Abstract
Acute stress has been shown to modulate memory for recently learned information, an effect attributed to the influence of stress hormones on medial temporal lobe (MTL) consolidation processes. However, little is known about which memories will be affected when stress follows encoding. One possibility is that stress interacts with encoding processes to selectively protect memories that had elicited responses in the hippocampus and amygdala, two MTL structures important for memory formation. There is limited evidence for interactions between encoding processes and consolidation effects in humans, but recent studies of consolidation in rodents have emphasized the importance of encoding “tags” for determining the impact of consolidation manipulations on memory. Here, we used fMRI in humans to test the hypothesis that the effects of post-encoding stress depend on MTL processes observed during encoding. We found that changes in stress hormone levels were associated with an increase in the contingency of memory outcomes on hippocampal and amygdala encoding responses. That is, for participants showing high cortisol reactivity, memories became more dependent on MTL activity observed during encoding, thereby shifting the distribution of recollected events toward those that had elicited relatively high activation. Surprisingly, this effect was generally larger for neutral, compared to emotionally negative, memories. The results suggest that stress does not uniformly enhance memory, but instead selectively preserves memories tagged during encoding, effectively acting as mnemonic filter.
Memories for individual moments are not formed in isolation but can be influenced by other events that occur around the same time. This kind of malleability is an important feature of memory systems, in that it supports the prioritization of memories that are followed by significant experiences. One type of experience that appears to drive changes in memory is acute stress, such as that experienced when giving an important presentation. The effects of acute stress on memory are complex and vary according to the timing and intensity of the stressor (Joëls, Fernandez, & Roozendaal, 2011; Schwabe, Joëls, Roozendaal, Wolf, & Oitzl, 2012), but one common finding is that acute stress can protect recently learned information from forgetting (Andreano & Cahill, 2006; Cahill, Gorski, & Le, 2003; McCullough & Yonelinas, 2013). In rodent models of stress and memory, stress hormone release has been shown to modulate hippocampal plasticity, thereby increasing the likelihood that recent experiences will be consolidated into long-term memory (McGaugh & Roozendaal, 2002).
Little is known, however, about which recent experiences will be remembered better when stress follows encoding. One possibility is that the effects of post-encoding stress depend on the emotional content of the memoranda. Prior studies of stress and memory in rodents have demonstrated that post-encoding modulation depends on noradrenergic arousal responses in the amygdala (Roozendaal, Nguyen, Power, & McGaugh, 1999), and, in particular, arousal experienced during learning (Okuda, Roozendaal, & McGaugh, 2004; Roozendaal, Okuda, Van der Zee, & McGaugh, 2006), suggesting that post-encoding stress may primarily affect emotional memories. In some human studies, post-encoding stress has been shown to preferentially benefit memory for emotional information (e.g., Cahill et al., 2003; Smeets, Otgaar, Candel, & Wolf, 2008), but in other studies, the effects have been equal to, or even larger, for neutral information (e.g., McCullough & Yonelinas, 2013; Preuß & Wolf, 2009), suggesting that emotional content cannot be the only critical factor.
An alternate possibility, which we set out to test in this study, is that the effects of post-encoding stress depend on processes that were engaged during encoding. That is, stress may act to filter memories based on their effect on neural processes during encoding. Processes in the amygdala and hippocampus, two structures in the medial temporal lobes (MTL), may be especially important for determining the effects of post-encoding stress on memory, due to these regions’ roles in prioritizing information in memory (Kensinger, 2009; Mather, Clewett, Sakaki, & Harley, 2015) and facilitating subsequent recollection (Diana, Yonelinas, & Ranganath, 2007; Yonelinas & Ritchey, 2015).
Interactions between encoding and consolidation processes are a key feature of tag-and-capture models of memory consolidation (Frey & Morris, 1998; Redondo & Morris, 2011; Viola, Ballarini, Martínez, & Moncada, 2014). In these models, memory traces are “tagged” during initial encoding, which allows the trace to capture plasticity-related products that become available around the time of encoding. Importantly, these products may arise from the target event itself or from other events occurring around the same time. Both tag and capture are necessary for consolidation into long-term memory, in that without capture, tagged memories would be quickly forgotten. Although these models have not typically been applied to studies of post-encoding stress (but see Korz & Frey, 2003; Mather et al., 2015; McIntyre, McGaugh, & Williams, 2012), they have been used to explain how other behavioral manipulations, such as exposure to novel environments, are able to drive changes in plasticity or in behavioral memory outcomes (Ballarini, Moncada, Martinez, Alen, & Viola, 2009; Moncada & Viola, 2007; Wang, Redondo, & Morris, 2010). Building on this literature, we hypothesized that stress would selectively enhance memory for tagged events, including those that would not typically be retained in the absence of stress, such as neutral items.
Here, we report a functional magnetic resonance imaging (fMRI) experiment in which we directly tested, for the first time, whether the effects of post-encoding stress on memory depend on neural processes during encoding. We predicted that changes in cortisol, a stress hormone, would increase the contingency of memory outcomes on amygdala and hippocampal processes during encoding.
Materials & Methods
Participants
Fifty males participated in the study. Of these participants, 25 were randomly assigned to the stress group (Mean age = 24.2 years, Mean years education = 16.6) and 25 to the control group (Mean age = 23.1 years, Mean years education = 15.6). Participants were excluded if any of the following were true: if there was more than 3 mm of head motion in any direction during an encoding scan (N=1), if there was an abnormality in the MR images (N=1), if there was an error with the experimental program during the memory test (N=2), or if they did not return for the memory test (N=1). Participants were additionally excluded if there were fewer than 5 trials in any condition of interest (N=10). After applying these criteria, 18 stress participants and 17 control participants were included in the analyses. One participant had an extreme cortisol reactivity value (more than 3 median absolute deviations from the median) and was excluded from regressions involving this measure, resulting in a total N of 34. The study was approved by the Institutional Review Board at the University of California, Davis, and all participants provided written informed consent prior to the experiment.
Stimuli & Materials
This study used a set of 312 pictures, half neutral and half negative, that was used in previous research (McCullough & Yonelinas, 2013). The pictures were selected primarily from the International Affective Photo Series (IAPS) based on their standard scores of emotional arousal and emotional valence (Lang, Bradley, & Cuthbert, 2008), as well as from an in-house set designed to balance the two sets for factors such as visual complexity, color, and the presence of people. Images were approximately 315 pixels square, with minor variation in size and shape. Eight of the images were used as example trials. In the encoding phase, 100 neutral and 100 negative images were presented to each participant in a random order. In the recognition test, each participant was presented with 200 studied images and 104 new images (52 neutral) in a random order.
Procedure
All participants were tested individually by a male experimenter starting between the hours of 8am and 5pm. The start time of the first session did not differ between groups, F(1,32)=.04, p=.85. An overview of the experimental design is shown in Figure 1a. In the first session, after providing informed consent, participants completed a safety screening form and a set of mood and trait questionnaires (not discussed; see McCullough et al., 2015) before providing a baseline saliva sample. The participant was offered a piece of gum and produced approximately 3mL of saliva into a Salivette tube. The participant was then provided with instructions for the picture rating task on a laptop computer, including presentation of two example pictures. The participant was put into the scanner, where they completed the incidental encoding task, in which 200 IAPS pictures (100 neutral, 100 negative) were presented, divided across 2 functional runs. Participants rated each picture for visual complexity on a scale of 1-6, using three buttons on each of two response boxes. These ratings were included to ensure that participants attended to each image, but were not analyzed. Each picture was presented for 1000 ms, after which the participant had an additional 1000 ms to respond. After an inter-trial interval that varied from 2-8 s (mean 2.98 s), the next trial was initiated. Trial order and timing was optimized with Optseq2 (https://surfer.nmr.mgh.harvard.edu/optseq/). Following the encoding task, there was a 7-minute resting-state scan, for which participants were instructed to remain awake and motionless.
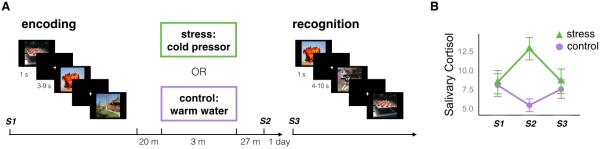
Figure 1. Experimental design.
A) On Day 1, participants rated the visual complexity of negative and neutral images while fMRI images were collected. The encoding task was followed by a short delay in which we obtained a resting-state scan, removed the participants from the scanner, and administered questionnaires. Then, half of the participants (N=25) completed a cold pressor task in which they submerged their arm in ice-cold water for 3 minutes, and the other half (N=25) completed a control task in which they submerged their arm in lukewarm water for 3 minutes. On Day 2, participants returned for a surprise recognition test, in which they rated whether they recollected the item or, if not, their confidence in whether it was new or old on a 5-point scale. Three saliva samples were obtained: one prior to encoding (S1), one approximately 27 minutes after the stress or control task (S2), and one prior to recognition (S3). B) The plot shows the mean salivary cortisol levels for each sample for the control (violet circles) and stress (green triangles) participants. Following the stress task (S2), stress participants had higher salivary cortisol than control participants. There were no differences before the encoding task (S1) or before the recognition task (S3). Error bars denote standard error of the mean.
Following the rest period, the participant was removed from the scanner, and completed questionnaires for approximately 10 min, providing demographic, medical, sleep, mood, and strategy-related information. Note that this short delay was consistent with prior work from our laboratory (McCullough & Yonelinas, 2013; Yonelinas, Parks, Koen, Jorgenson, & Mendoza, 2011). Each participant then completed either the cold-pressor test or control task. The participant submerged their non-dominant arm in either an ice-water bath (M = 0.06° C, SD = 0.12° C) or tepid water (M = 23.71° C, SD = 2.2° C). The participant was instructed to keep their arm submerged for 3 min, or as long as possible, and to refrain from talking during the task. Participants then completed additional mood and strategy questionnaires before returning to the MR scanner for to complete another set of task-free scans (i.e., a 7-minute resting-state scan, structural scans). The first session concluded with a second saliva sample, which took place, on average, 27 minutes after the end of the stress task.
The second session started approximately 24 hours after the beginning of the first session (plus or minus 0 to 90 minutes, with most participants starting within 20 minutes of the 24-hour mark). It began with the participant providing a third saliva sample and a set of mood ratings. Participants then completed a surprise recognition test, in which a mix of 200 studied images and 104 new images (52 negative) were presented for 1000 ms, after which the participant had an additional 2000 ms to respond. Participants rated each picture as either being Recollected, or on a familiarity scale of 1-5, in which 1 = Sure new and 5 = Sure old. After the participant responded, an inter-trial interval that varied from 2-8 s preceded the subsequent trial. The recognition test was divided into four phases of equal length, and participants were allowed a brief break in between phases. The recognition test was completed in the scanner; however, here we focus only on imaging data from the encoding task.
Cortisol reactivity
Saliva was assayed for salivary cortisol in two batches. The minimum detectable value of the first batch was 1.3854nmol/L, and one sample from a control participant fell below this threshold, so the minimum detectable value was substituted for that data point. We summarized individual differences in cortisol reactivity by measuring the difference between the pre-scan and post-stress samples. For some data visualizations, participants were divided into high and low reactivity groups based on a median split on cortisol reactivity.
fMRI Acquisition & Pre-Processing
Scanning was performed on a Siemens Skyra 3T scanner system with a 32-channel head coil. High-resolution T1-weighted structural images were acquired using a magnetization prepared rapid acquisition gradient echo (MPRAGE) pulse sequence (field of view = 25.6 cm, image matrix = 256 × 256, 208 axial slices with 1.0 mm thickness). Functional images were acquired using a gradient echo planar imaging (EPI) sequence (TR = 2000 ms; TE = 25 ms; FOV = 20.5 × 21.14 cm; image matrix = 64 × 66; flip angle = 90; 34 interleaved axial slices; voxel size = 3.20 × 3.20 × 3.20 mm). Field maps were also collected using the Siemens field map sequence with short TE=4.92 ms and long TE=7.38 ms and used to correct for geometric distortions due to magnetic field inhomogeneities.
SPM8 (http://www.fil.ion.ucl.ac.uk/spm/software/spm8/) was used to pre-process the images. Functional EPI images were corrected for slice timing, realigned to the first image, and unwarped (field map correction). The high-resolution T1 image was coregistered to the mean unwarped EPI, and parameters for nonlinear spatial normalization were obtained by segmenting the coregistered T1 and then applied to the T1 and functional EPIs, moving them into MNI space. Functional images were resliced to a resolution of 3 mm3 and smoothed with an 8-mm Gaussian kernel. Quality assurance included the identification of “suspect” time-points via the Artifact Detection Tools (ART; http://www.nitrc.org/projects/artifact_detect), defined as time-points marked by greater than .5 mm in movement or 1.5% global mean signal change. Participants were excluded if either encoding task run contained more than 3 mm total movement in any direction, resulting in one excluded participant.
ROI Definition
Anatomical ROIs were defined for the left and right hippocampus and amygdalae. An in-house probabilistic atlas of the MTL was used to define the hippocampal ROIs. The atlas was created by a) manual segmenting 55 T1 images according to the protocol outlined in Ritchey et al. 2015, b) registering the images to MNI space via the SPM8 DARTEL tool, and c) averaging the binary masks for each ROI. Thus, atlas values denote the probability that a given voxel was included in a manual segmentation of the target ROI, here thresholded at 50% probability. The left and right amygdalae were segmented on a template T1 image in MNI space, following the protocol outlined by Moore et al. (2014). ROIs are shown in Figure 2A.
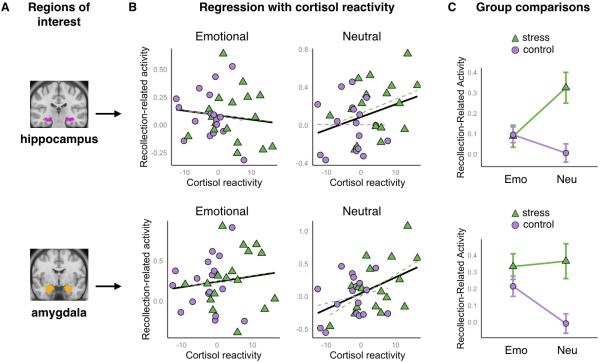
Figure 2. Effects of post-encoding stress on recollection-related activity in the hippocampus and amygdala.
A) Estimates for recollection-related activity (subsequently recollected – missed trials) were obtained for anatomical regions of interest (ROIs) for the hippocampus and amygdala, shown here on an MNI template. B) Scatterplots show the relationship between individual differences in cortisol reactivity (S2-S1) and recollection-related activity for emotional (left) and neutral (right) items. Participants in the control group are marked with violet circles and participants in the stress group are marked with green triangles. Solid black lines represent best linear fit across all participants, whereas the dotted violet and green lines represent the best linear fit within each group. For the hippocampus (top row), the relationship was stronger for neutral compared to emotionally negative memories. For the amygdala (bottom row), the relationship was significant and did not interact with valence. C) Line plots show average recollection-related activity across participants within the control (violet circles) and stress (green triangles) groups, separately for emotional (Emo) and neutral (Neu) items. For this plot, only stress responders (i.e., stress participants who showed in increase in salivary cortisol, N=13) were included. Error bars denote standard error of the mean.
fMRI Analyses
All imaging analyses were completed on data from the two encoding task runs. Two approaches were used: a subsequent memory analysis based on condition-level activity estimates and an analysis of single-trial activity estimates. Both models were estimated in SPM8 with event-related stick-function regressors modeling the trial onsets, convolved with the canonical hemodynamic response function. Six motion parameter regressors were included in addition to nuisance regressors that modeled out suspect timepoints, as defined above. Participants were excluded if they had fewer than 5 trials in any condition of interest.
For the subsequent memory analysis, trials were binned according to subsequent memory, such that each trial was labeled as subsequently recollected (R response), familiar (4 or 5 response) or missed (1, 2, or 3 response). The model contained regressors for the following trial types: emotional recollection, emotional familiarity, emotional miss, neutral recollection, neutral familiarity, neutral miss, and other (trials that received no responses during retrieval). Resulting parameter estimates were contrasted between conditions to yield voxel-wise contrast maps. For the ROI analyses, contrast values were averaged across voxels within each ROI. Based on prior evidence that the hippocampus and amygdala primarily support recollection processes (Diana et al., 2007; Yonelinas & Ritchey, 2015), we focused on measures of recollection-related activity, which were computed by taking the difference in activity for subsequently recollected and missed trials. Subsequently familiar trials were excluded from statistical analysis, but activity levels for familiarity trials generally fell between activity levels for recollected and missed trials. Recollection-related activity estimates were entered into an ANOVA model with a between-subjects regressor for cortisol reactivity and within-subjects factors for emotional valence (negative or neutral), region (hippocampus or amygdala), and hemisphere (left or right). Additional models were used to test the effects of the cold-pressor manipulation by including group assignment as a between-subjects factor.
Voxel-wise analyses were used to complement the ROI findings. Contrast maps were calculated for recollection-related activity (i.e., recollection – missed) separately for emotionally negative and neutral. Contrast maps were regressed onto individual differences in cortisol reactivity, and the resulting covariate contrast was tested with a one-sample t-test implemented in SPM8. T-maps were cluster corrected at p<.05 according to simulations implemented with AFNI's 3dClustSim tool (http://afni.nimh.nih.gov). Thresholds were determined for hypothesis-driven comparisons within the MTL, including the hippocampus, amygdala, and parahippocampal gyrus (voxel-wise p<.005, cluster size > 11 voxels), as well as for whole-brain comparisons (voxel-wise p<.001, cluster size > 36 voxels). Whole-brain comparisons were bounded by the coverage obtained in every participant and excluded the most ventral portion of the temporal lobes as well as the most dorsal portion of the frontal and parietal lobes.
For the single-trial analysis, we estimated a general linear model that included a separate regressor for every individual encoding trial. This model produced a beta image for every trial, which were then summarized into an average trial-series of beta values for each ROI. Within each trial-series, outlying trials were identified as trials more than 3 median absolute deviations from the median and removed from the analysis. Activity estimates were normalized by computing the z-score across all trials and averaged across hemispheres. Normalized activity estimates were aggregated across subjects to plot the distribution of trials across activity levels. The characteristics of the recollection trial distribution (i.e., mean, standard deviation, skew, and kurtosis) were evaluated for each subject, and values for the hippocampus and amygdala were entered into separate ANOVA models with a between-subjects regressor for cortisol reactivity and a within-subjects factor for emotional valence.
Results
Cortisol reactivity and recognition memory performance
We first sought to establish the efficacy of the stress manipulation by measuring its effects on salivary cortisol measures. To do so, we measured the change in salivary cortisol from pre-encoding to post-stress, a measure that we refer to as cortisol reactivity. Participants in the stress group showed greater cortisol reactivity than participants in the control group (Figure 1B), such that the groups were indistinguishable prior to encoding, F(1,32)=.03, p=.86, but differed following the stress manipulation, F(1,32)=19.45, p<.001. Although baseline salivary cortisol measures were correlated with the start time of the first session, r(32)=−.40, p=.02, consistent with an expected decline in cortisol levels over the course of a day, in this sample there was no relationship between cortisol reactivity and time of day, r(32)=.08, p=.70. Regardless, there was a great deal of variability in cortisol reactivity across participants, suggesting that there may exist important individual differences in the neurohormonal effects of stress on memory.
A detailed analysis of the relation between cortisol reactivity and recognition memory performance has been previously reported (McCullough, Ritchey, Ranganath, & Yonelinas, 2015) and is summarized here for convenience. In brief, increases in cortisol reactivity were associated with small but linear increases in recognition memory judgments that were associated with reports of familiarity. There was also a significant quadratic relationship between cortisol reactivity and recognition judgments associated with reports of recollection, such that moderate changes in cortisol were associated with the highest rates of recollection. Relationships between cortisol reactivity and memory were not affected by the emotional valence of the studied items. However, collapsing across all participants, emotionally negative events were recollected more often than neutral events, F(1,34)=23.8, p<.001, with no difference in familiarity, F(1,34)=.10, p=.75. We now turn to new results linking cortisol reactivity to fMRI activity related to subsequent memory, the focus of the present report.
Relationship between post-encoding stress and MTL encoding activity
We hypothesized that if post-encoding stress filters memories based on processes that were engaged during encoding, and if encoding-related MTL activity is related to these processes, then the relation between encoding activity and subsequent memory may be enhanced by post-encoding stress. More specifically, we expected that activity in the hippocampus and amygdala would be diagnostic of subsequent memory and that this effect would be related to the degree of stress experienced after encoding, as assessed by cortisol reactivity.
To test this hypothesis, we tested whether individual differences in cortisol reactivity were associated with changes in encoding activity related to subsequent recollection, focusing on activity in anatomically-defined masks of the hippocampus and amygdala (Figure 2A). For these ROIs, we measured the difference in activity related to subsequently recollected versus missed trials. This difference, which we refer to as “recollection-related activity,” measured the degree to which encoding activity was diagnostic of subsequent memory outcomes. The influence of cortisol reactivity on recollection-related activity was tested with an ANOVA model with a between-subjects factor for cortisol reactivity and within-subjects factors for emotional valence, region, and hemisphere.
Consistent with prior work, there was greater activity for subsequently recollected than missed items, as indicated by a significantly positive intercept, F(1,32)=9.76, p=.004. There was also a region by valence interaction, F(1,32)=15.00, p<.001, such that there were larger memory effects for emotional than neutral events in the amygdala, F(1,32)=6.91, p=.013, whereas the hippocampus tended to show the reverse pattern. Building on this core set of findings, we now turn to the effects of cortisol reactivity on recollection-related activity (Figure 2B). There was a significant region by cortisol reactivity interaction, F(1,32)=9.66, p=.004. Separate analyses of each region revealed that there was a significant positive relationship between cortisol reactivity and recollection-related activity in the amygdala, F(1,32)=5.89, p=.02. In the hippocampus, the main effect of cortisol reactivity was not significant, p>.1, but rather there was a significant valence by cortisol reactivity interaction, F(1,32)=4.43, p=.04, indicating a stronger relationship between recollection-related activity and cortisol reactivity for neutral compared to emotionally negative memories. The full set of ANOVA results is presented in Table 1. We additionally ran a model in which the cortisol reactivity term was squared to test for an inverted-U relationship between cortisol reactivity and recollection-related activity; none of these effects passed significance, ps>.1. In general, the results were consistent with our hypothesis that post-encoding stress would increase the dependence of memory outcomes on MTL encoding responses. Surprisingly, for the hippocampus, this effect was stronger for neutral memories.
Table 1.
ANOVA on recollection-related activity: Cortisol reactivity × Emotion (emotionally negative, neutral) × Region (hippocampus, amygdala) × Hemisphere (L, R)
| Full ANOVA | ||||
|---|---|---|---|---|
| Effect | DFn | DFd | F | p |
| (Intercept) | 1 | 32 | 9.765 | 0.004* |
| Cortisol reactivty | 1 | 32 | 3.562 | 0.068 |
| Emotion | 1 | 32 | 2.505 | 0.123 |
| Region | 1 | 32 | 5.714 | 0.023* |
| Hemisphere | 1 | 32 | 1.295 | 0.264 |
| Cortisol reactivty × Emotion | 1 | 32 | 4.020 | 0.053 |
| Cortisol reactivty × Region | 1 | 32 | 9.658 | 0.004* |
| Cortisol reactivity × Hemisphere | 1 | 32 | 0.377 | 0.544 |
| Emotion × Region | 1 | 32 | 15.002 | <0.001* |
| Emotion × Hemisphere | 1 | 32 | 1.511 | 0.228 |
| Region × Hemisphere | 1 | 32 | 0.017 | 0.896 |
| Cortisol reactivity × Emotion × Region | 1 | 32 | 0.001 | 0.981 |
| Cortisol reactivity × Emotion × Hemisphere | 1 | 32 | 0.303 | 0.586 |
| Cortisol reactivity × Region × Hemisphere | 1 | 32 | 0.186 | 0.669 |
| Emotion × Region × Hemisphere | 1 | 32 | 3.423 | 0.074 |
| Cortisol reactivity × Emotion × Region × Hemisphere | 1 | 32 | 1.071 | 0.308 |
| Hippocampus only | ||||
|---|---|---|---|---|
| Effect | DFn | DFd | F | p |
| (Intercept) | 1 | 32 | 7.009 | 0.012* |
| Cortisol reactivity | 1 | 32 | 0.969 | 0.332 |
| Emotion | 1 | 32 | 0.001 | 0.975 |
| Hemisphere | 1 | 32 | 1.800 | 0.189 |
| Cortisol reactivity × Emotion | 1 | 32 | 4.429 | 0.043* |
| Cortisol reactivity × Hemisphere | 1 | 32 | 1.456 | 0.236 |
| Emotion × Hemisphere | 1 | 32 | 0.188 | 0.668 |
| Cortisol reactivity × Emotion × Hemisphere | 1 | 32 | 0.151 | 0.700 |
| Amygdala only | ||||
|---|---|---|---|---|
| Effect | DFn | DFd | F | p |
| (Intercept) | 1 | 32 | 10.670 | 0.003* |
| Cortisol reactivity | 1 | 32 | 5.894 | 0.021* |
| Emotion | 1 | 32 | 6.911 | 0.013* |
| Hemisphere | 1 | 32 | 0.608 | 0.441 |
| Cortisol reactivity × Emotion | 1 | 32 | 2.854 | 0.101 |
| Cortisol reactivity × Hemisphere | 1 | 32 | 0.034 | 0.855 |
| Emotion × Hemisphere | 1 | 32 | 3.059 | 0.090 |
| Cortisol reactivity × Emotion × Hemisphere | 1 | 32 | 0.800 | 0.378 |
p<.05
The previous results supported the hypothesis that cortisol reactivity would be associated with increased dependence on MTL encoding processes for subsequent recollection. Moreover, plots of the relationship between cortisol reactivity and recollection-related activity suggest that this relationship might be driven by variance within the stress group (Figure 2B). To test explicitly the relation between cortisol reactivity and the cold-pressor task, we ran another model in which both cortisol reactivity and group assignment were included as between-subjects factors. In this model, there remained a significant cortisol reactivity by region interaction, F(1,30)=9.46, p=.005, but there were no significant effects of group assignment, ps>.10, nor were there any significant interactions between cortisol reactivity and group assignment, ps>.10. Thus, the effects of cortisol reactivity on recollection-related activity were not purely driven by the difference between the stress and control participants. However, as is readily apparent in Figure 2B, the relationship between these measures would not have been observed without the added variability in cortisol reactivity introduced by the stress manipulation. That is, there was no relationship between cortisol reactivity and recollection-related activity when only control participants were included in the analysis, ps>.10.
Finally, another approach to studying the effects of post-encoding stress is to compare participants in the stress and control groups directly. Participants in the stress group showed numerically greater recollection-related activity than participants in the control group, especially for neutral memories. However, these effects were not significant, ps>.1, perhaps due to the considerable variability in the stress response among participants (Table 2). The inclusion of stress non-responders (i.e., participants in the stress group who showed no increase in cortisol reactivity) may have obscured differences between the two groups. Indeed, when non-responders were excluded, there was a significant main effect of group on recollection-related activity, F(1,28)=5.65, p=.025, and a marginal group by valence interaction, F(1,28)=3.04, p=.09 (Figure 2C). These results converge with the cortisol reactivity analysis in showing that stress was associated with an increase in the extent to which hippocampal and amygdala activity was predictive of subsequent recollection, and that these effects were related to changes in stress hormone levels.
Table 2.
ANOVA on recollection-related activity: Group assignment (Stress, Control) × Emotion (emotionally negative, neutral) × Region (hippocampus, amygdala) × Hemisphere (L, R)
| All subjects | Excluding non-responders | |||||||
|---|---|---|---|---|---|---|---|---|
| Effect | DFn | DFd | F | p | DFn | DFd | F | p |
| (Intercept) | 1 | 33 | 12.297 | 0.001* | 1 | 28 | 17.586 | <0.001* |
| Group | 1 | 33 | 2.497 | 0.124 | 1 | 28 | 5.649 | 0.025* |
| Emotion | 1 | 33 | 0.313 | 0.580 | 1 | 28 | 0.016 | 0.899 |
| Region | 1 | 33 | 6.967 | 0.013* | 1 | 28 | 11.888 | 0.002* |
| Hemisphere | 1 | 33 | 1.421 | 0.242 | 1 | 28 | 1.716 | 0.201 |
| Group × Emotion | 1 | 33 | 2.514 | 0.122 | 1 | 28 | 3.037 | 0.092 |
| Group × Region | 1 | 33 | 0.443 | 0.510 | 1 | 28 | 2.521 | 0.124 |
| Group × Hemisphere | 1 | 33 | 0.170 | 0.682 | 1 | 28 | 0.057 | 0.813 |
| Emotion × Region | 1 | 33 | 13.389 | 0.001* | 1 | 28 | 12.174 | 0.002* |
| Emotion × Hemisphere | 1 | 33 | 1.288 | 0.265 | 1 | 28 | 1.018 | 0.322 |
| Region × Hemisphere | 1 | 33 | 0.037 | 0.848 | 1 | 28 | 0.040 | 0.842 |
| Group × Emotion × Region | 1 | 33 | 0.721 | 0.402 | 1 | 28 | 0.536 | 0.470 |
| Group × Emotion × Hemisphere | 1 | 33 | 0.001 | 0.972 | 1 | 28 | 0.000 | 0.985 |
| Group × Region × Hemisphere | 1 | 33 | 0.429 | 0.517 | 1 | 28 | 0.286 | 0.597 |
| Emotion × Region × Hemisphere | 1 | 33 | 3.552 | 0.068 | 1 | 28 | 2.537 | 0.122 |
| Group × Emotion × Region × Hemisphere | 1 | 33 | 0.986 | 0.328 | 1 | 28 | 0.653 | 0.426 |
p<.05
Stress-related changes in the distribution of recollection trials
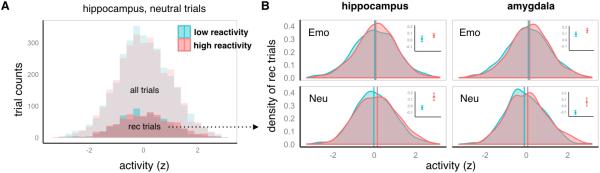
Because individual differences in cortisol reactivity were primarily driven by events that happened after encoding, it is unlikely that this measure would have been correlated with activity at the time of encoding. However, post-encoding stress could have affected subsequent memory outcomes and, importantly, the way in which these outcomes were related to encoding activity. This effect would explain the differences in recollection-related activity described above. Moreover, it could have led to changes in the way that recollection trials were distributed as a function of encoding activity, for instance, shifting the distribution such that recollection would be especially likely for trials eliciting relatively high compared to low encoding activity. To assess this hypothesis, we conducted a follow-up analysis that allowed us to characterize the distribution of recollection trials across different levels of encoding activity and to identify stress-related changes in this distribution. For this analysis, we obtained hippocampal and amygdala activity estimates for each individual encoding trial, then plotted the distribution of trial counts across levels of encoding activation for participants with relatively high or low cortisol reactivity, as defined by a median split (as illustrated in Figure 3A). We then examined the encoding strength distributions for the items that were subsequently recollected (Figure 3B). Note that the median split on cortisol reactivity was used to facilitate visualization in Figure 3, but that all statistical analyses were conducted on measures of cortisol reactivity, as in the previous section.
Figure 3. Stress-related shifts in the distribution of subsequent recollection.
Hippocampal and amygdala activity were estimated for each individual trial to permit investigation of the activity distribution of recollection trials. For visualization purposes, participants were divided into high reactivity (red) and low reactivity (blue) groups based on a median split on cortisol reactivity, and trials were aggregated across all participants in each group. However, note that the regression statistics reported in the text are based on continuous measures of cortisol reactivity. A) To illustrate the frequency of trials across different hippocampal activity levels, histograms are shown for all neutral trials (lighter shaded histograms in background) and for the subset of neutral trials that were subsequently recollected (darker shaded histograms in foreground). B) Probability density plots summarize the aggregated distribution of recollection trials for the negative (top) and neutral (bottom) conditions, separately for the hippocampus (left) and amygdala (right). Distribution means were calculated for each participant, and vertical lines denote the average of the participants’ distribution means within each group. These values are also displayed with error bars in the insets. Note the rightward shift in the recollection trial distribution for the high reactivity participants (in red), such that recollection was more likely for trials with high encoding activity compared to low encoding activity.
As shown in Figure 3B, cortisol reactivity was associated with a change in the activity distribution of recollection trials, such that there was a rightward shift for high relative to low reactivity participants. That is, especially for participants showing high cortisol reactivity, recollection tended to occur for events eliciting high relative to low encoding activity. This apparent shift was borne out as an effect of cortisol reactivity on mean hippocampal activity for recollection trials, F(1,32)=4.76, p=.037, and a marginal effect of cortisol reactivity on mean amygdala activity, F(1,32)=3.77, p=.06. The effects of cortisol reactivity on mean activity were similar within stress group alone, although in this smaller sample they did not reach significance (hippocampus: F(1,15)=3.14, p=.10; amygdala: F(1,15)=2.81, p=.11). There were no significant interactions with emotion in this analysis, although note that the average shift in strength was considerably larger for the neutral compared to the negative items. Cortisol reactivity had no significant effect on the standard deviation, skew or kurtosis of the recollection trial distributions, ps > .05. A control analysis showed that mean trial activity, irrespective of memory outcome, was not significantly modulated by cortisol reactivity for the hippocampus or the amygdala, ps > .1, indicating that this shift could not be explained by differences in baseline activity levels, but rather was induced by the experimental stressor.
Voxel-wise regression of recollection-related activity on cortisol reactivity
Finally, to evaluate whether the relationship between recollection-related activity and cortisol reactivity was observed across the entire brain or whether it was restricted to only certain areas (e.g., MTL), voxel-wise contrast maps of recollection-related activity were regressed onto individual differences in cortisol reactivity. For neutral memories, cortisol reactivity was positively associated with subsequent memory effects in a cluster spanning the left anterior hippocampus (local peak: −24, −7, −26) and amygdala (local peak: −24, −1, −20) (MTL cluster corrected p<.05, Figure 4). No clusters survived a whole-brain correction. When the whole-brain threshold was relaxed to match the thresholds used in the MTL correction, additional clusters emerged in the midbrain, putamen, nucleus accumbens, lateral orbitofrontal cortex, posterior thalamus, and left anterior temporal lobe. These results suggest that the relationship between subsequent memory effects and cortisol reactivity was relatively specific, in that clusters showing this effect were mostly limited to the MTL and, albeit to a lesser extent, other areas that have been associated with memory for salient events (Adcock, Thangavel, Whitfield-Gabrieli, Knutson, & Gabrieli, 2006; Gruber, Gelman, & Ranganath, 2014; Wittmann et al., 2005). Thus, the relationships between memory-related activity and cortisol reactivity were not a general property of the brain but appeared to be largest in areas important for memory prioritization. There were no significant clusters showing a relation between cortisol reactivity and subsequent memory effects for emotionally negative trials. Altogether, results from the ROI and voxel-wise analyses converge in suggesting that hormonal markers of post-encoding stress were associated with increased dependence of memory outcomes on MTL encoding activity, and that this effect was generally stronger for neutral memories.
Figure 4. Voxel-wise regression on cortisol reactivity.
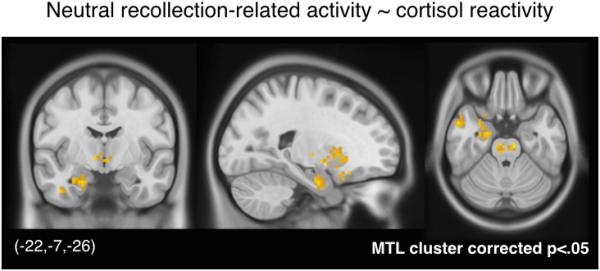
Cortisol reactivity was significantly associated with neutral recollection-related activity in an MTL cluster spanning the left anterior hippocampus and amygdala (cluster corrected p<.05 within the MTL). For completeness, other non-MTL clusters are shown at the same voxel and extent thresholds used for correction within the MTL, but they should be treated as exploratory results.
Discussion
In this study, we tested the hypothesis that the effects of post-encoding stress on memory would be contingent on neural processes engaged during encoding. Consistent with this hypothesis, we found that for participants who showed a large increase in stress hormone release after encoding, subsequent item recollection was more dependent on hippocampal and amygdala responses during encoding. This finding suggests that MTL responses during encoding were important for determining which memories would be affected by subsequent stress, resulting in a shift in the distribution of recollected trials. We also found that, for the hippocampus, this relationship was stronger for neutral compared to emotionally negative events, suggesting that the combination of hippocampal activity and post-encoding stress may make a bigger difference for memories that are not overtly emotional. These findings indicate that post-encoding stress does not uniformly influence memory for all preceding events, but rather filters memories according to their impact on neural processes during encoding.
Post-encoding stress as a mnemonic filter
Prior work in rodents and humans has shown that acute stress slows forgetting for recently learned information (McGaugh & Roozendaal, 2002; Schwabe et al., 2012). Here, we present novel evidence that stress interacts with memory by increasing the dependency of subsequent recollection on hippocampal and amygdala activity during encoding. We refer to this process as mnemonic filtering, whereby stress shifts the distribution of recollected trials to prioritize those events that had elicited strong MTL responses. This form of filtering may be achieved through the kinds of processes described in tag-and-capture models (Frey & Morris, 1998; Redondo & Morris, 2011; Viola et al., 2014), which propose that tags set during encoding determine which memories can benefit from periods of enhanced plasticity that occur around the time of encoding. Although functional neuroimaging data can provide only a macroscopic look at encoding processes in the MTL, the present results suggest that MTL engagement during encoding was involved in determining which memories would be most affected by post-encoding stress, consistent with the presence of a tagging-like mechanism in humans.
Evidence for tag-and-capture models in humans has been limited. One behavioral study recently demonstrated that memories were improved by fear conditioning following learning (Dunsmoor, Murty, Davachi, & Phelps, 2015). Critically, in that study, memory improvements were limited to items that were semantically related to the reinforced set and, compared to strongly encoded memories, were less likely to have been remembered otherwise. Another recent study demonstrated that increased cortisol during learning was associated with selective memory for items that had attracted the most visual attention (Bennion, Mickley Steinmetz, Kensinger, & Payne, 2013). Results from these studies were consistent with a tagging account, in that they show that modulatory states around the time of encoding can influence memory in ways that depend on processes engaged during encoding. However, the assumption that neural processes at encoding set the stage for post-encoding neurohormonal modulation had yet to be directly tested in humans, and in this way, the present results provide important new evidence in support of tag-and-capture models. Moreover, the present results extend the ideas of tag-and-capture to understanding the influence of stress on memory (c.f., Korz & Frey, 2003; Mather et al., 2015; McIntyre et al., 2012).
In contrast to the observed linear relationship between cortisol reactivity and recollection-related activity, we previously reported a non-monotonic relationship between cortisol reactivity and behavioral estimates of recollection (McCullough et al., 2015). This apparent discrepancy could simply reflect the fact that we may not have had sufficient power to detect a quadratic relationship, due to variability in activity estimates and the necessary exclusion of several participants from the fMRI analyses. Thus we cannot rule out the possibility that, like behavioral estimates of recollection, recollection-related activity might be modulated by cortisol reactivity in a non-monotonic way. This is an interesting question for future research, because behavioral estimates of recollection and recollection-related activity estimates are sensitive to different aspects of memory and thus may be affected by stress in different ways. Whereas recollection-related activity estimates are sensitive to the way in which recollection trials are distributed as a function of brain activity, behavioral recollection estimates are sensitive to the total number of items that are accurately endorsed with recollection. For instance, a participant might remember very few items but these items might comprise the subset of trials that had elicited the strongest MTL responses (i.e., low behavioral estimates but high recollection-related activity). Conversely, a participant might remember many items but these items might include some trials that had elicited relatively weak MTL responses, leading to a smaller difference in activity between remembered and forgotten items (i.e., high behavioral estimates but low recollection-related activity). Thus, behavioral and fMRI measures related to memory need not be affected in the same way by stress or other modulatory states.
Relation to stress and arousal effects during encoding
Whereas prior neuroimaging studies of stress and memory have investigated the effects of stress at the time of encoding (Henckens, Hermans, Pu, Joëls, & Fernández, 2009; Qin, Hermans, van Marle, & Fernández, 2012), this is the first study to our knowledge to investigate the relationship between encoding activity and stress occurring after encoding. Our results are broadly consistent with emerging evidence suggesting that stress and arousal may influence the selectivity of memory. For instance, stress and arousal during encoding have been shown to enhance memories for information at the focus of encoding but suppressing memories for other, less important or unrelated information (Kensinger, 2009; Mather et al., 2015; Mather & Sutherland, 2011). These effects have been attributed to the modulatory influence of noradrenaline on local glutamatergic processing (Mather et al., 2015), which results in “hot spots” of activity that initiate processes leading to long-term consolidation, similar to the tagging mechanism described above.
Based on the present findings, we hypothesize that neurohormonal manipulations during the post-encoding consolidation period help to stabilize or even amplify arousal-induced memory biases by boosting memory for tagged (i.e., high-activity) events and filtering out others. We attribute the increase in mnemonic filtering to changes that occurred after encoding. Although there is some possibility that arousal or stress experienced during encoding could have contributed to our measure of cortisol reactivity, changes during encoding were likely to have been quite small relative to the large changes elicited by the post-encoding stress manipulation. As can be seen in Figure 2, individual differences in cortisol reactivity were primarily driven by a large difference between the stress and control groups, as well as variability within the stress group itself. Prior work has additionally suggested that stress experienced during encoding might actually have the opposite effect on memory-related activity in the hippocampus, leading to stress-related reductions in hippocampal memory-related activity (Henckens et al., 2009; Qin et al., 2012). For these reasons, we conclude that the post-encoding stress event was crucially involved in drawing out the neurohormonal interactions observed here. Providing some support for this idea, Bennion et al. (2013) demonstrated that cortisol during learning predicted selective memory for visually attended items. However, this relationship was observed only for participants who slept during the retention interval, suggesting that sleep consolidation processes after learning played a role in drawing out these memory biases. Together with this prior literature, the present results suggest that the effects of stress on memory are not uniform but instead have selective effects on memory encoding and consolidation processes.
Differences in mnemonic filtering for neutral and emotional memories
A surprising aspect of the current results is that, for the hippocampus, the effects of stress on recollection-related activity were stronger for emotionally neutral than negative memories. Prior work on stress-related memory modulation has emphasized the dependence of stress effects on the experience of arousal during encoding (McGaugh, 2004; Roozendaal, McEwen, & Chattarji, 2009), leading to the expectation that the effects of stress might be larger for emotional materials. Even if there had been carry-over of arousal from the negative to neutral items, one might have expected that emotional and neutral memories would be similarly affected by stress. However, we found an altogether different pattern—that the effects of stress on recollection-related activity were more pronounced for neutral than emotional memories.
The principles of tag-and-capture may offer some insight into why activity-dependent stress effects were larger for neutral than emotional memories. In prior studies of tag-and-capture, a key finding has been that the modulatory event has the largest effect on memories that, otherwise, would not have been consolidated into long-term memory—e.g., events that can be recalled 30 minutes but not 1 day after learning (Moncada & Viola, 2007). Here, we found that neutral items tended to produce weaker memories in general than emotional items, but that they benefited the most from the combination of hippocampal activity during encoding and stress after encoding. Indeed, in the absence of post-encoding stress, hippocampal activity did not strongly predict subsequent recollection for neutral items, perhaps because of competition for encoding resources from adjacent emotional items (Mather & Sutherland, 2011) or perhaps to arousal-related suppression of weakly-encoded items (Mather et al., 2015). In contrast, emotional events may have initiated processes that were sufficient to drive long-term memory, regardless of whether stress occurred after encoding. In this way, neutral memories might have had more to gain from the added boost of plasticity following encoding.
Another possible interpretation is that post-encoding stress might affect only memories that have been associated with emotion. In the present design, because neutral items occurred close in time to many emotionally negative items, it may be that some neutral items were associated with emotion through inter-item binding processes supported by the hippocampus (Diana et al., 2007; Eichenbaum, Yonelinas, & Ranganath, 2007). In contrast, negative items are intrinsically emotional, and thus their association with emotion may be supported by extra-hippocampal binding processes, such as in the amygdala or perirhinal cortex (Yonelinas & Ritchey, 2015). This difference could explain why, in the present study, hippocampal encoding activity was a stronger determinant of stress-related memory effects for neutral items compared to negative items.
Future Directions
There are several remaining questions about the relationship between encoding processes and post-encoding stress. First, does post-encoding stress act as a filter by enhancing memory for events that were tagged by high MTL activity during encoding, by reducing interference from events that were not, or by some mixture of the two? Second, to what extent were the current results influenced by arousal during encoding? Some studies have suggested that arousal during encoding is necessary to observe the modulatory effects of stress on memory (e.g., Okuda et al., 2004). Furthermore, the localization of tag-like effects to the MTL could have been influenced by the use of emotionally arousing materials. Recent work has demonstrated that tag-and-capture effects can be regionally specific, as long as the modulatory event affects the same area or areas involved in initial encoding (Ballarini et al., 2009; Dunsmoor et al., 2015). Thus, it is possible that our findings were localized to the hippocampus and amygdala because these regions are involved in neutral and emotional recollection, respectively, and because they are particularly sensitive to the effects of stress (Kim & Diamond, 2002; Roozendaal et al., 2009). Future work using similar methods should test whether and how post-encoding stress filters memories when all preceding events were neutral or when preceding events are related or unrelated to the stressful event.
Finally, to what extent were the present results influenced by our inclusion of only male participants? We focused on male participants to reduce variability in the sample and to be consistent with the results of previous studies (Andreano & Cahill, 2006; McCullough & Yonelinas, 2013). However, prior work has demonstrated that there are sex differences in the stress response and its relationship to memory (Andreano & Cahill, 2006; Felmingham, Tran, Fong, & Bryant, 2012), and thus it will be essential for future work to determine whether female participants show a different profile of mnemonic filtering, particularly with respect to the effects of emotional valence (Felmingham et al., 2012).
Conclusion
In conclusion, this study offers new evidence for stress-related mnemonic filtering, a process by which post-encoding stress can selectively preserve memories that had attracted enhanced MTL processing during encoding. The ability to retroactively filter memories based on encoding signals is a key feature of an adaptive memory system, as it supports the prioritization of events based on the significance of their outcomes. Deeper understanding of this filtering process helps to clarify how stress impacts what we remember and what we forget.
Acknowledgements
This work was supported by National Institutes of Health grant R01MH083734 to CR and APY. MR was supported by NIH grant K99MH103401.
References
- Adcock RA, Thangavel A, Whitfield-Gabrieli S, Knutson B, Gabrieli JDE. Reward-motivated learning: mesolimbic activation precedes memory formation. Neuron. 2006;50(3):507–517. doi: 10.1016/j.neuron.2006.03.036. doi:10.1016/j.neuron.2006.03.036. [DOI] [PubMed] [Google Scholar]
- Andreano JM, Cahill L. Glucocorticoid release and memory consolidation in men and women. Psychol. Sci. 2006;17(6):466–470. doi: 10.1111/j.1467-9280.2006.01729.x. doi:10.1111/j.1467-9280.2006.01729.x. [DOI] [PubMed] [Google Scholar]
- Ballarini F, Moncada D, Martinez MC, Alen N, Viola H. Behavioral tagging is a general mechanism of long-term memory formation. Proc. Natl. Acad. Sci. U. S. A. 2009;106(34):14599–14604. doi: 10.1073/pnas.0907078106. doi:10.1073/pnas.0907078106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennion KA, Mickley Steinmetz KR, Kensinger EA, Payne JD. Sleep and Cortisol Interact to Support Memory Consolidation. Cereb. Cortex. 2013;25(3):646–657. doi: 10.1093/cercor/bht255. doi:10.1093/cercor/bht255. [DOI] [PubMed] [Google Scholar]
- Cahill L, Gorski L, Le K. Enhanced human memory consolidation with post-learning stress: interaction with the degree of arousal at encoding. Learn. Mem. 2003;10(4):270–274. doi: 10.1101/lm.62403. doi:10.1101/lm.62403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diana RA, Yonelinas AP, Ranganath C. Imaging recollection and familiarity in the medial temporal lobe: a three-component model. Trends in Cognitive Sciences. 2007;11(9):379–386. doi: 10.1016/j.tics.2007.08.001. doi: http://dx.doi.org/10.1016/j.tics.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Dunsmoor JE, Murty VP, Davachi L, Phelps EA. Emotional learning selectively and retroactively strengthens memories for related events. Nature. 2015;520(7547):345–348. doi: 10.1038/nature14106. doi:10.1038/nature14106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AP, Ranganath C. The Medial Temporal Lobe and Recognition Memory. Annual Review of Neuroscience. 2007;30(1):123–152. doi: 10.1146/annurev.neuro.30.051606.094328. doi:10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felmingham KL, Tran TP, Fong WC, Bryant RA. Sex differences in emotional memory consolidation: The effect of stress-induced salivary alpha-amylase and cortisol. Biological Psychology. 2012;89(3):539–544. doi: 10.1016/j.biopsycho.2011.12.006. doi: http://dx.doi.org/10.1016/j.biopsycho.2011.12.006. [DOI] [PubMed] [Google Scholar]
- Frey U, Morris RG. Synaptic tagging: implications for late maintenance of hippocampal long-term potentiation. Trends Neurosci. 1998;21(5):181–188. doi: 10.1016/s0166-2236(97)01189-2. doi:10.1016/s0166-2236(97)01189-2. [DOI] [PubMed] [Google Scholar]
- Gruber MJ, Gelman BD, Ranganath C. States of curiosity modulate hippocampus-dependent learning via the dopaminergic circuit. Neuron. 2014;84(2):486–496. doi: 10.1016/j.neuron.2014.08.060. doi:10.1016/j.neuron.2014.08.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henckens MJAG, Hermans EJ, Pu Z, Joëls M, Fernández G. Stressed memories: how acute stress affects memory formation in humans. J. Neurosci. 2009;29(32):10111–10119. doi: 10.1523/JNEUROSCI.1184-09.2009. doi:10.1523/jneurosci.1184-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joëls M, Fernandez G, Roozendaal B. Stress and emotional memory: a matter of timing. Trends Cogn. Sci. 2011;15(6):280–288. doi: 10.1016/j.tics.2011.04.004. doi:10.1016/j.tics.2011.04.004. [DOI] [PubMed] [Google Scholar]
- Kensinger EA. Remembering the Details: Effects of Emotion. Emot. Rev. 2009;1(2):99–113. doi: 10.1177/1754073908100432. doi:10.1177/1754073908100432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Diamond DM. The stressed hippocampus, synaptic plasticity and lost memories. Nat Rev Neurosci. 2002;3(6):453–462. doi: 10.1038/nrn849. doi:10.1038/nrn849. [DOI] [PubMed] [Google Scholar]
- Korz V, Frey JU. Stress-related modulation of hippocampal long-term potentiation in rats: Involvement of adrenal steroid receptors. J. Neurosci. 2003;23(19):7281–7287. doi: 10.1523/JNEUROSCI.23-19-07281.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather M, Clewett D, Sakaki M, Harley CW. Norepinephrine ignites local hot spots of neuronal excitation: How arousal amplifies selectivity in perception and memory. Behav. Brain Sci. 2015:1–100. doi: 10.1017/S0140525X15000667. doi:10.1017/s0140525x15000667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather M, Sutherland MR. Arousal-biased competition in perception and memory. Perspect. Psychol. Sci. 2011;6(2):114–133. doi: 10.1177/1745691611400234. doi:10.1177/1745691611400234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough AM, Ritchey M, Ranganath C, Yonelinas A. Differential effects of stress-induced cortisol responses on recollection and familiarity-based recognition memory. Neurobiol. Learn. Mem. 2015;123:1–10. doi: 10.1016/j.nlm.2015.04.007. doi:10.1016/j.nlm.2015.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough AM, Yonelinas AP. Cold-pressor stress after learning enhances familiarity-based recognition memory in men. Neurobiol. Learn. Mem. 2013;106:11–17. doi: 10.1016/j.nlm.2013.06.011. doi:10.1016/j.nlm.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu. Rev. Neurosci. 2004;27(1):1–28. doi: 10.1146/annurev.neuro.27.070203.144157. doi:10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- McGaugh JL, Roozendaal B. Role of adrenal stress hormones in forming lasting memories in the brain. Curr. Opin. Neurobiol. 2002;12(2):205–210. doi: 10.1016/s0959-4388(02)00306-9. [DOI] [PubMed] [Google Scholar]
- McIntyre CK, McGaugh JL, Williams CL. Interacting brain systems modulate memory consolidation. Neurosci. Biobehav. Rev. 2012;36(7):1750–1762. doi: 10.1016/j.neubiorev.2011.11.001. doi:10.1016/j.neubiorev.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncada D, Viola H. Induction of long-term memory by exposure to novelty requires protein synthesis: evidence for a behavioral tagging. J. Neurosci. 2007;27(28):7476–7481. doi: 10.1523/JNEUROSCI.1083-07.2007. doi:10.1523/jneurosci.1083-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore M, Hu Y, Woo S, O'Hearn D, Iordan AD, Dolcos S, Dolcos F. A comprehensive protocol for manual segmentation of the medial temporal lobe structures. J. Vis. Exp. 2014;(89) doi: 10.3791/50991. doi:10.3791/50991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda S, Roozendaal B, McGaugh JL. Glucocorticoid effects on object recognition memory require training-associated emotional arousal. Proc. Natl. Acad. Sci. U. S. A. 2004;101(3):853–858. doi: 10.1073/pnas.0307803100. doi:10.1073/pnas.0307803100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preuß D, Wolf OT. Post-learning psychosocial stress enhances consolidation of neutral stimuli. Neurobiol. Learn. Mem. 2009;92(3):318–326. doi: 10.1016/j.nlm.2009.03.009. doi:10.1016/j.nlm.2009.03.009. [DOI] [PubMed] [Google Scholar]
- Qin S, Hermans EJ, van Marle HJF, Fernández G. Understanding low reliability of memories for neutral information encoded under stress: alterations in memory-related activation in the hippocampus and midbrain. J. Neurosci. 2012;32(12):4032–4041. doi: 10.1523/JNEUROSCI.3101-11.2012. doi:10.1523/jneurosci.3101-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redondo RL, Morris RGM. Making memories last: the synaptic tagging and capture hypothesis. Nat. Rev. Neurosci. 2011;12(1):17–30. doi: 10.1038/nrn2963. doi:10.1038/nrn2963. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, McEwen BS, Chattarji S. Stress, memory and the amygdala. Nat. Rev. Neurosci. 2009;10(6):423–433. doi: 10.1038/nrn2651. doi:10.1038/nrn2651. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Nguyen BT, Power AE, McGaugh JL. Basolateral amygdala noradrenergic influence enables enhancement of memory consolidation induced by hippocampal glucocorticoid receptor activation. Proc. Natl. Acad. Sci. U. S. A. 1999;96(20):11642–11647. doi: 10.1073/pnas.96.20.11642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, Okuda S, Van der Zee EA, McGaugh JL. Glucocorticoid enhancement of memory requires arousal-induced noradrenergic activation in the basolateral amygdala. Proc. Natl. Acad. Sci. U. S. A. 2006;103(17):6741–6746. doi: 10.1073/pnas.0601874103. doi:10.1073/pnas.0601874103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabe L, Joëls M, Roozendaal B, Wolf OT, Oitzl MS. Stress effects on memory: an update and integration. Neurosci. Biobehav. Rev. 2012;36(7):1740–1749. doi: 10.1016/j.neubiorev.2011.07.002. doi:10.1016/j.neubiorev.2011.07.002. [DOI] [PubMed] [Google Scholar]
- Smeets T, Otgaar H, Candel I, Wolf OT. True or false? Memory is differentially affected by stress-induced cortisol elevations and sympathetic activity at consolidation and retrieval. Psychoneuroendocrinology. 2008;33(10):1378–1386. doi: 10.1016/j.psyneuen.2008.07.009. doi:10.1016/j.psyneuen.2008.07.009. [DOI] [PubMed] [Google Scholar]
- Viola H, Ballarini F, Martínez MC, Moncada D. The Tagging and Capture Hypothesis from Synapse to Memory. In: Zafar UK, Muly EC, editors. Progress in Molecular Biology and Translational Science. Vol. 122. Academic Press; 2014. pp. 391–423. [DOI] [PubMed] [Google Scholar]
- Wang S-H, Redondo RL, Morris RGM. Relevance of synaptic tagging and capture to the persistence of long-term potentiation and everyday spatial memory. Proc. Natl. Acad. Sci. U. S. A. 2010;107(45):19537–19542. doi: 10.1073/pnas.1008638107. doi:10.1073/pnas.1008638107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann BC, Schott BH, Guderian S, Frey JU, Heinze H-J, Düzel E. Reward-related FMRI activation of dopaminergic midbrain is associated with enhanced hippocampus-dependent long-term memory formation. Neuron. 2005;45(3):459–467. doi: 10.1016/j.neuron.2005.01.010. doi:10.1016/j.neuron.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP, Parks CM, Koen JD, Jorgenson J, Mendoza SP. The effects of post-encoding stress on recognition memory: examining the impact of skydiving in young men and women. Stress. 2011;14(2):136–144. doi: 10.3109/10253890.2010.520376. doi:10.3109/10253890.2010.520376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonelinas AP, Ritchey M. The slow forgetting of emotional episodic memories: an emotional binding account. Trends Cogn. Sci. 2015;19(5):259–267. doi: 10.1016/j.tics.2015.02.009. doi:10.1016/j.tics.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]