30], pancreas [31], non-Hodgkin lymphoma [32], head and neck [3]. We identified two new loci (rs3769823, rs10931936) mapping to a genomic region harboring CASP8 which associated with risk of both ESCC and lung cancer of Chinese Han. We are the first to report that SNPs mapping to ALS2CR12 and TRAK2 were significantly associated with ESCC and lung cancer of Chinese Han [33, 34]. Overall survival of allele rs10931936 TT genotype was increased compared with CT and CC genotype groups but without statistical significance (Fig 2, log-rank P = 0.101; Table 4). The cause may be due to the withdrawal of some cases from our follow-up and only informative 29 cases available for analysis. In addition, genotype CT in rs10931936 was borderline significantly associated with an increased risk in male subgroup (Table 5). Therefore, rs10931936 genotype group that map to CASP8 need further investigation to elucidate [9]. Further research should focus on the association between functional polymorphisms at 2q33 (rs13016963, rs3769823, rs10931936, rs2244438) and risk of lung cancer from different ethnics, other tumor types and as well as the association with chemotherapy treatments in China.
So far, at least three studies on GWAS reported that rs8034191 was involved in the susceptibility to lung cancer [15–17]. However, our data from particular Chinese region found that rs8034191 was not associated with the risk of lung cancer. Furthermore, other investigations provided similar evidence that other SNPs may not modify the genetic risk of lung cancer, which include rs8034191, rs4975616, rs2352028, rs1926293 [35–38]. The discrepancy could be due to differences of ethnic, region, genotyping platform or study sizes.
The limitations of the present study are threefold. First, our analysis was only limited to a couple of regions in Northern China. Second, the sample size in our validation phase was smaller than the original studies. Third, the follow-up time was short for overall survival investigation.
Conclusions
Our present results suggest that four genetic variants in 2q33 may contribute to the susceptibility to both lung and esophageal cancers in Han Chinese. However, we did not find an association between these four lung cancer risk variants with survival. In addition, our findings also showed that 16 common genetic variants that have been previously identified in lung cancer, ESCC or GA by GWAS were not associated with lung cancer susceptibility in the Han Chinese population.
Supporting information
(ZIP)
Acknowledgments
Our study was supported by the National Natural Science Foundation of China, and the Guangdong Provincial People’s Government of the Joint Natural Science Fund (U1301227) and Major Project of Science and Technology of Henan Province (161100311300). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The study has been funded by the National Natural Science Foundation of China, and the Guangdong Provincial People’s Government of the Joint Natural Science Fund (U1301227) and Major Project of Science and Technology of Henan Province (161100311300). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Gavine PR, Wang M, Yu D, Hu E, Huang C, Xia J, et al. Identification and validation of dysregulated MAPK7 (ERK5) as a novel oncogenic target in squamous cell lung and esophageal carcinoma. BMC Cancer. 2015;15:454–63. 10.1186/s12885-015-1455-y . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bass AJ, Watanabe H, Mermel CH, Yu S, Perner S, Verhaak RG, et al. SOX2 is an amplified lineage-survival oncogene in lung and esophageal squamous cell carcinoma. Nat Genet. 2009;41(11):1238–42. 10.1038/ng.465 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sun T, Gao Y, Tan W, Ma S, Shi Y, Yao J, et al. A six-nucleotide insertion-deletion polymorphism in the CASP8 promoter is associated with susceptibility to multiple cancers. Nat Genet. 2007;39(5):605–13. 10.1038/ng2030 . [DOI] [PubMed] [Google Scholar]
- 4.Nandy N, Dasanu CA. Incidence of second primary malignancies in patients with esophageal cancer: a comprehensive review. Curr Med Res Opin. 2013;29(9):1055–65. 10.1185/03007995.2013.816276 . [DOI] [PubMed] [Google Scholar]
- 5.Delahaye-Sourdeix M, Oliver J, Timofeeva MN, Gaborieau V, Johansson M, Chabrier A, et al. The 12p13.33/RAD52 locus and genetic susceptibility to squamous cell carcers of upper aerodigestive tract. PloS One. 2015;10(3):3 10.1371/journal.pone.0117639 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tan W, Chen GF, Xing DY, Song CY, Kadlubar FF, Lin DX. Frequency of CYP2A6 gene deletion and its relation to risk of lung and esophageal cancer in the Chinese population. Int J Cancer. 2001;95(2):96–101. . [DOI] [PubMed] [Google Scholar]
- 7.Huang XP, Zhou WH, Zhang YF. Genetic variation in the IGF-IGFR-IGFBP axis confer susceptibility to lung and esophageal cancer. Genetic Mol Res. 2014; 13(1):2107–19. 10.4238/2014.January.24.17 . [DOI] [PubMed] [Google Scholar]
- 8.Sato N, Koinuma J, Fujita M, Hosokawa M, Ito T, Tsuchiya E, et al. Activation of WD repeat and high-mobility group box DNA binding protein 1 in pulmonary and esophageal carcinogenesis. Clin Cancer Res. 2010; 16(1):226–39. 10.1158/1078-0432.CCR-09-1405 . [DOI] [PubMed] [Google Scholar]
- 9.Abnet CC, Wang Z, Song X, Hu N, Zhou FY, Freedman ND, et al. Genotypic variants at 2q33 and risk of esophageal squamous cell carcinoma in China: a meta-analysis of genome-wide association studies. Human Molecular Genetics. 2012; 21(9): 2132–41. 10.1093/hmg/dds029 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang LD, Zhou FY, Li XM, Sun LD, Song X, Jin Y, et al. Genome-wide association study of esophageal squamous cell carcinoma in Chinese subjects identifies susceptibility loci at PLCE1 and C20orf54. Nat Genet. 2010; 42(9): 759–63. 10.1038/ng.648 . [DOI] [PubMed] [Google Scholar]
- 11.Abnet CC, Freedman ND, Hu N, Wang Z, Yu K, Shu XO, et al. A shared susceptibility locus in PLCE1 at 10q23 for gastric adenocarcinoma and esophageal squamous cell carcinoma. Nat Genet. 2010; 42(9): 764–7. 10.1038/ng.649 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hinkes B, Wiggins RC, Gbadegesin R, et al. Positional cloning uncovers mutations in PLCE1 responsible for a nephrotic syndrome variant that may be reversible. Nat Genet. 2006; 38(12): 1397–405. 10.1038/ng1918 . [DOI] [PubMed] [Google Scholar]
- 13.Li Y, Sheu CC, Ye Y, de Andrade M, Wang L, Chang SC, et al. Genetic variants and risk of lung cancer in never smokers: a genome-wide association study. Lancet Oncology. 2010; 11(4): 321–30. 10.1016/S1470-2045(10)70042-5 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu Z, Wu C, Shi Y, Guo H, Zhao X, Yin Z, et al. A genome-wide association study identifies two new lung cancer susceptibility loci at 13q12.12 and 22q12.2 in Han Chinese. Nat Genet. 2011; 43(8): 792–6. 10.1038/ng.875 . [DOI] [PubMed] [Google Scholar]
- 15.Hung RJ, Mckay JD, Gaborieau V, Boffetta P, Hashibe M, Zaridze D, et al. A susceptibility locus for lung cancer maps to nicotinic acetylcholine receptor subunit genes on 15q25. Nat Genet. 2008; 452(7187): 633–7. 10.1038/nature06885 . [DOI] [PubMed] [Google Scholar]
- 16.Broderick P, Wang Y, Vijayakrishnan J, Matakidou A, Spitz MR, Eisen T, et al. Deciphering the Impact of Common Genetic Variation on Lung Cancer Risk: A Genome-Wide Association Study. Cancer Research. 2009; 69(16): 6634–41. 10.1158/0008-5472.CAN-09-0680 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amos CI, Wu X, Broderick P, Gorlov IP, Gu J, Eisen T, et al. Genome-wide association scan of tag SNPs identifies a susceptibility locus for lung cancer at 15q25.1. Nat Genet. 2008; 40: 616–22. 10.1038/ng.109 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mechanic LE, Bowman ED, Welsh JA, Khan MA, Hagiwara N, Enewold L, et al. Common genetic variation in TP53 is associated with lung cancer risk and prognosis in African American and somatic mutation in lung tumors. Cancer Epidemiol Biomarkers Prev. 2007; 16(2): 212–22. . [DOI] [PubMed] [Google Scholar]
- 19.Zhou L, Yuan Q, Yang M. A functional germline variant in the P53 polyadenylation signal and risk of esophageal squamous cell carcinoma. Gene. 2012; 506(2): 295–7. 10.1016/j.gene.2012.07.007 . [DOI] [PubMed] [Google Scholar]
- 20.Shi Y, Hu Z, Wu C, Dai J, Li H, Dong J, et al. A genome-wide association study identifies new susceptibility loci for non-cardia gastric cancer at 3q13.31 and 5p13.1. Nat Genet 2011; 43(12): 1215–8. 10.1038/ng.978 . [DOI] [PubMed] [Google Scholar]
- 21.Wang Y, Broderick P, Webb E, Wu X, Vijayakrishnan J, Matakidou A, et al. Common 5p15.33 and 6p21.33 variants influence lung cancer risk. Nat Genet. 2008; 40(12): 1407–9. 10.1038/ng.273 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cote ML, Liu M, Bonassi S, Neri M, Schwartz AG, Christiani DC, et al. Increased risk of lung cancer in individuals with a family history of the disease: A pooled analysis from the international lung Cancer Consortium. Eur J Cancer. 2012; 48(13): 1957–68. 10.1016/j.ejca.2012.01.038 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ichinose J, Murakawa T, Hino H, Konoeda C, Inoue Y, Kitano K, et al. Prognostic impact of the current Japanese nodal classification on outcomes in resected non-small cell lung cancer. Chest. 2014; 146(3): 644–9. 10.1378/chest.14-0159 . [DOI] [PubMed] [Google Scholar]
- 24.Lu Y, Ek WE, Whiteman D, Vaughan TL, Spurdle AB, Easton DF, et al. Most common ‘sporadic’ cancers have a significant germline genetic component. Human Molecular Genetics. 2014; 23(22): 6112–8. 10.1093/hmg/ddu312 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chung CC, Chanock SJ. Current status of genome-wide association studies in cancer. Hum Genet. 2011; 130(1): 59–78. 10.1007/s00439-011-1030-9 . [DOI] [PubMed] [Google Scholar]
- 26.Wu C, Wang Z, Song X, Feng XS, Abnet CC, He J, et al. Joint analysis of three genome-wide association studies of esophageal squamous cell carcinoma in Chinese populations. Nat Genet. 2014; 46(9): 1001–6. 10.1038/ng.3064 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao Y, Sui X. and Ren H. From procaspase-8 to caspase-8: revisiting structural functions of caspase-8. J Cell Physiol. 2010; 225(2): 316–20. 10.1002/jcp.22276 . [DOI] [PubMed] [Google Scholar]
- 28.Xue LY, Hu N, Song YM, Zou SM, Shou JZ, Qian LX, et al. Tissue microarray analysis reveals a tight correlation between protein expression pattern and progression of esophageal squamous cell carcinoma. BMC Cancer. 2006; 22(6): 296–302. 10.1186/1471-2407-6-296 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Son JW, Kang HK, Chae MH, Choi JE, Park JM, Lee WK, et al. Polymorphisms in the caspase-8 gene and the risk of lung cancer. Cancer Genet Cytogenet. 2006; 169(2): 121–7. 10.1016/j.cancergencyto.2006.04.001 . [DOI] [PubMed] [Google Scholar]
- 30.Cox A, Dunning AM, Garcia-Closas M, Balasubeamanian S, Reed MW, Pooley KA, et al. A common coding variant in CASP8 is associated with breast cancer risk. Nat Genet. 2007; 39(3): 352–8. 10.1038/ng1981 . [DOI] [PubMed] [Google Scholar]
- 31.Couch FJ, Wang X, McWilliams RR, Bamlet WR, de Andrade M, Petersen GM. Association of breast cancer susceptibility variants with risk of pancreatic cancer. Cancer Epidemiol Biomarker Prev. 2009; 18(11): 3044–8. 10.1158/1055-9965.EPI-09-0306 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lan Q, Morton LM, Armstrong B, Hartge P, Menashe I, Zheng T, et al. Genetic variation in caspase genes and risk of non-Hodgkin lymphoma: a pooled analysis of 3 population-based case-control studies. Blood. 2009; 114(2): 264–7. 10.1182/blood-2009-01-198697 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hadano S, Hand CK, Osuga H, Yanagisawa Y, Otomo A, Devon RS, et al. A gene encoding a putative GTPase regulator is mutated in familial amyotrophic lateral sclerosis 2. Nat Genet. 2001; 29(2): 166–73. 10.1038/ng1001-166 . [DOI] [PubMed] [Google Scholar]
- 34.Grishin A, Li H, Levitan ES and Zaks-Makhina E. Identification of gamma-aminobutyric acid receptor-interacting factor 1 (TRAK2) as a trafficking factor for the K+ channel Kir2. J Biol Chem. 2006; 281(40): 30104–11. 10.1074/jbc.M602439200 . [DOI] [PubMed] [Google Scholar]
- 35.He P, Yang XX, He XQ, Chen J, Li FX, Gu X, et al. CHRNA3 polymorphism modifies lung adenocarcinoma risk in the Chinese Han population. Int J Mol Sci. 2014; 15(4): 5446–57. 10.3390/ijms15045446 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun Y, Zhang YJ, Kong XM, et al. No association of XRCC1 and CLPTM1L polymorphisms with non-small cell lung cancer in a non-smoking Han Chinese population. Asian Pac J Cancer Prev. 2013; 14(9): 5171–4. . [DOI] [PubMed] [Google Scholar]
- 37.Liu L, Zhong R, Zou L, Fu J, Zhu B, Chen W, et al. Variants in the 5'-upstream region of GPC5 confer risk of lung caner in never smokers. Cancer Epidemiol. 2014; 38(1): 66–72. 10.1016/j.canep.2013.12.009 . [DOI] [PubMed] [Google Scholar]
- 38.Rafnar T, Sulem P, Besenbacher S, Gudbjartsson DF, Zanon C, Gudmundsson J, et al. Genome-wide significant association between a sequence variant at 15q15.2 and lung cancer risk. Cancer Res. 2011; 71(4): 1356–61. 10.1158/0008-5472.CAN-10-2852 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(ZIP)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.