Abstract
Background
Although Helicobacter pylori (H. pylori) infection is closely associated with the development of peptic ulcer, its involvement in pathophysiology in the lower intestinal tract and gastrointestinal (GI) motility remains unclear. Glucagon-like peptide-1 (GLP-1) is a gut hormone produced in the lower intestinal tract and involved in GI motility. Here, we investigated the effect of H. pylori infection on the link between GLP-1 expression and motility of the GI tract.
Methods
C57BL/6 mice were inoculated with a H. pylori strain. Twelve weeks later, the H. pylori-infected mice underwent H. pylori eradication treatment. GI tissues were obtained from the mice at various time intervals, and evaluated for the severity of gastric inflammatory cell infiltration and immunohistochemical expression of GLP-1 and PAX6 in the colonic mucosa. Gastrointestinal transit time (GITT) was measured by administration of carmine-red solution.
Results
GLP-1 was expressed in the endocrine cells of the colonic mucosa, and PAX6 immunoreactivity was co-localized in such cells. The numbers of GLP-1- and PAX6-positive cells in the colon were significantly increased at 12 weeks after H. pylori infection and showed a positive correlation with each other. The GITT was significantly longer in H. pylori-infected mice than in non-infected controls and showed a positive correlation with GLP-1 expression. When H. pylori-infected mice underwent H. pylori eradication, GITT and PAX6/GLP-1 expression did not differ significantly from those in untreated H. pylori-infected mice.
Conclusions
H. pylori infection may impair GI motility by enhancing the colonic GLP-1/PAX6 expression.
Introduction
Helicobacter pylori (H. pylori) is a bacterium capable of colonizing the gastric mucosa, causing chronic active gastritis. This continuous inflammation in the stomach plays a pivotal role in not only the development of gastric cancer but also dysfunction of the gastrointestinal (GI) tract. Indeed, chronic H. pylori infection is known to disrupt gastric acid secretion [1, 2] and impair GI motility [3, 4]. Furthermore, it is widely accepted that H. pylori infection significantly affects the profile of cytokines and endocrine cells in the stomach [4–6], and that these effects are likely linked to the observed impairment of gastric acid secretion or motility [1–4]. Recent evidence suggests that H. pylori infection plays a role in pathophysiology of not only the stomach but also other systemic organs including the lower GI tract [3, 7]. For instance, it is interesting that H. pylori infection is associated with the symptoms (abdominal pain or discomfort, etc.) of irritable bowels syndrome (IBS) [3, 7] as well as those (satiation, fullness, epigastric pain, etc.) of functional dyspepsia (FD) [8, 9], suggesting that H. pylori infection may be involved in dysfunction throughout the GI tract.
Glucagon-like peptide 1 (GLP-1) is an incretin hormone produced by intestinal endocrine cells [10] and regulates glucose homeostasis by stimulating insulin secretion from pancreatic β-cells [11, 12]. Furthermore, GLP-1 has been suggested to suppress gastric emptying [13, 14], to inhibit additional food intake and postprandial hyperglycemia. Likewise, GLP-1 plays important roles in not only metabolism but also GI motility [12, 15], and therefore this gut hormone has received considerable attention. However, it still remains to be clarified how GLP-1 is involved in alteration of motility throughout the GI tract and how H. pylori infection and its eradication affect the link between GLP-1 and GI motility. In the present study, therefore, we infected mice with H. pylori and examined the expression of GLP-1 and its transcriptional factor PAX6 [16] in the GI tract in relation to GI motility.
Methods
Animals and H. pylori strain
C57BL/6 mice (10-week-old females) were used in this study. They were housed in an air conditioned biohazard room with free access to food and water. The experimental protocol was approved by the Animal Use and Care Committee at Hyogo College of Medicine. H. pylori strain (Sydney strain 1) was grown on Skirrow agar plates containing 7% horse blood (NBLi; Tokyo, Japan) at 37°C for 5 days under microaerobic conditions. To prepare the bacterial suspension, bacterial colonies were scraped from the plates, transferred into Brucella broth (Becton Dickinson, Franklin Lakes, NJ, USA) containing 5% fetal bovine serum and incubated under microaerobic conditions.
Experimental design
Experimental schedule is shown in S1 Fig. C57BL/6 mice were inoculated with 200 μl of culture broth containing 1 x 108 colony-forming units H. pylori via a gastric tube daily for 3 days. Mice that received 200 μl of culture broth alone were employed as uninfected controls. Twelve weeks after inoculation, a proportion of the H. pylori-infected mice were orally administered lansoprazole, amoxicillin, and clarithromycin (30, 30, 30 mg/kg body weight, respectively) suspended in 0.5w/v% carboxymethylcellulose sodium solution once daily for 5 days [17]. Infected mice were killed at 4, 12 and 24 weeks after inoculation. The mice that had undergone H. pylori eradication were killed at 24 weeks after inoculation (i.e., 12 weeks after eradication). Gastrointestinal tissues were obtained from those mice after fasting for 12 hours. To confirm whether H. pylori infection and its eradication are successful, samples of gastric tissue were homogenized in Brucella broth and cultured on H. pylori-selective agar plates (Eiken Chemical Co., Ltd., Tokyo, Japan) as reported previously [18]. All mice used for analyses were qualified by this culture test.
Histopathological evaluation
The obtained GI tissues were fixed in 10% buffered formalin, sliced perpendicularly to the surface, embedded in paraffin, and cut into 4-μm sections. The sections were stained by haematoxylin and eosin for histopathological observations. The degree of inflammatory cell infiltration in the stomach was scored on a scale of 0 to 3 as previously described [19]: 0, normal; 1, mild; 2, moderate; 3, marked. The scores were evaluated in all of the slips from each stomach, and the results were averaged.
Immunohistochemistry
Immunohistochemical stainings for GLP-1 and paired box protein-6 (PAX6) were performed with an Envision Kit (Dako, Kyoto, Japan) according to the manufacturer’s protocol [20], using anti-GLP-1 antibody (Abcam, Cambridge, UK; dilution 1:500) and anti-PAX6 antibody (Merck KGaA, Darmstadt, Germany; dilution 1:500). As previously described [20], the sections were deparaffinized, rehydrated, placed in 0.01 M citrate buffer (pH 6.0), and treated by microwave heating for 20 min. The sections were then preincubated with 0.3% H2O2 in methanol for 20 min at room temperature to quench endogenous peroxidase activity. The sections were incubated with primary antibodies for 1 h at room temperature. Then, the slides were incubated with HRP-conjugated secondary antibody for 30 min, visualized by 3,3’-diaminobenzide tetrahydrochloride with 0.05% H2O2 for 3 min, and counterstained with Mayer’s haematoxylin. The number of GLP-1- or PAX6-positive epithelial cells was counted in a 500- or 1000-μm stretch of the entire length of well-oriented tissue sections in at least four different visual fields for each colon, and the average was calculated.
Immunohistochemical double staining was performed as previously described [20]. In brief, the sections were incubated with mouse anti-GLP-1 antibody or rabbit anti-PAX6 antibody for 60 min at room temperature. The sections were then incubated with fluorescein isothiocyanate-conjugated anti-mouse immunoglobulin (1: 200; Dako, Kyoto, Japan) and tetramethylrhodamine isothiocyanate-conjugated anti-rabbit immunoglobulin (1: 200; Dako, Kyoto, Japan) for 30 min at room temperature. After washing in phosphate-buffered saline, the sections were observed by fluorescence microscopy (DP72; Olympus, Tokyo, Japan).
Gastrointestinal transient time
GI transient time (GITT) was measured as previously described [21]. In brief, the mice received orally 0.3 mL of 0.5% methylcellulose solution including 6% carmine red (Wako, Osaka, Japan). After administration of the solution, mice were left free for food and water ad libitum until the first red fecal pellet appeared. GITT was determined as the time period between the gavage and the appearance of the first red fecal pellet.
Western blot analysis
Western blot analyses were performed using each primary antibody as previously described [22]. In brief, proteins were extracted from a whole tissue of the colon. Protein extract (20 μg) was fractionated by sodium dodecyl sulfate polyacrylamide gel electrophoresis, transferred to a polyvinylidene difluoride membrane, and detected using an enhanced chemiluminescence system (Amersham Biosciences, Buckinghamshire, UK). ImageJ software (NIH) was used for quantification of intensities of target bands. The staining intensity of β-actin was set as the internal control. The value in the individual test was expressed as fold of target protein/β-actin in the standard group.
Statistical analysis
All values were expressed as the mean ± SEM. Significance of differences between two animal groups was analyzed by Mann-Whitney U-test. Correlation among GITT, inflammatory cell infiltration, GLP-1 and PAX6 were assessed by linear regression analysis. Differences were considered to be significant at p < 0.05.
Results
Expression of GLP-1 and PAX6 in mice with H. pylori infection
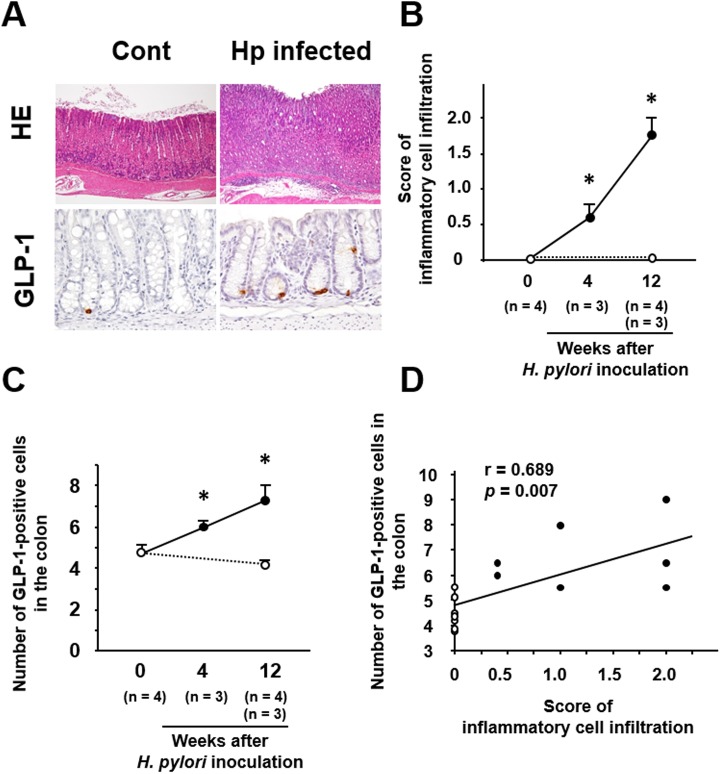
Infiltration of inflammatory cells was observed in the gastric mucosa of mice with H. pylori infection (Fig 1A) and its severity increased for up to 12 weeks after H. pylori inoculation (Fig 1B). However, no histopathological abnormality was observed in the small intestine and colon of mice with H. pylori infection (data not shown).
Fig 1. Effect of H. pylori infection on gastric inflammatory cell infiltration and colonic GLP-1 expression.
(A) Representative images of GI tissues in mice with H. pylori infection. Inflammatory cells are mainly infiltrating into the mucosal lamina propria in the stomach. GLP-1 is expressed in the cytoplasm of ovoid or pyramidal epithelial cells in the colonic mucosa and the number of these cells is increased relative to control mice without H. pylori infection. (B) Serial scores of inflammatory cell infiltration in the gastric mucosa of mice with H. pylori infection. (C) Serial counts of GLP-1-positive cells in the colonic mucosa of mice with H. pylori infection. (D) Correlation between scores for gastric inflammatory cell infiltration and number of colonic GLP-1-posiitve cells. ○, control without H. pylori infection; ●, H. pylori-infected mice. All the results are expressed as the mean ± SE. Significantly greater than control at start of the experiment: *P <0.05.
Immunoreactivity for GLP-1 was localized in the cytoplasm of ovoid or pyramidal epithelial cells in the colonic mucosa, the morphology being consistent with gut endocrine cells (Fig 1A). The number of GLP-1-positive cells in the colonic mucosa gradually increased in H. pylori-infected mice for up to 12 weeks after inoculation (Fig 1C). As the increase of gastric inflammation appeared to parallel the increase in GLP-1-positive cells, we investigated the correlation between them, and this revealed that the number of GLP-1-positive cells in the colon was positively correlated with the severity of inflammatory cell infiltration in the stomach (Fig 1D).
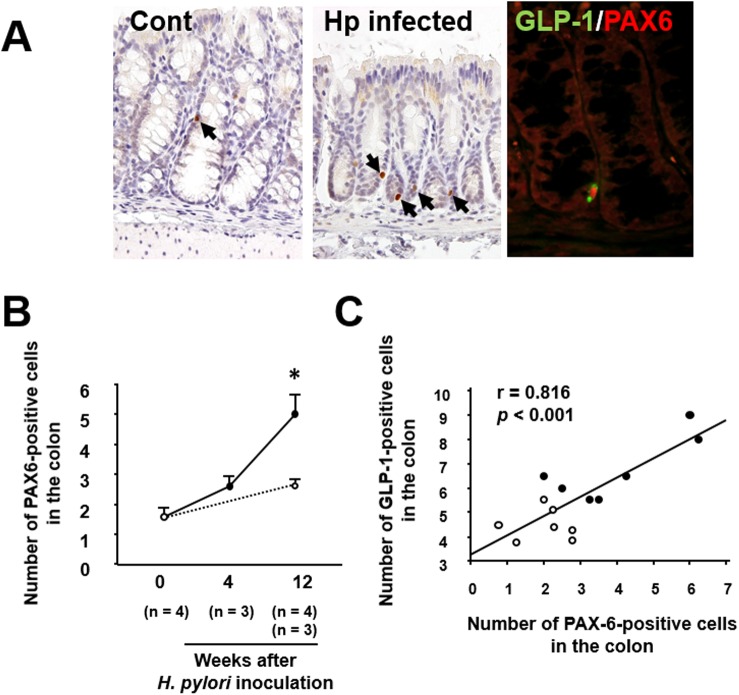
Immunoreactivity for PAX6 was localized in the nuclei of epithelial cells in the colonic mucosa (Fig 2A). Double immunostaining showed that PAX6 was co-expressed in GLP-1-positive epithelial cells in the colonic mucosa (Fig 2A). As shown in Fig 2B, the number of PAX6-positive cells was significantly increased in the colonic mucosa of mice with H. pylori infection. Furthermore, the number of GLP-1-positive cells was positively correlated with that of PAX6-positive cells in the colonic mucosa (Fig 2C).
Fig 2. Effect of H. pylori infection on colonic PAX6 expression.
(A) Immunostaining of PAX6 in the colonic mucosa of mice with H. pylori infection. PAX6 is expressed in the nuclei of colonic epithelial cells (arrows) and the number of cells expressing it is increased relative to control mice without H. pylori infection. Double immunostaining showing co-expression of GLP-1 (green) and PAX6 (red) in a colonic epithelial cell. (B) Serial counts of PAX6-posiitve cells in the colonic mucosa of mice with H. pylori infection. (C) Correlation between the numbers of PAX6- and GLP-1-positive cells. ○, control without H. pylori infection; ●, H. pylori-infected mice. All the results are expressed as the mean ± SE. Significantly greater than control at start of the experiment: *P <0.05.
Effect of H. pylori eradication on GLP-1/PAX6 expression and gastrointestinal motility in mice with H. pylori infection
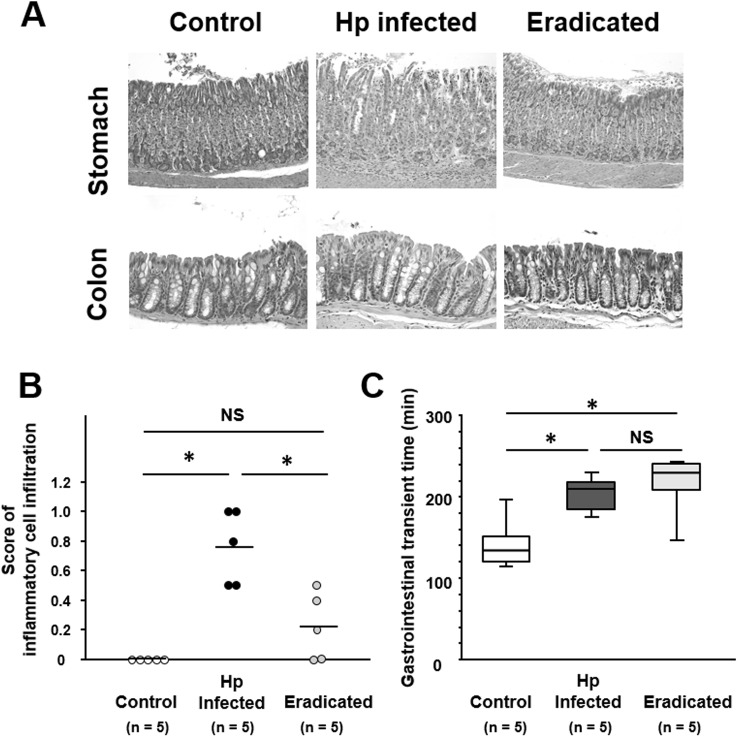
The severity of gastric inflammation was significantly greater in H. pylori-infected mice than in age-matched controls (Fig 3A and 3B). The increased severity of gastric inflammation in H. pylori-infected mice was significantly decreased by the eradication treatment (Fig 3A and 3B). In the colonic mucosa, no histopathological differences were evident between H. pylori-infected mice with eradication and those without (Fig 3A). GITT was significantly longer in mice with H. pylori infection than in controls (Fig 3C). When H. pylori-infected mice were given eradication treatment, their GITT did not alter significantly from that in H. pylori-infected mice without eradication treatment.
Fig 3. Effect of H. pylori infection and its eradication on GI motility.
(A) Histology of the gastric mucosa in mice with H. pylori infection and after eradication. When compared with untreated mice with H. pylori infection, the inflammatory cell infiltration is clearly suppressed in mice after eradication treatment. (B) Scores of inflammatory cell infiltration in the gastric mucosa of mice infected with H. pylori, and after H. pylori eradication. Mean values are shown as bars. (C) Gastrointestinal transit time in mice infected with H. pylori and after H. pylori eradication. Data are presented as medians and interquartile range (n = 5 in each group). Significantly different between two groups: *P <0.05. NS, not significant.
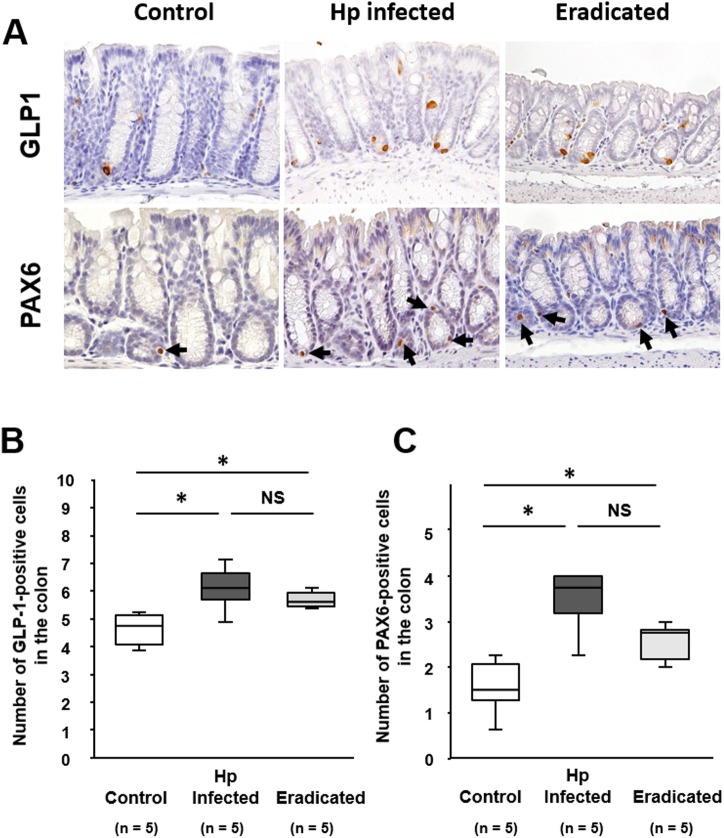
The number of GLP-1-positive cells was significantly increased in the colon of mice with H. pylori infection relative to that in age-matched non-inoculated controls (Fig 4A and 4B). On the other hand, the population of GLP-1-positive cells in H. pylori-infected mice was not affected by eradication treatment. Similarly, the number of PAX6-positive cells was significantly increased in the colonic epithelium of mice with H. pylori infection, and this increase was sustained after eradication treatment (Fig 4A and 4C).
Fig 4. Effect of H. pylori infection and its eradication on GLP-1 and PAX6 expression in the colonic mucosa.
(A) Immunostaining of GLP-1 and PAX6 in the colonic mucosa of mice infected with H. pylori, and after H. pylori eradication. Arrows indicate PAX6-positive cells. Numbers of cells expressing GLP-1 (B) and PAX6 (C) in the colonic mucosa of mice infected with H. pylori, and after H. pylori eradication. Data are presented as medians and interquartile range (n = 5 in each group). Significantly different between two groups: *P <0.05. NS, not significant.
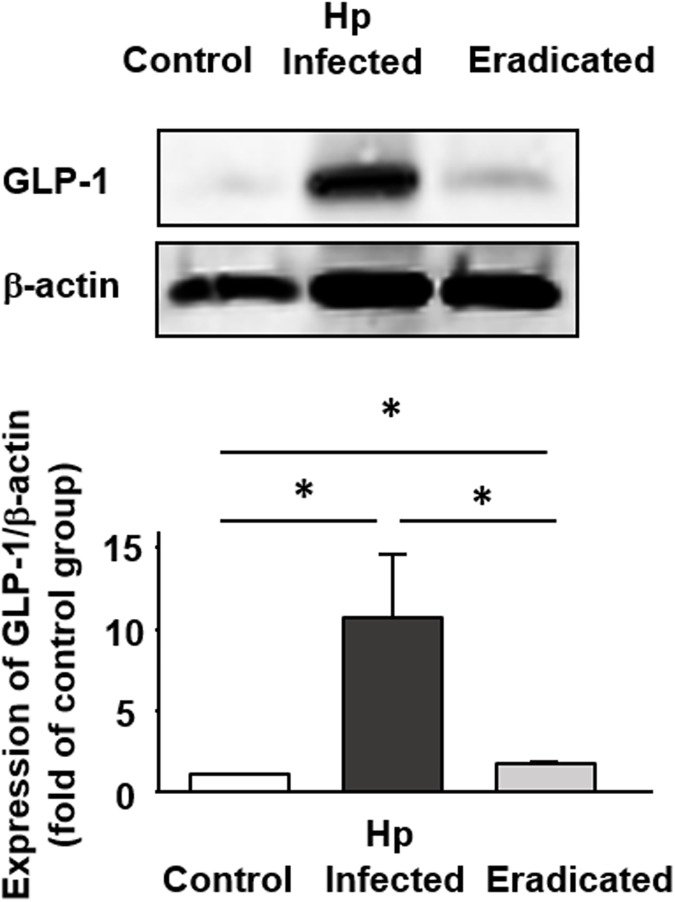
Moreover, we examined the expression level of GLP-1 protein in the colonic tissues (Fig 5). GLP-1 expression was significantly increased in H. pylori-infected mice than uninfected control, being compatible with immunohistochemical findings. This increase was inhibited by H. pylori eradication but the level of GLP-1 expression was still higher than that in uninfected control.
Fig 5. Quantitative evaluation of GLP-1 expression in the colon of mice infected with H. pylori and after H. pylori eradication.
Results are expressed as the mean ± SEM (n = 5). *Significantly different between two groups; P < 0.05.
Gastrointestinal transit time and its relationship to GLP-1/PAX6 expression in mice with H. pylori infection/eradication
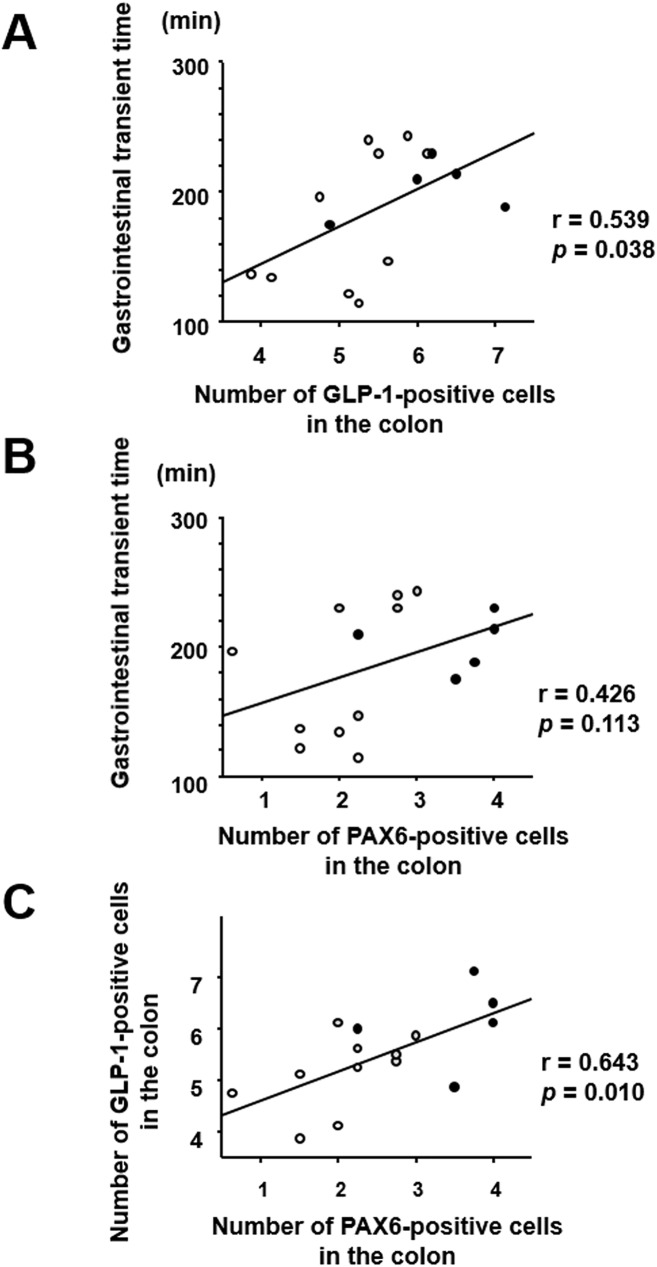
The patterns of change in GITT and GLP-1/PAX6 expression in mice with H. pylori infection/eradication were similar (Figs 3 and 4); therefore, we investigated the correlation between these two parameters (Fig 6). GITT was positively correlated with the number of GLP-1- and PAX6-positive cells in the colonic mucosa (Fig 6A and 6B). Moreover, the numbers of GLP-1- and PAX6-positive cells in the colonic mucosa were significantly correlated (Fig 6C).
Fig 6.
Correlation between gastrointestinal transit time and (A) colonic expression of GLP-1 or (B) PAX6. (C) Correlation between GLP-1 and PAX6 expression in the colon. White dots, controls; black dots, infected mice; gray dots, mice after eradication treatment.
Discussion
The pathophysiology of H. pylori-infected gastric mucosa has been intensively investigated worldwide, whereas that of the lower gastrointestinal tract in H. pylori-infected individuals has not been fully elucidated. In the present study, we observed that GLP-1 was expressed in colonic epithelial cells with a morphology characteristic of endocrine cells, being consistent with previous reports [10]. Interestingly, we clearly observed that GLP-1-positive cells were increased in number in the colonic mucosa of mice with H. pylori infection. Although it is difficult to speculate why H. pylori infection should affect the behavior of colonic GLP-1-positive cells, it is noteworthy that the number of such cells was significantly correlated with the severity of gastric inflammation. This suggests that gastric inflammation may be directly or indirectly associated with the behavior of colonic GLP-1-positive cells. GLP-1 is encoded by the proglucagon gene and its transcription may be enhanced by cytokine stimulation [23]. Although the pathway responsible for intracellular signaling in this process is not fully understood, PAX6 is reported to act as a significant transcription factor to activate the promoter of the proglucagon gene [16, 24]. Therefore, we investigated the expression of PAX6 in H. pylori-infected mice and found that PAX6 was co-expressed in the colonic epithelial cells. Furthermore, we showed that PAX6 as well as GLP-1 expression was increased in H. pylori-infected mice, and that the expression of PAX6 was significantly correlated with that of GLP-1. These findings suggest that the PAX6/GLP-1 axis is certainly activated in H. pylori-infected mice, although the exact mechanism responsible remains to be elucidated.
Patients with gastritis due to H. pylori infection often have functional disorder in the area from the upper to the lower GI tract [3, 4, 7–9]. To examine the effect of H. pylori infection on gastrointestinal motility, we measured the GITT in mice with H. pylori infection and found that it was significantly prolonged. This finding was consistent with the increase of GLP-1 expression in the H. pylori-infected group, as the GLP-1 signal is known to have an inhibitory effect on gastrointestinal motility [12–15]. Indeed, in the present study, the delay of GITT was significantly correlated with the increase of GLP-1 expression. However, the mechanism by which GLP-1 inhibits gastrointestinal motility is still not fully clear, partly because of the current lack of data on GLP-1 receptor localization in vivo. Although the specificity of available anti-GLP-1 receptor antibodies remains debatable [25], a few investigators have reported that immunoreactivity for the GLP-1 receptor is localized in the neural cells in the GI walls [15, 26, 27]. Therefore, it is tempting to speculate that GLP-1 signaling affects the nervous system in the GI wall, and that this may explain why H. pylori-infected mice have increased GLP-1 expression and delayed GI motility.
In the present study, we examined the effect of H. pylori eradication on GLP-1 expression and GI motility. Eradication treatment significantly improved the inflammation in the gastric mucosa but had no effect on the increased number of GLP-1-positive cells in the colonic mucosa. In addition, we showed that eradication treatment did not affect either PAX6 expression or GITT in mice with H. pylori infection. Thus, although we had speculated a possible link between gastric inflammation and these factors, our data seem to suggest that this is unlikely. Thus, against our expectation, eliminated gastritis did not link to the reduction of GLP-1 expression. However, when we investigate GLP-1 expression in eradicated mice, we have to consider the effect of not only eliminated gastritis but also antibiotics themselves. Indeed, recent studies have reported that some antibiotics treatments increased plasma GLP-1 level and/or the number of GLP-1-positive cells in the intestine of animal model [28–30]. On the other hand, a few studies have reported that eradication does not affect the level of pre-prandial plasma GLP-1 in human [31, 32], not conflicting to our immunohistological data. However, on the whole, it may be difficult to understand the regulation of GLP-1 expression in subjects who underwent eradication for H. pylori infection. In contrast, however, it is interesting that the pattern of change in GITT and GLP-1/PAX6 expression in mice with H. pylori infection/eradication was similar, and that these factors were well correlated with each other. In humans, H. pylori eradication treatment is useful for relieving the symptoms in a very small subgroup of patients with functional gastrointestinal disorders such as functional dyspepsia [33] and irritable bowels syndrome [3, 34], paradoxically suggesting that the treatment has less effect on functional gastrointestinal disorders in patients with H. pylori infection. Thus, our finding that eradication treatment did not affect GITT in mice with H. pylori infection appears to reflect the above findings in humans. It is difficult to speculate why functional gastrointestinal disorder in patients with H. pylori infection is mostly irreversible. However, it is well known that GI bacterial infection can trigger functional gastrointestinal disorder and that the dysfunctions of GI motility continues even after the infection has been eliminated [35, 36]. These observations suggest that bacterial infection may be involved in the initiation of irreversible dysfunction of GI motility, and in this regard, our experimental model may be useful for studying the pathophysiology of functional gastrointestinal disorder in patients with H. pylori infection.
In summary, we have shown that H. pylori infection increases the expression of PAX6/GLP-1 in colonic epithelial cells and prolongs GITT in mice, and that furthermore GITT is significantly correlated with GLP-1 expression. We also observed that H. pylori eradication treatment eliminated gastric inflammation but had no effect on the increased number of PAX6/GLP-1-positive cells and delayed GITT resulting from H. pylori infection. The mechanism by which H. pylori infection is involved in the enhancement of PAX6/GLP-1 expression and the delay of GITT remains to be elucidated. To approach these issues, we will need in vitro studies using GLP-1 producing cells in future. However, we have shown that H. pylori infection plays a role in pathophysiology, not only in the stomach but also the lower GI tract, subsequently affecting GI motility.
Supporting information
Mice received either Brucella broth containing H. pylori (black triangle) or Brucella broth alone (white triangle). A subgroup of the infected mice underwent H. pylori eradication using lansoprazole, amoxicillin, and clarithromycin (gray arrow). Parentheses indicating the number of animals in each group examined at each time point. The data until 12 weeks was presented in Figs 1 and 2. The data at 24 weeks was presented in Figs 3–6.
(TIF)
(PDF)
Acknowledgments
We thank Mayumi Yamada and Chiyomi Itoh (Hyogo College of Medicine) for their technical assistance. This work was supported in part by Grants-in-aid for Scientific Research 26460953 from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported in part by Grants-in-aid for Scientific Research 26460953 from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
References
- 1.El-Omar EM, Oien K, El-Nujumi A, Gillen D, Wirz A, Dahill S, et al. Helicobacter pylori infection and chronic gastric acid hyposecretion. Gastroenterology. 1997; 113(1):15–24. [DOI] [PubMed] [Google Scholar]
- 2.Schubert ML, Peura DA. Control of gastric acid secretion in health and disease. Gastroenterology. 2008; 134(7):1842–60. doi: 10.1053/j.gastro.2008.05.021 [DOI] [PubMed] [Google Scholar]
- 3.Su YC, Wang WM, Wang SY, Lu SN, Chen LT, Wu DC, et al. The association between Helicobacter pylori infection and functional dyspepsia in patients with irritable bowel syndrome. Am J Gastroenterol. 2000; 95(8):1900–5. doi: 10.1111/j.1572-0241.2000.02252.x [DOI] [PubMed] [Google Scholar]
- 4.Budzyński J, Kłopocka M. Brain-gut axis in the pathogenesis of Helicobacter pylori infection. World J Gastroenterol. 2014; 20(18):5212–25. doi: 10.3748/wjg.v20.i18.5212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blaser MJ, Atherton JC. Helicobacter pylori persistence: biology and disease. J Clin Invest. 2004; 113(3):321–33. doi: 10.1172/JCI20925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D'Elios MM, Czinn SJ. Immunity, inflammation, and vaccines for Helicobacter pylori. Helicobacter. 2014; 19(Suppl 1):19–26. [DOI] [PubMed] [Google Scholar]
- 7.Gerards C, Leodolter A, Glasbrenner B, Malfertheiner P. H. pylori infection and visceral hypersensitivity in patients with irritable bowel syndrome. Dig Dis. 2001; 19(2):170–3. doi: 50673 [DOI] [PubMed] [Google Scholar]
- 8.Moayyedi P, Forman D, Braunholtz D, Feltbower R, Crocombe W, Liptrott M, et al. The proportion of upper gastrointestinal symptoms in the community associated with Helicobacter pylori, lifestyle factors, and nonsteroidal anti-inflammatory drugs. Leeds HELP Study Group. Am J Gastroenterol. 2000; 95(6):1448–55. doi: 10.1111/j.1572-0241.2000.2126_1.x [DOI] [PubMed] [Google Scholar]
- 9.Mizuta Y, Shikuwa S, Isomoto H, Mishima R, Akazawa Y, Masuda J, et al. Recent insights into digestive motility in functional dyspepsia. J Gastroenterol. 2006; 41(11):1025–40. doi: 10.1007/s00535-006-1966-z [DOI] [PubMed] [Google Scholar]
- 10.Eissele R, Göke R, Willemer S, Harthus HP, Vermeer H, Arnold R, et al. Glucagon-like peptide-1 cells in the gastrointestinal tract and pancreas of rat, pig and man. Eur J Clin Invest. 1992; 22(4):283–91. [DOI] [PubMed] [Google Scholar]
- 11.Holst JJ. Gromada J. Role of incretin hormones in the regulation of insulin secretion in diabetic and nondiabetic humans. Am J Physiol Endocrinol Metab. 2004; 287(2):E199–E206. doi: 10.1152/ajpendo.00545.2003 [DOI] [PubMed] [Google Scholar]
- 12.Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007; 87:1409–39. doi: 10.1152/physrev.00034.2006 [DOI] [PubMed] [Google Scholar]
- 13.Imeryüz N, Yeğen BC, Bozkurt A, Coşkun T, Villanueva-Peñacarrillo ML, Ulusoy NB. Glucagon-like peptide-1 inhibits gastric emptying via vagal afferent-mediated central mechanisms. Am J Physiol. 1997; 273(4 Pt 1):G920–7. [DOI] [PubMed] [Google Scholar]
- 14.Nauck MA, Niedereichholz U, Ettler R, Holst JJ, Orskov C, Ritzel R, et al. Glucagon-like peptide 1 inhibition of gastric emptying outweighs its insulinotrophic effects in healthy humans. Am J Physiol Endocrinol Metab. 1997; 273(5 Pt 1):E981–8. [DOI] [PubMed] [Google Scholar]
- 15.Amoto A, Cinci L, Rotondo A, Serio R, Faussone-Pellegrini MS, Vannucchi MG, et al. Peipheral motor action of glucagon-like peptide-1 through enteric neuronal receptors. Neurogastroenterol Motil. 2010; 22(6):664–e203. doi: 10.1111/j.1365-2982.2010.01476.x [DOI] [PubMed] [Google Scholar]
- 16.Trinh DKY, Zhang K, Hossain M, Brubaker PL, Drucker DJ. Pax-6 activates endogenous proglucagon gene expression in the rodent gastrointestinal epithelium. Diabetes. 2003; 52(2):425–33. [DOI] [PubMed] [Google Scholar]
- 17.Watanabe K, Murakami K, Sato R, Kashimura K, Miura M, Ootsu S, et al. Effect of sucralfate on antibiotic therapy for Helicobacter pylori infection in mice. Antimicrob Agents Chemother. 2004; 48(12):4582–8. doi: 10.1128/AAC.48.12.4582-4588.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hifumi E, Morihara F, Hatiuchi K, Okuda T, Nishizono A, Uda T. Catalytic features and eradication ability of antibody light-chain UA15-L against Helicobacter pylori. J Biol Chem. 2008; 283(2):899–907. doi: 10.1074/jbc.M705674200 [DOI] [PubMed] [Google Scholar]
- 19.Fukui H, Franceschi F, Penland RL, Sakai T, Sepulveda AR, Fujimori T, et al. Effects of Helicobacter pylori infection on the link between regenerating gene expression and serum gastrin levels in Mongolian gerbils. Lab Invest. 2003; 83(12):1777–86. [DOI] [PubMed] [Google Scholar]
- 20.Fukui H, Zhang X, Sun C, Hara K, Kikuchi S, Yamasaki T, et al. IL-22 produced by cancer-associated fibroblasts promotes gastric cancer cell invasion via STAT3 and ERK signaling. Br J Cancer. 2014; 111(4):763–71. doi: 10.1038/bjc.2014.336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Welch MG, Margolis KG, Li Z, Gershon MD. Oxytocin regulates gastrointestinal motility, inflammation, macromolecular permeability, and mucosal maintenance in mice. Am J Physiol Gastrointest Liver Physiol. 2014; 307(8):G848–62. doi: 10.1152/ajpgi.00176.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fukui H, Sekikawa A, Tanaka H, Fujimori Y, Katake Y, Fujii S, et al. DMBT1 is a novel gene induced by IL-22 in ulcerative colitis. Inflamm Bowel Dis. 2011; 17(5):1177–88. doi: 10.1002/ibd.21473 [DOI] [PubMed] [Google Scholar]
- 23.Ellingsgaard H, Hauselmann I, Schuler B, Habib AM, Baggio LL, Meier DT, et al. Interleukin-6 enhances insulin secretion by increasing glucagon-like peptide-1 secretion from L cells and alpha cells. Nat Med. 2011; 17(11):1481–9. doi: 10.1038/nm.2513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gosmain Y, Cheyssac C, Heddad Masson M, Dibner C, Philippe J. Glucagon gene expression in the endocrine pancreas: the role of the transcription factor Pax6 in α-cell differentiation, glucagon biosynthesis and secretion. Diabetes Obes Metab. 2011; 13(Suppl 1):31–8. [DOI] [PubMed] [Google Scholar]
- 25.Panjwani N, Mulvihill EE, Longuet C, Yusta B, Campbell JE, Brown TJ, et al. GLP-1 receptor activation indirectly reduces hepatic lipid accumulation but does not attenuate development of atherosclerosis in diabetic male ApoE-/- mice. Endocrinology. 2013; 154(1):127–39. doi: 10.1210/en.2012-1937 [DOI] [PubMed] [Google Scholar]
- 26.Körner M, Stöckli M, Waser B, Reubi JC. GLP-1 receptor expression in human tumors and human normal tissues: potential for in vivo targeting. J Nucl Med. 2007; 48(5):736–43. doi: 10.2967/jnumed.106.038679 [DOI] [PubMed] [Google Scholar]
- 27.Pyke C, Heller RS, Kirk RK, Ørskov C, Reedtz-Runge S, Kaastrup P, et al. GLP-1 receptor localization in monkey and human tissue: novel distribution revealed with extensively validated monoclonal antibody. Endocrinology. 2014; 155(4):1280–90. doi: 10.1210/en.2013-1934 [DOI] [PubMed] [Google Scholar]
- 28.Wichmann A, Allahyar A, Greiner TU, Plovier H, Lundén GÖ, Larsson T, et al. Microbial modulation of energy availability in the colon regulates intestinal transit. Cell Host Microbe. 2013; 14(5):582–90. doi: 10.1016/j.chom.2013.09.012 [DOI] [PubMed] [Google Scholar]
- 29.Hwang I, Park YJ, Kim YR, Kim YN, Ka S, Lee HY, et al. Alteration of gut microbiota by vancomycin and bacitracin improves insulin resistance via glucagon-like peptide 1 in diet-induced obesity. FASEB J. 2015; 29(6):2397–411. doi: 10.1096/fj.14-265983 [DOI] [PubMed] [Google Scholar]
- 30.Rajpal DK, Klein JL, Mayhew D, Boucheron J, Spivak AT, Kumar V, et al. Selective Spectrum Antibiotic Modulation of the Gut Microbiome in Obesity and Diabetes Rodent Models. PLoS One. 2015; 10(12):e0145499 doi: 10.1371/journal.pone.0145499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Francois F, Roper J, Joseph N, Pei Z, Chhada A, Shak JR, et al. The effect of H. pylori eradication on meal-associated changes in plasma ghrelin and leptin. BMC Gastroenterol. 2011; 11:37 doi: 10.1186/1471-230X-11-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yap TW, Leow AH, Azmi AN, Francois F, Perez-Perez GI, Blaser MJ, et al. Changes in metabolic hormones in Malaysian young adults following Helicobacter pylori eradication. PLoS One. 2015; 10(8):e0135771 doi: 10.1371/journal.pone.0135771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moayyedi P, Soo S, Deeks J, Delaney B, Harris A, Innes M, et al. Eradication of Helicobacter pylori for non-ulcer dyspepsia. Cochrane Database Syst Rev. 2006; (2):CD002096 doi: 10.1002/14651858.CD002096.pub4 [DOI] [PubMed] [Google Scholar]
- 34.Xiong F, Xiong M, Ma Z, Huang S, Li A, Liu S. Lack of association found between Helicobacter pylori infection and diarrhea-predominant irritable bowel syndrome: A multicenter retrospective study. Gastroenterol Res Pract. 2016: 2016:3059201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Talley NJ, Holtmann G, Walker MM. Therapeutic strategies for functional dyspepsia and irritable bowel syndrome based on pathophysiology. J Gastroenterol. 2015; 50(6):601–13. doi: 10.1007/s00535-015-1076-x [DOI] [PubMed] [Google Scholar]
- 36.DuPont AW. Postinfectious irritable bowel syndrome. Clin Infect Dis. 2008; 46(4):594–9. doi: 10.1086/526774 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Mice received either Brucella broth containing H. pylori (black triangle) or Brucella broth alone (white triangle). A subgroup of the infected mice underwent H. pylori eradication using lansoprazole, amoxicillin, and clarithromycin (gray arrow). Parentheses indicating the number of animals in each group examined at each time point. The data until 12 weeks was presented in Figs 1 and 2. The data at 24 weeks was presented in Figs 3–6.
(TIF)
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.