Abstract
Gamma-secretase (GS) is an enzyme complex that cleaves numerous substrates, and it is best known for cleaving amyloid precursor protein (APP) to form amyloid-beta (Aβ peptides. Aberrant cleavage of APP can lead to Alzheimer’s disease, so much research has been done to better understand GS structure and function in hopes of developing therapeutics for Alzheimer’s. Therefore, most of the attention in this field has been focused on developing modulators that reduce pathogenic forms of Aβ while leaving Notch and other GS substrates intact, but GS provides multiple avenues of modulation that could improve AD pathology. GS has complex regulation, through its essential subunits and other associated proteins, providing other targets for AD drugs. Therapeutics can also alter GS trafficking and thereby improve cognition, or move beyond Aβ entirely, effecting Notch and neural stem cells. GS also cleaves substrates that affect synaptic morphology and function, presenting another window by which GS modulation could improve AD pathology. Taken together, GS presents a unique cross road for neural processes and an ideal target for AD therapeutics.
Keywords: Alzheimer’s disease, Gamma-secretase, Amyloid-beta
1. Background
Alzheimer’s disease (AD) is a global health crisis. It is a neurodegenerative disorder characterized by amyloid plaques made of amyloid beta aggregates (Aβ and neurofibrillary tangles (NFT) made of hyper-phosphorylated tau protein. Symptoms include memory loss and behavioral deficits (Musiek and Holtzman, 2015), and there is no cure for this disease. This is especially troubling as the number one risk factor for AD is age, and the western world has an aging population.
While NFT align more closely with disease stage (Arriagada et al., 1992; Giannakopoulos et al, 2003), researchers believe amyloid beta (Aβ to be causative in the disorder, mainly because of the genetic evidence (Musiek and Holtzman, 2015). Autosomal dominant AD is caused by mutations in amyloid precursor protein (APP) or presenilin, the catalytic subunit of gamma-secretase (GS) (Ahn et al, 2010; Bettens et al., 2013), and these mutations lead to an increase in Aβ and downstream dementia.
Amyloid precursor protein (APP) can be cleaved by two pathways, the non-amyloidogenic versus the amyloidogenic pathway (Zheng and Koo, 2011). In the non-amyloidogenic pathway, APP is first cleaved by alpha-secretase and then by GS. In the amyloidogenic pathway, however, the first cleavage is done by beta-secretase (BACE) then. This second pathway releases Aβ of varying lengths (Fig. 1), with GS first cleaving the β-CTF into long forms of Aβ, either Aβ48 or Aβ49. GS then makes step-wise cleavages every three amino acids, preferring Aβ40 and Aβ42 (Barnwell et al., 2014; Li et al., 2016; Qi-Takahara et al., 2005; Takami et al., 2009). Aβ42 production may also not relate solely to the cleavage of the longer Aβ forms, but instead may depend on the dissociation rate of Aβ42 from the complex. If it remains in the active site, it may cleave further into shorter forms (Okochi et al., 2013). APP cleavage is a more complicated process than was originally described.
Fig. 1.
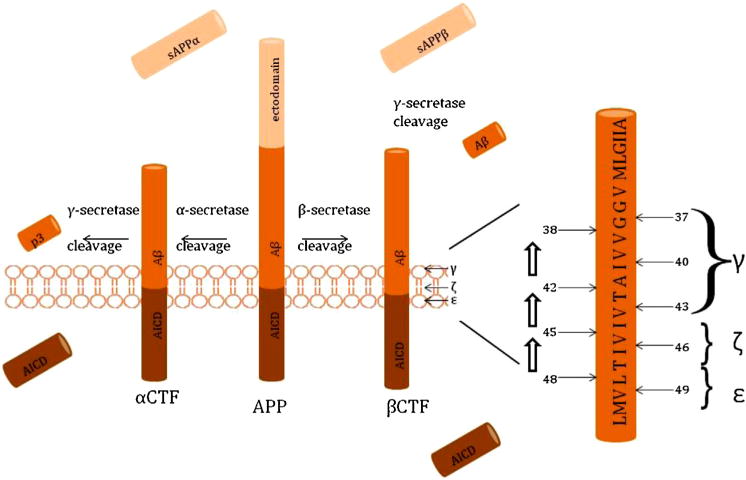
Amyloid precursor protein (APP) can be cleaved in two major pathways. If it is first cleaved by alpha-secretase, then subsequent cleavage by gamma-secretase results in the intracellular domain (AICD) and p3, a non-amyloidogenic by-product. However, if beta-secretase performs the initial cleavage, then gamma-secretase cleaves the beta-CTF at multiple sites, sequentially releasing the AICD and Aβ peptides of varying lengths, which can oligomerize. The gamma-secretase cleavage sites are referred to as ε, ζ, and γ, and the starting amino acid, either 48 or 49, determines the end product, either Aβ338/42 or 37/40.
Of the Aβ lengths, Aβ42 is believed to be more toxic then Aβ40. Scientists measure the ratio of Aβ42: Aβ40, and when this ratio increases, like in genetic forms of AD, Aβ peptides oligomerize more readily (Iwatsubo et al., 1994). After oligomerization, the Aβ species then aggregate, eventually leading to downstream neurotoxicity. The original hypothesis for AD, the Aβ hypothesis coined by Hardy and Higgins (Hardy and Selkoe, 2002; Hardy and Higgins, 1992) has been updated to place Aβ not as the sole instigator of a direct cascade, but instead as an initiator for a series of changes, many through tau protein and inflammation, that eventually lead to neurodegeneration (Musiekand Holtzman, 2015).
Because of its role in the cleavage of Aβ and the fact that many genetic forms of AD are caused by mutations in the enzyme, GS has long been a target for drug development, though previous clinical trials of Semagacestat, a GS inhibitor, have failed due to an increase in skin cancer, and a decrease in cognitive performance (Doody et al, 2013; Herrmann et al., 2011; Niva et al, 2013). GS has more than 90 identified substrates, and gamma-secretase inhibitors (GSI) block the action of GS on all of these, likely resulting in those unwanted side effects (De Strooper and Chavez Gutierrez, 2015; Henley et al., 2014). Researchers then developed a reported Notch-sparing inhibitor, Avagacestat (Gillman et al., 2010). This compound had the similar toxicity issues Semagacestat. However, multiple studies indicated that Avagacestat was actually not Notch-sparing, having a similar potency for both Notch and APP (Chavez-Gutierrez et al., 2012; Crump et al, 2012). Because of the large-scale failures, Aβ as a target then fell out of favor.
Recently, there has been revitalization for Aβ as a therapeutic target for two main reasons. First, Biogen has shown preliminary clinical evidence that their Aβ antibody improves cognition in patients (Underwood, 2015). Second, scientists found a protective mutation in APP, showing that modulation of Aβ can protect a patient from developing AD by reducing the beta cleavage of APP (Jonsson et al., 2012). With this renewed vigor, researchers are turning their attention back to the APP cleavage pathway. By better understanding the complex regulation and modulation of GS, researchers can develop better therapeutics that reduce Aβ toxicity. It is also crucial to understand how GS affects other neuronal substrates, for GS-directed compounds can influence a range of pathways beyond amyloidogenesis. This review will highlight a few of the most important roles and regulators of GS, in hopes of highlighting the unique position of GS in AD pathology.
2. Gamma-secretase complex
If the goal is to create GS directed therapeutics, it is first important to understand the subunit structure and composition of this enzyme. GS is an enzyme complex, composed of 4 required sub-units that form a 1:1:1:1 heterodimer (Li et al., 2014; Sato et al., 2007): presenilin (PS), nicastrin (NCT), anterior pharynx-defective 1 (APH-1), and presenilin enhancer 2 (PEN2) (Francis et al., 2002; Goutte et al., 2002; Yu et al., 2000) (Fig. 2). It is an aspartyl protease, accountable for cleaving over 90 integral membrane proteins after they have undergone ectodomain shedding (Haapasalo and Kovacs, 2011).
Fig. 2.
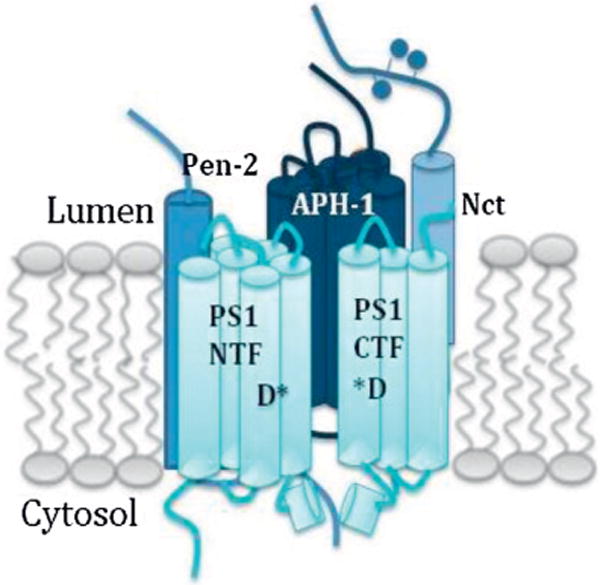
Gamma-secretase is composed of four essential subunits: presenilin (PS1), Nicastrin (Nct), Pen-2, and APH-1. Presenilin must be endoproteolysed into the N and C terminal fragments to become active, and the catalytic residues (D*) are present in this subunit.
Of the subunits, PS is the most important for activity and therefore the most studied. PS contains the catalytic subunit for the complex (Ahn et al., 2010; Esler et al., 2000; Li et al., 2000), with nine transmembrane helixes (Doan et al., 1996; Laudon et al., 2005). The two catalytic aspartyl residues are located in transmembrane domains 6 and 7 (Wolfe et al., 1999). PS has two forms in mammals, PS1 and PS2. PS must be endoproteolysed to form the N-terminal and C-terminal fragments to become active, with the exception of the exon 9 deletion mutant PS that is active but not cleaved (Thinakaran et al., 1996). Mutations in PS lead to changes in either the ratio of Aβ peptides, with a shift towards more amyloidogenic forms, or an increase in the total amount of Aβ generated (Citron et al., 1997; Scheuner et al., 1996). These familial mutations lead to the heritable form of Alzheimer’s disease (Chavez-Gutierrez et al., 2012). Of note, whether loss of or gain of function of PS1 mutations leads to AD has been questioned (Shen and Kelleher, 2007; Xia et al., 2015).
The other three subunits help form the mature enzyme. NCT, with its large extracellular domain, transmembrane helix and smaller cytoplasmic domain (Yu et al, 2000), is involved in substrate recognition. Extracellular domain antibodies disrupt NCT binding to substrates (Zhang et al., 2012), but this role is controversial (Chavez-Gutierrez et al., 2008; Dries et al, 2009; Zhao et al., 2010), as NCT is not absolutely required for GS activity (Shah et al., 2005). The final two subunits, APH-1 and PEN2, are less well studied. APH-1 helps form a scaffold, and PEN2 works in enzyme maturation (Niimura et al., 2005; Prokop et al., 2004). APH-1 has two different isoforms from two paralogous genes on chromosomes 1 (APH-1A) and 15 (APH-1B). The structure of PEN2 also presents some controversy, as biochemical studies have shown it to only have one transmembrane domain with a reentrant loop (Zhang et al., 2015), differing from previous models of a subunit with two transmembrane domains. More work is needed to fully understand the role of these two subunits in GS activity and specificity.
Even with all of the subunits present, the complex must also be correctly assembled for the enzyme to function properly. The complex is first assembled in the endoplasmic reticulum, with NCT and APH-1 binding together. They form the initial scaffold, so full-length PS can attach itself, and finally PEN2 associates and causes the endoproteolysis of PS into the N-terminal fragment (NTF) and C-terminal fragment (CTF). The active complex is then shuttled to the Golgi where it is glycosylated (Takasugi et al., 2003). Only after the assembly of all four subunits and the glycosylation will GS become active, and even then, not all present complexes are active (Beher et al, 2003; Lai et al., 2003). The disconnect between the presenilin level and the activation of γ-secretase complex remain a large area of research for the GS community.
To help address these questions about GS structure, much work has focused on generating high-resolution structures for GS. Many groups have recently published structures using electron microscopy techniques, building on previous work giving a GS structure at a lower resolution (Lazarov et al, 2006). The group by Li et al. published a 17 Å model, showing a large base with a smaller head, and NCT’s extracellular domain is in the smaller head (Li et al., 2014). This study was followed up by a 3.4 Å resolution structure that showed GS in the same shape as the previous report, with APH-1 and Pen-2 holding PS1 under the NCT extracellular domain, while leaving PS1 flexible (Bai et al., 2015; Lu et al., 2014). Other EM papers have also shown that GS exists in multiple conformations (Bai et al., 2015; Elad et al., 2015). In order to measure the distinct conformations, Bai et al. used a GSI, DAPT, to lock the enzyme in place. The sixth transmembrane helix of PS1 can exist in 3 shapes, potentially providing the range of enzyme activities. The group also found a single helix in the cavity of PS1 that does not belong to any of the subunits, and mass spectrometry identified a mixture of proteins. This mixture could include potential regulatory subunits. The structural information from the EM work will serve as a jumping off point for future rational drug design, as well as highlighting the importance of enzyme regulation.
3. Gamma-secretase regulation
An enzyme complex with such a large range of substrates and functions requires tight regulation. It is important to keep in mind that only a small fraction of GS complexes are active (Beher et al., 2003; Gu et al., 2004; Lai et al., 2003). All active complexes have all four subunits, and previously it was thought that activity cannot be increased by only overexpressing PS (Levitan et al., 2001)-all subunits must be increased (Edbauer et al., 2003). However, when these studies moved into a mouse model, overexpression of PS alone was able to increase GS activity (Li et al., 2011). This discrepancy between cells and animal models show that GS regulation in vivo is much more complicated than originally anticipated. GS is regulated by layers of control, from subunit composition to associated proteins that may regulate the complex in specific tissues or disease situations.
There are multiple GS complexes since PS and APH-1 have 2 isoforms, and APH-1 also has two splice variants, APH-1A and APH-1B (Lai et al., 2003; Shirotani et al., 2004). These variants can exist at the same time in the same tissue, and isoforms sometimes compete for substrates (Placanica et al., 2009a,b). APH-1A regulates Notch during embryogenesis (Ma et al., 2005; Serneels et al., 2005), and APH-1B contributes to the production of longer Aβ fragments. By targeting APH-1B, researchers can reduce aggregates without Notch-related toxicity (Serneels et al., 2009). More work is required to fully understand how GS activity is regulated by its subunits.
GS is also regulated by associated proteins. It can form differential complexes with modulatory proteins. One example is GSAP, which complexes with GS and APP, giving preference to APP cleavage over Notch. GSAP knockdown mice reduce Aβ when crossed with an AD model (Chu et al., 2014; He et al., 2010), and there is a GSAP SNP associated with AD (Zhu et al., 2014). However, the precise mechanism is unknown. Recent work has also shown that GS can be regulated by Hif1α, long known as the master regulator of hypoxia (Villa et al., 2014). Hif1α normally acts as a transcription factor, stabilized in low oxygen conditions, and turning on several genes in response. However, Hif1α binds directly to GS and increases its Notch activity by shifting inactive complexes to their active form, independent of Hif1 α’s ability to act as a transcription factor.
To alter GS activity, one can alter not only the active site, but also by altering its subunits and associated proteins. Because GS has a wide range of substrates and functions, it needs a wide range of controls on activity.
4. Gamma-secretase trafficking
GS activity for APP can be influenced by sub-cellular trafficking, as APP cleavage differs depending on its localization. APP is synthesized in the ER and transported to the trans-Golgi network (Annaert et al., 1999; Pasternak et al, 2003; Ray et al., 1999a; Rechards et al., 2003; Vassaretal., 1999). If itisonthe cell surface, it can be cleaved by α-secretase (Parvathy et al, 1999). APP can also be internalized and cleaved by β-secretase (BACE) and GS in the ER, Golgi, and the endosomal system. The goal of some therapeutics is to shift localization to the cell surface to decrease amyloidogenic processing. Therefore, it is important to understand what factors alter APP localization.
Cholesterol and lipid metabolism can affect this trafficking. APP needs to interact with lipids and associated proteins to change its localization. For example, LRP1 binds to APP and mediates Aβ clearance (Deane et al, 2004; Herz and Bock, 2002; Zerbinatti et al, 2006). It is found near plaques (Rebeck et al., 1993) and polymorphisms are linked with increased risk for AD(Kang et al., 1997, 2000). Disrupting the interaction between LRP1 and APP decreases Aβ production by increasing cell surface APP (Ulery et al, 2000). LRP1 is also a GS substrate (Lleo et al., 2005), so modulating GS cleavage of LRP1 can shift APP trafficking.
SorLA is another GS substrate implicated in APP trafficking. SorLA is decreased in AD brains (Scherzer et al, 2004) and binds APP directly (Andersen et al., 2005). Its overexpression shifts the APP to the Golgi, thereby altering Aβ production (Offe et al., 2006). SorLA was also implicated as an AD risk gene through gene variants identified in a GWAS study (Rogaeva et al., 2007).
Finally, GS activity itself is regulated by low levels of cholesterol (Grimm et al., 2005, 2008). Shifting APP to lipid rafts increases Aβ, where GS is localized (Fuentealba et al, 2007). Cholesterol lowering drugs decrease this interaction (Ehehalt et al., 2003; Kojro et al., 2001). An increase in cholesterol shift PS1 to late endosomes and is associated with an increase in GS activity (Burns et al., 2003). However, the interplay between GS and cholesterol is still controversial. Modulating lipid metabolism and GS activity could provide a new therapeutic target for AD.
5. Notch and neural stem cells
Notch was the second GS substrate identified (De Strooper et al., 1999; Ray et al., 1999a,b). Importantly, Notch can act as a proto-oncogene or tumor suppressor in some cancers (Lobry et al., 2011). It is also involved in neural differentiation, which is especially crucial during neural development.
Notch must be cleaved to become active (Kopan and Goate, 2000). Ligand binding triggers the sequential cleavage, first by ADAM metalloproteases and then by GS. This releases the Notch intracellular domain (NICD), which acts as a transcription factor for a host of genes, many involved in cell survival and differentiation (Kopan and Ilagan, 2009). All four Notch receptors are GS substrates (Saxena et al., 2001), so any therapeutic that inhibits GS activity completely will also block the action of Notch.
Neural stem cells exist in both the adult and the embryonic brain, defined as any cell that can both replicate and also has the potential to differentiate into neurons or glia (Altman and Das, 1965). These neural stem cells give rise to either other neural stem cells or neural progenitors cells, which have a limited ability to divide and cannot self-renew (Bonaguidi et al., 2011). Neural progenitor cells give rise to new adult neurons, and this is correlated with improved spatial memory (Sahay et al, 2011; Stone et al., 2011). In AD, there is a decrease in neurogenesis, so understanding the complex mechanisms that regulate this process may open a new therapeutic window for the treatment of AD (Lazarov et al., 2010). Strikingly, GS and its substrates are at the heart of these pathways.
Notch signaling maintains the balance between neural stem cells and neural progenitors (Aguirre et al., 2010; Basak and Taylor, 2007; Mizutani et al., 2007). Conditional knock out of Notch causes depletion in the progenitor pool, showing that Notch is required for the maintenance of neural stem cells (Basak et al., 2012). Notch inhibits the differentiation of progenitors into neurons and promotes the differentiation of glia progenitors into astrocytes (Bai et al., 2007; Shimojo et al., 2008). Scientists, looking to accelerate neural differentiation of induced pluripotent stem cells (iPSCs), took advantage of Notch signaling. By adding a gamma-secretase inhibitor, researchers are able to drive iPSCs to neurons (Borghese et al., 2010; Chambers et al, 2012; Chen et al., 2014; Wang et al., 2015) (Fig. 3).
Fig. 3.
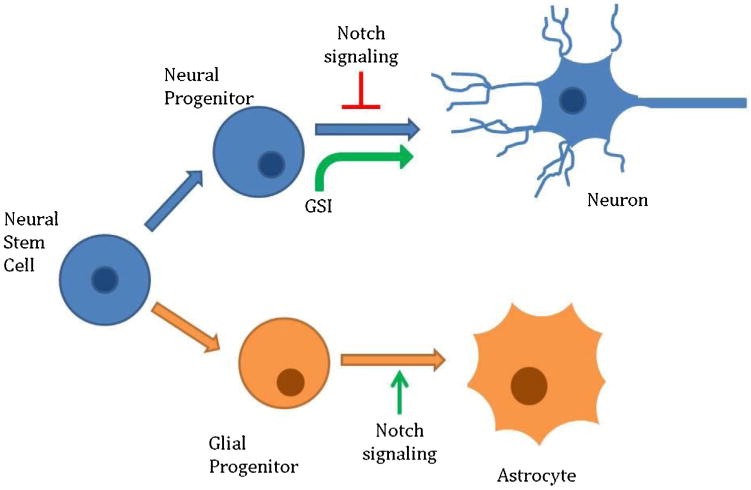
Notch is crucial to maintaining neural progenitors. Notch inhibition maintains the progenitor pool for neurons, while Notch signaling drives glial progenitors to become astrocytes. By applying gamma-secretase inhibitors (GSIs) to induced pluripotent stem cells, researchers can drive progenitors into neurons.
PS1 is also involved in adult neural stem cells. Scientists knocked down PS1 and saw a decrease in neurogenesis in the subgranular zone of the hippocampus (Gadadhar et al., 2011). Also, by knocking-in PS1 familial mutations or using a transgenic mouse expressing a chimeric human-mouse version of APP with the Swedish mutation, a severe and well studied familial mutant, researchers observed a decrease neural proliferation (Haughey et al., 2002; Wang et al., 2004). Many groups have seen a similar effect with other PS1-dependent AD mouse models, including PS1 mutants and PS1 knock downs (Bonds et al., 2015; Choi et al, 2008; Demars et al, 2010; Donovan et al., 2006; Rodriguez et al, 2008; Wen et al., 2004; Zhang et al, 2007). Conditional knock-out of PS1 in forebrain alone is enough to reduce neurogenesis (Saura et al, 2005), highlighting the importance of PS1 in the maintenance of neural stem cells.
GS regulation is crucial to the maintenance and proliferation of adult neural stem cells, and modulating GS activity could improve cognitive outcomes based on its effect on these specific cell types. While most of the focus of GS modulators has been on its effects on Aβ, altering Notch signaling could also improve cognitive outcomes by maintaining neural stem cells, and Notch-focused therapies still need to be investigated.
6. Gamma-secretase in neuronal structures: beyond Aβ and notch
GS, through its other substrates, is involved in many neuronal processes. One such process is axonal guidance, and many GS substrates are involved. For example, the Netrin receptor, DCC, must be processed for axons to recognize midline guidance cues (Serafini et al., 1994). GS inhibition leads to an accumulation of DCC—CTF (Taniguchi et al., 2003) and increased neurites. Another GS substrate, ephrinB and its receptor are involved in spine maturation and synaptogenesis (Barthet et al., 2013). It contributes to deficits in neuronal circuits, and acts with BACE to regulate growth cone collapse and recovery in axon path finding through CHL1 processing (Barao et al, 2015).
GS is also crucial for synaptic morphology. Synaptic dysfunction precedes neurodegeneration and is crucial to brain health. GS substrates include cell adhesion molecules-neuroligin in the post synaptic membrane is involved in cell signaling (Marballi et al., 2012). Disruptions in this pathway are implicated in schizophrenia (Mei and Xiong, 2008). Finally, GS activity regulates dendritic spine density. EphrinA4 is cleaved by GS and regulates dendritic spine morphology. EphrinA4 cleavage is disrupted by FAD mutations, and its activity is increased by synaptic activity (Inoue et al., 2009).
By looking outside canonical substrates, APP and Notch, researchers could develop GS-directed therapeutics spine maturation, synaptic morphology, and dendritic spine density, all processes dysregulated in AD.
7. Conclusion
Gamma-secretase is an enzyme complex involved in multiple signaling pathways through its numerous substrates (Fig. 4). GS is best known for its role in AD, where aberrant cleavage of APP can cause neurodegeneration. Developing therapeutics that block amyloidogenic cleavage, without altering other substrates would decrease Aβ peptides in the brain without the negative side effects associated with other GS substrates.
Fig. 4.
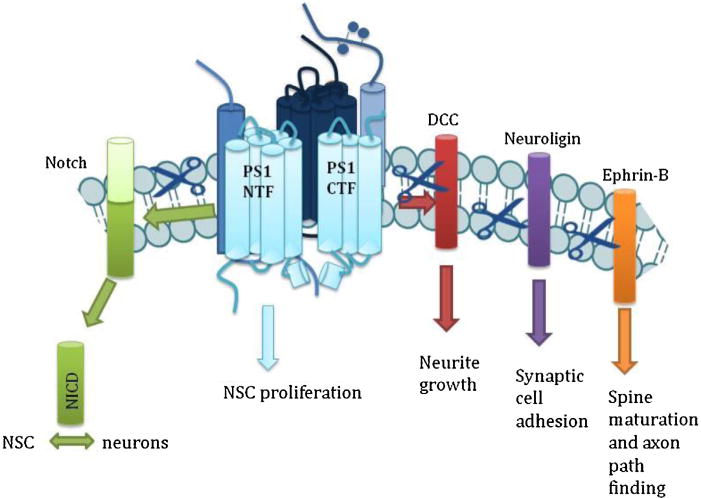
Gamma-secretase is vital to multiple neuronal processes. Through Notch signaling, GS is involved in the balance between neural stem cells (NSC) and neurons. GS also cleaves DCC, which regulates neurite outgrowth, neuroligin, which controls synaptic adhesion, and ephrin-B, which is involved in spine maturation.
However, GS as an enzyme holds more process in AD therapeutics then just canonical inhibitors and modulators. GS is regulated by its subunits and associated proteins, so drugs that influence those components can decrease Aβ as well. Therapeutics can also alter GS trafficking and thereby improve cognition, or move beyond Aβ entirely, effecting Notch and neural stem cells. GS cleavage of DCC, neuroligin, and ephrin control neuronal morphology and function, presenting another avenue by which GS modulation could improve AD pathology. Taken together, GS presents a unique hub for neural processes and an ideal target for AD therapeutics.
Footnotes
Conflict of interest
The authors declare that there are no conflicts of interest.
References
- Aguirre A, Rubio ME, Gallo V. Notch and EGFR pathway interaction regulates neural stem cell number and self-renewal. Nature. 2010;467:323–327. doi: 10.1038/nature09347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn K, Shelton CC, Tian Y, Zhang X, Gilchrist ML, Sisodia SS, Li YM. Activation and intrinsic γ-secretase activity of presenilin 1. Proc Natl Acad Sci U S A. 2010;107:21435–21440. doi: 10.1073/pnas.1013246107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124:319–335. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- Andersen OM, Reiche J, Schmidt V, Gotthardt M, Spoelgen R, Behlke J, von Arnim CA, Breiderhoff T, Jansen P, Wu X, Bales KR, Cappai R, Masters CL, Gliemann J, Mufson EJ, Hyman BT, Paul SM, Nykjaer A, Willnow TE. Neuronal sorting protein-related receptor sorLA/LR11 regulates processing of the amyloid precursor protein. Proc Natl Acad Sci U S A. 2005;102:13461–13466. doi: 10.1073/pnas.0503689102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annaert WG, Levesque L, Craessaerts K, Dierinck I, Snellings G, Westaway D, George-Hyslop PS, Cordell B, Fraser P, De Strooper B. Presenilin 1 controls gamma-secretase processing of amyloid precursor protein in pre-golgi compartments of hippocampal neurons. J Cell Biol. 1999;147:277–294. doi: 10.1083/jcb.147.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arriagada PV, Growdon JH, Hedley-Whyte ET, Hyman BT. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer’s disease. Neurology. 1992;42:631–639. doi: 10.1212/wnl.42.3.631. [DOI] [PubMed] [Google Scholar]
- Bai G, Sheng N, Xie Z, Bian W, Yokota Y, Benezra R, Kageyama R, Guillemot F, Jing N. Id sustains Hes1 expression to inhibit precocious neurogenesis by releasing negative autoregulation of Hes1. Dev Cell. 2007;13:283–297. doi: 10.1016/j.devcel.2007.05.014. [DOI] [PubMed] [Google Scholar]
- Bai XC, Yan C, Yang G, Lu P, Ma D, Sun L, Zhou R, Scheres SH, Shi Y. An atomic structure of human gamma-secretase. Nature. 2015;525:212–217. doi: 10.1038/nature14892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barao S, Gartner A, Leyva-Diaz E, Demyanenko G, Munck S, Vanhoutvin T, Zhou L, Schachner M, Lopez-Bendito G, Maness PF, De Strooper B. Antagonistic effects of BACE1 and APH1B-gamma-secretase control axonal guidance by regulating growth cone collapse. Cell Rep. 2015;12:1367–1376. doi: 10.1016/j.celrep.2015.07.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnwell E, Padmaraju V, Baranello R, Pacheco-Quinto J, Crosson C, Ablonczy Z, Eckman E, Eckman CB, Ramakrishnan V, Greig NH, Pappolla MA, Sambamurti K. Evidence of a novel mechanism for partial gamma-secretase inhibition induced paradoxical increase in secreted amyloid beta protein. PLoS One. 2014;9:e91531. doi: 10.1371/journal.pone.0091531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthet G, Dunys J, Shao Z, Xuan Z, Ren Y, Xu J, Arbez N, Mauger G, Bruban J, Georgakopoulos A, Shioi J, Robakis NK. Presenilin mediates neuroprotective functions of ephrinB and brain-derived neurotrophic factor and regulates ligand-induced internalization and metabolism of EphB2 and TrkB receptors. Neurobiol Aging. 2013;34:499–510. doi: 10.1016/j.neurobiolaging.2012.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basak O, Taylor V. Identification of self-replicating multipotent progenitors in the embryonic nervous system by high Notch activity and Hes5 expression. Eur J Neurosci. 2007;25:1006–1022. doi: 10.1111/j.1460-9568.2007.05370.x. [DOI] [PubMed] [Google Scholar]
- Basak O, Giachino C, Fiorini E, Macdonald HR, Taylor V. Neurogenic subventricular zone stem/progenitor cells are Notch1-dependent in their active but not quiescent state. J Neurosci. 2012;32:5654–5666. doi: 10.1523/JNEUROSCI.0455-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beher D, Fricker M, Nadin A, Clarke EE, Wrigley JD, Li YM, Culvenor JG, Masters CL, Harrison T, Shearman MS. In vitro characterization of the presenilin-dependent gamma-secretase complex using a novel affinity ligand. Biochemistry. 2003;42:8133–8142. doi: 10.1021/bi034045z. [DOI] [PubMed] [Google Scholar]
- Bettens K, Sleegers K, Van Broeckhoven C. Genetic insights in Alzheimer’s disease. Lancet Neurol. 2013;12:92–104. doi: 10.1016/S1474-4422(12)70259-4. [DOI] [PubMed] [Google Scholar]
- Bonaguidi MA, Wheeler MA, Shapiro JS, Stadel RP, Sun GJ, Ming GL, Song H. In vivo clonal analysis reveals self-renewing and multipotent adult neural stem cell characteristics. Cell. 2011;145:1142–1155. doi: 10.1016/j.cell.2011.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonds JA, Kuttner-Hirshler Y, Bartolotti N, Tobin MK, Pizzi M, Marr R, Lazarov O. Presenilin-1 dependent neurogenesis regulates hippocampal learning and memory. PLoS One. 2015;10:e0131266. doi: 10.1371/journal.pone.0131266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghese L, Dolezalova D, Opitz T, Haupt S, Leinhaas A, Steinfarz B, Koch P, Edenhofer F, Hampl A, Brustle O. Inhibition of notch signaling in human embryonic stem cell-derived neural stem cells delays G1/S phase transition and accelerates neuronal differentiation in vitro and in vivo. Stem Cells. 2010;28:955–964. doi: 10.1002/stem.408. [DOI] [PubMed] [Google Scholar]
- Burns M, Gaynor K, Olm V, Mercken M, LaFrancois J, Wang L, Mathews PM, Noble W, Matsuoka Y, Duff K. Presenilin redistribution associated with aberrant cholesterol transport enhances beta-amyloid production in vivo. J Neurosci. 2003;23:5645–5649. doi: 10.1523/JNEUROSCI.23-13-05645.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers SM, Qi Y, Mica Y, Lee G, Zhang XJ, Niu L, Bilsland J, Cao L, Stevens E, Whiting P, Shi SH, Studer L. Combined small-molecule inhibition accelerates developmental timing and converts human pluripotent stem cells into nociceptors. Nat Biotechnol. 2012;30:715–720. doi: 10.1038/nbt.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez-Gutierrez L, Tolia A, Maes E, Li T, Wong PC, de Strooper B. Glu(332) in the Nicastrin ectodomain is essential for gamma-secretase complex maturation but not for its activity. J Biol Chem. 2008;283:20096–22105. doi: 10.1074/jbc.M803040200. [DOI] [PubMed] [Google Scholar]
- Chavez-Gutierrez L, Bammens L, Benilova I, Vandersteen A, Benurwar M, Borgers M, Lismont S, Zhou L, Van Cleynenbreugel S, Esselmann H, Wiltfang J, Serneels L, Karran E, Gijsen H, Schymkowitz J, Rousseau F, Broersen K, De Strooper B. The mechanism of gamma-secretase dysfunction in familial Alzheimer disease. EMBOJ. 2012;31:2261–2274. doi: 10.1038/emboj.2012.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CY, Liao W, Lou YL, Li Q, Hu B, Wang Y, Deng ZF. Inhibition of Notch signaling facilitates the differentiation of human-induced pluripotent stem cells into neural stem cells. Mol Cell Biochem. 2014;395:291–298. doi: 10.1007/s11010-014-2130-3. [DOI] [PubMed] [Google Scholar]
- Choi SH, Veeraraghavalu K, Lazarov O, Marler S, Ransohoff RM, Ramirez JM, Sisodia SS. Non-cell-autonomous effects of presenilin 1 variants on enrichment-mediated hippocampal progenitor cell proliferation and differentiation. Neuron. 2008;59:568–580. doi: 10.1016/j.neuron.2008.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu J, Lauretti E, Craige CP, Pratico D. Pharmacological modulation of GSAP reduces amyloid-beta levels and tau phosphorylation in a mouse model of Alzheimer’s disease with plaques and tangles. J Alzheimers Dis. 2014;41:729–737. doi: 10.3233/JAD-140105. [DOI] [PubMed] [Google Scholar]
- Citron M, Westaway D, Xia W, Carlson G, Diehl T, Levesque G, Johnson-Wood K, Lee M, Seubert P, Davis A, Kholodenko D, Motter R, Sherrington R, Perry B, Yao H, Strome R, Lieberburg I, Rommens J, Kim S, Schenk D, Fraser P, St George Hyslop P, Selkoe DJ. Mutant presenilins of Alzheimer’s disease increase production of 42-residue amyloid beta-protein in both transfected cells and transgenic mice. Nat Med. 1997;3:67–72. doi: 10.1038/nm0197-67. [DOI] [PubMed] [Google Scholar]
- Crump CJ, Castro SV, Wang F, Pozdnyakov N, Ballard TE, Sisodia SS, Bales KR, Johnson DS, Li YM. BMS-708,163 targets presenilin and lacks notch-sparing activity. Biochemistry. 2012;51:7209–7211. doi: 10.1021/bi301137h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Strooper B, Chavez Gutierrez L. Learning by failing: ideas and concepts to tackle gamma-secretases in Alzheimer’s disease and beyond. Annu Rev Pharmacol Toxicol. 2015;55:419–437. doi: 10.1146/annurev-pharmtox-010814-124309. [DOI] [PubMed] [Google Scholar]
- De Strooper B, Annaert W, Cupers P, Saftig P, Craessaerts K, Mumm JS, Schroeter EH, Schrijvers V, Wolfe MS, Ray WJ, Goate A, Kopan R. A presenilin-1-dependent gamma-secretase-like protease mediates release of Notch intracellular domain. Nature. 1999;398:518–522. doi: 10.1038/19083. [DOI] [PubMed] [Google Scholar]
- Deane R, Wu Z, Sagare A, Davis J, Du Yan S, Hamm K, Xu F, Parisi M, LaRue B, Hu HW, Spijkers P, Guo H, Song X, Lenting PJ, Van Nostrand WE, Zlokovic BV. LRP/amyloid beta-peptide interaction mediates differential brain efflux of Abeta isoforms. Neuron. 2004;43:333–344. doi: 10.1016/j.neuron.2004.07.017. [DOI] [PubMed] [Google Scholar]
- Demars M, Hu YS, Gadadhar A, Lazarov O. Impaired neurogenesis is an early event in the etiology of familial Alzheimer’s disease in transgenic mice. J Neurosci Res. 2010;88:2103–2117. doi: 10.1002/jnr.22387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doan A, Thinakaran G, Borchelt DR, Slunt HH, Ratovitsky T, Podlisny M, Selkoe DJ, Seeger M, Gandy SE, Price DL, Sisodia SS. Protein topology of presenilin 1. Neuron. 1996;17:1023–1030. doi: 10.1016/s0896-6273(00)80232-9. [DOI] [PubMed] [Google Scholar]
- Donovan MH, Yazdani U, Norris RD, Games D, German DC, Eisch AJ. Decreased adult hippocampal neurogenesis in the PDAPP mouse model of Alzheimer’s disease. J Comp Neurol. 2006;495:70–83. doi: 10.1002/cne.20840. [DOI] [PubMed] [Google Scholar]
- Doody RS, Raman R, Farlow M, Iwatsubo T, Vellas B, Joffe S, Kieburtz K, He F, Sun X, Thomas RG, Aisen PS, Alzheimer’s Disease Cooperative Study Steering, C. Siemers E, Sethuraman G, Mohs R, Semagacestat Study, G A phase 3 trial of semagacestat for treatment of Alzheimer’s disease. N Engl J Med. 2013;369:341–350. doi: 10.1056/NEJMoa1210951. [DOI] [PubMed] [Google Scholar]
- Dries DR, Shah S, Han YH, Yu C, Yu S, Shearman MS, Yu G. Glu-333 of nicastrin directly participates in gamma-secretase activity. J Biol Chem. 2009;284:29714–29724. doi: 10.1074/jbc.M109.038737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edbauer D, Winkler E, Regula JT, Pesold B, Steiner H, Haass C. Reconstitution of gamma-secretase activity. Nat Cell Biol. 2003;5:486–488. doi: 10.1038/ncb960. [DOI] [PubMed] [Google Scholar]
- Ehehalt R, Keller P, Haass C, Thiele C, Simons K. Amyloidogenic processing of the Alzheimer beta-amyloid precursor protein depends on lipid rafts. J Cell Biol. 2003;160:113–123. doi: 10.1083/jcb.200207113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elad N, De Strooper B, Lismont S, Hagen W, Veugelen S, Arimon M, Horre K, Berezovska O, Sachse C, Chavez-Gutierrez L. The dynamic conformational landscape of gamma-secretase Nadav. J Cell Sci. 2015;128:589–598. doi: 10.1242/jcs.164384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esler WP, Kimberly WT, Ostaszewski BL, Diehl TS, Moore CL, Tsai JY, Rahmati T, Xia W, Selkoe DJ, Wolfe MS. Transition-state analogue inhibitors of gamma-secretase bind directly to presenilin-1. Nat Cell Biol. 2000;2:428–434. doi: 10.1038/35017062. [DOI] [PubMed] [Google Scholar]
- Francis R, McGrath G, Zhang J, Ruddy DA, Sym M, Apfeld J, Nicoll M, Maxwell M, Hai B, Ellis MC, Parks AL, Xu W, Li J, Gurney M, Myers RL, Himes CS, Hiebsch R, Ruble C, Nye JS, Curtis D. aph-1 and pen-2 are required for Notch pathway signaling gamma-secretase cleavage of betaAPP, and presenilin protein accumulation. Dev Cell. 2002;3:85–97. doi: 10.1016/s1534-5807(02)00189-2. [DOI] [PubMed] [Google Scholar]
- Fuentealba RA, Barria MI, Lee J, Cam J, Araya C, Escudero CA, Inestrosa NC, Bronfman FC, Bu G, Marzolo MP. ApoER2 expression increases Abeta production while decreasing Amyloid Precursor Protein (APP) endocytosis: possible role in the partitioning of APP into lipid rafts and in the regulation of gamma-secretase activity. Mol Neurodegener. 2007;2:14. doi: 10.1186/1750-1326-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadadhar A, Marr R, Lazarov O. Presenilin-1 regulates neural progenitor cell differentiation in the adult brain. J Neurosci. 2011;31:2615–2623. doi: 10.1523/JNEUROSCI.4767-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannakopoulos P, Herrmann FR, Bussiere T, Bouras C, Kovari E, Perl DP, Morrison JH, Gold G, Hof PR. Tangle and neuron numbers but not amyloid load, predict cognitive status in Alzheimer’s disease. Neurology. 2003;60:1495–1500. doi: 10.1212/01.wnl.0000063311.58879.01. [DOI] [PubMed] [Google Scholar]
- Gillman KW, Starrett JE, Jr, Parker MF, Xie K, Bronson JJ, Marcin LR, McElhone KE, Bergstrom CP, Mate RA, Williams R, Meredith JE, Jr, Burton CR, Barten DM, Toyn JH, Roberts SB, Lentz KA, Houston JG, Zaczek R, Albright CF, Decicco CP, Macor JE, Olson RE. Discovery and evaluation of BMS-708163 a potent, selective and orally bioavailable gamma-Secretase inhibitor. ACS Med Chem Lett. 2010;1:120–124. doi: 10.1021/ml1000239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goutte C, Tsunozaki M, Hale VA, Priess JR. APH-1 is a multipass membrane protein essential for the Notch signaling pathway in Caenorhabditis elegans embryos. Proc Natl Acad Sci U S A. 2002;99:775–779. doi: 10.1073/pnas.022523499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm MO, Grimm HS, Patzold AJ, Zinser EG, Halonen R, Duering M, Tschape JA, De Strooper B, Muller U, Shen J, Hartmann T. Regulation of cholesterol and sphingomyelin metabolism by amyloid-beta and presenilin. Nat Cell Biol. 2005;7:1118–1123. doi: 10.1038/ncb1313. [DOI] [PubMed] [Google Scholar]
- Grimm MO, Grimm HS, Tomic I, Beyreuther K, Hartmann T, Bergmann C. Independent inhibition of Alzheimer disease beta- and gamma-secretase cleavage by lowered cholesterol levels. J Biol Chem. 2008;283:11302–11311. doi: 10.1074/jbc.M801520200. [DOI] [PubMed] [Google Scholar]
- Gu Y, Sanjo N, Chen F, Hasegawa H, Petit A, Ruan X, Li W, Shier C, Kawarai T, Schmitt-Ulms G, Westaway D, St George-Hyslop P, Fraser PE. The presenilin proteins are components of multiple membrane-bound complexes that have different biological activities. J Biol Chem. 2004;279:31329–31336. doi: 10.1074/jbc.M401548200. [DOI] [PubMed] [Google Scholar]
- Haapasalo A, Kovacs DM. The many substrates of presenilin/gamma-secretase. J Alzheimers Dis. 2011;25:3–28. doi: 10.3233/JAD-2011-101065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy JA, Higgins GA. Alzheimer’s disease: the amyloid cascade hypothesis. Science. 1992;256:184–185. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Haughey NJ, Nath A, Chan SL, Borchard AC, Rao MS, Mattson MP. Disruption of neurogenesis by amyloid beta-peptide, and perturbed neural progenitor cell homeostasis, in models of Alzheimer’s disease. J Neurochem. 2002;83:1509–1524. doi: 10.1046/j.1471-4159.2002.01267.x. [DOI] [PubMed] [Google Scholar]
- He G, Luo W, Li P, Remmers C, Netzer WJ, Hendrick J, Bettayeb K, Flajolet M, Gorelick F, Wennogle LP, Greengard P. Gamma-secretase activating protein is a therapeutic target for Alzheimer’s disease. Nature. 2010;467:95–98. doi: 10.1038/nature09325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henley DB, Sundell KL, Sethuraman G, Dowsett SA, May PC. Safety profile of semagacestat, a gamma-secretase inhibitor: iDENTITY trial findings. Curr Med Res Opin. 2014;30:2021–2032. doi: 10.1185/03007995.2014.939167. [DOI] [PubMed] [Google Scholar]
- Herrmann N, Chau SA, Kircanski I, Lanctot KL. Current and emerging drug treatment options for Alzheimer’s disease: a systematic review. Drugs. 2011;71:2031–2065. doi: 10.2165/11595870-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Herz J, Bock HH. Lipoprotein receptors in the nervous system. Annu Rev Biochem. 2002;71:405–434. doi: 10.1146/annurev.biochem.71.110601.135342. [DOI] [PubMed] [Google Scholar]
- Inoue E, Deguchi-Tawarada M, Togawa A, Matsui C, Arita K, Katahira-Tayama S, Sato T, Yamauchi E, Oda Y, Takai Y. Synaptic activity prompts gamma-secretase-mediated cleavage of EphA4 and dendritic spine formation. J Cell Biol. 2009;185:551–564. doi: 10.1083/jcb.200809151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwatsubo T, Odaka A, Suzuki N, Mizusawa H, Nukina N, Ihara Y. Visualization of A beta 42(43) and A beta 40 in senile plaques with end-specific A beta monoclonals: evidence that an initially deposited species is A beta 42(43) Neuron. 1994;13:45–53. doi: 10.1016/0896-6273(94)90458-8. [DOI] [PubMed] [Google Scholar]
- Jonsson T, Atwal JK, Steinberg S, Snaedal J, Jonsson PV, Bjornsson S, Stefansson H, Sulem P, Gudbjartsson D, Maloney J, Hoyte K, Gustafson A, Liu Y, Lu Y, Bhangale T, Graham RR, Huttenlocher J, Bjornsdottir G, Andreassen OA, Jonsson EG, Palotie A, Behrens TW, Magnusson OT, Kong A, Thorsteinsdottir U, Watts RJ, Stefansson K. A mutation in APP protects against Alzheimer’s disease and age-related cognitive decline. Nature. 2012;488:96–99. doi: 10.1038/nature11283. [DOI] [PubMed] [Google Scholar]
- Kang DE, Saitoh T, Chen X, Xia Y, Masliah E, Hansen LA, Thomas RG, Thal LJ, Katzman R. Genetic association of the low-density lipoprotein receptor-related protein gene (LRP), an apolipoprotein E receptor, with late-onset Alzheimer’s disease. Neurology. 1997;49:56–61. doi: 10.1212/wnl.49.1.56. [DOI] [PubMed] [Google Scholar]
- Kang DE, Pietrzik CU, Baum L, Chevallier N, Merriam DE, Kounnas MZ, Wagner SL, Troncoso JC, Kawas CH, Katzman R, Koo EH. Modulation of amyloid beta-protein clearance and Alzheimer’s disease susceptibility by the LDL receptor-related protein pathway. J Clin Invest. 2000;106:1159–1166. doi: 10.1172/JCI11013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojro E, Gimpl G, Lammich S, Marz W, Fahrenholz F. Low cholesterol stimulates the nonamyloidogenic pathway by its effect on the alpha -secretase ADAM 10. Proc Natl Acad Sci U S A. 2001;98:5815–5820. doi: 10.1073/pnas.081612998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopan R, Goate A. A common enzyme connects notch signaling and Alzheimer’s disease. Genes Dev. 2000;14:2799–2806. doi: 10.1101/gad.836900. [DOI] [PubMed] [Google Scholar]
- Kopan R, Ilagan MX. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell. 2009;137:216–233. doi: 10.1016/j.cell.2009.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai MT, Chen E, Crouthamel MC, DiMuzio-Mower J, Xu M, Huang Q, Price E, Register RB, Shi XP, Donoviel DB, Bernstein A, Hazuda D, Gardell SJ, Li YM. Presenilin-1 and presenilin-2 exhibit distinct yet overlapping gamma-secretase activities. J Biol Chem. 2003;278:22475–22481. doi: 10.1074/jbc.M300974200. [DOI] [PubMed] [Google Scholar]
- Laudon H, Hansson EM, Melen K, Bergman A, Farmery MR, Winblad B, Lendahl U, von Heijne G, Naslund J. A nine-transmembrane domain topology for presenilin 1. J Biol Chem. 2005;280:35352–35360. doi: 10.1074/jbc.M507217200. [DOI] [PubMed] [Google Scholar]
- Lazarov VK, Fraering PC, Ye W, Wolfe MS, Selkoe DJ, Li H. Electron microscopic structure of purified, active gamma-secretase reveals an aqueous intramembrane chamber and two pores. Proc Natl Acad Sci U S A. 2006;103:6889–6894. doi: 10.1073/pnas.0602321103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarov O, Mattson MP, Peterson DA, Pimplikar SW, van Praag H. When neurogenesis encounters aging and disease. Trends Neurosci. 2010;33:569–579. doi: 10.1016/j.tins.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitan D, Lee J, Song L, Manning R, Wong G, Parker E, Zhang L. PS1 N-and C-terminal fragments form a complex that functions in APP processing and Notch signaling. Proc Natl Acad Sci U S A. 2001;98:12186–12190. doi: 10.1073/pnas.211321898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YM, Lai MT, Xu M, Huang Q, DiMuzio-Mower J, Sardana MK, Shi XP, Yin KC, Shafer JA, Gardell SJ. Presenilin 1 is linked with gamma-secretase activity in the detergent solubilized state. Proc Natl Acad Sci U S A. 2000;97:6138–6143. doi: 10.1073/pnas.110126897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Li YM, Ahn K, Price DL, Sisodia SS, Wong PC. Increased expression of PS1 is sufficient to elevate the level and activity of gamma-secretase in vivo. PLoS One. 2011;6:e28179. doi: 10.1371/journal.pone.0028179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Lu SH, Tsai CJ, Bohm C, Qamar S, Dodd RB, Meadows W, Jeon A, McLeod A, Chen F, Arimon M, Berezovska O, Hyman BT, Tomita T, Iwatsubo T, Johnson CM, Farrer LA, Schmitt-Ulms G, Fraser PE, St George-Hyslop PH. Structural interactions between inhibitor and substrate docking sites give insight into mechanisms of human PS1 complexes. Structure. 2014;22:125–135. doi: 10.1016/j.str.2013.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Liu K, Qiu Y, Ren Z, Dai R, Deng Y, Qing H. Effect of presenilin mutations on APP cleavage; insights into the pathogenesis of FAD. Front Aging Neurosci. 2016;8:51. doi: 10.3389/fnagi.2016.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lleo A, Waldron E, von Arnim CA, Herl L, Tangredi MM, Peltan ID, Strickland DK, Koo EH, Hyman BT, Pietrzik CU, Berezovska O. Low density lipoprotein receptor-related protein (LRP) interacts with presenilin 1 and is a competitive substrate of the amyloid precursor protein (APP) for gamma-secretase. J Biol Chem. 2005;280:27303–27309. doi: 10.1074/jbc.M413969200. [DOI] [PubMed] [Google Scholar]
- Lobry C, Oh P, Aifantis I. Oncogenic and tumor suppressor functions of Notch in cancer: it’s NOTCH what you think. J Exp Med. 2011;208:1931–1935. doi: 10.1084/jem.20111855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu P, Bai XC, Ma D, Xie T, Yan C, Sun L, Yang G, Zhao Y, Zhou R, Scheres SH, Shi Y. Three-dimensional structure of human gamma-secretase. Nature. 2014;512:166–170. doi: 10.1038/nature13567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma G, Li T, Price DL, Wong PC. APH-1a is the principal mammalian APH-1 isoform present in gamma-secretase complexes during embryonic development. J Neurosci. 2005;25:192–198. doi: 10.1523/JNEUROSCI.3814-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marballi K, Cruz D, Thompson P, Walss-Bass C. Differential neuregulin 1 cleavage in the prefrontal cortex and hippocampus in schizophrenia and bipolar disorder: preliminary findings. PLoS One. 2012;7:e36431. doi: 10.1371/journal.pone.0036431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei L, Xiong WC. Neuregulin 1 in neural development, synaptic plasticity and schizophrenia. Nat Rev Neurosci. 2008;9:437–452. doi: 10.1038/nrn2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizutani K, Yoon K, Dang L, Tokunaga A, Gaiano N. Differential Notch signalling distinguishes neural stem cells from intermediate progenitors. Nature. 2007;449:351–355. doi: 10.1038/nature06090. [DOI] [PubMed] [Google Scholar]
- Musiek ES, Holtzman DM. Three dimensions of the amyloid hypothesis: time, space and ‘wingmen’. Nat Neurosci. 2015;18:800–806. doi: 10.1038/nn.4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niimura M, Isoo N, Takasugi N, Tsuruoka M, Ui-Tei K, Saigo K, Morohashi Y, Tomita T, Iwatsubo T. Aph-1 contributes to the stabilization and trafficking of the gamma-secretase complex through mechanisms involving intermolecular and intramolecular interactions. J Biol Chem. 2005;280:12967–12975. doi: 10.1074/jbc.M409829200. [DOI] [PubMed] [Google Scholar]
- Niva C, Parkinson J, Olsson F, van Schaick E, Lundkvist J, Visser SA. Has inhibition of Abeta production adequately been tested as therapeutic approach in mild AD? A model-based meta-analysis of gamma-secretase inhibitor data. Eur J Clin Pharmacol. 2013;69:1247–1260. doi: 10.1007/s00228-012-1459-3. [DOI] [PubMed] [Google Scholar]
- Offe K, Dodson SE, Shoemaker JT, Fritz JJ, Gearing M, Levey AI, Lah JJ. The lipoprotein receptor LR11 regulates amyloid beta production and amyloid precursor protein traffic in endosomal compartments. J Neurosci. 2006;26:1596–1603. doi: 10.1523/JNEUROSCI.4946-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okochi M, Tagami S, Yanagida K, Takami M, Kodama TS, Mori K, Nakayama T, Ihara Y, Takeda M. gamma-secretase modulators and presenilin 1 mutants act differently on presenilin/gamma-secretase function to cleave Abeta42 and Abeta43. Cell Rep. 2013;3:42–51. doi: 10.1016/j.celrep.2012.11.028. [DOI] [PubMed] [Google Scholar]
- Parvathy S, Hussain I, Karran EH, Turner AJ, Hooper NM. Cleavage of Alzheimer’s amyloid precursor protein by alpha-secretase occurs at the surface of neuronal cells. Biochemistry. 1999;38:9728–9734. doi: 10.1021/bi9906827. [DOI] [PubMed] [Google Scholar]
- Pasternak SH, Bagshaw RD, Guiral M, Zhang S, Ackerley CA, Pak BJ, Callahan JW, Mahuran DJ. Presenilin-1, nicastrin, amyloid precursor protein, and gamma-secretase activity are co-localized in the lysosomal membrane. J Biol Chem. 2003;278:26687–26694. doi: 10.1074/jbc.m304009200. [DOI] [PubMed] [Google Scholar]
- Placanica L, Tarassishin L, Yang G, Peethumnongsin E, Kim SH, Zheng H, Sisodia SS, Li YM. Pen2 and presenilin-1 modulate the dynamic equilibrium of presenilin-1 and presenilin-2 gamma-secretase complexes. J Biol Chem. 2009a;284:2967–2977. doi: 10.1074/jbc.M807269200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Placanica L, Zhu L, Li YM. Gender- and age-dependent gamma-secretase activity in mouse brain and its implication in sporadic Alzheimer disease. PLoS One. 2009b;4:e5088. doi: 10.1371/journal.pone.0005088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokop S, Shirotani K, Edbauer D, Haass C, Steiner H. Requirement of PEN-2 for stabilization of the presenilin N-/C-terminal fragment heterodimer within the gamma-secretase complex. J Biol Chem. 2004;279:23255–23261. doi: 10.1074/jbc.M401789200. [DOI] [PubMed] [Google Scholar]
- Qi-Takahara Y, Morishima-Kawashima M, Tanimura Y, Dolios G, Hirotani N, Horikoshi Y, Kametani F, Maeda M, Saido TC, Wang R, Ihara Y. Longer forms of amyloid beta protein: implications forthe mechanism of intramembrane cleavage by gamma-secretase. J Neurosci. 2005;25:436–445. doi: 10.1523/JNEUROSCI.1575-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray WJ, Yao M, Mumm J, Schroeter EH, Saftig P, Wolfe M, Selkoe DJ, Kopan R, Goate AM. Cell surface presenilin-1 participates in the gamma-secretase-like proteolysis of Notch. J Biol Chem. 1999a;274:36801–36807. doi: 10.1074/jbc.274.51.36801. [DOI] [PubMed] [Google Scholar]
- Ray WJ, Yao M, Nowotny P, Mumm J, Zhang W, Wu JY, Kopan R, Goate AM. Evidence for a physical interaction between presenilin and Notch. Proc Natl Acad Sci U S A. 1999b;96:3263–3268. doi: 10.1073/pnas.96.6.3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebeck GW, Reiter JS, Strickland DK, Hyman BT. Apolipoprotein E in sporadic Alzheimer’s disease: allelic variation and receptor interactions. Neuron. 1993;11:575–580. doi: 10.1016/0896-6273(93)90070-8. [DOI] [PubMed] [Google Scholar]
- Rechards M, Xia W, Oorschot VM, Selkoe DJ, Klumperman J. Presenilin-1 exists in both pre- and post-Golgi compartments and recycles via COPI-coated membranes. Traffic. 2003;4:553–565. doi: 10.1034/j.1600-0854.2003.t01-1-00114.x. [DOI] [PubMed] [Google Scholar]
- Rodriguez JJ, Jones VC, Tabuchi M, Allan SM, Knight EM, LaFerla FM, Oddo S, Verkhratsky A. Impaired adult neurogenesis in the dentate gyrus of a triple transgenic mouse model of Alzheimer’s disease. PLoS One. 2008;3:e2935. doi: 10.1371/journal.pone.0002935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogaeva E, Meng Y, Lee JH, Gu Y, Kawarai T, Zou F, Katayama T, Baldwin CT, Cheng R, Hasegawa H, Chen F, Shibata N, Lunetta KL, Pardossi-Piquard R, Bohm C, Wakutani Y, Cupples LA, Cuenco KT, Green RC, Pinessi L, Rainero I, Sorbi S, Bruni A, Duara R, Friedland RP, Inzelberg R, Hampe W, Bujo H, Song YQ, Andersen OM, Willnow TE, Graff-Radford N, Petersen RC, Dickson D, Der SD, Fraser PE, Schmitt-Ulms G, Younkin S, Mayeux R, Farrer LA, St George-Hyslop P. The neuronal sortilin-related receptor SORL1 is genetically associated with Alzheimer disease. Nat Genet. 2007;39:168–177. doi: 10.1038/ng1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahay A, Scobie KN, Hill AS, O’Carroll CM, Kheirbek MA, Burghardt NS, Fenton AA, Dranovsky A, Hen R. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature. 2011;472:466–470. doi: 10.1038/nature09817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, Diehl TS, Narayanan S, Funamoto S, Ihara Y, De Strooper B, Steiner H, Haass C, Wolfe MS. Active gamma-secretase complexes contain only one of each component. J Biol Chem. 2007;282:33985–33993. doi: 10.1074/jbc.M705248200. [DOI] [PubMed] [Google Scholar]
- Saura CA, Chen G, Malkani S, Choi SY, Takahashi RH, Zhang D, Gouras GK, Kirkwood A, Morris RG, Shen J. Conditional inactivation of presenilin 1 prevents amyloid accumulation and temporarily rescues contextual and spatial working memory impairments in amyloid precursor protein transgenic mice. J Neurosci. 2005;25:6755–6764. doi: 10.1523/JNEUROSCI.1247-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena MT, Schroeter EH, Mumm JS, Kopan R. Murine notch homologs (N1–4) undergo presenilin-dependent proteolysis. J Biol Chem. 2001;276:40268–40273. doi: 10.1074/jbc.M107234200. [DOI] [PubMed] [Google Scholar]
- Scherzer CR, Offe K, Gearing M, Rees HD, Fang G, Heilman CJ, Schaller C, Bujo H, Levey AI, Lah JJ. Loss of apolipoprotein E receptor LR11 in Alzheimer disease. Arch Neurol. 2004;61:1200–1205. doi: 10.1001/archneur.61.8.1200. [DOI] [PubMed] [Google Scholar]
- Scheuner D, Eckman C, Jensen M, Song X, Citron M, Suzuki N, Bird TD, Hardy J, Hutton M, Kukull W, Larson E, Levy-Lahad E, Viitanen M, Peskind E, Poorkaj P, Schellenberg G, Tanzi R, Wasco W, Lannfelt L, Selkoe D, Younkin S. Secreted amyloid beta-protein similartothat in the senile plaques of Alzheimer’s disease is increased in vivo by the presenilin 1 and 2 and APP mutations linked to familial Alzheimer’s disease. Nat Med. 1996;2:864–870. doi: 10.1038/nm0896-864. [DOI] [PubMed] [Google Scholar]
- Serafini T, Kennedy TE, Galko MJ, Mirzayan C, Jessell TM, Tessier-Lavigne M. The netrins define a family of axon outgrowth-promoting proteins homologous to C elegans UNC-6. Cell. 1994;78:409–424. doi: 10.1016/0092-8674(94)90420-0. [DOI] [PubMed] [Google Scholar]
- Serneels L, Dejaegere T, Craessaerts K, Horre K, Jorissen E, Tousseyn T, Hebert S, Coolen M, Martens G, Zwijsen A, Annaert W, Hartmann D, De Strooper B. Differential contribution of the three Aph1 genes to gamma-secretase activity in vivo. Proc Natl Acad Sci U S A. 2005;102:1719–1724. doi: 10.1073/pnas.0408901102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serneels L, Van Biervliet J, Craessaerts K, Dejaegere T, Horre K, Van Houtvin T, Esselmann H, Paul S, Schafer MK, Berezovska O, Hyman BT, Sprangers B, Sciot R, Moons L, Jucker M, Yang Z, May PC, Karran E, Wiltfang J, D’Hooge R, De Strooper B. Gamma-secretase heterogeneity in the Aph1 subunit: relevance for Alzheimer’s disease. Science. 2009;324:639–642. doi: 10.1126/science.1171176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah S, Lee SF, Tabuchi K, Hao YH, Yu C, LaPlant Q, Ball H, Dann CE, 3rd, Sudhof T, Yu G. Nicastrin functions as a gamma-secretase-substrate receptor. Cell. 2005;122:435–447. doi: 10.1016/j.cell.2005.05.022. [DOI] [PubMed] [Google Scholar]
- Shen J, Kelleher RJ., 3rd The presenilin hypothesis of Alzheimer’s disease: evidence for a loss-of-function pathogenic mechanism. Proc Natl Acad Sci U S A. 2007;104:403–409. doi: 10.1073/pnas.0608332104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimojo H, Ohtsuka T, Kageyama R. Oscillations in notch signaling regulate maintenance of neural progenitors. Neuron. 2008;58:52–64. doi: 10.1016/j.neuron.2008.02.014. [DOI] [PubMed] [Google Scholar]
- Shirotani K, Edbauer D, Prokop S, Haass C, Steiner H. Identification of distinct gamma-secretase complexes with different APH-1 variants. J Biol Chem. 2004;279:41340–41345. doi: 10.1074/jbc.M405768200. [DOI] [PubMed] [Google Scholar]
- Stone SS, Teixeira CM, Devito LM, Zaslavsky K, Josselyn SA, Lozano AM, Frankland PW. Stimulation of entorhinal cortex promotes adult neurogenesis and facilitates spatial memory. J Neurosci. 2011;31:13469–13484. doi: 10.1523/JNEUROSCI.3100-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takami M, Nagashima Y, Sano Y, Ishihara S, Morishima-Kawashima M, Funamoto S, Ihara Y. Gamma-secretase: successive tripeptide and tetrapeptide release from the transmembrane domain of beta-carboxyl terminal fragment. J Neurosci. 2009;29:13042–13052. doi: 10.1523/JNEUROSCI.2362-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takasugi N, Tomita T, Hayashi I, Tsuruoka M, Niimura M, Takahashi Y, Thinakaran G, Iwatsubo T. The role of presenilin cofactors in the gamma-secretase complex. Nature. 2003;422:438–441. doi: 10.1038/nature01506. [DOI] [PubMed] [Google Scholar]
- Taniguchi Y, Kim SH, Sisodia SS. Presenilin-dependent gamma-secretase processing of deleted in colorectal cancer (DCC) J Biol Chem. 2003;278:30425–30428. doi: 10.1074/jbc.C300239200. [DOI] [PubMed] [Google Scholar]
- Thinakaran G, Borchelt DR, Lee MK, Slunt HH, Spitzer L, Kim G, Ratovitsky T, Davenport F, Nordstedt C, Seeger M, Hardy J, Levey AI, Gandy SE, Jenkins NA, Copeland NG, Price DL, Sisodia SS. Endoproteolysis of presenilin 1 and accumulation of processed derivatives in vivo. Neuron. 1996;17:181–190. doi: 10.1016/s0896-6273(00)80291-3. [DOI] [PubMed] [Google Scholar]
- Ulery PG, Beers J, Mikhailenko I, Tanzi RE, Rebeck GW, Hyman BT, Strickland DK. Modulation of beta-amyloid precursor protein processing by the low density lipoprotein receptor-related protein (LRP). Evidence that LRP contributes to the pathogenesis of Alzheimer’s disease. J Biol Chem. 2000;275:7410–7415. doi: 10.1074/jbc.275.10.7410. [DOI] [PubMed] [Google Scholar]
- Underwood E. NEUROSCIENCE. Alzheimer’s amyloid theory gets modest boost. Science. 2015;349:464. doi: 10.1126/science.349.6247.464. [DOI] [PubMed] [Google Scholar]
- Vassar R, Bennett BD, Babu-Khan S, Kahn S, Mendiaz EA, Denis P, Teplow DB, Ross S, Amarante P, Loeloff R, Luo Y, Fisher S, Fuller J, Edenson S, Lile J, Jarosinski MA, Biere AL, Curran E, Burgess T, Louis JC, Collins F, Treanor J, Rogers G, Citron M. Beta-secretase cleavage of Alzheimer’s amyloid precursor protein by the transmembrane aspartic protease BACE. Science. 1999;286:735–741. doi: 10.1126/science.286.5440.735. [DOI] [PubMed] [Google Scholar]
- Villa JC, Chiu D, Brandes AH, Escorcia FE, Villa CH, Maguire WF, Hu CJ, de Stanchina E, Simon MC, Sisodia SS, Scheinberg DA, Li YM. Nontranscriptional role of Hif-1alpha in activation of gamma-secretase and notch signaling in breast cancer. Cell Rep. 2014;8:1077–1092. doi: 10.1016/j.celrep.2014.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Dineley KT, Sweatt JD, Zheng H. Presenilin 1 familial Alzheimer’s disease mutation leads to defective associative learning and impaired adult neurogenesis. Neuroscience. 2004;126:305–312. doi: 10.1016/j.neuroscience.2004.03.048. [DOI] [PubMed] [Google Scholar]
- Wang J, Ye Z, Zheng S, Chen L, Wan Y, Deng Y, Yang R. Lingo-1 shRNA and Notch signaling inhibitor DAPT promote differentiation of neural stem/progenitor cells into neurons. Brain Res. 2015 doi: 10.1016/j.brainres.2015.11.029. [DOI] [PubMed] [Google Scholar]
- Wen PH, Hof PR, Chen X, Gluck K, Austin G, Younkin SG, Younkin LH, DeGasperi R, Gama Sosa MA, Robakis NK, Haroutunian V, Elder GA. The presenilin-1 familial Alzheimer disease mutant P117L impairs neurogenesis in the hippocampus of adult mice. Exp Neurol. 2004;188:224–237. doi: 10.1016/j.expneurol.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Wolfe MS, Xia W, Ostaszewski BL, Diehl TS, Kimberly WT, Selkoe DJ. Two transmembrane aspartates in presenilin-1 required for presenilin endoproteolysis and gamma-secretase activity. Nature. 1999;398:513–517. doi: 10.1038/19077. [DOI] [PubMed] [Google Scholar]
- Xia D, Watanabe H, Wu B, Lee SH, Li Y, Tsvetkov E, Bolshakov VY, Shen J, Kelleher RJ., 3rd Presenilin-1 knockin mice reveal loss-of-function mechanism for familial Alzheimer’s disease. Neuron. 2015;85:967–981. doi: 10.1016/j.neuron.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu G, Nishimura M, Arawaka S, Levitan D, Zhang L, Tandon A, Song YQ, Rogaeva E, Chen F, Kawarai T, Supala A, Levesque L, Yu H, Yang DS, Holmes E, Milman P, Liang Y, Zhang DM, Xu DH, Sato C, Rogaev E, Smith M, Janus C, Zhang Y, Aebersold R, Farrer LS, Sorbi S, Bruni A, Fraser P, St George-Hyslop P. Nicastrin modulates presenilin-mediated notch/glp-1 signal transduction and betaAPP processing. Nature. 2000;407:48–54. doi: 10.1038/35024009. [DOI] [PubMed] [Google Scholar]
- Zerbinatti CV, Wahrle SE, Kim H, Cam JA, Bales K, Paul SM, Holtzman DM, Bu G. Apolipoprotein E and low density lipoprotein receptor-related protein facilitate intraneuronal Abeta42 accumulation in amyloid model mice. J Biol Chem. 2006;281:36180–36186. doi: 10.1074/jbc.M604436200. [DOI] [PubMed] [Google Scholar]
- Zhang C, McNeil E, Dressler L, Siman R. Long-lasting impairment in hippocampal neurogenesis associated with amyloid deposition in a knock-in mouse model of familial Alzheimer’s disease. Exp Neurol. 2007;204:77–87. doi: 10.1016/j.expneurol.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Hoey RJ, Lin G, Koide A, Leung B, Ahn K, Dolios G, Paduch M, Ikeuchi T, Wang R, Li YM, Koide S, Sisodia SS. Identification of a tetratricopeptide repeat-like domain in the nicastrin subunit of gamma-secretase using synthetic antibodies. Proc Natl Acad Sci U S A. 2012;109:8534–8539. doi: 10.1073/pnas.1202691109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Yu CJ, Sisodia SS. The topology of pen-2, a gamma-secretase subunit, revisited: evidence for a reentrant loop and a single pass transmembrane domain. Mol Neurodegener. 2015;10:39. doi: 10.1186/s13024-015-0037-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao G, Liu Z, Ilagan MX, Kopan R. Gamma-secretase composed of PS1/Pen2/Aph1acan cleave notch and amyloid precursor protein in the absence of nicastrin. J Neurosci. 2010;30:1648–1656. doi: 10.1523/JNEUROSCI.3826-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Koo EH. Biology and pathophysiology of the amyloid precursor protein. Mol Neurodegener. 2011;6:27. doi: 10.1186/1750-1326-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu M, Tao Y, He Q, Gao H, Song F, Sun YM, Li HL, Wu ZY, Saffen D. Common GSAP promoter variant contributes to Alzheimer’s disease liability. Neurobiol Aging. 2014;35(265):e1–7. doi: 10.1016/j.neurobiolaging.2014.05.018. [DOI] [PubMed] [Google Scholar]


