Abstract
Background
The study was conducted to investigate potential association between MTHFR genotypes and diabetic peripheral neuropathy (DPN) in Puerto Ricans with type-2 diabetes mellitus (T2DM) treated with metformin. The prevalence of major MTHFR polymorphisms in this cohort was also ascertained.
Methods
DNAs from 89 metformin-treated patients with T2DM and DPN were genotyped using the PCR-based RFLP assay for MTHFR677C > T and 1298A > C polymorphisms. Frequency distributions of these variants in the study cohort were compared to those reported for three reference populations (HapMap project) and controls (400 newborn specimens). Chi-square (or Fischer’s exact) tests and odds ratios (OR) were used to assess association with DPN susceptibility risk (patients vs. controls) and biochemical markers (wild types vs. carriers).
Results
Sixty-seven percent (67%) of participants carry at least one of these MTHFR polymorphisms. No deviations from Hardy-Weinberg equilibrium were detected. The genotype and allele frequencies showed statistically significant differences between participants and controls (p < 0.0001 and p = 0.03, respectively). Results suggest that 1298A > C but not 677C > T is associated with DPN susceptibility in this cohort (p = 0.018). Different patterns of allelic dissimilarities are observed when comparing our cohort vs. the three parental ancestries. After sorting individuals by their carrier status, no significant associations were observed between these genetic variants (independently or combined) and any of the biochemical markers (HbA1c, folate, vitamin B12, homocysteine).
Conclusions
Prevalence of major MTHFR variants in Puerto Rican patients with T2DM is first time ever reported. The study provides further evidence on the use of this genetic marker as an independent risk factor for DPN.
Keywords: diabetic peripheral neuropathy, methylenetetrahydrofolate reductase (MTHFR), pharmacogenetics, Puerto Ricans, type-2 diabetes mellitus
Introduction
Diabetic peripheral neuropathy (DPN) is a common, chronic complication of type-2 diabetes mellitus (T2DM), where significant damage to peripheral nerves in extremities occurs and is usually associated with microvascular injuries [1, 2]. DPN affects many T2DM patients, causing sensory, motor, and autonomic dysfunctions [3]. At least one manifestation of DPN is present in ~ 20% of adults with T2DM. Several epidemiological studies assessed DPN among patients with T2DM and reported prevalence rates of 26%–47% [3]. In Puerto Rico, the prevalence of T2DM is much higher than in US mainland and other countries, achieving levels of 12.9% during 2009–2014 [4–6]. It is estimated that, from a total population of approximately 3.5 million, nearly 100,000 patients could have DPN [4–6]. The total annual cost of DPN and its complications was estimated to be between 4.6 and 13.7 billion US dollars. Up to 27% of the direct medical cost of T2DM may be attributed to DPN [7]. In the US, workers who have T2DM with neuropathic symptoms lose the equivalent of 3.65 billion US dollars/year in health-related lost productive time (LPT) [8].
Symptomatic treatments are available for the manifestations of DPN and autonomic neuropathy [1]. More effective disease-modifying treatments should be directed to correct the underlying pathogenesis of DPN, which has not yet been fully understood. The causes of DPN are thought to be multi-factorial, encompassing both metabolic and ischemic processes related to hyperglycemia, which increasingly deteriorates tissues. Vascular complications are among the most common causative factors in T2DM morbimortality, and they have also been related to DPN [9]. A risk factor for vasculopathies in T2DM is the elevated blood levels of homocysteine (Hcy). Some earlier reports suggest an association between hyperhomocysteinemia in T2DM patients and the prevalence of DPN [9–12].
Methylenetetrahydrofolate reductase (MTHFR) is the key enzyme in the metabolic pathway that converts Hcy back to methionine via remethylation [13]. Therefore, a reduced MTHFR activity increases the odds for a hyperhomocysteinemia [14]. The polymorphic MTHFR gene, encoding for the MTHFR enzyme, is mapped to chromosome 1p36.3. Genetic variations in this gene influence susceptibility to many health conditions [14–18], and common mutations at this locus are associated with MTHFR deficiency [19–21]. The most common, naturally occurring nonsynonymous coding single nucleotide polymorphisms (SNPs) on MTHFR are two-point “missense” mutations (i.e. dbSNP rs1801133: C → T substitution at nucleotide 677, p.Ala222Val, and dbSNP rs1801131: A → C substitution at position 1298, p.Glu429Ala) resulting in up to 50% reduction of enzymatic activity. Although the 677C > T and 1298A > C variants were initially reported to occur at nucleotides 677 and 1298, respectively, and continue to be referred to as such in the literature, the actual locations are at nucleotide 665 (i.e. c.665C>T) and at nucleotide 1289 (i.e. c.1286A>C) of the coding region.
Homozygous for 677C > T only shows 10%–20% efficiency in processing folic acid, resulting in high homocysteine and low vitamin B12 and folate levels. A recent meta-analysis by Wu et al. [22] showed that the 677C > T polymorphism may be the main risk factor for DPN in Turkish but not in Japanese and Pakistani. Associations between this mutation and DPN were found significant under both the allele (OR = 1.43, 95% CI: 1.08–1.90, p = 0.014) and the dominant models (p < 0.05). In addition, a study by Yigit et al. [9] found a significant association between the MTHFR677C > T mutation and DPN in Turkish patients with T2DM (p = 0.04). However, no studies have been focused on this association in Caribbean Hispanics. This study was aimed at assessing the potential association of MTHFR677C > T and 1298A > C polymorphisms with diabetic neuropathy in Puerto Rican patients with T2DM treated with metformin. Additionally, we ascertained the frequency distribution of these two SNPs in the study cohort and investigated the possible relationship between observed MTHFR genotypes and some biochemical markers related to DPN.
Materials and methods
Participants
A convenience sample of 101 metformin-treated Puerto Rican patients suffering from T2DM and DPN was selected. All participants were recruited at Farmacia San José in Lares, Puerto Rico, through the pharmaceutical care and diabetes education service (PCDES). Protocol approval (#A4070112) was granted by the university of Puerto Rico medical sciences campus (UPR-MSC) Institutional Committee for protection of human participants in clinical research (IRB). All experimental procedures were conducted according to the principles expressed in the Declaration of Helsinki. Written informed consent was obtained from each participant prior to enrollment.
Patients met eligibility criteria if they are Puerto Ricans having T2DM diagnosis, DPN, and metformin treatment for at least the last 6 months. Patients were individually diagnosed by licensed well-experienced endocrinologists, based on medical judgments and criteria that correspond to the ICD-9-CM Diagnosis Code 250.60/337.1 for diabetes with neurological manifestations, type II or unspecified type (equivalent to ICD-10-CM E11.40). Patients were excluded if they are women of childbearing potential not using contraception and with uncontrolled hypertension, renal disease, or impaired hepatic function. Patients with severe complications related to poor glucose control as amputations, foot ulcers, dialysis, and legal blindness related to sever diabetic retinopathy were also excluded. Upon recruitment, participants were asked to complete an on-site questionnaire and have their medical records reviewed.
A 5-mL small whole fresh blood sample was collected from each participant in EDTA vacutainers tube at the time of routinely scheduled glucose testing. Human genomic DNA was extracted and purified from fresh whole blood collected from peripheral leukocytes using the corresponding GentraPuregene Blood Kit (Qiagen-Inc., Valencia, CA, USA), following the manufacturer’s protocol. Extracted DNA samples were separated into fractions, and duplicates were stored at – 80 °C in TRIS-EDTA (TE) buffer for retrieval as needed. Quantification of DNA was performed by NanoDrop® Spectrophotometer (ThermoScientific, Wilmington, DE, USA). The concentration of extracted DNA was adjusted to 90 ng/μL (volume is 50 μL; OD260 = 0.25 absorbance unit per cm) in DNase-free distilled water. Investigators were blinded as to genotype information, as DNA analysis was not performed until data abstraction was completed.
Genotyping
The DNA analysis for the most common polymorphisms in the 5,10 methylenetetrahydrofolate reductase (MTHFR) gene (677C > T and 1298A > C) was performed by restriction fragment length polymorphisms (RFLP) using restriction enzyme digestion of the PCR product (e.g. HinF1), as described by Frosst et al. [14]. Experimental errors were circumvented by ensuring proper DNA extraction and sample’s handling (using master and working stocks), performing duplicates and testing departure from HWE under the null hypothesis of the predictable segregation ratio of specific matching genotypes (p > 0.05) by use of χ2 goodness-of-fit test.
Clinical variables
All relevant clinical non-genetic and demographic data, including initiation of therapy (fixed date), metformin dose (mg), duration of T2DM and DPN, age, ethnicity, gender, weight, socioeconomic status (SES), concurrent medications, and co-morbidities, were collected during the interview or retrieved from medical records. Continuous variables are presented as means (± SD), and dichotomous or categorical variables, as percentages (%). Comparison of baseline clinical and demographical parameters between carriers and wild types (Table 1) was performed using unpaired t-test (or Mann-Whitney test if normal distribution assumption is not met) for continuous variables and by Pearson’s χ2 (or Fisher’s exact test when applicable) for categorical variables.
Table 1.
Baseline clinical and demographical features of the 89 Puerto Rican patients with T2DM in this study.
| Characteristics | Total, n = 89 | Wild types (WT), n = 21 (24%) | Carriers, n = 68 (76%) | p-Valuea |
|---|---|---|---|---|
| Gender, # female/male (%) | 56/33 (63/37) | 14/7 (67/33) | 42/26 (62/38) | 0.682 |
| Age, years (mean ± SD) | 66 ± 11 | 65 ± 10 | 66 ± 11 | 0.912 |
| Height, cm (mean ± SD) | 162.4 ± 8.9 | 161 ± 7.3 | 162.6 ± 9.5 | 0.828 |
| Weight, kg (mean ± SD) | 78.2 ± 25.04 | 74 ± 14.5 | 79.6 ± 27.8 | 0.772 |
| BMI, kg/m2 (mean ± SD) | 29.6 ± 8.9 | 28.3 ± 5.3 | 30.0 ± 9.9 | 0.806 |
| Disease, T2DM duration, years (mean ± SD) | 13.8 ± 9.5 | 12.9 ± 10.1 | 14.1 ± 9.4 | 0.887 |
| History of DPN, years (mean ± SD) | 6.3 ± 5.7 | 4.18 ± 3.25 | 7.23 ± 6.42 | 0.503 |
| Smoking, n (%) | 12 (13.5) | 2 (9.5) | 10 (14.7) | 0.541 |
| History of HBP, n (%) | 67 (75.3) | 15 (71.4) | 52 (76.5) | 0.638 |
| Metformin use, years (mean ± SD) | 7.6 ± 6.4 | 8.38 ± 7.61 | 7.35 ± 6.04 | 0.863 |
BMI, body mass index; DPN, diabetes peripheral neuropathy; HBP, high blood pressure (hypertension); T2DM, type-2 diabetes mellitus. p-Values represent significance differences between carriers and wild types after comparison by using unpaired t-test (continuous variables) or χ2-test (categorical variables).
The results are not statistically different (p > 0.05) between carriers and wild types, at a significant level of 5%.
In addition, blood samples from a portion of the participants (n = 59) were used to measure four surrogate biochemical markers (i.e. homocysteine, glycosylated hemoglobin A1c, cyanocobalamin, and folate) at a CLIA-certified laboratory. Results are reported as continuous variables (means ± SD).
Data analysis
The 95% confidence intervals (CI) for allele and genotype frequencies (%) were calculated according to the method of Newcombe and Altman. Data were used for comparing the frequency distributions of observed genotypes in our study cohort to the frequencies published in available HapMap database, release#28, for three non-His-panic populations of reference (i.e. African-Americans, Americans, and Europeans) using z-test for independent proportions. Existing data pertaining to the reference populations were retrieved from SPSmart (i.e. the SNPs-for-Population-Studies-mart interface, http://spsmart.cesga.es/) web-based resource by user-defined queries [23]. In addition, genotypes and allele frequency distributions (95% CI) observed in our study cohort (cases) were also compared to those in an unmatched control group of 400 newborn samples (local repository) previously screened for these variants [unpublished data]. Chi-square (χ2) test and unadjusted odd ratios (OR) with 95% CIs were used to assess association of these MTHFR variants with DPN susceptibility risk between cases and controls. Noteworthy, DNA specimens from the newborn biobank that were used as controls in this study came from individuals with no family history of T2DM + DPN, who have not been diagnosed later on with any of these conditions.
The Wright’s Fst to SNP allele frequencies was applied to characterize differentiation at these two specific SNPs on MTHFR locus [24]. For the purpose of this study, population 1 corresponds to the Puerto Rican cohort of patients with T2DM, whereas population two corresponds to the reference parental population of African-Americans, Americans, or Europeans from the HapMap project, release #28 [23].
The extent of pharmacogenetic divergence between any two populations is denoted according to Wright’s quality criteria: Fst values less than 0.05 represent low genetic divergences; values between 0.05 and 0.15 represent moderate divergence; values between 0.15 and 0.25 represent large divergence; and values greater than 0.25, very large divergence. The statistical significance of differences in the distribution of polymorphism-specific Fst values across populations is calculated by ANOVA followed by pairwise Tukey test (5% significance level).
Relationships between MTHFR genotypes combined and each surrogate biochemical markers were independently evaluated using association analyses. For this purpose, the values were dichotomized (high/low levels) as follows: HbA1c levels ≥ or < 8%; folate levels ≥ or < 17.5 ng/mL; Hcy levels ≥ or < 12 μM; and vitamin B12 ≥ or < 450 pg/mL. Two groups were formed based on carrier status: wild types and carriers. No further stratification by type of carriers (i.e. single, double, triple, etc.) was made due to sample size limitations. Contingency tables measuring 2 × 2 were constructed, and the χ2 or Fischer’s exact tests were performed. The corresponding ORs and 95% CIs were calculated. Covariates such as metformin dose, gender, and age were added to the model one at a time to determine and, subsequently, adjust for potential confounders. The STATA statistical package (ver.11) was used for statistical analyses. Significance level was set at 5%.
Results
One hundred and one eligible patients with T2DM, who were receiving metformin orally over the last 6 weeks, were approached to participate in the study. All these subjects agreed to participate, resulting in 101 enrolled after completing a fully written consent process. A total of 12 patients were excluded from further genotyping analyses due to poor DNA quality or poor genotyping call rates (i.e. specimens from 89 patients were assessed). Regarding the association tests, results from only 59 samples are presented due to lack of complete data of biochemical markers from the rest of the patients. Table 1 summarizes the basic demographic and clinical non-genetic characteristics of our study cohort (n = 89). It is a single-center study, with participants coming from one geographic region of Puerto Rico (i.e. Mayaguez Health Region). Participants were of both genders. We enrolled patients with a similar socioeconomic status and health insurance coverage.
Loss-of-function 677C > T and 1298A > C polymorphisms were found in the study cohort at frequencies slightly different to those previously reported for Puerto Ricans [25]. Tables 2 and 3 show the allelic and genotype frequency distributions of these two polymorphisms on the MTHFR gene locus. Among all the patients, 60 (67%) had at least one polymorphism in MTHFR, and 5 (5.6%) are carriers of the two variants. Three patients are triple carriers of allele variants on this locus (i.e. TT at 677 + AC at 1298). None was a quadruple carrier. Concerning the 677C > T variant, there were 72 (80% ± 8.31%, 95% CI: 71.69% to 88.31%) participants with the wild-type CC genotype, eight heterozygous or single carrier of the variant genotype (9.9% ± 6.2%, 95% CI: 3.7% to 16.1%), and nine double carriers (TT) of the allele variant (10.1% ± 6.26%, 95% CI: 3.84% to 16.36%). The observed frequency of the T allele for this SNP was 14.6% (± 5.19, 95% CI: 9.41% to 19.79%).
Table 2.
Comparison of MTHFR677C > T genotypes and alleles between the study cohort and controls.
| MTHFR677C > T | Patients n = 89, % (95% CI) | Controls n = 400, % (95% CI) | Unadjusted-OR (95% CI) | p-Value |
|---|---|---|---|---|
| Genotypes | ||||
| CC | 72 (80.9; 71–88) | 184 (46.0; 41–51) | < 0.0001 | |
| CT | 8 (8.9; 4–17) | 159 (39.8; 35–45) | ||
| TT | 9 (10.1; 5–18) | 57 (14.2; 11–18) | ||
| CC + CT : TT | 80 (89.9; 81–95):9 (10.1; 5–18) | 343 (85.8; 82–89):57 (14.2; 11–18) | 0.68 (0.32–1.42) | 0.39 |
| CC:CT + TT | 72 (80.9; 71–88):17 (19.1; 12–29) | 184 (46.0; 41–51):216 (54.0; 49–59) | 0.20 (0.11–0.35) | < 0.0001 |
| Alleles | ||||
| C | 152 (85.4; 79–90) | 527 (65.9; 62–69) | 0.33 (0.21–0.51) | < 0.0001 |
| T | 26 (14.6; 10–21) | 273 (34.1; 31–37) |
Two-sided 95% CI for the single proportion includes continuity correction.
Table 3.
Comparison of MTHFR 1298A > C genotypes and alleles between the study cohort and controls.
| MTHFR 1298A > C | Patients n = 89, % (95% CI) | Controls n = 40, % (95% CI) | Unadjusted-OR (95% CI) | p-Value |
|---|---|---|---|---|
| Genotypes | ||||
| AA | 41 (46.1; 36–57) | 251(62.7; 58–67) | 0.03 | |
| AC | 43 (48.3; 37–59) | 138 (34.5; 30–39) | ||
| CC | 1 (1.1; 0.2–6) | 11 (2.8; 1.5–5) | ||
| Unknown | 4 (4.5; 1.5–11) | |||
| AA + AC:CC | 84 (94.4; 87–98):1 (1.1; 0.2–6) | 389 (97.2; 95–98):11 (2.8; 1.5–5) | 0.42 (0.05–3.30) | 0.49 |
| AA: AC + CC | 41 (46.1; 36–57):44 (49.4; 39–60) | 251 (62.7; 58–67):149 (37.3; 32–42) | 1.81 (1.13–2.90) | 0.0182 |
| Alleles | ||||
| A | 124 (73.0; 65–79) | 640 (80.0; 77–82) | 1.48 (1.01–2.17) | 0.05 |
| C | 46 (27.0; 20 – 34) | 160 (20.0; 17–23) | ||
| Combined heterozygosity | ||||
| 1298AC + 677CT | 2 (2.2; 0.4–8) | 48 (12.0; 9–15) | 0.18 (0.04–0.74) | 0.0139 |
| Combined genotypes | ||||
| 1298AA/677CC: 1298AC + CC/677CT + TT | 27 (32.0; 22–43): 5 (5.6; 2–13) | N/A |
N/A, not available. Combined heterozygosity and genotypes also shown. Two-sided 95% CI for the single proportion includes continuity correction.
For the 1298A > C variant, a lower proportion of double carriers but a larger frequency of single carriers than those carrying the 677C > T variant were found. Indeed, we observed a frequency of 27% ( ± 9.07, 95% CI: 17.93% to 36.07%) for the C allele in the SNP at position 1298 of the MTHFR gene. Furthermore, the frequency distribution of genotypes at this SNP was calculated as follows: 48% of wild-type AA ( ± 10.21, 95% CI: 37.79% to 58.21%), 51% of single carriers or heterozygous AC ( ± 10.21, 95% CI: 40.79% to 61.21%), and 1% of CC double carrier ( ± 2.03, 95% CI: − 1.03% to 3.03%). No deviations from Hardy-Weinberg equilibrium were detected (χ2 = 36.4, p < 0.005).
Statistically significant differences in genotype and allele frequency distribution of these two common MTHFR gene variants were found between T2DM patients with DPN and the control group (p < 0.0001, OR = 0.33, 95% CI: 0.21–0.51, Table 2; p = 0.03 and 0.05, OR = 1.48, 95% CI: 1.01–2.17, Table 3, respectively). Significance was also noted when patients and controls were compared with respect to their carrier status (i.e. 677CC vs. CT + TT genotypes, p < 0.0001, OR = 0.20, 95% CI: 0.11–0.35, Table 2, and 1298AA vs. AC + CC genotypes, p = 0.0182, OR = 1.81, 95% CI: 1.13–2.90, Table 3, respectively).
Table 4 shows the results of comparative analyses (z-tests) of the MTHFR677C > T and 1298A > C minor allele frequencies (MAF) between the study cohort of Puerto Rican patients and the corresponding reference values in Africans, Americans, and Europeans populations from the HapMap project database [23]. For the 677C > T polymorphism, the observed MAF better resembled that in Africans (− 0.6077, p = 0.5434) but not those early reported in Americans (0.3347, p < 0.0001) and Europeans (4.562, p < 0.0001). On the other hands, z-test results for the MAF at the MTHFR1298A > CSNP revealed a slightly but still significant difference between Puerto Ricans and Africans (2.1214, p = 0.0339), although not statistically significant differences with respect to Americans (1.6548, p = 0.098) and Europeans (0.9596, p = 0.3373) were found. Interestingly, the two MTHFR polymorphisms showed different patterns of allelic dissimilarities when comparing our study cohort of T2DM patients vs. the three main parental ancestries, a result that seems to confirm locus-specific admixture of Puerto Ricans.
Table 4.
Comparative analysis of MTHFR677C > T and 1298A > C MAFs: Puerto Ricans vs. African, American, and European Populations (n = 89) using Z-test, 5% significance.
| MTHFR locus | Alleles | Puerto Ricans vs. African-Americans
|
Puerto Ricans vs. Americans
|
Puerto Ricans vs. Europeans
|
|||
|---|---|---|---|---|---|---|---|
| Fst/Wright’s criterion | Z-statistic/p-value | Fst/Wright’s criterion | Z-statistic/p-value | Fst/Wright’s criterion | Z-statistic/p-value | ||
| 677 | C → T | < 0.05 | − 0.608 | > 0.25 | 0.335 | > 0.25 | 4.562 |
| Low divergence | 0.5434 | Very large divergence | < 0.0001 | Very large divergence | < 0.0001 | ||
| 1298 | A → C | 0.05–0.15 | 2.12 | 0.05–0.15 | 1.65 | < 0.05 | 0.96 |
| Moderate divergence | 0.034 | Moderate divergence | 0.098 | Low divergence | 0.34 | ||
Frequencies in these reference parental populations were taken from the HapMap project database [23]. Δ Fst values and the corresponding Wright’s qualitative criterion (i.e. extent of pharmacogenetic divergence between any two populations) are presented.
Table 5 represents the distribution in the study cohort of observed haplotypes for these two SNPs on the MTHFR locus. There are 27 wild types for both polymorphisms (CC/AA, 32%), 40 single carriers for the 1298A > C variant (CC/AC, 47%), and six single carriers for the 677C > TSNP (CT/AA, 7%). Among double carriers, two correspond to combined heterozygous for both variant alleles (CT/AC, 2%), whereas six are homozygous for the allele variant at position 677 (TT/AA, 7%) and one homozygous for the variant at position 1298 (CC/CC, 1%). Finally, three participants in the study were sorted out as TT/AC triple carriers (4%), but none of them were homozygous for the allele variants at both SNP positions combined (i.e. quadruple carriers, TT/CC). Neither did we identify any CT/CC triple carrier within the study sample. Haplotype analysis suggested that these two polymorphisms are not in strong linkage disequilibrium (R < 0.5, D = 0), they rather segregate independently from each other.
Table 5.
Haplotype distribution in the study cohort for the two common SNPs on the MTHFR locus.
| Diplotypes | Counts | Frequency, % (95% CI) |
|---|---|---|
| CC/AA | 27 | 30.3% (21.0–41.0) |
| CC/AC | 40 | 45% (35.0–56.0) |
| CC/CC | 1 | 1.12% (0.2–7.0) |
| CT/AA | 6 | 6.74% (3.0–14.0) |
| CT/AC | 2 | 2.25% (0.4–8.0) |
| CT/CC | 0 | |
| TT/AA | 6 | 6.74% (3.0–14.0) |
| TT/AC | 3 | 3.37% (0.9–10.0) |
| TT/CC | 0 | |
| Unknown | 4 | 4.5% (1.5–11.0) |
Frequencies are represented as percentage (%) of the overall group of patients with T2DM (n = 89). Two-sided 95% CI for the single proportion includes continuity correction.
Association analysis between MTHFR genotypes and biochemical markers
Table 6 shows the results of testing associations between 677C > T and 1298A > CMTHFR genotypes combined and the high/low levels of HbA1c, folate, vitamin B12, and Hcy laboratory biomarkers, respectively. The odds ratio (OR) of the association with HbA1c was 3.9, suggesting that patients who carry at least one of these two polymorphisms might hold an increased risk of having HbA1c level higher than 8%. However, such an association was not found to be significant under the current experimental conditions as determined by the Fisher’s exact test (p = 0.09). Although not statistically significant yet (p = 0.33), a similar trend was observed for the folate biomarker, with an OR of 1.9, which suggests an increased risk of almost double to have folate deficiency (i.e. < 17.5 ng/mL) in patients with at least one polymorphism at MTHFR. The two other biomarkers (i.e. low vitamin B12 and high Hcy levels) did not show any significant association with MTHFR polymorphisms (p values of 0.25 and 0.73, respectively).
Table 6.
Average metrics (overall and by carrier status) of blood levels corresponding to relevant DPN-related biochemical markers in a sub-set of the study cohort (i.e. n = 59 Puerto Rican patients with T2DM) and association analyses between these biochemical markers and the combinatorial MTHFR677C > T and 1298A > C haplotypes (last two columns).
| Biomarkers | Total | Wild types (WT) | Carriers | Odds ratio (OR), 95% CI | p-Value |
|---|---|---|---|---|---|
| HbA1C, % | 7.60 ± 1.07 | 7.31 ± 0.87 | 7.75 ± 1.12 | 3.9 (0.77–19.72) | 0.09 |
| Vitamin B12, pg/mL | 454.34 ± 310.6 | 488.29 ± 408.59 | 432.44 ± 273.5 | 2.22 (0.57–8.65) | 0.25 |
| Folate, ng/mL | 18.97 ± 3.43 | 19.36 ± 3.31 | 18.84 ± 3.78 | 1.89 (0.51–6.96) | 0.33 |
| Hcy, μM | 11.84 ± 5.13 | 11.6 ± 3.88 | 12.09 ± 6.04 | 0.82 (0.25–2.58) | 0.73 |
χ2 or Fisher’s exact tests were used for the association analyses. Odd ratios and 95% CI were calculated. Values a given as mean ± SD. HbA1C, glycosylated hemoglobin; Hcy, homocysteine.
After stratifying by polymorphisms, the estimated ORs for the risk of having decreased levels of vitamin B12 or elevated levels of Hcy in blood were 1.17 and 1.78, respectively, in subjects carrying the 677C > T but not the 1298A > C SNP. Strikingly, the 1298A > C polymorphism seems to be protective for the risk of having decreased levels of vitamin B12 (OR = 0.42) or elevated levels of Hcy (OR = 0.68) in the bloodstream of these patients. Small sample size is likely the main reason to account for such contradictory findings in 1298A > C carriers and the explanation for insufficient statistical power to find significance of the observed associations between the markers and genotypes, mainly with the 677C > T SNP. However, we cannot discard a likely effect of differential vitamin supplementation on individual blood levels of these biochemical markers as this potential confounder was not properly controlled in the current experiment.
Discussion
Results suggest that the MTHFR gene 1298A > C mutation is associated with DPN susceptibility in this cohort of Puerto Rican patients with T2DM. We have postulated that, under risk conditions, low folate intake affects individuals with the 677TT + CT and 1298CC + AC genotypes to a greater extent than those with the 677CC or 1298AA genotypes. In this study, carriers of the 1298CC + AC genotypes were indeed at higher risk of suffering from DPN (Table 3). Moreover, 677TT (but not 677CC + CT) individuals with lower blood folate levels are at higher risk for elevated blood Hcy levels.
These common MTHFR gene mutations (i.e. 677C > T and 1298A > C) have been previously associated with a thermolabile form of the corresponding enzyme and, therefore, been postulated as risk factors for many disorders, ranging from cardiovascular to neural tube defects (NTD) [26]. The correlation between the frequency of myocardial infarction (MI) and NTD with high T allele frequency at the 677 position is consistent with the hypothesis that the 677C > T mutation is a risk factor for serious diseases [26]. According to early reports, there seems to be a genetic component that may play a role in the development of DPN [14, 16, 27].
The 677C > T SNP is considered a major genetic marker for elevated Hcy levels and DPN in other populations [9, 22, 28]. Homozygotes for the 677C > T mutation (i.e. 677TT individuals) are predisposed to hyperhomocysteinemia and, therefore, an increased risk of cardiovascular disease and neuropathy [9, 28]. It is because they seem to have less active MTHFR enzymes to produce the required 5-methyltetrahydrofolate to decrease Hcy [19].
Although the 1298A > C mutation seems to be not directly associated with either low plasma folate or high Hcy levels, this variant is also deemed a risk factor when combined with the 677C > T gene variant [unpublished data]. Together, they both explained the high incidence of NTD in Puerto Ricans, resulting in twice the cases vs. the US population. It has also been early found that individuals with MTHFR1298A > C allele variant, but not 677C > T, have been associated with metabolic syndrome [29].
Metformin therapy for more than 6 months in patients with T2DM who are carriers of any dysfunctional MTHFR polymorphism could be considered a pharmacogenetic cause for exacerbation of DPN, suggesting an earlier switch to insulin. A clear understanding of its role necessarily awaits further research on this matter. Several studies have reported increased risk of DPN with the use of metformin [30–32]. Even though the exact role of MTHFR polymorphisms on the manifestation of such risk is unknown, its use represents an important potential “genetic-iatrogenic” contributor to the development and severity of DPN. Noteworthy is that there are no published data so far indicating clinical associations of MTHFR alleles with metformin-induced worsening of DPN in patients with T2DM [27]. However, the implications for the metabolic consequences of metformin and the risk of neuropathy suggest a role for supplementation of the active folate, especially in patients with some polymorphic variants [27, 30–32].
Previous in vitro assays have demonstrated an effect of hyperhomocysteinemia on nervous functions either by direct cytotoxicity or by oxidative damage to endothelial cells that provoke an occlusive arteriosclerosis in small vessels [9, 33, 34]. Accordingly, the hyperhomocysteinemia-induced microvascular damage could explain nerve lesions in patients suffering from DPN. In a study by Ambrosch et al. [11], they observed a significantly higher frequency of hyperhomocysteinemia in German patients with T2DM suffering from DPN as compared to those without neuropathy. Authors also found that vitamin B12 levels showed a statistically significant tendency to diminish in the DPN patients [11]. Other studies have revealed an association between the use of metformin, B12 depletion, and clinical neuropathy in patients with T2DM [35, 36]. Likewise, blood Hcy levels were also found to be independently associated with DPN in Chinese [12].
The focus on the Puerto Rican population follows from the urgent need for developing prescription guidelines in Hispanics, the largest minority population in the US. Mainland Puerto Ricans currently represent 11% of Hispanics, the second largest Latino group in the US [37]. The island of Puerto Rico is endowed with a distinctive population in terms of its gene flow, genomic structure, population stratification, and admixture patterns [38]. Therefore, it has been speculated that Puerto Ricans will significantly differ from other earlier characterized populations with respect to the frequency distribution of allelic variants and their combinations, as well as their linkage disequilibrium (LD) patterns and haplotype blocks, in genes associated with metformin response [39, 40]. Previous studies suggested that LD across the MTHFR gene is very different in different ethnic groups, with over 20 haplotypes that are differentially represented in White (Caucasian), African American, Asian, and Latino (Mexican American) populations [41]. A differential haplotype structure and LD pattern in the study cohort might explain the observed association of 1298A > C, but not of 677C > T, with DPN susceptibility in these patients.
We have provided, for the first time ever, some relevant data on prevalence of major MTHFR alleles and genotypes in a cohort of Puerto Rican patients with T2DM taking metformin. There are some limitations in the study, including a relatively small sample size (n < 100) that was underpowered to detect statistical difference between carriers and wild types in terms of the biochemical markers. Furthermore, the study cohort is not representative of the entire Puerto Rican population (i.e. it is rather a convenience sample to conduct a “proof-of-principle” analysis). However, all participants were recruited from the Mayaguez Health Region, which resulted with the highest frequencies for both the 677C > T and 1298A > C MTHFR gene mutations in a previous report on MTHFR gene frequencies distribution among the different health regions in Puerto Rico [25].
Although the baseline clinical and demographic characteristics of the participants shown in Table 1 were found to be not significantly different between carriers and wild types, we were not able to control for other baseline characteristics (including dietary intake, folate and vitamin B12 supplementation, and certain comorbidities and co-medications) given the naturalistic and quasi-experimental strategy followed in this study for recruitments, with minimal protocol-based constraints. Moreover, chances of confounding (in terms of altered baseline levels of biomarkers as well as neuropathic event rates during the period prior to the date of index prescription with metformin) cannot be controlled because of the lack of such information in the medical records.
The study found an association between the MTH-FR1298A > C variant and a susceptibility to DPN. Some biochemical markers related to folate metabolism such as plasma homocysteine were measured, but no associations were found. As other markers are also related to the folate metabolic pathway (e.g. nitric oxide, Figure 1), the effects of polymorphisms in the endothelial nitric oxide synthase gene, as well as in additional folate metabolizing genes, on the concentration of serum nitrate need further attention [42]. Future assessments of these and other markers could shed some light on the significance of the observed association.
Figure 1.
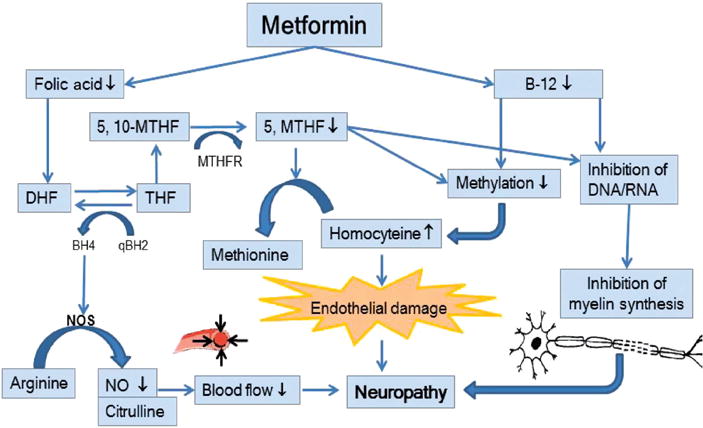
Representation of candidate markers involved in a biochemical pathway related to folate metabolism, including endothelial nitric oxide synthase (NOS), serum nitrate/nitric oxide (NO), folate, and plasma total homocysteine to illustrate the proposed mechanisms of metformin-induced neuropathy.
Available vitamin B12 and folate are depleted causing a cascade of reactions that affect nerve structure (i.e. myelin) and function (i.e. blood flow: oxygen and nutrients). Active folate is reduced by metformin, resulting in less NO and, therefore, reduced blood flow to nerve. In addition, lowering folate also contributes to elevation of plasma homocysteine. Reduced B12 diminishes methylation, which inhibits homocysteine metabolism and provokes vascular endothelial damage. Moreover, reduction of folate and B12 reduces DNA/RNA synthesis, which inhibits myelin synthesis. Inability to maintain intact neuron myelin sheath reduces nerve neurotransmission and leads to neuropathy.
This survey provides further evidence in favor of the use of this genetic marker as an independent risk factor for DPN predisposition. Future studies are warranted in order to elucidate the clinical utility and validity of genotyping Puerto Ricans for MTHFR polymorphisms and give foundation to a DNA-guided strategy for better clinical management of patients with T2DM who develop or worsen DPN.
Acknowledgments
The material presented herein is the result of work supported with resources from and the use of facilities for performing genetic assays at the UPR-MSC RCMI Center for Genomics in Health Disparities and Rare Disorders. The authors also want to thank Miguel Ayala, Luis A Robles de Jesus, Hasna Abboud-Chalhoub, Zoriely Amador-Ríos, Edmarielis González-González, Isaúl Hernández-Diaz, Ana P. Lozada-Barea, Brenda I. Mén-dez-Guzmán, Nydia A. Moreno-Rivera, Vanessa G. Pozzi-Lorenzo, Rafael E. Ramos-Franco, Jomir Rivera-Rivera, Ivonne M. Rosario-Calderon, Beverly N. Santos-Sierra, and Glendalis Vargas-Vargas for their help in this survey. Finally, we want to thank all the patients for their participation in this study.
Research funding: This investigation was supported in part by the Research Center in Minority Institutions (RCMI) grants from the National Center for Research Resources (2G12-RR003051) and the National Institute on Minority Health and Health Disparities (8G12-MD007600) of the National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH, owners, and employees at Farmacia San José or the United States government.
Footnotes
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
Employment or leadership: None declared.
Honorarium: None declared.
Competing interests: The funding organization(s) played no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the report for publication.
Authors have no relevant affiliation or financial involvement with any organization or entity with a financial interest in or conflicts of interest with the subject matter or materials discussed in the article that need to be disclosed. No writing assistance was utilized in the production of this manuscript.
Contributor Information
Francisco J. Jiménez-Ramírez, School of Pharmacy, Department of Pharmacy Practice, University of Puerto Rico Medical Sciences Campus, San Juan, Puerto Rico
Liza M. Castro, School of Pharmacy, Department of Pharmacy Practice, University of Puerto Rico Medical Sciences Campus, San Juan, Puerto Rico
Clarymar Ortiz, School of Pharmacy, Department of Pharmaceutical Sciences, University of Puerto Rico Medical Sciences Campus, San Juan, Puerto Rico.
Jennifer Concepción, School of Pharmacy, Department of Pharmaceutical Sciences, University of Puerto Rico Medical Sciences Campus, San Juan, Puerto Rico.
Jessicca Y. Renta, School of Medicine, Department of Biochemistry, University of Puerto Rico Medical Sciences Campus, San Juan, Puerto Rico
Raúl H. Morales-Borges, Ashford Institute of Hematology and Oncology, Ashford Medical Center, San Juan, Puerto Rico.
Jorge R. Miranda-Massari, School of Pharmacy, Department of Pharmacy Practice, University of Puerto Rico Medical Sciences Campus, San Juan, Puerto Rico.
Jorge Duconge, School of Pharmacy, Department of Pharmaceutical Sciences, University of Puerto Rico Medical Sciences Campus, Pharmacy Bldg., 3rd Floor, Room 325-14, PO Box 365067 San Juan, Puerto Rico 00936-5067.
References
- 1.Boulton AJ, Vinik AI, Arezzo JC, Bril V, Feldman EL, Freeman R, et al. Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes Care. 2005;28:956–62. doi: 10.2337/diacare.28.4.956. [DOI] [PubMed] [Google Scholar]
- 2.Deshpande AD, Harris-Hayes M, Schootman M. Epidemiology of diabetes and diabetes-related complications. Phys Ther. 2008;88:1254–64. doi: 10.2522/ptj.20080020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barrett AM, Lucero MA, Le T, Robinson RL, Dworkin RH, Chappell AS. Epidemiology, public health burden, and treatment of diabetic peripheral neuropathic pain: a review. Pain Med. 2007;8(Suppl 2):S50–62. doi: 10.1111/j.1526-4637.2006.00179.x. [DOI] [PubMed] [Google Scholar]
- 4.Albers JW, Herman WH, Pop-Busui R, Feldman EL, Martin CL, Cleary PA, et al. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group Effect of prior intensive insulin treatment during the Diabetes Control and Complications Trial (DCCT) on peripheral neuropathy in type-1 Diabetes during the Epidemiology of Diabetes Interventions and Complications (EDIC) Study. Diabetes Care. 2010;33:1090–6. doi: 10.2337/dc09-1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Behavioral Risk Factor Surveillance System (BRFSS) Database. Available at: http://www.cdc.gov/brfss/brfssprevalence/index.html. Accessed in October 2016.
- 6.Departamento de Salud. Gobierno de Puerto Rico, Informe de la Salud en Puerto Rico. 2014:141–144. Available at: https://www.salud.gov.pr/Estadisticas-Registros-y-Publicaciones/Estadisticas%20Vitales/Informe%20de%20la%20Salud%20en%20PUerto%20Rico%202014.pdf.
- 7.Gordois A, Scuffham P, Shearer A, Oglesby A, Tobian JA. The health care costs of diabetic peripheral neuropathy in the US. Diabetes Care. 2003;26:1790–5. doi: 10.2337/diacare.26.6.1790. [DOI] [PubMed] [Google Scholar]
- 8.Stewart WF, Ricci JA, Chee E, Hirsch AG, Brandenburg NA. Lost productive time and costs due to Diabetes and diabetic neuropathic pain in the US workforce. J Occup Environ Med. 2007;49:672–9. doi: 10.1097/JOM.0b013e318065b83a. [DOI] [PubMed] [Google Scholar]
- 9.Yigit S, Karakus N, Inanir A. Association of MTHFR gene C677T mutation with diabetic peripheral neuropathy and diabetic retinopathy. Mol Vis. 2013;19:1626–30. [PMC free article] [PubMed] [Google Scholar]
- 10.Buysschaert M, Dramais AS, Wallemacq PE, Hermans MP. Hyperhomocysteinemia in type 2 diabetes: Relationship to macroangiopathy, nephropathy, and insulin resistance. Diabetes Care. 2000;23:1816–22. doi: 10.2337/diacare.23.12.1816. [DOI] [PubMed] [Google Scholar]
- 11.Ambrosch A, Dierkes J, Lobmann R, Kühne W, König W, Luley C, et al. Relation between homocysteinaemia and diabetic neuropathy in patients with Type 2 diabetes mellitus. Diabet Med. 2001;18:185–92. doi: 10.1046/j.1464-5491.2001.00445.x. [DOI] [PubMed] [Google Scholar]
- 12.Jianbo L, Yuche C, Ming S, Jingrong T, Qing D, Yu Z, et al. Association of homocysteine with peripheral neuropathy in Chinese patients with type 2 diabetes. Diabetes Res Clin Pract. 2011;93:38–42. doi: 10.1016/j.diabres.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 13.Toffoli G, Russo A, Innocenti F, Corona G, Tumolo S, Sartor F, et al. Effect of methylenetetrahydrofolate reductase 677CRT polymorphism on toxicity and homocysteine plasma level after chronic methotrexate treatment of ovarian cancer patients. Int J Cancer. 2003;103:294–9. doi: 10.1002/ijc.10847. [DOI] [PubMed] [Google Scholar]
- 14.Frosst P, Blom HJ, Milos R, Goyette P, Sheppard CA, Matthews RG, et al. A candidate genetic risk factor for vascular disease: a common mutation in methylene-tetrahydrofolate reductase. Nat Genet. 1995;10:111–3. doi: 10.1038/ng0595-111. [DOI] [PubMed] [Google Scholar]
- 15.Skibola CF, Smith MT, Kane E, Roman E, Rollinson S, Cartwright RA, et al. Polymorphisms in the methylene-tetrahydrofolate reductase gene are associated with susceptibility to acute leukemia in adults. Proc Natl Acad Sci USA. 1999;96:12810–5. doi: 10.1073/pnas.96.22.12810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schwahn B, Rozen R. Polymorphisms in the methylene-tetrahydrofolate reductase gene: clinical consequences. Am J Pharmacogenomics. 2001;1:189–201. doi: 10.2165/00129785-200101030-00004. [DOI] [PubMed] [Google Scholar]
- 17.Iqbal MP, Frossard PM. Methylene-tetrahydrofolate reductase gene and coronary artery disease. J Pak Med Assoc. 2003;53:33–6. [PubMed] [Google Scholar]
- 18.Muntjewerff JW, Kahn RS, Blom HJ, den Heijer M. Homocysteine, methylene-tetrahydrofolate reductase and risk of schizophrenia: a meta-analysis. Mol Psychiatry. 2006;11:143–9. doi: 10.1038/sj.mp.4001746. [DOI] [PubMed] [Google Scholar]
- 19.Matthews RG. Methylene-tetrahydrofolate reductase: a common human polymorphism and its biochemical implications. Chem Record. 2003;2:4–12. doi: 10.1002/tcr.10006. [DOI] [PubMed] [Google Scholar]
- 20.Kalow W. Pharmacogenetics of drug metabolism. 1st. Elsevier Science Pub Co; 1992. p. 1. [Google Scholar]
- 21.Engen RM, Marsh S, Van Booven DJ, McLeod HL. Ethnic differences in pharmacogenetically relevant genes. In: Llerena A, Licinio J, editors. Current drug targets; pharmacogenetic and pharmacogenomics. Vol. 7. 2006. pp. 1641–8. [DOI] [PubMed] [Google Scholar]
- 22.Wu S, Han Y, Hu Q, Zhang X, Cui G, Li Z, et al. Effects of common polymorphisms in the MTHFR and ACE genes on diabetic peripheral neuropathy progression: a meta-analysis. Mol Neurobiol. 2015;52:1885. doi: 10.1007/s12035-016-9823-4. [DOI] [PubMed] [Google Scholar]
- 23.Amigo J, Salas A, Phillips C, Carracedo A. SPSmart: adapting population based SNP genotype databases for fast and comprehensive web access. BMC Bioinformatics. 2008;9:428. doi: 10.1186/1471-2105-9-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen J, Teo YY, Toh DS, Sung C. Interethnic comparisons of important pharmacology genes using SNP databases: potential application to drug regulatory assessments. Pharmacogenomics. 2010;11:1077–94. doi: 10.2217/pgs.10.79. [DOI] [PubMed] [Google Scholar]
- 25.García-Fragoso L, García-García I, Leavitt G, Renta JY, Ayala AM, Cadilla CL. MTHFR polymorphisms in Puerto Rican children with isolated congenital heart disease and their mothers. Int J Genet Mol Biol. 2010;2:43–7. [PMC free article] [PubMed] [Google Scholar]
- 26.Van der Put NM, Eskes TK, Blom HJ. Is the common 677C>T mutation in the methylene-tetrahydrofolate reductase gene a risk factor for neural tube defects? A meta-analysis. Q J Med. 1997;90:111–5. doi: 10.1093/qjmed/90.2.111. [DOI] [PubMed] [Google Scholar]
- 27.Ukinc K, Ersoz HO, Karahan C, Erem C, Eminagaoglu S, Hacihasanoglu AB, et al. Methyltetrahydrofolate reductase C677T gene mutation and hyperhomocysteinemia as a novel risk factor for diabetic nephropathy. Endocrine. 2009;36:255–61. doi: 10.1007/s12020-009-9218-7. [DOI] [PubMed] [Google Scholar]
- 28.Seshadri N, Robinson K. Homocysteine, B vitamins and coronary artery disease. Med Clin North Am. 2000;84:215–7. doi: 10.1016/s0025-7125(05)70215-7. [DOI] [PubMed] [Google Scholar]
- 29.Van Winkel R, Rutten BP, Peerbooms O, Peuskens J, van Os J, De Hert M. MTHFR and risk of metabolic syndrome in patients with schizophrenia. Schizophr Res. 2010;121:193–8. doi: 10.1016/j.schres.2010.05.030. [DOI] [PubMed] [Google Scholar]
- 30.Palomba S, Falbo A, Giallauria F, Russo T, Tolino A, Zullo F, et al. Effects of metformin with or without supplementation with folate on homocysteine levels and vascular endothelium of woman with polycystic ovarian syndrome. Diabetes Care. 2010;33:246–51. doi: 10.2337/dc09-1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Jager J, Kooy A, Lehert P, Wulffelé MG, van der Kolk J, Bets D, et al. Long term treatment with metformin in patients with type 2 Diabetes and risk of vitamin B-12 deficiency: randomized placebo controlled trial. Br Med J. 2010;340:c2181. doi: 10.1136/bmj.c2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wulffele MG, Kooy A, Lehert P, Bets D, Ogterop JC, Borger van der Burg B, et al. Effects of short-term treatment with metformin on serum concentrations of homocysteine, folate and vitamin B12 in type 2 diabetes mellitus: a randomized, placebo-controlled trial. J Intern Med. 2003;254:455–63. doi: 10.1046/j.1365-2796.2003.01213.x. [DOI] [PubMed] [Google Scholar]
- 33.Schlüssel E. Homocysteine-induced oxidative damage: mechanisms and possible roles in neurodegenerative and atherogenic processes. Z Naturforsch. 1995;50:699–707. doi: 10.1515/znc-1995-9-1017. [DOI] [PubMed] [Google Scholar]
- 34.Weir DG, Scott JM. The biochemical basis of neuropathy in cobalamin deficiency. Baillieres Clin Haematol. 1995;8:479–97. doi: 10.1016/s0950-3536(05)80217-3. [DOI] [PubMed] [Google Scholar]
- 35.Singh AK, Kumar A, Karmakar D, Jha RK. Association of B12 deficiency and clinical neuropathy with metformin uses in type 2 diabetes patients. J Postgrad Med. 2013;59:253–7. doi: 10.4103/0022-3859.123143. [DOI] [PubMed] [Google Scholar]
- 36.Ahmed MA, Muntingh G, Rheeder P. Vitamin B12 deficiency in metformin-treated type-2 diabetes patients, prevalence and association with peripheral neuropathy. BMC Pharmacol Toxicol. 2016;17:44. doi: 10.1186/s40360-016-0088-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Population Statistics for Racial/Ethnic Minorities in the United States. Source: population projections program Population Division, U.S. Bureau of the Census. U.S. Department of Commerce; 2010. Available at: https://www.census.gov/prod/cen2010/briefs/c2010br-04.pdf. Accessed in December 2016. [Google Scholar]
- 38.Suarez-Kurtz G, Pena S. Pharmacogenomics in the Americas: the impact of genetic admixture. Curr Drug Targets. 2006;7:1649–58. doi: 10.2174/138945006779025392. [DOI] [PubMed] [Google Scholar]
- 39.Gonzalez-Burchard E, Borrell LN, Choudhry S, Naqvi M, Tsai HJ, Rodriguez-Santana JR, et al. Latino populations: a unique opportunity for the study of race, genetics, and social environment in epidemiological research. Am J Public Health. 2005;95:2161–68. doi: 10.2105/AJPH.2005.068668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schneider JA, Rees DC, Liu YT, Clegg JB. Worldwide distribution of a common methylene-tetrahydrofolate reductase mutation. Am J Hum Genet. 1998;62:1258–60. doi: 10.1086/301836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martin YN, Salavaggione OE, Eckloff BW, Wieben ED, Schaid DJ, Weinshilboum RM. Human methylenetetrahydrofolate reductase pharmacogenomics: gene resequencing and functional genomics. Pharmacogenet Genomics. 2006;16:265–77. doi: 10.1097/01.fpc.0000194423.20393.08. [DOI] [PubMed] [Google Scholar]
- 42.Cabo R, Hernes S, Slettan A, Haugen M, Ye S, Blomhoff R, et al. Effects of polymorphisms in endothelial nitric oxide synthase and folate metabolizing genes on the concentration of serum nitrate, folate, and plasma total homocysteine after folic acid supplementation: a double-blind crossover study. Nutrition. 2015;31:337–44. doi: 10.1016/j.nut.2014.08.009. [DOI] [PubMed] [Google Scholar]


