Abstract
We recently observed that dysregulation of the complement system may be involved in the pathogenesis of hematopoietic stem cell transplantation–associated thrombotic microangiopathy (HSCT-TMA). These findings suggest that the complement inhibitor eculizumab could be a therapeutic option for this severe HSCT complication with high mortality. However, the efficacy of eculizumab in children with HSCT-TMA and its dosing requirements are not known. We treated 6 children with severe HSCT-TMA using eculizumab and adjusted the dose to achieve a therapeutic level >99 μg/mL. HSCT-TMA resolved over time in 4 of 6 children after achieving therapeutic eculizumab levels and complete complement blockade, as measured by low total hemolytic complement activity (CH50). To achieve therapeutic drug levels and a clinical response, children with HSCT-TMA required higher doses or more frequent eculizumab infusions than currently recommended for children with atypical hemolytic uremic syndrome. Two critically ill patients failed to reach therapeutic eculizumab levels, even after dose escalation, and subsequently died. Our data indicate that eculizumab may be a therapeutic option for HSCT-TMA, but HSCT patients appear to require higher medication dosing than recommended for other conditions. We also observed that a CH50 level ≤ 4 complement activity enzyme units correlated with therapeutic eculizumab levels and clinical response, and therefore CH50 may be useful to guide eculizumab dosing in HSCT patients as drug level monitoring is not readily available.
Keywords: Thrombotic microangiopathy, Eculizumab, CH50, Eculizumab pharmacokinetics, Hematopoietic stem cell transplant
INTRODUCTION
Hematopoietic stem cell transplantation–associated thrombotic microangiopathy (HSCT-TMA) is a challenging post-transplant complication associated with long-term morbidity and high mortality [1–3]. HSCT-TMA shares features with other TMAs where endothelial injury affects the kidney and other organs. Mild HSCT-TMA may have a benign course that requires no therapy or only modification of calcineurin inhibitor dosing [4,5]. However, a proportion of cases develop a systemic vascular injury that manifests as kidney damage, serositis, pulmonary hypertension, and multisystem organ failure [6–9]. In the most severe form of HSCT-TMA, mortality rates approach 90%, whereas milder cases have an increased risk of chronic kidney disease [3,8,10]. Targeted therapy is urgently needed for patients with severe HSCT-TMA in whom mortality is the highest.
Recently, we showed that patients with HSCT-TMA have evidence of complement dysregulation, including complement factor H autoantibodies and renal C4d deposition [11,12]. HSCT-TMA is a multifactorial disease in which either the classical or alternative complement pathways may become activated, resulting in tissue damage from micro-vessel thrombosis [13]. Currently available therapeutic options in patients with HSCT-TMA include therapeutic plasma exchange (TPE), rituximab, and withdrawal of calcineurin inhibitors. However, clinical response is often limited, especially in patients with severe HSCT-TMA. Furthermore, altering immunosuppressive therapy may increase the risk of graft-versus-host disease (GVHD), which itself is associated with high mortality [5,14,15].
Eculizumab, a humanized monoclonal antibody against the complement component C5 that prevents tissue damage by blocking formation of the membrane attack complex, is increasingly prescribed in the treatment of other diseases presenting with TMA [16–19]. Eculizumab has a low toxicity profile and has been well tolerated in patients with paroxysmal nocturnal hemoglobinuria and atypical hemolytic uremic syndrome (aHUS). It has been approved for use in children with aHUS, and a pediatric weight-based dosing schedule has been established [20]. It is unknown if eculizumab is effective in children with HSCT-TMA and if the existing dosing regimen adequately blocks complement in these patients. Therefore, we describe our clinical experience in a cohort of children treated with eculizumab for severe HSCT-TMA and propose an algorithm to adjust the dosage and monitor therapeutic response based on our observations.
METHODS
Patient Population
Six patients with severe HSCT-TMA were treated with eculizumab (Alexion, Cheshire, CT) at Cincinnati Children’s Hospital Medical Center (CCHMC) between January 2012 and May 2013. The CCHMC Institutional Review Board approved retrospective chart review. Patient demographics, therapy characteristics, and HSCT complications were abstracted from the medical record. HSCT-TMA was diagnosed using current diagnostic criteria and included elevated lactate dehydrogenase above normal for age, haptoglobin below the lower limit of normal, schistocytes on peripheral blood smear, anemia, thrombocytopenia, a negative Coombs test, and acute kidney injury, defined as a doubling of the serum creatinine or a 50% decline in the cystatin C–estimated glomerular filtration rate (eGFR) from each subject’s pre-HSCT baseline [5,21,22]. Proteinuria was identified using a random spot urine protein-to-creatinine ratio (normal <.2 mg/mg, nephrotic range >2 mg/mg) [23,24]. Kidney biopsy results, if available, were reviewed for histology of TMA.
Each subject’s legal guardian signed informed consent for treatment with eculizumab. The decision to start eculizumab was at the discretion of the treating physician but generally included severe TMA presenting with multiorgan impairment, uncontrolled hypertension, worsening renal function, and a lack of response to TPE and withdrawal of calcineurin inhibitors in allogeneic transplant recipients.
Outcomes
A hematologic response to eculizumab was defined as normalization of lactate dehydrogenase and haptoglobin, resolution of the need for RBC and platelet transfusions, and disappearance of schistocytes. A complete responsewas defined as normalization of the hematologic parameters noted above combined with a doubling of the cystatin C–eGFR and improvement of proteinuria to values below the nephrotic range, as defined by a random spot urine protein-to-creatinine ratio <2 mg/mg [25].
Complement Assessments
Assessment of the complement profile was performed at the time of HSCT-TMA diagnosis. Testing included measurement of complement proteins (Table 1), soluble terminal complement complex activity (sC5b-9), and autoantibodies to complement factor H (CFH) [11,26,27]. ADAMTS13 activity was measured to rule out thrombotic thrombocytopenic purpura. These studies were performed at the CCHMC clinical laboratory. Circulating CFH-related 1 protein (CFHR1) testing was performed on recipients’ plasma to exclude a CFHR1 homozygous deletion. CFHR1 was detected by immunoblotting (Division of Nephrology, The Hospital for Sick Children, Toronto, Ontario, Canada), as previously described [28,29]. CFH-related genes 3 and 1 (CFHR3-CFHR1) were examined in stored pretransplant (recipient) DNA samples by multiplex ligation-dependent probe amplification testing (MORL Laboratory, University of Iowa, Iowa City, IA) [30,31].
Table 1.
Patient Demographics and Disease Characteristics
| Responders | Non-responders | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | |
| Gender | F | F | M | F | M | M |
| Age at HSCT, y | 4.9y | 5.1y | 2.4y | 4.3y | 7.2y | 10.9y |
| Weight, kg | 4.9 | 5.1 | 2.4 | 4.3 | 7.2 | 10.9 |
| Diagnosis | NBL | NBL | WAS | CID | WAS | CID |
| Stem cell source | Autologous | Autologous | Allogeneic | Allogeneic | Allogeneic | Allogeneic |
| HSCT conditioning regimen | MA | MA | MA | RIC | MA | RIC |
| Day post-HSCT when TMA was diagnosed | 13 | 68 | 390 | 6 | 41 | 57 |
| Cystatin C-eGFR (mL/min) at TMA diagnosis | 48 | 20 | 16 | 30 | 15 | 17 |
| Renal replacement therapy at TMA diagnosis | No | No | Yes | No | No | Yes |
| Urine protein-to-creatinine ratio (normal <.2 mg/ mg, nephrotic >2 mg/mg) | 81.6 | 11.3 | 4.5 | 10.6 | 14.1 | 6.4 |
| HSCT-TMA–related complications | HTN, PH, pericardial effusion | HTN, pericardial effusion | HTN, pericardial tamponade | HTN, PRES, seizures, pericardial effusion | HTN, pulmonary bleeding | HTN, pericardial effusion |
| Complement profile* | ↑C2, ↑C6, ↑CFB | ↓C4 | ↓C4 | ↑C5, ↑C7, ↑C4BP | ↑ C8, ↑C1Inhib, ↓ CFH | ↑ C1Q, ↑C4, ↑C8 |
| sC5b-9 (normal, 119 to 17 5 ng/mL) | 283 | 307 | 139(on TPE) | 328 | 432 | 375 |
| CFH antibody | Absent | Absent | Absent | Absent | Absent | Absent |
| CFHR3-CFHR1 (by MLPA) | Not tested | Normal | Heterozygous deletion | Normal | Not tested | Not tested |
| CFHR1 protein | Present | Present | Present | Present | Present | Present |
| Renal biopsy/autopsy | Not done | TMA, C4d deposits in arterioles | Not done | Not done | Not done | TMA |
| Infections | None | None | None | None | None | Adenovirus, BK, and HSV viremia |
| Acute GVHD | n/a | n/a | Yes (skin) | No | Yes (skin, gut) | Yes (skin, gut) |
| Eculizumab start from HSCT-TMA diagnosis, days | 3 | 40 | 122 | 26 | 24 | 97 |
| Number of eculizumab doses given | 9 | 13 | 6 | 4 | 7 | 2 |
| Number of TPE sessions done before starting eculizumab | None | 32 | 79 | 15 | 24† | 17† |
NBL indicates neuroblastoma; WAS, Wiskott-Aldridge syndrome; CID, combined immunodeficiency; MA, myeloablative regimen; RIC, reduced intensity regimen; HTN, systemic hypertension; PH, pulmonary hypertension; PRES, posterior reversible encephalopathy syndrome; HSV, herpes simplex 1 virus; MLPA, multiplex ligation-dependent probe amplification; Del (CFHR3-CFHR1), heterozygous deletion of CFH gene 3 and 1; n/a, not applicable.
Complement profile includes C1inhib, complement component 1 inhibitor; C1Q, complement component 1q complex; C2–C9, complement components 2–9; C4BP, C4d binding protein; complement factors (CF) F, B, H, and I.
Died while on eculizumab therapy.
Eculizumab Treatment and Monitoring
In patients receiving TPE, treatment was stopped before starting eculizumab so as not to remove the drug. The first dose of eculizumab was given according to recommendations for children with aHUS [20]. Eculizumab was infused via central venous access over 60 minutes. Because immunocompromised HSCT recipients do not respond to meningococcal vaccination, all patients were maintained on ciprofloxacin or penicillin VK prophylaxis until eculizumab was cleared and the CH50 levels normalized.
Because dosing recommendations to guide eculizumab therapy in HSCT recipients are not available, eculizumab serum levels were tested at least twice a week after each dose and always included a trough level drawn before each dose. Eculizumab drug levels were performed at Cambridge Biomedical, Inc. (Boston, MA), and we adjusted the dose to maintain a trough concentration >99 μg/mL, because this drug level has been considered therapeutic in patients with aHUS [16]. Patients weighing <40 kg started with 600 mg i.v., and patients weighing ≥ 40 kg started with 900 mg i.v. as a first dose. Subsequent dose adjustments were as follows: If a trough level was reported as therapeutic before the next weekly dose, the same induction dose was continued weekly. If the patient was due for the next weekly dose and the eculizumab trough concentration was subtherapeutic, the dose was increased by 300 mg/dose. If a subtherapeutic result was reported 4 to 5 days after the prior dose, an extra induction dose was given when the result was obtained. If results were not available for dose adjustment, the same eculizumab induction dose was continued weekly until the trough eculizumab concentration was documented to be above the therapeutic level. Laboratory markers of TMA were documented daily during inpatient stay and at least twice weekly in the outpatient setting.
Weekly induction therapy was continued until patients achieved a hematologic TMA response and had documented eculizumab levels >99 μg/mL, at which point a maintenance schedule was started (see Figure 3B). The criteria for stopping eculizumab included normalization of hematologic TMA parameters and improvement in renal function.
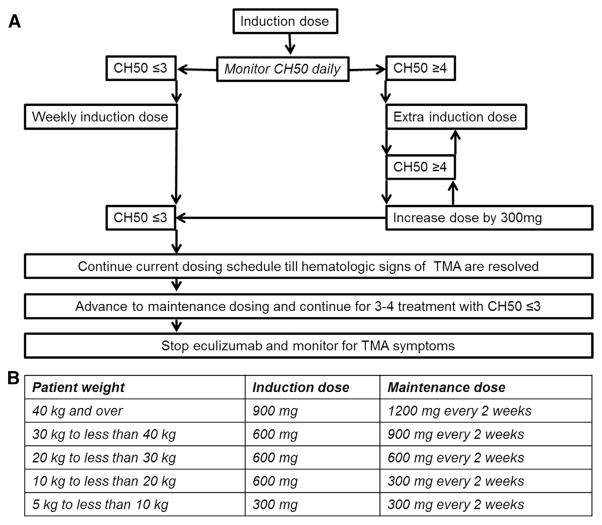
Figure 3.
Eculizumab dosing optimization in HSCT patients with TMA. (A) Suggested eculizumab dose adjustments based on CH50 level and clinical TMA response. (B) Eculizumab induction and maintenance doses based on patient’s weight (modified from dosing schedule for children with aHUS [20]). CH50 should be monitored daily after the first eculizumab induction dose is given with the goal to maintain CH50 level of 0 to 3 CAE units that corresponds to a therapeutic eculizumab dose >99 μg/mL. If CH50 remains 0 to 3 CAE units after start of therapy, the induction dose should be given weekly until full resolution of hematologic parameters of TMA before advancing to maintenance schedule. If CH50 is ≥ 4 CAE units earlier than 7 days after starting therapy, an additional induction dose should be given. If CH50 raises again to ≥4 CAE units, the next dose should be increased by 300 mg to achieve a steady therapeutic level as soon as possible. The dose of eculizumab that maintains a CH50 level ≤3 CAE units is the required weekly induction dose for the particular patient. During maintenance therapy, CH50 should be monitored at least twice a week to ensure a therapeutic drug level.
Pharmacokinetic and Pharmacodynamic Analyses
Total hemolytic complement activity (CH50) was measured in serum during eculizumab therapy as a pharmacodynamic marker of eculizumab-induced complement blockage, at the same time points as eculizumab drug levels. We correlated eculizumab drug levels with the degree of complement blockade as measured by CH50. A normal CH50 level was 60 to 144 complement activity enzyme (CAE) units (ARUP Laboratories, Salt Lake City, UT) [32].
The pharmacokinetic profiling of eculizumab was performed at the Division of Clinical Pharmacology, CCHMC, and was described by a 1-compartment model because the eculizumab concentrations showed an exponential decline over time. The elimination rate constant (kel) and concentration at 0 (C0) were estimated by linear least-squares regression of the log-transformed concentrations versus time in the log-linear phase of the disposition profile. Apparent volume of distribution (Vd) was estimated based on dose/C0 in the first treatment. Apparent systemic clearance (CL) was calculated by the formula CL (L/h) = kel × Vd. Maximum eculizumab concentrations in the nth dose (Cmax,n) were determined by the following equation: Cmax,n = Ctrough, n-1 + dose/Vd. Receiver operating characteristic curve analysis and Youden’s index were used to find the best cut-off value of CH50 that predicted a therapeutic eculizumab trough level > 99 μg/mL.
RESULTS
Patient Characteristics
Six children with severe HSCT-TMA received eculizumab therapy; patient characteristics are shown in Table 1. Median patient age was 5 years (range, 2.4 to 10.9 years). HSCT-TMA was diagnosed within 100 days (range, 6 to 69 days) of transplant in 5 patients. One patient was diagnosed 1 year post-transplant after presenting with acute HSCT-TMA and renal failure requiring hemodialysis. At diagnosis of HSCT-TMA, all patients had impaired renal function with a median serum cystatin C–eGFR of 18.5 mL/min (range, 15 to 48 mL/min) and nephrotic range proteinuria with a median random urine protein-to-creatinine ratio of 11 mg/mg (range, 4.5 to 81.6 mg/mg). Two patients required renal replacement therapy. All patients had severe hypertension requiring 6 to 9 antihypertensive medications at initiation of eculizumab therapy. ADAMTS13 activity level was normal (>67%) in all patients, ruling out thrombotic thrombocytopenic purpura.
More specifically, patient 1 was treated with eculizumab without attempting TPE because she had acute development of HSCT-TMA with uncontrolled hypertension and was not considered a suitable candidate for apheresis line placement under anesthesia. Patient 2 had progressive renal function decline and was approaching the need for renal replacement therapy. Renal biopsy showed >90% of glomeruli affected by TMA. Diffuse C4d staining was evident in TMA affected renal arterioles indicating complement deposits, as previously described [12]. Patient 3 had exacerbation of HSCT-TMA symptoms and progressive hypertension with each attempt to wean TPE. Patient 4 developed seizures and posterior reversible encephalopathy syndrome and was on 9 antihypertensive medications with blood pressures still above 99% for her age and height before initiation of eculizumab. Patients 5 and 6 were critically ill with acute GVHD, viral infections, and multiorgan failure in addition to HSCT-TMA before initiation of eculizumab.
Cyclosporine was discontinued in all 4 allogeneic transplant recipients at diagnosis of HSCT-TMA due to kidney injury or the development of posterior reversible encephalopathy syndrome/seizures and was replaced with steroids and/or mycophenolate mofetil. Three of these patients had active acute GVHD at diagnosis of TMA. Patient 3 had acute stage III skin GVHD (overall grade II GVHD) and was treated with mycophenolate mofetil and methylprednisolone 1 mg/kg/day. GVHD improved sufficiently by the end of eculizumab therapy to allow weaning of anti-GVHD medications. Patient 5 had acute ocular GVHD, acute stage III skin GVHD, and acute stage II gut GVHD (overall grade III GVDH) at TMA diagnosis and was treated with mycophenolate mofetil, methylprednisolone 2 mg/kg/day, and infliximab with resolution of all but ocular GVHD by the time he died from multiorgan failure. Patient 6 had acute stage II gut GVHD with intermittent intestinal bleeding at HSCT-TMA diagnosis and was treated with methylprednisolone 2 mg/kg/day and infliximab. Intestinal bleeding persisted during eculizumab therapy until patient’s family opted to withdraw clinical support. At autopsy, intestinal bleeding was attributed to gut GVHD coincident with severe intestinal TMA.
Complement Assessments
Terminal complement complex activity (sC5b-9) was elevated in 5 of 6 patients (Table 1). The patient with a normal sC5b-9 level was receiving TPE when the blood sample was obtained. CFH autoantibody was not detected in any patient. Two patients had decreased C4 levels and 1 decreased CFH levels. All patients had detectable CFHR1 protein, ruling out a homozygous deletion of CFHR3-CFHR1. One of 3 patients tested with multiplex ligation-dependent probe amplification testing had a heterozygous CFHR3-CFHR1 deletion. Renal biopsy performed on patient 2 showed severe TMA with diffuse C4d staining in affected arterioles [12]. Patient 6 had renal TMA confirmed on autopsy.
Clinical Response to Eculizumab
Patients 1 to 4 had complete clinical responses to eculizumab after achieving therapeutic eculizumab levels (Table 2). A median of 7 doses (range, 4 to 13 doses) of eculizumab were required to resolve HSCT-TMA. All surviving patients weighing < 40 kg received eculizumab 600 mg i.v. and those weighing ≥ 40 kg received 900 mg i.v. at least weekly for at least 4 weeks before any consideration was given to advance the dosing schedule. A hematologic TMA response in these 4 patients was observed a median of 28.5 days (range,15 to 45 days) after initiation of eculizumab.
Table 2.
Response to Eculizumab Therapy in Patients with HSCT-TMA
| Responders | Non-responders | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | |
| Response to eculizumab | ||||||
| Therapeutic eculizumab level (trough) after first dose | No | No | Yes | Yes | No | No |
| Therapeutic eculizumab level (trough) after subsequent doses | Yes | Yes | Yes | Yes | No* | No* |
| Normalization of LDH, d | 16 | 18 | 27 | 15 | NR | NR |
| Normalization of haptoglobin, d | 25 | 43 | 15 | 15 | 8 | NR |
| Disappearance of schistocytes, d | 30 | 45 | 14 | 7 | NR | NR |
| Normalization of hemoglobin without transfusions, d | 20 | 43 | 23 | 7 | NR | NR |
| Recovery of platelets to 20,000 K/μL, d | 18 | 43 | 14 | 7 | NR | NR |
| Recovery of platelets to 50,000 K/μL, d | 30 | 45 | 14 | 7 | NR | NR |
| Doubling of cystatin C–eGFR, d | 31 | 95 | 141 | 16 | NR | NR |
| Urine protein-to-creatinine ratio <2 mg/mg, d | 19 | 108 | 63 | 29 | NR | NR |
| Number of anti-HTN medication at the start of eculizumab therapy | 7 | 6 | 6 | 9 | 6 | 7 |
| Number of anti-HTN medication at the end of eculizumab therapy | 0 | 1 | 2 | 2 | 4 | 2 |
| Hematologic HSCT-TMA response, d | 30 | 45 | 27 | 15 | NR | NR |
| Complete resolution of HSCT-TMA, d | 31 | 108 | 141 | 29 | NR | NR |
| Outcomes | ||||||
| Current status | Alive | Alive | Alive | Alive | Dead | Dead |
| Time from starting eculizumab therapy to the last encounter, weeks | 38 | 55 | 40 | 72 | 5 | <2 |
| Most current cystatin C–eGFR, mL/min | 138 | 48 | 41 | 150 | n/a | n/a |
| Most current random urine protein-to-creatinine ratio (normal <.2 mg/mg, nephrotic range >2 mg/mg) | .2 | .9 | .48 | .14 | n/a | n/a |
LDH, lactate dehydrogenase; HTN, hypertension; NR, no response.
Did not achieve therapeutic eculizumab level even with the dose escalation.
All 4 responders had a dramatic improvement in hypertension with a reduction in the number of antihypertensive medications from 6 to 9 at the start of eculizumab therapy to 0 to 2 medications at eculizumab therapy completion. The median time for a complete response of all HSCT-TMA parameters, including doubling in cystatin C–eGFR and improvement of proteinuria below 2 mg/mg, was 69.5 days (range, 29 to 141 days) after eculizumab initiation. All responders were doing well at a median of 47.5 weeks (range, 38 to 72 weeks) post-diagnosis of HSCT-TMA. Patients 1 and 4 completely recovered renal function as evidenced by normal cystatin C–eGFR >100 mL/min and the absence of proteinuria and hypertension. Patients 2 and 3, both of whom had prolonged severe kidney injury due to HSCT-TMA at the start of eculizumab therapy, remain with chronic kidney disease and a cystatin C–eGFR <50 mL/min but are off hemodialysis and normotensive on losartan therapy.
Patients 5 and 6 died from multisystem organ failure and active HSCT-TMA while receiving eculizumab. These 2 patients were older (and weigh more) than the 4 patients who responded to treatment. Both patients were critically ill with severe multiorgan injury at the time of starting eculizumab therapy, and neither achieved sustained therapeutic eculizumab trough levels or complement blockade, as measured by CH50 (Figure 1). All 6 patients tolerated eculizumab therapy without any side effects or reactions, and there were no meningococcal infections or other bacterial infections.
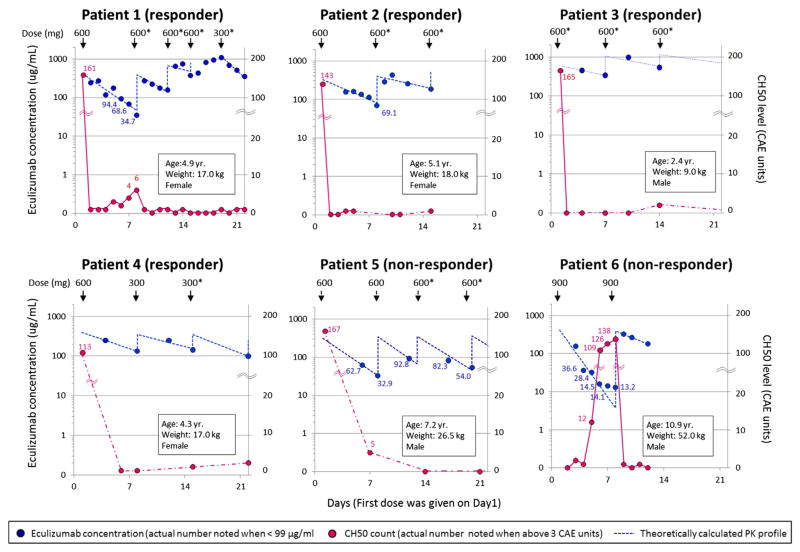
Figure 1.
Pharmacokinetic and pharmacodynamic analyses of eculizumab therapy in 6 patients with HSCT-TMA during the first 3 weeks of treatment are displayed. The left and right y axes show eculizumab concentrations and total complement activity (CH50) levels, respectively. The x axis shows time as days from the start of eculizumab therapy with the first eculizumab dose given on day 1. Dosage (mg) and the timing of administration are indicated with arrows on the top of each figure. Asterisk (*) marks eculizumab doses that are either higher than currently recommended for children with aHUS based on dosing weight or given more frequently [20]. Blue circles represent observed eculizumab concentrations. Actual measured values are noted beside the circles only when eculizumab concentration is below 99 μg/mL. Blue dashed lines represent predicted eculizumab pharmacokinetic profiles based on a 1-compartment analysis (see Methods for detail). Red circles represent the CH50 level. The actual values are listed on the graph only when the CH50 level is above 3 CAE units. Red circles are connected with red solid or dashed lines when measurement of CH50 levels are continuous (daily) or intermittent, respectively. In patient 6, the first 3 measurements days 2, 3, and 4 were used to estimate pharmacokinetic parameters (kel, CL) because concentrations of eculizumab irregularly showed a bi-exponential decline with very low concentrations at the last 3 measurements on days 5, 6s and 7. The Vd for this patient was calculated based on the second treatment.
Eculizumab Pharmacokinetics
Eculizumab dosing and drug levels during the first 3 weeks of therapy are shown in Figure 1. Four of 6 patients had subtherapeutic eculizumab through levels after the first dose. Notably, the first eculizumab dose for patient 3 was higher (600 mg) than recommended (300 mg) for his weight of 9 kg, producing a therapeutic level after the first dose. This child was the only patient who achieved and maintained a therapeutic eculizumab level after the first dose, which may have been associated with a faster clinical response. Therapeutic trough levels of eculizumab >99 μg/mL were eventually achieved in all 4 responders (patients 1 to 4) using either extra doses or doses that were higher than currently recommended (Figure 1). Eculizumab trough levels remained subtherapeutic in the 2 nonresponding cases (patients 5 and 6). Patient 5 did not achieve a therapeutic trough level during 5 weeks of therapy, despite significant dose escalation to 900 mg twice weekly, starting on day 24 of therapy (data not shown in Figure 1). Patient 6 received 2 weekly induction doses of 900 mg, as recommended for his weight, before he died. In both nonresponders, the eculizumab level dropped below therapeutic 3 days after the first dose, but results were pending so dosing could not be adjusted in a timely manner. Elimination rate constants and systemic clearance of eculizumab showed significant variability among patients, with the most rapid clearance in patients 5 and 6 who were most ill at the time of treatment and perhaps more highly catabolic than the responding patients (data not shown).
Relationship between Eculizumab Levels and CH50
Eculizumab and CH50 levels that were measured at the same time points strongly correlated with each other (Figures 1 and 2). Specifically, a CH50 level of 0 to 3 CAE units corresponded with an eculizumab concentration >99 μg/mL, except for 1 measurement from patient 6, drawn during an extremely rapid elimination phase in the first week. Conversely, at all sampling time points with a CH50 count ≥ 4 CAE units, the patients’ eculizumab concentrations were below therapeutic at <99 μg/mL (P = .0001).
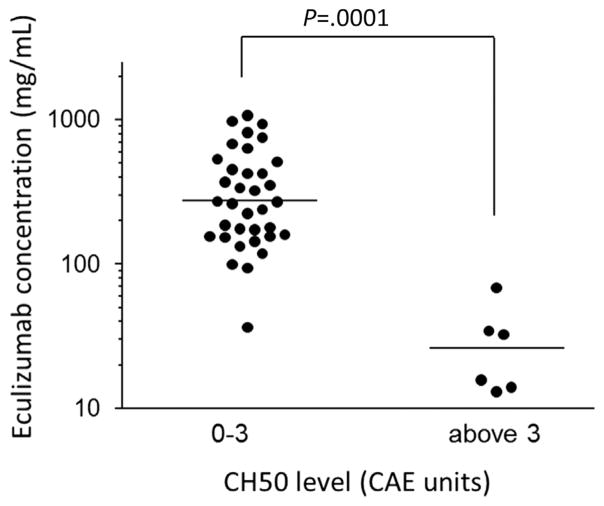
Figure 2.
Relationship between eculizumab concentration and total complement activity (CH50) level. Using the reported therapeutic eculizumab trough cut-off level of >99 or ≤99 μg/mL, the receiver operating characteristic curve and Youden’s Index were used to determine the optimal CH50 cut-off level of 3.5 CAE units. The y axis shows eculizumab concentrations in log-scale. The x axis shows the CH50 level as a group. Horizontal lines represent medians. A CH50 level of 0 to 3 CAE units corresponded with an eculizumab concentration of >99 μg/mL in all except 1 measurement (drawn from patient 6 during an extremely rapid elimination phase in the first week of therapy). Conversely, at all sampling time points, a CH50 level >3 CAE units corresponded with a subtherapeutic level < 99 μg/mL (P = .0001, Mann-Whitney U test).
Complete blockade of complement activity (CH50 < 3 CAE units) was achieved in all 4 responders (patients 1 to 4) after the second dose of therapy (Figure 1). Complement blockade was incomplete in the 2 nonresponding cases (patients 5 and 6).
DISCUSSION
We report the first use of the terminal complement inhibitor eculizumab in children with severe HSCT-TMA. We observed that 4 of 6 children had complete resolution of severe HSCT-TMA after achieving steady therapeutic eculizumab levels. The 2 remaining patients, who were critically ill when therapy was initiated, died without achieving therapeutic drug levels or complement blockade, despite dose escalation. The 2 nonresponders were also older than the 4 responders, an observation that deserves future study. All children with HSCT-TMA required higher eculizumab dosing and/or more frequent drug administrations to reach and maintain therapeutic eculizumab trough levels > 99 μg/mL compared with the dosing regimen currently recommended for children with aHUS [20].
Measurement of eculizumab levels in our patient cohort was of significant value in understanding the drug’s pharmacokinetics in children after HSCT. Pharmacokinetic analysis showed that 3 of 6 patients had eculizumab subtherapeutic drug concentrations < 99 μg/mL as soon as 4 to 5 days after the first eculizumab infusion and required an additional eculizumab dose to achieve a therapeutic drug trough level. Also, the rate of eculizumab elimination was not constant after every treatment but decreased over time and stabilized as represented by a declining slope of the log-transformed eculizumab concentration, which became shallower after multiple treatments indicating that doses could be administered with longer intervals. After steady therapeutic eculizumab serum concentrations were achieved, younger patients in our cohort were able to maintain a much higher drug level than targeted (>99 μL/mL) without any adverse events and with notable improvement in TMA-related symptoms. The current literature reports no significant adverse events attributed to high eculizumab levels, outside the known risk of infection. Unfortunately, eculizumab drug levels are only available from 1 laboratory in the United States (Cambridge Biomedical, Inc.), and results are often not available for 1 to 2 weeks. This long turnaround time may delay needed dose adjustment and prevent the achievement of complement blockade in HSCT patients treated with eculizumab. We speculate that earlier adjustments to dosing may have allowed treatment to be more effective in controlling TMA and preserving organ function in some of our patients.
In contrast, CH50 represents a pharmacodynamic measure of complement activity and is more readily available in most institutions, with a result turnaround time of 1 to 2 days. Therefore, CH50 testing during eculizumab therapy in HSCT patients may offer a more rapid assessment of complement blockade, allowing for practical dose adjustments in a more timely fashion. Our proposed dosing schedule modification based on CH50 level is displayed in Figure 3. In our study, only a CH50 level < 4 CAE units strongly correlated with a therapeutic eculizumab level > 99 μg/mL and clinical TMA response, indicating successful complement blockade and adequate drug dosing. Our observations suggest that low CH50 levels of 0 to 3 CAE units (or CH50 activity below 5%) should be maintained during eculizumab treatment to ensure the best therapeutic response.
Our study also provides important data regarding the time to clinical response with eculizumab in children with HSCT-TMA. Even though resolution of severe TMA took several weeks in responding patients, similar to aHUS, the fastest and most complete response was achieved in patients who started eculizumab therapy promptly after the diagnosis of HSCT-TMA and sustained steady therapeutic eculizumab levels and complement blockade [16]. However, we were able to achieve a hematologic response and control of hypertension even in patients with a prolonged course of HSCT-TMA that was previously refractory to TPE. In cases with prolonged HSCT-TMA before eculizumab therapy, renal recovery was incomplete and the time to recovery was longer, but all responders exhibited continuous, time-dependent improvement in renal function, suggesting that early use of eculizumab may maximize benefit in cases of severe HSCT-TMA.
Based on our clinical and pharmacologic observations, we suggest at least 4 to 6 weeks of induction therapy with a documented therapeutic eculizumab trough level or adequate complement blockade (low CH50) before calling a patient a nonresponder. If a patient is determined to be a rapid eliminator after the first induction dose (ie, 3 to 4 days after therapy initiation), an additional eculizumab induction dose should be considered to achieve a therapeutic trough level as quickly as possible. The CH50 level should be measured daily during induction therapy. In patients who fail to achieve full complement blockade, we suggest increasing the eculizumab dose by 300 mg above the currently recommended induction dose (Figure 3). As demonstrated by our 2 nonresponding subjects, those with additional complications such as GVHD and viral infections may be highly catabolic with concomitant activation of terminal complement. It may be challenging to achieve therapeutic levels of eculizumab needed to block complement and control TMA. Administration of eculizumab in critically ill HSCT patients without proper monitoring may provide only intermittent or no complement blockade, leading to the conclusion that eculizumab does not work in controlling TMA while in reality inadequate dose is being administered.
Five patients in our cohort had elevated sC5b-9, indicating an activated terminal complement pathway that could be caused by either alternative or classic complement dysregulation as previously described by our group of patients with HSCT-TMA [11,12]. Differences in the pathogenesis of HSCT-TMA occurring after allogeneic and autologous HSCT remain to be examined. We observed that autologous HSCT recipients present with evidence of classic pathway activation with a low C4 level, C4d deposition in the kidney, and severe multiorgan injury that may result from direct damage to the vascular endothelium from conditioning chemotherapy. Conversely, after allogeneic HSCT, we observed that some patients have evidence of alternative pathway activation with the presence of CFH antibodies. Nevertheless, complement activated by either the alternative or classic pathway can be blocked by the terminal complement blocker eculizumab, making this medication an appropriate therapy regardless of the complement pathway involved in pathogenesis of HSCT-TMA (Figure 4) [20,33].
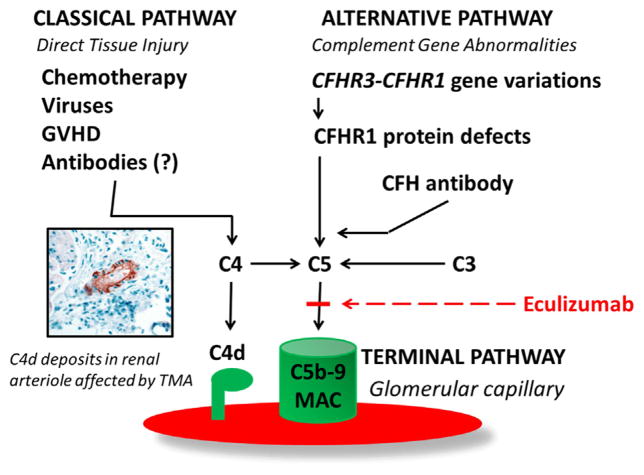
Figure 4.
Complement activation in pathogenesis of HSCT-TMA. Factors involved in classic and alternative pathway activation in patients with HSCT-TMA. The classic pathway can be activated by direct tissue injury from high-dose chemotherapy, viruses, and GVHD. C4d deposits, as the evidence of classic complement pathway activation, can be detected in renal arterioles of HSCT patients affected with TMA [12]. Alternative pathway dysregulation might present as a formation of the pathogenic autoantibody against complement factor H (CFH ab) due to either defects in CFHR3-CFHR1 genes associated with defective or absent CFHR1 protein or immune dysregulation after HSCT without genetic predisposition [11]. Dysregulation in either the classic or alternative pathway of complement results in terminal complement activation detected as elevated sC5b-9 (MAC) that can be blocked by eculizumab, which inhibits the cleavage of C5 to C5a and C5b by the C5 convertase, which prevents the generation of the terminal complement complex C5b-9 and tissue injury.
Although patients with paroxysmal nocturnal hemoglobinuria and aHUS are often committed to life-long therapy with eculizumab, some HSCT patients may develop TMA due to infections or temporary immune dysregulation without a genetic predisposition, suggesting that eculizumab therapy can be discontinued in these patients after TMA is controlled. We were able to stop eculizumab therapy in all 4 responders after achieving a hematologic and renal response. The shortest successful course was 4 weeks, and the longest therapy was 15 weeks. There were no recurrences of HSCT-TMA in the 4 responders after stopping therapy.
To consider stopping eculizumab therapy, we first suggest achieving full resolution of hematologic TMA markers during induction therapy with the steady therapeutic eculizumab level and full complement blockade documented by low a CH50 level. After achieving hematologic TMA control and improvement in renal function, eculizumab can be advanced to a maintenance schedule. Hypertension must be well controlled in all patients with a target systolic blood pressure below the 95th percentile for age and height [34]. Only after ensuring TMA is well controlled on a maintenance dosing regimen, eculizumab may be stopped while TMA markers are closely monitored (Figure 3).
In conclusion, eculizumab is a promising therapeutic option for pediatric patients with severe HSCT-TMA. In our practice, we consider eculizumab as a first-line therapy in patients who present with acute-onset HSCT-TMA with significant microangiopathic hemolysis, severe hypertension, or evidence of multiorgan involvement or in patients with subacute TMA whose symptoms progress within 2 weeks of observation and supportive care. We believe that early therapy initiation with eculizumab may prevent irreversible organ damage, and toxicity is low. Eculizumab dosing in children with HSCT-TMA should be guided by pharmacokinetic or pharmacodynamic testing, and more aggressive dosing schedules need to be explored in the most ill and catabolic patients. CH50 monitoring may aid in timely dose adjustments, especially in highly catabolic patients, likely requiring drug administration more than once a week. Controlled studies of this approach in both children and adults after HSCT are warranted to further examine clinical response and the benefit of therapeutic monitoring.
Acknowledgments
The authors thank the physicians, nurses, care managers, transplant coordinators, and other care providers and staff at Cincinnati Children’s Hospital Medical Center, and especially the patients and their families.
Conflict of interest statement: SJ has an Alexion Investigator-Sponsored Research Grant (as PI) for complement gene testing in HSCT patients from Alexion Pharmaceuticals, Inc. as is co-PI on NIH P50DK096418 grant for acute kidney injury biomarker testing in HSCT-TMA. BPD has financial relationship with both Alexion Pharmaceuticals, Inc. and Novartis Pharmaceuticals via consultancy and paid speaking. CL has financial relationship with Alexion Pharmaceuticals, Inc. via consultancy, paid speaking and unrestricted research grants. BLL is supported by an American Society for Blood and Marrow Transplantation/Genentech New Investigator Award. None of these funding sources had any input in the study design, analysis, manuscript preparation, or decision to submit for publication. SD, TF, AV, KM, JG, AT, FGP, LL have no competing financial interests to report.
Footnotes
Financial disclosure: The authors have nothing to disclose.
References
- 1.Laskin BL, Goebel J, Davies SM, Jodele S. Small vessels, big trouble in the kidneys and beyond: hematopoietic stem cell transplantation-associated thrombotic microangiopathy. Blood. 2011;118:1452–1462. doi: 10.1182/blood-2011-02-321315. [DOI] [PubMed] [Google Scholar]
- 2.Batts ED, Lazarus HM. Diagnosis and treatment of transplantation-associated thrombotic microangiopathy: real progress or are we still waiting? Bone Marrow Transplant. 2007;40:709–719. doi: 10.1038/sj.bmt.1705758. [DOI] [PubMed] [Google Scholar]
- 3.Hingorani S. Chronic kidney disease in long-term survivors of hematopoietic cell transplantation: epidemiology, pathogenesis, and treatment. J Am Soc Nephrol. 2006;17:1995–2005. doi: 10.1681/ASN.2006020118. [DOI] [PubMed] [Google Scholar]
- 4.Nakamae H, Yamane T, Hasegawa T, et al. Risk factor analysis for thrombotic microangiopathy after reduced-intensity or myeloablative allogeneic hematopoietic stem cell transplantation. Am J Hematol. 2006;81:525–531. doi: 10.1002/ajh.20648. [DOI] [PubMed] [Google Scholar]
- 5.Ho VT, Cutler C, Carter S, et al. Blood and marrow transplant clinical trials network toxicity committee consensus summary: thrombotic microangiopathy after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2005;11:571–575. doi: 10.1016/j.bbmt.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 6.Jodele S, Bleesing JJ, Mehta PA, et al. Successful early intervention for hyperacute transplant-associated thrombotic microangiopathy following pediatric hematopoietic stem cell transplantation. Pediatr Transplant. 2012;16:E39–E42. doi: 10.1111/j.1399-3046.2010.01408.x. [DOI] [PubMed] [Google Scholar]
- 7.Jodele S, Hirsch R, Laskin B, et al. Pulmonary arterial hypertension in pediatric patients with hematopoietic stem cell transplant-associated thrombotic microangiopathy. Biol Blood Marrow Transplant. 2013;19:202–207. doi: 10.1016/j.bbmt.2012.08.022. [DOI] [PubMed] [Google Scholar]
- 8.Glezerman IG, Jhaveri KD, Watson TH, et al. Chronic kidney disease, thrombotic microangiopathy, and hypertension following T cell-depleted hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2010;16:976–984. doi: 10.1016/j.bbmt.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoffmeister PA, Hingorani SR, Storer BE, et al. Hypertension in long-term survivors of pediatric hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2010;16:515–524. doi: 10.1016/j.bbmt.2009.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen EP, Drobyski WR, Moulder JE. Significant increase in end-stage renal disease after hematopoietic stem cell transplantation. Bone Marrow Transplant. 2007;39:571–572. doi: 10.1038/sj.bmt.1705643. [DOI] [PubMed] [Google Scholar]
- 11.Jodele S, Licht C, Goebel J, et al. Abnormalities in the alternative pathway of complement in children with hematopoietic stem cell transplant-associated thrombotic microangiopathy. Blood. 2013;122:2003–2007. doi: 10.1182/blood-2013-05-501445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laskin BL, Maisel J, Goebel J, et al. Renal arteriolar C4d deposition: a novel characteristic of hematopoietic stem cell transplantation-associated thrombotic microangiopathy. Transplantation. 2013;96:217–223. doi: 10.1097/TP.0b013e31829807aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ricklin D, Cines DB. TMA: beware of complements. Blood. 2013;122:1997–1999. doi: 10.1182/blood-2013-07-512707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jodele S, Laskin BL, Goebel J, et al. Does early initiation of therapeutic plasma exchange improve outcome in pediatric stem cell transplant-associated thrombotic microangiopathy? Transfusion. 2013;53:661–667. doi: 10.1111/j.1537-2995.2012.03776.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Worel N, Greinix HT, Leitner G, et al. ABO-incompatible allogeneic hematopoietic stem cell transplantation following reduced-intensity conditioning: close association with transplant-associated microangiopathy. Transfus Apher Sci. 2007;36:297–304. doi: 10.1016/j.transci.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 16.Legendre CM, Licht C, Muus P, et al. Terminal complement inhibitor eculizumab in atypical hemolytic-uremic syndrome. N Engl J Med. 2013;368:2169–2181. doi: 10.1056/NEJMoa1208981. [DOI] [PubMed] [Google Scholar]
- 17.Hillmen P, Muus P, Roth A, et al. Long-term safety and efficacy of sustained eculizumab treatment in patients with paroxysmal nocturnal haemoglobinuria. Br J Haematol. 2013;162:62–73. doi: 10.1111/bjh.12347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fakhouri F, Vercel C, Fremeaux-Bacchi V. Obstetric nephrology: AKI and thrombotic microangiopathies in pregnancy. Clin J Am Soc Nephrol. 2012;7:2100–2106. doi: 10.2215/CJN.13121211. [DOI] [PubMed] [Google Scholar]
- 19.Peffault de Latour R, Xhaard A, Fremeaux-Bacchi V, et al. Successful use of eculizumab in a patient with post-transplant thrombotic microangiopathy. Br J Haematol. 2013;161:279–280. doi: 10.1111/bjh.12202. [DOI] [PubMed] [Google Scholar]
- 20.Schmidtko J, Peine S, El-Housseini Y, et al. Treatment of atypical hemolytic uremic syndrome and thrombotic microangiopathies: a focus on eculizumab. Am J Kidney Dis. 2013;61:289–299. doi: 10.1053/j.ajkd.2012.07.028. [DOI] [PubMed] [Google Scholar]
- 21.Cho BS, Yahng SA, Lee SE, et al. Validation of recently proposed consensus criteria for thrombotic microangiopathy after allogeneic hematopoietic stem-cell transplantation. Transplantation. 2010;90:918–926. doi: 10.1097/TP.0b013e3181f24e8d. [DOI] [PubMed] [Google Scholar]
- 22.Laskin BL, Nehus E, Goebel J, et al. Cystatin C-estimated glomerular filtration rate in pediatric autologous hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2012;18:1745–1752. doi: 10.1016/j.bbmt.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 23.Levey AS, Coresh J, Balk E, et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139:137–147. doi: 10.7326/0003-4819-139-2-200307150-00013. [DOI] [PubMed] [Google Scholar]
- 24.Bellomo R, Ronco C, Kellum JA, et al. Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204–R212. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rule AD, Bergstralh EJ, Slezak JM, et al. Glomerular filtration rate estimated by cystatin C among different clinical presentations. Kidney Int. 2006;69:399–405. doi: 10.1038/sj.ki.5000073. [DOI] [PubMed] [Google Scholar]
- 26.Skerka C, Zipfel PF, Muller D, et al. The autoimmune disease DEAP-hemolytic uremic syndrome. Semin Thromb Hemost. 2010;36:625–632. doi: 10.1055/s-0030-1262884. [DOI] [PubMed] [Google Scholar]
- 27.Bresin E, Rurali E, Caprioli J, et al. Combined complement gene mutations in atypical hemolytic uremic syndrome influence clinical phenotype. J Am Soc Nephrol. 2013;24:475–486. doi: 10.1681/ASN.2012090884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heinen S, Hartmann A, Lauer N, et al. Factor H-related protein 1 (CFHR-1) inhibits complement C5 convertase activity and terminal complex formation. Blood. 2009;114:2439–2447. doi: 10.1182/blood-2009-02-205641. [DOI] [PubMed] [Google Scholar]
- 29.Abarrategui-Garrido C, Martinez-Barricarte R, Lopez-Trascasa M, et al. Characterization of complement factor H-related (CFHR) proteins in plasma reveals novel genetic variations of CFHR1 associated with atypical hemolytic uremic syndrome. Blood. 2009;114:4261–4271. doi: 10.1182/blood-2009-05-223834. [DOI] [PubMed] [Google Scholar]
- 30.Holliday EG, Smith AV, Cornes BK, et al. Insights into the genetic architecture of early stage age-related macular degeneration: a genome-wide association study meta-analysis. PloS One. 2013;8:e53830. doi: 10.1371/journal.pone.0053830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jozsi M, Licht C, Strobel S, et al. Factor H autoantibodies in atypical hemolytic uremic syndrome correlate with CFHR1/CFHR3 deficiency. Blood. 2008;111:1512–1514. doi: 10.1182/blood-2007-09-109876. [DOI] [PubMed] [Google Scholar]
- 32.Nilsson B, Ekdahl KN. Complement diagnostics: concepts, indications, and practical guidelines. Clin Dev Immunol. 2012;2012:962702. doi: 10.1155/2012/962702. http://dx.doi.org/10.1155/2012/962702. Epub 2012 Nov 14. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Campistol JM, Arias M, Ariceta G, et al. An update for atypical haemolytic uraemic syndrome: diagnosis and treatment. A consensus document. Nefrologia. 2013;33:27–45. doi: 10.3265/Nefrologia.pre2012.Nov.11781. [DOI] [PubMed] [Google Scholar]
- 34.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114:555–576. [PubMed] [Google Scholar]