Abstract
Background
Electronic cigarette (EC) aerosols contain unique compounds in addition to toxicants and carcinogens traditionally found in tobacco smoke. Studies are warranted to understand the public health risks of ECs.
Objective
The aim of this study was to determine the genotoxicity and the mechanisms induced by EC aerosol extracts on human oral and lung epithelial cells.
Methods
Cells were exposed to EC aerosol or mainstream smoke extracts and DNA damage was measured using the primer anchored DNA damage detection assay (q-PADDA) and 8-oxo-dG ELISA assay. Cell viability, reactive oxygen species (ROS) and total antioxidant capacity (TAC) were measured using standard methods. mRNA and protein expression were evaluated by RT-PCR and western blot, respectively.
Results
EC aerosol extracts induced DNA damage in a dose-dependent manner, but independently of nicotine concentration. Overall, EC aerosol extracts induced significantly less DNA damage than mainstream smoke extracts, as measured by q-PADDA. However, the levels of oxidative DNA damage, as indicated by the presence of 8-oxo-dG, a highly mutagenic DNA lesion, were similar or slightly higher after exposure to EC aerosol compared to mainstream smoke extracts. Mechanistically, while exposure to EC extracts significantly increased ROS, it decreased TAC as well as the expression of 8-oxoguanine DNA glycosylase (OGG1), an enzyme essential for the removal of oxidative DNA damage.
Conclusions
Exposure to EC aerosol extracts suppressed the cellular antioxidant defenses and led to significant DNA damage. These findings emphasize the urgent need to investigate the potential long-term cancer risk of exposure to EC aerosol for vapers and the general public.
Introduction
Electronic cigarettes (ECs) are battery-powered devices that heat up a solution of chemicals (e-liquid) with or without nicotine and turn it into an inhalable aerosol. Whether ECs are a safer alternative to combustible tobacco products and/or assist patients with smoking cessation are still major controversies [1–6]. Nonetheless, the use of ECs has increased sharply since 2003 [7–9]. In a 2015 survey, about 10% of U.S. adults reported to use ECs [10]. Disturbingly, the use of ECs among middle and high school students has had a 4-fold increase between 2013 and 2014 reaching 3.9% and 13.4%, respectively [11]. The retail EC industry is projected to reach $50 billion USD by 2025 [12]. The U.S. Food and Drug Administration (FDA) has called for additional scientific research to inform the development of effective EC regulations [13] and has extended its regulatory authority to cover all tobacco products, including ECs [14]. The high prevalence of EC use, the polarized views on the subject, and the limited toxicology data available on EC aerosols, all stress the urgent need for rigorous evaluation of the health effects of EC aerosols to ensure public safety and support evidence-based public health policies and regulations.
The potential long-term human health effects of EC aerosols are unknown. EC aerosol constituents comprise some toxicants and carcinogens present in cigarette smoke, in addition to other unique, and potentially harmful compounds such as silicate beads, tin, and flavorants, most of which are not yet well characterized [15–18]. Chemicals identified in EC aerosols include the most potent carcinogenic tobacco-specific nitrosamines [nicotine metabolites: 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) and N'-nitrosonornicotine (NNN)], aldehydes, volatile organic compounds, phenolic compounds, polycyclic aromatic hydrocarbons, tobacco alkaloids, heavy metals, flavors, and nicotine [15–23].
Initial studies, focused mainly on first generation and/or low power devices, reported that the levels of potentially toxic compounds in EC aerosol (e.g. formaldehyde, acetaldehyde, acrolein, and toluene) are significantly lower (9- to 450-fold lower) than those in cigarette smoke [20, 24], and in many cases (e.g., NNN and NNK) comparable with the trace amounts present in nicotine replacement products [25–27]. However, recent studies, have shown that specific toxicants and carcinogens present in EC aerosols can reach levels equal (e.g., acetaldehyde and chromium) to or exceeding (e.g., formaldehyde, and nickel) to those found in cigarette smoke, particularly as the power of the device increases [15, 22]. Formaldehyde is a human carcinogen causally associated with many cancers, including oral and lung cancer [28]. Acetaldehyde is classified as possibly carcinogenic to humans [28]. Lead, nickel, and chromium are in the FDA's “harmful and potentially harmful chemicals” list [29] and tin is a potential lung carcinogen [30, 31]. EC aerosols also contain high levels of free radicals [32–34] and have been shown to induce oxidative stress and inflammation in mouse models [32, 33]. These data suggest that EC aerosols expose users and bystanders to toxic and carcinogenic substances, which have the potential to induce DNA damage and increase cancer risk.
In this work, we investigated the effects of short-term and long-term exposure to EC aerosol extracts on the levels of DNA damage in human oral and lung epithelial cells. Given the promoted potential role of EC as a harm reduction strategy for smoking cessation, we also performed side-by-side toxicology and mechanistic studies to characterize the genotoxicity associated with exposure to EC aerosol and tobacco smoke extracts. In this study, several systems important for understanding the mechanisms of genotoxicity (e.g., total cellular antioxidant capacity, cellular reactive oxygen species, and distinct DNA repair mechanisms) were investigated taking into consideration the potential use of EC as a smoking cessation aid. Our studies identified significant differences and similarities in the mechanisms and the levels of DNA damage induced by EC aerosol and mainstream tobacco smoke. Of high public health significance, our study is the first to suggest that even very low levels of exposure to EC aerosol can lead to significant DNA damage and potentially increase cancer risk. Our study also emphasizes the urgent need to further investigate the health consequences of exposure to EC aerosols and highlights the extreme importance of regulating ECs for the purpose of protecting the public.
Material and methods
E-cigarette aerosol and tobacco smoke extracts
Tobacco smoke extracts were prepared from Marlboro 100s (16 mg tar and 1.2 mg nicotine, Philip Morris) cigarettes as previously described [35]. According to 2015 sales data, Marlboro is the most popular cigarette brand in the United States, with sales greater than the next eight leading competitors combined [36]. Smoking conditions were two 50 mL puffs per minute, until the cigarette burned to 3mm short of the filter. This puff regimen (volume and interval) mimics the reported human puffing profiles for cigarettes with more than 14 mg tar [37] and is similar to the Health Canada Intensive (HCI) smoking standard conditions (two 55±5 ml puffs per minute). A similar puffing regimen of two 55mL puffs per minute has been recommended for EC aerosol studies [38]. Mainstream (MS) smoke is the material drawn from the mouth end of a cigarette during puffing and inhaled by smokers. A modification of the smoke extraction apparatus was used to produce ECs extracts as previously described [39]. The changes in mass observed for ECs were consistent with the amount of EC liquid consumed by experienced EC users [40]. Five distinct EC extracts were prepared from two distinct device types: NJoy [OneJoy, Traditional Flavor, propylene glycol/vegetable glycerin (PG/VG) 50:50, undisclosed power], and eGo-T (OKC Vapes, Desert Sands Flavor, PG/VG 50:50, 6 W) and based on the commercially available nicotine concentrations for each e-liquid: N12 (NJoy 12 mg/ml nicotine), N18 (NJoy 18 mg/ml nicotine), E0 (eGo 0 mg/ml nicotine), E12 (eGo 12 mg/ml nicotine), and E18 (eGo 18 mg/ml nicotine). An e-liquid without nicotine was not commercially available for the NJoy device used in this study. Identical extraction apparatus were used for each EC device (NJoy and eGo-T). The extraction apparatus used for each device were extensively cleaned between extractions and the lines carrying EC aerosol were replaced. To assure extract stability, extracts were aliquoted and frozen at -80°C immediately after preparation. A new aliquot was thawed just before cells were to be exposed. For our two weeks exposure experiments, aliquots were maintained at -80°C, and a new aliquot was thawed every other day, just before media preparation and exchange.
Nicotine concentration was determined by Gas Chromatography Mass Spectroscopy (GCMS) analysis essentially as previously described [41], except for the use of a smaller (500 μl) sample volume (full details in S1 File). Analysis was conducted using an Agilent 6890 GC with 5973 quadrapole Mass Selective Detector. The method detection limit (MDL) of 0.076 μg/ml was estimated from 11 replicate analyses of nicotine in HEPES at 0.39 μg/ml using the EPA approach. Lower limit of quantification was set a 3MDL = 0.227 μg/ml. The nicotine concentrations present in our stock (10 puffs/100 ml) extract solutions were determined to be the following: E0, below limit of detection; N12 = 0.254±0.026; E12 = 0.535±0.021; E18 = 1.715± 0.009; N18 = 1.957±0.030; MS = 15.419±0.134 μg/ml. The average nicotine yield for one cigarette was 1.542 mg, which is within the range described for reference 3R4F cigarettes smoked under International Standards Organization (0.707 to 1.84 mg) or HCI (1.90 mg) smoking conditions [27, 42, 43].
Cell culture
All cell lines were cultured under standard conditions [44]. Human epithelial normal bronchial cells (Nuli1) were cultured in serum free Airway Epithelial Cell Basal Medium (ATCC CRL-4011), with Bronchial Epithelial Cell Growth Kit additives (ATCC PCS-300-040). The human premalignant dysplastic oral mucosal keratinocyte cells (POE9n) were cultured in keratinocyte serum free medium supplemented with 25 μg of bovine pituitary extract (BPE) per ml, 0.2 ng of EGF per ml, and 0.4 mM CaCl2 [45, 46]. Human oral squamous cell carcinoma (UM-SCC-1) cells were cultured in high glucose Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum [47]. Cell line verification was performed by short-tandem repeat-based DNA profiling (CellCheck Cell line authentication, IDEXX Bioresearch, Columbia, MO, USA).
E-cigarette aerosol and tobacco smoke exposure
For short-term experiments, epithelial cancer and non-cancer cells were exposed for 1 h to diverse doses of EC aerosol extract (equivalent to 1, 10 or 100 puffs/5 L). The EC doses used include doses representative of the approximate number of puffs reported by EC users: 13 puffs/vaping session [40] or 120–180/day [7, 48] and are indicated as puffs per 5 L (the typical blood volume of an adult). To mimic chronic genotoxic exposure [49–51], cells were treated every other day for 2 weeks with 10 puffs/5 L of EC aerosol extract. Control cells were exposed to vehicle only. Mainstream smoke extract was used for comparison at a dose equivalent to 10 puffs/5 L (~ 1 cigarette), which we have previously shown to cause significant DNA damage [52].
Quantification of DNA damage
Total genomic DNA was isolated according to Mullenbach et al. [53], and following previously described steps to reduce artifactual DNA damage [54]. DNA damage was quantified using two distinct methods: a PCR based assay (q-PADDA) which detects many types of DNA damage with high sensitivity [52, 54], and a colorimetric based assay (HT 8-oxo-dG ELISA Kit II, Trevigen, MD) which detects exclusively 8-hydroxy-2’-deoxyguanosine (8-oxo-dG) lesions. 8-oxo-dG is one of the major products of DNA oxidation. The primer-anchored DNA damage detection assay (q-PADDA) was performed as we previously described [52]. We chose to quantify DNA damage within the transcribed strand (TS) and non-transcribed strand (NTS) of TP53 (commonly referred as p53), because p53 is the most frequently mutated gene in human cancer [55] and is mutated in nearly all smoking related cancers.[56, 57] For DNA damage quantification, as well as for all other analysis described below, we performed three independent experiments, each with 3–6 technical replicates.
ROS, TAC and MTT assays
The activity of hydroxyl, peroxyl and other reactive oxygen species (ROS) within the cell was determined using a standard 2’,7’–dichlorofluorescin diacetate (DCFDA) assay (Abcam, MA). The total cellular antioxidant activity was measured using a standard kit (Cayman Chemical Company, MI), which relies on the ability of antioxidants in the sample to inhibit the oxidation of ABTS (2,2'-azino-di-[3-ethylbenzthiazoline sulphonate]). Cell viability was measured by MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) assay (Invitrogen, CA) as we previously described [52]. All assays were performed as recommended by the respective manufacturer. Three independent experiments were performed, each with at least three technical replicates.
Real-time RT-PCR analysis
Total RNA was isolated from cells using TRIzol reagent (Invitrogen) and subjected to reverse transcription with SuperscriptTM II RNase H—Reverse Transcriptase and random hexanucleotide primers (Invitrogen). The cDNA was subsequently used for real-time RT-PCR using gene specific primers (ERCC1-512F, GGCGACGTAATTCCCGACT; ERCC1-596R, TAGCGGAGGCTGAGGAACA; OGG1-714F, AAATTCCAAGGTGTGCGACTG; OGG1-796R, GCGATGTTGTTGTTGGAGGA). β-actin expression was used as a normalization control as we previously described [58]. The changes in mRNA were expressed as fold change relative to untreated cells.
Western blot analysis
Protein was extracted in radioimmuno precipitation assay buffer (RIPA), sonicated, and centrifuged at 12,000 r.p.m. for 10 min at 4°C. Total protein (40 μg) from each sample was fractionated on 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and electrotransferred on to a PVDF (polyvinylene difluride) membrane. The membranes were blocked for 1 h with 5% non-fat milk and incubated overnight at 4°C with primary antibodies [ERCC1 (sc-56673), OGG1 (sc-376935) or actin (sc-1616); Santa Cruz Biotechnology, CA]. The membranes were then washed thrice in Tris-Buffered Saline and Tween 20 (TBS-T). This was followed by incubation with secondary antibodies coupled with HRP (Santa Cruz Biotechnology) for 1 h at room temperature, and thrice washed in TBS-T. Immunoreactive antibody–antigen complexes were visualized with the enhanced chemiluminescence reagents (Pierce Biotechnology, IL). The signals were detected using the ChemiDocTM touch imaging system (BioRad, CA) and quantified using Image lab software (BioRad, CA). Protein expression was normalized using actin as control.
Statistical analysis
Data were compiled in Excel (Microsoft) files and statistical analyses were performed using SAS/STAT Version 9.1 (SAS Institute Inc.). Independent means were compared using unpaired Student's t tests whose degrees of freedom were corrected, when appropriate, for inequality of variance. We considered p < 0.05 to be statistically significant.
Results
EC aerosols induce a dose-dependent increase in DNA damage
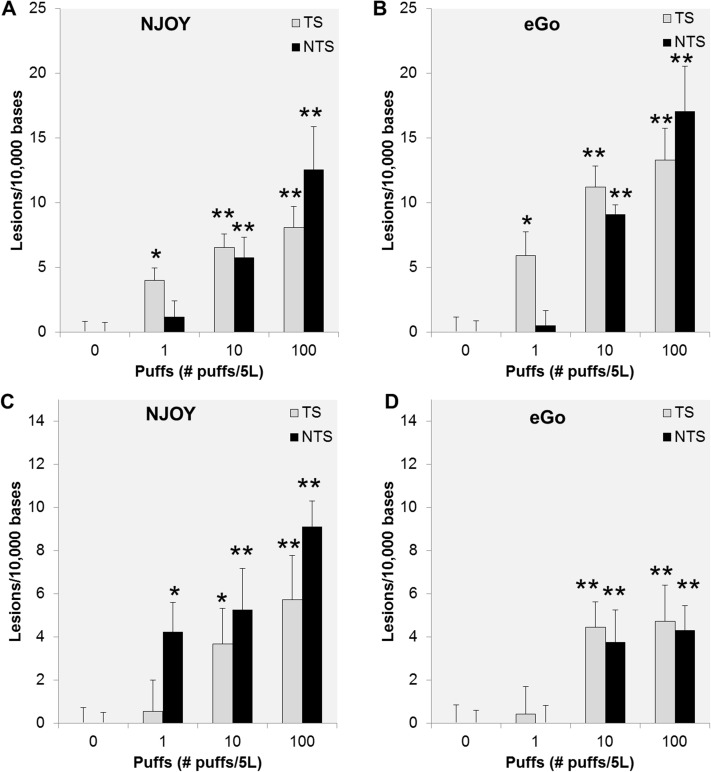
EC aerosols contain several potential toxicants and have been reported to share various adverse effects with tobacco smoke including: causing oxidative stress [32, 33] and eliciting bronchial patterns of gene expression similar to tobacco smoke [59, 60]. Therefore, it is essential to determine whether exposure to EC aerosol can be a significant source of DNA damage to oral and lung epithelial cells. We have previously reported that exposure of human oral epithelial cancer cells (UM-SCC-1) to very low doses of mainstream smoke extract causes significant DNA damage measurable by q-PADDA [52]. Our initial aim was to assess whether EC aerosol has a similar effect on oral and lung epithelial cells. Hence, we first exposed UM-SCC-1 cells for 1 h to increasing doses of aerosol extracts obtained from two distinct brands of ECs and measured DNA damage using q-PADDA. This assay has high sensitivity to detect many types of DNA damage including alkylative and oxidative lesions [52, 54], potentially caused by formaldehyde and reactive oxygen species present in EC aerosol. Both EC aerosol extracts induced significant DNA damage (p<0.001) in UM-SCC-1 cells when compared to the unexposed control cells (Fig 1A and 1B). A significant increase in DNA damage was also observed in NuLi1 cells, an immortalized cell line established from normal bronchus epithelium, after 1 h exposure to either EC aerosol extract (Fig 1C and 1D). Remarkably, the increase in DNA damage (p<0.001) was consistently observed in both p53 DNA strands and in both cell lines for doses equivalent to 10 or more EC puffs/5L (Fig 1). Moreover, a dose-dependent increase in DNA damage was also observed in both cell lines and for both EC extracts (Fig 1). No cell death was observed for either of the doses used (S1 Fig). By comparison with our previously published data [52], the levels of DNA damage induced by EC extracts are lower than those induced by mainstream smoke extracts. Altogether, our data show that EC aerosol can cause significant DNA damage in human oral and lung epithelial cells.
Fig 1. Dose-dependent increase in DNA damage in cells exposed to EC aerosol extracts.
UM-SCC-1 (A and B) and NuLi1 (C and D) cells were exposed for 1 h to increasing doses of NJOY (N18) or eGo (E18) and DNA damage quantified by q-PADDA within the transcribed (TS) and non-transcribed (NTS) strands of the TP53 gene. Data are represented as mean ± SEM. *p<0.05, **p<0.01.
DNA damage induced by EC aerosols is nicotine independent
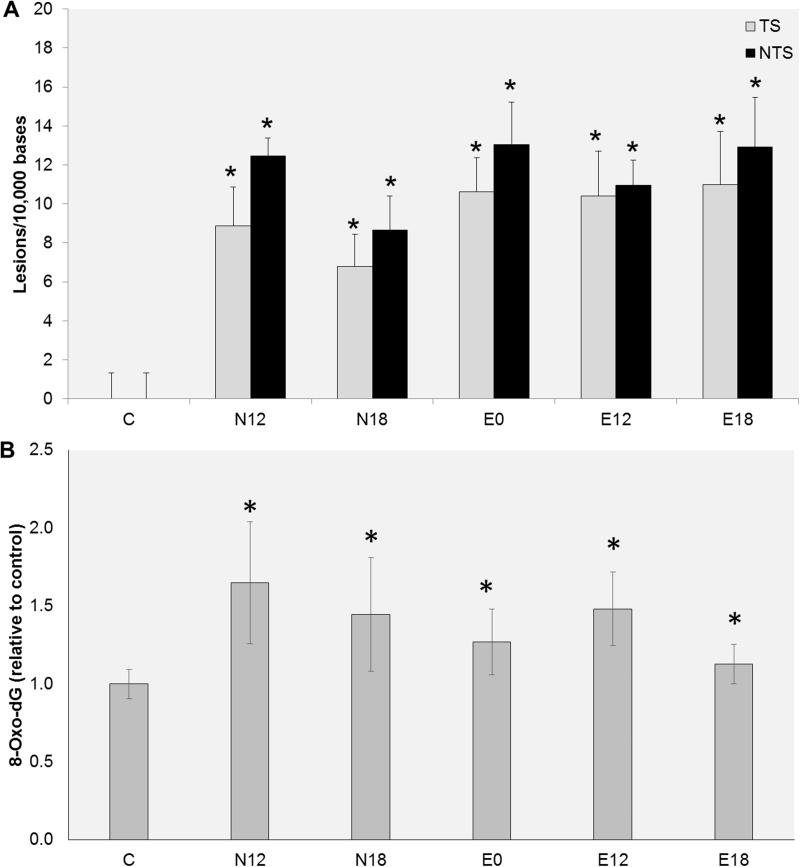
To determine whether the observed DNA damage is dependent of the level of nicotine present in the EC aerosol, cells were exposed to extracts obtained from e-liquids containing diverse nicotine concentrations (0, 12 or 18 mg/ml). All extracts induced significant levels of DNA damage within p53 (Fig 2A). Yet, EC aerosols obtained from e-liquids lacking nicotine caused levels of DNA damage similar to those obtained from e-liquids containing 12 or 18 mg/ml of nicotine (Fig 2A). EC aerosol have been reported to contain high levels of reactive oxygen species [32, 33]. Therefore, we determined whether exposure to EC aerosol extracts causes oxidative damage. For that purpose we chose to quantify the levels of 8-oxo-dG, one of the most frequent and most mutagenic oxidative DNA lesions. As shown in Fig 2B, all EC extracts induced significant levels of 8-oxo-dG. Consistent with the data observed with q-PADDA, the levels of damage induced by the diverse extracts were similar and independent of the concentration of nicotine present in the e-liquid from which the aerosols were derived (Fig 2B). Altogether, these data suggest that other components, rather than nicotine, are responsible for the DNA damage induced by EC aerosol extracts.
Fig 2. Effect of nicotine on DNA damage levels.
UM-SCC-1 cells were exposed for 1 h to EC aerosol extracts obtained from e-liquids with different nicotine concentrations and DNA damage quantified by q-PADDA (A) or ELISA (B). Data are represented as mean ± SEM. *p<0.05.
Chronic exposure to EC aerosols causes significant oxidative DNA damage
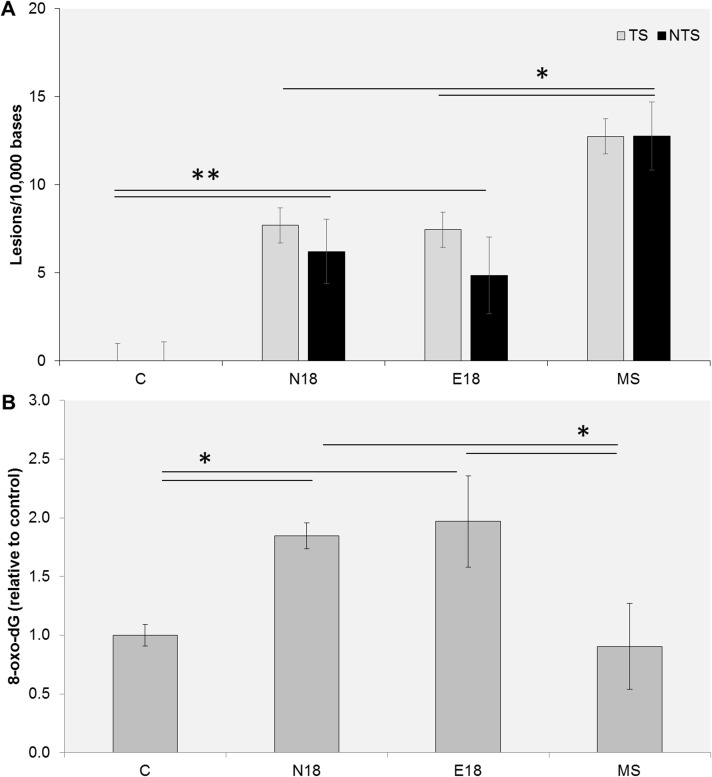
DNA damage is the main initiator of cancer [61] and plays a key role in the pathogenesis of aging, neurodegenerative, pulmonary, and cardiovascular diseases [62, 63]. To compare the potential DNA damaging effects of long-term exposure to EC aerosols and tobacco smoke extracts, we measured overall DNA damage within p53 and quantified total 8-oxo-dG DNA lesions in cells exposed for 2 weeks to either EC aerosol (N18 or E18) or mainstream (MS) smoke extracts. Significant levels of DNA damage within p53 were observed in cells exposed to EC extracts relative to unexposed control cells (Fig 3A). Consistent with the data observed after acute exposure, we also observed significantly lower levels of DNA damage within p53 in cells exposed to EC aerosol extracts than in cells exposed to mainstream smoke extracts. Cells exposed to EC aerosol extract also had significantly higher levels of 8-oxo-dG, than unexposed cells (Fig 3B). These data show that chronic exposure to EC aerosol can induce significant DNA damage, including highly mutagenic oxidative damage. Intriguingly, the observed levels of 8-oxo-dG in UM-SCC-1 cells were higher after long-term exposure to EC aerosol than to mainstream smoke extract (Fig 3B). These data suggest that either EC aerosols cause more oxidative stress than mainstream tobacco smoke, or cells exposed to EC fail to activate cellular pathways that prevent oxidative DNA damage.
Fig 3. DNA damage levels measured after long-term exposure to EC aerosol or MS smoke extracts.
UM-SCC-1 cells were exposed for 2 weeks to EC aerosol (N18 and E18) or MS smoke extracts and DNA damage quantified by q-PADDA (A) or ELISA (B). Data are represented as mean ± SEM. *p<0.05; **p<0.01.
EC aerosols induce significant ROS and decrease the cellular total antioxidant capacity
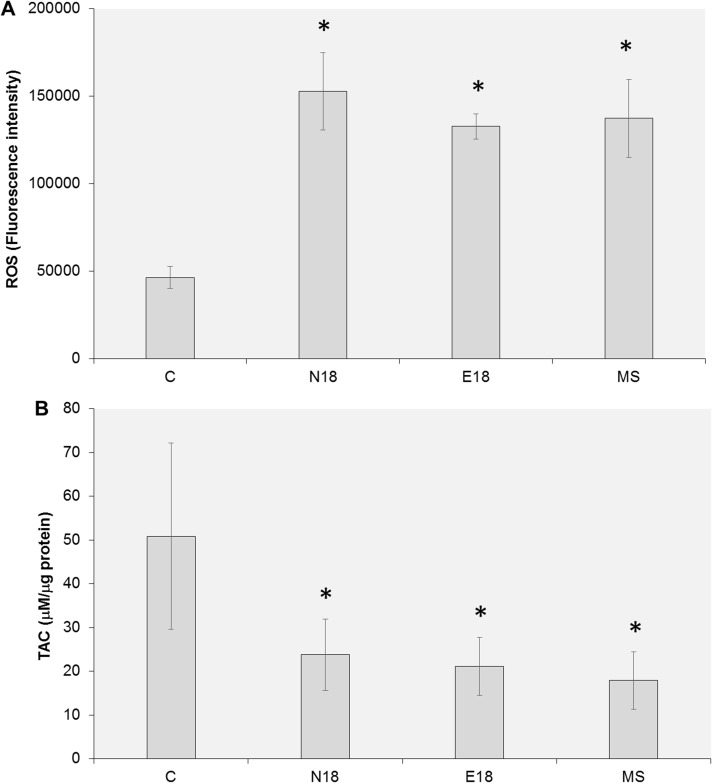
To investigate the mechanisms contributing to our observations, we quantified the intracellular levels of ROS in UM-SCC-1 cells exposed to EC aerosol or mainstream smoke extracts for 2 weeks. We observed a significant increase in the intracellular levels of ROS after exposure to EC extracts (Fig 4A). Similar levels of ROS were also observed after long-term exposure to mainstream smoke (Fig 4A). Then, we measured the cellular total antioxidant capacity after long-term exposure to EC aerosol or mainstream smoke extracts. Consistent with the observed increase in cellular ROS, we observed a significant decrease in the total antioxidant capacity of cells exposed to EC aerosol (Fig 4B). There was no significant difference in TAC levels between EC exposed cells and MS exposed cells (Fig 4B). These data suggest that at the doses tested, EC and mainstream smoke induce similar levels of oxidative stress.
Fig 4. Exposure to EC aerosol increases cellular ROS and decreases TAC.
UM-SCC-1 cells were exposed for 2 weeks to EC aerosol (N18 and E18) or MS smoke extracts and the cellular levels of ROS (A) and TAC (B) were measured using standard methods. Data are represented as mean ± SEM. *p<0.05.
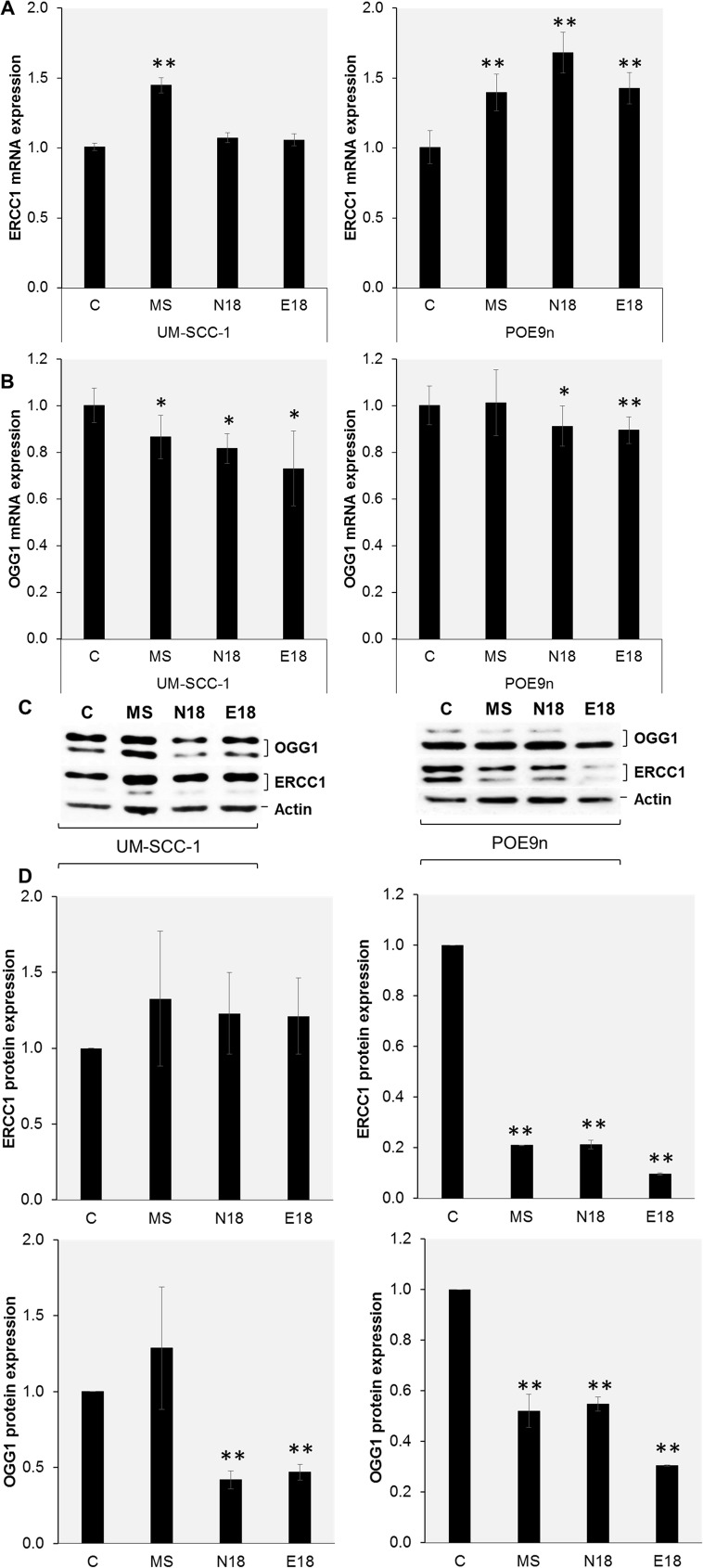
EC aerosols decrease the levels of proteins essential for the removal of DNA damage
Aldehydes and free radicals, such as those reported in EC aerosols and tobacco smoke, are known to cause diverse types of DNA damage which are repaired mainly by nucleotide excision repair (NER) and base excision repair (BER). Hence, we assessed whether chronic exposure to EC aerosol and mainstream smoke extracts modifies the expression of ERCC1 (excision repair cross-complementation group 1), a NER protein essential for the removal of bulky DNA damage, and OGG1 (8-oxoguanine-DNA glycosylase), a BER protein essential for the removal of oxidative DNA lesions. Using real-time RT-PCR analysis, we observed that ERCC1 mRNA expression was significantly increased in cells after exposure to MS smoke, compared to unexposed control cells (Fig 5A). A similar trend was observed after exposure to EC extracts, but the data only reached significance in POE9n cells (Fig 5A). In contrast, we observed that exposure to EC aerosol (N18 and E18) caused a significant decrease (p<0.01) in the expression of OGG1 mRNA in both cell lines, compared to unexposed control cells (Fig 5B). Exposure to MS smoke lead to a significant decrease in OGG1 mRNA only in UM-SCC-1 cells (Fig 5B). To assess whether exposure to EC aerosol and MS smoke extracts had an impact on protein availability for the removal of the diverse types of DNA damage, we performed western blot analysis. We observed that chronic exposure to EC aerosol or mainstream smoke extracts caused a significant reduction in ERCC1 protein in POE9n cells, but did not significantly change the levels of ERCC1 protein expression in UM-SCC-1 cells (Fig 5C). Most importantly, consistent with our mRNA analysis, chronic exposure of either cell line to EC aerosol extracts led to a significant decrease in the expression of the OGG1 protein (Fig 5C and 5D). Chronic exposure of POE9n to MS smoke extracts also lead to a significant decrease in the expression of OGG1 protein (Fig 5C and 5D). No significant changes in OGG1 protein levels were observed after exposure of UM-SCC-1 cells to MS smoke extracts (Fig 5C and 5D). These data suggest that the significant decrease in the expression of OGG1 protein might lead to a significant decrease in the repair of 8-oxo-dG, and contribute to the observed high levels of 8-oxo-dG lesions observed after chronic exposure to EC aerosol extracts. Overall, these data show that chronic exposure to EC aerosols can lead to a significant reduction in the level of OGG1 and ERCC1, and consequently might reduce the overall repair capacity of the two main pathways responsible for the repair of oxidative and bulky DNA damage: basic excision and nucleotide excision repair.
Fig 5. Exposure to EC aerosol changes the expression of DNA repair proteins.
UM-SCC-1 and POE9n cells were exposed for 2 weeks to EC aerosol or MS smoke extracts and the levels of mRNA (A,B) and protein (C) were measured by real-time RT-PCR and western blot, respectively. Protein expression after chronic exposure to EC aerosol or MS smoke extracts was quantified and compared to non-exposed cells (D). Data are represented as mean ± SD. *p<0.05; **p<0.01.
Discussion
Active and passive smoking constitute a significant public health problem, as tobacco smoke is the leading preventable cause of morbidity and mortality [64]. ECs have been promoted as a smoking cessation aid and, based on limited data, are perceived as less harmful to one’s health than traditional cigarettes. However, the study of the potential long-term health effects of EC use is lacking. In this study, we examined the effects of exposure to EC aerosol extracts on DNA damage in human oral and lung epithelial cells. Moreover, we investigated the effects of long-term exposure to EC aerosol extracts on the main cellular mechanisms that modulate the levels of oxidative stress and DNA damage: total antioxidant capacity, cellular ROS and DNA damage repair. Parallel studies with mainstream smoke allowed for side-by-side comparison. Of major public health relevance, all the EC aerosols used in this study induced significant DNA damage, including high levels of 8-oxo-dG, a highly mutagenic DNA lesion.
The puffing regimen used for EC aerosol and MS smoke extracts is similar to the puffing regimen that has been recommended for EC aerosol studies [38]. The smoking regimen used to obtain MS smoke extract (two 50 ml puffs/minute) mimics the reported human puffing profiles for cigarettes with more than 14 mg tar [37] and is similar to the Health Canada Intensive smoking standard conditions (two 55±5 ml puffs per minute). These conditions have been reported to generate higher amount of smoke per cigarette than the ISO smoking conditions (one 35 ml puff per minute), however the amount of toxicants and the in vitro toxicity per mg of nicotine have been reported to be generally lower under HCI than under ISO smoking conditions [43].
The aerosol doses used in our study (1 to 100 puffs/5 L) encompass those obtained by experienced EC users during a vaping session. For example, it has been reported that eGo EC users vape on average 13 puffs and consume 62 ± 16 mg e-liquid in 5 min (similar to the time needed to smoke one tobacco cigarette) vaping session [40]. For chronic exposure, we chose to use EC aerosol extracts at a dose equivalent to 10 puffs/5L. Based on the nicotine measured in our extracts, which varied with the EC device and the e-liquid nicotine concentration, 10 puffs of EC aerosol extract per 5L lead to approximately zero (E0), 5.1 (N12), 10.7 (E12), 34.3 (E18), or 39.1 (N18) ng/ml of nicotine. These doses are representative of the nicotine concentrations we observed in the plasma of EC users immediately after a 10 puff session (4.5–30.4 ng/ml) or a two hour ad libitum vaping session (10.7–36.4 ng/ml) [65]. Moreover, they encompass the nicotine levels reported in the plasma of smokers and are significantly lower than the average dose present in the oral cavity of vapers and smokers, which due to nicotine concentration in saliva can be up to 100-fold higher than in the plasma [66].
To the best of our knowledge, our study is the first to show that short-term exposure to increasing doses of EC aerosol induces a dose-dependent increase in DNA damage. Moreover, we have shown that long-term exposure to doses of EC extract, similar to those experienced by EC users [65], leads to significant levels of DNA damage. Previously, a single study has reported that exposure to EC aerosol extracts caused extensive DNA strand-breaks [67]. However, the biological relevance of this study is hampered by the fact that the authors used an extremely high dose of EC aerosol extracts (1% by volume), reported by the authors to be equivalent to 500 μM of nicotine and to cause very high cell death (49% to 66% in non-cancer cells). Cell death leads to DNA breakage and is a major confounding variable when DNA damage is measured exclusively in function of detectable strand breaks, as it was reported by Yu et al. (2016). Using doses up to 100 puffs/5L, which is well above the average puffing at a single vaping session, we observed no cell death in cancer and non-cancer cells. Our data is consistent with all other toxicology studies using EC aerosols, which have shown that, in contrast with EC e-liquids [68–71], EC aerosols cause little or no cell death [39, 68, 72].
In our study, we used two completely distinct approaches to measure DNA damage: a very sensitive PCR based assay (q-PADDA) that quantifies a broad spectra of DNA lesions (including oxidative, alkylative and bulky lesions) [52, 54], and an ELISA assay that detects a specific type of oxidative DNA damage (8-oxo-dG). DNA damage precedes mutation and varies significantly by genomic area. Therefore, for q-PADDA assay, we chose to quantify damage in p53, because p53 is the most frequently mutated gene in human cancer [55], and is mutated in nearly all smoking related cancers [56]. Our data consistently showed that both short-term and long-term exposure to EC aerosol extracts led to a significant increase in DNA damage which is detectable by both methods, ELISA and q-PADDA.
There have been no previous studies comparing side-by-side the effects of similar doses of EC aerosol and mainstream smoke extracts on overall DNA damage. Nonetheless, a single study has reported that tobacco smoke causes significantly higher levels of double-strand breaks (a specific type of DNA damage) than EC aerosol [73]. Tobacco smoke is a complex mixture of more than 7000 chemicals: hundreds of these are hazardous, and at least 69 are known to damage DNA and cause cancer [74]. EC aerosols have been reported to contain potentially toxic compounds, but overall the number of genotoxic substances and their concentration, for the most part, has been reported to be significantly lower than those in cigarette smoke [20, 24–26]. In agreement with these data, we showed that both short-term and long-term exposure to EC extract led to overall less DNA damage than the equivalent exposure to mainstream smoke.
A single cigarette puff of tobacco smoke contains over a 1014 free radicals [75], and causes a 35–50% increase in oxidative DNA damage [74]. EC aerosols have also been reported to contain high levels of free radicals [32–34] and shown to cause oxidative stress and inflammatory responses [32, 33, 39, 76]. Yet, no previous studies have reported whether or not EC aerosols cause oxidative DNA damage. Here, we showed that both short-term and long-term exposure to EC aerosol extracts induced a significant increase in the levels of 8-oxo-dG, one of the major DNA lesions formed from reactive oxygen species (ROS). These data are of high concern, as 8-oxo-dG base modification is highly mutagenic and if not recognized and repaired it may cause GC to TA transversions [77, 78]. Moreover, oxidative DNA lesions have been proposed to cause the vast majority of human mutations [79]. Our data is in apparent contradiction with the fact that ECs have been deemed non-mutagenic to Ames Salmonella tester strains TA98 and TA100 [80, 81]. However, none of the previous studies included the strains TA102 or TA104 which have the highest sensitivity for the detection of mutagenesis induced by oxidative or cross-linking agents [82]. TA104 has also been reported to be particularly sensitive to carbonyl based compounds [83], as those reported to be present in EC aerosol. Moreover, the Organization for Economic Co-operation and Development (OECD) recommends testing a battery of at least five tester strains to capture the full range of chemical interactions and mutagenic events acting via different modes of action[81]. Another variable to consider, is the fact that toxicant yield varies with EC brand, product model, e-liquid flavor, e-liquid solvent, and device power [19, 21, 23, 84, 85] so it is not possible to directly compare our study to others using different ECs or e-liquids. Nonetheless, altogether these data stress the need to further investigate whether the oxidative damage induced by EC aerosol increases mutagenicity.
Oxidative DNA damage reflects not only exposure to specific genotoxics, but also the cellular capacity for antioxidant detoxification and DNA repair. Tobacco smoke has been previously shown to reduce the antioxidant capacity of tissues [86]. Here, we showed that like MS smoke, long-term exposure to EC aerosol significantly increases cellular ROS and decreases total cellular antioxidant capacity. These observations are very important as they pinpoint additional mechanisms, besides the genotoxic content of EC aerosols, by which exposure to EC aerosol might contribute to increase DNA damage.
To deal with the large variety of DNA lesions induced by endogenous and exogenous genotoxics, human cells have developed a network of DNA repair mechanisms, involving more than 150 genes. Among the major types of DNA repair mechanisms, nucleotide excision repair (NER) is the most versatile repair pathway in the cell. NER is the primary mechanism for the removal of bulky DNA adducts that significantly distort the DNA helix structure, such as those induced by tobacco smoke [87, 88]. NER also plays an important role in the repair of oxidative damage. Basic excision repair (BER) is the main pathway for the removal of small base lesions caused by reactive oxygen and nitrogen species, as well as by alkylating agents [87], such as those reported in EC aerosol and tobacco smoke [15, 22]. Tobacco smoke has previously been shown to reduce the rate of DNA damage repair [89]. Here, we show for the first time that exposure to EC aerosol extracts can also lead to a significant decrease in the expression of ERCC1 and OGG1, two DNA excision repair proteins that are rate limiting for the efficiency of NER and BER, respectively. ERCC1 forms a complex with XPF protein forming a nuclease that is essential for incision of a DNA lesion during nucleotide excision repair. The ERCC1-XPF nuclease also participates in the repair of DNA double-strand breaks and crosslinks. NER deficiency results in the skin cancer-prone inherited disease xeroderma pigmentosum (XP) [87]. Polymorphic variations on ERCC1 gene and low levels of the ERCC1 enzyme have also been shown to be a marker for susceptibility to tobacco-related cancers [90]. OGG1 is the main enzyme responsible for the removal of 8-oxo-dG. Alteration in the levels of OGG1 and polymorphisms in the OGG1 gene modulate BER capacity and are known risk factors for human cancer [91]. The fact that EC aerosols used in this study, like MS smoke, can lead to a significant decrease in the expression of ERCC1 and OGG1, suggest yet another mechanism by which exposure to EC aerosol can contribute to DNA damage, and therefore increase cancer risk.
These observations have significant implications not only for the repair of DNA damage induced by exposure to EC aerosol, but also for the repair of DNA damage induced by other toxicants that EC users and bystanders might be exposed to.
Due to the high number of variables studied and length of exposure, our study used established cell lines instead of primary cells or tissues. Established cell lines might lack metabolic competency which can affect their ability to bioactivate and/or detoxify xenobiotics. All cell lines used in this study have an intact wild-type p53 gene [92], but due to the presence of HPV E6/E7 or a splice mutation (UM-SCC-1) express low levels of wild-type p53 protein [93] and might have impaired DNA repair capacity. Cell lines with defective DNA repair are anticipated to have higher levels of basal DNA damage than those with effective DNA repair, making it harder to detect small increases in DNA damage. Yet, our data clearly shows that under the conditions tested exposure to EC aerosol induces significant DNA damage. Whether these observations reflect mainly an in vitro exposure or is a common event occurring in EC users is currently being investigated in our laboratory.
In summary, here we report for the first time that exposure to EC aerosol extracts can cause significant levels of DNA damage, including high levels of 8-oxo-dG, an oxidative and highly mutagenic DNA damage lesion. Even so, the overall EC-induced DNA damage levels were in general lower than the levels of damage induced by mainstream smoke extracts. By examining the mechanisms that modulate DNA damage, we identified an increase in cellular ROS, a decrease in TAC, and a decrease in the expression of proteins essential for DNA damage repair as novel mechanisms by which exposure to EC aerosol can cause DNA damage and potentially increase cancer risk. Our study emphasizes the further need to investigate the health consequences of exposure to EC aerosols and highlights the extreme importance in regulating ECs and the exposure to EC aerosol.
Supporting information
UM-SCC-1 and NuLi1 cells were continuously exposed to 10 or 100 puffs/5L of EC aerosol extracts and cell viability was determined by MTT assay at 96 h. Data are represented as mean ± SD.
(TIF)
(PDF)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the Oklahoma Tobacco Research Center (LQ), the Presbyterian Health Foundation (LQ) and by the Oklahoma Center for the Advancement of Science & Technology (LQ). Dr. Queimado holds a Presbyterian Health Foundation Endowed Chair in Otorhinolaryngology Position. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Hajek P. Electronic cigarettes have a potential for huge public health benefit. BMC Med. 2014;12:225 PubMed Central PMCID: PMC4260378. doi: 10.1186/s12916-014-0225-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaplan A. Rebuttal: Can electronic cigarettes assist patients with smoking cessation? Yes. Can Fam Physician. 2015;61(6):e255 [PMC free article] [PubMed] [Google Scholar]
- 3.Kaplan A. Can electronic cigarettes assist patients with smoking cessation? Yes. Can Fam Physician. 2015;61(6):499–500. [PMC free article] [PubMed] [Google Scholar]
- 4.Levitz S. Rebuttal: Can electronic cigarettes assist patients with smoking cessation? No. Can Fam Physician. 2015;61(6):e256 [PMC free article] [PubMed] [Google Scholar]
- 5.Levitz S. Can electronic cigarettes assist patients with smoking cessation? No. Can Fam Physician. 2015;61(6):500–1. [PMC free article] [PubMed] [Google Scholar]
- 6.e-cigarettes an evidence update https://www.gov.uk/government/publications/e-cigarettes-an-evidence-update: Public Health England; 2015 [updated Aug 19, 2015; cited 2016 12/28/2016].
- 7.Etter JF, Bullen C. Electronic cigarette: users profile, utilization, satisfaction and perceived efficacy. Addiction. 2011;106(11):2017–28. doi: 10.1111/j.1360-0443.2011.03505.x [DOI] [PubMed] [Google Scholar]
- 8.Wagener TL, Siegel M, Borrelli B. Electronic cigarettes: achieving a balanced perspective. Addiction. 2012;107(9):1545–8. doi: 10.1111/j.1360-0443.2012.03826.x [DOI] [PubMed] [Google Scholar]
- 9.Pearson JL, Richardson A, Niaura RS, Vallone DM, Abrams DB. e-Cigarette awareness, use, and harm perceptions in US adults. Am J Public Health. 2012;102(9):1758–66. PubMed Central PMCID: PMC3474361. doi: 10.2105/AJPH.2011.300526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mincer J. E-cigarette usage surges in past year: Reuters/Ipsos poll. 2015.
- 11.Arrazola RA, Singh T, Corey CG, Husten CG, Neff LJ, Apelberg BJ, et al. Tobacco Use Among Middle and High School Students—United States, 2011–2014. Morbidity and Mortality Weekly Report. 2015;64(14):381–5. [PMC free article] [PubMed] [Google Scholar]
- 12.Wood L. Research and Markets: E-Cigarette & Vaporizer Market to Reach USD 50 Billion by 2025—Global Analysis & Forecast Report 2015–2025 http://www.businesswire.com/news/home/20151125005434/en/Research-Markets-E-Cigarette-Vaporizer-Market-Reach-USD2015 [cited 2016 January 20].
- 13.(FDA) UFaDA. Deeming Tobacco Products To Be Subject to the Federal Food, Drug, and Cosmetic Act, as Amended by the Family Smoking Prevention and Tobacco Control Act; Regulations on the Sale and Distribution of Tobacco Products and Required Warning Statements for Tobacco Products. http://www.regulations.gov/#!documentDetail;D=FDA-2014-N-0189-00012014. [PubMed]
- 14.Kux L. Deeming Tobacco Products To Be Subject to the Federal Food, Drug, and Cosmetic Act, as Amended by the Family Smoking Prevention and Tobacco Control Act; Restrictions on the Sale and Distribution of Tobacco Products and Required Warning Statements for Tobacco Products https://www.federalregister.gov/articles/2016/05/10/2016-10685/deeming-tobacco-products-to-be-subject-to-the-federal-food-drug-and-cosmetic-act-as-amended-by-the: FDA; 2016 [updated 5/5/16; cited 2016 5/17/2016]. [PubMed]
- 15.Williams M, Villarreal A, Bozhilov K, Lin S, Talbot P. Metal and silicate particles including nanoparticles are present in electronic cigarette cartomizer fluid and aerosol. PLoS One. 2013;8(3):e57987 PubMed Central PMCID: PMC3603976. doi: 10.1371/journal.pone.0057987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Administration USFaD. Summary of results: Laboratory analysis of electronic cigarettes conducted by FDA. FDA, US, page 1. FDA Consumer Health Information. 2014 (updated in):1.
- 17.FDA warns of health risks posed by e-cigarettes. [Internet]. http://www.fda.gov/downloads/ForConsumers/ConsumerUpdates/Updates/UCM173430.pdf: FDA; 2009
- 18.McCauley L, Markin C, Hosmer D. An unexpected consequence of electronic cigarette use. Chest. 2012;141(4):1110–3. doi: 10.1378/chest.11-1334 [DOI] [PubMed] [Google Scholar]
- 19.Goniewicz ML, Gupta R, Lee YH, Reinhardt S, Kim S, Kim B, et al. Nicotine levels in electronic cigarette refill solutions: A comparative analysis of products from the US, Korea, and Poland. Int J Drug Policy. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Westenberger B. Evaluation of e-cigarettes. In: U.S. Food and Drug Administration, editor. Washington, DC: http://www.fda.gov/downloads/drugs/scienceresearch/ucm173250.pdf (accessed 9 June 2015). 2009.
- 21.Bekki K, Uchiyama S, Ohta K, Inaba Y, Nakagome H, Kunugita N. Carbonyl compounds generated from electronic cigarettes. Int J Environ Res Public Health. 2014;11(11):11192–200. PubMed Central PMCID: PMC4245608. doi: 10.3390/ijerph111111192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kosmider L, Sobczak A, Fik M, Knysak J, Zaciera M, Kurek J, et al. Carbonyl compounds in electronic cigarette vapors: effects of nicotine solvent and battery output voltage. Nicotine Tob Res. 2014;16(10):1319–26. doi: 10.1093/ntr/ntu078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng T. Chemical evaluation of electronic cigarettes. Tob Control. 2014;23 Suppl 2:ii11–7. PubMed Central PMCID: PMC3995255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goniewicz ML, Knysak J, Gawron M, Kosmider L, Sobczak A, Kurek J, et al. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob Control. 2014;23(2):133–9. doi: 10.1136/tobaccocontrol-2012-050859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cahn Z, Siegel M. Electronic cigarettes as a harm reduction strategy for tobacco control: a step forward or a repeat of past mistakes? J Public Health Policy. 2011;32(1):16–31. doi: 10.1057/jphp.2010.41 [DOI] [PubMed] [Google Scholar]
- 26.McAuley TR, Hopke PK, Zhao J, Babaian S. Comparison of the effects of e-cigarette vapor and cigarette smoke on indoor air quality. Inhal Toxicol. 2012;24(12):850–7. doi: 10.3109/08958378.2012.724728 [DOI] [PubMed] [Google Scholar]
- 27.Margham J, McAdam K, Forster M, Liu C, Wright C, Mariner D, et al. Chemical Composition of Aerosol from an E-Cigarette: A Quantitative Comparison with Cigarette Smoke. Chem Res Toxicol. 2016;29(10):1662–78. doi: 10.1021/acs.chemrestox.6b00188 [DOI] [PubMed] [Google Scholar]
- 28.Agents classified by the IARC Monographs, [Internet]. World Organization; 2012. [cited June 22, 2015]. Available from: http://monographs.iarc.fr/ENG/Classification/index.php. [Google Scholar]
- 29.Administration USFaD. Harmful and Potentially Harmful Constituents in Tobacco Products and Tobacco Smoke: Established List. In: Services DoHaP, editor Rockville. 2012.
- 30.Bian L, He YW, Tang RZ, Ma LJ, Wang CY, Ruan YH, et al. Induction of lung epithelial cell transformation and fibroblast activation by Yunnan tin mine dust and their interaction. Med Oncol. 2011;28 Suppl 1:S560–9. [DOI] [PubMed] [Google Scholar]
- 31.Ruan YH, Hua HR, Gao Q, Song JL, Liang R, Jin KW. [Pathological study of lung cancer induced by Yunnan tin mine dusts in F344 rats]. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2007;25(6):331–5. [PubMed] [Google Scholar]
- 32.Lerner CA, Sundar IK, Yao H, Gerloff J, Ossip DJ, McIntosh S, et al. Vapors produced by electronic cigarettes and e-juices with flavorings induce toxicity, oxidative stress, and inflammatory response in lung epithelial cells and in mouse lung. PLoS One. 2015;10(2):e0116732 PubMed Central PMCID: PMC4319729. doi: 10.1371/journal.pone.0116732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sussan TE, Gajghate S, Thimmulappa RK, Ma J, Kim JH, Sudini K, et al. Exposure to electronic cigarettes impairs pulmonary anti-bacterial and anti-viral defenses in a mouse model. PLoS One. 2015;10(2):e0116861 PubMed Central PMCID: PMC4317176. doi: 10.1371/journal.pone.0116861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lerner CA, Sundar IK, Watson RM, Elder A, Jones R, Done D, et al. Environmental health hazards of e-cigarettes and their components: Oxidants and copper in e-cigarette aerosols. Environ Pollut. 2015;198:100–7. PubMed Central PMCID: PMCPMC4323666. doi: 10.1016/j.envpol.2014.12.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rubenstein D, Jesty J, Bluestein D. Differences between mainstream and sidestream cigarette smoke extracts and nicotine in the activation of platelets under static and flow conditions. Circulation. 2004;109(1):78–83. doi: 10.1161/01.CIR.0000108395.12766.25 [DOI] [PubMed] [Google Scholar]
- 36.CDC. Tobacco Brand Preferences https://www.cdc.gov/tobacco/data_statistics/fact_sheets/tobacco_industry/brand_preference/: Centers for Disease Control and Prevention; 2016 [cited 2016 Dec 28].
- 37.Marian C, O'Connor RJ, Djordjevic MV, Rees VW, Hatsukami DK, Shields PG. Reconciling human smoking behavior and machine smoking patterns: implications for understanding smoking behavior and the impact on laboratory studies. Cancer Epidemiol Biomarkers Prev. 2009;18(12):3305–20. PubMed Central PMCID: PMCPMC2789355. doi: 10.1158/1055-9965.EPI-09-1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tayyarah R. 2014 Electronic Cigarette Aerosol Parameters Study. CORESTA website: Cooperation Centre for Scientific Research Relative to Tobacco, 2015 March 2015. Report No.
- 39.Rubenstein DA, Hom S, Ghebrehiwet B, Yin W. Tobacco and e-cigarette products initiate Kupffer cell inflammatory responses. Mol Immunol. 2015. [DOI] [PubMed] [Google Scholar]
- 40.Farsalinos KE, Romagna G, Tsiapras D, Kyrzopoulos S, Voudris V. Evaluation of electronic cigarette use (vaping) topography and estimation of liquid consumption: implications for research protocol standards definition and for public health authorities' regulation. Int J Environ Res Public Health. 2013;10(6):2500–14. PubMed Central PMCID: PMC3717749. doi: 10.3390/ijerph10062500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gellner CA, Reynaga DD, Leslie FM. Cigarette Smoke Extract: A Preclinical Model of Tobacco Dependence. Curr Protoc Neurosci. 2016;77:9 54 1–9 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thorne D, Dalrymple A, Dillon D, Duke M, Meredith C. A comparative assessment of cigarette smoke aerosols using an in vitro air-liquid interface cytotoxicity test. Inhal Toxicol. 2015;27(12):629–40. PubMed Central PMCID: PMCPMC4732453. doi: 10.3109/08958378.2015.1080773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roemer E, Schramke H, Weiler H, Buettner A, Kausche S, Weber S, et al. Mainstream Smoke Chemistry and in Vitro and In Vivo Toxicity of the Reference Cigarettes 3R4F and 2R4F. Beitrage zur Tabakforschung International Contributions to Tobacco Research(25(1)):316–35. [Google Scholar]
- 44.Queimado L, Lopes C, Du F, Martins C, Fonseca I, Bowcock AM, et al. In vitro transformation of cell lines from human salivary gland tumors. Int J Cancer. 1999;81(5):793–8. [DOI] [PubMed] [Google Scholar]
- 45.Dickson MA, Hahn WC, Ino Y, Ronfard V, Wu JY, Weinberg RA, et al. Human keratinocytes that express hTERT and also bypass a p16(INK4a)-enforced mechanism that limits life span become immortal yet retain normal growth and differentiation characteristics. Mol Cell Biol. 2000;20(4):1436–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rheinwald JG, Hahn WC, Ramsey MR, Wu JY, Guo Z, Tsao H, et al. A two-stage, p16(INK4A)- and p53-dependent keratinocyte senescence mechanism that limits replicative potential independent of telomere status. Mol Cell Biol. 2002;22(14):5157–72. doi: 10.1128/MCB.22.14.5157-5172.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brenner JC, Graham MP, Kumar B, Saunders LM, Kupfer R, Lyons RH, et al. Genotyping of 73 UM-SCC head and neck squamous cell carcinoma cell lines. Head Neck. 2010;32(4):417–26. PubMed Central PMCID: PMCPMC3292176. doi: 10.1002/hed.21198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tackett AP, Lechner WV, Meier E, Grant DM, Driskill LM, Tahirkheli NN, et al. Biochemically verified smoking cessation and vaping beliefs among vape store customers. Addiction. 2015;110(5):868–74. doi: 10.1111/add.12878 [DOI] [PubMed] [Google Scholar]
- 49.Nyunoya T, Monick MM, Klingelhutz A, Yarovinsky TO, Cagley JR, Hunninghake GW. Cigarette smoke induces cellular senescence. Am J Respir Cell Mol Biol. 2006;35(6):681–8. PubMed Central PMCID: PMC2643295. doi: 10.1165/rcmb.2006-0169OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nyunoya T, Monick MM, Klingelhutz AL, Glaser H, Cagley JR, Brown CO, et al. Cigarette smoke induces cellular senescence via Werner's syndrome protein down-regulation. Am J Respir Crit Care Med. 2009;179(4):279–87. PubMed Central PMCID: PMC2643077. doi: 10.1164/rccm.200802-320OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kanaji N, Basma H, Nelson A, Farid M, Sato T, Nakanishi M, et al. Fibroblasts that resist cigarette smoke-induced senescence acquire profibrotic phenotypes. Am J Physiol Lung Cell Mol Physiol. 2014;307(5):L364–73. doi: 10.1152/ajplung.00041.2014 [DOI] [PubMed] [Google Scholar]
- 52.Ganapathy V, Ramachandran I, Rubenstein DA, Queimado L. Detection of in vivo DNA damage induced by very low doses of mainstream and sidestream smoke extracts using a novel assay. American journal of preventive medicine. 2015;48(1 Suppl 1):S102–10. [DOI] [PubMed] [Google Scholar]
- 53.Mullenbach R, Lagoda PJ, Welter C. An efficient salt-chloroform extraction of DNA from blood and tissues. Trends in Genetics. 1989;5(12):391 [PubMed] [Google Scholar]
- 54.Reis AM, Mills WK, Ramachandran I, Friedberg EC, Thompson D, Queimado L. Targeted detection of in vivo endogenous DNA base damage reveals preferential base excision repair in the transcribed strand. Nucleic Acids Research. 2012;40(1):206–19. Epub 2011/09/14. PubMed Central PMCID: PMC3245927. doi: 10.1093/nar/gkr704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kandoth C, McLellan MD, Vandin F, Ye K, Niu B, Lu C, et al. Mutational landscape and significance across 12 major cancer types. Nature. 2013;502(7471):333–9. PubMed Central PMCID: PMC3927368. doi: 10.1038/nature12634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cancer Genome Atlas N. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature. 2015;517(7536):576–82. PubMed Central PMCID: PMC4311405. doi: 10.1038/nature14129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cancer Genome Atlas Research N. Comprehensive genomic characterization of squamous cell lung cancers. Nature. 2012;489(7417):519–25. PubMed Central PMCID: PMC3466113. doi: 10.1038/nature11404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ramachandran I, Ganapathy V, Gillies E, Fonseca I, Sureban SM, Houchen CW, et al. Wnt inhibitory factor 1 suppresses cancer stemness and induces cellular senescence. Cell death & disease. 2014;5:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cressey D. E-cigarettes affect cells. Nature. 2014;508(7495):159 doi: 10.1038/508159a [DOI] [PubMed] [Google Scholar]
- 60.Park SJ, Walser TC, Perdomo C, Wang T, Pagano PC, Liclican EL, et al. Abstract B16: The effect of e-cigarette exposure on airway epithelial cell gene expression and transformation. Clinical Cancer Research. 2014;20(2 Supplement):B16. [Google Scholar]
- 61.Doll R, Peto R. The causes of cancer: quantitative estimates of avoidable risks of cancer in the United States today. J Natl Cancer Inst. 1981;66(6):1191–308. [PubMed] [Google Scholar]
- 62.Shukla PC, Singh KK, Yanagawa B, Teoh H, Verma S. DNA damage repair and cardiovascular diseases. The Canadian journal of cardiology. 2010;26 Suppl A:13A–6A. PubMed Central PMCID: PMC2827216. [DOI] [PubMed] [Google Scholar]
- 63.Sundar IK, Yao H, Rahman I. Oxidative stress and chromatin remodeling in chronic obstructive pulmonary disease and smoking-related diseases. Antioxid Redox Signal. 2013;18(15):1956–71. PubMed Central PMCID: PMC3624634. doi: 10.1089/ars.2012.4863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bettcher DW, Sanda LS. Clinical cancer control and prevention. Eliminating tobacco-induced cancers: a worldwide challenge. Ann Oncol. 2008;19 Suppl 7:vii230–3. Epub 2008/09/20. [DOI] [PubMed] [Google Scholar]
- 65.Wagener TL, Floyd EL, Stepanov I, Driskill LM, Frank SG, Meier E, et al. Have combustible cigarettes met their match? The nicotine delivery profiles and harmful constituent exposures of second-generation and third-generation electronic cigarette users. Tob Control. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lindell G, Lunell E, Graffner H. Transdermally administered nicotine accumulates in gastric juice. Eur J Clin Pharmacol. 1996;51(3–4):315–8. [DOI] [PubMed] [Google Scholar]
- 67.Yu V, Rahimy M, Korrapati A, Xuan Y, Zou AE, Krishnan AR, et al. Electronic cigarettes induce DNA strand breaks and cell death independently of nicotine in cell lines. Oral Oncol. 2016;52:58–65. doi: 10.1016/j.oraloncology.2015.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Farsalinos KE, Romagna G, Allifranchini E, Ripamonti E, Bocchietto E, Todeschi S, et al. Comparison of the cytotoxic potential of cigarette smoke and electronic cigarette vapour extract on cultured myocardial cells. Int J Environ Res Public Health. 2013;10(10):5146–62. doi: 10.3390/ijerph10105146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Farsalinos KE, Polosa R. Safety evaluation and risk assessment of electronic cigarettes as tobacco cigarette substitutes: a systematic review. Therapeutic advances in drug safety. 2014;5(2):67–86. PubMed Central PMCID: PMC4110871. doi: 10.1177/2042098614524430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Behar RZ, Davis B, Wang Y, Bahl V, Lin S, Talbot P. Identification of toxicants in cinnamon-flavored electronic cigarette refill fluids. Toxicol In Vitro. 2014;28(2):198–208. [DOI] [PubMed] [Google Scholar]
- 71.Bahl V, Lin S, Xu N, Davis B, Wang YH, Talbot P. Comparison of electronic cigarette refill fluid cytotoxicity using embryonic and adult models. Reproductive toxicology. 2012;34(4):529–37. doi: 10.1016/j.reprotox.2012.08.001 [DOI] [PubMed] [Google Scholar]
- 72.Romagna G, Allifranchini E, Bocchietto E, Todeschi S, Esposito M, Farsalinos KE. Cytotoxicity evaluation of electronic cigarette vapor extract on cultured mammalian fibroblasts (ClearStream-LIFE): comparison with tobacco cigarette smoke extract. Inhal Toxicol. 2013;25(6):354–61. doi: 10.3109/08958378.2013.793439 [DOI] [PubMed] [Google Scholar]
- 73.Thorne D, Larard S, Baxter A, Meredith C, Gaa M. The comparative in vitro assessment of e-cigarette and cigarette smoke aerosols using the gammaH2AX assay and applied dose measurements. Toxicol Lett. 2017;265:170–8. doi: 10.1016/j.toxlet.2016.12.006 [DOI] [PubMed] [Google Scholar]
- 74.Services USDoHaH. How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General. In: U.S. Department of Health and Human Services CfDCaP, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, editor. Atlanta, GA2010. [PubMed]
- 75.Pryor WA, Stone K. Oxidants in cigarette smoke. Radicals, hydrogen peroxide, peroxynitrate, and peroxynitrite. Ann N Y Acad Sci. 1993;686:12–27; discussion -8. [DOI] [PubMed] [Google Scholar]
- 76.Ji EH, Sun B, Zhao T, Shu S, Chang CH, Messadi D, et al. Characterization of Electronic Cigarette Aerosol and Its Induction of Oxidative Stress Response in Oral Keratinocytes. PLoS One. 2016;11(5):e0154447 PubMed Central PMCID: PMCPMC4880184. doi: 10.1371/journal.pone.0154447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang D, Kreutzer DA, Essigmann JM. Mutagenicity and repair of oxidative DNA damage: insights from studies using defined lesions. Mutat Res. 1998;400(1–2):99–115. [DOI] [PubMed] [Google Scholar]
- 78.(ESCODD) TESCoODD. Measurement of DNA oxidation in human cells by chromatographic and enzymic methods. Free Radic Biol Med. 2003;34(8):1089–99. [DOI] [PubMed] [Google Scholar]
- 79.De Bont R, van Larebeke N. Endogenous DNA damage in humans: a review of quantitative data. Mutagenesis. 2004;19(3):169–85. [DOI] [PubMed] [Google Scholar]
- 80.Misra M, Leverette RD, Cooper BT, Bennett MB, Brown SE. Comparative in vitro toxicity profile of electronic and tobacco cigarettes, smokeless tobacco and nicotine replacement therapy products: e-liquids, extracts and collected aerosols. Int J Environ Res Public Health. 2014;11(11):11325–47. PubMed Central PMCID: PMC4245615. doi: 10.3390/ijerph111111325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Thorne D, Crooks I, Hollings M, Seymour A, Meredith C, Gaca M. The mutagenic assessment of an electronic-cigarette and reference cigarette smoke using the Ames assay in strains TA98 and TA100. Mutat Res. 2016;812:29–38. doi: 10.1016/j.mrgentox.2016.10.005 [DOI] [PubMed] [Google Scholar]
- 82.Mortelmans K, Zeiger E. The Ames Salmonella/microsome mutagenicity assay. Mutat Res. 2000;455(1–2):29–60. [DOI] [PubMed] [Google Scholar]
- 83.Dillon D, Combes R, Zeiger E. The effectiveness of Salmonella strains TA100, TA102 and TA104 for detecting mutagenicity of some aldehydes and peroxides. Mutagenesis. 1998;13(1):19–26. [DOI] [PubMed] [Google Scholar]
- 84.Behar RZ, Luo W, Lin SC, Wang Y, Valle J, Pankow JF, et al. Distribution, quantification and toxicity of cinnamaldehyde in electronic cigarette refill fluids and aerosols. Tob Control. 2016;25(Suppl 2):ii94–ii102. doi: 10.1136/tobaccocontrol-2016-053224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Leigh NJ, Lawton RI, Hershberger PA, Goniewicz ML. Flavourings significantly affect inhalation toxicity of aerosol generated from electronic nicotine delivery systems (ENDS). Tob Control. 2016;25(Suppl 2):ii81–ii7. doi: 10.1136/tobaccocontrol-2016-053205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Maritz GS. Are nicotine replacement therapy, varenicline or bupropion options for pregnant mothers to quit smoking? Effects on the respiratory system of the offspring. Ther Adv Respir Dis. 2009;3(4):193–210. Epub 2009/08/27. doi: 10.1177/1753465809343712 [DOI] [PubMed] [Google Scholar]
- 87.Friedberg Errol C., Walker Graham C., Siede Wolfram, Wood Richard D., Schultz Roger A., Ellenberger T. DNA Repair and Mutagenesis. 2nd ed. Washington, DC: American Society Microbiology; 2005. [Google Scholar]
- 88.Marteijn JA, Lans H, Vermeulen W, Hoeijmakers JH. Understanding nucleotide excision repair and its roles in cancer and ageing. Nat Rev Mol Cell Biol. 2014;15(7):465–81. doi: 10.1038/nrm3822 [DOI] [PubMed] [Google Scholar]
- 89.Fracasso ME, Doria D, Franceschetti P, Perbellini L, Romeo L. DNA damage and repair capacity by comet assay in lymphocytes of white-collar active smokers and passive smokers (non- and ex-smokers) at workplace. Toxicology Letters. 2006;167(2):131–41. doi: 10.1016/j.toxlet.2006.09.003 [DOI] [PubMed] [Google Scholar]
- 90.Neumann AS, Sturgis EM, Wei Q. Nucleotide excision repair as a marker for susceptibility to tobacco-related cancers: a review of molecular epidemiological studies. Mol Carcinog. 2005;42(2):65–92. doi: 10.1002/mc.20069 [DOI] [PubMed] [Google Scholar]
- 91.Tudek B. Base excision repair modulation as a risk factor for human cancers. Mol Aspects Med. 2007;28(3–4):258–75. doi: 10.1016/j.mam.2007.05.003 [DOI] [PubMed] [Google Scholar]
- 92.Bouaoun L, Sonkin D, Ardin M, Hollstein M, Byrnes G, Zavadil J, et al. TP53 Variations in Human Cancers: New Lessons from the IARC TP53 Database and Genomics Data. Hum Mutat. 2016;37(9):865–76. doi: 10.1002/humu.23035 [DOI] [PubMed] [Google Scholar]
- 93.Zhou G, Wang J, Zhao M, Xie TX, Tanaka N, Sano D, et al. Gain-of-function mutant p53 promotes cell growth and cancer cell metabolism via inhibition of AMPK activation. Mol Cell. 2014;54(6):960–74. PubMed Central PMCID: PMCPMC4067806. doi: 10.1016/j.molcel.2014.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
UM-SCC-1 and NuLi1 cells were continuously exposed to 10 or 100 puffs/5L of EC aerosol extracts and cell viability was determined by MTT assay at 96 h. Data are represented as mean ± SD.
(TIF)
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.