Abstract
Background
Bronchiectasis is characterized by permanent dilatation of the bronchial tree caused by recurrent airway infection and inflammation. The association of atherosclerosis and inflammation is well established. However, studies on the relationship between bronchiectasis and stroke are scant.
Objective
We conducted a population-based cohort study to investigate the incidence and risk of ischemic stroke in patients with bronchiectasis.
Methods
Data of 1,295 patients newly diagnosed as bronchiectasis between 2000 and 2008 were retrieved from the Taiwan National Health Insurance Research Database. A total of 6,475 controls without bronchiectasis at a ratio of 5:1 were randomly selected from the general population based on frequency-matched age and sex to the patients. All participants were followed up to the date of ischemic stroke development, censoring, or the end of 2010. The Cox proportional hazard model was used to identify the risk of ischemic stroke in patients with bronchiectasis compared with those without bronchiectasis.
Results
The patients with bronchiectasis exhibited a higher incidence rate of ischemic stroke (9.18 vs 4.66 per 1,000 person-years) than the patients without bronchiectasis, with an adjusted hazard ratio of 1.74 (95% confidence interval =1.28–2.35). The patients with bronchiectasis and any comorbidities exhibited a 2.66-fold adjusted hazard ratio of ischemic stroke compared with those with neither bronchiectasis nor comorbidity (95% confidence interval =1.85–3.84). The patients with bronchiectasis carried a dose response of ischemic stroke according to the number of emergency visits and hospitalizations per year.
Conclusion
This study indicated that bronchiectasis is an independent risk factor of ischemic stroke.
Keywords: bronchiectasis, ischemic stroke, cohort study
Plain language summary
Bronchiectasis is characterized by recurrent airway infection and inflammation. The association of atherosclerosis and inflammation is well established. However, studies on the relationship between bronchiectasis and stroke are scant. Therefore, we conducted a population-based cohort study to determine the incidence and risk of ischemic stroke in patients with bronchiectasis.
The patients with bronchiectasis exhibited a higher incidence rate of ischemic stroke (9.18 vs 4.66 per 1,000 person-years) than the patients without bronchiectasis, with an adjusted hazard ratio (aHR) of 1.74 (95% confidence interval [CI] =1.28–2.35). The patients with bronchiectasis carried a dose response of ischemic stroke according to the number of emergency visits and hospitalizations per year.
This study indicated that bronchiectasis is an independent risk factor of ischemic stroke. Clinicians should take proactive strategies to carefully assess risk factors of stroke, and holistically treat patients with bronchiectasis.
Introduction
Bronchiectasis is characterized by a permanent dilatation and thickening of the bronchial tree caused by recurrent airway infection and inflammation. Common symptoms of bronchiectasis include viscid sputum production, hemoptysis, dyspnea, and weight loss.1,2 High-resolution computed tomography (HRCT) is the recognized gold standard for the diagnosis of bronchiectasis.3,4
Bronchiectasis can cause substantial impact not only on patients’ physical and psychosocial well-being but also on morbidity and mortality.2,5 A recent study from the United Kingdom reported an association between bronchiectasis and increased prevalence of coronary heart disease and stroke.6 The probable pathology is that leukocyte recruitment and inflammatory mediators may activate atherogenesis.7,8 Libby reported that leukocyte recruitment and expression of proinflammatory cytokines characterize early atherogenesis and that malfunction of inflammatory mediators mutes atheroma formation in mice.7,8 It was also observed that autoimmune diseases such as rheumatoid arthritis, a chronic systemic inflammation, predispose to atherosclerosis and cardiovascular disease.7,8 Systemic inflammation can increase intima medial thickness in carotid artery and the presence of carotid plaque.9,10 Hence, patients who have bronchiectasis and experience frequent respiratory infections may have an increased risk of ischemic stroke.
Stroke is the rapid development of a focal neurologic deficit caused by a disruption of blood supply to the corresponding area of brain. It can lead to the most devastating impairment of physical and physiological function and therefore result in a great social and economic burden worldwide.11 In addition, stroke remains the second leading cause of death in the world and in Taiwan as well.12–14 Medical comorbid disorders, such as hypertension, diabetes, hyperlipidemia, coronary artery disease (CAD), congestive heart disease (CHF), and atrial fibrillation (AF), are well-established risk factors of stroke.15–19 Recent studies have indicated that chronic obstructive pulmonary disease (COPD) is also associated with an increased risk of stroke.20,21 However, studies on the relationship between bronchiectasis and stroke for an Asian population are scant. Therefore, in this study, we conducted a nationwide population-based cohort study to evaluate the incidence and risk of ischemic stroke in patients with bronchiectasis in Taiwan.
Methods
Data source
Single-payer National Health Insurance (NHI) program started from March 1, 1995, in Taiwan. More than 99.9% of Taiwan’s citizens have been enrolled in this program in 2015.22 The National Health Insurance Research Database (NHIRD) was provided by the National Health Insurance Administration (NHIA, formerly named Bureau of National Health Insurance) and managed by the National Health Research Institute. We used the Longitudinal Health Insurance Database (LHID), a sub-database of NHIRD, comprising data of 1 million randomly selected beneficiaries of the NHI program in 2000. The database has been released for public research after deidentification of the beneficiaries. A variety of outpatient and inpatient medical records are included in the LHID. Diagnoses are coded according to the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). Previous studies have validated the diagnosis of ICD-9-CM codes in the database.23–25 This study was approved by the Institutional Review Board of Tsaotun Psychiatric Center, Ministry of Health and Welfare (IRB No 105002). The National Health Research Institute de-identified the study participants before NHIRD release for public research. Therefore, the Institutional Review Board waived the requirement for patient written informed consent for this study.
Study patients
The patients with bronchiectasis (ICD-9-CM Code 494) newly confirmed by HRCT examination and with age ranging from 20 to 90 years visiting health care centers (outpatients or inpatients) within 2000–2008 constituted a bronchiectasis cohort. The date of bronchiectasis first diagnosed was used as the index year. The comparison cohort consisted of patients without bronchiectasis randomly selected from general population and 5:1 ratio frequency-matched with bronchiectasis cohort according to sex, age, and index year. Patients with ischemic stroke (ICD-9-CM Code 433-435) diagnosed before the index date were excluded in both cohorts.
Outcome measures
The outcome variable was ischemic stroke (ICD-9-CM Code 433-435) diagnosed and confirmed by CT or magnetic resonance image (MRI) examinations. CT or MRI is widely used to distinguish ischemic stroke from hemorrhagic stroke. The follow-up person-years (PYs) were calculated based on the interval between the index date and the date the ischemic stroke event first occurred or was censored: withdrawal from the NHI program, death, or the end of 2010 when the study terminated.
Covariates and comorbid diseases
The patients were divided into the three groups according to age: age <50 years as young adults, age 50–64 years as middle-aged adults, and age ≥65 years as elderly adults. The comorbid diseases reported to be risk factors of stroke, including hypertension (ICD-9-CM codes 401-405); diabetes (ICD-9-CM codes 250); hyperlipidemia (ICD-9-CM codes 272); CAD (ICD-9-CM codes 410-414) including myocardial infarction (ICD-9-CM codes 410 and 412), ischemic heart disease (ICD-9-CM codes 411 and 414), and angina pectoris (ICD-9-CM code 413); CHF (ICD-9-CM 428); COPD (ICD-9-CM 491, 492, 496); and AF (ICD-9-CM 427.31) were considered in this study.15–19
Statistical analyses
The Statistical Package for the Social Sciences (SPSS) Version 22.0 (IBM Corp., Armonk, NY, USA) was adopted for all statistical analyses. The χ2 test was used to compare and test difference in the proportional distribution of the demographic characteristics and comorbidities in both cohorts. The Student’s two sample t-test was used to measure and compare the mean ages of both cohorts. We evaluated the overall, age-specific, and comorbidity-specific incidence of ischemic stroke in both cohorts by follow-up PYs. Cox proportional hazard regression models were used to compare the hazard ratio (HR) and 95% CI of ischemic stroke development between patients with bronchiectasis and those without bronchiectasis. Nelson–Aalen analysis was adopted to evaluate the difference of cumulated probability of ischemic stroke between the two cohorts using XLSTAT add-on statistic software (Addinsoft Inc., Chicago, IL, USA). The significance level was set at P<0.05 at 2-tail.
Results
Demographic characteristics and comorbidities of patients with and without bronchiectasis
A total of 7,770 patients, including 1,295 patients with bronchiectasis (585 men and 710 women) and 6,475 patients without bronchiectasis (2,925 men and 3,550 women), have 6,312.70 and 34,958.00 follow-up PYs, respectively. The age and sex distribution of the patients in both cohorts were the same. The mean age of the patients in both cohorts was 62.0±15.3 years. The bronchiectasis cohort exhibited a significantly higher prevalence of CAD (16.8% vs 13.1%), CHF (8.0% vs 2.8%), COPD (48.8% vs 8.5%), and AF (1.9% vs 1.1%) than did the non-bronchiectasis cohort (Table 1). The patients with bronchiectasis had a significantly lower prevalence of being not comorbid with any medical disorders when compared with the patients without bronchiectasis (28.9% vs 48.7%, P<0.001).
Table 1.
Demographic characteristics and comorbidity in patients with and without bronchiectasis
| Variable | Bronchiectasis
|
P-value | |
|---|---|---|---|
| No
|
Yes
|
||
| N=6,475 | N=1,295 | ||
| Sex, n (%) | 1 | ||
| Female | 3,550 (54.8) | 710 (54.8) | |
| Male | 2,925 (45.2) | 585 (45.2) | |
| Age, mean (SD)a | 62.0 (15.3) | 62.0 (15.3) | 1 |
| Age stratification, n (%) | 1 | ||
| <50 years | 1,445 (22.3) | 289 (22.3) | |
| 50–64 years | 2,075 (32.0) | 415 (32.0) | |
| ≥65 years | 2,955 (45.6) | 591 (45.6) | |
| Comorbidity, n (%) | |||
| Hypertension | 2,399 (37.1) | 476 (36.8) | 0.842 |
| Diabetes | 1,018 (15.7) | 208 (16.1) | 0.759 |
| Hyperlipidemia | 986 (15.2) | 171 (13.2) | 0.62 |
| CAD | 849 (13.1) | 217 (16.8) | 0.001 |
| CHF | 181 (2.8) | 103 (8.0) | <0.001 |
| COPD | 553 (8.5) | 632 (48.8) | <0.001 |
| AF | 73 (1.1) | 25 (1.9) | 0.018 |
| Without any comorbidity | 3,151 (48.7) | 374 (28.9) | <0.001 |
Notes: χ2 test;
Student’s two sample t-test.
Abbreviations: SD, standard deviation; CAD, coronary artery disease; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; AF, atrial fibrillation.
Comparison of incidence and risk of ischemic stroke stratified by sex, age, and comorbidity between bronchiectasis and non-bronchiectasis patients
As indicated in Table 2, the patients with bronchiectasis exhibited a higher incidence rate of ischemic stroke (9.18 vs 4.66 per 1,000 PYs) than those without bronchiectasis with a crude HR of 1.93 (95% CI =1.43–2.61) and an adjusted HR (aHR) of 1.74 (95% CI =1.28–2.35) after controlling for age, sex, and comorbidities. The incidence rates of ischemic stroke increased with age in both cohorts. After adjusting for covariates, the risk of stroke was higher in the middle-aged patients (aHR =4.79, 95% CI =1.88–12.17) and elderly patients (aHR =11.76, 95% CI =4.76–29.07) compared to the younger patients. The risk of ischemic stroke was significantly higher in the bronchiectasis cohort than those in non-bronchiectasis cohort in both sexes (crude HR =1.98, 95% CI =1.32–2.97 for men and crude HR =1.89, 95% CI =1.21–2.96 for women). After adjusting for covariates, the risk of stroke was not significantly different in both sexes despite men exhibiting higher incidence of ischemic stroke than women.
Table 2.
Comparison of incidence and hazard ratio of ischemic stroke stratified by sex, age, and comorbidity between patients with and without bronchiectasis
| Variables | Bronchiectasis
|
Crude HRb (95% CI) | aHRc (95% CI) | |||||
|---|---|---|---|---|---|---|---|---|
| No
|
Yes
|
|||||||
| Event | PYs | Ratea | Event | PYs | Ratea | |||
| All | 163 | 34,958.00 | 4.66 | 58 | 6,312.70 | 9.18 | 1.93 (1.43–2.61)*** | 1.74 (1.28–2.35)*** |
| Sex | ||||||||
| Female | 73 | 19,220.60 | 3.80 | 26 | 3,559.90 | 7.30 | 1.89 (1.21–2.96)* | 1 |
| Male | 90 | 15,737.40 | 5.72 | 32 | 2,752.80 | 11.62 | 1.98 (1.32–2.97)** | 1.31 (0.99–1.70) |
| Age stratification, years | ||||||||
| <50 | 4 | 8,040.40 | 0.50 | 1 | 1,548.20 | 0.65 | 1.29 (0.14–11.57) | 1 |
| 50–64 | 34 | 11,290.80 | 3.01 | 9 | 2,098.10 | 4.29 | 1.42 (0.68–2.96) | 4.79 (1.88–12.17)** |
| ≥65 | 125 | 15,626.80 | 7.99 | 48 | 2,666.40 | 18.0 | 2.18 (1.56–3.04)*** | 11.76 (4.76–29.07)*** |
| Comorbidityd | ||||||||
| No | 34 | 17,260.50 | 1.97 | 4 | 1,970.30 | 2.03 | 1.02 (0.36–2.88) | 1 |
| Yes | 129 | 17,697.50 | 7.29 | 54 | 4,342.40 | 12.43 | 1.66 (1.21–2.28)** | 2.66 (1.85–3.84)*** |
Notes:
Incidence rate per 1,000 PYs;
relative hazard ratio;
multivariable analysis including age, sex, and comorbidities;
only have one of the comorbidities: hypertension, diabetes, hyperlipidemia, CAD, CHF, COPD, and AF.
P<0.05,
P<0.01, and
P<0.001.
Abbreviations: HR, hazard ratio; aHR, adjusted HR; CI, confidence interval; PYs, person-years; CAD, coronary artery disease; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; AF, atrial fibrillation.
Comparison of incidence and HR of ischemic stroke by individual comorbidity between bronchiectasis and non-bronchiectasis patients
The patients with bronchiectasis had a higher incidence of stroke than did the patients without bronchiectasis regardless of comorbidities. The incidence of stroke was higher in patients with one or more comorbidities, including hypertension, diabetes, hyperlipidemia, CAD, CHF, COPD, and AF, compared with the patients without the corresponding comorbidity. However, after adjustment for covariates, hypertension, diabetes, and AF remained independent risk factors of stroke development (Table 3).
Table 3.
Comparison of incidence and HR of ischemic stroke between patients with and without bronchiectasis by considering individual comorbidities
| Variables | Bronchiectasis
|
Crude HRb (95% CI) | aHRc (95% CI) | |||||
|---|---|---|---|---|---|---|---|---|
| No
|
Yes
|
|||||||
| Event | PYs | Ratea | Event | PYs | Ratea | |||
| Hypertension | ||||||||
| No | 63 | 22,409.60 | 2.81 | 18 | 4,179.70 | 4.31 | 1.52 (0.90–2.57) | 1 |
| Yes | 100 | 12,548.40 | 7.97 | 40 | 2,133.00 | 18.75 | 2.27 (1.57–3.27)*** | 1.62 (1.21–2.17)** |
| Diabetes | ||||||||
| No | 107 | 29,613.70 | 3.61 | 38 | 5,331.50 | 7.12 | 1.94 (1.34–2.81)*** | 1 |
| Yes | 56 | 5,344.30 | 10.47 | 20 | 981.20 | 21.02 | 1.90 (1.14–3.17)* | 1.97 (1.47–2.65)*** |
| Hyperlipidemia | ||||||||
| No | 132 | 29,857.40 | 4.42 | 46 | 5,545.20 | 8.29 | 1.84 (1.31–2.57)*** | 1 |
| Yes | 31 | 5,100.60 | 6.07 | 12 | 767.50 | 15.63 | 2.55 (1.31–4.98)*** | 0.88 (0.62–1.62) |
| CAD | ||||||||
| No | 120 | 30,369.50 | 3.95 | 38 | 5,337.50 | 7.12 | 1.77 (1.23–2.56)* | 1 |
| Yes | 43 | 4,588.50 | 9.37 | 20 | 975.20 | 22.55 | 2.08 (1.22–3.55)*** | 1.34 (0.98–1.83) |
| CHF | ||||||||
| No | 152 | 33,980.90 | 4.47 | 49 | 5,888.30 | 8.32 | 1.83 (1.32–2.52)*** | 1 |
| Yes | 11 | 977.10 | 11.25 | 9 | 424.40 | 21.22 | 1.79 (0.73–4.34) | 1.17 (0.72–1.91) |
| COPD | ||||||||
| No | 136 | 31,793.20 | 4.27 | 24 | 3,278.70 | 7.31 | 1.68 (1.09–2.60)** | 1 |
| Yes | 27 | 3,164.80 | 8.53 | 34 | 3,034.0 | 11.20 | 1.23 (0.74–2.05) | 1.14 (0.81–1.59) |
| AF | ||||||||
| No | 158 | 34,589.70 | 4.56 | 52 | 6,202.10 | 8.38 | 1.80 (1.31–2.46)*** | 1 |
| Yes | 5 | 368.30 | 13.57 | 6 | 110.60 | 54.24 | 3.84 (1.17–12.62)* | 1.92 (1.02–3.61)* |
Notes:
Incidence rate per 1,000 person-years;
relative hazard ratio;
multivariable analysis including age, sex, hypertension, diabetes, hyperlipidemia, CAD, COPD, AF, and bronchiectasis.
P<0.05,
P<0.01, and
P<0.001.
Abbreviations: HR, hazard ratio; aHR, adjusted HR; CI, confidence interval; PYs, person-years; CAD, coronary artery disease; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; AF, atrial fibrillation.
Joint effect of bronchiectasis and comorbidity on the risk of ischemic stroke
Table 4 lists the joint effect of bronchiectasis and individual comorbidity on the risk of ischemic stroke. Patients with bronchiectasis comorbid with diabetes, AF, or hypertension exhibited a multiplicative risk of ischemic stroke compared with patients with neither bronchiectasis nor comorbidity. The odds ratios of ischemic stroke associated with bronchiectasis and covariates, including sex, age, and comorbidity, are shown in Table 5. Patients with bronchiectasis, older age, or other comorbidity had significantly higher risk of ischemic stroke.
Table 4.
Cox proportional hazard regression analysis for the risk of ischemic stroke associated with the joint effect of bronchiectasis and individual comorbidity
| Variables | N | Event n | aHRa (95% CI) |
|
|---|---|---|---|---|
| Bronchiectasis | Diabetes | |||
| No | No | 5,457 | 107 | 1 |
| Yes | No | 1,087 | 38 | 2.00 (1.38–2.90)*** |
| No | Yes | 1,018 | 56 | 2.20 (1.59–3.05)*** |
| Yes | Yes | 208 | 20 | 4.42 (2.74–7.14)*** |
| Bronchiectasis | AF | |||
| No | No | 6,402 | 158 | 1 |
| Yes | No | 1,270 | 52 | 1.88 (1.37–2.57)*** |
| No | Yes | 73 | 5 | 1.89 (0.77–4.61) |
| Yes | Yes | 25 | 6 | 7.02 (3.10–15.9)*** |
| Bronchiectasis | Hypertension | |||
| No | No | 4,076 | 63 | 1 |
| Yes | No | 819 | 18 | 1.61 (0.95–2.72) |
| No | Yes | 2,399 | 100 | 1.79 (1.30–2.47)*** |
| Yes | Yes | 476 | 40 | 4.02 (2.69–6.02)*** |
Notes:
Multivariable analysis including age and sex;
P<0.001.
Abbreviations: aHR, adjusted hazard ratio; CI, confidence interval; AF, atrial fibrillation.
Table 5.
Odds ratios of ischemic stroke associated with bronchiectasis and covariates
| Variable | Crude odds ratio (95% CI) | Adjusted odds ratioa (95% CI) |
|---|---|---|
| Bronchiectasis | ||
| No | 1 | 1 |
| Yes | 1.82 (1.34–2.47)*** | 1.62 (1.19–2.22)** |
| Sex | ||
| Female | 1 | 1 |
| Male | 1.51 (1.16–1.98)** | 1.3 (0.99–1.71) |
| Age stratification, years | ||
| <50 | 1 | 1 |
| 50–64 | 6.08 (2.40–12.70)*** | 4.75 (1.86–12.09)** |
| ≥65 | 17.74 (7.28–43.23)*** | 11.5 (4.63–28.5)*** |
| Comorbidityb | ||
| No | 1 | 1 |
| Yes | 4.13 (2.91–5.88)*** | 2.29 (1.59–3.31)*** |
Notes:
Multivariable analysis including bronchiectasis, age, sex, and comorbidities;
only have one of the comorbidities: hypertension, diabetes, hyperlipidemia, CAD, CHF, COPD, and AF.
P<0.01 and
P<0.001.
Abbreviations: CI, confidence interval; CAD, coronary artery disease; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; AF, atrial fibrillation.
Association of risk of ischemic stroke and number of emergency department visits and hospitalizations in patients with bronchiectasis
Table 6 presents the incidence and risk of ischemic stroke in patients without bronchiectasis and patients with bronchiectasis based on the number of emergency department visits and hospitalizations a year. The risk of ischemic stroke in patients with bronchiectasis increased with the annual number of emergency department visits and hospitalizations (aHR =4.31, 95% CI =2.53–7.35 for 1–3 times/year; aHR =6.24, 95% CI =2.30–16.89 for >3 times/year) compared with those without bronchiectasis.
Table 6.
Comparison of ischemic stroke risk between bronchiectasis and comparison cohorts by annual number of emergency room visits and hospitalization due to acute respiratory infection
| Ischemic stroke
|
HR (95% CI)
|
|||
|---|---|---|---|---|
| n/N | Crude | Adjusteda | ||
| Non-bronchiectasis | 163/6,475 | 1 (Reference) | 1 (Reference) | |
| Bronchiectasis | ||||
| <1 | 39/1,128 | 1.44 (1.01–2.04) | 1.37 (0.96–1.95) | |
| 1–3 | 15/136 | 6.45 (3.80–11.0)*** | 4.31 (2.53–7.35)*** | |
| >3 | 4/31 | 8.13 (3.01–22.0)*** | 6.24 (2.30–16.89)*** | |
| P for trend | <0.001 | <0.001 | ||
Notes:
Multivariable analysis including age, sex, and comorbidities.
P<0.001.
Abbreviations: HR, hazard ratio; CI, confidence interval.
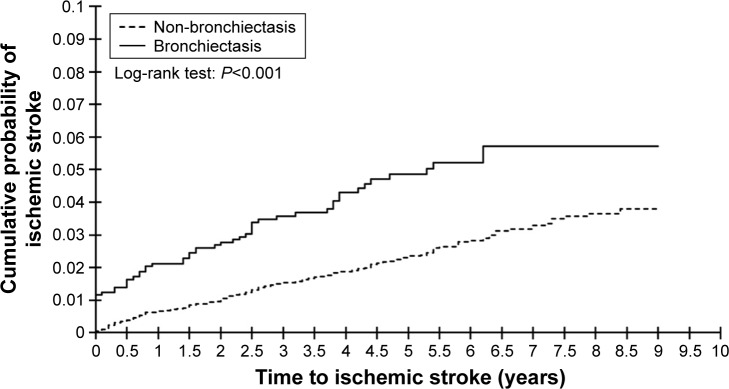
Cumulative probability of ischemic stroke between patients with and without bronchiectasis during follow-up periods
Figure 1 illustrated that the cumulative probability of ischemic stroke was significantly higher in the bronchiectasis cohort than in the comparison cohort (log-rank test, P<0.001).
Figure 1.
Nelson–Aalen analysis comparing the cumulative probabilities of ischemic stroke between patients with and without bronchiectasis.
Discussion, limitations, and conclusion
This longitudinal cohort study demonstrated that the incidence of ischemic infarction was higher in the bronchiectasis cohort than in the non-bronchiectasis cohort (9.18 vs 4.66 per 1,000 PYs). The patients with bronchiectasis exhibited a significantly (P<0.05) higher prevalence of CAD, CHF, COPD, and AF than did the patients without bronchiectasis, which is consistent to previous studies.15–19 After adjustment for sex, age, and comorbid disorders, the bronchiectasis cohort had a 1.74-fold increased risk of developing ischemic stroke compared with the non-bronchiectasis cohort. Similarly, Navaratnam et al6 also reported that people with bronchiectasis exhibited a 1.92-fold higher risk of stroke compared with those without bronchiectasis. However, in contrast to our study, they used a cross-sectional design rather than the cohort study to evaluate the prevalence of stroke in patients with bronchiectasis.6
The possible mechanisms for the increased risk of ischemic stroke need further elucidation. Bronchiectasis is characterized by chronic inflammation and inability to clear airway secretion, which results in recurrent infection. Previous studies have reported that patients with bronchiectasis are related to increased systemic inflammation.26,27 Inflammation also plays a pivotal role in the initiation and progression of atherosclerosis28 and is associated with vulnerable atherosclerotic plaque and subsequent thromboembolic events.29,30 The vulnerable atheromatous plaque in carotid arteries is responsible for acute ischemic stroke.31 In addition, observational studies have demonstrated that acute respiratory tract infections like influenza and Chlamydia pneumoniae infection are associated with the increased incidence and risk of stroke.32–36
The bronchiectasis cohort exhibited higher incidence of ischemic stroke than did the non-bronchiectasis cohort irrespective of sex, age subgroups, and comorbidity. Most patients with bronchiectasis were women (54.8%), a finding which is consistent with previous studies.37,38 The incidence of stroke increased with age. As presented in this study, 78.3% (173/221) of ischemic stroke occurred in elderly adults, which is consistent with previous reports.39,40 Compared to the young adults, middle-aged and elderly adults had 4.79- and 11.76-fold increased risks of stroke development, respectively. As evident in a previous study, the elderly have a high incidence of carotid artery stenosis, which is a major risk factor of ischemic stroke.41 In addition, watershed stroke is more common in the elderly adults than in the young adults, which is associated with the fact that the elderly have a higher prevalence of AF, hypertension, cardiovascular dysfunction, and other medical disorders.40,42–44
Hypertension, diabetes, and AF remained the independent risk factors of stroke development in the bronchiectasis and non-bronchiectasis cohorts. Bronchiectasis combined with diabetes, AF, or hypertension exhibited a multiplicative risk of ischemic stroke (Table 3). Furthermore, regarding COPD, the incidence rate of stroke was significantly higher in bronchiectasis patients without COPD than in non-bronchiectasis patients without COPD (7.31 vs 4.27 per 1,000 PYs) (Table 3). Although not significant, the incidence rate of stroke was also higher in bronchiectasis patients with COPD than in non-bronchiectasis patients with COPD (11.20 vs 8.53 per 1,000 PYs). The bronchiectasis cohort still carried a 1.74-fold increased risk of developing ischemic stroke compared with the non-bronchiectasis cohort after adjustment for covariates, despite 50% of the bronchiectasis cohort having COPD in our dataset.
Furthermore, symptom overlap between COPD and bronchiectasis may complicate discriminative diagnoses of these two diseases. As evident in this study, among the patients with bronchiectasis, up to 48.8% also had COPD (Table 1). A similar observation was reported in previous studies that bronchiectasis coexisted in 30%–57% of patients with COPD,45–48 which significantly increased the duration of ICU stay and hospital admission46 as well as the mortality rate.4,48 On the other hand, a Germany study showed that, with bronchiectasis as the primary diagnosis, 39% of the hospitalized patients were found to be comorbid with COPD and emphysema.49 COPD and bronchiectasis share many features in common, presenting a similar inflammatory profile with symptoms of chronic cough and sputum production, and both sometimes overlap, complicating their discriminative diagnoses and treatments.50 Bronchiectasis is generally diagnosed with the presence of airway dilation and airway wall thickness based on HRCT examination, whereas COPD is diagnosed based on the manifestation of poorly reversible airflow obstruction, symptoms of shortness of breath, and exposure to cigarette smoke and pollutants.50 However, according to a UK study, some COPD patients also exhibited airway wall abnormality, while 81% of patients diagnosed with bronchiectasis were current smokers or had a history of smoking.45 Moreover, a recent study confirmed airway wall abnormality, typically manifested in bronchiectasis, in patients with COPD exacerbation.48 High prevalence rate and great severity of bronchiectasis presented in COPD patients suggested bronchiectasis as a pathological phenotype of COPD, which may have prognostic and therapeutic implications.51
Patients with bronchiectasis who had respiratory infection-related ED visits and hospitalization exhibited a dose–response effect on the risk of ischemic stroke. The finding may explain the relationship of severity of bronchiectasis and frequent respiratory infections with an increased risk of developing stroke.
Several limitations should be addressed in the current study. First, the detailed smoking habits and physical activity levels are not available in the LHID. Hypertension and COPD are well-recognized comorbidities associated with cigarette smoking.52,53 Therefore, we controlled for hypertension and COPD to mediate the influence of smoking. Second, although diagnoses based on ICD-9-CM codes may cause misclassification, we used HRCT to help validate the diagnosis of bronchiectasis and ischemic stroke. Furthermore, the NHIA routinely audits the claims data through an administrative and peer-review process to minimize medical fraud.
The strength of our study is that it is a large population-based sample: this is the first study on Asian people to evaluate the incidence and risk of ischemic stroke in patients with bronchiectasis. All NHI beneficiaries are assigned personal identification numbers that facilitate follow-up of the study patients.
In conclusion, this cohort study of 1,295 patients (6,312.70 PYs) with bronchiectasis and 6,475 patients (34,958.00 PYs) without bronchiectasis demonstrated that bronchiectasis patients carried a 1.74-fold increased risk of ischemic stroke compared with those without bronchiectasis. Patients with bronchiectasis experiencing frequent respiratory infections are at a substantial risk of ischemic stroke. Clinicians should take proactive strategy to carefully assess risk factors of stroke and holistically treat patients with bronchiectasis.
Acknowledgments
This study was supported in part by Ministry of Science and Technology of Taiwan (MOST104-2410-H-166-005 and MOST105-2410-H-166-006) Taichung Hospital, Ministry of Health and Welfare, as well as Central Taiwan University of Science and Technology. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Author contributions
All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.McShane PJ, Naureckas ET, Tino G, Strek ME. Non-cystic fibrosis bronchiectasis. Am J Respir Crit Care Med. 2013;188(6):647–656. doi: 10.1164/rccm.201303-0411CI. [DOI] [PubMed] [Google Scholar]
- 2.Chalmers JD, Goeminne P, Aliberti S, et al. The bronchiectasis severity index. An international derivation and validation study. Am J Respir Crit Care Med. 2014;189(5):576–585. doi: 10.1164/rccm.201309-1575OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dodd JD, Lavelle LP, Fabre A, Brady D. Imaging in cystic fibrosis and non-cystic fibrosis bronchiectasis. Semin Respir Crit Care Med. 2015;36(2):194–206. doi: 10.1055/s-0035-1546749. [DOI] [PubMed] [Google Scholar]
- 4.Goeminne PC, Nawrot TS, Ruttens D, Seys S, Dupont LJ. Mortality in non-cystic fibrosis bronchiectasis: a prospective cohort analysis. Respir Med. 2014;108(2):287–296. doi: 10.1016/j.rmed.2013.12.015. [DOI] [PubMed] [Google Scholar]
- 5.Lavery K, O’Neill B, Elborn JS, Reilly J, Bradley JM. Self-management in bronchiectasis: the patients’ perspective. Eur Respir J. 2007;29(3):541–547. doi: 10.1183/09031936.00057306. [DOI] [PubMed] [Google Scholar]
- 6.Navaratnam V, Millett ER, Hurst JR, et al. Bronchiectasis and the risk of cardiovascular disease: a population-based study. Thorax. 2017;72(2):161–166. doi: 10.1136/thoraxjnl-2015-208188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Libby P. Inflammation in atherosclerosis. Nature. 2002;420(6917):868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 8.Del Rincon I, O’Leary DH, Freeman GL, Escalante A. Acceleration of atherosclerosis during the course of rheumatoid arthritis. Atherosclerosis. 2007;195(2):354–360. doi: 10.1016/j.atherosclerosis.2006.09.027. [DOI] [PubMed] [Google Scholar]
- 9.Del Rincon I, Williams K, Stern MP, Freeman GL, O’Leary DH, Escalante A. Association between carotid atherosclerosis and markers of inflammation in rheumatoid arthritis patients and healthy subjects. Arthritis Rheum. 2003;48(7):1833–1840. doi: 10.1002/art.11078. [DOI] [PubMed] [Google Scholar]
- 10.Kobayashi H, Giles JT, Polak JF, et al. Increased prevalence of carotid artery atherosclerosis in rheumatoid arthritis is artery-specific. J Rheumatol. 2010;37(4):730–739. doi: 10.3899/jrheum.090670. [DOI] [PubMed] [Google Scholar]
- 11.Di Carlo A. Human and economic burden of stroke. Age Ageing. 2009;38(1):4–5. doi: 10.1093/ageing/afn282. [DOI] [PubMed] [Google Scholar]
- 12.Mukherjee D, Patil CG. Epidemiology and the global burden of stroke. World Neurosurg. 2011;76(Suppl 6):S85–S90. doi: 10.1016/j.wneu.2011.07.023. [DOI] [PubMed] [Google Scholar]
- 13.WHO . The Top 10 Causes of Death. Geneva: World Health Organization; 2014. [Google Scholar]
- 14.Hsiao AJ, Chen LH, Lu TH. Ten leading causes of death in Taiwan: a comparison of two grouping lists. J Formos Med Assoc. 2015;114(8):679–680. doi: 10.1016/j.jfma.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 15.Wolf PA, D’Agostino RB, Belanger AJ, Kannel WB. Probability of stroke: a risk profile from the Framingham Study. Stroke. 1991;22(3):312–318. doi: 10.1161/01.str.22.3.312. [DOI] [PubMed] [Google Scholar]
- 16.Air EL, Kissela BM. Diabetes, the metabolic syndrome, and ischemic stroke: epidemiology and possible mechanisms. Diabetes Care. 2007;30(12):3131–3140. doi: 10.2337/dc06-1537. [DOI] [PubMed] [Google Scholar]
- 17.Shindo A, Tomimoto H. Diabetes and ischemic stroke. Brain Nerve. 2014;66(2):107–119. [PubMed] [Google Scholar]
- 18.Lewis A, Segal A. Hyperlipidemia and primary prevention of stroke: does risk factor identification and reduction really work? Curr Atheroscler Rep. 2010;12(4):225–229. doi: 10.1007/s11883-010-0117-4. [DOI] [PubMed] [Google Scholar]
- 19.Mitchell AB, Cole JW, McArdle PF, et al. Obesity increases risk of ischemic stroke in young adults. Stroke. 2015;46(6):1690–1692. doi: 10.1161/STROKEAHA.115.008940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Donaldson GC, Hurst JR, Smith CJ, Hubbard RB, Wedzicha JA. Increased risk of myocardial infarction and stroke following exacerbation of COPD. Chest. 2010;137(5):1091–1097. doi: 10.1378/chest.09-2029. [DOI] [PubMed] [Google Scholar]
- 21.Portegies ML, Lahousse L, Joos GF, et al. Chronic obstructive pulmonary disease and the risk of stroke. The Rotterdam Study. Am J Respir Crit Care Med. 2016;193(3):251–258. doi: 10.1164/rccm.201505-0962OC. [DOI] [PubMed] [Google Scholar]
- 22.NHIA . National Health Insurance. 2015. [Accessed June 13, 2016]. [Google Scholar]
- 23.Hsieh CY, Chen CH, Li CY, Lai ML. Validating the diagnosis of acute ischemic stroke in a National Health Insurance claims database. J Formos Med Assoc. 2015;114(3):254–259. doi: 10.1016/j.jfma.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 24.Cheng CL, Kao YH, Lin SJ, Lee CH, Lai ML. Validation of the National Health Insurance Research Database with ischemic stroke cases in Taiwan. Pharmacoepidemiol Drug Saf. 2011;20(3):236–242. doi: 10.1002/pds.2087. [DOI] [PubMed] [Google Scholar]
- 25.Chung WS, Lin CL, Kao CH. Bronchiectasis and the risk of cancer: a nationwide retrospective cohort study. Int J Clin Pract. 2015;69(6):682–688. doi: 10.1111/ijcp.12599. [DOI] [PubMed] [Google Scholar]
- 26.Wilson CB, Jones PW, O’Leary CJ, et al. Systemic markers of inflammation in stable bronchiectasis. Eur Respir J. 1998;12(4):820–824. doi: 10.1183/09031936.98.12040820. [DOI] [PubMed] [Google Scholar]
- 27.Martinez-Garcia MA, Perpina-Tordera M, Roman-Sanchez P, et al. The association between bronchiectasis, systemic inflammation, and tumor necrosis factor alpha. Archivos de bronconeumologia. 2008;44(1):8–14. doi: 10.1016/s1579-2129(08)60003-8. [DOI] [PubMed] [Google Scholar]
- 28.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105(9):1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 29.Tang TY, Howarth SP, Miller SR, et al. Comparison of the inflammatory burden of truly asymptomatic carotid atheroma with atherosclerotic plaques contralateral to symptomatic carotid stenosis: an ultra small superparamagnetic iron oxide enhanced magnetic resonance study. J Neurol Neurosurg Psychiatry. 2007;78(12):1337–1343. doi: 10.1136/jnnp.2007.118901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nighoghossian N, Derex L, Douek P. The vulnerable carotid artery plaque: current imaging methods and new perspectives. Stroke. 2005;36(12):2764–2772. doi: 10.1161/01.STR.0000190895.51934.43. [DOI] [PubMed] [Google Scholar]
- 31.Naghavi M, Libby P, Falk E, et al. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: Part II. Circulation. 2003;108(15):1772–1778. doi: 10.1161/01.CIR.0000087481.55887.C9. [DOI] [PubMed] [Google Scholar]
- 32.Paganini-Hill A, Lozano E, Fischberg G, et al. Infection and risk of ischemic stroke: differences among stroke subtypes. Stroke. 2003;34(2):452–457. doi: 10.1161/01.str.0000053451.28410.98. [DOI] [PubMed] [Google Scholar]
- 33.Heuschmann PU, Neureiter D, Gesslein M, et al. Association between infection with Helicobacter pylori and Chlamydia pneumoniae and risk of ischemic stroke subtypes: results from a population-based case-control study. Stroke. 2001;32(10):2253–2258. doi: 10.1161/hs1001.097096. [DOI] [PubMed] [Google Scholar]
- 34.Elkind MS, Lin IF, Grayston JT, Sacco RL. Chlamydia pneumoniae and the risk of first ischemic stroke: The Northern Manhattan Stroke Study. Stroke. 2000;31(7):1521–1525. doi: 10.1161/01.str.31.7.1521. [DOI] [PubMed] [Google Scholar]
- 35.Asghar Z, Coupland C, Siriwardena N. Influenza vaccination and risk of stroke: self-controlled case-series study. Vaccine. 2015;33(41):5458–5463. doi: 10.1016/j.vaccine.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 36.Siriwardena AN, Asghar Z, Coupland CC. Influenza and pneumococcal vaccination and risk of stroke or transient ischaemic attack-matched case control study. Vaccine. 2014;32(12):1354–1361. doi: 10.1016/j.vaccine.2014.01.029. [DOI] [PubMed] [Google Scholar]
- 37.Pasteur MC, Bilton D, Hill AT. British Thoracic Society guideline for non-CF bronchiectasis. Thorax. 2010;65(Suppl 1):i1–i58. doi: 10.1136/thx.2010.136119. [DOI] [PubMed] [Google Scholar]
- 38.Trow TK. Clinical year in review II: occupational lung disease, pulmonary vascular disease, bronchiectasis, and chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2006;3(7):557–560. doi: 10.1513/pats.200606-135TT. [DOI] [PubMed] [Google Scholar]
- 39.Feigin VL, Lawes CM, Bennett DA, Anderson CS. Stroke epidemiology: a review of population-based studies of incidence, prevalence, and case-fatality in the late 20th century. Lancet Neurol. 2003;2(1):43–53. doi: 10.1016/s1474-4422(03)00266-7. [DOI] [PubMed] [Google Scholar]
- 40.Chen RL, Balami JS, Esiri MM, Chen LK, Buchan AM. Ischemic stroke in the elderly: an overview of evidence. Nat Rev Neurol. 2010;6(5):256–265. doi: 10.1038/nrneurol.2010.36. [DOI] [PubMed] [Google Scholar]
- 41.de Weerd M, Greving JP, de Jong AW, Buskens E, Bots ML. Prevalence of asymptomatic carotid artery stenosis according to age and sex: systematic review and metaregression analysis. Stroke. 2009;40(4):1105–1113. doi: 10.1161/STROKEAHA.108.532218. [DOI] [PubMed] [Google Scholar]
- 42.Kannel WB, Benjamin EJ. Status of the epidemiology of atrial fibrillation. Med Clin North Am. 2008;92(1):17–40. ix. doi: 10.1016/j.mcna.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hammami S, Mehri S, Hajem S, Koubaa N, Souid H, Hammami M. Prevalence of diabetes mellitus among non institutionalized elderly in Monastir City. BMC Endocr Disord. 2012;12:15. doi: 10.1186/1472-6823-12-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ruff CT. Cardiology patient page: stroke prevention in atrial fibrillation. Circulation. 2012;125(16):e588–e590. doi: 10.1161/CIRCULATIONAHA.111.067843. [DOI] [PubMed] [Google Scholar]
- 45.O’Brien C, Guest PJ, Hill SL, Stockley RA. Physiological and radiological characterisation of patients diagnosed with chronic obstructive pulmonary disease in primary care. Thorax. 2000;55(8):635–642. doi: 10.1136/thorax.55.8.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gursel G. Does coexistence with bronchiectasis influence intensive care unit outcome in patients with chronic obstructive pulmonary disease? Heart Lung. 2006;35(1):58–65. doi: 10.1016/j.hrtlng.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 47.Patel IS, Vlahos I, Wilkinson TM, et al. Bronchiectasis, exacerbation indices, and inflammation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2004;170(4):400–407. doi: 10.1164/rccm.200305-648OC. [DOI] [PubMed] [Google Scholar]
- 48.Martinez-Garcia MA, de la Rosa Carrillo D, Soler-Cataluna JJ, et al. Prognostic value of bronchiectasis in patients with moderate-to-severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;187(8):823–831. doi: 10.1164/rccm.201208-1518OC. [DOI] [PubMed] [Google Scholar]
- 49.Ringshausen FC, de Roux A, Pletz MW, Hamalainen N, Welte T, Rademacher J. Bronchiectasis-associated hospitalizations in Germany, 2005–2011: a population-based study of disease burden and trends. PloS one. 2013;8(8):e71109. doi: 10.1371/journal.pone.0071109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hurst JR, Elborn JS, De Soyza A. COPD-bronchiectasis overlap syndrome. Eur Respir J. 2015;45(2):310–313. doi: 10.1183/09031936.00170014. [DOI] [PubMed] [Google Scholar]
- 51.Stockley RA. Bronchiectasis with chronic obstructive pulmonary disease: association or a further phenotype? Am J Respir Crit Care Med. 2013;187(8):786–788. doi: 10.1164/rccm.201302-0203ED. [DOI] [PubMed] [Google Scholar]
- 52.Virdis A, Giannarelli C, Neves MF, Taddei S, Ghiadoni L. Cigarette smoking and hypertension. Curr Pharm Des. 2010;16(23):2518–2525. doi: 10.2174/138161210792062920. [DOI] [PubMed] [Google Scholar]
- 53.Eisner MD, Anthonisen N, Coultas D, et al. An official American Thoracic Society public policy statement: novel risk factors and the global burden of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2010;182(5):693–718. doi: 10.1164/rccm.200811-1757ST. [DOI] [PubMed] [Google Scholar]