Abstract
Fragile X syndrome (FXS) is associated with intellectual disability and behavioral dysfunction, including anxiety, ADHD symptoms, and autistic features. Although individuals with FXS are largely considered healthy and lifespan is not thought to be reduced, very little is known about the long-term medical health of adults with fragile X syndrome and no systematically collected information is available on standard laboratory measures from metabolic screens. During the course of follow up of a large cohort of patients with FXS we noted that many patients had low cholesterol and HDL values and thus initiated a systematic chart review of all cholesterol values present in charts from a clinic cohort of over 500 patients with FXS. Total cholesterol (TC), low density lipoprotein (LDL) and high density lipoprotein (HDL) were all significantly reduced in males from the FXS cohort relative to age-adjusted population normative data. This finding has relevance for health monitoring in individuals with FXS, for treatments with cholesterol-lowering agents that have been proposed to target the underlying CNS disorder in FXS based on work in animal models, and for potential biomarker development in FXS.
Keywords: Fraglle X syndrome, FMRP, FMR1, cholesterol, HDL, LDL, Lovastatin
INTRODUCTION
Fragile X syndrome (FXS) is the most common known inherited cause of intellectual disability (ID) and the most common identifiable genetic cause of autism spectrum disorder (ASD), with a prevalence of about 1/4000 [Crawford et al., 2001]. FXS results from a CGG repeat expansion mutation of >200 repeats (full mutation) in the promoter of FMR1 (fragile X mental retardation 1) which leads to hypermethylation and transcriptional silencing of FMR1 and reduction of expression of the FMR1 gene product, FMRP (fragile X mental retardation protein) [Pieretti et al., 1991]. In the brain, loss of FMRP, an RNA binding protein which is a negative regulator of group I mGluR and other receptor-activated dendritic translation, results in increased synthesis of proteins normally regulated by FMRP, dysregulation of synaptic signal transduction and channel function, excess mGluR signaling, and aberrant dendritic arborization and synaptic plasticity [Berry-Kravis et al., 2011, Gross et al., 2012,Darnell and Klann, 2013]. In addition to cognitive disability, FXS is associated with mildly dysmorphic features such as large ears, long face, and macrocephaly in a percentage of patients, macroorchidism in adult males, and prominent behavioral symptoms in most patients that include varying degrees of distractibility, hyperactivity, autistic symptoms, anxiety, gaze aversion, and in some cases, irritability and aggression [Grossman et al., 2002, Hagerman et al., 2009].
A number of medical problems have been reported to be increased in frequency in children with FXS relative to the general population, including seizures, frequent otitis media, strabismus, gastroesophageal reflux, and loose stools. A number of these problems are thought to be due to loose connective tissue, and indeed loose skin over the palms and hyperflexible joints are seen in a large fraction of patients [Grossman et al., 2002, Hagerman et al., 2009], suggesting that regulation of cellular function by FMRP is not limited to the CNS. There is little information on long-term health risks or medical problems in adults with FXS. Some studies have focused on hormonal function in FXS or the FXS mouse model [Wilson et al., 1988, Bregman et al., 1990, Qin and Smith, 2008], but definitive abnormalities in lab values have not been identified in humans with FXS. A single study evaluated medical features of adults with FXS over age 40 and identified obesity as a major health risk although rates of obesity were not elevated over those for the general US population [Utari et al., 2010]. The rate of Parkinsonism seemed to be increased in older individuals with FXS [Utari et al., 2010].
No studies have been published describing cholesterol levels in individuals with FXS. We have observed that patients with FXS attending our large FXS clinic seemed to very frequently have low high density lipoprotein (HDL),low density lipoprotein (LDL), and total cholesterol (TC) levels. Based on these observations we performed a chart review to systematically collect information on cholesterol levels from all patients with fragile X syndrome attending the clinic program at our institution.
METHODS
Charts were reviewed from all patients who had been seen at the Fragile X Clinic and Research Program at Rush University Medical Center from 1992 through 2013. Lab records were obtained for clinical management or as a result of transfer of records to clinic charts after participation in a clinical trial. Within the patient population, 87.4% were Caucasian, 6.8% were African American, 2.9% were Asian, and 2.9% were Asian and Caucasian. All TC, HDL, and LDL determinations for which records were available were recorded in excel spreadsheets. When multiple determinations were present for a given patient, the most recent data were used. BMI and medications that patients were taking at the time of the cholesterol determination were recorded. Values were compared to age- and gender-based normative data from a large National Health and Nutrition Examination Survey (NHANES) 1988–1994 population study and were categorized as being below or above the 10th centile for age group [Hickman et al., 1998]. The NHANES 1988–1994 study population was about 70% Caucasian as compared to 90% in the fragile X group, however since TC, HDL and LDL means and medians for the entire NHANES 1988–1994 study population differed only by 0–2 mg/dL (<4%) from those for the Caucasian only population for all values in both the age 4–19 and the age 20+ group, the data from the entire population was used for comparison. Effects of ethnicity within the FXS group could not be examined due to the fact that sample sizes from non-Caucasian groups were to small for meaningful comparison. Binomial test was performed to compare the percentage of FXS individuals with values below the 10th centile relative to the normative group. The relationship between BMI and lipid levels in FXS was explored by classifying FXS males as normal weight, overweight or obese based on age-related CDC normative data. Mean lipid levels between weight groups were compared using the analysis of variance and the fraction of individuals with lipid values less than the 10th centile of the normative data for each weight group was compared using Chi-square or Fisher’s exact test. Centile curves for TC, HDL and LDL were generated from the FXS data, for children and adolescents (age 4–19), and adults (age 20+). Age categories were based on categories for the published cohort from which normative data was derived [Hickman et al., 1998].
An additional set of comparisons of data from the FXS cohort to more recently obtained and published 2007–2010 NHANES population data [Go et al., 2014] was performed to ensure results were similar to the comparisons from the 1988–1994 NHANES data. The data from the Caucasian population in this study was used for comparison given the FXS population was predominantly Caucasian and there was more difference in the cholesterol values between Caucasian and non-Caucasian groups in this later study. The data presented in this more recent population study was more limited than in the earlier study and so comparisons were limited to comparisons of mean TC, HDL and LDL values in the child/adolescent (age 4–19) group and comparisons of percentages of adult FXS and control samples with TC>200, HDL<40, and LDL>130 mg/dL, as well as a comparison of mean adult LDL values.
All statistical analyses were performed with SAS 9.2 (SAS Institute Inc, Cary NC, USA). A p-value < 0.05 was considered significant.
RESULTS
Data on TC, HDL and LDL were available for 88, 67, and 51 male patients with FXS and for 15, 9, and 6 females with FXS, respectively. Descriptive data for the study cohort is provided in Table 1. There was data from 56 male children and adolescents with FXS age 4–19, and 32 male adults with FXS patient age 20 and over. Numbers of patients falling below the 10th centile for 1988–1994 NHANES normative data for each age range for TC, HDL and LDL are shown in Table 2. The percent of individuals with values below the normative data 10th centile was significantly increased for males in both FXS age groups for both TC and HDL (p<0.0001 in all cases except p=0.025 for adult TC) and in adults for LDL (p=0.0004).
Table 1.
Descriptive Data for FXS Cohort
| Characteristic | Males | Females |
|---|---|---|
| Total | 88 | 15 |
| 4–19 | 56 | 9 |
| 20+ | 32 | 6 |
|
| ||
| BMI | - Total (4–19, 20+) | - |
| - Normal | - 41 (27,14) | - 4 |
| - Overweight | - 24 (12,12) | - 3 |
| - Obese | - 23 (17, 6) | - 8 |
|
| ||
| Behavioral Medication | 69 (47, 22) | 12 |
| Antipsychotics | 24 (15, 9) | 3 |
| Stimulants | 16 (13, 3) | 3 |
| Antipsychotics and Stimulants | 11 (9, 2) | 2 |
| Other | 18 (10, 8) | 4 |
Table 2.
Fraction of FXS cohort With TC, HDL, and LDL Below the 10th Centile.
| TC | HDL | LDL | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Group | # (%<10%ile) | CI | P | # (% <10%ile) | CI | p | # (% 10%ile) | CI | P |
| Males 4–19 | 16/56 (28.6) | 16.7–40.4 | <0.0001 | 17/45 (37.8) | 23.6–51.9 | <0.0001 | 5/30 (16.7)* | 3.3–30 | 0.22 |
| Males 20+ | 7/32 (21.9) | 7.5–36.2 | 0.025 | 9/22 (40.9) | 20.4–61.4 | <0.0001 | 7/21 (33.3) | 13.2–53.5 | 0.0004 |
| All Males | 23/88 (26.1) | 17–35.3 | <0.0001 | 26/67 (38.8) | 27.1–50.5 | <0.0001 | 12/51 (23.5) | 11.9–35.2 | 0.001 |
| Females | 5/15 (33.3) | 11.8–61.6 | 0.03 | 1/9 (11.1) | 0.3–48.3 | 1.0 | 2/6 (33.3) | 4.3–77.7 | 0.23 |
The normal LDL values for children under 12 in the general population were not available so only LDL data from the FXS cohort of patients 12 and over was used for the analysis.
Rates of overweight/obesity based on BMI were not different in the adult FXS group from population rates (58% of FXS group overweight or obese versus 69% of the US population [Ogden et al., 2014], p=0.2) although the 4–19 year FXS group had an increased rate of overweight/obesity over the general US population (51% of FXS group versus 32% in US population [Ogden et al., 2014], p=0.0005). Despite the increased BMI in the child/adolescent FXS group, there was no significant difference in the fraction of individuals with a TC, HDL, or LDL level <10%ile between weight groups (normal, overweight, obese) for either the 4–19 age group or the adults. There was no significant difference in the mean TC, HDL or LDL values between the normal, overweight and obese groups for adult males, and no difference in mean HDL and LDL values between the weight groups for male children/adolescents, although TC for male children was borderline significantly higher in the higher weight groups (p=0.04). There was no significant correlation between BMI and TC, HDL, or LDL levels in either the male child/adolescent or adult FXS group, except for a borderline correlation between TC and BMI in the child adolescent group (r=0.3, p=0.03).
Females with FXS of all ages were grouped together due to small sample size (Table 2). Only TC showed a significant increase in the number of females with FXS having values below the 10th centile (p=0.04). There was no significant relationship in females between BMI and TC (correlation coefficient=0.25, p=0.43), HDL (correlation coefficient=−0.16, p=0.71) and LDL (correlation coefficient=0.31, p=0.45). Due to the small sample size and expectation that females would be less affected than males, no further analyses were done on the female group.
Centile curves for the male FXS cohort for all age ranges showed a shift to the left (lower values) for TC, HDL and LDL levels across the entire range of values (Figure 1). Two males and one female were treated with statins. Removal of these individuals from all data analyses had no effect on the overall result. Although effects of individual medications or classes of psychotropic medication could not be analyzed due to small sample sizes, overall treatment with psychotropic medication for behavior had no impact on the frequency of individuals with FXS and TC, HDL or LDL levels <10th%ile for either the adult or the child/adolescent group.
Figure 1.
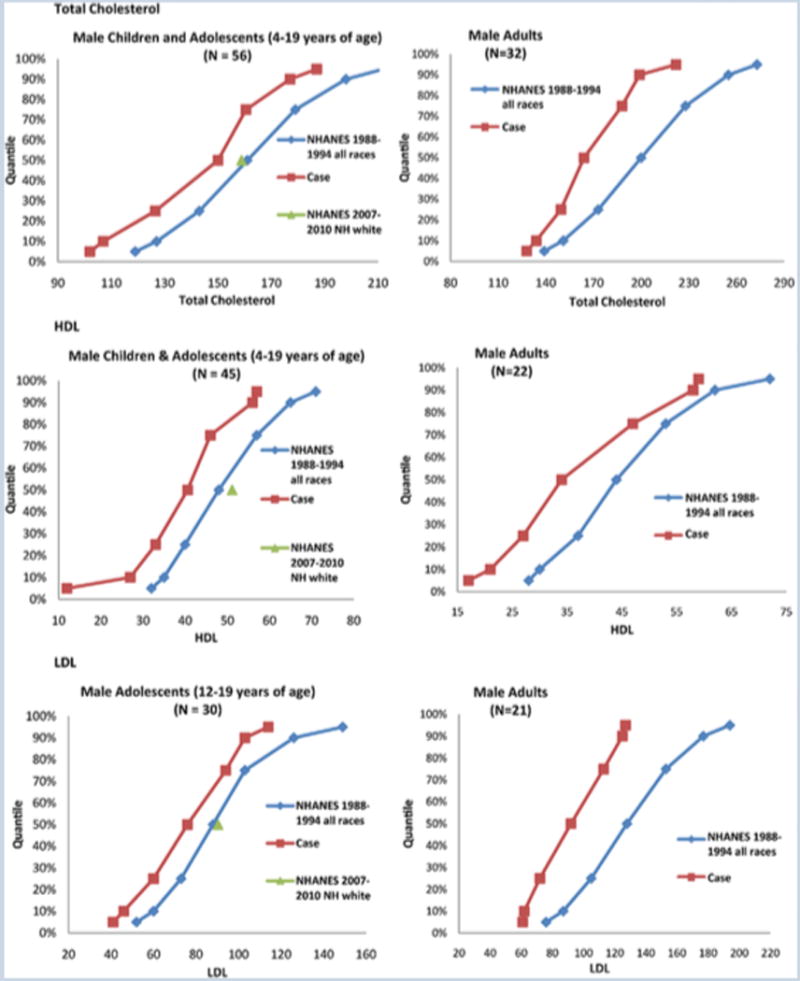
Figures of empirical quantiles (5th, 10th, 25th, 50th,75th,90th,95th) for FXS cases and the general population from the 1988–1994 NHANES cohort for male children/adolescents age 4–19 (left panels) and adults >age 19 (right panels) for total cholesterol (top panels), HDL (middle panels), and LDL (bottom panels). All centile curves were shifted to lower numbers for all lipid levels across all age ranges in patients with FXS. The N value shown in the figure refers to the number of patients with FXS in the cohort and the general population data was taken from Hickman et al. (12) The normal LDL values for children under 12 in the general population were not available so only LDL data from the FXS cohort of patients 12 and over was used for the analysis. The mean values for the 2007–2010 NHANES Caucasian population cohort are shown at the 50%ile for reference on the child/adolescent data plots. This data was not available for adults in the 2007–2010 NHANES published data and thus is only presented in the child/adolescent plots.
Comparison of the TC, HDL and LDL values in mg/dL from the FXS group to those from the limited data available from the more recent 2007–2010 NHANES normative population data [Go et al., 2014] for Caucasians only confirmed that mean values were significantly lower for children/adolescents age 4–19 in the FXS group (TC 144.1±25.78 FXS vs 158.85 control, p<0.001; HDL 38.4±14.76 FXS vs 51.15 control, p<0.001; LDL 76.93±20.71 FXS vs 90.4 control, p=0.001). In adults 9.38% of FXS samples versus 40.5% of control samples had TC greater than 200 (p=0.0002); 59.09% of FXS samples versus 33.1% of control samples had HDL<40 (p=0.02); 4.76% FXS samples versus 30.1% of control samples had LDL>130 (p=0.01); and mean LDL in FXS samples was 93.81±28.78 mg/dL versus 115.1 in control samples (p=0.003). In combination these comparisons confirm that TC, HDL, and LDL levels in the FXS group are lower than normative population data.
DISCUSSION
This retrospective study strongly suggests that TC, HDL and LDL levels are lower in males with FXS than in the general population. Overall it appears that in males with FXS, TC, LDL, and HDL centiles are all shifted to lower numbers across all age ranges. There is a suggestion that HDL is more significantly reduced relative to the normative male population than TC or LDL. It is likely that TC is also reduced in females with FXS but findings in females are not definitive in this study due to small sample size. It is expected that a sample size larger than for males would be needed to demonstrated abnormalities in females due to the milder more variable manifestations of the condition in females. It will be important to confirm this finding in a larger cohort of males and females in which fasting lipid levels including triglycerides are systematically collected prospectively.
TC, HDL and LDL are not related to BMI at all in adult FXS males, and only TC shows a minimal relationship with BMI in male children/adolescents, which may be an effect dependent on age and higher than typical BMI in the child/adolescent group, given TC levels go up with age and the excess of overweight and obese individuals in the 4–19 age group tended to be at the older end of the age range. This is supported by the lack of a relationship between BMI and the fraction of male children/adolescents with TC <10%ile for age. The general lack of relationship between BMI and lipids levels in FXS is unlike findings in the general population that show correlations between BMI and lipid levels in Caucasian cohorts [Bennasar-Veny et al., 2013, Abbasi et al., 2013]. This suggests that the low TC, HDL and LDL levels may be an FXS-related characteristic that occurs independently of BMI and other relationships seen in the general population.
This finding has implications for health monitoring and treatment practices. Adult individuals with FXS undergoing standard health screening including a lipid profile are sometimes put on statins despite low TC because the low HDL results in an apparently unfavorable lipid ratio. Given the overall low levels of TC and LDL also seen in FXS, it is not clear this treatment is necessary or the best health practice [Boekholdt et al., 2013]. On the other hand, the low HDL may represent a vascular or cardiac risk factor and since limited data is available on aging in FXS this relationship is yet to be determined through standard monitoring of lipid profiles and health trajectories in adults with FXS.
Recent basic research has suggested excessive activity of ERK in the brain of the FXS mouse due to absence of FMRP and overactivity of translational signaling [Gross et al., 2012, Darnell and Klann, 2013]. Lovastatin was used to indirectly inhibit ERK through a mechanism involving reduction of Ras isoprenylation and activity with downstream effects on ERK activity [Osterweil et al., 2013], resulting in rescue of the audiogenic seizure phenotype seen in the FXS mouse. This pre-clinical finding has prompted a pilot open-label trial of lovastatin in humans with FXS [www.clincaltrials.gov]. As lovastatin is expected to lower TC, LDL and HDL levels, it will be important to monitor for problems associated with excessively low lipid levels in individuals with FXS who may already have very low levels. Particularly if young children with FXS were exposed to this treatment, consideration would need to be given to possible effects on CNS myelination, which can be affected by low cholesterol levels [Werner et al., 2013].
It has been challenging to find consistent biomarkers that could give readouts for treatment effects or predict those more likely to respond to treatment, yet such markers are badly needed [Berry-Kravis et al., 2013]. TC, LDL and HDL are easily measured blood components which presumably are low in FXS because of misregulation of synthesis or processing when FMRP is absent or reduced. Thus TC, LDL and HDL may provide markers of treatment effects for pharmacotherapies in FXS that target correction of activity in pathways in which FMRP normally functions.
FMRP is an RNA binding protein that regulates stimulus-dependent translation of a large number of other proteins in cells [Berry-Kravis et al., 2011, Darnell and Klann, 2012, Gross et al., 2012]. It seems likely that misregulation of one or multiple proteins normally regulated by FMRP is responsible for the lower TC, LDL and HDL levels in FXS. A recent screen for genes responsible for determining lipid levels in humans revealed that several known and novel FMRP target genes are linked to regulation of lipid levels [Global Lipids Genetics Consortium, 2013], including APOE and GSK3B [Darnell et al., 2011], as well as SETD2, MTMR3, MARCH8 and ABCA1 (JC Darnell, unpublished data). While each of these candidates is linked to various subsets of the 4 lipid levels measured in the genetic study it is likely that the lipid profile of fragile X patients results from the combined effect of loss of translational regulation of multiple FMRP targets [Global Lipids Genetics Consortium, 2013]. Other lipid regulating genes may be targets of FMRP regulation but cannot be identified in current data from screens for FMRP -associated mRNAs, because these were performed in brain where there is very low level of expression for many of these genes. In light of the overlap between genes for autism/ID and FMRP targets [Darnell et al., 2011], it is interesting that TC and LDL were found to be low in individuals with ASD and ID [Moses et al., 2013] although the findings were not significant after correction for age and BMI, and Bonferroni correction. HDL was not low in these groups, suggesting a somewhat different disease-specific problem with regulation of cholesterol levels in FXS.
This study is limited by the retrospective design, relatively small cohort size, lack of precise ethnicity matching in comparison data, and random timing of the lipid levels obtained. However the magnitude of the significance of the findings in an FXS cohort of this size relative to two different population cohorts of Caucasian and mixed ethnicity support a real difference in TC, LDL and HDL levels in persons with FXS. Further study is needed to confirm the results in a larger prospective study in multiple ethnicity groups, in which apolipoprotein E and fasting triglyceride levels could also be measured, to fully define the health implications, mechanism, and utility as a marker of disease for the abnormal lipid levels identified in FXS in this study.
References
- Abbasi F, Blasey C, Reaven G. Cardiometabolic risk factors and obesity: does it matter whether BMI or waist circumference is the index of obesity? Am J Clin Nutr. 2013;98(3):637–640. doi: 10.3945/ajcn.112.047506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcobra Ltd. A 6-week, Study of MG01CI Low Dose and High Dose Compared With Placebo in Adults and Adolescents With Fragile X Syndrome. http://www.clinicaltrials.gov/ct2/show/NCT02126995?term=Fragile+X+Syndrome&rank=7.
- Bennasar-Veny M, Lopez-Gonzalez A, Tauler P, Cespedes M, Vicente-Herrero T, Yanez A, Tomas-Salva M, Aguilo A. Body adiposity index and cardiovascular health risk factors in Caucasians: a comparison with the body mass index and others. PLoS One. 2013;8(5):e63999. doi: 10.1371/journal.pone.0063999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry-Kravis E, Hessl D, Abbeduto L, Reiss A, Beckel-Mitchener A, Urv T. Outcome measures for clinical trials in Fragile X syndrome. J Dev Behav Pediatr. 2013;34:508–522. doi: 10.1097/DBP.0b013e31829d1f20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry-Kravis E, Knox A, Hervey C. Targeted treatments for fragile X syndrome. J Neurodevelop Disord. 2011;3:193–210. doi: 10.1007/s11689-011-9074-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boekholdt SM, Arsenault BJ, Hovingh GK, Mora S, Pedersen TS, Larosa JC, Welch KM, Amarenco P, Demicco DA, Tonkin AM, Sullivan DR, Kirby A, Colhoun HM, Hitman GA, Betteridge DJ, Durrington PN, Clearfield MB Downs, JR, Gotto AM, Jr, Ridger PM, Kastelein JJ. Levels and changes of HDL cholesterol and apolipoprotein A-I in relation to risk of cardiovascular events among statin-treated patients: a meta-analysis. Circulation. 2013;128:1504–1512. doi: 10.1161/CIRCULATIONAHA.113.002670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bregman J, Leckman J, Ort S. Thyroid function in Fragile-X syndrome males. The Yale Journal of Biology and Medicine. 1990;63:293–299. [PMC free article] [PubMed] [Google Scholar]
- Crawford D, Acuna J, Sherman S. FMR1 and the fragile X syndrome: human genome epidemiology review. Genet Med. 2001;3(5):359–371. doi: 10.1097/00125817-200109000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell J, Klann E. The translation of translational control by FMRP: Therapeutic targets for FXS. J Nat Neurosci. 2013;16:1530–1536. doi: 10.1038/nn.3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell J, Van Driesche S, Zhang C, Hung K, Mele A, Fraser C, Stone E, Chen C, Fak J, Chi S, Licatalosi D, Richter J, Darnell R. FMRP stalls ribosomal translocation on mRNAs linked to synaptic function and Autism. Cell. 2011;146:247–261. doi: 10.1016/j.cell.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Global Lipids Genetics Consortium. Discovery and refinement of loci associated with lipid levels. Nat Genet. 2013;45(11):1274–1282. doi: 10.1038/ng.2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, 3rd, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB, American Heart Association Statistics Committee and Stroke Statistics Subcommittee Executive Summary: Heart Disease and Stroke Statistics–2014 Update: A Report From the American Heart Association. Circulation. 2014;129:399–410. doi: 10.1161/01.cir.0000442015.53336.12. [DOI] [PubMed] [Google Scholar]
- Gross C, Berry-Kravis E, Bassell G. Therapeutic strategies in fragile X syndrome: dysregulated mGluR signaling and beyond. Neuropsychopharmacology Reviews. 2012;37:178–195. doi: 10.1038/npp.2011.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman A, Berry-Kravis E, Crnic L, Greenough W. Understanding fragile X mental retardation syndrome. Current Pediatrics. 2002;12:316–324. [Google Scholar]
- Hagerman R, Berry-Kravis E, Kaufmann W, Ono M, Tartaglia N, Lachiewicz A, Kronk R, Delahunty C, Hessl D, Visootsak J, Picker J, Gane L, Tranfaglia M. Advances in the treatment of Fragile X syndrome. Pediatrics. 2009;123:378–390. doi: 10.1542/peds.2008-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickman T, Briefel R, Carroll M, Rifkind B, Cleeman J, Maurer K, Johnson C. Distributions and trends of serum lipid levels among United States children and adolescents ages 4–19 years: data from the Third National Health and Nutrition Examination Survey. Preventive Medicine. 1998;27:879–890. doi: 10.1006/pmed.1998.0376. [DOI] [PubMed] [Google Scholar]
- Moses L, Katz N, Weizman A. Metabolic profiles in adults with autism spectrum disorder and intellectual disabilities. Eur Psychiatry. 2013 doi: 10.1016/j.eurpsy.2013.05.005. pii: S0924-9338(13)00071-0. [DOI] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of Childhood and Adult Obesity in the United States, 2011–2012. JAMA. 2014;311:806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterweil E, Chuang S, Chubykin A, Sidorov M, Bianchi R, Wong R, Bear M. Lovastatin corrects excess protein synthesis and prevents Epileptogenesis in a mouse model of Fragile X syndrome. Neuron. 2013;77:243–250. doi: 10.1016/j.neuron.2012.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieretti M, Zhang F, Fu Y, Warren S, Oostra B, Caskey C, Nelson D. Absence of expression of the FMR-1 gene in Fragile X syndrome. Cell. 1991;66:817–822. doi: 10.1016/0092-8674(91)90125-i. [DOI] [PubMed] [Google Scholar]
- Qin M, Smith C. Unaltered hormonal response to stress in a mouse model of Fragile X syndrome. Psychoneuroendocrinology. 2008;33:883–889. doi: 10.1016/j.psyneuen.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utari A, Adams E, Berry-Kravis E, Chavez A, Scaggs F, Ngotran L, Boyd A, Hessl D, Gane L, Tassone F, Tartaglia N, Leehey M, Hagerman R. Aging in Fragile X syndrome. J Neurodevelop Disord. 2010;2:70–76. doi: 10.1007/s11689-010-9047-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner H, Kramer-Albers E, Strenzke N, Saher G, Tenzer S, Ohno-Iwashita Y, De Monasterio-Schrader P, Mobius W, Moser T, Griffiths I, Nave K. A critical role for cholesterol-associated proteolipids PLP and M6B in myelination of the central nervous system. GLIA. 2013;61:567–586. doi: 10.1002/glia.22456. [DOI] [PubMed] [Google Scholar]
- Wilson D, Carpenter N, Berkovitz G. Thyroid function in men with Fragile X-linked MR. American Journal of Medical Genetics. 1988;31:733–734. doi: 10.1002/ajmg.1320310402. [DOI] [PubMed] [Google Scholar]


