Abstract
Objective
COPD is associated with significant economic burden. The objective of this study was to explore the direct and indirect costs associated with COPD and identify the key cost drivers of disease management in Greece.
Methods
A Delphi panel of Greek pulmonologists was conducted, which aimed at eliciting local COPD treatment patterns and resource use. Resource use was translated into costs using official health insurance tariffs and Diagnosis-Related Groups (DRGs). In addition, absenteeism and caregiver’s costs were recorded in order to quantify indirect COPD costs.
Results
The total costs of managing COPD per patient per year were estimated at €4,730, with direct (medical and nonmedical) and indirect costs accounting for 62.5% and 37.5%, respectively. COPD exacerbations were responsible for 32% of total costs (€1,512). Key exacerbation-related cost drivers were hospitalization (€830) and intensive care unit (ICU) admission costs (€454), jointly accounting for 85% of total exacerbation costs. Annual maintenance phase costs were estimated at €835, with pharmaceutical treatment accounting for 77% (€639.9). Patient time costs were estimated at €146 per year. The average number of sick days per year was estimated at 16.9, resulting in productivity losses of €968. Caregiver’s costs were estimated at €806 per year.
Conclusion
The management of COPD in Greece is associated with intensive resource use and significant economic burden. Exacerbations and productivity losses are the key cost drivers. Cost containment policies should focus on prioritizing treatments that increase patient compliance as these can lead to reduction of exacerbations, longer maintenance phases, and thus lower costs.
Keywords: COPD, burden, direct cost, indirect cost, Greece
Introduction
COPD is associated with significant clinical, societal and economic burden.1 In 2012, it was the third leading cause of death, accounting for 5.6% of all deaths.2 The impairment of health-related quality of life (HRQoL) in patients with COPD is considerable and has been shown to increase with disease severity.3 The prevalence of COPD varies globally between 8% and 10% in the adult population.4 In Greece, it has been estimated at 8.4% of the population older than 35 years of age (and with smoking history),5 while newer data suggest a prevalence of 10.6% of the population aged ≥40 years.6
In addition to the increased morbidity and mortality, COPD has a significant economic impact, which consists of both direct and indirect costs.7 Annual direct costs per patient have been estimated to range widely between $504 in South Korea and $9,981 in the USA, while the percentage of patients unable to work due to COPD ranges from 6% in Italy to 52% in the USA and the UK.8 Indirect per-patient costs exceed direct costs in most countries worldwide, accounting for 61%, 82%, and 83% in Italy, the Netherlands, and the UK, respectively.8 In 2010, total COPD-related burden in the USA was estimated at $36 billion, with the direct medical costs accounting for 89.2% ($32.1 billion) and indirect costs (absenteeism costs) accounting for 10.8% ($3.9 billion).9
In Greece, the cost of COPD has not been adequately explored. Previous studies have estimated the cost of severe exacerbations at €1,711 per exacerbation.10 However, other direct costs, such as maintenance phase costs, as well as indirect costs, ie, absenteeism and productivity losses, have not been investigated. The objective of this study was to explore the per-patient direct and indirect costs associated with COPD and identify the key cost drivers of disease management in Greece. We hypothesized that direct costs would be the main cost driver in Greece.
Methods
A questionnaire was developed to elicit data on the treatment patterns and resource use associated with the management of COPD. The questionnaire was developed based on a review of the international literature and clinical guidelines,11 and it was distributed electronically to 12 leading pulmonologists, members of the COPD Working Group of the Hellenic Thoracic Society. Expert opinion was sought throughout the development and validation process of the questionnaire. The study was approved by the Research Committee of the University of Peloponnese and written informed consent was received from the participants.
Study participants represented all health care settings (public and university hospitals, private clinics, and private offices) and were geographically obtained from three out of the seven health care districts of Greece, covering the largest part of the population in the country. The questionnaire included questions on drug dosage, frequency and duration of administration, frequency of visits to physicians, laboratory and imaging tests, as well as frequency and duration of hospitalization. Use of additional resources, such as oxygen therapy and nebulizers at home, was also collected. All resource use components were collected for both the maintenance phase of the disease and the exacerbations. In addition, data on patients’ time (including traveling and waiting time), as well as nonpaid caregivers’ time (from patients’ family and friendly environment) and absence from work due to illness, were collected. Moreover, questions exploring the impact of the economic crisis on patients’ compliance, as well as its determinants, were included in the questionnaire.
The data elicitation method was the Delphi technique, which aims at consensus building via multiple rounds of questionnaire completion.12 Questionnaires were completed remotely, data collected upon completion of first round were analyzed, and results were presented to study participants during a face-to-face meeting. As discrepancies were minimal, consensus was reached during the second (face-to-face) round. The questionnaire completion was conducted between October 2015 and February 2016.
Unit costs were retrieved from publicly available sources and were subsequently assigned to resource use in order to estimate total costs per patient. Direct medical costs were estimated at the prices reimbursed by the National Organization of Health Care Services Provision (EOPYY), the largest public sector payer in Greece, currently covering 90% of the Greek population.13 Pharmaceutical costs were calculated based on reimbursement prices published in the Positive Reimbursement List.14 Hospitalization costs were based on the Diagnosis-Related Groups (DRGs),15 intensive care unit (ICU) costs were based on the Decree published by the Ministry of Health,16 and laboratory and imaging test costs were estimated using prices reimbursed by EOPYY.17 The cost base year was 2015. Indirect costs were estimated based on Organization for Economic Cooperation and Development (OECD) data for Greece’s per capita gross domestic product (GDP), projected to 2015 based on historical average annual growth rate (2008–2014) and used as a proxy for daily wage rate (€57.4).18 All cost estimates refer to the average patient, ie, the values have been weighted with the probability of use of each resource item. As a proxy of the probability, the percentage of patients requiring the specific health care resource was used.
Results
Direct medical costs
Of the patients experiencing an exacerbation, 36.8% visit hospital emergency rooms (ERs) and are subsequently followed up by their managing physician, at one of the following places: the hospital outpatient clinics (42.3%), the physician’s private office (37.0%), or the patient’s home (20.7%) (Figure 1). Irrespective of the health care setting, patients are managed mainly by pulmonologists (60.2%) and secondarily by general practitioners (GPs) and internists (jointly accounting for 39.8%). During the maintenance phase, the percentages of patients managed in a specific health care setting and by specialty do not differ from the respective percentages during management of exacerbations.
Figure 1.
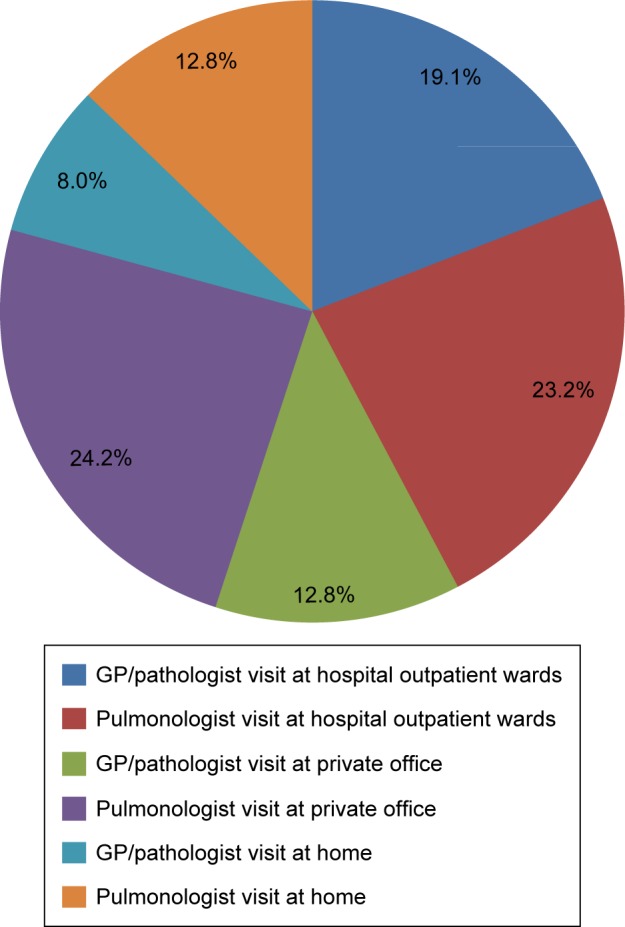
Management of COPD patients.
Abbreviation: GP, general practitioner.
Moreover, 34.5% of the patients suffering an exacerbation required hospitalization, and 41.9% of these were hospitalized under DRG code A25M (COPD with comorbidities and/or complications), with an average length of stay (LOS) of 9 days and a cost of €1,446, while 58.1% were hospitalized under DRG code A25X (COPD without comorbidities and/or complications), with an average LOS of 5 days and mean cost of €863. Of the patients hospitalized, 9.8% required hospitalization in an ICU, with an LOS of 10.7 days and an additional cost of €2,130 per patient. The average cost per exacerbation – weighted with the probability of requiring hospitalization in an ICU – was estimated at €209.1. During the disease maintenance phase, only 7.3% of the patients required hospitalization, without necessitating admissions to the ICU.
Laboratory tests during both exacerbations and maintenance phase included complete blood count (CBC), biochemical tests, C-reactive protein (CRP) level, arterial blood gases (ABG), erythrocyte sedimentation rate (ESR), and urine analysis. Functional and imaging tests included spirometry, electrocardiography, chest radiography (X-ray), and computed tomography (CT). The frequency and percentage of patients undergoing each test per exacerbation and during the maintenance phase, as well as the unit costs per test, are presented in Table 1.
Table 1.
Laboratory and imaging tests
| Tests | Exacerbations
|
Maintenance phase
|
Unit cost (€) | ||
|---|---|---|---|---|---|
| % of patients | Frequency of test per exacerbation | % of patients | Frequency of test per year | ||
| Laboratory tests | |||||
| CBC | 81.8 | 2.2 | 45.7 | 1.0 | 2.9 |
| Biochemical tests* | 67.8 | 2.0 | 51.5 | 1.0 | 21.7 |
| ABG | 50.0 | 2.0 | – | – | 12.4 |
| CRP | 33.3 | 1.0 | – | – | 10.0 |
| ESR | 33.3 | 2.0 | – | – | 1.8 |
| Urine analysis | 38.6 | 1.0 | 13.8 | 0.6 | 1.8 |
| Sputum culture | 50.0 | 1.0 | – | – | 5.2 |
| Functional/imaging tests | |||||
| Spirometry | 57.1 | 0.8 | 85.8 | 1.1 | 44.0 |
| Electrocardiography | 65.0 | 1.2 | 54.2 | 1.1 | 4.1 |
| Chest CT | 21.7 | 0.9 | 22.5 | 0.8 | 45.0 |
| Chest radiography | 84.1 | 1.5 | 53.5 | 0.9 | 4.1 |
Note:
Includes urea, creatinine, SGOT, SGPT, K, Na, Ca, and LDH assays.
Abbreviations: ABG, arterial blood gases; CBC, complete blood count; CRP, C-reactive protein; CT, computed tomography; ESR, erythrocyte sedimentation rate; LDH, lactate dehydrogenase; SGOT, serum glutamic oxaloacetic transaminase; SGPT, serum glutamic pyruvic transaminase.
Pharmaceutical treatment during the maintenance phase mainly included long-acting muscarinic antagonists (LAMAs; 63% of patients); long-acting beta2 agonist (LABA) + LAMA (indacaterol + glycopyrronium) (23% of patients); LABA + inhaled corticosteroid (ICS) (13% of patients); and LABA monotherapy (indacaterol) (10% of patients). The annual cost of drugs during the maintenance phase was estimated at €639.9, while the respective figure for the management of exacerbations, which are mainly treated with prednisolone (45% of patients), budesonide (31%), ipratropium (19%), salbutamol (15%), and antibiotics (13%), was estimated at €22.6.
The mean number of COPD exacerbations per patient was estimated at 2.17 per year. Based on this, the annual cost of managing exacerbations was estimated at €1,512, 85% of which relates to hospitalization and admissions to the ICU. Maintenance costs were estimated at €835 per year, mainly reflecting pharmaceutical costs (76.6%) (Table 2).
Table 2.
Annual per-patient costs (€) during exacerbations and maintenance phase
| Resource use item | Annual costs associated with the management of exacerbations* (% of total) | Annual costs of the maintenance phase (% of total) |
|---|---|---|
| Pharmaceutical treatment | 22.6 (1.5) | 639.9 (76.6) |
| Medical treatment | 6.8 (0.4) | 53.6 (6.4) |
| Hospitalization | 829.9 (54.9) | 74.5 (8.9) |
| ICU admission | 453.8 (30.0) | – |
| Laboratory tests | 113.3 (7.5) | 12.1 (1.4) |
| Functional/imaging tests | 86.0 (5.7) | 54.9 (6.6) |
| Total costs, € | 1,512.4 | 835.0 |
Notes:
Based on the mean number of exacerbations per year (2.17). Numbers have been rounded to the nearest euro, thus percentages might not sum up to 100%.
Abbreviation: ICU, intensive care unit.
Furthermore, 37.7% and 26.7% of patients with COPD use nebulizer and oxygen therapy at home, respectively. Additional resources used are portable oxygen bottles (7.4% of the patients), humidifiers (11%), special chairs or beds (8.9%), and mobility aids (10.3%). The average cost of all additional resources was estimated at €462 per year.
Direct nonmedical costs
The average patient time for medical follow-up, including travel and waiting time, ranged from 1.3 hours for the GP private office to 2.7 hours for the hospital outpatient clinic. Given that patients visit their physician ~1.1 times per month during the maintenance phase, the total number of medical follow-up visits was estimated at 13.2 per year, and thus the mean patient time was estimated to range between 17.3 and 35.1 hours per year. The mean annual cost of patient time (based on the mean daily wage rate and weighted for the percentage of patients per health care setting) was estimated at €146.
Indirect costs
The average number of work-loss days per year was estimated at 16.9 days, leading to productivity losses of €968. In addition, ~33% of patients received help from family, relatives, and/or friendly environment for their daily activities, occupying ~3.6 hours per day, 4.1 days per week, of the non-paid caregivers’ time. Moreover, 43% of the caregivers are economically active, adding €806 per year to productivity losses associated with the disease management.
Total costs per year
Total costs of managing COPD per patient per year were estimated at €4,730, one-third of which reflected the management of COPD exacerbations. Direct medical costs accounted for 59.4%, whereas indirect costs accounted for 37.5% of total costs (Table 3).
Table 3.
Total per-patient costs (€)
| Cost type | Annual cost, € | % of total cost |
|---|---|---|
| Direct medical cost | 2,809.7 | 59.4 |
| Cost of exacerbations | 1,512.4 | 32.0 |
| Costs of maintenance phase | 835.0 | 17.7 |
| Costs of additional resources | 462.3 | 9.8 |
| Direct nonmedical cost | 146.1 | 3.1 |
| Cost of patients’ time | 146.1 | 3.1 |
| Productivity losses | 1,774.1 | 37.5 |
| Cost of work loss days | 968.4 | 20.5 |
| Cost of nonpaid caregivers’ time | 805.7 | 17.0 |
| Total cost | 4,729.9 | 100 |
Note: The lines in bold are the sum of the rows that follow them, therefore, when total cost is to be estimated, only the bold numbers should be added.
Parameters affecting patient compliance with treatment
The expert panel also provided input on the key factors affecting patient compliance. The economic crisis, patients’ smoking history, and the frequency and duration of exacerbations had a negative impact on compliance, while improvement of patients’ socioeconomic status was recorded as a factor positively affecting compliance.
Discussion
This study explored the direct and indirect costs associated with the management of COPD patients in Greece. We found that the annual per-patient direct costs of COPD were €2,810, 54% of which reflected the management of exacerbations. The key cost driver of exacerbations was inpatient hospitalization and admissions to the ICU, while during the maintenance phase, COPD was mainly managed by pharmaceutical treatment, which accounted for 77% of total maintenance costs. When indirect and patient time costs were added to direct costs, the total annual burden of COPD in Greece was estimated at €4,730 per patient.
In the international literature, there are significant variations of COPD annual costs across countries. Societal per-patient costs have been found to vary widely, from $1,721 in Russia to $30,826 in the USA.8 Such variations are mainly attributed to geographic differences and measurement methods of COPD prevalence, hospitalization, and mortality.19 However, there is a consistent pattern across countries, showing that increased costs are associated with more severe disease and a greater number of comorbidities.8
Pharmaceutical treatment patterns identified through our study are in accordance with another recent study conducted in Greece, which investigated prescribing patterns for COPD with real-world data.20 In addition, hospitalizations and oxygen therapy, which were identified as significant cost parameters in our study, have also been identified as key cost drivers of direct costs in a multinational study by Foo et al.8
The annual cost of exacerbations (on average 2.17 per year), which was estimated in our study at €1,512, does not match the respective estimate by Geitona et al,10 where the cost of one exacerbation was estimated at €1,711. However, the latter reflects the cost of severe exacerbations only, whereas our findings refer to mean costs of both severe and nonsevere exacerbations. Finally, the significance of indirect costs has been highlighted in the international literature. In the USA, it has been estimated that 16.4 million days of work are lost because of COPD.9
Our study has certain limitations. First, study results are based on expert opinion rather than patient-level data. However, given the lack of a registry for COPD in Greece, the Delphi panel survey could be considered to be the second-best method to collect data that could map the current clinical practice and resource use associated with the disease management. Second, resource use data were elicited from pulmonologists only, while the study revealed that the disease is currently being managed by both pulmonologists (60%) and GPs/internists (40% of total patients). Thus, the results only reflect the management of the disease in Greece by specialists (who are, however, the majority of treating physicians). In addition, pulmonologists treat, on average, more number of severe COPD patients, and therefore, the annual prevalence of exacerbations and associated costs might have been overestimated and might not fully correspond to that in the general COPD population.
On the other hand, there are some burden-of-disease parameters that have not been taken into consideration in the current study and, thus, might have led to an underestimation of its true cost. Cost estimates do not include disability pensions or other health care benefits COPD patients might receive. In addition, COPD is recognized as a risk factor for multiple chronic conditions (cardiovascular disease, pneumonia, and depression, to name but a few of these),9 and therefore, its total burden should include the cost of comorbidities. Because the perspective adopted is that of the EOPYY, out-of-pocket payments have not been incorporated in the analysis, leading to cost underestimation, especially in the case of physician visits, the costs for which are mainly covered through out-of-pocket payments. The unit cost for physician visits used in the analysis is the price reimbursed by EOPYY, which is, however, very low and does not reflect true costs. Overall, there is some evidence that results might have been both over- and underestimated, these two effects counterbalancing each other, at least to some extent.
Another limitation of our study is that it does not incorporate productivity losses associated with premature death due to COPD. Premature death is a significant component of indirect costs, especially in the case of diseases such as COPD that are associated with significant mortality. However, it was out of the scope of the current analysis, as we only aimed to capture the costs associated with absence from work due to illness.
The study also showed that compliance is a parameter negatively affected by the current socioeconomic conditions in Greece. Compliance reduces the economic burden of the disease, as it can keep patients under maintenance, reducing exacerbations that increase resource use and respective costs. The study by Toy et al21 has shown that improved compliance, measured as a 5% increase in the proportion of days covered by treatment, reduced the annual number of hospitalizations by 2.5% and the ER visits by 1.8%, leading to a net reduction in annual costs of ~$300 per patient in the USA. This finding, together with the fact that our study showed that the annual cost of maintenance was almost half the cost of exacerbations, can provide significant insight into and input for health care decision making, especially under the current difficult economic era in Greece.
To the best of our knowledge, this is the first study that aimed to capture a complete picture of the total COPD costs, including direct medical as well as nonmedical, indirect, and societal costs. It is also the first study to map resource use for both exacerbations and the maintenance phase. Results of this study can contribute to discussions around cost rationalization policies in the field of health care, targeting patient stabilization, increasing maintenance phase duration, reducing exacerbations, and thus minimizing costs. Such policies could significantly reduce the burden of COPD on social insurance funds, patients, and their families.
Conclusion
The management of COPD in Greece is associated with intensive resource use and significant economic burden. Exacerbations and productivity losses are the key cost drivers. In the current era of economic crisis, cost containment policies should target treatment strategies that increase patient compliance, as these can lead to reduction of exacerbations, longer maintenance phases, and lower costs.
Acknowledgments
The authors thank the members of the Hellenic Thoracic Society for their contribution to the study. The University of Peloponnese received a grant from the Hellenic Thoracic Society to undertake this project.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Punekar YS, Shukla A, Müllerova H. COPD management costs according to the frequency of COPD exacerbations in UK primary care. Int J Chron Obstruct Pulmon Dis. 2014;9:65–73. doi: 10.2147/COPD.S54417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization [webpage on the Internet] The top 10 causes of death-Fact Sheet 310 [updated May 2014] [Accessed November 11, 2016]. Available from: http://www.who.int/mediacentre/factsheets/fs310/en/
- 3.Wacker ME, Jörres RA, Karch A, et al. COSYCONET-Consortium Assessing health-related quality of life in COPD: comparing generic and disease-specific instruments with focus on comorbidities. BMC Pulm Med. 2016;16(1):70. doi: 10.1186/s12890-016-0238-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diaz-Guzman E, Mannino D. Epidemiology and prevalence of chronic obstructive pulmonary disease. Clin Chest Med. 2014;35(1):7–16. doi: 10.1016/j.ccm.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 5.Tzanakis N, Anagnostopoulou U, Filaditaki V, Christaki P, Siafakas N. Prevalence of COPD in Greece. Chest. 2004;125(3):892–900. doi: 10.1378/chest.125.3.892. [DOI] [PubMed] [Google Scholar]
- 6.Kourlaba G, Hillas G, Vassilakopoulos T, Maniadakis N. The disease burden of chronic obstructive pulmonary disease in Greece. Int J Chron Obstruct Pulmon Dis. 2016;11:2179–2189. doi: 10.2147/COPD.S110373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guarascio AJ, Ray SM, Finch CK, Self TH. The clinical and economic burden of chronic obstructive pulmonary disease in the USA. Clinicoecon Outcomes Res. 2013;5:235–245. doi: 10.2147/CEOR.S34321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Foo J, Landis SH, Maskell J, et al. Continuing to confront COPD international patient survey: economic impact of COPD in 12 countries. PLoS One. 2016;11(4):e0152618. doi: 10.1371/journal.pone.0152618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ford E, Murphy L, Khavjou O, Giles W, Holt J, Croft J. Total and state-specific medical and absenteeism costs of COPD among adults aged $18 years in the United States for 2010 and projections through 2020. Chest. 2015;147(1):31–45. doi: 10.1378/chest.14-0972. [DOI] [PubMed] [Google Scholar]
- 10.Geitona M, Hatzikou M, Steiropoulos P, Alexopoulos EC, Bouros D. The cost of COPD exacerbations: a university hospital – based study in Greece. Respir Med. 2011;105(3):402–409. doi: 10.1016/j.rmed.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 11.National Institute for Health and Care Excellence [webpage on the Internet] CG101: Chronic obstructive pulmonary disease in over 16s: diagnosis and management. 2010. [Accessed November 11, 2016]. Available from: http://guidance.nice.org.uk/CG101/Guidance/pdf/English. [PubMed]
- 12.Hsu C-C, Sandford BA. The Delphi Technique: making sense of consensus. Pract Assessment Res Eval. 2007;12(10):1. [Google Scholar]
- 13.OECD . Greece: Reform of Social Welfare Programmes (Greek Version) 2013. [Google Scholar]
- 14.Greek Ministry of Health . Positive Reimbursement List. Feb 16, 2016. (Government Gazette 333). [Google Scholar]
- 15.Greek Ministry of Health [webpage on the Internet] Diagnosis Related Groups March 2012. [Accessed November 11, 2016]. Available from: http://www.moh.gov.gr/articles/health/domes-kai-draseis-gia-thn-ygeia/kwdikopoihseis/709-kleista-enopoihmena-noshlia-1.
- 16.Ministerial Decree . Government Gazette 2150. Sep 27, 2011. [Google Scholar]
- 17.National Organization for Health Care Services Provision [homepage on the Internet] [Accessed November 11, 2016]. Available from: www.eopyy.gov.gr.
- 18.OECD [webpage on the Internet] Economic References-Gross Domestic Product. [Accessed April 1, 2016]. Available from: http://stats.oecd.org/index.aspx?DatasetCode=HEALTH_ECOR.
- 19.Ford E, Croft J, Mannino D, Wheaton A, Zhang X, Giles W. COPD surveillance – United States, 1999–2011. Chest. 2013;144(1):284–305. doi: 10.1378/chest.13-0809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Souliotis K, Kani C, Papageorgiou M, Lionis D, Gourgoulianis K. Using big data to assess prescribing patterns in Greece: the case of chronic obstructive pulmonary disease. PLoS One. 2016;11(5):e0154960. doi: 10.1371/journal.pone.0154960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Toy E, Beaulieu N, McHale J, et al. Treatment of COPD: relationships between daily dosing frequency, adherence, resource use, and costs. Respir Med. 2011;105(3):435–441. doi: 10.1016/j.rmed.2010.09.006. [DOI] [PubMed] [Google Scholar]


