Abstract
Aims
Combined left ventricular assist device (LVAD) and pharmacological therapy has been proposed to favour myocardial recovery in patients with end-stage heart failure (HF). Clenbuterol (Clen), a β2-adrenoceptor (β2-AR) agonist, has been used as a part of this strategy. In this study, we investigated the direct effects of clenbuterol on unloaded myocardium in HF.
Methods and results
Left coronary artery ligation or sham operation was performed in male Lewis rats. After 4–6 weeks, heterotopic abdominal transplantation of the failing hearts into normal recipients was performed to induce LV unloading (UN). Recipient rats were treated with saline (Sal) or clenbuterol (2 mg/kg/day) via osmotic minipumps (HF + UN + Sal or HF + UN + Clen) for 7 days. Non-transplanted HF animals were treated with Sal (Sham + Sal, HF + Sal) or clenbuterol (HF + Clen). LV myocytes were isolated and studied using optical, fluorescence, and electrophysiological techniques. Clenbuterol treatment improved in vivo LV function measured with echocardiography (LVEF (%): HF 35.9 ± 2 [16], HF + Clen 52.1 ± 1.4 [16]; P < 0.001; mean ± SEM [n]). In combination with unloading, clenbuterol increased sarcomere shortening (amplitude (µm): HF + UN + Clen 0.1 ± 0.01 [50], HF + UN + Sal 0.07 ± 0.01 [38]; P < 0.001) by normalizing the depressed myofilament sensitivity to Ca2+ (slope of the linear relationship between Ca2+ transient and sarcomere shortening hysteresis loop during relaxation (μm/ratio unit): HF + UN + Clen 2.13 ± 0.2 [52], HF + UN + Sal 1.42 ± 0.13 [38]; P < 0.05).
Conclusion
Clenbuterol treatment of failing rat hearts, alone or in combination with mechanical unloading, improves LV function at the whole-heart and cellular levels by affecting cell morphology, excitation–contraction coupling, and myofilament sensitivity to calcium. This study supports the use of this drug in the strategy to enhance recovery in HF patients treated with LVADs and also begins to elucidate some of the possible cellular mechanisms responsible for the improvement in LV function.
Keywords: Clenbuterol, Unloading, Calcium, Myofilament sensitivity, Heart failure
1. Introduction
End-stage heart failure (HF) has a high incidence in the community with a mortality of 45% after 1 year.1 Heart transplantation is the most effective treatment for this disease but is hindered due to an inadequate availability of donor organs. Among other treatments, left ventricular assist devices (LVADs) have been shown to be a suitable alternative.2 LVAD support is primarily used as a ‘bridge to transplantation’ where the failing heart can be supported by mechanical circulatory assistance until a suitable donor organ becomes available.3 In few patients, the LVAD also acts as ‘bridge to recovery’ since it induces substantial functional improvement that allows explantation of the device without the need of transplantation.4 However, the rate for explantation of the LVAD is low with an incidence of only 4–24%.5–7 In addition, the functional improvement observed during LVAD treatment seems to be a transient phenomenon.7
The main consequence of the use of LVAD on the heart is mechanical unloading leading to functional, structural, signalling, and molecular changes in HF.8 One important effect is the development of myocardial atrophy with possible detrimental consequences. Mechanical unloading in animal studies shows time-dependent changes in myocyte contractile function and excitation–contraction (EC) coupling9 that can impair cardiac performance after prolonged treatment with LVADs.
We have recently shown that using a combination of mechanical unloading and pharmacological therapy leads to a substantially improved recovery rate in patients with dilated cardiomyopathy.10,11 Together with other drugs, the β2-adrenoceptor (β2-AR) agonist clenbuterol has been included in the protocol with the aim to prevent unloading-induced atrophy and consequent myocardial dysfunction. Clenbuterol is known to produce skeletal12 and cardiac hypertrophy, to affect EC coupling and cellular metabolism,13 gene expression and myocardial function14–16 in normal hearts. Clenbuterol has been shown to affect myocardial structure, function, metabolism, gene and protein expression at whole-heart and cellular level in normal,13–18 failing,12,19 and unloaded hearts.12,20 In the setting of the successful combination of mechanical unloading and pharmacological therapy for the treatment of patients with HF, the specific role of clenbuterol in the recovery process remains controversial.
The pathological process involved in the development of HF has been termed ‘ventricular remodelling’, a concept relating to a complex of anatomic, functional, cellular, and molecular changes that the myocardium undergoes in response to the injury and increased wall stress.1 LVAD support has been shown to affect and sometimes reverse the remodelling process (‘reverse remodelling’).21 Whether clenbuterol enhances the process of reverse remodelling in unloaded failing hearts is unknown.
In the present study, we have investigated the role and possible mechanisms of clenbuterol in enhancing reverse remodelling during mechanical unloading in a murine chronic HF model. Specifically, we have investigated the effects of clenbuterol on whole-heart function, cell size, EC coupling, myofilament sensitivity to Ca2+, and electrophysiological properties in a rat model of HF, induced by left coronary artery (LCA) ligation, and unloading, using abdominal heart transplantation.
2. Methods
Detailed description of methods can be found in Supplementary material.
2.1. Animal models
The investigation conforms with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85–23, revised 1996). Rat model of chronic post-infarction cardiomyopathy was used as it is a well established reproducible model of dysfunction.22,23 Syngeneic rat strains avoid the need for immunosuppression after abdominal transplantation. Therefore, male Lewis rats (200–300 g) were used for all experiments. HF was induced by permanent LCA ligation as previously described.22,23 Sham operated animals underwent left thoracotomy and the needle was passed under the LCA and removed without ligation. Change in LV function was assessed by transthoracic echocardiography (Acuson Sequoia™ 256; Acuson, USA) over a period of 4–6 weeks, as previously described.24
To test the effect of clenbuterol in HF, Sham and HF animals received an osmotic minipump (Model 2001, Alzet) containing clenbuterol (2 mg/kg body weight/day) or normal saline (Sal) for 7 days (Sham + Sal, HF + Sal or HF + Clen), implanted subcutaneously.
To test the effects of mechanical unloading (UN), the failing hearts were heterotopically transplanted25 into the abdomen of control Lewis rats and the recipients then received an osmotic minipump containing clenbuterol or Sal for 7 days (HF + UN + Sal or HF + UN + Clen). Briefly the failing heart was removed from the donor rat following cardioplegic arrest and the donor aorta was anastamosed to the recipient abdominal aorta and the donor pulmonary artery to the recipient inferior vena cava.
2.2. Ex vivo left ventricular function assessment
Ex vivo LV function of the transplanted heart was assessed by measuring the pressure–volume relationship using an intra-ventricular balloon as described previously.26 Hearts were perfused with normal Tyrode (NT) solution at 37°C on a Langendorff apparatus and paced with platinum electrodes on the right ventricle to maintain a heart rate of ∼300 bpm.
2.3. Cardiomyocyte studies
LV myocytes were isolated following a previously described method27 using collagenase (1 mg/mL, Worthington) and hyaluronidase (0.6 mg/mL, Sigma) for 8–10 min. All cellular experiments were performed at 37°C, within 7–8 h of cell isolation. Cell size was assessed from digital images of the cardiomyocytes. The projected two-dimensional area for each cell was measured using ImageJ software (NIH, USA).
2.3.1. Sarcomere shortening and cytoplasmic calcium measurement
Cells were superfused with a NT solution containing (in mM): NaCl 140, KCl 6, MgCl2 1, glucose 10, HEPES 10, CaCl2 1; pH 7.4, field-stimulated at 1 Hz and illuminated by red light (wavelength of >600 nm), enabling the cardiomyocyte in the field of view to be visualized for simultaneous measurement of Indo-1 fluorescence (Indo-1 AM) and sarcomere shortening using a Ionoptix system (Ionoptix Corporation, USA) as described in data supplement. Sarcoplasmic reticulum (SR) Ca2+ content was assessed by rapid application of 20 mM caffeine and amplitude of caffeine-induced Indo-1 transient taken as an index of SR Ca2+ content (see Supplementary material for protocol). Time constant (τ) of the transient was assessed by fitting a mono-exponential curve on the decay phase to assess the contribution of sodium–calcium exchanger (NCX) to Ca2+ extrusion.
2.3.2. Assessment of myofilament sensitivity to calcium
Cardiomyocyte myofilament sensitivity to Ca2+ was assessed using a technique described by Bailey et al.28 Hysteresis loops were generated for all cells studied and a constant region of the declining phase of the loop (the region between 20 and 80% from the peak of the sarcomere shortening) was selected and the slope value of this line was calculated by fitting a linear regression line. Only slope values of the regression lines with a goodness of fit (r2) greater than 0.8 were used for this analysis and data from each treatment group were compared to assess changes in myofilament sensitivity to Ca2+.
2.3.3. Electrophysiological studies
Cardiomyocytes were superfused with NT solution. Action potentials (APs) were measured in current-clamp mode using ∼30 MΩ resistance pipettes (containing (in mM) KCl 2000, HEPES 5, EGTA 0.1; pH 7.2) using a 1 ms, 1.2–1.4 nA pulse. For the measurement of NCX current (INCX), cells were superfused with a solution containing (in mM) NaCl 140, HEPES 10, glucose 10, MgCl2 1, CaCl2 1, CsCl 6; 0.01 strophanthidin; 0.01 nifedipine; pH 7.4. The pipette-filling solution contained (in mM): CsCl 45, HEPES 20, MgCl2 11, Na2ATP 10, CsOH 100, EGTA 50, CaCO3 25; pH 7.2. Pipette resistance was 2.3–3.5 MΩ. A 3 s descending ramp was applied from +80 to −120 mV at 0.1 Hz, from a holding potential of −40 mV. The INCX was taken as the 5 mM Ni2+-sensitive component. For the measurement of L-type calcium current (ICa,L), the same hardware configuration and pipette-filling solution, as for AP were used, but in voltage-clamp mode as previously reported.29,30 Current–voltage (I–V) relationships for ICa,L were built using 450 ms depolarization steps from a holding potential of −40 mV (range −45 to +50 mV, in 5 mV increments). The measured current is nifedipine-sensitive and 4-aminopyridine-insensitive, hence attributable to ICa,L. To study time-dependent inactivation, a bi-exponential of the ICa,L measured at 0 mV was fitted to the decay, and fast and slow components of ICa,L inactivation identified (τfast and τslow). The voltage-dependent steady-state activation profile (G/GMAX) and steady-state inactivation (F/FMAX) of the ICa,L was studied as previously described.31 All data were analysed using Clampfit© software (Axon Instruments, CA, USA).
2.4. Gene expression analysis
RT-PCR was performed as described previously32 (see Supplementary material). Primers and standard Taqman probes for α- and β-myosin heavy chain (MHC) were designed in-house using Primer Express v.1 5 (α-MHC: Forward primer: 5′-caaactcatggccacactcttct-3′, reverse primer: 5′-ggaaggatgagcctttcttcttg-3′, probe: 5′-FAM-ccactgtcaccggtatcagcagaagcata -3′. β-MHC: Forward primer: 5′-agctcctaagtaatctgtttgccaa-3′, reverse primer: 5′-aaaggatgagcctttctttgct-3′, probe 5′-FAM-cttgtctacaggtgcatcagctccagcat-3′). Data were analysed using the comparative Ct method with target gene expression normalized to 18S ribosomal RNA levels.
2.5. Statistical analysis
Statistical comparison of data was performed using one-way analysis of variance followed by Newman–Keuls post hoc test for individual significant differences or Student’s t-test where appropriate. All statistical analyses were performed using Prism 4 software (GraphPad Software, Inc.) and P < 0.05 was considered significant. Data are expressed as mean ± SEM [n], where n is the number of cells unless otherwise specified.
3. Results
3.1. The effects of clenbuterol on left ventricular function of failing hearts in vivo
LV function of failing and sham operated animals, treated with clenbuterol or saline, was assessed in vivo by echocardiography. LCA ligation produced LV dysfunction after 4–6 weeks at whole-heart level characterized by reduced LVEF, reduced LV fractional shortening, LV wall thinning, and LV chamber dilation on echocardiography (Figure 1B and D, Table 1). Clenbuterol treatment of failing hearts increased LVEF by ∼16%, increased LV fractional shortening by ∼6%, and improved thickening of the viable posterior wall by ∼20% (Figure 1C and D, Table 1). These results indicate that clenbuterol affects LV function of failing hearts.
Figure 1.
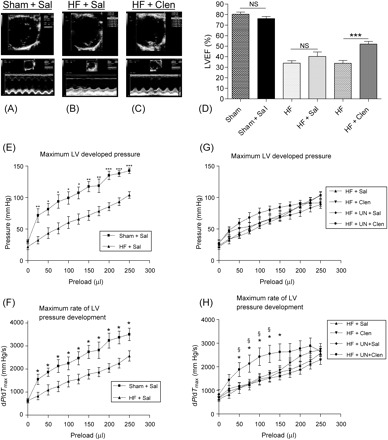
Representative two-dimensional (top) and M-mode (bottom) echocardiography traces at mid-papillary level of a normal control heart (A), a failing heart treated with saline showing a kinetic anterior scar and reduced contractility and thinning of posterior wall (B). A failing heart treated with clenbuterol shows improved posterior wall contractility with unchanged anterior scar movement (C). Sham operated and heart failure animals treated with saline showed no change in LVEF but clenbuterol treatment of failing hearts improved LVEF by ∼18% (D) (data shown in Table 1). (***P < 0.001 HF vs. HF + Clen). Pressure–volume relationship of sham operated and heart failure groups showing a reduced LV developed pressure (E) and dP/dTmax (F) of failing hearts treated with saline. The combination of clenbuterol treatment and mechanical unloading improved dP/dTmax (H) but did not affect the LV developed pressure (G). (*P < 0.05, **P < 0.01, ***P < 0.001 HF + Sal vs. Sham + Sal; §P < 0.05 HF + UN + Sal vs. HF + UN + Clen).
Table 1.
Echocardiographic parameters measured in experimental groups
| Sham | Sham + Sal | HF | HF + Sal | HF | HF + Clen | ||
|---|---|---|---|---|---|---|---|
| LV ejection fraction (%) | 79.0 ± 1.4 [12] | 81.1 ± 1.9 [11] | 35.9 ± 2.4 [11]*** | 41.7 ± 1.8 [10]*** | 35.9 ± 2 [16]*** | 52.1 ± 1.4 [16]***††† | |
| LV fractional shortening (%) | 44.7 ± 1.8 [12] | 48.3 ± 2.1 [10] | 18.9 ± 1.6 [11]*** | 18.0 ± 1.3 [9]*** | 18.9 ± 0.9 [15]*** | 24.7 ± 1.1 [16]***†† | |
| LV diameter (cm) | |||||||
| Diastole | 0.71 ± 0.02 [12] | 0.73 ± 0.02 [11] | 0.98 ± 0.02 [11]*** | 0.98 ± 0.01 [9]*** | 0.96 ± 0.02 [15]*** | 1.02 ± 0.03 [16]*** | |
| Systole | 0.39 ± 0.02 [12] | 0.37 ± 0.02 [11] | 0.79 ± 0.02 [11]*** | 0.81 ± 0.02 [9]*** | 0.78 ± 0.02 [15]*** | 0.78 ± 0.03 [16]*** | |
| LV posterior wall thickness (cm) | |||||||
| Diastole | 0.23 ± 0.02 [12] | 0.23 ± 0.01 [11] | 0.18 ± 0.01 [11]* | 0.21 ± 0.02 [9] | 0.18 ± 0.01 [15]* | 0.20 ± 0.01 [16] | |
| Systole | 0.31 ± 0.01 [12] | 0.32 ± 0.01 [11] | 0.25 ± 0.01 [11]** | 0.27 ± 0.02 [9] | 0.25 ± 01 [15]*** | 0.30 ± 0.01 [16]††† | |
| LV anterior wall thickness (cm) | |||||||
| Diastole | 0.17 ± 0.01 [12] | 0.16 ± 0.01 [11] | 0.10 ± 0.01 [11]*** | 0.10 ± 0.01 [9]*** | 0.13 ± 0.01 [15]*** | 0.12 ± 0.01 [16] | |
| Systole | 0.29 ± 0.01 [12] | 0.30 ± 0.01 [11] | 0.12 ± 0.01 [11]*** | 0.11 ± 0.01 [9]*** | 0.14 ± 0.01 [15]*** | 0.15 ± 0.01 [16]*** |
*P < 0.05, **P < 0.01, ***P < 0.001 vs. Sham; ††P < 0.01, †††P < 0.001 HF vs. HF + Clen. (data in [] indicates number of hearts studied).
3.2. The effects of clenbuterol on left ventricular function ex vivo
LV function of normally loaded and mechanically unloaded failing hearts, treated with clenbuterol or saline, was assessed by ex vivo pressure–volume relationship studies. LCA ligation reduced LV developed pressure and dP/dTmax (Figure 1E and F). Clenbuterol treatment or mechanical unloading alone did not affect these parameters but the combination of both clenbuterol treatment and mechanical unloading of failing hearts improved LV dP/dTmax (Figure 1G and H). These results indicate that the combination of clenbuterol and mechanical unloading enhances LV functional recovery of failing hearts.
3.3. The effects of clenbuterol on the unloading-induced cell size reduction
Two-dimensional cell surface area, length, and width of LV cardiomyocytes from all experimental groups were assessed by planimetry. After LCA ligation, cardiomyocyte hypertrophy developed, as shown by an increase in cell surface area ((µm2) Sham + Sal 3251 ± 64 [173], HF + Sal 4129 ± 92 [106]; P < 0.001), increase in length ((µm): Sham + Sal 128 ± 2 [173], HF + Sal 147 ± 2 [106]; P < 0.001) and increase in width ((µm): Sham + Sal 35 ± 1 [173], HF + Sal 40 ± 1 [106]; P < 0.001). As shown in Figure 2, clenbuterol treatment alone did not further increase cell size. Mechanical unloading alone reduced cell size to normal values (cell area (µm2): Sham + Sal 3251 ± 64 [173] vs. HF + UN + Sal 3276 ± 63 [181]; P = NS) but clenbuterol treatment during unloading limited this effect (cell area (µm2): HF + UN + Sal 3276 ± 63 [181], HF + UN + Clen 3547 ± 59 [232]; P < 0.01; HF + Sal vs. HF + UN + Clen; P < 0.001). These results indicate that clenbuterol limits unloading-induced cell size reduction and supports the rationale of its use to limit unloading-induced myocardial atrophy.
Figure 2.
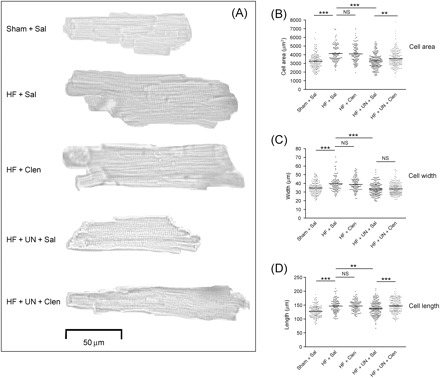
Representative pictures of left ventricular myocytes showing changes induced by heart failure, mechanical unloading and clenbuterol treatment (A). Heart failure induced myocyte hypertrophy, indicated by an increased cell area (B), width (C) and length (D). Clenbuterol treatment limited unloading-induced normalization of cell area by retaining cell length. (**P < 0.01, ***P < 0.001).
3.4. The effects of clenbuterol on cardiomyocyte contractility
Cardiomyocyte contractility was assessed during field-stimulation by real time changes of the sarcomere length. Sarcomere shortening and relaxation were impaired after LCA ligation (Figure 3A, Table 2) and were unaffected by mechanical unloading alone (Figure 3C, Table 2). Clenbuterol treatment alone or in combination with mechanical unloading improved sarcomere shortening and relaxation (Figure 3BandD, Table 2). These results indicate that mechanical unloading alone did not affect the depressed cardiomyocyte contractility but clenbuterol combined with mechanical unloading enhanced functional recovery.
Figure 3.

Simultaneously acquired sarcomere shortening and Indo-1 transients showing defective EC coupling in heart failure (A). Clenbuterol treatment, with and without mechanical unloading, improved myocyte contractility and Ca2+ transient kinetics (B–D). Arrows highlight statistically significant differences between groups (data shown in Table 2).
Table 2.
Contractile and calcium handling parameters in experimental groups
| Sham + Sal | HF + Sal | HF + Clen | HF + UN + Sal | HF + UN + Clen | ||
|---|---|---|---|---|---|---|
| 1 Hz Sarcomere shortening | ||||||
| Baseline SL (µm) | 1.63 ± 0.02 [46] | 1.59 ± 0.01 [68]* | 1.64 ± 0.01 [69]†† | 1.59 ± 0.01 [38] | 1.64 ± 0.01 [50]††,§ | |
| Amplitude (µm) | 0.08 ± 0.01 [46] | 0.07 ± 0.01 [68]* | 0.1 ± 0.01 [69]††† | 0.07 ± 0.01 [38] | 0.1 ± 0.01 [50]†††,§§§ | |
| Time to peak (ms) | 86.9 ± 3.3 [46] | 102.6 ± 2.6 [68]*** | 84.9 ± 2.3 [69]††† | 97.8 ± 2.7 [38] | 71.9 ± 1.7 [50]†††,§§§ | |
| Time to 50% relaxation (ms) | 70.5 ± 5.7 [42] | 91.2 ± 6.1 [65]* | 53.6 ± 2.4 [68]††† | 90.0 ± 6.2 [38] | 46.0 ± 2.2 [50]†††,§§§ | |
| 1 Hz Ca2+ transient | ||||||
| Baseline (Indo-1 ratio units) | 0.31 ± 0.01 [45] | 0.33 ± 0.01 [67]** | 0.33 ± 0.01 [69] | 0.31 ± 0.01 [38] | 0.32 ± 0.01 [48] | |
| Amplitude (Indo-1 ratio units) | 0.12 ± 0.01 [45] | 0.15 ± 0.01 [67]** | 0.14 ± 0.01 [69] | 0.14 ± 0.01 [38] | 0.11 ± 0.01 [48]†††,§§ | |
| Time to peak (ms) | 33.3 ± 1.1 [45] | 36.4 ± 0.6 [67]** | 34.6 ± 0.7 [69] | 34.9 ± 1.1 [38] | 31.2 ± 0.7 [47]†††,§§ | |
| Time to 50% decay (ms) | 70.9 ± 1.5 [45] | 75.4 ± 1.4 [66]* | 68.5 ± 1.1 [69]††† | 74.3 ± 1.9 [38] | 62.5 ± 1.4 [48]†††,§§§ | |
| Indo-1 decay, τ (ms) | 76.4 ± 2.9 [45] | 88.2 ± 3.4 [66]* | 65.8 ± 2.2 [69]††† | 83.1 ± 3.8 [38] | 58.5 ± 1.3 [48]†††,§§§ | |
| Caffeine induced Ca2+ transient | ||||||
| SR Ca2+ content (Indo-1 ratio units) | 0.11 ± 0.01 [29] | 0.14 ± 0.01 [43]** | 0.14 ± 0.004 [41] | 0.14 ± 0.01 [26] | 0.11 ± 0.01 [34]†,§ | |
| Indo-1 decay, τ (s) | 1.21 ± 0.08 [22] | 1.44 ± 0.06 [42]* | 1.21 ± 0.07 [35]†† | 1.20 ± 0.07 [23]† | 1.14 ± 0.07 [26]†† | |
| Myofilament sensitivity to Ca2+ | ||||||
| Slope value (µm/ratio unit) | 2.23 ± 0.27 [35] | 1.65 ± 0.12 [66]* | 2.36 ± 0.18 [69]†† | 1.42 ± 0.13 [38] | 2.13 ± 0.2 [52]†,§ |
*P < 0.05, **P < 0.01, ***P < 0.001 HF + Sal vs. Sham + Sal; †P < 0.05, ††P < 0.01, †††P < 0.001 vs. HF + Sal; §P < 0.05, §§P < 0.01, §§§P < 0.001, HF + UN + Sal vs. HF + UN + Clen (data in [] indicates number of cells studied).
3.5. The effects of clenbuterol on calcium cycling
Cytoplasmic [Ca2+] was assessed by measuring Indo-1 fluorescence ratio. Cardiomyocyte Ca2+ cycling, following LCA ligation, was characterized by delayed Ca2+ release and extrusion. However, the Ca2+ transient amplitude was paradoxically increased (Figure 3A, Table 2). The increased Ca2+ transient amplitude was accompanied by increased SR Ca2+ content with delayed removal in caffeine-induced Ca2+ transient (Table 2) suggesting a reduced NCX contribution to Ca2+ extrusion from the cytoplasm. Clenbuterol treatment alone did not affect Ca2+ transient amplitude or SR Ca2+ content but normalized the delayed stimulation-induced and caffeine-induced Ca2+ transient decline (Figure 3B, Table 2). Mechanical unloading alone did not affect the Ca2+ transient kinetics or SR Ca2+ content but induced a faster decline of the caffeine-induced Ca2+ transient (Table 2). The combination of clenbuterol treatment and mechanical unloading of HF normalized Ca2+ handling at cytoplasmic and SR levels (Figure 3D, Table 2). These results indicate that the combination of clenbuterol and mechanical unloading normalized the deranged cardiomyocyte Ca2+ cycling.
3.6. The effects of clenbuterol on myofilament sensitivity to calcium
Myofilament sensitivity to Ca2+ was assessed by sarcomere length-[Ca2+]i relationship from simultaneously recorded Indo-1 fluorescence changes and sarcomere shortening of LV cardiomyocytes. The sarcomere shortening-Ca2+ transient hysteresis loops showed that less contractility was produced for the same [Ca2+]i following LCA ligation, suggesting reduced myofilament sensitivity to Ca2+ (Figure 4E, Table 2). Clenbuterol treatment alone or in combination with mechanical unloading normalized this parameter. Mechanical unloading did not affect the depressed myofilament sensitivity to Ca2+ (Figure 4E, Table 2).
Figure 4.
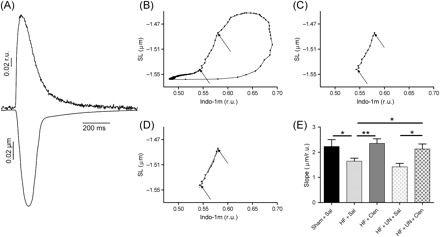
Simultaneously acquired sarcomere shortening and Indo-1 transient traces (A) were used to generate hysteresis loops (B) showing the relationship of sarcomere length and cytoplasmic Ca2+ concentration, measured by Indo-1 (r.u.—ratio units). The slope of a regression line, fitted through the linear portion of the loop (C, D), was used as a measure of myofilament sensitivity to Ca2+. Clenbuterol treatment improved the depressed myofilament sensitivity to Ca2+ in heart failure, with and without mechanical unloading (E, data shown in Table 2). (*P < 0.05, **P < 0.01, ***P < 0.001).
3.7. The effects of clenbuterol on action potential prolongation
APD was assessed in current-clamp mode as described above. Following LCA ligation, electrophysiological studies demonstrated increased AP duration at all frequencies compared to sham-operated controls (Figure 5A). Clenbuterol treatment did not affect the prolonged AP duration. Mechanical unloading alone further prolonged the AP duration at higher stimulation frequency (APD90 at 5 Hz (ms): HF + Sal 44.7 ± 3.0 [51], HF + UN + Sal 63.0 ± 4.8 [26]; P < 0.01) (Figure 5A). The combination of clenbuterol treatment and mechanical unloading normalized the prolonged AP duration in HF at all frequencies (Figure 5A). These results indicate that clenbuterol combined with mechanical unloading enhances recovery of deranged AP properties.
Figure 5.
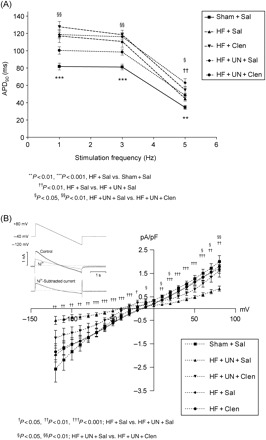
Action potential duration was prolonged in heart failure and unaffected by clenbuterol treatment (A). Mechanical unloading of failing hearts further prolonged action potential duration at higher stimulation frequency which was normalized by clenbuterol treatment. Clenbuterol normalized the unloading-induced reduction of INCX current density (B). Inset showing the voltage-clamp protocol used.
3.8. The effects of clenbuterol on sodium–calcium exchanger current
Clenbuterol normalized the delayed stimulation-induced and caffeine-induced Ca2+ transient decline suggesting an improved NCX contribution to Ca2+ extrusion from the cytoplasm. Therefore, INCX was measured to test if clenbuterol affected the biophysical properties of NCX. The INCX was unchanged at all voltages tested in cardiomyocytes from hearts following LCA ligation and unaffected by clenbuterol treatment alone (Figure 5B). Mechanical unloading reduced INCX but this current was partially restored by clenbuterol treatment (at +80 mV (pA/pF): HF + Sal 1.7 ± 0.2 [30], HF + UN + Sal 0.9 ± 0.1 [25], HF + UN + Clen 1.6 ± 0.3 [21]; HF + Sal vs. HF + UN + Sal, P < 0.01; HF + Sal vs. HF + UN + Clen, P= NS) (Figure 5B). These results indicate that clenbuterol combined with mechanical unloading improves NCX activity.
3.9. The effects of clenbuterol on L-type calcium current
HF reduced peak ICa,L (Figure 6B) and resulted in slower inactivation times (Figure 6E and F) which may partially account for delayed Ca2+ release of the Ca2+ transient and the prolonged APD90, respectively. Clenbuterol treatment alone or in combination with the mechanical unloading did not affect the ICa,L properties. Mechanical unloading alone of failing hearts did not affect the peak ICa,L but normalized slower ICa,L inactivation times seen in HF (τfast (ms): Sham + Sal 7.2 ± 0.6 [43], HF + UN + Sal 5.5 ± 0.4 [23]; P = NS; τslow (ms): Sham + Sal 31.2 ± 2.0 [43], HF + UN + Sal 27.3 ± 2.0 [23]; P = NS) (Figure 6E and F). These results indicate that clenbuterol does not affect L-type calcium current.
Figure 6.
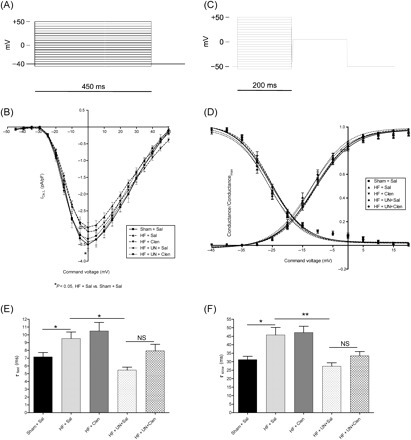
I Ca,L activation and inactivation properties were studied using the protocols A and C, respectively. Heart failure reduced peak ICa,L as shown by I–V relationship (B) prolonged inactivation times (E, F) which were unaffected by clenbuterol treatment. Mechanical unloading normalized the prolonged inactivation times with no additional affect by clenbuterol treatment. The conductance/conductancemax relationship of all groups was unchanged (D). (*P < 0.05, **P < 0.01).
3.10. The effects of clenbuterol on myosin heavy chain isoform changes
HF33 and mechanical unloading34 are known to induce a shift in the expression of MHC isoforms. This can be a reason for changes in the myofilament sensitivity to Ca2+. In this study, we show that clenbuterol normalizes the depressed myofilament sensitivity to Ca2+ (Figure 4E). To clarify this point, gene expression of α- and β-MHC were measured by RT-PCR. There was a reduction in α-MHC RNA expression in HF, which was further reduced by mechanical unloading ((relative mRNA expression) Sham + Sal 1.58 ± 0.14,6 HF + Sal 0.93 ± 0.06;6P < 0.01; HF + UN + Sal 0.31 ± 0.135 vs. HF + Sal; P < 0.001). β-MHC expression was unchanged in HF and mechanical unloading. The ratio of α-MHC/β-MHC confirmed a reduction in relative α-MHC expression in HF and mechanical unloading ((relative mRNA expresion) Sham + Sal 2.05 ± 0.31,6 HF + Sal 0.83 ± 0.25;6P < 0.05; HF + UN + Sal 0.14 ± 0.055 vs. HF + Sal; P < 0.05). Clenbuterol treatment had no further effect on the relative α-MHC isoform expression changes ((relative mRNA expresion) HF + Sal vs. HF + Clen 0.63 ± 0.12;5P = NS; HF + UN + Sal vs. HF + UN + Clen 0.15±0.05;4P= NS). This suggests that the improved myofilament sensitivity to Ca2+ seen after clenbuterol treatment is unlikely to be due to changes in MHC isoform expression.
4. Discussion
This study demonstrates that the depressed whole-heart and cellular contractile function observed in HF can be improved by chronic administration of β2-AR agonist clenbuterol. At whole-heart level in vivo clenbuterol improves LVEF and, when combined with mechanical unloading, it also improves dP/dTmaxex vivo. At a cellular level, depressed sarcomere contractility is restored to normal values in HF by clenbuterol treatment, with or without mechanical unloading. Clenbuterol affects cell size, EC coupling, ion channel function and AP properties but the cellular functional improvement can be ascribed particularly to the normalization of myofilament sensitivity to Ca2+. Mechanical unloading alone has no effects on the depressed sarcomere shortening or whole-heart function.
4.1. β2-AR agonists and heart failure
HF causes derangements of β-AR signalling pathways.35 There is evidence that the β2-AR-Gi subtype specific stimulation has cardioprotective effects and beneficial functional consequences in rodent ischaemic cardiomyopathy and may also be enhanced when combined with β1-AR blockade.19,23,36 In the present study, we demonstrate whole-heart and cellular function improvement with the β2-AR agonist clenbuterol without mechanical unloading. Xydas et al.19 have shown an improvement in ex vivo end diastolic pressure–volume relationship in failing hearts treated for 9 weeks with clenbuterol compared to β1 blocker metoprolol. In a preliminary study, we have found that clenbuterol predominantly activates the β2-AR-Gi pathway37 and this may explain the cardioprotective effects observed. More studies are required to elucidate this point. The role of β2-AR stimulation during mechanical unloading is also complex. Although mechanical unloading is known to restore β-AR responsiveness and density in HF38 and increase β2-AR mRNA expression,39 it has been shown that adenoviral gene transfer of a β2-AR transgene in unloaded rabbit hearts further improved myocardial function.40 In the present study, the combination of β2-AR agonist clenbuterol and mechanical unloading improved whole-heart and cardiomyocyte contractile function, affected cell morphology, normalized deranged Ca2+ cycling, myofilament sensitivity to Ca2+, AP duration and NCX current density. Our study further supports the use of β2-AR stimulation during mechanical unloading to improve myocardial function.
The ability of clenbuterol to induce structural changes to the myocardium is controversial. Burniston et al.41 have shown a dose-dependent, β1-AR mediated, clenbuterol-induced myocardial apoptosis, necrosis, and collagen content in the heart and skeletal muscle of the rat after clenbuterol administration. We did not selectively block the β1-AR in our study but it is possible to predict that this strategy would further improve the beneficial effects of clenbuterol during mechanical unloding in HF. On the basis of the above rationale to maximize the β2-AR mediated beneficial effects of clenbuterol during mechanical unloading, bisoprolol a selective β1 blocker, is used in combination with clenbuterol in the patients treated with LVADs.11 Other studies have shown that clenbuterol administration has a beneficial effect on fibrosis and apoptosis. Wong et al. have shown that clenbuterol administration in conjunction with pressure overload by aortic banding in rats increases LV mass with less fibrosis16 and collagen content15 than banding alone. George et al.12 report no change in collagen content in HF patients treated with clenbuterol during mechanical unloading by LVAD.
4.2. Clenbuterol and unloading-induced myocyte atrophy
Myocardial atrophy is an important consequence of mechanical unloading and has been demonstrated in both human7 and animal42 studies. This is likely to be the main limiting factor to improve myocardial recovery and forms the basis for use of clenbuterol in patients treated with LVADs in order to improve explantation rate for ‘bridge to recovery’.10 We have shown in a previous study that clenbuterol increases cardiomyocyte size in normal hearts13 and, in this study, limits cell size reduction after mechanical unloading in HF. In contrast, Tsuneyoshi et al.20 found that clenbuterol could not prevent atrophy during mechanical unloading of a normal rat heart for 2 weeks. This may suggest that myocyte hypertrophy, induced by HF, may be a prerequisite for the effects of clenbuterol during mechanical unloading. It should be noted that we only assessed unloading after 1 week. It is known that longer duration of unloading in failing human7 and animal34 hearts can deleteriously affect whole-heart and cardiomyocyte function, despite regression of myocyte hypertrophy. Testing whether clenbuterol administration is beneficial when used during longer periods of mechanical unloading of failing hearts would be of interest. Clenbuterol may have an important role in providing an early phase of protection, delaying the onset or preventing unloading-induced atrophy. To elucidate these points, further studies are required.
We have shown that clenbuterol treatment during unloading (HF + UN + Clen) limits cardiomyocyte atrophy by a preferential retention of cell length than width. This may indicate a different form of hypertrophy induced by clenbuterol, which has been proposed to be more ‘physiological’.14–17,43,44 In addition, in the unloaded myocardium, there is an association with the atrophic response of the myocytes and this can explain the morphological differences observed. More studies are required to investigate this point.
4.3. Clenbuterol and excitation–contraction coupling
Twitch Ca2+ transient amplitude and SR Ca2+ content are generally reduced in human45 and animal models of HF.46 However, some studies in animal models have also shown unchanged47 or increased22 Ca2+ transient amplitude with altered contractility. As in the present study, Loennechen et al.22 have shown an increased diastolic and systolic Ca2+ transient at 1, 4, and 13 weeks post-infarction in a rat model. Vahl et al.48 have demonstrated the after-load dependence of the Ca2+ transient amplitude in human HF. They attribute this to an increased dissociation of Ca2+ from the contractile proteins, defective Ca2+ reuptake of the SR or an increased Ca2+ inflow via Ca2+ channels. The differing results in Ca2+ transient kinetics in the above studies may depend on the model of HF and experimental conditions. The paradoxical increased Ca2+ transient amplitude and SR Ca2+ content with reduced sarcomere shortening amplitude seen in our model of post-infarction cardiomyopathy may indicate an intermediate stage in the development of HF with pathological hypertrophy, reduced contractile function and defective myofilament sensitivity to Ca2+.
An increased Ca2+ transient may result from a reduced function of NCX and consequent increased Ca2+ load of the SR in the presence of maintained SR Ca2+ uptake. The mechanisms by which NCX is involved in the improvement are unclear. It is possible that NCX has a compensatory rather than a causal role in the improvement observed. Other Ca2+ regulatory mechanisms, such as SR Ca2+ uptake, and factors that regulate NCX activity, such as [Na+]i49,50 and phosphorylation,51 can be involved. NCX mediated-Ca2+ extrusion was faster in unloaded myocytes with or without clenbuterol. In the clenbuterol-treated group, the biophysical properties of NCX (assessed as INCX) were directly affected. More studies are required to investigate this point.
The prolongation of the AP in HF may also contribute to the increased SR Ca2+ loading and increase the twitch Ca2+ transient amplitude30 as seen in our study. Mechanical unloading alone did not affect the deranged cardiomyocyte contractility or Ca2+ cycling of failing hearts in our study. This might be due to the short duration of unloading as suggested by Ito et al.9
We found a delayed time course of both twitch and caffeine-induced Ca2+ removal in myocytes from failing hearts which was improved by clenbuterol treatment alone and in combination with mechanical unloading. A number of factors can explain this behaviour. In the group treated with clenbuterol alone, the NCX contribution to Ca2+ removal may be increased due to the changes in intracellular [Na+] as discussed above. In the group with clenbuterol and mechanical unloading, the shortening of the AP and increased NCX current density could also be important. Finally the contribution of the SR Ca2+ uptake, which has not been addressed in this study, may also play an important role.
4.4. Clenbuterol and myofilament sensitivity to calcium
Abnormal myofilament function contributes significantly to contractile dysfunction in chronic HF although its relative importance compared to altered Ca2+ homeostasis is currently debated.52 In the model used in this study, we have demonstrated depressed myofilament Ca2+ sensitivity. Clenbuterol treatment normalized this depressed myofilament Ca2+ sensitivity irrespective of whether it was associated to mechanical unloading. This may be a key mechanism involved in the ability of clenbuterol to improve contractile function at cellular level. Our results suggest that the improved myofilament sensitivity to Ca2+ seen after clenbuterol treatment is not due to the effects of clenbuterol on MHC expression. It is possible that clenbuterol affects other key mechanisms like the phophorylation states of troponin-I and myosin binding protein-C.
One possible limitation in our study is that we have estimated myofilament sensitivity to Ca2+ by assessing the relationship between sarcomere shortening and Indo-1 fluorescence. We did not calibrate the fluorescence signals and quantify cytoplasmic [Ca2+]. Although this can be a possible source of error, the relationship between Indo-1 fluorescence ratio and cytoplasmic [Ca2+] is sufficiently linear in the range of measurements reported (20–80% of Ca transients)53,54 to allow comparisons between groups.
4.5. Conclusions
In our study, clenbuterol affects cell size, EC coupling, ion channel function, and AP properties and normalizes myofilament sensitivity to Ca2+. The combination of all the above changes, rather than changes in a single parameter, may lead to the improved cellular and whole-heart function. The temporal and causal relationship of the changes in relation to contractile functional improvement is a challenging point that requires further studies.
In conclusion, the treatment of failing hearts with clenbuterol, alone or in combination with mechanical unloading, improves LV function at whole-heart and cellular level. This study supports the use of this drug in the strategy to enhance recovery in HF patients treated with LVADs and also begins to elucidate some of the possible cellular mechanisms responsible for the improvement in LV function.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Funding
We are grateful to the Magdi Yacoub Institute (HSC 87/04) and the British Heart Foundation for financial support (BHF PG05/005/18163).
Conflict of interest: none declared.
Supplementary Material
References
- 1.Jessup M, Brozena S. Heart failure. N Engl J Med. 2003;348:2007–2018. doi: 10.1056/NEJMra021498. [DOI] [PubMed] [Google Scholar]
- 2.Rose EA, Gelijns AC, Moskowitz AJ, Heitjan DF, Stevenson LW, Dembitsky W, et al. Long-term mechanical left ventricular assistance for end-stage heart failure. N Engl J Med. 2001;345:1435–1443. doi: 10.1056/NEJMoa012175. [DOI] [PubMed] [Google Scholar]
- 3.Clegg AJ, Scott DA, Loveman E, Colquitt JL, Royle P, Bryant J. Clinical and cost-effectiveness of left ventricular assist devices as a bridge to heart transplantation for people with end-stage heart failure: a systematic review and economic evaluation. Eur Heart J. 2006;27:2929–2938. doi: 10.1093/eurheartj/ehi857. [DOI] [PubMed] [Google Scholar]
- 4.Frazier OH, Myers TJ. Left ventricular assist system as a bridge to myocardial recovery. Ann Thorac Surg. 1999;68:734–741. doi: 10.1016/s0003-4975(99)00801-2. [DOI] [PubMed] [Google Scholar]
- 5.Dandel M, Weng Y, Siniawski H, Potapov E, Lehmkuhl HB, Hetzer R. Long-term results in patients with idiopathic dilated cardiomyopathy after weaning from left ventricular assist devices. Circulation. 2005;112:I37–I45. doi: 10.1161/CIRCULATIONAHA.104.525352. [DOI] [PubMed] [Google Scholar]
- 6.Mancini DM, Beniaminovitz A, Levin H, Catanese K, Flannery M, DiTullio M, et al. Low incidence of myocardial recovery after left ventricular assist device implantation in patients with chronic heart failure. Circulation. 1998;98:2383–2389. doi: 10.1161/01.cir.98.22.2383. [DOI] [PubMed] [Google Scholar]
- 7.Maybaum S, Mancini D, Xydas S, Starling RC, Aaronson K, Pagani FD, et al. Cardiac improvement during mechanical circulatory support: a prospective multicenter study of the LVAD Working Group. Circulation. 2007;115:2497–2505. doi: 10.1161/CIRCULATIONAHA.106.633180. [DOI] [PubMed] [Google Scholar]
- 8.Wohlschlaeger J, Schmitz KJ, Schmid C, Schmid KW, Keul P, Takeda A, et al. Reverse remodeling following insertion of left ventricular assist devices (LVAD): a review of the morphological and molecular changes. Cardiovasc Res. 2005;68:376–386. doi: 10.1016/j.cardiores.2005.06.030. [DOI] [PubMed] [Google Scholar]
- 9.Ito K, Nakayama M, Hasan F, Yan X, Schneider MD, Lorell BH. Contractile reserve and calcium regulation are depressed in myocytes from chronically unloaded hearts. Circulation. 2003;107:1176–1182. doi: 10.1161/01.cir.0000051463.72137.96. [DOI] [PubMed] [Google Scholar]
- 10.Yacoub MH. A novel strategy to maximize the efficacy of left ventricular assist devices as a bridge to recovery. Eur Heart J. 2001;22:534–540. doi: 10.1053/euhj.2001.2613. [DOI] [PubMed] [Google Scholar]
- 11.Birks EJ, Tansley PD, Hardy J, George RS, Bowles CT, Burke M, et al. Left ventricular assist device and drug therapy for the reversal of heart failure. N Engl J Med. 2006;355:1873–1884. doi: 10.1056/NEJMoa053063. [DOI] [PubMed] [Google Scholar]
- 12.George I, Xydas S, Mancini DM, Lamanca J, DiTullio M, Marboe CC, et al. Effect of clenbuterol on cardiac and skeletal muscle function during left ventricular assist device support. J Heart Lung Transplant. 2006;25:1084–1090. doi: 10.1016/j.healun.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 13.Soppa GK, Smolenski RT, Latif N, Yuen AH, Malik A, Karbowska J, et al. Effects of chronic administration of clenbuterol on function and metabolism of adult rat cardiac muscle. Am J Physiol Heart Circ Physiol. 2005;288:H1468–H1476. doi: 10.1152/ajpheart.00624.2004. [DOI] [PubMed] [Google Scholar]
- 14.Petrou M, Wynne DG, Boheler KR, Yacoub MH. Clenbuterol induces hypertrophy of the latissimus dorsi muscle and heart in the rat with molecular and phenotypic changes. Circulation. 1995;92:II483–II489. doi: 10.1161/01.cir.92.9.483. [DOI] [PubMed] [Google Scholar]
- 15.Wong K, Boheler KR, Bishop J, Petrou M, Yacoub MH. Clenbuterol induces cardiac hypertrophy with normal functional, morphological and molecular features. Cardiovasc Res. 1998;37:115–122. doi: 10.1016/s0008-6363(97)00190-9. [DOI] [PubMed] [Google Scholar]
- 16.Wong K, Boheler KR, Petrou M, Yacoub MH. Pharmacological modulation of pressure-overload cardiac hypertrophy: changes in ventricular function, extracellular matrix, and gene expression. Circulation. 1997;96:2239–2246. doi: 10.1161/01.cir.96.7.2239. [DOI] [PubMed] [Google Scholar]
- 17.Hon JK, Steendijk P, Petrou M, Wong K, Yacoub MH. Influence of clenbuterol treatment during six weeks of chronic right ventricular pressure overload as studied with pressure-volume analysis. J Thorac Cardiovasc Surg. 2001;122:767–774. doi: 10.1067/mtc.2001.114354. [DOI] [PubMed] [Google Scholar]
- 18.Petrou M, Clarke S, Morrison K, Bowles C, Dunn M, Yacoub M. Clenbuterol increases stroke power and contractile speed of skeletal muscle for cardiac assist. Circulation. 1999;99:713–720. doi: 10.1161/01.cir.99.5.713. [DOI] [PubMed] [Google Scholar]
- 19.Xydas S, Kherani AR, Chang JS, Klotz S, Hay I, Mutrie CJ, et al. {beta}-2 Adrenergic stimulation attenuates left ventricular remodeling, decreases apoptosis, and improves calcium homeostasis in a rodent model of ischemic cardiomyopathy. J Pharmacol Exp Ther. 2006;317:553–561. doi: 10.1124/jpet.105.099432. [DOI] [PubMed] [Google Scholar]
- 20.Tsuneyoshi H, Oriyanhan W, Kanemitsu H, Shiina R, Nishina T, Matsuoka S, et al. Does the beta2-agonist clenbuterol help to maintain myocardial potential to recover during mechanical unloading? Circulation. 2005;112:I51–I56. doi: 10.1161/CIRCULATIONAHA.104.525097. [DOI] [PubMed] [Google Scholar]
- 21.Margulies KB. Reversal mechanisms of left ventricular remodeling: lessons from left ventricular assist device experiments. J Card Fail. 2002;8:S500–S505. doi: 10.1054/jcaf.2002.129264. [DOI] [PubMed] [Google Scholar]
- 22.Loennechen JP, Wisloff U, Falck G, Ellingsen O. Cardiomyocyte contractility and calcium handling partially recover after early deterioration during post-infarction failure in rat. Acta Physiol Scand. 2002;176:17–26. doi: 10.1046/j.1365-201X.2002.01011.x. [DOI] [PubMed] [Google Scholar]
- 23.Ahmet I, Krawczyk M, Heller P, Moon C, Lakatta EG, Talan MI. Beneficial effects of chronic pharmacological manipulation of beta-adrenoreceptor subtype signaling in rodent dilated ischemic cardiomyopathy. Circulation. 2004;110:1083–1090. doi: 10.1161/01.CIR.0000139844.15045.F9. [DOI] [PubMed] [Google Scholar]
- 24.Yang XP, Liu YH, Rhaleb NE, Kurihara N, Kim HE, Carretero OA. Echocardiographic assessment of cardiac function in conscious and anesthetized mice. Am J Physiol. 1999;277:H1967–H1974. doi: 10.1152/ajpheart.1999.277.5.H1967. [DOI] [PubMed] [Google Scholar]
- 25.Ono K, Lindsey ES. Improved technique of heart transplantation in rats. J Thorac Cardiovasc Surg. 1969;57:225–229. [PubMed] [Google Scholar]
- 26.Smolenski RT, Raisky O, Slominska EM, Abunasra H, Kalsi KK, Jayakumar J, et al. Protection from reperfusion injury after cardiac transplantation by inhibition of adenosine metabolism and nucleotide precursor supply. Circulation. 2001;104:I246–I252. doi: 10.1161/hc37t1.094712. [DOI] [PubMed] [Google Scholar]
- 27.Hering S, Bodewei R, Wollenberger A. Sodium current in freshly isolated and in cultured single rat myocardial cells: frequency and voltage-dependent block by mexiletine. J Mol Cell Cardiol. 1983;15:431–444. doi: 10.1016/0022-2828(83)90263-8. [DOI] [PubMed] [Google Scholar]
- 28.Bailey BA, Dipla K, Li S, Houser SR. Cellular basis of contractile derangements of hypertrophied feline ventricular myocytes. J Mol Cell Cardiol. 1997;29:1823–1835. doi: 10.1006/jmcc.1997.0422. [DOI] [PubMed] [Google Scholar]
- 29.Terracciano CM, Hardy J, Birks EJ, Khaghani A, Banner NR, Yacoub MH. Clinical recovery from end-stage heart failure using left-ventricular assist device and pharmacological therapy correlates with increased sarcoplasmic reticulum calcium content but not with regression of cellular hypertrophy. Circulation. 2004;109:2263–2265. doi: 10.1161/01.CIR.0000129233.51320.92. [DOI] [PubMed] [Google Scholar]
- 30.Terracciano CM, Tweedie D, MacLeod KT. The effects of changes to action potential duration on the calcium content of the sarcoplasmic reticulum in isolated guinea-pig ventricular myocytes. Pflugers Arch. 1997;433:542–544. doi: 10.1007/s004240050312. [DOI] [PubMed] [Google Scholar]
- 31.Keung EC, Toll L, Ellis M, Jensen RA. L-type cardiac calcium channels in doxorubicin cardiomyopathy in rats morphological, biochemical, and functional correlations. J Clin Invest. 1991;87:2108–2113. doi: 10.1172/JCI115241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Felkin LE, Birks EJ, George R, Wong S, Khaghani A, Yacoub MH, et al. A quantitative gene expression profile of matrix metalloproteinases (MMPS) and their inhibitors (TIMPS) in the myocardium of patients with deteriorating heart failure requiring left ventricular assist device support. J Heart Lung Transplant. 2006;25:1413–1419. doi: 10.1016/j.healun.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 33.Gupta MP. Factors controlling cardiac myosin-isoform shift during hypertrophy and heart failure. J Mol Cell Cardiol. 2007;43:388–403. doi: 10.1016/j.yjmcc.2007.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oriyanhan W, Tsuneyoshi H, Nishina T, Matsuoka S, Ikeda T, Komeda M. Determination of optimal duration of mechanical unloading for failing hearts to achieve bridge to recovery in a rat heterotopic heart transplantation model. J Heart Lung Transplant. 2007;26:16–23. doi: 10.1016/j.healun.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 35.Lohse MJ, Engelhardt S, Eschenhagen T. What is the role of beta-adrenergic signaling in heart failure? Circ Res. 2003;93:896–906. doi: 10.1161/01.RES.0000102042.83024.CA. [DOI] [PubMed] [Google Scholar]
- 36.Ahmet I, Lakatta EG, Talan MI. Pharmacological stimulation of beta (2)-adrenergic receptors (beta (2)AR) enhances therapeutic effectiveness of beta (1)AR blockade in rodent dilated ischemic cardiomyopathy. Heart Fail Rev. 2005;10:289–296. doi: 10.1007/s10741-005-7543-3. [DOI] [PubMed] [Google Scholar]
- 37.Siedlecka U, Arora M, Soppa GKR, Lee J, Stagg MA, Harding SE, et al. Clenbuterol affects rat ventricular myocyte contractility via an inhibitory G protein-mediated pathway. J Mol Cell Cardiol. 2007;42:S49. (Abstract) [Google Scholar]
- 38.Ogletree-Hughes ML, Stull LB, Sweet WE, Smedira NG, McCarthy PM, Moravec CS. Mechanical unloading restores beta-adrenergic responsiveness and reverses receptor downregulation in the failing human heart. Circulation. 2001;104:881–886. doi: 10.1161/hc3301.094911. [DOI] [PubMed] [Google Scholar]
- 39.Tsuneyoshi H, Oriyanhan W, Kanemitsu H, Shiina R, Nishina T, Ikeda T, et al. Heterotopic transplantation of the failing rat heart as a model of left ventricular mechanical unloading toward recovery. ASAIO J. 2005;51:116–120. doi: 10.1097/01.mat.0000150325.05589.8b. [DOI] [PubMed] [Google Scholar]
- 40.Tevaearai HT, Eckhart AD, Walton GB, Keys JR, Wilson K, Koch WJ. Myocardial gene transfer and overexpression of beta2-adrenergic receptors potentiates the functional recovery of unloaded failing hearts. Circulation. 2002;106:124–129. doi: 10.1161/01.cir.0000020220.79105.fd. [DOI] [PubMed] [Google Scholar]
- 41.Burniston JG, Tan LB, Goldspink DF. Beta2-adrenergic receptor stimulation in vivo induces apoptosis in the rat heart and soleus muscle. J Appl Physiol. 2005;98:1379–1386. doi: 10.1152/japplphysiol.00642.2004. [DOI] [PubMed] [Google Scholar]
- 42.Rakusan K, Heron MI, Kolar F, Korecky B. Transplantation-induced atrophy of normal and hypertrophic rat hearts: effect on cardiac myocytes and capillaries. J Mol Cell Cardiol. 1997;29:1045–1054. doi: 10.1006/jmcc.1996.0350. [DOI] [PubMed] [Google Scholar]
- 43.Ghorayeb N, Batlouni M, Pinto IM, Dioguardi GS. Left ventricular hypertrophy of athletes: adaptative physiologic response of the heart. Arq Bras Cardiol. 2005;85:191–197. doi: 10.1590/s0066-782x2005001600008. [DOI] [PubMed] [Google Scholar]
- 44.Kong SW, Bodyak N, Yue P, Liu Z, Brown J, Izumo S, et al. Genetic expression profiles during physiological and pathological cardiac hypertrophy and heart failure in rats. Physiol Genomics. 2005;21:34–42. doi: 10.1152/physiolgenomics.00226.2004. [DOI] [PubMed] [Google Scholar]
- 45.Gwathmey JK, Copelas L, MacKinnon R, Schoen FJ, Feldman MD, Grossman W, et al. Abnormal intracellular calcium handling in myocardium from patients with end-stage heart failure. Circ Res. 1987;61:70–76. doi: 10.1161/01.res.61.1.70. [DOI] [PubMed] [Google Scholar]
- 46.Li P, Park C, Micheletti R, Li B, Cheng W, Sonnenblick EH, et al. Myocyte performance during evolution of myocardial infarction in rats: effects of propionyl-L-carnitine. Am J Physiol. 1995;268:H1702–H1713. doi: 10.1152/ajpheart.1995.268.4.H1702. [DOI] [PubMed] [Google Scholar]
- 47.Anand IS, Liu D, Chugh SS, Prahash AJ, Gupta S, John R, et al. Isolated myocyte contractile function is normal in postinfarct remodeled rat heart with systolic dysfunction. Circulation. 1997;96:3974–3984. doi: 10.1161/01.cir.96.11.3974. [DOI] [PubMed] [Google Scholar]
- 48.Vahl CF, Bonz A, Timek T, Hagl S. Intracellular calcium transient of working human myocardium of seven patients transplanted for congestive heart failure. Circ Res. 1994;74:952–958. doi: 10.1161/01.res.74.5.952. [DOI] [PubMed] [Google Scholar]
- 49.Bers DM, Despa S, Bossuyt J. Regulation of Ca2+ and Na+ in normal and failing cardiac myocytes. Ann N Y Acad Sci. 2006;1080:165–177. doi: 10.1196/annals.1380.015. [DOI] [PubMed] [Google Scholar]
- 50.Pieske B, Houser SR. [Na+]i handling in the failing human heart. Cardiovasc Res. 2003;57:874–886. doi: 10.1016/s0008-6363(02)00841-6. [DOI] [PubMed] [Google Scholar]
- 51.Wei SK, Ruknudin A, Hanlon SU, McCurley JM, Schulze DH, Haigney MC. Protein kinase A hyperphosphorylation increases basal current but decreases beta-adrenergic responsiveness of the sarcolemmal Na+-Ca2+ exchanger in failing pig myocytes. Circ Res. 2003;92:897–903. doi: 10.1161/01.RES.0000069701.19660.14. [DOI] [PubMed] [Google Scholar]
- 52.Day SM, Westfall MV, Metzger JM. Tuning cardiac performance in ischemic heart disease and failure by modulating myofilament function. J Mol Med. 2007;85:911–921. doi: 10.1007/s00109-007-0181-6. [DOI] [PubMed] [Google Scholar]
- 53.Bassani JW, Bassani RA, Bers DM. Calibration of indo-1 and resting intracellular [Ca]i in intact rabbit cardiac myocytes. Biophys J. 1995;68:1453–1460. doi: 10.1016/S0006-3495(95)80318-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Levi AJ, Li J, Spitzer KW, Bridge JH. Effect on the indo-1 transient of applying Ca2+ channel blocker for a single beat in voltage-clamped guinea-pig cardiac myocytes. J Physiol. 1996;494:653–673. doi: 10.1113/jphysiol.1996.sp021522. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


