Abstract
Background
Non-communicable disease (NCD) prevention strategies now prioritise four major risk factors: food, tobacco, alcohol and physical activity. Dietary salt intake remains much higher than recommended, increasing blood pressure, cardiovascular disease and stomach cancer. Substantial reductions in salt intake are therefore urgently needed. However, the debate continues about the most effective approaches. To inform future prevention programmes, we systematically reviewed the evidence on the effectiveness of possible salt reduction interventions. We further compared “downstream, agentic” approaches targeting individuals with “upstream, structural” policy-based population strategies.
Methods
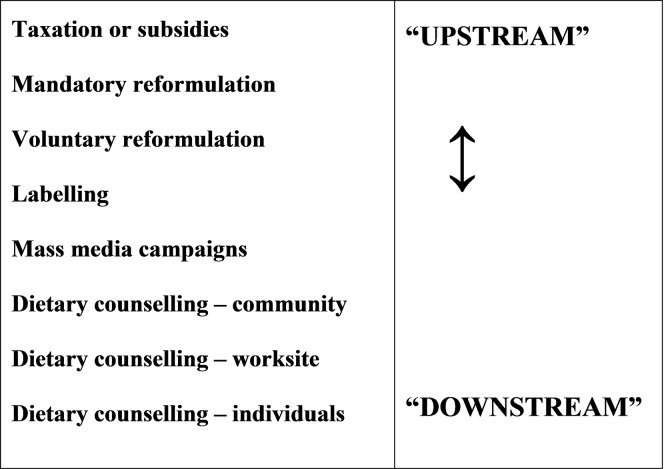
We searched six electronic databases (CDSR, CRD, MEDLINE, SCI, SCOPUS and the Campbell Library) using a pre-piloted search strategy focussing on the effectiveness of population interventions to reduce salt intake. Retrieved papers were independently screened, appraised and graded for quality by two researchers. To facilitate comparisons between the interventions, the extracted data were categorised using nine stages along the agentic/structural continuum, from “downstream”: dietary counselling (for individuals, worksites or communities), through media campaigns, nutrition labelling, voluntary and mandatory reformulation, to the most “upstream” regulatory and fiscal interventions, and comprehensive strategies involving multiple components.
Results
After screening 2,526 candidate papers, 70 were included in this systematic review (49 empirical studies and 21 modelling studies). Some papers described several interventions. Quality was variable. Multi-component strategies involving both upstream and downstream interventions, generally achieved the biggest reductions in salt consumption across an entire population, most notably 4g/day in Finland and Japan, 3g/day in Turkey and 1.3g/day recently in the UK. Mandatory reformulation alone could achieve a reduction of approximately 1.45g/day (three separate studies), followed by voluntary reformulation (-0.8g/day), school interventions (-0.7g/day), short term dietary advice (-0.6g/day) and nutrition labelling (-0.4g/day), but each with a wide range. Tax and community based counselling could, each typically reduce salt intake by 0.3g/day, whilst even smaller population benefits were derived from health education media campaigns (-0.1g/day). Worksite interventions achieved an increase in intake (+0.5g/day), however, with a very wide range. Long term dietary advice could achieve a -2g/day reduction under optimal research trial conditions; however, smaller reductions might be anticipated in unselected individuals.
Conclusions
Comprehensive strategies involving multiple components (reformulation, food labelling and media campaigns) and “upstream” population-wide policies such as mandatory reformulation generally appear to achieve larger reductions in population-wide salt consumption than “downstream”, individually focussed interventions. This ‘effectiveness hierarchy’ might deserve greater emphasis in future NCD prevention strategies.
Introduction
Non-communicable diseases (NCDs) kill over 35 million people annually. Common cancers, cardiovascular diseases, diabetes, respiratory diseases and dementia together now account for over two thirds of the entire global burden of disability and death.[1,2] These NCDs are mainly attributable to just four major risk factors. Furthermore, the contribution from poor diet exceeds the combined contribution from alcohol, tobacco and physical inactivity.[3] This poor diet mainly reflects a predominantly unhealthy global food environment, dominated by processed foods high in sugar, saturated fat, trans-fat and, crucially, salt.[3]
In the UK and other high income countries, over 70% of dietary salt is consumed in processed foods such as bread, breakfast cereals, processed meats, snack foods, soups and sauces.[4–6] This food environment contributes to excessive salt intake among adults, on average 10g/day or more,[7] far in excess of what the body actually needs.[8] High salt intake is a major risk factor for increasing blood pressure,[9–11] cardiovascular disease,[12–14] stroke,[15,16] and stomach cancer.[17–19] Moreover, a reduction in salt intake would substantially reduce this risk.[10]
WHO recommends a maximum adult salt intake of 5g/day.[20] Different strategies and policy options have been proposed to achieve this goal. Individual level interventions often involve behavioural approaches, for example dietary counselling, leaflets or medical advice. These are sometimes termed “downstream” or “agentic” interventions, and are dependent on the individual responding. [21,22] Conversely, “upstream” structural interventions take place at the population level and typically involve policies such as regulatory approaches, taxes or subsidies. Finally, intermediate interventions target subgroups in worksites, schools or communities.[23]
National salt reduction strategies were identified in 75 countries in 2015, a substantial increase from 32 in 2010.[24] However, the debate regarding the most effective and acceptable salt reduction strategy continues.
Notable policy approaches have been seen in Finland,[25] Japan,[26] and more recently, the United Kingdom.[27] In the UK, a combination of awareness campaigns, agreed target settings, voluntary reformulation from industry and population monitoring of salt consumption have led to a 1.4g per day reduction in population salt intake between 2001 and 2011 (the campaign started in 2003).[27] However, health inequalities in salt consumption have persisted.[28,29] Furthermore, the introduction of the UK Responsibility Deal in 2010 shifted emphasis to ‘downstream’ interventions, coupled with ineffective voluntary agreements and, controversially, the direct involvement of the industry in policy decisions.[30,31]
Geoffrey Rose famously advocated population wide approaches rather than targeting high-risk individuals.[32] Furthermore, there seems to be some evidence for a public health ‘effectiveness hierarchy’ whereby “upstream” structural interventions consistently achieve larger improvements in population health, are more equitable and often reduce health inequalities[33,34] compared to “downstream” agentic interventions targeting individuals, for instance in tobacco control and alcohol policies.[35,36] Emerging evidence suggests that a comparable effectiveness hierarchy might also exist for salt reduction strategies, whereby upstream interventions apparently achieve bigger reductions in salt intake.[37,38]. To test this hypothesis and hence inform future preventive health strategies, we have systematically reviewed the evidence for studies focusing on the effectiveness of salt interventions to reduce salt intake.
Methods
Study design
We conducted a systematic review of interventions intended to decrease population dietary salt intake. To ensure proper conduct, we adhered to the PRISMA checklist (Preferred Reporting Items for Systematic Reviews and Meta-Analyses)(S1 Table).[39] We used a narrative synthesis and formally investigated evidence to support or refute an effectiveness hierarchy. The research protocol can be found in S1 File.
Search strategy
We first identified exemplar studies to define and refine search terms needed for targeted searches. The search strategy consisted of a combination of four sets of key words:
1) salt, sodium; 2) health promotion, nutrition education, campaigns, dietary counselling, regulation, legislation, tax, self-regulation, reformulation, social marketing, promotion, provision, labelling, marketing control, primary care advice, food industry; 3) public policy, health policy, nutrition policy, policies, interventions, strategies, initiatives, programmes, policy option, actions; and 4) effectiveness, effect, intake, consumption, reduction, cost-benefit analysis, and cardiovascular diseases.
A pilot search was conducted to determine appropriate databases, identify relevant studies and highlight potential issues to be addressed. This process identified six databases which were then used for the targeted searches: Ovid MEDLINE, Science Citation Index, SCOPUS, Cochrane Database of Systematic Reviews, The Campbell Collaboration Library of Systematic Reviews and the CRD Wider Public Health database. We searched for all studies published in the last four decades (from 1975 onwards). The final searches were conducted on 30 October 2015. All papers identified by the searches were imported into the Zotero data management programme to identify duplicates and help screen titles, abstracts and full texts as appropriate. The reference lists of included studies were scanned for potential additional papers and topic experts (FPC and SC) were also consulted for additional data sources.[40,41]
Study selection and inclusion criteria
Studies were included if they investigated the effectiveness of specific interventions on population dietary salt intake and contained quantitative outcomes. Only studies in English were included. We included a wide range of study designs including meta-analyses, trials, observational studies and natural experiments. Empirical studies and modelling studies were analysed separately, in view of their profound differences. The retrieved studies were assessed using the PICOS approach (Participants, Interventions, Comparators, Outcomes and Study design), summarised in Table 1. The primary outcome was salt intake (g/day). Studies reporting urinary sodium excretion (mmol/day) or sodium mg/day were converted to g/day. Where necessary, we simultaneously considered studies reporting solely on salt intake data in a specific population with the corresponding studies describing the interventions during that same time period.
Table 1. PICOS; Inclusion/exclusion criteria.
| Participants | |
| Include | Exclude |
| Studies for all age groups from all populations, from high-, middle- and low-income countries | Studies on animals, cells and pregnant women |
| Interventions | |
| Systematic Reviews and primary studies evaluating the effects of actions to promote salt reduction by government policy or adopted in specific real or experimental settings | Studies evaluating the effect of a general or specific diet |
| Comparators | |
| Systematic and non-systematic reviews where actions to promote salt reduction were evaluated or compared | No comparisons of different actions to promote salt reduction presented |
| Outcomes | |
| Primary outcome of interest was dietary salt intake (g/day). Studies including urinary sodium excretion as an outcome were converted to g/day. Secondary outcomes included changes in clinical/physiological indicators related to NCDs and behaviours associated with a healthy diet | Process evaluations reporting on implementation of interventions/policies without any quantitative outcome data; feasibility or acceptability without an assessment or primary outcomes (intake); studies on individuals as opposed to populations; data on cost only and BMI |
| Study design | |
| Primary studies, RCTs, Systematic Reviews (SRs), empirical observational studies, natural experiments, and modelling studies, secondary analysis, and before vs. after interventions | Commentary/opinion articles and purely qualitative evaluations with no quantitative assessment |
One reviewer (LH) conducted the searches; extracted potential papers and removed duplicates. Two reviewers (LH and AEG) then independently screened titles and abstracts for eligibility using the inclusion and exclusion criteria. Full text was retrieved for all papers deemed potentially eligible and these were also screened independently by the two reviewers. Any discrepancies were resolved by consensus or by involving the senior author (SC).
Data extraction and management
Pre-designed and pre-piloted tables were used to extract data from all included studies. To ensure that all relevant information was captured, extracted data included: first author; year of publication; funder(s); study aim(s); sample size; study design; methods; participants; policies analysed; geographical scope; length of follow-up; outcomes, effect and response; authors’ assessment of limitations and our own assessment of potential risk of bias. The sources referenced for the effect sizes used in each modelling study were also specified in the tables (recognising that some modelling studies are based on empirical studies, potentially some included in this review). This data extraction was done independently by two reviewers (LH and AEG).
Quality assessment of included studies
Two reviewers (LH and AEG) independently assessed the methodological quality of each study (poor, fair or good). We used the National Heart, Lung and Blood Institute (NHLBI) tools specific for each research design (i.e. RCTs, cross-sectional studies, before and after studies, and systematic reviews).[42] Several questions were asked for each study design (varying from 8 to 14) and depending on the points scored, the studies were labelled as good, fair or poor. However, we also took into consideration as to which questions points were allocated. For example, if an RCT scored 10 out of 14 points, but did not conduct an intention to treat analysis, it would be rated as fair rather than good. Modelling studies were independently assessed by two modelling experts (MOF & CK) using a different tool adapted from Fattore et al. (2014).[43] Discrepancies in quality assessment were reconciled by consensus or by involving a third, senior member of the team (SC or HB).
Data synthesis and effectiveness hierarchy continuum
The evidence was summarised as a narrative synthesis according to intervention type, ranging from downstream to upstream interventions, to facilitate comparisons between the interventions. Summary tables of the studies included in this review can be found in Tables 2–10 for empirical studies and Table 11 for modelling studies. A more detailed data extraction of these studies can be found in S2 Table. We defined UPSTREAM interventions as those targeting the entire population (not a subset, however large) and creating structural changes (effectively removing individual choice from the equation). This accorded with the Nuffield’s ladder taxonomy,[44] and with McLaren’s structural/agentic continuum.[21] Conversely, we defined DOWNSTREAM interventions as those where the principal mechanism of action is “agentic”, being dependent on an individual altering their behaviour.
Table 2. Dietary counselling (individuals).
| Study | Study type | Geographical scope | Aim and main outcomes | Policies analysed | Relevant results | Quality assessment |
|---|---|---|---|---|---|---|
| Hooper et al. (2002)45 | SR and meta-analysis of RCTs | US, Australia, New Zealand, UK | Aim: to assess the long term effects of advice to restrict dietary sodium in adults with and without hypertension. Outcomes: salt intake as measured by urinary sodium excretion | Dietary advice |
Meta-analysis (11 studies included). They found reductions in salt intake at both intermediate, <12 months (2.8g/day) and late follow up, 13–60 months (2.0g/day). |
Good |
| Appel et al. (2003)46 | Randomised trial | US |
Aim: to determine the effect on BP of 2 multicomponent, behavioral interventions Outcomes: salt intake as measured by urinary sodium excretion |
Dietary advice | Only the reduction in the established group differed significantly from that of advice only group. 24-hour dietary recall data indicated both behavioral interventions significantly reduced sodium intake in comparison with advice only group (P value = 0.01). Advice group • Baseline = 10.0g/day • 6 months = 8.8g/day • Mean difference = -1.2g/day Intervention group • Established: mean difference = -1.82 g/day • Established + DASH: mean difference = -1.83 g/day |
Good |
| Brunner et al. (1997)47 | Meta-analysis of RCTs | UK, US, Netherlands and Australia | Aim: to evaluate the effectiveness of dietary advice in primary prevention of chronic disease. Outcomes: salt intake | Dietary advice | Overall mean net reduction of 1.8g/day which is a 20% reduction in salt intake. The heterogeneity test was highly significant (P < .0005) for the 3- to 6-month trials, with a net reduction of 3.4 (95% CI = 45, 72) g/day. Summary effect of the two trials with SE was somewhat larger at 9–18 months than at 3–6 months. | Fair |
| Francis & Taylor (2009)48 | Randomised control group study | US | Aim: to implement a health-healthy diet-education programme. Outcomes: salt intake | Dietary counselling | Intervention salt consumption decreased significantly (P0.020) from record 1 to record 3. The reduction in control group participants’ sodium intake was not significant Intervention: (Mean ± SEM (g/day); P-value) • Record 1: 7.0 ± 0.5; 0.020e • Record 2: 5.9 ± 0.3; 0.067 • Record 3: 5.9 ± 0.4; 0.937 Control (Mean ± SEM (g/day), P-value) • Record 1: 6.2 ± 0.5; 0.323 • Record 2: 6.1 ± 0.4; 0.880 • Record 3: 5.7 ± 0.4; 0.284 Mean effect size:- 0.6g/day |
Fair |
| Parekh et al. (2012)49 | RCT | Australia | Aim: to evaluate the effectiveness of a minimal intervention on multiple lifestyle factors including diet using computer tailored feedback. Outcomes: salt intake (%) | Health promotion–computer tailored advice | Salt (%) Intervention +5.43 net change. Control +1.23 net change. Significant changes between groups were observed for reduced salt intake (OR 1.19, CI 1.05–1.38). The intervention group were 20% more likely to reduce salt intake | Fair |
| Petersen et al. (2013)50 | RCT | Australia | Aim: to investigate whether urinary sodium excretion can be reduced by educating people with T2DM to read food labels and choose low sodium products. Outcomes: salt intake | Nutrition education | Baseline reported salt intake: 6.8 ± 3.2 g/day Intervention • Baseline: 10.0 ± 0.7 • 3 months: 10.1 ± 0.7 • Change: +0.06 ± 0.9 Control • Baseline: 9.6 ± 0.9 • 3 months: 9.3 ± 0.7 • Change: -0.3 ± 0.8 There was no between group difference (p > 0.05) |
Fair |
| Kokanović et al. (2014)51 | Before and after study | Croatia |
Aim: to assess eating habits of adolescent population diagnosed with one or more cardiovascular risks before and after two months of individual dietary intervention Outcomes: salt intake |
Nutrition education | Difference in intake on initial and control examination statistically significant for intake of sodium p = 0.013. Salt intake g/day. Initial examination: 18.9d/day; Control examination: 15.4g/day; Difference: -3.5g/day (= -18.8%) |
Fair |
| Heino et al. (2000)52 | Prospective randomized trial | Finland | Aim: to examine sodium intake of 1-5-y-old children in a CHD prevention trial, focused on dietary fat modification. Outcomes: salt intake | Dietary counselling | Intervention children (+1.5g/day) • 13 months: 4.1 ± 1.2 • 3 years: 4.9 ± 1.2 • 5 years: 5.6 ± 1.3 Control children (+1.6g/day) • 13 months: 3.9 ± 1.4 • 3 years: 4.7 ± 1.3 • 5 years: 5.5 ± 1.4 No significant differences between the intervention and control group found |
Poor |
| Wang et al. (2013)53 | RCT | US |
Aim: one year dietary intervention study to examine patterns and amount of daily sodium intake among participants with metabolic syndrome Outcomes: salt intake |
Dietary counselling | Intervention arm at one year follow-up found participants who consumed sodium greater than 5.8g/day declined from 75% at baseline to 59%. Those consumed higher than 3.8g/day declined from 96% (at baseline) to 85%. Average salt intake decreased from 7.5 g/day at baseline to 6.4 g/day at one-year (P<0.001). At one-year visit, salt intake was consistently reduced; significant difference only observed between males (7.6± 0.4 g/day) and females (6.0 ± 0.2 g/day; p < 0.001) | Poor |
Table 10. Salt intake outcomes with interventions detailed in other publications.
| Study | Study type | Geographical scope | Aim and main outcomes | Policies analysed | Relevant results | Quality assessment |
|---|---|---|---|---|---|---|
| Laatikainen et al. (2006)25 | Cross-sectional population surveys | Finland |
Aim: to present trends in urinary sodium and potassium excretion from 1979 to 2002 Outcomes: salt intake as measured by urinary sodium excretion |
1) Reformulation 2) Mass media campaigns 3) Labelling |
Between 1979 and 2002 salt intake as measured by sodium excretion decreased from over 12.7g/day to less than 9.8g/day among men and from nearly 10.4 to less than 7.5g/day among women. In 1979 the most educated North Karelian men had lower salt intake compared to the least educated being 11.4 g in the highest education tertile and 13.1 g in the lowest tertile. Respectively, in 2002, the salt intake in southwestern Finland among women in the highest education tertile was 6.7g compared to 8.1g in the lowest tertile | Good |
| Otsuka et al. (2011)101 | Longitudinal study | Japan |
Aim: to describe salt intake over 8 years according to age groups. Also to examine whether salt intake changes over time in middle-aged and elderly Japanese subjects Outcomes: salt intake |
In stratified analyses by age, mean salt intake in men decreased 0.08 g/year among 40- to 49-year-olds, 0.09 g/year among 50- to 59-year-olds, 0.16 g/year among 60- to 69-year-olds, and 0.14 g/year among 70- to 79-year-olds. For women, mean salt intake decreased 0.08 g/year among 70- to 79-year-olds (P0.098). | Fair | |
| Du et al. (2014)102 | Ongoing open cohort study | China | Aim: to analyse the patterns and trends of dietary sodium intake, potassium intake and the Na/K ratio and their relations with incident hypertension. Outcomes: salt intake as measured per 24h dietary recalls | Labelling & media campaign | Salt intake decreased from 16.5g/day in 1991 to 11.8g/day in 2009 | Fair |
| Miura et al. (2000)103 | Report | Japan |
Aim: to present the status of salt consumption, salt-reducing measures/guidance methods in individual and population strategies to reduce salt intake Outcomes: salt intake |
The National Health and Nutrition Survey in 2010 reported that the mean salt intake in adults was 10.6 g/day. There was an ~4 g decrease in comparison with that in 1972 (14.5 g), when salt intake was investigated for the first time in the National Nutrition Survey |
Poor |
Table 11. Modelling studies included in the systematic review.
| Study | Study type | Geographical scope | Aim and main outcomes | Policies analysed | Relevant results | Quality assessment | |
|---|---|---|---|---|---|---|---|
| Salt | Cobiac et al. (2010)54 | Modelling study | Australia | Aim: to evaluate population health benefits and cost-effectiveness of interventions for reducing salt in the diet. Outcomes: DALYs and proportion of DALYs averted | 1) Voluntary reformulation 2) Mandatory reformulation 3) Dietary advice |
Mandatory reformulation: could avert 18% of the disease burden (110,000 DALYs). Dietary advice: might avert less than 0.5% of the disease burden (1,700–2,600 DALYs) Voluntary Reformulation: modelled for breads, margarines and cereals would avert less than 1% of the disease burden(5,300 DALYs) Voluntary reformulation and mandatory salt reduction had a 100% probability of being dominant (i.e., cost saving to the health sector) under all modelled scenarios. Dietary advice had zero probability of being cost-effective. |
Good |
| Cobiac et al. (2012)55 | Modelling study | Australia |
Aim: to evaluate the optimal mix of lifestyle, pharmaceutical and population-wide interventions for primary prevention of cardiovascular disease Outcomes: DALYs |
1) Mandatory reformulation 2) Community heart health programme 3) Dietary advice |
Mandatory reformulation in breads, margarines and cereals is easily the most effective and cost-effective strategy for primary prevention of CVD; (80,000 DALYs) and cost saving (dominant). Community heart health program (3,000 DALYs; $44,000) Dietary advice (180–370 DALYs) are least cost-effective of all the primary prevention strategies ($ 1,000,000 to $1,400,000) |
Good | |
| Nghiem et al. (2015)56 | Modelling study | New Zealand |
Aim: to compare the impact of eight sodium reduction interventions Outcomes: QALYs |
1) Dietary counselling 2) Labelling 3) Mandatory 3G reformulation (breads, processed meats and sauces) 4) Mandatory ‘All’ reformulation 5) UK package (multiple policies) 6) Mass media campaign 7) Tax |
QALYs gained in order of effectiveness: 1) Salt tax (195,000) 2) Mandatory ‘all’ reformulation (110,000) 3) UK package (85,100) 4) Mandatory 3G reformulation (61,700) 5) Mass media campaign as per the UK one (25,200) 6) Voluntary labelling (7,900) 7) Dietary counselling (200) |
Good | |
| Collins et al. (2014)68 | Modelling study | UK | Aim: to evaluate the cost-effectiveness of four population health policies to reduce dietary salt intake on an English population to prevent coronary heart disease (CHD). Outcomes: life years gained and salt intake | 1) Health promotion campaign 2) Labelling 3) Voluntary salt reformulation 4) Mandatory salt reformulation |
Primary outcomes: Salt intake reductions: Campaign = 0.16g/d; Labelling = 0.16g/d; Voluntary reformulation = 1.21g/d; Mandatory reformulation = 1.62g/d Secondary outcomes: Gains: Change4life and labelling might each gain approximately = 1960 life-years; Voluntary reformulation = 14,560 life-years; and Mandatory reformulation 19,320 life-years. |
Good | |
| Gillespie et al. (2015)69 | Modelling study | England |
Aim: to forecast the potential impact on English adults of policies implemented during the 2015 UK parliament, projecting the health consequences to 2025 Outcomes: salt intake, CHD deaths prevented, LYG |
1) Mandatory reformulation 2) Voluntary reformulation 3) Social marketing 4) Nutrition labelling |
Mandatory reformulation (30% reduction in salt content) • Salt intake = -1.45g/day • SBP = -0.81mmHg • CHD deaths = 4.500 prevented or postponed • LYG = 44.000 Mandatory reformulation (10% reduction in salt content) • Salt intake = -0.48g/day • SBP = -0.27mmHg • CHD deaths = 1.500 prevented or postponed • LYG = 15.000 Voluntary reformulation • Salt intake = -0.48g/day • SBP = -0.27mmHg • CHD deaths = 1.500 prevented or postponed • LYG = 14.000 Social marketing (50% impact) • Salt intake = -0.13g/day • SBP = -0.078mmHg • CHD deaths = 400–500 prevented or postponed • LYG = 5.000 Social marketing (10% impact) • Salt intake = -0.027g/day • SBP = -0.015mmHg • CHD deaths = 100 prevented or postponed • LYG = 780 Nutrition labelling (50% impact) • Salt intake = -0.16g/day • SBP = -0.091mmHg • CHD deaths = 500 prevented or postponed • LYG = 5.000 Nutrition labelling (10% impact) • Salt intake = -0.031g/day • SBP = -0.018mmHg • CHD deaths = 100 prevented or postponed • LYG = 1.000 |
Good | |
| Wilcox et al. (2014)70 | Modelling study | Syria | Aim: to present a cost-effectiveness analysis of salt reduction policies to lower coronary heart disease in Syria. Outcomes: salt intake, deaths prevented and life years gained | 1) Health promotion 2) Labelling 3) Reformulation |
Health promotion campaign– 5% reduction in salt intake. 252 deaths prevented; 5.679 LYG Labelling– 10% reduction in salt intake. 497 deaths prevented; 11.192 LYG Reformulation– 10% reduction in salt intake. 497 deaths prevented; 11.192 LYG Reformulation + HP & Reformulation + Labelling– 15% reduction in salt intake. 735 deaths prevented; 16.543 LYG All 3 policies– 30% reduction in salt intake. 1.413 deaths prevented; 31.674 LYG |
Good | |
| Mason et al. (2014)71 | Modelling study | Tunisia, Syria, Palestine and Turkey |
Aim: to present an economic evaluation of population based salt reduction policies in Tunisia, Syria, Palestine and Turkey. Outcomes: life years gained |
1) Health promotion campaign (HP) 2) Labelling (L) 3) Mandatory reformulation (R) |
Tunisia: HP = 1.151 LYG; L = 2.272 LYG; R = 2.272 LYG; All 3 policies = 6.455 LYG Syria: HP = 5.679 LYG; L = 11.192 LYG; R = 11.192 LYG; All 3 policies = 31.674 LYG Palestine: HP = 479 LYG; L = 945 LYG; R = 945 LYG; All 3 policies = 2.682 LYG Turkey: HP = 68.816 LYG; L = 135.221 LYG; R = 135.221 LYG; All 3 policies = 199.303 LYG |
Fair | |
| Pietinen et al. (2008)74 | Modelling study | Finland | Aim: to estimate the impact of choosing food products labelled either as low or high in salt Outcomes: salt intake | Salt labelling | If the entire population were to choose low-salt breads, cheeses, processed meat and fish, fat spreads, and breakfast cereals, then salt intake could be lowered by 1.5 g in men and by 0.9 g in women. If everybody was to select high-salt products, then salt intake would go up by 1.9 g in men and by 1.2 g in women. Thus, the potential difference between the low and the high alternatives would be 3.4 g in men and 2.9 g in women. If all prepared foods had a reduced salt content, the mean salt intake would go further down by 2.3 g in men and by 1.7 g in women. Excluding under reporters, the mean salt intake was 11.1 g in men and would go down to 9.5 g if all men chose lightly salted products and further down to 6.8 g if also all prepared foods would have a lower salt content. In women, the respective numbers are 7.8, 6.7 and 4.9 g | Fair | |
| Temme et al. (2010)75 | Modelling study | Netherlands |
Aim: to evaluate the effects of changed food compositions according to health logo criteria on the intake of saturated fat, sugar and sodium Outcomes: salt intake |
Labelling (health logos) | At baseline salt intake was 7.3 (95% CI 2.8, 2.9) g/day. For salt, in a 100% market share scenario (scenario II), salt reduction expected is 0.3g/day (4% reduction; non-significant). In scenario III, when all non-complying foods are replaced with foods complying with health logo criteria, sodium intake reduced by 23% to 5.5g/d (-1.8g/day). | Fair | |
| De Menezes et al. (2013)76 | Modelling study | Brazil | Aim: to evaluate the impact of introducing products that are in agreement with the Choices criteria in the usual diet. Outcomes: salt intake | Food Labelling | Salt would still be considered an important reduction, 36% in relation to the typical menus (TM), but it would be 7.6 g/day, which is above the recommended by the program (6.5 g/day). Salt (g/day) • Typical menus (TM): 11.9 ± 1.2 • Choices menus (CM): 6.3 ± 0.1 • Choices menus energy (CME)–same as CM, but adjusted for energy of TM: 7.6 ± 0.9 |
Fair | |
| Roodenburg et al. (2013)77 | Modelling study | The Netherlands |
Aim: to evaluate these nutritional criteria by investigating the potential effect on nutrient intakes Outcomes: salt intake |
Labelling | A reduction of -23% for sodium was seen for sodium compared to the ‘actual scenario’. Salt (g/day) • Actual: 7.4 • Choices: 5.7 • Choices energy adjusted: 6.5 • Snacks (partially replaced): 5.8 • Snacks (not replaced): 5.9 |
Fair | |
| Choi et al. (2015)41 | Modelling study | US |
Aim: to estimate the cardio-vascular impact of the expanded NSRI among different segments of the US population and under varying possible producer and consumer responses to the initiative Outcomes: sodium intake, MIs, stroke and hypertension |
Reformulation (restaurants and manufacturers) | Restaurants and manufacturers reaching agreed upon sodium targets. Expansion of the initiative to ensure all restaurants and manufacturers reach agreed-upon sodium targets would be expected to avert from 0.9 to 3.0 MIs (a 1.6%–5.4% reduction) and 0.5 to 2.8 strokes (a 1.1%–6.2% reduction) per 10,000 Americans per year over the next decade, after incorporating consumption patterns and variations in the effect of sodium reduction on blood pressure among different demographic groups. The expanded NSRI covering both packaged and restaurant food items would be expected to reduce mean daily sodium intake by 447 mg per person per day on average, or 13.0%. If the NSRI included only restaurant food items, the program would lower MI and stroke mortality by an estimated 2.7% (95% CI, 1.4–4.0) and 2.1% (95% CI, 0.4–3.9), respectively. Hence, most of the benefit from the program would likely be due to sodium changes among packaged foods | Good | |
| Murray et al. (2003)79 | Modelling study | South East Asia (SEA), Latin America (LA), Europe (EU) |
Aim: to report estimates of the population health effects, and costs of selected interventions to reduce the risks associated with high cholesterol and blood pressure in areas of the world with differing epidemiological profiles Outcomes: DALYs |
1) Voluntary reformulation 2) Mandatory reformulation |
Measures to decrease salt intake appeared cost effective. Legislation appeared more cost effective than voluntary agreements with assumption it would lead to a larger reduction in dietary salt intake. A 15% reduction in mean population salt intake could avert 8.5 million cardiovascular deaths Voluntary reformulation: • EU: 7 X106 DALYs averted ($44 per DALY) • SEA: 5X106 DALYs averted ($37 per DALY) • LA: 3 X106 DALYs averted ($24 per DALY) Mandatory reformulation • EU: 13x106 DALYs averted ($23 per DALY) • SEA: 10 x106 DALYs averted ($19 per DALY) • LA: 6 x106 DALYs averted ($13 per DALY) |
Good | |
| Rubinstein et al. (2010)80 | Modelling study | Argentina | Aim: to estimate the burden of acute CHD and stroke and the cost-effectiveness of preventative population-based and clinical interventions. Outcomes: DALYs | Salt reduction in bread |
Reducing salt in bread is cost-saving • DALY averted: 672.80 • % of DALY saved: 0.11% • International Dollars per DALY saved: 1,406.93 |
Good | |
| Smith-Spangler et al. (2010)81 | Modelling study | US | Aim: to assess the cost-effectiveness of two population strategies to reduce sodium intake Outcomes: strokes and MIs averted, life years and QALYs gained | 1) Voluntary reformulation 2) Sodium tax |
Collaboration with the industry: a 9.5% reduction in sodium intake resulted in • Averted strokes = 513 885 s • Averted MIs = 480 358 • LYG = 1.3 million • QALYs = 2 million Sodium tax: would lead to a 6% decrease in sodium intake. • Averted Strokes = 327 892 • Averted MIs = 306 137 • LYG = 840 113 • QALYs = 1.3 million |
Good | |
| Konfino et al. (2013)82 | Modelling study | Argentina | Aim: to use Argentina-specific data to project impact of Argentina’s sodium reduction policies under two scenarios—the 2-year intervention currently being undertaken or a more persistent 10 year sodium reduction strategy. Outcomes: salt intake as measured by urinary sodium excretion, systolic blood pressure, deaths and cases averted, mortality | Reformulation |
Scenario 1: current initiative (2 year intervention) • Projected to reduce mean salt consumption by 0.96 g/day in men and 0.79 g/day in women • SBP would reduce by 0.93 mmHg to 1.81 mmHg depending on population subgroup • 19.000 deaths, 6.000 CHD deaths and 2.000 stroke deaths, 13.000 MIs and 10.000 stroke cases averted • Overall mortality reduction of 0.6% in adults >35 years, 1.5% in total MIs, 1% in total stroke cases in the next decade Scenario 2: current initiative maintained for 10 years • Projected to reduce mean salt consumption by 4.83 g/day in men and 3.98 g/day in women • SBP would reduce by 4.66 mmHg to 9.04 mmHg depending on subgroup • 55.000 deaths, 16.000 CHD deaths and 5.000 stroke deaths, 38.000 MIs and 27.000 strokes averted • Overall mortality decreased by 2% in adults > 35 years, 4.3% MIs and 2.7% stroke cases in the next decade |
Good | |
| Rubinstein et al. (2009)83 | Modelling study | Argentina | Aim: to use generalised cost-effectiveness analysis to identify the most efficient interventions to decrease CVD. Outcomes: cost-effectiveness; DALYs | Reformulation in bread | Lowering salt intake in the population through reducing salt in bread was found to be the most cost-effective ($17 per DALY averted). Less salt in bread • Total Cost per year (ARS$): $ 9.644 • DALY Age weighted, 3% discounted per year: 579 • DALY No age-weight 3% discounted per year: 713 • DALY # age-weight, undiscounted per year: 1,107 • ARS$ (1)/DALY (2): $ 17 |
Fair | |
| Hendriksen et al. (2014)84 | Modelling study | Netherlands | Aim: to evaluate the health benefits of salt-reduction strategies related to processed foods. Outcomes: AMI, CHF and CVA averted, life expectancy and DALYs gained, salt intake (g/day) | 1) Reformulation 2) Substitution of high salt foods with low salt foods 3)Adherence to the recommended intake |
If salt intake is reduced to the recommended maximum salt intake (6 g/d): Prevented cases: • 31.800 cases of AMI; • 15.300 cases of CHF; • 51.900 cases of CVA. • Mortality reduction: 0.7%. • LE increased by 0.15 years • 56000 DALYs gained Salt reduction processed foods scenario: median salt intake would decrease by -2.3g/d (28%) Prevented Cases: • 29.200 AMI cases; • 16.600 CHF cases; • 53.400 CVA. • Mortality Reduction: 0.8% • LE increased by 0.15 years • 56.400 DALYs gained Substitution: median salt intake would decrease by - 3.0g/day (35%). Prevented Cases: • 35.5000 cases of AMI; • 20.000 cases of CHF; • 64.300 cases of CVA • LE increased by 0.18 years • 67.900 DALYs gained |
Fair | |
| Ni Mhurchu et al. (2015)87 | Modelling study | New Zealand | Aim: to estimate the effects of health-related food taxes and subsidies Outcomes: deaths prevented or postponed | Tax on major dietary sodium products | A 20% tax on major dietary sources of sodium might result in 2,000 (1300 to 2,700) DPP (6.8%) | Good | |
| Asaria et al. (2007)104 | Modelling study | 23 low and middle income countries | Aim: to investigate potential deaths averted over 10 years by implementation of selected population-based interventions. Outcomes: CVD deaths averted, salt reduction (g/day) | Combined: 1) Mass media campaign 2) Voluntary reformulation |
8.5 million deaths would be averted by implementation of the salt-reduction strategy (15%) alone. Salt interventions: 15% reduction in mean salt intake • risk factor reduction of 1.69g/day • 8.4 million CVD deaths averted 30% reduction in mean intake • risk factor reduction of 3.38g/day • 16.0 million CVD deaths averted Reducing salt intake to 5g/d • risk factor reduction of 6.28g/d ay • 28.3 million CVD deaths averted Reduction in deaths • CVD = 75.6% Respiratory disease = 15·4% Cancer = 8·7% |
Good | |
| Dodhia et al. (2012)105 | Modelling study | England | Aim: to assess the impact of cost-effective interventions in terms of the avoidable CVD burden and costs by comparing these strategies to the current situation Outcomes: IHD and stroke events and deaths avoided, DALYs | Combined 1) Health promotion 2) Reformulation |
30% reformulation through agreement with the food industry. Interventions: Na– 2mmHg • IHD events avoided: 56.116 • Stroke events avoided: 98.497 • IHD deaths avoided: 26.781 • Stroke deaths avoided: 39.557 • DALYs averted: 238.043 • Cost per DALY ($): -4.228 Na– 5mmHg • IHD events avoided: 120.138 • Stroke events avoided: 257.508 • IHD deaths avoided: 57.322 • Stroke deaths avoided: 103.492 • DALYs averted: 579.869 • Cost per DALY ($): -5.021 Reducing salt intake to 6g/day through reformulation NA–MRC review • IHD events avoided: 80.366 • Stroke events avoided: 128.032 • IHD deaths avoided: 38.372 • Stroke deaths avoided: 51.419 Reducing salt intake in the population with a 5 mmHg reduction in SBP had the greatest population impact and cost-saving to the NHS. |
Good | |
| Gase et al. (2011)106 | Modelling study | US | Aim: to examine approaches to reduce sodium content of food served in settings operated or funded by the government of the County of Los Angeles, California Outcomes: salt intake and BP | Combined: 1) Labelling 2) Promotion 3) Subsidy 4) Provide low sodium food options |
Hospital cafeterias: Average sodium reduction of 1.8g/day (23%). Overall SBP: 1.59 County government cafeterias: Average sodium reduction of 0.7g/day (11%). Overall SBP: 0.63 |
Fair | |
| Ha & Chrisholm (2011)107 | Modelling study | Vietnam | Aim: to assess costs, health effects and cost-effectiveness prevention strategies to reduce CVD Outcomes: DALYs | Combined 1) Mass media campaign 2) Voluntary reformulation |
Media salt campaign • Cost per year (USD, million): 4.1 • DALYs averted per year: 45.939 • VND per DALY saved: 89.2 |
Fair | |
| Barton et al. (2011) 120 | Modelling study | England and Wales | Aim: to estimate the potential cost effectiveness of a population-wide risk factor reduction programme aimed at preventing cardiovascular disease. Outcomes: BP, CVD deaths averted | Salt legislation | Reducing salt intake by 3 g/day might reduce mean population systolic blood pressure by approximately 2.5 mm Hg preventing approximately 4450 deaths from cardiovascular disease |
Good |
Interventions were then categorised according to their position in the McLaren et al. (2010) continuum from “upstream” to “downstream” (Fig 1).[21]
Fig 1. Interventions classified on the upstream / downstream continuum.
Multi-component interventions were considered separately.
Patient involvement
Individual patients were not involved in this research; this is a secondary analysis of published data.
Results
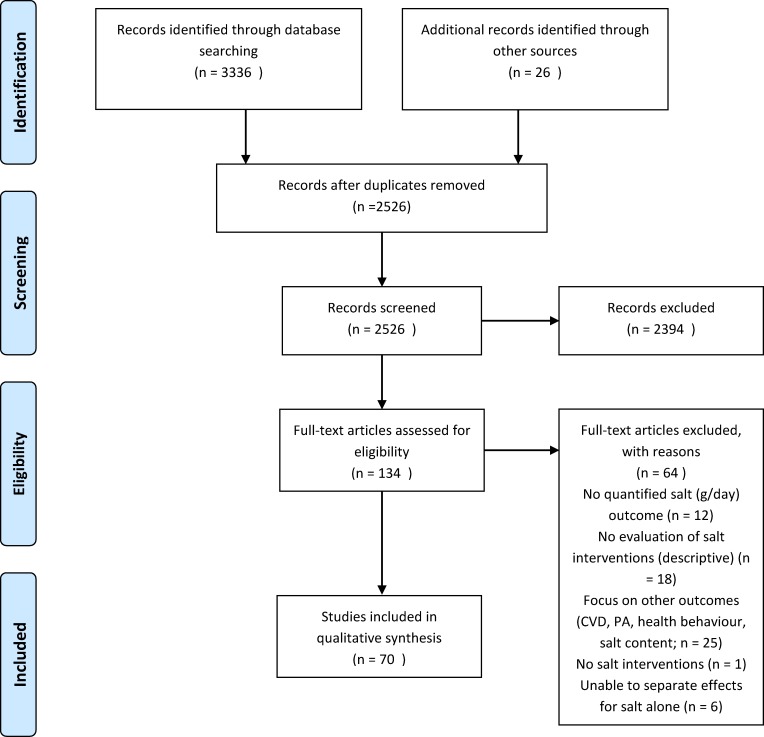
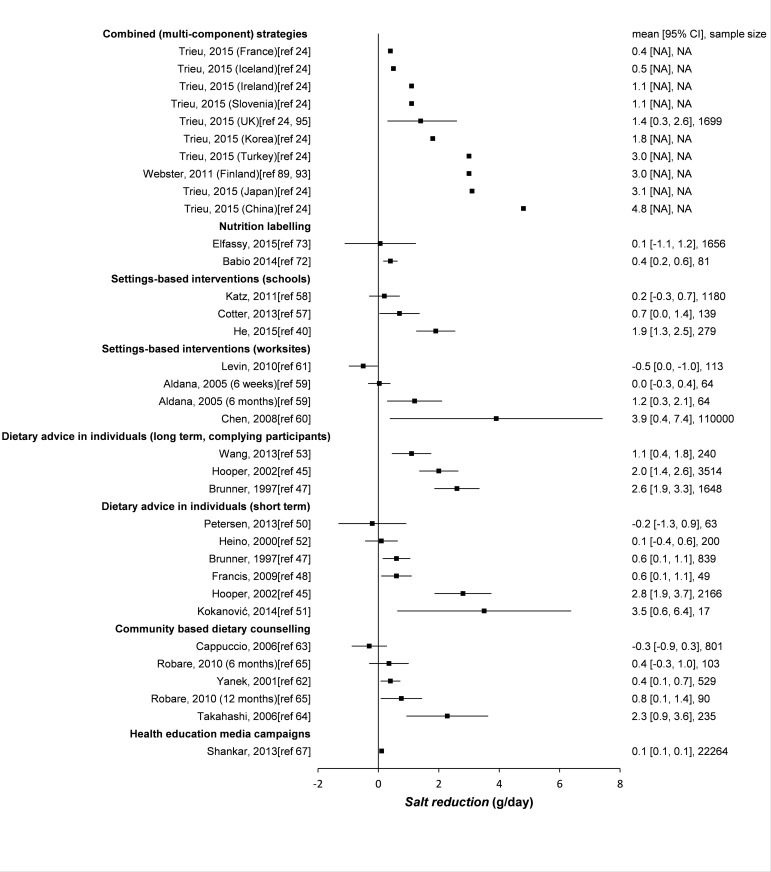
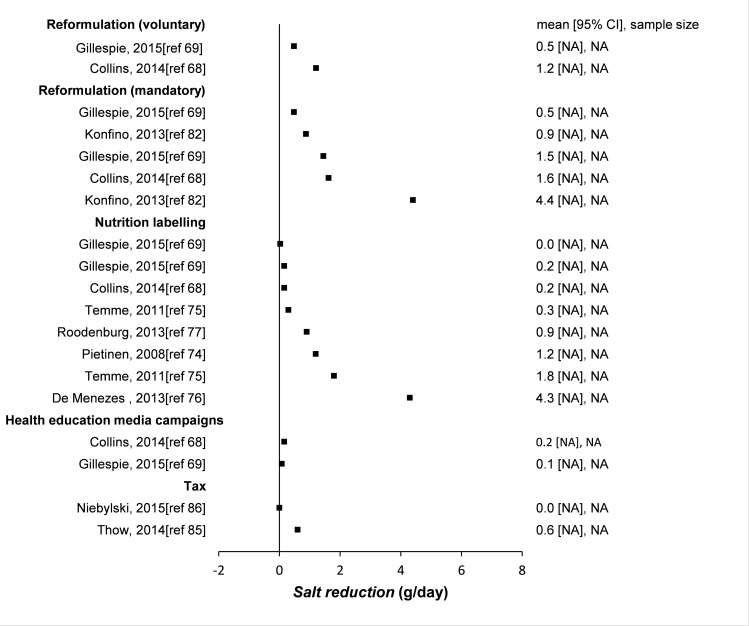
The literature search identified 3336 potentially relevant papers. An additional 26 papers were identified through other sources, including reference lists and key informants. After removing 836 duplicates, 2526 publications were left to be screened by title and abstract, after which 134 full-text papers were assessed for eligibility. A total of 70 papers were finally included (49 empirical studies and 21 modelling studies, Fig 2). The interventions and their effect sizes are presented in Fig 3 (empirical studies) and Fig 4 (modelling studies).
Fig 2. PRISMA flowchart.
Fig 3. Effectiveness of interventions to reduce salt intake (empirical studies).
Forest plot of the empirical studies that were included in this systematic review. Negative values of salt reduction are interpreted as reported increase in salt consumption. For most combined interventions the sample size and confidence intervals were not reported. NA denotes not applicable or not reported.
Fig 4. Effectiveness of interventions to reduce salt intake (modelling studies).
Forest plot of the modelling studies that were included in this systematic review. Because of the different modelling approaches in these studies, their uncertainty measures are not comparable. Therefore we do not plot them in this graph. Different scenarios were considered for different studies. NA denotes not applicable or not reported.
Dietary counselling–individual level (Table 2)
Nine empirical studies (two of good quality;[45–46] five of fair quality;[47–51] and two of poor quality [52–53]), and three modelling studies (all of good quality [54–56]) investigated the effect on salt intake of dietary counselling targeted at consenting individuals.
Two separate meta-analyses investigated the effect of dietary advice on salt intake. The first included eleven randomised controlled trials (RCTs) and found a 1.8g/day salt reduction after up to 18 months of dietary advice.[47] The second meta-analysis included eight RCTs and reported an overall reduction in salt consumption of 2.8g/day at 12 months and 2g/day up to 60 months.[45] The two meta-analyses overlapped in respect of only three studies.
One additional RCT found a statistically significant net reduction of 0.6g/day between the groups,[48] whilst a second RCT found no effect between the control and intervention group.[50]
All three modelling studies predicted that dietary advice is less effective in reducing the disease burden of high salt intake, only gaining 180–2,600 quality-adjusted life years (QALYs) compared to other interventions (7,900–195,000 QALYs).[54–56]
Dietary counselling–school based and worksite interventions (Table 3)
Table 3. Dietary counselling (worksite/schools).
| Study | Study type | Geographical scope | Aim and main outcomes | Policies analysed | Relevant results | Quality assessment |
|---|---|---|---|---|---|---|
| He et al. (2015)40 | Cluster RCT | China |
Aim: to determine whether an education programme targeted at schoolchildren could lower salt intake in children and their families Outcomes: salt intake as measured by urinary excretion |
Health education | At baseline, the mean salt intake in children was 7.3 (SE 0.3) g/day in the intervention group and 6.8 (SE 0.3) g/day in the control group. The mean effect on salt intake for intervention versus control group was −1.9 g/day (95% confidence interval −2.6 to −1.3 g/day; P<0.001). In adult family members the salt intakes were 12.6 (SE 0.4) and 11.3 (SE 0.4) g/day, respectively. During the study there was a reduction in salt intake in the intervention group, whereas in the control group salt intake increased. The mean effect on salt intake for intervention versus control group was −2.9 g/day (−3.7 to −2.2 g/day; P<0.001) | Good |
| Cotter et al. (2013)57 | School based RCT | Portugal |
Aim: to examine the influence on salt intake and blood pressure of three different educational interventions for 6 months Outcomes: salt intake as measured by urinary sodium excretion |
Nutrition education |
Baseline: mean salt intake of 7.8 ± 2.5 g per day. Estimated salt intake (g/d): CRT • Baseline: 7.7 ± 2.0 • Final: 7.4 ± 3.0 • Change: 0.35 ± 2.42 THEOR • Baseline: 8.1 ± 3.0 • Final: 7.5 ± 3.0 • Change: 0.60 ± 3.24 PRACT • Baseline: 7.5 ± 2.4 • Final: 6.4 ± 2.2 • Change: 1.08 ± 2.47* |
Fair |
| Katz et al. (2011)58 | School based RCT | US | Aim: to evaluate the effects of a nutrition education programme in distinguishing between healthful and less healthful choices in diverse food categories. Outcomes: salt intake | Nutrition education | There were no statistically significant improvements in dietary patterns from baseline between the intervention (-0.23g/day) and control groups (-0.04g/day) for salt intake (p = .44) |
Poor |
| Aldana et al. (2005)59 | RCT | US |
Aim: to determine behavioral and clinical impact of a worksite chronic disease prevention program Outcomes: salt intake |
Health education |
Intervention group (salt g/day) • Baseline: 7.5 • ∆6 weeks: -0.5 • ∆6 months: -1.7 Control group (salt g/day) • Baseline: 6.3 • ∆6 weeks: -0.5 • ∆6 months: -0.5 Significant differences in mean change scores were not observed at 6 weeks (P = 0.88) but they were seen at 6 months (P = 0.0097) |
Fair |
| Chen et al. (2008)60 | Intervention control trial | China |
Aim: to report the effects of these two programmes on blood pressure and changes in morbidity and mortality from CHD and stroke Outcomes: salt intake |
Health education | Mean daily salt intake declined from 16.0 to 10.6 g d-1 in the intervention factory, compared with the control factory from 16.9 to 15.4 g d-1, with the net reduction of 3.9 g d-1, which was significantly different (P < 0.05). | Fair |
| Levin et al. (2009)61 | Worksite based dietary intervention | US |
Aim: to examine whether a worksite nutrition programme using a low-fat vegan diet could significantly improve nutritional intake Outcomes: salt intake |
Dietary counselling | Intervention group participants significantly increased the reported intake and mean intake (P = 0.04) of salt compared to the control group. Salt (g/day) Intervention group • Baseline: 4.1 ± 0.1 • 22 weeks: 5.0 ± 0.2 • Mean difference: 0.9 ± 0.2 Control group • Baseline: 4.5 ± 0.2 • 22 weeks: 4.9 ± 0.2 • Mean difference: 0.4 ± 0.2 Mean effect size: +0.5 (95% CI 9.2, 394.4; P = 0.04) |
Fair |
Three school-based interventions (one of good quality;[40] one of fair quality;[57] one of poor quality [58]) and three worksite-based studies (all of fair quality) were included.[59–61] No modelling studies were identified for this section.
Schools
A nutrition programme in schools aimed at distinguishing between healthy and less healthy choices reported a non-significant reduction.[58] In the second school based RCT, the practical intervention group achieved a significant net reduction of 0.7g/day compared with the control group.[57] In a cluster RCT in China, education and training significantly reduced salt intake by a mean of −1.9 g/day in 279 school children (and −2.9 g/day in adult family members).[40]
Worksites
A randomised trial of a chronic disease prevention programme achieved a net reduction of 1.2g/day between the intervention and control group (P = 0.01).[59] A factory-based intervention study in China assessed health education aimed at altering diet, together with a high-risk strategy of hypertension control. Salt intake was reduced by 3.9g/day from a mean of 16g/day (P<0.05).[60]
Dietary counselling–community level (Table 4)
Table 4. Dietary counselling (community).
| Study | Study type | Geographical scope | Aim and main outcomes | Policies analysed | Relevant results | Quality assessment |
|---|---|---|---|---|---|---|
| Yanek et al. (2001)62 | RCT | US |
Aim: to test the impact on cardiovascular risk profiles after one year of participation in one of three church-based nutrition and physical activity strategies Outcomes: salt intake |
Health promotion–education | Salt (g/day) Combined standard and spiritual intervention groups • Baseline: 6.7 ±2.5 • Change: -0.4 ±0.06 Self-help control group • Baseline: 7.4 ±3.0 • Change: -0.02 ±0.09 Between group P value = 0.0167 |
Fair |
| Cappuccio et al. (2006)63 | Community-based cluster randomised trial | Ghana |
Aim: to establish the feasibility of salt reduction as a way of reducing BP Outcomes: salt intake |
Health education | Sodium intake as measured by sodium excretion fell in four out of six villages in the intervention group and in 5 out of six villages in the control group. The net intervention effect was non-significant. Control Intervention Baseline: 6.0 g/day Baseline: 5.8 g/day 3 months: 5.6 g/day 3 months: 5.4 g/day 6 months: 5.2 g/day 6 months: 5.3 g/day |
Fair |
| Takahashi et al. (2006)64 | Community based open randomizer controlled cross-over trial | Japan | Aim: to assess whether dietary intervention in free-living healthy subjects is effective in improving blood pressure levels. Outcomes: salt intake as measured by urinary sodium excretion | Dietary education | Salt intake as measured by sodium excretion, collected at two points, in the intervention group decreased by 2.8 (95% CI: -3.6, -2.1) and 0.6 g/day (-1.4, +0.2) in the control group. This difference in change between the two groups was statistically significant (P < 0.001). Dietary counselling for 1 year reduced salt intake by 2.2 g/day as measured by 24-h urinary sodium | Fair |
| Robare et al. (2010)65 | Community based intervention trial | US |
Aim: to evaluate a dietary Na reduction trial in a community setting Outcomes: salt intake as measured by urinary sodium excretion |
Nutrition education | Salt intake decreased by 0.3g/day (7.8 to 7.5g/day) from baseline to 6 months follow up which was not significant (p = 0.30). When comparing baseline with 12 months follow up, salt intake decreased by 0.7g/day (7.8 to 7.2g/day) which was significant (p = 0.03) |
Fair |
| Van de Vijver et al. (2012)66 | Review | Ghana and China |
Aim: to evaluate the effectiveness of the community-based interventions for CVD prevention programmes in LMIC Outcomes: BP and salt intake (g/day and n, %) |
Health education |
Cappuccio et al. (2006) • BP: reduction SBP 2.5 mmHg (1.45 to 6.54), DBP 3.9 mmHg (0.78 7.11)* vs control • Salt: no significant reduction in salt intake vs control Chen, Wu, and Gu (2008) (urban) • BP: reduction SBP 1.9 mmHg, reduction DBP 2.2 mmHg* vs control • Salt: reduction in salt intake of 3.9 g/day* vs control Yu et al. (1999) • BP: reduction among men in prevalence in HT 2%,* SBP 0%, among women prevalence of HT 2%,* SBP 2 mmHg • Salt: reduction in salt intake 6.0% Huang et al. (2011) • BP: reduction prevalence HT 12.9%* pre vs post • Salt: reduction in salt intake 30%* (n, %) |
Fair |
Four empirical studies and one review, all of fair quality,[62–66] investigated community based dietary counselling. One study reported a statistically significant difference of -0.4g/day in salt intake between the intervention and control groups.[62] Two intervention trials of nutrition education reported significant reductions of 0.7g/day and 2.2g/day reductions respectively in salt intake after 12 months.[63–64] One RCT reported a favourable trend; however, this was non-significant and could have been caused by contamination between the groups.[63]
Mass media campaigns (Table 5)
Table 5. Media campaigns.
| Study | Study type | Geographical scope | Aim and main outcomes | Policies analysed | Relevant results | Quality assessment |
|---|---|---|---|---|---|---|
| Shankar et al. (2012)67 | Cross-sectional | UK |
Aim: to examine the trend in salt intake over a set period and deduce the effects of the policy on the intake of socio-demographic groups Outcomes: salt intake as measured by spot urinary sodium readings |
Salt campaign (and potential effect on reformulation and table salt use) | The results are consistent with a previous hypothesis that the campaign reduced salt intakes by approximately 10%. The impact is shown to be stronger among women than among men. Salt as measured by spot urinary sodium readings • 2003: 6.3 g/day • 2004: 6.4 g/day • 2005: 5.7 g/day • 2006: 5.6 g/day • 2007: 5.4 g/day Difference in g/day between 2003–2007 = 0.9 g/day = 13.5% |
Fair |
One empirical study of fair quality [67] and five modelling studies; four of good quality[56, 68–70] and one of fair quality[71] were included.
The UK FSA salt reduction programme involved media campaigns to discourage table salt use, plus sustained pressure on industry to reformulate. Although salt consumption declined by 0.9g/day using spot urinary sodium readings from 2003–2007, the media contribution was unclear but likely modest.[67]
The modelling studies likewise suggested media campaigns were generally considered less effective than food labelling or reformulation.[56, 69–71] The Change4Life campaign in the UK was predicted to reduce salt intake by 0.16g/day, less than labelling or reformulation.[68] Gillespie et al. (2015) similarly estimated that social marketing might modestly reduce salt consumption by 0.03g/day to 0.13g/day.[69]
Nutrition labelling (Table 6)
Table 6. Labelling.
| Study | Study type | Geographical scope | Aim and main outcomes | Policies analysed | Relevant results | Quality assessment |
|---|---|---|---|---|---|---|
| Babio et al. (2013)72 | Randomised cross-over trial | Spain | Aim: to compare two models of front-of-pack guideline daily amounts (GDA) and the ability to choose a diet that follows the nutritional recommendations. Outcomes: salt intake based on choices | Labelling | Participants using the multiple-traffic-light GDA system chose significantly less salt (0.4g/day; P <0.001) than those using the monochrome GDA labels |
Poor |
| Elfassy et al. (2015)73 | Cross-sectional | US |
Aim: to examine independent association between hypertension and frequency use of NF label for sodium information and whether this was associated with differences in intake Outcomes: salt intake as measured by urinary sodium excretion |
Labelling (use) | Daily sodium intake was not lower in those who reported frequent vs non-frequent use of the NF label for sodium information (7.7g/day vs 7.6g/day; P = 0.924) | Poor |
Two empirical studies, both of poor quality, investigated the effect of nutrition labelling on salt intake [72–73]. Reduced salt intake was not observed in participants who reported frequent vs. non-frequent label use (7.7g/day vs. 7.6g/day).[73]
Ten modelling studies also examined labelling, four of good quality[56,68–70] and two of fair quality.[71, 74–77] These suggested that labelling might modestly reduce UK salt intake by 0.03g/day to 0.16g/day [68, 69]; much less than the 0.9g/day estimated by Roodenburg et al. (2013).[77] Another study suggested that salt intake might be lowered by 1.2g/day if the population were to choose products labelled as low-salt, or increased by 1.6g/day if they choose products labelled as high salt content.[74]
Reformulation (Table 7)
Table 7. Reformulation.
| Study | Study type | Geographical scope | Aim and main outcomes | Policies analysed | Relevant results | Quality assessment |
|---|---|---|---|---|---|---|
| Chang et al. (2006)78 | Cluster RCT | Taiwan | Aim: to examine the effects of potassium-enriched salt on CVD mortality and medical expenditures in elderly veterans. Outcomes: incidence, CVD mortality, LYG | Reformulation–low sodium salt | The incidence of CVD-related deaths was 13.1 per 1000 persons (27 deaths in 2057 person-years) and 20.5 per 1000 (66 deaths in 3218 person years) for the experimental and control groups, respectively A significant reduction in CVD mortality (age-adjusted hazard ratio: 0.59; 95% CI: 0.37, 0.95) was observed in the experimental group. Persons in the experimental group lived 0.3–0.90 y longer | Fair |
Very few studies which focused on reformulation included quantified results of salt intake. In one empirical Taiwanese study of fair quality,[78] salt was enriched with potassium in the intervention group and their outcomes were an apparent reduction in cardiovascular deaths by 41%, compared to the control group rather than salt intake. Furthermore, people in the intervention group lived 0.3–0.9 years longer.[78]
Fourteen modelling studies evaluated reformulation, eleven of good quality[41, 54–56, 68–70, 79–82] and three of fair quality[71, 83, 84]. Mandatory reformulation could consistently achieve bigger salt reductions than voluntary reformulation; 1.6g/day compared with 1.2g/day;[68] and 1.4g/day versus 0.5g/day.[69] Mandatory reformulation might also achieve higher reductions in disability-adjusted life years (DALYs) and QALYs compared to voluntary reformulation.[54, 56, 79]
In the Netherlands, reformulation of processed foods was predicted to reduce median salt intake by 2.3g/day,[84] compared with a 0.9g/day from a two-year salt reformulation initiative in Argentina.[82]
Fiscal interventions (Table 8)
Table 8. Taxes.
| Study | Study type | Geographical scope | Aim and main outcomes | Policies analysed | Relevant results | Quality assessment |
|---|---|---|---|---|---|---|
| Thow et al. (2014)85 | Systematic Review | US (with UK data) |
Aim: to assess the effect of food taxes on consumption Outcomes: sodium consumption |
Sodium tax | A modelling study predicted that a sodium tax increasing the price of salty foods by 40% would reduce sodium consumption by 6% | Fair |
| Niebylski et al. (2015)86 | SystematicReview | France and US | Aim: to evaluate the evidence base to assess the effect of unhealthy food taxation. Outcomes: energy intake | 1) Tax on salty snacks 2) Tax on cheese/butter |
1) Modelling study of tax on chips/salty snacks on energy intake in US. Predicted a 1% tax had no effect on consumption or body weight 2) Modelling study of effect of 1% VAT on cheese/butter, sugar, and fat products along with ready-made meals in France. Predicted proposed taxes reduced saturated fat, cholesterol, sodium, and energy intake but suggest 1% is insufficient to have positive health effect. |
Fair |
Two systematic reviews of fair quality [85, 86] included three modelling studies eligible for this review. Furthermore, three additional tax modelling studies were included, all of good quality.[56, 81, 87] Two studies included in Niebylski et al’s. systematic review (2015) modelled a 1% tax on salty snacks or on cheese and butter; neither reduced salt consumption.[86] Another modelling study suggested that a very high (40%) tax might achieve a 6% reduction in salt consumption (0.6g/day).[81]
One modelling study predicted that a 20% tax on major dietary sodium sources might prevent or postpone 2000 deaths annually,[87] whilst Nghiem et al. (2015) predicted that a sodium tax might gain more QALYs than other interventions.[56]
Multi-component interventions (Table 9 and Table 10)
Table 9. Multi-component interventions.
| Study | Study type | Geographical scope | Aim and main outcomes | Policies analysed | Relevant results | Quality assessment |
|---|---|---|---|---|---|---|
| He et al. (2014)88 | Comprehensive analysis | UK |
Aim: to analyse the UK salt reduction programme Outcomes: salt intake as measured by urinary sodium excretion |
1) Reformulation 2) Labelling 3) Health promotion campaigns |
15% decrease, there have been a steady fall in salt intake at a rate of ~2% per year since the introduction of the salt reduction strategy. The 0.9g/day reduction in salt intake achieved by 2008 led to E 6000 fewer CVD deaths per year. • 2000–2001: salt intake = 9.5g/day • 2005–2006: salt intake = 9.0g/day • 2008: salt intake = 8.6g/day • 2011: salt intake = 8.1g/day |
Good |
| Mozaffarian et al. (2012)89 | Systematic review | Finland and China | Aim: to systematically review and grade the current scientific evidence for effective population approaches to improve dietary habits. Outcomes: salt intake as measured by urinary sodium excretion | 1) Education 2) Combined effects of labelling, reformulation and campaigns |
Tian et al. (1995) 1) Education: In the intervention neighborhoods, mean sodium intake decreased by 1.3 and 0.6 mmol/day in men and women, respectively, compared with increases of 1.0 and 0.2 mmol/day, respectively, in the control neighborhoods (P0.001 for men, P0.065 for women) Pekka et al. (2002) + Puska & Stahl (2010) 2) From the 1970s to the late 1990s, mean daily salt consumption in Finland declined from approximately 14.5 g in men (unknown in women) to approximately 11 g in men and 7 g in women; mean diastolic blood pressure declined by 5% in men and 13% in women |
Good |
| Fattore et al. (2014)43 | Systematic review | Australia, US and Vietname |
Aim: to summarize and critically assess economic evaluation studies conducted on direct (e.g., counseling) or indirect (e.g., food labeling) interventions aimed at promoting voluntary dietary improvements through reduction of fat intake Outcomes: DALYs |
1) Voluntary reformulation, mandatory reformulation and dietary advice 2) Reduction in daily caloric intake of 100 to 500 kcal below current estimated energy requirements 3) A set of personal (e.g., individual treatment of SBP >160 mmHg) and non-personal (e.g., a mass media campaign for reducing consumption of salt) prevention strategies to reduce CVD 4) Voluntary reformulation and sodium tax |
1) Cobiac et al. (2010) 610,000 DALYs averted (95%CI: 480,000–740,000) if everyone reduced their salt intake to recommended limits. Dietary advice: <0.5% disease burden (IHD & stroke cases) averted; Tick program: <1%; making Tick limits mandatory: 18% 2) Dall et al. (2009) 400 mg/d sodium intake reduction 3) Ha & Chisholm (2011) A health education program to reduce salt intake (VND 1,945,002 or USD 118 per DALY averted) & individual treatment of SBP >160 mmHg (VND 1,281,596 or USD 78 per DALY averted) are the most cost-effective measures 4) Smith-Spangler (2010) (1) vs. (2): 1.25-mm Hg vs. 0.93-mm Hg decrease in mean SBP; 513,885 vs. 327,892 strokes averted; 480,358 vs. 306,137 MIs averted; 1.3 million vs. 840,113 years LE increase. Collaboration with industry: 2.1 million QALYs gained; USD 32.1 billion medical cost savings. Tax on sodium: 1.3 million QALYs gained; USD 22.4 billion medical cost savings |
Fair |
| He & MacGregor (2009)90 | Review | Japan, Finland and UK | Aim: to provide an update on the current experience of worldwide salt reduction programmes. Outcomes: salt intake, blood pressure, stroke & CHD mortality and life expectancy | 1) Reformulation to reduce the salt content of all foods 2) Health promotion campaigns 3) Labelling to highlight salt content |
Japan. The Japanese Government initiated a campaign to reduce salt intake. Over the following decade salt intake was reduced from an average of 13.5 to 12.1 g/day. However, in the north of Japan salt intake fell from 18 to 14 g/day. Paralleling this reduction in salt intake, there was an 80% reduction in stroke mortality despite large increases in population fat intake, cigarette smoking, alcohol consumption and an increase in BMI. Finland. Since the 1970s, Finland aimed to reduce salt intake by reformulation and raising general awareness of the harmful effects of salt on health. This led to a significant reduction in salt intake of 3g/day from 1979 to 2002 (12 to 9g/day) as measured by urinary sodium. This was accompanied by a fall of over 10mmHg in both systolic and diastolic BP, a pronounced decrease of 75–80% in both stroke and CHD mortality, and a remarkable increase of 5–6 years in life expectancy. UK. Salt added to cooking or at the table: estimated that 15% of the total 9.5g/day consumed was added (1.4g/day). Naturally present in food: approximately 5% (0.6g/day). Reformulation: 80% (7.5g/day) was added by the food industry. The UK salt reduction strategy started in 2003/2004 and the adult daily salt intake has already fallen, as measured by urinary sodium, from an average of 9.5 g/day to 8.6 g/day by May 2008 |
Fair |
| Pietinen et al. (2010)91 | Before and after study | Finland | Aim: to describe the main actions in Finnish nutrition policy during the past decades. Outcomes: salt intake | 1) Education 2) Voluntary reformulation 3) Labelling |
1981; Eastern Finland: salt intake was about 13 g in men and 11 g in women. Salt intake has decreased continuously to a level of about 9 g in men and 7 g in women in 2007 | Fair |
| Wang et al. (2011)92 | Literature review | US |
Aim: to summarize cost-effectiveness evidence on selected interventions to reduce sodium intake that would be intended as population-wide approaches to control hypertension Outcomes: stroke and MI averted |
1) Reformulation 2) Sodium tax |
Smith-Spangler et al. For US adults aged 40–85 years, collaboration with industry that decreased mean intake of sodium by 9.5% was estimated to avert 513 885 strokes and 480 358 myocardial infarctions over their lifetimes and to save US$ 32.1 billion in annual medical costs. Over the same period, a tax on sodium that decreased the population’s intake of sodium by 6% was projected to save US$ 22.4 billion in such costs |
Fair |
| Webster et al. (2011)93 | Review | Finland, France, Japan and UK | Aim: to provide an overview of national salt reduction initiatives around the world and describe core characteristic. Outcomes: salt intake, LYG, CHD and stroke mortality | 1) Reformulation 2) Labelling 3) Health promotion campaigns |
Finland: started salt reduction strategy in 1978 (reformulation, labelling and mass media campaigns) and by 2002 had demonstrated a 3 g reduction in average population salt intake (from 12 to 9 g/person per day). During the same period there was a corresponding 60% fall in CHD and stroke mortality UK: the Food Standards Agency (FSA) started working with the food industry in 2003 and launched its consumer education campaign in 2005. By 2008 the UK had achieved an average 0.9 g/person per day reduction in daily salt consumption, which is predicted to be saving some 6000 lives a year. France: the Food Safety Authority recommended a reduction in population salt consumption in 2000 and has since reported a decline in intake provided by foods from 8.1 to 7.7 g/day in the overall adult population. Focus was on bread reformulation and nutrition campaigns Japan: 60s started a salt campaign through a sustained public education campaign. Over the following decade average salt intake was reduced from 13.5 to 12.1 g/day with a parallel fall in blood pressure in adults and children, and an 80% reduction in stroke mortality despite large adverse changes in a range of other cardiovascular risk factors. |
Fair |
| Wang & Bowman (2013)94 | Literature review | US, UK |
Aim: to summarize recent economic analyses of interventions to reduce sodium intake. Outcomes: SBP, hypertension, cardiovascular events |
1) reducing the sodium content of all foods 2) reducing sodium content by labelling foods and by promoting, subsidizing, and providing low sodium food options 3) Legislation |
US (1&2): If the sodium-reduction strategies were implemented, adults in the county would reduce their intake of sodium by 233 mg per day, on average, in 2010. This would correspond to an average decrease of 0.71 mmHg in SBP among adults with hypertension, 388 fewer cases of uncontrolled hypertension, and a decrease per year of $629,724 in direct health care costs UK (3): Legislation or other measures to reduce the intake of salt by 3 g per person per day (in a population where the current mean intake was about 8.5 g per person per day) would reduce the mean population SBP by approximately 2.5 mmHg, prevent about 30,000 cardiovascular events and approximately 4,450 deaths, and produce discounted savings overall of approximately £347 million (about $684 million) over a decade, which would be equivalent to annual savings of approximately £40 million |
Fair |
| He et al. (2014)95 | Cross-sectional | England |
Aim: to determine the relationship between the reduction in salt intake that occurred in England, and BP, as well as mortality from stroke and IHD Outcomes: salt intake as measured by urinary sodium excretion |
Combined 1) Reformulation 2) Health promotion campaigns 3) Labelling |
From 2003 to 2011, salt intake decreased by 1.4 g/day (15%, p<0.05 for the downward trend). From 2003 to 2011, stroke mortality decreased from 128/1 000 000 to 82/1 000 000 (36% reduction, p<0.001) and IHD mortality decreased from 423/1 000 000 to 272/1 000 000 (36% reduction, p<0.001). • 2003: 9.5g/day • 2005/2006: 9.0g/day • 2008: 8.6g/day • 2011: 8.1g/day |
Fair |
| Enkhtungalag et al. (2015)96 | Before and after study | Mongolia | Aim: to reduce salt intake of the employees of three of the main food producing factories. Outcomes: salt intake as measured by 24h urine excretion | Education on salt consumption and provision of reduced salt foods | Salt intake reduced from 11.5g/day in 2011 to 8.7g/day in 2013 | Fair |
| Trieu et al. (2015)24 | Systematic review | 75 countries | Aim: to quantify progress with the initiation of salt reduction strategies around the world in the context of the global target to reduce population salt intake by 30% by 2025. Outcomes: salt (g/day) | Labelling, mass media campaigns, education, reformulation |
Denmark: from 2006 to 2010 salt intake reduced from 10.7 to 9.9g/day in men and 7.5g to 7.0g/day in women (7%) Japan: salt intake reduced from 13.5in 1997 to 10.4g/day in 2012 (23%) Korea: salt intake reduced from 13.4g in 2005 to 11.6g/day in 2012 (13.6%) Slovenia: salt intake reduced from 12.4g in 2007 to 11.3g/day in 2012 (8.9%) Du et al. (2014) China: salt intake reduced from 16.8g in 1999 to 12g/day in 2009 (28%) Pietinen et al. (2010) & Laatikanen et al. (2006) Finland: from 1979 to 2007 salt intake reduced from 13g to 8.3g/day in men and 11g to 7.0g/day in women (36%) European commission (2008) France: salt intake reduced from 8.1g in 1999 to 7.7g/day in 2007(4.9%) WHO (2013) Iceland: salt intake reduced from 8.4g in 2002 to 7.9g/day in 2010 (6%) Walton (2013) Ireland: salt intake reduced from 8.1g in 2001 to 7g/day in 2011(13.6%) National Food and Veterinary Risk Assessment Institute Lithuania: salt intake reduced from 10.8g in 1997 to 8.8g/day in 2007(18.6%) WHO (2013) Turkey: salt intake reduced from 18.0g in 2008 to 15g/day in 2012(16.7%) Sadler et al. (2011) UK: Salt intake reduced from 9.5g in 2001 to 8.1g/day in 2011(14.7%) |
Fair |
| Luft et al. (1997)97 | Review | Finland and US | Aim: to discuss the approaches used in a community-wide salt-reduction project. Outcomes: salt intake as measured by urinary excretion | 1) Nutrition education 2) Reformulation |
Pietinen et al. (1984)—Health education & reformulation. After 3 y salt intake had not changed significantly. Hypertensive subjects Men Women 1979: 13.8 ± 5.3 1979: 10.4 ± 4.7 1982: 13.7 ± 5.5 1982: 10.0 ± 4.1 Normotensive subjects Men Women 1979: 12.4 ± 4.8 1979: 9.8 ± 3.8 1982: 12.2 ± 4.8 1982: 9.1 ± 3.6 Lang et al. (1985)—Dietary counselling. Women reduced their salt intake from 7.5 ± 0.4 to 3.6 ± 0.2 g/day and men reduced their salt intake from 10.3 ± 0.8 to 4.7 ± 0.3 g/day. Wassertheil-Smoller et a. (1992)–Education. Salt intake as measured by urinary sodium excretion was reduced from 7.9 to 1 6.4 g/day. Analysis of 3-d food records indicated that sodium intake decreased from 8.1 to 4.9 g/day. Hypertension prevention collaborative research group (1992)—Nutrition education. Salt intake as measured by urinary sodium excretion Intervention Control Baseline: 8.9 ± 3.4 Baseline: 9.0 ± 3.5 Change: -3.2 ± 4.4 Change: -0.6 ± 4.4 |
Poor |
| Mohan et al. (2009)98 | Review | UK | Aim: to review the evidence related to dietary sodium and health in the context of the Ottawa Charter for health promotion. Outcomes: salt intake, stroke, CVD & coronary artery mortality | 1) Reformulation 2) Labelling 3) Health promotion campaign |
UK: Consumer-friendly labelling indicating sodium content in processed foods by use of a colour system implemented in several UK food chains. Together with other efforts population salt intake decreased from 9.5g/day in 2004 to 8.6g/day in 2008 | Poor |
| He & MacGregor et al. (2010)99 | Comprehensive review | Japan, Finland and UK |
Aim: to provide an update on the current salt reduction programmes that have been successfully carried out Outcomes: salt intake |
1) Reformulation 2) Labelling 3) Health promotion campaigns |
Japan: over a decade national salt intake fell from 13.5g/day to 12.1g/day. In the North, salt intake was reduced from 18 to 14g/day. There was also an 80% reduction in stroke mortality despite large increases in fat intake, cigarette smoking, alcohol consumption, and obesity Finland: reformulation, labelling and campaigns led to a significant reduction in salt from 12g/day in 1979 to 9g/day in 2002 UK: salt reduction strategy started in 2003/2004 and salt intake has already fallen from 9.5 to 8.6 g/d by May 2008 |
Poor |
| Wyness et al. (2012)100 | Literature review | UK |
Aim: to describe the UK Food Standards Agency's (FSA) salt reduction programme undertaken between 2003 and 2010 and to discuss its effectiveness Outcomes: salt intake |
1) Health promotion campaigns 2) Voluntary reformulation 3) Labelling |
• 2000–2001: salt intake = 9.5g/day • 2005–2006: salt intake = 9.0g/day • 2008: : salt intake = 8.6g/day |
Poor |
Fifteen papers were included under multi-component interventions. Most studies came from Japan, Finland and the UK. Two were of good quality;[88, 89] ten of fair quality;[24, 43, 89–96] and four of poor quality.[97–100]
Four studies were included which presented dietary salt intake and linked to papers describing the interventions; (one of good quality;[25]; two of fair quality;[101, 102] and one of poor quality.[103]
Japan
The Japanese government initiated a sustained campaign in the 1960s.[26] Over the following decade, mean salt intake fell from 13.5g/day to 12.1g/day overall (and from 18g/day to 14g/day in Northern Japan). Miura et al. (2000) reported that salt intake subsequently decreased from 14.5g/day in 1972 to 10.6g/day in 2010, a fall of almost 4g/day [103]. Stroke mortality was predicted to fall by 80%.[90, 93]
Finland
Starting in 1978, Finland pursued a comprehensive salt reduction strategy using mass media campaigns, mandatory labelling and voluntary reformulation by the food industry. Population salt consumption was monitored regularly by using 24h urinary assessment and dietary survey data.[72] By 2007, salt intake had reduced by approximately 4g/day, from 13 to 8.3g/day in men, and from 11 to 7g/day in women.[24, 25] Stroke and coronary heart disease (CHD) mortality fell by over 75% during that period.[90]
United Kingdom
The UK salt reduction strategy included voluntary reformulation, a consumer awareness campaign, food labelling, target settings and population monitoring.[95] By 2011, population salt intake, measured by 24h urinary sodium excretion, had decreased by 1.4g/day (9.5g/day to 8.1g/day)[88]. He et al. (2014b) estimated that this might reduce stroke and coronary heart disease mortality by some 36%.[88]
Other countries have implemented several strategies including labelling, media campaigns and voluntary reformulation and effect sizes ranged from -0.4g/day in France [24, 93] to -4.8g/day in China [24, 102].
Modelling studies of combined interventions
Six modelling studies investigated the effect of multi-component interventions, three were of good quality;[70, 104, 105] whilst three others were of fair quality.[70, 106, 107]
Several modelling studies consistently suggested that multi-component salt reduction strategies (e.g. labelling, health promotion and reformulation) would be more effective than any single intervention.[70, 71] For instance, Gase et al. (2011) suggested that using labelling, promotion, subsidies and provision of low sodium options could lead to a 0.7–1.8g/day reduction.[106]
Discussion
Main results
This systematic review of salt reduction interventions suggests that comprehensive strategies could generally achieve the biggest reductions in salt consumption across an entire population, approximately 4g/day in Finland and Japan, 3g/day in Turkey and 1.3g/day recently in the UK. Mandatory reformulation alone could achieve a reduction of approximately 1.4g/day, followed by voluntary reformulation (median 0.7g/day) school interventions (0.7g/day) and worksite interventions (+0.5g/day). Smaller population benefits were generally achieved by short-term dietary advice (0.6g/day), community-based counselling (0.3g/day), nutrition labelling (0.4g/day), and health education media campaigns (-0.1g/day). Although dietary advice to individuals achieved a -2g/day reduction, this required optimal research trial conditions (smaller reductions might be anticipated in unselected individuals).
Comparison with other research
Geoffrey Rose famously argued that a greater net benefit came from the population-wide approach, (achieving a small effect in a large number of people) when compared with targeting high risk individuals (a large effect but only achieved in a small number of people).[32]
Multi-component interventions
Multi-component salt reduction strategies involving a series of structural initiatives together with campaigns to increase population awareness have been successful in Japan and Finland where they substantially reduced dietary salt consumption, and associated high stroke and cardiovascular disease mortality rates. In Finland, some credit should also go to other dietary changes e.g. fat quality.[108]
Between 2003 and 2010, a multi-component approach in the UK including voluntary reformulation and political pressure on industry to agree category-specific targets achieved some success (1.3g/day reduction in population salt consumption over 8 years to 8.1g/day in 2011). Interestingly, pre-existing health inequalities in salt consumption persisted.[29] However, from 2010, the Responsibility Deal simply advocated a voluntary scheme. This was ineffective, and MacGregor therefore subsequently recommended mandatory reformulation.[31] Other useful reductions were demonstrated in other countries mostly using dietary surveys and some from grey literature. However, the -4.8g/day reduction reported in China appears extra-ordinarily large and perhaps merits some caution [24]. Multi-component interventions clearly have more potential than single interventions, and synergies might be anticipated. [13,93] Similarly powerful benefits have also been observed with comprehensive strategies for tobacco control and alcohol reduction.[35,36]
Reformulation
In high income countries, the majority of dietary salt intake comes in processed food (75%) and reformulation can be very effective in reducing salt consumption.[109] Though mandatory reformulation is more powerful, most countries currently use voluntary reformulation.[54,56,68,69,110] Success may then be very dependent on the degree of political pressure applied to the food industry and on regular, independent monitoring, as recently achieved in the UK. [111,112]
Food labelling
Nutrition labelling can be potentially effective, as demonstrated in Finland [72] and Brazil.[74] Nutrition labelling allows consumers to make informed choices whilst also putting pressure on the food industry to reformulate.[89] However, interpretation of labels depends on health literacy and different labelling systems may confuse consumers,[113] and reinforce inequalities.[29]. Consumers generally want simple (traffic light) labels which are easier to understand.[76,77,113,114]
Dietary interventions in diverse settings: communities, worksites, schools and homes.
Dietary interventions can be delivered at different levels, such as communities, worksites, schools or to individuals. However, effectiveness varies widely.[45,47,50] Furthermore, the benefits of dietary counselling decrease over time and are thus generally not sustainable; much smaller reductions might therefore be anticipated in unselected individuals in the general population.[44] Furthermore, for many individuals, issues such as competing priorities and financial constraints might reduce compliance and adherence,[8,13,21,22] and thus reduce net population benefits.
Mass media campaigns
Few empirical studies have examined salt media campaigns. However, benefits appear to be generally modest.[56, 67,68,69,115] or negligible.[111] Many individuals may not perceive any personal relevance and hence fail to engage in any behaviour change.[22,116,117]
Taxation
Price increases can powerfully reduce consumption of tobacco or alcohol.[35,36] However, salt is cheap, and a substantial tax of at least 40% might be needed to reduce consumption by just 6%.[81,118]
Public health benefits and cost-effectiveness
Most economic analyses have consistently predicted substantial reductions in cardiovascular mortality, and consequent gains in life-years, QALYs, DALYs and healthcare savings. This is consistent with the growing evidence that population-wide prevention policies can often be powerful, rapid, equitable and cost-saving.[38,119–122]
Several modelling studies also investigated the cost-effectiveness of the salt interventions described above. Mandatory and voluntary reformulation appeared far more cost-effective than labelling or [54,55,68] dietary advice targeting individuals.[122]
Strengths and limitations
This systematic review has multiple strengths. Firstly, two independent reviewers screened all papers and assessed quality using appropriate validated tools. Secondly, the inclusion of modelling studies (presented separately) adds value by allowing the evaluation of certain interventions where empirical studies failed (e.g. labelling). In addition, we recorded the effect size used in each modelling paper together with the source reference. Furthermore, most of the better quality modelling studies confirmed the superiority of upstream approaches. Finally, the studies reviewed included a wide variety of interventions, thus providing a useful spread of estimates.
Our review also has limitations. We were unable to conduct a formal meta-analysis due to the profound heterogeneity of the diverse studies, many of which included multiple interventions. Furthermore, studies were only included if the full text was available in English (15 non-English papers were excluded). We also had to exclude two potentially relevant studies which lacked the full text.[123,124] Publication bias remains possible, potentially over-estimating the true effect of some interventions. The primary outcome of this study was dietary intake (consumption); we excluded studies considering other dietary behaviours such as awareness, knowledge, preferences or purchasing behaviour. Also, the positive benefits of policy changes may sometimes appear larger if favourable underlying secular trends have not been formally considered. Furthermore, we did not contact authors for missing data. However, all the key information was presented in all but two papers. [123,124] Finally, generalization of the results should be cautioned as countries may vary in baseline salt intake.
Socio-economic Inequalities
More deprived groups more often consume foods high in salt, (and sugar and fat); all are associated with poor health.[125–127] These inequalities persist in Britain [28,29] and Italy.[128]
Downstream interventions focused on individuals typically widen inequalities whereas upstream “structural” interventions may reduce inequalities.[33,129,130]
Future research
This review highlights the greater power of combined (multi-component) strategies, mandatory reformulation and traffic light labelling. Most were cost-effective and many were cost-saving. However, the feasibility of implementing policy changes also deserves further study. Many factors can facilitate or obstruct successful policy development, notably including political feasibility and stakeholder influence.[114,131,132]
Stoeckle and Zola’s “upstream”/”downstream” concept was disseminated by John McKinlay,[133] critiqued by Krieger,[134] and then refined as a structural/agentic continuum by McLaren et al 2010.[21] To test our effectiveness hierarchy hypothesis, one ideally needs to quantify the “average” effect of each category of salt reduction intervention. Yet, the limited number and heterogeneity of these studies precludes a formal meta-analysis. However, the consistency with the effectiveness hierarchies demonstrated by tobacco and alcohol control interventions is encouraging. The effectiveness hierarchy hypothesis now clearly needs to be tested in other fields.
Conclusions
There are clear implications for public health. The biggest population-wide reductions in salt consumption were consistently achieved by comprehensive multi-component strategies involving “upstream” population-wide policies (regulation, mandatory reformulation, and food labelling).”Downstream” individually-based interventions appear relatively weak (e.g. dietary counselling to individuals and school children, and media campaigns in isolation).
This ‘effectiveness hierarchy’ might deserve greater emphasis on the agendas of the WHO and other global health organizations reviewing action plans for NCD prevention.
Supporting information
(DOCX)
(DOC)
(DOCX)
Acknowledgments
We thank Mark Petticrew and Cecile Knai for their very helpful comments. FPC contributed under the remit of the Terms of Reference of the World Health Organization Collaborating Centre for Nutrition of the University of Warwick.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
Lirije Hyseni, Rory McGill and Lois Orton were funded by LiLaC/NIHR. This article presents independent research funded by the National Institute for Health Research’s School for Public Health Research (NIHR SPHR). The School for Public Health Research (SPHR) is funded by the National Institute for Health Research (NIHR). SPHR is a partnership between the Universities of Sheffield, Bristol, Cambridge, Exeter, UCL; The London School for Hygiene and Tropical Medicine; the LiLaC collaboration between the Universities of Liverpool and Lancaster and Fuse; The Centre for Translational Research in Public Health. The work was carried out under the remit of the WHO Collaborating Centre for Nutrition of the University of Warwick. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, the Department of Health or the World Health Organization. All other authors were funded by HEFCE. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional moratlity from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859): 2095–128. doi: 10.1016/S0140-6736(12)61728-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vos T, Barber RM, Bell B, Bertozzi-Villa A, Biryukov S, Bolligeret I, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. In press, corrected proof. [DOI] [PMC free article] [PubMed]
- 3.Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. The Lancet. 2012;380: 2224–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson CA, Appel LJ, Okuda N, Brown IJ, Chan Q, Zhao L, et al. Dietary Sources of Sodium in China, Japan, the United Kingdom, and the United States, Women and Men Aged 40 to 59 Years: The INTERMAP Study. J Am Diet Assoc. 2010;110(5): 736–45. doi: 10.1016/j.jada.2010.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Webster JL, Dunford EK, Neal BC. A systematic survey of the sodium contents of processed foods. Am J Clin Nutr. 2010;91(2): 413–420. doi: 10.3945/ajcn.2009.28688 [DOI] [PubMed] [Google Scholar]
- 6.Ni Mhurchu C, Capelin C, Dunford EK, Webster JL, Neal BC, Jebb SA. Sodium content of processed foods in the United Kingdom: analysis of 44,000 foods purchased by 21,000 households. Am J Clin Nutr. 2011;93(3): 594–600. doi: 10.3945/ajcn.110.004481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization. Salt reduction [online], WHO 2010. Available at: http://www.who.int/mediacentre/factsheets/fs393/en/
- 8.Cappuccio FP & Capewell S. Facts, Issues, and Controversies in Salt Reduction for the Prevention of Cardiovascular Disease. Functional Food Reviews. 2015;7: 41–61. [Google Scholar]
- 9.He FJ & MacGregor GA. Effect of modest salt reduction on blood pressure: a meta-analysis of randomized trials. Implications for public health. J Hum Hypertens. 2002;16: 761–770. doi: 10.1038/sj.jhh.1001459 [DOI] [PubMed] [Google Scholar]
- 10.Aburto NJ, Ziolkovska A, Hooper L, Elliott P, Cappuccio FP, Meerpohl JJ. Effect of lower sodium intake on health: systematic review and meta-analyses. BMJ. 2013;346: f1326 doi: 10.1136/bmj.f1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He FJ, Li J, MacGregor GA. Effect of longer term modest salt reduction on blood pressure: Cochrane systematic review and meta-analysis of randomised trials. BMJ. 2013;346: f1325 doi: 10.1136/bmj.f1325 [DOI] [PubMed] [Google Scholar]
- 12.Cook NR, Cutler JA, Obarzanek E, Buring JE, Rexrode KM, Kumanyika SK, et al. Long term effects of dietary sodium reduction on cardiovascular disease outcomes: observational follow-up of the trials of hypertension prevention (TOHP). BMJ. 2007;334: 885 doi: 10.1136/bmj.39147.604896.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He FJ & MacGregor GA. A comprehensive review on salt and health and current experience of worldwide salt reduction programmes. J Hum Hypertens. 2009;23(6): 363–84. doi: 10.1038/jhh.2008.144 [DOI] [PubMed] [Google Scholar]
- 14.Strazzullo P, D’Elia L, Kandala NB, Cappuccio FP. Salt intake, stroke, and cardiovascular disease: meta-analysis of prospective studies. BMJ. 2009;339: b4567 doi: 10.1136/bmj.b4567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perry IJ & Beevers DG. Salt intake and stroke: a possible direct effect. J Hum Hypertens. 1992; 6(1): 23–5. [PubMed] [Google Scholar]
- 16.Karpannen H & Mervaala E. Sodium intake and hypertension. Prog Cardiovasc Dis. 2006;49(2): 59–75. doi: 10.1016/j.pcad.2006.07.001 [DOI] [PubMed] [Google Scholar]
- 17.Joossens JV, Hill MJ, Elliott P, Stamler R, Lesaffre E, Dyer A et al. Dietary salt, nitrate and stomach cancer mortality in 24 countries. European Cancer Prevention (ECP) and the INTERSALT Cooperative Research Group. Int J Epidemiol. 1996;25: 494–504. [DOI] [PubMed] [Google Scholar]
- 18.Wang X- Q, Terry PD, Yan H. Review of salt consumption and stomach cancer risk: Epidemiological and biological evidence. World J Gastroenterol. 2009;15(18): 2204–13. doi: 10.3748/wjg.15.2204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.D'Elia L, Rossi G, Ippolito R, Cappuccio FP, Strazzullo P. Habitual salt intake and risk of gastric cancer: a meta-analysis of prospective studies. Clin Nutr. 2012;31: 489–98. doi: 10.1016/j.clnu.2012.01.003 [DOI] [PubMed] [Google Scholar]
- 20.WHO. Guideline: Sodium intake for adults and children. Geneva, World Health Organization (WHO),2012 [PubMed] [Google Scholar]
- 21.McLaren L, McIntyre L & Kirkpatrick S. Rose’s population strategy of prevention need not increase social inequalities in health. Int J Epidemiology. 2010;39: 372–377. [DOI] [PubMed] [Google Scholar]
- 22.Adams J, Mytton O, White M, Monsivais P. Why Are Some Population Interventions for Diet and Obesity More Equitable and Effective Than Others? The Role of Individual Agency. PLoS Med (2016); 13 (4): e1001990 doi: 10.1371/journal.pmed.1001990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brownson RC, Seiler R, Eyler AA. Measuring the impact of public health policy. Prev Chronic Dis. 2010;7(4): A77 [PMC free article] [PubMed] [Google Scholar]
- 24.Trieu K, Neal B, Hawkes C, Dunford E, Campbell N, Rodriguez-Fernandez R, et al. Salt initiatives around the world–A systematic review of progress towards the global target. Plos One. 2015;10(7): e0130247 doi: 10.1371/journal.pone.0130247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laatikainen T, Pietinen P, Valsta L, Sundvall J, Reinivuo H, Tuomilehto J. Sodium in the Finnish diet: 20-year trends in urinary sodium excretion among the adult population. Eur J Clin Nutr. 2006;60(8): 965–70. doi: 10.1038/sj.ejcn.1602406 [DOI] [PubMed] [Google Scholar]
- 26.Sasaki N. The salt factor in apoplexy and hypertension: epidemiological studies in Japan In: Yamori Y, editor. Prophylactic approach to hypertensive diseases. New York: Raven Press; 1979. p. 467–74. [Google Scholar]
- 27.He FJ, Pombo-Rodrigues S, Macgregor GA. Salt reduction in England from 2003 to 2011: its relationship to blood pressure, stroke and ischaemic heart disease mortality. BMJ Open. 2014a;4(4): e004549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ji C, Kandala N- B & Cappuccio FP. Spatial variation of salt intake in Britain and association with socio-economic status. BMJ Open. 2013;3: e002246 doi: 10.1136/bmjopen-2012-002246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ji C & Cappuccio FP. Socio-economic inequality in salt intake in Britain 10 years after a national salt reduction programme. BMJ Open. 2014;4: e005683 doi: 10.1136/bmjopen-2014-005683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knai C, Petticrew M, Durand MA, Eastmure E, James L, Mehrotra A, et al. Has a public–private partnership resulted in action on healthier diets in England? An analysis of the Public Health Responsibility Deal food pledges. Food Policy. 2015;54: 1–10. [Google Scholar]
- 31.MacGregor GA, He FJ, Pombo-Rodrigues S. Food and the responsibility deal: how the salt reduction strategy was derailed by Andrew Lansley and the coalition government. Br Med J. 2015;350: h1936. [DOI] [PubMed] [Google Scholar]
- 32.Rose G. Sick individuals and sick populations. International Journal of Epidemiology. 2001;30:427–432 [DOI] [PubMed] [Google Scholar]
- 33.Capewell S & Graham H. Will cardiovascular disease prevention widen health inequalities? PLoS Med. 2010;7(8): e1000320 doi: 10.1371/journal.pmed.1000320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hogberg L, Cnattingius S, Lundholm C, Sparén P, Iliadou AN. Intergenerational social mobility and the risk of hypertension. J Epidemiol Community Health. 2012;66(6): e9 doi: 10.1136/jech.2010.130567 [DOI] [PubMed] [Google Scholar]
- 35.Joossens L & Raw M. The Tobacco Control Scale: a new scale to measure country activity. Tob Control. 2006;15: 247–253. doi: 10.1136/tc.2005.015347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anderson P, Chisholm D, Fuhr DC. Alcohol and Global Health 2. Effectiveness and cost-effectiveness of policies and programmes to reduce the harm caused by alcohol. Lancet. 2009;373: 2234–46. doi: 10.1016/S0140-6736(09)60744-3 [DOI] [PubMed] [Google Scholar]
- 37.Cappuccio FP, Capewell S, Lincoln P, McPherson K. Population salt reduction to prevent cardiovascular disease: identifying policy options. BMJ. 2011;343: d4995 doi: 10.1136/bmj.d4995 [DOI] [PubMed] [Google Scholar]
- 38.NICE Public Health Guidance: Prevention of cardiovascular disease at population level. 2010. Reviewed and updated in 2014: http://guidance.nice.org.uk/PH25/Review
- 39.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for the systematic reviews and meta-analysis: the PRISMA statement. BMJ. 2009;339: b2535 doi: 10.1136/bmj.b2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.He FJ, Wu Y, Feng X- X, Ma J, Ma Y, Wang H, et al. School based education programme to reduce salt intake in children and their families (School-EduSalt): cluster randomised controlled trial. BMJ. 2015;350: h770 doi: 10.1136/bmj.h770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Choi SE, Brandeau ML, Basu S. Expansion of the National Salt Reduction Initiative: A Mathematical Model of Benefits and Risks of Population-Level Sodium Reduction. Medical Decision Making 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.National institute of Health. Quality assessment tools. 2014. Accessed from https://www.nhlbi.nih.gov/health-pro/guidelines/in-develop/cardiovascular-risk-reduction/tools
- 43.Fattore G, Ferre F, Meregaglia M, Fattore E, Agostoni C. Critical review of economic evaluation studies of interventions promoting low-fat diets. Nutr Rev. 2014;72(11): 691–706. doi: 10.1111/nure.12142 [DOI] [PubMed] [Google Scholar]
- 44.Nuffield Council on Bioethics. Policy process and practice In: Public Health: Ethical issues. London, UK: Nuffield Council on Bioethics; 2007: 31–47. [Google Scholar]
- 45.Hooper L, Bartlett C, Davey Smith G, Ebrahim S. Systematic review of long term effects of advice to reduce dietary salt in adults. BMJ. 2002;325(7365): 628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Appel LJ. Effects of Comprehensive Lifestyle Modification on Blood Pressure Control: Main Results of the PREMIER Clinical Trial. JAMA. 2003;289(16): 2083–93. doi: 10.1001/jama.289.16.2083 [DOI] [PubMed] [Google Scholar]
- 47.Brunner E, White I, Thorogood M, Bristow A, Curle D, Marmot M. Can dietary interventions change diet and cardiovascular risk factors: a meta-analysis of randomized controlled trials. Am J Public Health. 1997;87(9): 1415–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Francis S, Taylor M. A social marketing theory-based diet-education program for women ages 54 to 83 years improved dietary status. J Am Diet Assoc. 2009;109(12): 2052–6. doi: 10.1016/j.jada.2009.09.002 [DOI] [PubMed] [Google Scholar]
- 49.Parekh S, Vandelanotte C, King D, Boyle FM. Improving diet, physical activity and other lifestyle behaviours using computer-tailored advice in general practice: a randomised controlled trial. Int J Behav Nutr Phys Act. 2012;9: 108 doi: 10.1186/1479-5868-9-108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Petersen K, Torpy D, Chapman I, Guha S, Clifton P, Turner K, et al. Food label education does not reduce sodium intake in people with type 2 diabetes mellitus. A randomised controlled trial. Appetite. 2013;68: 147–51. doi: 10.1016/j.appet.2013.04.028 [DOI] [PubMed] [Google Scholar]
- 51.Kokanović A, Mandić ML, Banjari I. Does individual dietary intervention have any impact on adolescents with cardiovascular health risks? Medicinski Glasnik. 2014;11(1): 234–7. [PubMed] [Google Scholar]
- 52.Heino T, Kallio K, Jokinen E, Lagström H, Seppänen R, Välimäki I, et al. Sodium intake of 1 to 5-year-old children: the STRIP project. The Special Turku Coronary Risk Factor Intervention Project. Acta paediatrica. 2000;89(4): 406–10. [DOI] [PubMed] [Google Scholar]
- 53.Wang J, Olendzki BC, Wedick NM, Persuitte GM, Culver AL, Li W, et al. Challenges in sodium intake reduction and meal consumption patterns among participants with metabolic syndrome in a dietary trial. Nutrition Journal. 2013, 12:163 doi: 10.1186/1475-2891-12-163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cobiac LJ, Vos T, Veerman JL. Cost-effectiveness of interventions to reduce dietary salt intake. Heart. 2010;96(23): 1920–5. doi: 10.1136/hrt.2010.199240 [DOI] [PubMed] [Google Scholar]
- 55.Cobiac LJ, Magnus A, Lim S, Barendregt JJ, Carter R, Vos T. Which interventions offer best value for money in primary prevention of cardiovascular disease? PLoS One. 2012;7: e41842 doi: 10.1371/journal.pone.0041842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nghiem N, Blakely T, Cobiac LJ, Pearson AL, Wilson N. Health and economic impacts of eight different dietary salt reduction interventions. PLoS ONE. 2015;10(4): e0123915 doi: 10.1371/journal.pone.0123915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cotter J, Cotter MJ, Oliveira P, Cunha P, Polónia J. Salt intake in children 10–12 years old and its modification by active working practices in a school garden. J Hypertens. 2013;31(10): 1966–71. doi: 10.1097/HJH.0b013e328363572f [DOI] [PubMed] [Google Scholar]
- 58.Katz DL, Katz CS, Treu JA, Reynolds J, Njike V, Walker J, et al. Teaching healthful food choices to elementary school students and their parents: the Nutrition DetectivesTM program. J Sch Health. 2011;81(1): 21–8. doi: 10.1111/j.1746-1561.2010.00553.x [DOI] [PubMed] [Google Scholar]
- 59.Aldana SG, Greenlaw RL, Diehl, Salberg A, Merrill RM, Ohmine S. The effects of a worksite Chronic disease prevention program. J Occup Environ Med. 2005;47: 558–564. [DOI] [PubMed] [Google Scholar]
- 60.Chen J, Wu X, Gu D. Hypertension and cardiovascular diseases intervention in the capital steel and iron company and Beijing Fangshan community. Obes Rev. 2008;9 Suppl 1:142–5. [DOI] [PubMed] [Google Scholar]
- 61.Levin S, Ferdowsian H, Hoover V, Green AA, Barnard ND. A worksite programme significantly alters nutrient intakes. Public Health Nutr. 2010;13(10): 1629–35. doi: 10.1017/S136898000999303X [DOI] [PubMed] [Google Scholar]
- 62.Yanek LR, Becker DM, Moy TF, Gittelsohn J, Koffman DM. Project Joy: faith based cardiovascular health promotion for African American women. Public Health Rep. 2001;116 Suppl 1:68–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cappuccio FP, Kerry SM, Micah FB, Plange-Rhule J, Eastwood JB. A community programme to reduce salt intake and blood pressure in Ghana [ISRCTN88789643]. BMC Public Health. 2006;6: 13 doi: 10.1186/1471-2458-6-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Takahashi Y, Sasaki S, Okubo S, Hayashi M, Tsugane S. Blood pressure change in a free-living population-based dietary modification study in Japan. J Hypertension. 2006;24(3): 451–8. [DOI] [PubMed] [Google Scholar]
- 65.Robare JF, Milas NC, Bayles CM, Williams K, Newman AB, Lovalekar MT, et al. The key to life nutrition program: results from a community-based dietary sodium reduction trial. Public Health Nutr. 2010;13(5): 606–14. doi: 10.1017/S1368980009991583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Van de Vijver S, Oti S, Addo J, de Graft-Aikins A, Agyemang C. Review of community-based interventions for prevention of cardiovascular diseases in low- and middle-income countries. Ethn Health. 2012;17(6): 651–76. doi: 10.1080/13557858.2012.754409 [DOI] [PubMed] [Google Scholar]
- 67.Shankar B, Brambila-Macias J, Traill B, Mazzocchi M, Capacci S. An evaluation of the UK Food Standards Agency’s salt campaign. Health Econ. 2013;22(2): 243–50. doi: 10.1002/hec.2772 [DOI] [PubMed] [Google Scholar]
- 68.Collins M, Mason H, O’Flaherty M, Guzman-Castillo M, Critchley J, Capewell S. An economic evaluation of salt reduction policies to reduce coronary heart disease in England: A policy modeling study. Value in Health. 2014;17(5): 517–24. doi: 10.1016/j.jval.2014.03.1722 [DOI] [PubMed] [Google Scholar]
- 69.Gillespie D, Allen K, Guzman-Castillo M, Bandosz P, Moreira P, McGill R et al. The health equity and effectiveness of policy options to reduce dietary salt intake in England: policy forecast. PLoS ONE. 2015;10(7): e0127927 doi: 10.1371/journal.pone.0127927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wilcox ML, Mason H, Fouad FM, Rastam S, al Ali R, Page TF, et al. Cost-effectiveness analysis of salt reduction policies to reduce coronary heart disease in Syria, 2010–2020. Int J Public Health. 2015;60: S23–30. doi: 10.1007/s00038-014-0577-3 [DOI] [PubMed] [Google Scholar]
- 71.Mason H, Shoaibi A, Ghandour R, O’Flaherty M, Capewell S, Khatib R, et al. A cost effectiveness analysis of salt reduction policies to reduce coronary heart disease in four Eastern Mediterranean countries. PLoS ONE. 2014;9(1): e84445 doi: 10.1371/journal.pone.0084445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Babio N, Vicent P, López L, Benito A, Basulto J, Salas-Salvadó J. Adolescents’ ability to select healthy food using two different front-of-pack food labels: a cross-over study. Public Health Nutr. 2014;17(6): 1403–9. doi: 10.1017/S1368980013001274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Elfassy T, Yi S, Eisenhower D, Lederer A, Curtis CJ. Use of sodium information on the nutrition facts label in New York city adults with hypertension. J Acad Nutr Diet. 2015;115(2): 278–83. doi: 10.1016/j.jand.2014.08.027 [DOI] [PubMed] [Google Scholar]
- 74.Pietinen P, Valsta LM, Hirvonen T, Sinkko H. Labelling the salt content in foods: a useful tool in reducing sodium intake in Finland. Public Health Nutr. 2008;11(4): 335–40. doi: 10.1017/S1368980007000249 [DOI] [PubMed] [Google Scholar]
- 75.Temme EHM, van der Voet H, Roodenburg AJC, Bulder A, van Donkersgoed G, van Klaveren J. Impact of foods with health logo on saturated fat, sodium and sugar intake of young Dutch adults. Public Health Nutr. 2011;14(4): 635–44. doi: 10.1017/S1368980010002089 [DOI] [PubMed] [Google Scholar]
- 76.De Menezes EW, Lopes TDVC, Mazzini ER, Dan MCT, Godoy C, Giuntini EB. Application of Choices criteria in Brazil: Impact on nutrient intake and adequacy of food products in relation to compounds associated to the risk of non-transmissible chronic diseases. Food Chem. 2013;140(3): 547–52. doi: 10.1016/j.foodchem.2013.02.031 [DOI] [PubMed] [Google Scholar]
- 77.Roodenburg AJC, van Ballegooijen AJ, Dötsch-Klerk M, van der Voet H, Seidell JC. Modelling of Usual Nutrient Intakes: Potential Impact of the Choices Programme on Nutrient Intakes in Young Dutch Adults. PLoS ONE. 2013;8(8): e72378 doi: 10.1371/journal.pone.0072378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chang HY, Hu YW, Yue CS, Wen YW, Yeh WT, Hsu LS, et al. Effect of potassium enriched salt on cardiovascular mortality and medical expenses of elderly men. Am J Clin Nutr. 2006;83: 1289–96. [DOI] [PubMed] [Google Scholar]
- 79.Murray CJ, Lauer JA, Hutubessy RC, Niessen L, Tomijima N, Rodgers A, et al. Effectiveness and costs of interventions to lower systolic blood pressure and cholesterol: a global and regional analysis on reduction of cardiovascular-disease risk. The Lancet. 2003;361(9359): 717–25. [DOI] [PubMed] [Google Scholar]
- 80.Rubinstein A, Colantonio L, Bardach A, Caporale J, Martí SG, Kopitowski K, et al. Estimation of the burden of cardiovascular disease attributable to modifiable risk factors and cost-effectiveness analysis of preventative interventions to reduce this burden in Argentina. BMC Public Health. 2010;10: 627 doi: 10.1186/1471-2458-10-627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Smith-Spangler CM, Juusola JL, Enns EA, Owens DK, Garber AM. Population strategies to decrease sodium intake and the burden of cardiovascular disease: A cost-effectiveness analysis. Ann Intern Med. 2010;152(8): 481–7. doi: 10.7326/0003-4819-152-8-201004200-00212 [DOI] [PubMed] [Google Scholar]
- 82.Konfino J, Mekonnen TA, Coxson PG, Ferrante D, Bibbins-Domingo K. Projected impact of a sodium consumption reduction initiative in Argentina: an analysis from the CVD policy model—Argentina. PLoS ONE. 2013;8(9): e73824 doi: 10.1371/journal.pone.0073824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rubinstein A, García Martí S, Souto A, Ferrante D, Augustovski F. Generalized cost-effectiveness analysis of a package of interventions to reduce cardiovascular disease in Buenos Aires, Argentina. Cost Eff Resour Alloc. 2009;7(1): 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hendriksen MAH, Hoogenveen RT, Hoekstra J, Geleijnse JM, Boshuizen HC, van Raaij JMA. Potential effect of salt reduction in processed foods on health. Am J Clin Nutr. 2014;99(3): 446–53. doi: 10.3945/ajcn.113.062018 [DOI] [PubMed] [Google Scholar]
- 85.Thow AM, Downs S, Jan S. A systematic review of the effectiveness of food taxes and subsidies to improve diets: understanding the recent evidence. Nutr Rev. 2014;72(9): 551–65. doi: 10.1111/nure.12123 [DOI] [PubMed] [Google Scholar]
- 86.Niebylski ML, Redburn KA, Duhaney T, Campbell NR. Healthy food subsidies and unhealthy food taxation: A systematic review of the evidence. Nutrition. 2015;31(6): 787–95. doi: 10.1016/j.nut.2014.12.010 [DOI] [PubMed] [Google Scholar]
- 87.Mhurchu CN, Eyles H, Genc M, Scarborough P, Rayner M, Mizdrak A, et al. Effects of health-related food taxes and subsidies on mortality from diet-related disease in New Zealand: An econometric-epidemiologic modelling study. PLoS ONE 2015;10(7): e0128477 doi: 10.1371/journal.pone.0128477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.He FJ, Brinsden HC, Macgregor GA. Salt reduction in the United Kingdom: A successful experiment in public health. J Hum Hypertens. 2014a;28(6): 345–52. [DOI] [PubMed] [Google Scholar]
- 89.Mozaffarian D, Ashkan A, Benowitz NL, Bittner V, Daniels SR, Franch HA, et al. Population Approaches to Improve Diet, Physical Activity, and Smoking Habits. A Scientific Statement From the American Heart Association. Circulation. 2012; 126(12): 1514–1563 doi: 10.1161/CIR.0b013e318260a20b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.He FJ, MacGregor GA. A comprehensive review on salt and health and current experience of worldwide salt reduction programmes. J Hum Hypertens. 2009;23(6): 363–84. doi: 10.1038/jhh.2008.144 [DOI] [PubMed] [Google Scholar]
- 91.Pietinen P, Mannisto S, Valsta LM, Sarlio-Lähteenkorva S. Nutrition policy in Finland. Public Health Nutr. 2010;13(6A): 901–6. doi: 10.1017/S1368980010001072 [DOI] [PubMed] [Google Scholar]
- 92.Wang G, Labarthe D. The cost-effectiveness of interventions designed to reduce sodium intake. J Hypertens. 2011;29(9): 1693–9. doi: 10.1097/HJH.0b013e328349ba18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Webster JL, Dunford EK, Hawkes C, Neal BC. Salt reduction initiatives around the world. J Hypertens. 2011;29(6): 1043–50. doi: 10.1097/HJH.0b013e328345ed83 [DOI] [PubMed] [Google Scholar]
- 94.Wang G, Bowman BA. Recent economic evaluations of interventions to prevent cardiovascular disease by reducing sodium intake. Curr Atheroscler Rep. 2013;15(9): 349 doi: 10.1007/s11883-013-0349-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.He FJ, Pombo-Rodrigues S, Macgregor GA. Salt reduction in England from 2003 to 2011: its relationship to blood pressure, stroke and ischaemic heart disease mortality. BMJ Open. 2014b;4(4): e004549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Enkhtungalag B, Batjargal J, Chimedsuren O, Tsogzolmaa B, Anderson CS, Webster J. Developing a national salt reduction strategy for Mongolia. Cardiovasc Diagn Ther. 2015;5(3): 229–37. doi: 10.3978/j.issn.2223-3652.2015.04.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Luft FC, Morris CD, Weinberger MH. Compliance to a low-salt diet. Am J Clin Nutr. 1997;65(2): 698S–703S. [DOI] [PubMed] [Google Scholar]
- 98.Mohan S, Campbell NRC, Willis K. Effective population-wide public health interventions to promote sodium reduction. CMAJ. 2009;181(9): 605–9. doi: 10.1503/cmaj.090361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.He FJ & MacGregor GA. Reducing population salt intake worldwide: from evidence to implementation. Prog Cardiovasc Dis. 2010;52(5): 363–82. doi: 10.1016/j.pcad.2009.12.006 [DOI] [PubMed] [Google Scholar]
- 100.Wyness LA, Butriss JL, Stanner SA. Reducing the population’s sodium intake: The UK Food Standards Agency’s salt reduction programme. Public Health Nutr. 2012;15(2): 254–61. doi: 10.1017/S1368980011000966 [DOI] [PubMed] [Google Scholar]
- 101.Otsuka R, Kato Y, Imai T, Ando F, Shimokata H. Decreased salt intake in Japanese men aged 40 to 70 years and women aged 70 to 79 years: an 8-year longitudinal study. J Am Diet Assoc. 2011;111(6): 844–50. doi: 10.1016/j.jada.2011.03.020 [DOI] [PubMed] [Google Scholar]
- 102.Du S, Batis C, Wang H, Zhang B, Zhang J, Popkin BM. Understanding the patterns and trends of sodium intake, potassium intake, and sodium to potassium ratio and their effect on hypertension in China. Am J Clin Nutr. 2014;99(2): 334–43. doi: 10.3945/ajcn.113.059121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Miura K, Ando K, Tsuchihashi T, Yoshita K, Watanabe Y, Kawarazaki H, et al. [Scientific Statement] Report of the Salt Reduction Committee of the Japanese Society of Hypertension(2) Goal and strategies of dietary salt reduction in the management of hypertension. Hypertens Res. 2013;36(12): 1020–5. doi: 10.1038/hr.2013.105 [DOI] [PubMed] [Google Scholar]
- 104.Asaria P, Chisholm D, Mathers C, Ezzati M, Beaglehole R. Chronic disease prevention: health effects and financial costs of strategies to reduce salt intake and control tobacco use. Lancet. 2007;370(9604): 2044–53. doi: 10.1016/S0140-6736(07)61698-5 [DOI] [PubMed] [Google Scholar]
- 105.Dodhia H, Phillips K, Zannou M- I, Airoldi M, Bevan G. Modelling the impact on avoidable cardiovascular disease burden and costs of interventions to lower SBP in the England population. J Hypertens. 2012;30(1): 217–26. doi: 10.1097/HJH.0b013e32834d86ee [DOI] [PubMed] [Google Scholar]
- 106.Gase LN, Kuo T, Dunet D, Schmidt SM, Simon PA, Fielding JE. Estimating the potential health impact and costs of implementing a local policy for food procurement to reduce the consumption of sodium in the county of Los Angeles. Am J Public Health. 2011;101(8): 1501–7. doi: 10.2105/AJPH.2011.300138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ha DA, Chisholm D. Cost-effectiveness analysis of interventions to prevent cardiovascular disease in Vietnam. Health Policy Plan. 2011;26(3): 210–22. doi: 10.1093/heapol/czq045 [DOI] [PubMed] [Google Scholar]
- 108.Laatikainen T, Critchley J, Vartiainen E, Salomaa V, Ketonen M, Capewell S. Explaining the Decline in Coronary Heart Disease Mortality in Finland between 1982 and 1997. Am. J. Epidemiol., 2005; 162: 764–773 doi: 10.1093/aje/kwi274 [DOI] [PubMed] [Google Scholar]
- 109.Van Vliet BN, Campbell NRC, Canadian Hypertension Education Program. Efforts to reduce sodium intake in Canada: why, what, and when? Can J Cardiol. 2011;27(4): 437–45. doi: 10.1016/j.cjca.2011.04.012 [DOI] [PubMed] [Google Scholar]
- 110.Bech-Larsen T & Aschemann-Witzel J. A Macromarketing Perspective on Food Safety Regulation The Danish Journal of Macromarketing. 2012; 32(2) 208–219 [Google Scholar]
- 111.Lloyd-Williams F, Bromley H, Orton L, Hawkes C, Taylor-Robinson D, O'Flaherty M, et al. Smorgasbord or symphony? Assessing public health nutrition policies across 30 European countries using a novel framework. BMC Public Health. 2014;14: 1195 doi: 10.1186/1471-2458-14-1195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.MacGregor GA, He FJ, Pombo-Rodrigues S. Food and the responsibility deal: how the salt reduction strategy was derailed by Andrew Lansley and the coalition government. Br Med J. 2015;350: h1936. [DOI] [PubMed] [Google Scholar]
- 113.Campos S, Doxey J, Hammond D. Nutrition labels on pre-packaged foods: a systematic review. Public Health Nutr. 2011;14(8): 1496–506. doi: 10.1017/S1368980010003290 [DOI] [PubMed] [Google Scholar]
- 114.Cowburn G & Stockley L. Consumer understanding and use of nutrition labelling: a systematic review. Public Health Nutr. 2005;8(01): 21–8. [DOI] [PubMed] [Google Scholar]
- 115.Wakefield MA, Loken B, Hornik RC. Use of mass media campaigns to change health behaviour. The Lancet. 2010; 376:1261–1271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mols F, Haslam SA, Jetten J, Steffens NK. Why a Nudge is Not Enough: A Social Identity Critique of Governance by Stealth. Euro J Polit Res. 2014; 54(1): 87–98. [Google Scholar]
- 117.Fransen ML, Smit EG, Verlegh PWJ. Strategies and motives for resistance to persuasion: an integrative framework. Front Psychol. 2015;6: 1201 doi: 10.3389/fpsyg.2015.01201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Forshee RA. Innovative regulatory approaches to reduce sodium consumption: could a cap-and-trade system work? Nutr Rev. 2008;66(5): 280–5. doi: 10.1111/j.1753-4887.2008.00033.x [DOI] [PubMed] [Google Scholar]
- 119.Bibbins-Domingo K, Chertow GM, Coxson PG, Moran A, Lightwood JM, Pletcher MJ, et al. Projected effect of dietary salt reductions on future cardiovascular disease. N Eng J Med. 2010;362(7): 590–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Barton P, Andronis L, Briggs A, McPherson K, Capewell S. Effectiveness and cost effectiveness of cardiovascular disease prevention in whole populations: Modelling study. BMJ. 2011;343: d4044 doi: 10.1136/bmj.d4044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cobiac LJ, Veerman L, Vos T. The role of cost-effectiveness analysis in developing nutrition policy. Annu Rev Nutr. 2013;33: 373–93. doi: 10.1146/annurev-nutr-071812-161133 [DOI] [PubMed] [Google Scholar]
- 122.Owen L, Morgan A, Fischer A, Ellis S, Hoy A, Kelly MP. The cost-effectiveness of public health interventions. J Public Health. 2011;34(1): 37–45. [DOI] [PubMed] [Google Scholar]
- 123.Beckmann S, Os I, Kjeldsen S, Eide I, Westheim A, Hjermann I. Effect of dietary counselling on blood pressure and arterial plasma catecholamines in primary hypertension. Am J hypertens. 1995;8(7): 704–11. [DOI] [PubMed] [Google Scholar]
- 124.Tian HG, Guo ZY, Hu G, Yu SJ, Sun W, Pietinen P, et al. Changes in sodium intake and blood pressure in a community-based intervention project in China. J Hum Hypertens. 1995;9(12): 959–68. [PubMed] [Google Scholar]
- 125.Cappuccio FP. Salt and cardiovascular disease. Br Med J. 2007;334: 859–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rodriguez-Fernandez R, Siopa M, Simpson SJ, Amiya RM, Breda J, Cappuccio FP. Current salt reduction policies across gradients of inequality-adjusted human development in the WHO European region: minding the gaps. Public Health Nutr. 2014;17: 1894–904. doi: 10.1017/S136898001300195X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Fair Society, Healthy Lives. The Marmot Review. Strategic Review of Health Inequalities in England post-2010. Published by The Marmot Review; 2010;1–238. [Google Scholar]
- 128.Cappuccio FP, Ji C, Donfrancesco C, Palmieri L, Ippolito R, Vanuzzo D et al. Geographic and socioeconomic variation of sodium and potassium intake in Italy: results from the MINISAL-GIRCSI programme. BMJ Open. 2015;5: e007467 doi: 10.1136/bmjopen-2014-007467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.McGill R, Anwar E, Orton L, Bromley H, Lloyd-Williams F, O'Flaherty M, et al. Are interventions to promote healthy eating equally effective for all? Systematic review of socioeconomic inequalities in impact. BMC Public Health. 2015;15: 457 doi: 10.1186/s12889-015-1781-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lorenc T, Petticrew M, Welch V, Tugwell P. What types of interventions generate inequalities? Evidence from systematic reviews. J Epidemiol Community Health. 2012;67(2): 190–3. doi: 10.1136/jech-2012-201257 [DOI] [PubMed] [Google Scholar]
- 131.Orton L, Lloyd-Williams F, Taylor-Robinson D, O'Flaherty M, Capewell S. The Use of Research Evidence in Public Health Decision Making Processes: Systematic Review. PLoS ONE. 2011;6(7): e21704 doi: 10.1371/journal.pone.0021704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Oliver K, Innvar S, Lorenc T, Woodman J, Thomas J. A systematic review of barriers to and facilitators of the use of evidence by Policymakers. BMC Health Services Research. 2014;14: 2 doi: 10.1186/1472-6963-14-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.McKinlay JB & Marceau LD. Upstream healthy public policy: Lessons from the battle of tobacco. Int J Health Serv. 2000;30(1): 49–69. doi: 10.2190/2V5H-RHBR-FTM1-KGCF [DOI] [PubMed] [Google Scholar]
- 134.Krieger N. Proximal, Distal, and the Politics of Causation: What's Level Got to Do With It? Am J Public Health. 2008; 98: 221–230. doi: 10.2105/AJPH.2007.111278 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOC)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.