Abstract
Background
Sarcopenia is an aging and disease-related syndrome characterized by progressive and generalized loss of skeletal muscle mass and strength, with the risk of frailty and poor quality of life. Sarcopenia is diagnosed by a decrease in skeletal muscle index (SMI) and reduction of either handgrip strength or gait speed. However, measurement of SMI is difficult for general physicians because it requires special equipment for bioelectrical impedance assay or dual-energy X-ray absorptiometry. The purpose of this study was, therefore, to explore a novel, simple diagnostic method of sarcopenia evaluation in patients with cardiovascular diseases (CVD).
Methods
We retrospectively investigated 132 inpatients with CVD (age: 72±12 years, age range: 27–93 years, males: 61%) Binomial logistic regression and correlation analyses were used to assess the associations of sarcopenia with simple physical data and biomarkers, including muscle-related inflammation makers and nutritional markers.
Results
Sarcopenia was present in 29.5% of the study population. Serum concentrations of adiponectin and sialic acid were significantly higher in sarcopenic than non-sarcopenic CVD patients. Stepwise multivariate binomial logistic regression analysis revealed that adiponectin, sialic acid, sex, age, and body mass index were independent factors for sarcopenia detection. Sarcopenia index, obtained from the diagnostic regression formula for sarcopenia detection including the five independent factors, indicated a high accuracy in ROC curve analysis (sensitivity 94.9%, specificity 69.9%) and the cutoff value for sarcopenia detection was -1.6134. Sarcopenia index had a significant correlation with the conventional diagnostic parameters of sarcopenia.
Conclusions
Our new sarcopenia index using simple parameters would be useful for diagnosing sarcopenia in CVD patients.
Introduction
Sarcopenia is an age-related syndrome characterized by progressive and generalized loss of skeletal muscle mass, weakening strength and decreasing physical performance with a risk of frailty that increases poor health outcomes, including falls, incident disability, hospitalization and mortality [1]. Sarcopenia can be categorized as ‘primary’(age-related) or ‘secondary’ (disease-related) sarcopenia. Disease-related sarcopenia is associated with advanced organ failure and chronic inflammatory diseases, such as chronic heart failure and chronic kidney disease secondary to cardiovascular diseases (CVD) [2]. In elderly patients with CVD, sarcopenia can be considered not only age-related, but also disease-related sarcopenia associated with CVD. Our previous article showed that sarcopenia with CVD was frequently found in elderly, female, small body mass index and chronic kidney disease patients [3].
Nutrition and chronic inflammation play an important role in the manifestation and progression of sarcopenia. The International Working Group on Sarcopenia recommended inflammation-related markers, oxidative stress markers, nutritional markers, antioxidant markers and hormones as biomarkers of sarcopenia [2]. For example, daily protein intake correlates with skeletal muscle index (SMI), handgrip strength and gait speed [3]. Adiponectin is an anti-inflammatory adipokine that is known to be associated with CVD and skeletal muscle function [4–8]. The conference report of ‘Cachexia in heart disease: highlights from the ESC 2010’ suggested that adiponectin might be a marker of muscle wasting in chronic heart failure [9].
The conventional diagnostic criteria of sarcopenia include loss of skeletal muscle mass, as assessed by the SMI, together with either weakened muscle strength, assessed by handgrip strength, or low physical performance, assessed by gait speed [2,10]. Although SMI is the most important component in the diagnosis of sarcopenia, it is difficult for general physicians to measure SMI routinely because the measurement requires either bioelectrical impedance assay or dual-energy X-ray absorptiometry. The purpose of this study was to explore a novel diagnostic method of sarcopenia assessment in patients with CVD, using simple parameters that do not need special equipment.
Methods
Study patients
We retrospectively studied 132 consecutive inpatients (age: 72±12 years, age range: 27–93 years, 80 males) with CVD and/or undergoing cardiovascular surgery who were admitted to our hospital between April 2013 and December 2015. These patients were divided into sarcopenic and non-sarcopenic groups. Patients with pacemaker implantation were excluded because bioelectric impedance assay could not be performed in them. Written informed consent was obtained from all patients, and the study was approved by the Ethics Committee of Kurume University.
Diagnosis of sarcopenia
Sarcopenia was diagnosed by measuring muscle mass, muscle strength and physical performance according to the recommended diagnostic algorithm of the Asian Working Group for Sarcopenia (AWGS) guidelines [10]. Sarcopenia was defined as low SMI (< 7.0 kg/m2 in males; < 5.7 kg/m2 in females) associated with either low handgrip strength (< 26 kgf in males; < 18 kgf in females) or low gait speed (< 0.8m/sec). Non-sarcopenia was diagnosed when subjects had normal SMI (≥ 7.0 kg/m2 in males; ≥ 5.7 kg/m2 in females) or when they had normal handgrip strength (≥ 26 kgf in males; ≥ 18 kgf in females) and normal gait speed (≥ 0.8m/sec). The age criterion of more than 65 years old was not adopted for sarcopenia diagnosis in this study, because age is a possible confounding factor of disease-related (secondary) sarcopenia and, hence, CVD patients less than 65 years old were included in this study.
Muscle mass measurements
Muscle mass was measured by bioelectrical impedance assay using the InBody S10 body composition analyzer (Biospace, Tokyo, Japan). This system applies electricity at frequencies of 1, 5, 50, 250, 500 kHz, and 1 MHz through the body. Whole-body impedance was measured using an ipsilateral foot-hand electrical pathway. The recommended conditions for bioelectrical impedance assay measurements, as explained to the subjects, were: (i) fasting for 4 hours before measurements; (ii) bladder voided before measurements; and (iii) no exercise in the 8-hour period prior to measurements [11]. Obviously edematous patients were examined after an improvement in their edema. Appendicular muscle mass was calculated as the sum of the muscle mass of the arms and legs. Absolute appendicular muscle mass was converted to SMI by dividing the value by the square of the height in meters (kg/m2).
Muscle strength measurements
Muscle strength was assessed as handgrip strength using a Smedley hand dynamometer MY-2080 (Matsumiya Ikaseiki Seisakusho Co. Ltd., Tokyo, Japan). One trial was performed for each hand, and the result from the stronger hand was used for sarcopenia diagnosis.
Physical performance measurements
Physical performance was assessed as usual gait speed. We referenced and modified a previous technique reported by Tanimoto et al [12]. Patients were asked to walk straight ahead for 12 meters at their usual speed for measurement of 10-meter walk time. The walking speed reached a steady speed within the first 2 meters. Gait speed (m/sec) was calculated by dividing the distance covered (10 meters (m)) by the 10-meter walk time (sec).
Data collection
Data on admission were collected from hospital charts and databases. Blood was drawn from the antecubital vein early in the morning, after at least 8-hours of fasting, and subjected to biochemical and immunological measurements, including high sensitivity C-reactive protein (hsCRP), interleukin-6 (IL-6), sialic acid and adiponectin. General nutritional condition was assessed using body mass index (BMI) and the controlling nutritional status (CONUT) score, which was determined using serum albumin, total cholesterol and total lymphocyte count [13].
Statistical analysis
All data were statistically analyzed using the Ekuseru-Toukei 2012 software purchased from Social Survey Research Information Co., Ltd. (Tokyo, Japan). Numerical data were expressed as mean ± standard deviation or as median with 95% confidence interval (CI). Student’s t-test and Mann-Whitney u-test were appropriately used to compare the two groups. Proportional data was analyzed using chi-square test or Fisher’s exact test. Univariate and stepwise multivariate logistic regression analyses were performed to determine independent factors for sarcopenia diagnosis. Receiver operating characteristic (ROC) curve analysis was calculated to predict the cutoff value for sarcopenia diagnosis. Statistical significance was considered at the level of p<0.05.
Results
Characteristics of sarcopenic patients
As shown in Table 1, 39 of 132 patients (29.5%) were diagnosed as having sarcopenia. Sarcopenic patients were older and had lower estimated glomerular filtration rates (eGFR) and lower Barthel index, and included higher numbers of females and subjects with chronic kidney disease as compared with non-sarcopenic patients. The distributions of eGFR and Barthel index were not biphasic in sarcopenia and non-sarcopenia groups (S1 Fig). The prevalence of symptomatic heart failure, baseline CVD, comorbidities and left ventricular ejection fraction did not differ between the two groups. SMI, handgrip strength and gait speed were significantly lower in sarcopenic patients than those in non-sarcopenic patients (Table 2).
Table 1. Baseline characteristics of sarcopenic and non-sarcopenic patients.
| Non-sarcopenic n = 93 | Sarcopenic n = 39 | p value | |
|---|---|---|---|
| Age, years, (range) | 70 ± 13 (27–93) | 77 ± 8 (54–92) | 0.0001 |
| Male, n (%) | 67 (72.0) | 13 (33.3) | < 0.0001 |
| Symptomatic heart failure (ACC/AHA CHF stage C and D), n (%) |
27 (29.0) | 16 (41.0) | 0.2226 |
| Cardiovascular disease, n (%) | |||
| CAD (including CABG) | 53 (57.0) | 17 (43.6) | 0.1837 |
| Hypertensive heart disease | 8 (8.6) | 5 (12.8) | 0.5252 |
| Idiopathic cardiomyopathy | 6 (6.5) | 2 (5.2) | 1.0000 |
| Arrhythmia | 12 (12.9) | 3 (7.7) | 0.5513 |
| Valvular heart disease | 9 (9.7) | 8 (20.5) | 0.1514 |
| Peripheral artery disease | 3 (3.2) | 2 (5.1) | 0.6319 |
| PCS (excluding CABG) | 2 (2.2) | 2 (5.1) | 0.5812 |
| Comorbidity, n (%) | |||
| Hypertension | 60 (64.5) | 24 (61.5) | 0.8432 |
| Diabetes mellitus | 37 (39.8) | 12 (30.8) | 0.4300 |
| Dyslipidemia | 34 (36.6) | 13 (33.3) | 0.8427 |
| Hyperuricemia | 9 (9.7) | 3 (7.7) | 1.0000 |
| Chronic kidney disease | 10 (10.8) | 11 (28.2) | 0.0184 |
| Stroke | 11 (11.8) | 2 (5.1) | 0.3432 |
| eGFR, mL/min/1.73m2 | 64.3 (62.1–71.0) | 51.2 (41.0–59.0) | 0.0028 |
| Left ventricular ejection fraction, % | 64.0 (62.0–67.0) | 62.0 (59.0–65.0) | 0.1494 |
| Barthel index | 100 (90–100) | 90 (85–100) | 0.0171 |
Numerical data are expressed as mean ± standard deviation or as median (95% confidence interval). The numbers in parenthesis denote the percentage. ACC/AHA CHF stage = American College of Cardiology/American Heart Association chronic heart failure stage; CABG = coronary artery bypass grafting; CAD = coronary artery disease; eGFR = estimated glomerular filtration rate; PCS = post-cardiovascular surgery.
Table 2. Baseline values of the diagnostic components for sarcopenia in sarcopenic and non-sarcopenic patients.
| Non-sarcopenic | Sarcopenic | p value | |
|---|---|---|---|
| Skeletal muscle index, kg/m2 | 7.36 ± 1.24 (n = 85) | 5.20 ± 0.71 (n = 39) | <0.0001 |
| Handgrip strength, kgf | 30.0 ± 12.7 (n = 69) | 12.6 ± 5.9 (n = 34) | <0.0001 |
| Gait speed, m/sec | 1.16 ± 0.40 (n = 58) | 0.75 ± 0.27 (n = 26) | <0.0001 |
Numerical data are expressed as mean ± standard deviation.
Muscle-related inflammatory and nutritional markers
Serum levels of IL-6, adiponectin and sialic acid were significantly higher in sarcopenic than in non-sarcopenic patients (p<0.01, p<0.0001 and p<0.05, respectively) (Table 3). There was no significant difference in hsCRP between the 2 groups. Sarcopenic patients had significantly lower BMI and serum albumin than non-sarcopenic patients (p<0.0001 and p<0.05, respectively). In total cholesterol, total lymphocyte count and CONUT score, there were no significant differences between sarcopenic and non-sarcopenic groups.
Table 3. Muscle-related inflammatory and nutritional markers.
| Non-sarcopenic n = 93 | Sarcopenic n = 39 | P value | |
|---|---|---|---|
| Muscle-related inflammatory markers | |||
| hs-CRP, ng/mL | 1280 (907–2290) | 2440 (915–6970) | 0.1913 |
| Interleukin-6, pg/mL | 4.20 (3.50–4.80) | 5.70 (4.30–10.10) | 0.0072 |
| Adiponectin, μg/mL | 3.61 (3.22–4.50) | 8.32 (6.52–11.50) | <0.0001 |
| Sialic acid, mg/dL | 62.0 (59.0–65.0) | 65.0 (60.0–76.0) | 0.0247 |
| Nutritional markers | |||
| Body mass index, kg/m2 | 25.6 ± 4.8 | 21.7 ± 2.5 | <0.0001 |
| Serum albumin, g/dL | 4.11 ± 0.46 | 3.93 ± 0.39 | 0.0332 |
| Total cholesterol, mg/dL | 173 ± 45 | 170 ± 31 | 0.7061 |
| Total lymphocyte count, /μL | 1540 ± 689 | 1333 ± 542 | 0.0972 |
| CONUT | 2.0 (1.0–2.0) | 2.0 (2.0–3.0) | 0.2132 |
Data were expressed as mean ± standard deviation or as median (95% confidence interval). CONUT = controlling nutritional status score; hs-CRP = high sensitivity c-reactive protein.
Sarcopenia index
To determine the index for diagnosing sarcopenia, binomial logistic regression analyses for the presence of sarcopenia were performed in the entire patient cohort, obtained by assessing sarcopenic and non-sarcopenic groups together. Univariate binomial logistic regression analyses revealed that age (positively), female gender (positively), adiponectin (positively), sialic acid (positively), BMI (inversely), albumin (inversely), and eGFR (inversely) significantly correlated with sarcopenia (Table 4). In stepwise multivariate binomial logistic regression analysis using the above 7 significant variables, adiponectin, sialic acid, age, BMI and sex were independent factors for sarcopenia (Table 5). According to the partial regression coefficients of the independent factors, the following diagnostic regression formula is considered as a diagnostic index for sarcopenia:
Table 4. Single binomial logistic regression analysis.
| Biomarkers | SE | β | p value |
|---|---|---|---|
| Age | 0.0223 | 0.8214 | 0.0023 |
| Sex, (male = 1, female = 2) | 0.4108 | 0.8012 | 0.0001 |
| Estimated glomerular filtration rate | 0.0093 | —0.4546 | 0.0294 |
| Muscle-related inflammatory markers | |||
| Adiponectin | 0.0410 | 0.7844 | 0.0001 |
| Sialic acid | 0.0180 | 0.5424 | 0.0057 |
| High-sensitivity C reactive protein | 0.1196 | 0.2998 | 0.1224 |
| Interleukin-6 | 0.0175 | 0.1217 | 0.4973 |
| Nutritional markers | |||
| Body mass index | 0.0761 | —1.5047 | 0.0001 |
| Albumin | 0.4494 | —0.4081 | 0.0398 |
| Controlling nutritional status | 0.1063 | 0.1127 | 0.5481 |
| Total lymphocyte count | 0.0003 | —0.3326 | 0.0990 |
| Total cholesterol | 0.0046 | —0.0627 | 0.7435 |
β = standardized partial regression coefficient; SE = standard error.
Table 5. Stepwise multivariate binomial logistic regression analysis for sarcopenia.
| Independent variables | Partial regression coefficient | SE | β | p value |
|---|---|---|---|---|
| Body mass index | —0.3430 | 0.1034 | —1.5674 | 0.0009 |
| Sialic acid | 0.0840 | 0.0287 | 0.9121 | 0.0034 |
| Sex, (male = 1, female = 2) | 1.5751 | 0.5457 | 0.7696 | 0.0039 |
| Age | 0.0843 | 0.0328 | 1.0212 | 0.0101 |
| Adiponectin | 0.1117 | 0.0563 | 0.5551 | 0.0472 |
| Constant term | —7.8072 | 3.9208 | - | 0.0465 |
β = standardized partial regression coefficient; SE = standard error. There were no significant correlations for the following variables: estimated glomerular filtration rate and albumin.
Sarcopenia index = 0.1117 x [adiponectin (μg/mL)] + 0.0840 x [sialic acid (mg/dL)] + 1.5751 x [sex (male = 1, female = 2)] + 0.0843 x [age (years)]—0.3430 x [BMI (kg/m2)]—7.8072.
Sarcopenia index and conventional diagnostic criteria for sarcopenia
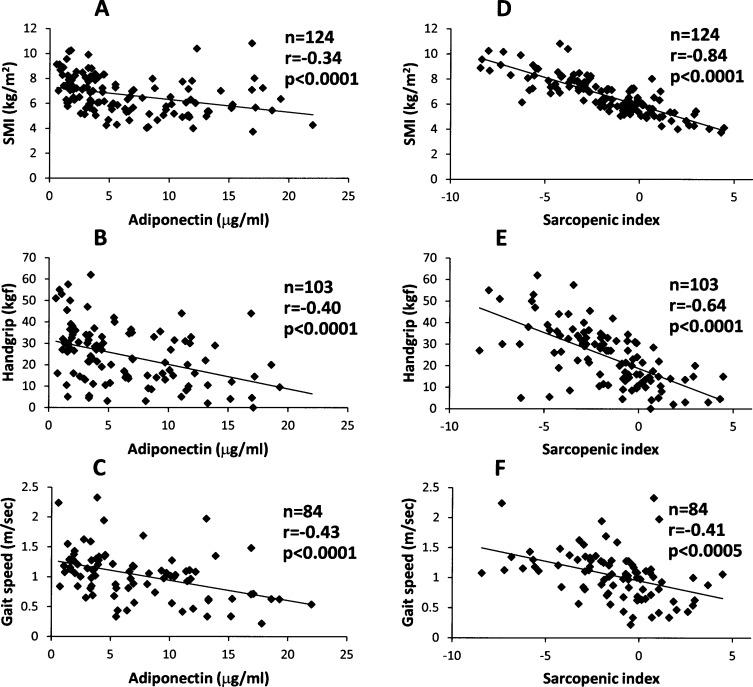
As shown in Fig 1A, 1B and 1C, adiponectin had weak negative correlations with SMI (r = -0.34) and handgrip strength (r = -0.40), and a modest correlation with gait speed (r = -0.43), whereas sialic acid weakly correlated with only SMI (r = -0.21) (S2 Fig). In contrast, the sarcopenia index showed a strong negative correlation with SMI (r = -0.84), and modest negative correlations with handgrip strength (r = -0.64) and gait speed (r = -0.41) (Fig 1D, 1E and 1F).
Fig 1.
Correlations of adiponectin (A, B and C) and sarcopenia index (D, E and F) with skeletal muscle index (A and D, n = 124), handgrip strength (B and E, n = 103), and gait speed (C and F, n = 84) in patients with cardiovascular disease. r = correlation coefficient. Sarcopenia index = 0.1117 x [adiponectin (μg/mL)] + 0.0840 x [sialic acid (mg/dL)] + 1.5751 x [sex (male = 1, female = 2)] + 0.0843 x [age (years)]—0.3430 x [BMI (kg/m2)]—7.8072.
Cutoff value of sarcopenia index for sarcopenia diagnosis
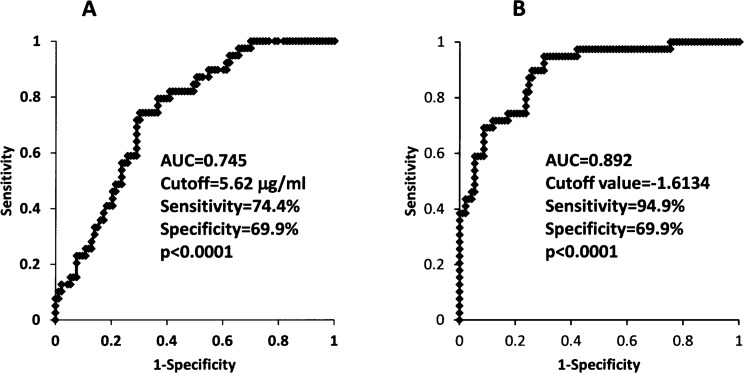
In ROC curve analysis, the cutoff value of adiponectin for detection of sarcopenia was 5.62 μg/ml, with a sensitivity and specificity of 74.4% and 69.9%, respectively (Fig 2A). In contrast, sarcopenia index significantly improved the diagnostic accuracy for sarcopenia: ROC analysis showed that the diagnostic accuracy of the sarcopenia index had a sensitivity of 94.9% and specificity of 69.9%. The cutoff value for sarcopenia detection was -1.6134 (Fig 2B).
Fig 2.
Receiver operating characteristic curve analyses of adiponectin (A) and sarcopenia index (B) for the detection of sarcopenia in patients with cardiovascular diseases. AUC = area under the curve.
Discussion
In this study, we proposed the sarcopenia index as a new diagnostic tool for sarcopenia in CVD patients, which is independent of the conventional diagnostic criteria, i.e. skeletal muscle mass, handgrip force and gait speed. The sarcopenia index is calculated by a regression formula including the five components of age, sex, BMI, serum adiponectin and serum sialic acid, which are independent factors for detecting sarcopenia, based on stepwise multivariate binomial logistic regression analysis.
BMI, which represents age and nutritional status, is an important indicator of the progression and manifestation of sarcopenia, as previously reported [2]. Generally, elderly people more than 65 years old are the subjects of studies on the diagnosis of primary sarcopenia [2,10]. We excluded the age criterion in this study because age was expected to be a possible confounding factor in disease-related sarcopenia secondary to CVD, and hence, CVD patients less than 65 years old were included in this study. Indeed, stepwise multivariate binomial logistic regression analysis revealed that age was one of the independent factors for sarcopenia diagnosis in CVD patients, as shown in Table 5. Since the diagnostic criteria of SMI are reportedly different between males and females [2,10], and the proportion of females with sarcopenia was higher than those in the non-sarcopenia group, as shown in Table 1, gender cannot be ignored when diagnosing sarcopenia.
Elevation of the concentration of adiponectin has been reported as a marker of weakened skeletal muscle force [6,7]. On the other hand, however, adiponectin is known to protect the skeletal muscle against inflammation and injury in dystrophinopathies [14]. Also, in in vitro analyses, treatment with globular adiponectin induced differentiation and fusion of skeletal muscle cells [15], and activated motility and regenerative traits in muscle satellite cells [16]. Hence, the up-regulation of adiponectin in sarcopenia may be a compensatory response to the stress of muscle wasting, as shown in functional overloading-associated muscle hypertrophy or regrowth of unloading-associated atrophied muscle [17].
Sialic acid, a derivative of neuraminic acid, has multiple physiological functions. Sialic acid is necessary for maintenance of skeletal muscle motor performance [18,19], and loss of sialic acid leads to myopathy [20]. The concentration of sialic acid in muscle is reportedly a marker of skeletal muscle aging [21]. We speculate that sialic acid may be up-regulated to compensate for reduced skeletal muscle motor performance in sarcopenia. Accordingly, it is considered that both adiponectin and sialic acid play an important role in skeletal muscle function and maintenance.
It was noteworthy that the sarcopenia index more strongly correlated with the three conventional diagnostic components of sarcopenia as compared with serum adiponectin, which is an established biomarker of sarcopenia [4–8], as shown in Fig 1. Moreover, ROC curve analysis demonstrated that the sarcopenia index had a greater AUC and higher sensitivity for detecting sarcopenia than serum adiponectin (Fig 2). Taken together, this suggests that sarcopenia index could be a new, accurate and reliable diagnostic tool for evaluation of sarcopenia in CVD patients.
Further, the sarcopenia index can be calculated by measurement of adiponectin and sialic acid and simple physical data (age, sex, weight and height). Since specific equipment and skill are required to evaluate the conventional diagnostic criteria, such as skeletal muscle mass and physical performance (handgrip strength and gait speed), it is sometimes difficult for general physicians to diagnose sarcopenia in their daily clinical practice. Therefore, sarcopenia index may have an advantage over conventional diagnostic methods in the screening of sarcopenia in CVD patients.
Limitations
The first limitation of this study was the small number of subjects and biomarkers investigated. Future large-scaled studies investigating a greater spectrum of biomarkers will improve the sensitivity and specificity of sarcopenia index. Next, we focused on CVD-related sarcopenia in this study. It is possible that the sarcopenia index for CVD-related sarcopenia might not be applicable for ‘primary’ (or age-related) sarcopenia and ‘secondary‘ sarcopenia related to other diseases. Second, as shown in new S1 and S2 Tables, the characteristic features of the baseline characteristics, nutritional markers and circulating levels of inflammatory markers in the females were almost similar to those in the whole patient group, whereas the characteristic features in the males seemed different from those in the females. Thus, it is possible that the gender difference would be important issue. The gender difference of the sarcopenia index should be addressed in future study including larger number of patients with enough statistic power.
Conclusion
We proposed a new diagnostic index for sarcopenia in CVD patients, which is calculated using values of circulating concentrations of adiponectin and sialic acid and simple physical data (i.e. sex, age, height and body weight). We believe that this sarcopenia index could be a simple and useful tool for diagnosing sarcopenia in CVD patients.
Supporting information
eGFR = estimated glomerular filtration rate.
(TIFF)
SMI = skeletal muscle index.
(TIFF)
(TIF)
(TIF)
Acknowledgments
We thank Misako Ando for assessment of nutritional status and measurement of SMI, and Kanae Matsuzaki, Yuya Tsukada and Michiya Kishimoto for obtaining physical performance data.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Xue QL. The frailty syndrome: Definition and natural history. Clin Geriatr Med 2011; 27: 1–15. doi: 10.1016/j.cger.2010.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis. Report of the European Working Group on Sarcopenia in Older People. Age and Ageing 2010; 39: 412–423. doi: 10.1093/ageing/afq034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harada H, Kai H, Niiyama H, Nishiyama Y, Katoh A, Yoshida N, et al. Effectiveness of Cardiac Rehabilitation for Prevention and Treatment of Sarcopenia in Patients with Cardiovascular Disease—a Retrospective Cross-sectional Analysis. J Nutr Health Aging 2016; [DOI] [PubMed] [Google Scholar]
- 4.Hui X, Lam KSL, Vanhoutte PM, Xu A. Adiponectin and cardiovascular health: an update. British Journal of Pharmacology 2012; 165: 574–590. doi: 10.1111/j.1476-5381.2011.01395.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shibata R, Murohara T, Ouchi N. Protective Role of Adiponectin in Cardiovascular Disease. Current Medicinal Chemistry 2012; 19: 5459–5466. [DOI] [PubMed] [Google Scholar]
- 6.Karvonen-Gutierrez CA, Zheng H, Mancuso P, Harlow SD. Higher Leptin and Adiponectin Concentrations Predict Poorer Performance-based Physical Functioning in Midlife Women: the Michigan Study of Women's Health Across the Nation. J Gerontol A Biol Sci Med Sci 2015; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang C, Tomata Y, Kakizaki M, Sugawara Y, Hozawa A, Momma H, et al. High circulating adiponectin levels predict decreased muscle strength among older adults aged 70 years and over: A prospective cohort study. Nutrition, Metabolism & Cardiovascular Diseases 2015; 25: 594–601. [DOI] [PubMed] [Google Scholar]
- 8.Sente T, Van Berendoncks AM, Hoymans VY, Vrints CJ. Adiponectin resistance in skeletal muscle: pathophysiological implications in chronic heart failure. J Cachexia Sarcopenia Muscle 2016; 7: 261–274. doi: 10.1002/jcsm.12086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kung T, Szabó T, Springer J, Doehner W, Anker SD, von Haehling S. Cachexia in heart disease: highlights from the ESC 2010. J Cachexia Sarcopenia Muscle 2011; 2: 63–69. doi: 10.1007/s13539-011-0020-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen LK, Liu LK, Woo J, Assantachai P, Auyeung T-W, Bahyah KS, et al. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc 2014; 15: 95–101. doi: 10.1016/j.jamda.2013.11.025 [DOI] [PubMed] [Google Scholar]
- 11.Kyle UG, Bosaeus I, De Lorenzo AD, Deurenberg P, Elia M, Go´mez JM, et al. Bioelectrical impedance analysis—part II: utilization in clinical practice. Clinical Nutrition 2004; 23: 1430–1453. doi: 10.1016/j.clnu.2004.09.012 [DOI] [PubMed] [Google Scholar]
- 12.Tanimoto Y, Watanabe M, Sun W, Sugiura Y, Tsuda Y, Kimura M, et al. Association between sarcopenia and higher-level functional capacity in daily living in community-dwelling elderly subjects in Japan. Archives of Gerontology and Geriatrics 2012; 55: e9–e13. doi: 10.1016/j.archger.2012.06.015 [DOI] [PubMed] [Google Scholar]
- 13.Ignacio de Ulíbarri J, González-Madroño A, de Villar NG, González P, González B, Mancha A, et al. CONUT: a tool for controlling nutritional status. First validation in a hospital population. Nutr Hosp 2005; 20: 38–45. [PubMed] [Google Scholar]
- 14.Abou-Samra M, Lecompte S, Schakman O, Noel L, Many MC, Gailly P, et al. Involvement of adiponectin in the pathogenesis of dystrophinopathy. Skeletal Muscle 2015; 5: 25 doi: 10.1186/s13395-015-0051-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fiaschi T, Cirelli D, Comito G, Gelmini S, Ramponi G, Serio M, et al. Globular adiponectin induces differentiation and fusion of skeletal muscle cells, Cell Research 2009; 19: 584–597. doi: 10.1038/cr.2009.39 [DOI] [PubMed] [Google Scholar]
- 16.Fiaschi T, Giannoni E, Taddei ML, Chiarugi P. Globular Adiponectin Activates Motility and Regenerative Traits of Muscle Satellite Cells. PLOS ONE 2012; www.plosone.org 2012; 7: e34782 doi: 10.1371/journal.pone.0034782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goto A, Ohno Y, Ikuta A, Suzuki M, Ohira T, Egawa T, et al. Up-Regulation of Adiponectin Expression in Antigravitational Soleus Muscle in Response to Unloading Followed by Reloading, and Functional Overloading in Mice. PLOS ONE 2013; www.plosone.org 8: e81929 doi: 10.1371/journal.pone.0081929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Malicdan MCV, Noguchi S, Nonaka I, Hayashi YK, Nishino I. A Gne knockout mouse expressing human V572L mutation develops features similar to distal myopathy with rimmed vacuoles or hereditary inclusion body myopathy. Human Molecular Genetics 2007; 16: 115–128. doi: 10.1093/hmg/ddl446 [DOI] [PubMed] [Google Scholar]
- 19.Malicdan MCV, Noguchi S, Nonaka I, Hayashi YK, Nishino I. A Gne knockout mouse expressing human GNE D176V mutation develops features similar to distal myopathy with rimmed vacuoles or hereditary inclusion body myopathy. Human Molecular Genetics 2007; 16: 2669–2682. doi: 10.1093/hmg/ddm220 [DOI] [PubMed] [Google Scholar]
- 20.Celeste FV, Vilboux T, Ciccone C, de Dios JK, Malicdan MCV, Leoyklang P, et al. Mutation Update for GNE Gene Variants Associated with GNE Myopathy. Hum Mutat 2014; 35: 915–926. doi: 10.1002/humu.22583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hanisch F, Weidemann W, Großmann M, Joshi PR, Holzhausen H-J, Stoltenburg G, et al. Sialylation and Muscle Performance: Sialic Acid Is a Marker of Muscle Ageing. PLOS ONE 2013; www.plosone.org 8: e80520 doi: 10.1371/journal.pone.0080520 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eGFR = estimated glomerular filtration rate.
(TIFF)
SMI = skeletal muscle index.
(TIFF)
(TIF)
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.