Abstract
Migraine frequently co-occurs with depression. Using a large sample of Australian twin pairs, we aimed to characterise the extent to which shared genetic factors underlie these two disorders. Migraine was classified using three diagnostic measures, including self-reported migraine, the ID migraine™ screening tool, or migraine without aura (MO) and migraine with aura (MA) based on International Headache Society (IHS) diagnostic criteria. Major depressive disorder (MDD) and minor depressive disorder (MiDD) were classified using the Diagnostic and Statistical Manual of Mental Disorders (DSM) criteria. Univariate and bivariate twin models, with and without sex-limitation, were constructed to estimate the univariate and bivariate variance components and genetic correlation for migraine and depression. The univariate heritability of broad migraine (self-reported, ID migraine or IHS MO/MA) and broad depression (MiDD or MDD) was estimated at 56% (95% confidence interval [CI]: 53–60%) and 42% (95% CI: 37–46%), respectively. A significant additive genetic correlation (rG=0.36, 95% CI: 0.29–0.43) and bivariate heritability (h2=5.5%, 95% CI: 3.6–7.8%) was observed between broad migraine and depression using the bivariate Cholesky model. Notably, both the bivariate h2 (13.3%, 95% CI: 7.0–24.5%) and rG (0.51, 95% CI: 0.37–0.69) estimates significantly increased when analysing the more narrow clinically-accepted diagnoses of IHS MO/MA and MDD. Our results indicate that for both broad and narrow definitions, the observed comorbidity between migraine and depression can be explained almost entirely by shared underlying genetically determined disease mechanisms.
Keywords: migraine, depression, twin study, heritability, genetic correlation, bivariate heritability
Migraine and depression are common complex disorders that share a higher than expected co-occurrence (Saraceno, 2002; Vos et al., 2012). Previous longitudinal studies (Breslau et al., 1994; Breslau et al., 2003; Breslau et al., 2000; Modgill et al., 2012; Mongini et al., 2003) identified a bi-directional association between migraine and depression, with one disorder increasing the relative risk for the other, and vice versa. However, the etiology underlying the two disorders is still poorly understood.
Previous twin and family studies consistently observed a moderate genetic effect on susceptibilities of the two disorders, with heritability (h2) estimates of around 30–50% for both migraine (Honkasalo et al., 1995; Mulder et al., 2003; Svensson et al., 2003) and depression (Bierut et al., 1999; Duncan et al., 2014; Nes et al., 2013; Sullivan et al., 2000). Several studies also found evidence for shared genetic components between migraine and depression (Ligthart et al., 2014; Ligthart et al., 2010; Schur et al., 2009; Stam et al., 2010). Firstly, Schur et al. (Schur et al., 2009) utilised bivariate structural equation modelling (SEM) in a US sample of 758 monozygotic (MZ) and 306 dizygotic (DZ) female twin pairs to estimate a trait-specific heritability of 52% (95% confidence interval [CI]: 11–66%) for self-reported doctor’s diagnosis of depression and 44% (95% CI: 18–55%) for self-reported doctor’s diagnosis of migraine headache, and the authors estimated that 20% of the variance in depression and migraine is due to shared genetics (i.e., bivariate heritability of 20%) and 4% of the unique environmental component is shared. A second twin study using 223 MZ male, 100 DZ male, 602 MZ female, 286 DZ female, and 280 DZ opposite sex Dutch twin pairs (Ligthart et al., 2010) estimated a heritability of 45% for latent class analysis (LCA)-derived migrainous headache and 55% for anxious depression—a measure consisting of a factor score based on several measures of anxiety, depression, and neuroticism—and estimated a genetic correlation (rG) between migrainous headache and anxious depression of 0.30 (95% CI: 0.18–0.43). Additionally, a family-based study in a large Dutch genetic isolate utilised International Headache Society (IHS) diagnostic criteria and reported significant heritability estimates for migraine without aura (MO) (h2=0.77, 95% CI: 0.38–1.00), migraine with aura (MA) (h2=0.96, 95% CI: 0.51–1.00), and all migraine (h2=0.56, 95% CI: 0.26–0.86), which all decreased (albeit non-significantly) after adjustment for symptoms of depression or use of antidepressant medication (Stam et al., 2010). Interestingly, a comparison of the heritability scores for depression between patients with migraine and controls found evidence for shared genetic factors only between MA and depression. Lastly, a significant correlation in genetic risk across migraine and major depressive disorder (MDD) was revealed by a recent genetic risk score (GRS) analysis (Ligthart et al., 2014).
Although providing consistent evidence for shared genetic components between migraine and depression, the twin and family studies performed to date utilised a wide variety of approaches and diagnostic measures which complicate their interpretation and comparison. Furthermore, the shared genetic variance might be described by either the same genetic and environmental factors (i.e., same genetic and environmental factors account for susceptibility of both migraine and depression [pleiotropy]), or their potential causation (i.e., genetic and environmental factors cause a primary disorder which results in a secondary disorder) (De Moor et al., 2008). In addition, some studies (Bierut et al., 1999; Larsson et al., 1995) report a higher genetic basis for migraine and depression in females compared to males, suggesting sex may play a role in the variation of the shared genetic components.
Therefore, this study utilised a variety of diagnostic measures and performed SEM (Rijsdijk & Sham, 2002) with and without sex-limitation in a large Australian population-based twin sample to i) estimate the trait-specific heritability of migraine and depression; ii) estimate the shared genetic components between migraine and depression; and iii) investigate whether the shared genetic components are due to the same genetic factors or due to one disorder causing the other.
Materials and methods
Samples
As detailed in Supplement Figure S1, participants were drawn from three Australian twin cohorts based at QIMR Berghofer Medical Research Institute (Heath et al., 2001; Wright & Martin, 2004). Subjects with migraine and depression status were selected and constituted the “merged migraine sample” (N=38279) and “merged depression sample” (N=60170), respectively. Definitions of migraine and depression were homogenised across the cohorts. Subjects also answered questions regarding demographic characteristics (e.g., sex, date of birth, zygosity), via semi-structured telephone interview and/or questionnaire. After combining the two merged samples and removal of non-twins and twins with missing status of either migraine or depression, a total of 5319 twin pairs (2456 MZ and 2863 DZ pairs) remained for analysis.
Assessment of migraine
Migraine symptom information ranged from single answer self-report (‘yes’ or ‘no’) of migraine, the ID Migraine™ Screener (Lipton et al., 2003)—three questions shown to accurately identify 93% of people with migraines, to detailed IHS diagnostic criteria (the International Classification of Headache Disorders, ICHD-3) (Headache Classification Committee of the International Headache, 2013). For the collection of detailed ICHD-3 diagnostic criteria (see Table S1), participants answering ‘yes’ to ever having ‘migraine or recurrent attacks of headache’ (screening positive), then answered a number of questions relating to their symptoms. Diagnoses were determined for the two major varieties of migraine: 1.1 migraine without aura (MO) and 1.2 migraine with aura (MA, primarily comprising 1.2.1 Typical aura with migraine headache), which account for 90–95% of all IHS migraines (Launer et al., 1999).
After careful merging of all available migraine information, lifetime diagnoses for migraine were made subject to data availability, according to i) self-reported migraine, ii) the ID Migraine™ Screener, or iii) IHS ICHD-3 MO/MA diagnostic criteria. Hence, migraine status was measured in four categories according to these three criteria: non-migraine, self-report migraine (i.e., participants with positive status of self-reported measurement but negative or unknown status of the other two criteria), ID migraine (i.e., participants with positive status ID migraineTM Screener criteria but negative or unknown IHS-based migraine status) and IHS MO/MA (i.e., participants with positive status of IHS-based migraine). The ‘broad’ migraine status (i.e., any migraine) was defined when participants had reported at least one positive migraine status; and the ‘narrow’ migraine status was restricted to the clinically-accepted diagnosis of IHS MO/MA.
Assessment of depression
Participants were first asked two screening questions “Has there ever been two weeks or more when you were depressed or down most of the day, nearly every day?” and “Has there ever been two weeks or more when you were a lot less interested in most things or unable to enjoy the things you used to enjoy, most of the day, nearly every day?”. With at least one positive response, participants then answered additional questions (see Table S1). Lifetime depression was diagnosed according to the third and revised edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-III-R) (Diagnostic and Statistical Manual of Mental Disorders Fourth Edition, 1994) criteria: during two-week period, participants who had positive responses of more than five symptoms were diagnosed suffering MDD and participants who had 2–4 positive responses were diagnosed suffering minor depressive disorder (MiDD). Therefore, in our study, depression status was measured in three categories: non-depressed, MiDD, and MDD. Participants with either MiDD or MDD were defined to have the ‘broad’ depression status (i.e., any depression), and the MDD status was used as the narrow clinically-accepted diagnostic measure of depression.
Statistical analysis
All the analyses were performed in RStudio (RStudio Team, 2014). The concordance of migraine and depression for MZ and DZ twins were calculated by polychoric correlation—a measurement quantifying association between two ordinal variables that have an underlying bivariate normal distribution. Polychoric correlations were estimated using the R package polycor (https://cran.r-project.org/web/packages/polycor/index.html). The polychoric correlation, assumes that underlying the observed polychotomous distribution of affection status, there exists a continuous, normally distributed latent (non-observable) liability. That is, the polychoric correlation is an estimate of the correlation between two latent variables, where each latent variable is assumed to have a bivariate normal distribution. A χ2 goodness-of-fit test is used to test whether the multiple threshold model provides a good fit to the observed data (i.e., compares the observed frequencies to those predicted by the model).
A classical twin study was used to estimate the genetic and environmental contributions to the susceptibility of a target trait. The proportion of the phenotypic variance due to genetic differences is termed the heritability (Almasy & Blangero, 2010). The model assumes the total phenotypic variance is comprised of the variances from additive genetic factors (A), non-additive (dominance) genetic factors (D), non-unique (shared) environmental factors (C) and unique environmental factors (E). MZ twins share 100% of their A, D and C, while DZ twins share 50% of A, 25% of D, and 100% of C on average (Boomsma et al., 2002). According to these principles, the proportions of genetic and environmental contributions can be estimated by comparing concordance between MZ and DZ twins using structural equation modelling (SEM) (Rijsdijk & Sham, 2002). Using the R package OpenMx (Boker et al., 2011) twin models were initially generated for the broad definitions of migraine and depression, and subsequent models utilised more specific and narrow definitions by excluding individuals from the analysis.
Univariate analyses were first used to examine model fit and characteristics, and estimate trait-specific variance components for migraine and depression. The liability threshold model, adjusted by age and sex, was applied to each trait. Specifically, when analysing broad migraine and broad depression, univariate models were built independently as a comparison: the first (‘one-threshold’) model was built using one threshold to separate two phenotype categories: non-migraine and broad migraine; and non-depressed and broad depression. The second (‘two-threshold’) model was built using two thresholds to separate three phenotype categories: non-migraine, self-report/ID migraine and IHS MO/MA; and non-depressed, MiDD and MDD. When analysing IHS MO/MA and MDD, one threshold was utilised to separate two phenotype categories for migraine (i.e., non-migraine and IHS MO/MA) and depression (i.e., non-depressed and MDD). Starting with the full model (ACE/ADE), the reduced models (AE, CE) were then built by systematic removal of latent variables from the model. We selected the best fitting model using the likelihood-ratio test and comparing the Akaike’s information criterion (AIC) values.
The univariate model can then be extended to the bivariate Cholesky model (Meanderthal, 2012), to estimate the shared genetic variance components between two traits by calculating their genetic correlation and bivariate heritability. The genetic correlation (rG) between two traits is defined as , where represents the genetic covariance between two traits, and and are the heritability of trait 1 and trait 2. The bivariate heritability (h2) stands for the proportion of genetic overlap between the heritability of trait 1 and trait 2. The bivariate Cholesky models were built referring to the best fitting models of univariate analyses for migraine and depression. By comparing results from analysis of broad migraine and depression to results using more narrow diagnoses, we can examine the influence of using different diagnostic measures on the univariate and bivariate estimates of genetic and environmental variance components.
In addition, sex-limitation models (Neale et al., 2006) for both univariate and bivariate analyses were constructed to explore the potential influence of sex-specific effects on the genetic liability to migraine and depression. A general non-scalar sex-limitation model was firstly built, including latent variables for females (i.e., Af, Cf and Ef) and males (i.e., Am, Cm and Em) and an specific additive genetic component for males which does not correlate with the female component (i.e., AmS). The reduced non-scalar sex-limitation model was then constructed by removing the additional specific additive genetic variable. A restricted scalar effects sex-limitation model was also built, which assumes that all the latent variables for males are linked with the latent variables for females by a scalar effect k (i.e., Af=kAm, Cf=kCm, Ef=kEm). The best fitting model was selected by the likelihood ratio test and comparing the AIC values.
Following the bivariate analyses, two approaches were used to test whether potential causation or the same genetic factors between migraine and depression best explain the shared genetic components between the two traits (De Moor et al., 2008). Firstly, under the hypothesis of causation, one disorder would increase risk for the other via both genetic and environmental factors. Hence, both the genetic and environmental correlations between two conditions should be significant; otherwise, the hypothesis of causation is invalid. A second approach focuses on checking the presence of causal environmental factors. Briefly, in MZ twins the intrapair differences in trait 1 should be associated with intrapair differences in trait 2. The absence of this association between the intrapair differences in trait 1 and trait 2 falsifies the hypothesis of direct causation (i.e., one trait causing the other), whereas the presence of this association would support the causal hypothesis because it excludes confounding by genetic factors (i.e., the twins are genetically identical). Using this ‘MZ twin intrapair differences’ model, we computed the differences of migraine status of an MZ twin and his or her co-twin. For example, non-migraine, self-report/ID migraine and IHS MO/MA were coded as 0, 1 and 1; and non-depressed, MiDD and MDD were coded as 0, 1 and 1, respectively. For each MZ twin pair, the intrapair difference was then calculated as either −1, 0 or 1 for broad migraine and −1, 0 or 1 for broad depression. The intrapair differences for migraine were then regressed on the intrapair differences for depression. Significant regression coefficients would be compatible with the causal hypothesis, whereas nonsignificant regression analysis would falsify this hypothesis.
Results
Demographics and twin concordance of migraine and depression
The mean age of 10638 individuals was 36±11 years when they participated in the survey, with a range from 18 to 89 years (Table S2). Age was used as a covariate in model fitting. The lifetime prevalence was evaluated at 45% for broad migraine (53% of females, 33% of males) and 42% for broad depression (45% of females, 37% of males), respectively. Whereas, 14% of participants (18% of females, 8% of males) were diagnosed suffering IHS MO/MA and 34% of participants (38% of females, 29% of males) were diagnosed as MDD.
The polychoric correlations for MZ twins were always higher than DZ twins for the broad diagnoses of migraine and depression (Table 1), suggesting genetic contributions to both disorders. Importantly, none of the multiple-threshold model goodness-of-fit tests (one for each zygosity group) were significant at the 5% level. Therefore, these results support the validity of the multiple threshold model for the migraine and depression classifications, and indicate that they can both be conceptualized as different levels of severity on a single dimension of liability. The polychoric correlations estimated for the three-category migraine and depression classifications (i.e., non-migraine, self-report/ID migraine and IHS MO/MA; and non-depressed, MiDD and MDD) were higher than those estimated for the two-category classifications (i.e., non-migraine and broad migraine; and non-depressed and broad depression), indicating the three-category classifications better capture the familial aggregation of the two disorders. Similar to the analysis of broad diagnoses, the estimated correlations for the more narrow clinically-accepted diagnoses of IHS MO/MA and MDD were higher within MZ than DZ twins. Furthermore, the increased difference between MZ and DZ correlations indicates a stronger genetic influence to IHS MO/MA and MDD.
Table 1.
Polychoric correlations for migraine and depression according to twin zygosity
| Two category broad classification* | |||||
|
| |||||
| Broad migraine | |||||
|
| |||||
| Zygosity [N] | MZFF [1623] | MZMM [833] | DZFF [1064] | DZMM [589] | DZFM [1210] |
| ρpolychoric (sd) | 0.45 (0.03) | 0.43 (0.05) | 0.31 (0.05) | 0.25 (0.07) | 0.12 (0.05) |
| 95% CI | (0.39–0.52) | (0.33–0.53) | (0.23–0.40) | (0.12–0.38) | (0.03–0.21) |
|
| |||||
| Broad depression | |||||
|
| |||||
| Zygosity [N] | MZFF [1623] | MZMM [833] | DZFF [1064] | DZMM [589] | DZFM [1210] |
| ρpolychoric (sd) | 0.43 (0.03) | 0.37 (0.05) | 0.10 (0.05) | 0.34 (0.06) | 0.13 (0.05) |
| 95% CI | (0.37–0.50) | (0.27–0.47) | (3×10−3–0.19) | (0.22–0.46) | (0.04–0.21) |
|
| |||||
| Three category broad classification^ | |||||
|
| |||||
| Broad migraine | |||||
|
| |||||
| Zygosity [N] | MZFF [1623] | MZMM [833] | DZFF [1064] | DZMM [589] | DZFM [1210] |
| ρpolychoric (sd) | 0.52 (0.02) | 0.42 (0.04) | 0.36 (0.03) | 0.30 (0.06) | 0.28 (0.04) |
| 95% CI | (0.47–0.57) | (0.33–0.51) | (0.30–0.43) | (0.19–0.41) | (0.21–0.36) |
|
| |||||
| Broad depression | |||||
|
| |||||
| Zygosity [N] | MZFF [1623] | MZMM [833] | DZFF [1064] | DZMM [589] | DZFM [1210] |
| ρpolychoric (sd) | 0.46 (0.03) | 0.37 (0.05) | 0.12 (0.04) | 0.29 (0.06) | 0.13 (0.04) |
| 95% CI | (0.40–0.52) | (0.28–0.47) | (0.04–0.21) | (0.17–0.40) | (0.05–0.22) |
|
| |||||
| Two category narrow classification# | |||||
|
| |||||
| IHS MO/MA | |||||
|
| |||||
| Zygosity [N] | MZFF [722] | MZMM [498] | DZFF [583] | DZMM [348] | DZFM [642] |
| ρpolychoric (sd) | 0.84 (0.03) | 0.69 (0.08) | 0.48 (0.06) | 0.50 (0.11) | 0.60 (0.06) |
| 95% CI | (0.79–0.89) | (0.54–0.84) | (0.36–0.59) | (0.29–0.71) | (0.49–0.71) |
|
| |||||
| MDD | |||||
|
| |||||
| Zygosity [N] | MZFF [1401] | MZMM [695] | DZFF [902] | DZMM [512] | DZFM [1024] |
| ρpolychoric (sd) | 0.53 (0.03) | 0.45 (0.06) | 0.15 (0.05) | 0.30 (0.07) | 0.16 (0.05) |
| 95% CI | (0.47–0.60) | (0.34–0.55) | (0.05–0.25) | (0.16–0.44) | (0.06–0.26) |
ρ polychoric: polychoric correlation; 95% CI: 95% confidence interval; sd: standard deviation; MZFF: female MZ twins; MZMM: male MZ twins; DZFF: female DZ twins; DZMM: male DZ twins; DZFM: opposite-sex DZ twins; N: number of twin pairs.
Two phenotype categories for broad migraine: non-migraine and self-report/ID migraine/IHS MO/MA; two phenotype categories for broad depression: non-depressed and MiDD/MDD.
Three phenotype categories for broad migraine: non-migraine, self-report/ID migraine and IHS MO/MA; three phenotype categories for broad depression: non-depressed, MiDD and MDD.
Two phenotype categories for narrow migraine: non-migraine and IHS MO/MA; two phenotype categories for narrow depression: non-depressed and MDD.
Results from broad diagnoses analyses
Univariate analyses
Four SEMs (ACE, AE, CE and ADE) were built to examine the genetic architecture of broad migraine and depression, based on either the one-threshold (two-category classification) model or two-threshold (three-category classification) model. No differences in threshold liability distributions were observed within twin pairs and across zygosity groups for migraine and depression. Constraining the threshold distributions to be equal in males and females resulted in a significant deterioration in fit for migraine (p-value=5.16×10−61 for the one-threshold model; p-value=1.58×10−68 for the two-threshold model) and depression (p-value=7.64×10−11 for the one-threshold model; p-value=8.82×10−14 for the two-threshold model) when compared to a model of separate sex thresholds. We also observed that the sex differences in threshold distributions could be accounted for by a single set of liability thresholds for males plus a displacement to account for the observed higher prevalence in females for migraine and depression.
For both migraine and depression, the AE model provided a more plausible and parsimonious fit than other models (Table S3). In line with the polychoric correlation results, the two-threshold model consistently produced higher heritability estimates for migraine and depression compared to the one-threshold model. We therefore concentrate on results from the two-threshold model. For the two-threshold AE model (Table 2), the univariate heritability was estimated at 56% (95% CI: 53–60%) for migraine and 42% (95% CI: 37–46%) for depression. The non-scalar sex-limitation model without specific additive genetic effects for males provided the best fit to the data. Females had a higher heritability estimate for migraine and depression compared to males, however the sex difference did not reach statistical significance at the 5% level, and the male-female genetic correlation was estimated at 0.92 (95% CI: 0.64–1.0) for migraine and 0.60 (95% CI: 0.20–1.0) for depression.
Table 2.
Univariate analyses for broad migraine and broad depression with and without sex-limitation based on two-threshold model
| Disorders | Total sample | Sex-limitation | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Female | Male | |||||
|
| ||||||
| A | E | A | E | A | E | |
| Broad migraine^ | 0.57 (0.53–0.60) | 0.44 (0.40–0.47) | 0.60 (0.55–0.64) | 0.40 (0.36–0.45) | 0.49 (0.40–0.55) | 0.52 (0.45–0.60) |
| Broad depression^ | 0.42 (0.37–0.46) | 0.58 (0.54–0.63) | 0.44 (0.38–0.50) | 0.56 (0.50–0.62) | 0.40 (0.32–0.48) | 0.60 (0.52–0.68) |
A: additive genetic factors; E: unique environmental factors.
Three phenotype categories for broad migraine: non-migraine, self-report/ID migraine and IHS MO/MA; three phenotype categories for broad depression: non-depressed, MiDD and MDD.
Bivariate analyses
Bivariate Cholesky SEMs were utilised to estimate variance components shared between migraine and depression. As for the univariate SEM analyses, the two-threshold AE/AE model best fit the data. The trait-specific heritability of broad migraine (56%) and depression (42%) were essentially the same as the estimates from the univariate analyses (Table 2 and Table 3). Importantly, a significant additive genetic correlation (rG=0.36, 95% CI: 0.29–0.43) and bivariate heritability (h2=5.5%, 95% CI: 3.6–7.8%) was observed between migraine and depression. The unique environmental correlation was not significant (rE=0.05, 95% CI: −0.01–0.11) and the bivariate unique environmental variance was very small (VE=0.15%, 95% CI: 0.00034–0.72%). Under the sex-limitation model, no significant difference in rG and h2 was observed between females and males.
Table 3.
Bivariate analyses for broad migraine and broad depression with and without sex-limitation based on two-threshold model
| Disorders | Total sample | Sex-limitation | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Female | Male | |||||
|
| ||||||
| A | E | A | E | A | E | |
| Broad migraine^ | 0.56 (0.53–0.60) | 0.44 (0.40–0.47) | 0.60 (0.55–0.64) | 0.40 (0.36–0.45) | 0.49 (0.41–0.56) | 0.51 (0.44–0.59) |
| Broad depression^ | 0.42 (0.37–0.47) | 0.58 (0.53–0.63) | 0.44 (0.38–0.50) | 0.56 (0.50–0.62) | 0.40 (0.31–0.48) | 0.60 (0.52–0.69) |
| Bivariate variance | 0.055 (0.036–0.078) | 1.5×10−3 (3.4×10−6–7.1×10−3) | 0.047 (0.026–0.074) | 4.1×10−6 (4.1×10−6–4.2×10−6) | 0.058 (0.024–0.11) | 7.3×10−3 (2.1×10−5–0.028) |
| Correlation | 0.36 (0.29–0.43) | 0.050 (−0.011–0.11) | 0.32 (0.25–0.41) | 2.7×10−3 (−0.075–0.080) | 0.38 (0.25–0.52) | 0.11 (5.4×10−3–0.21) |
A: additive genetic factors; E: unique environmental factors.
Three phenotype categories for broad migraine: non-migraine, self-report/ID migraine and IHS MO/MA; three phenotype categories for broad depression: non-depressed, MiDD and MDD.
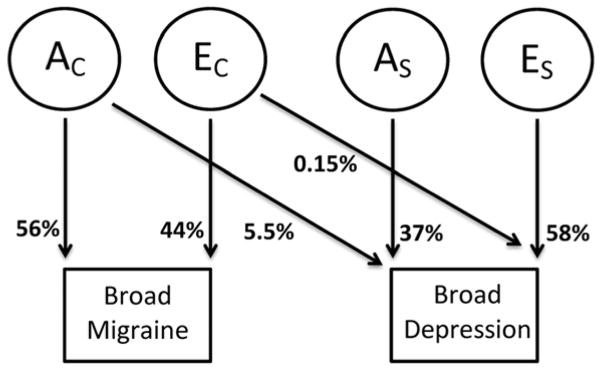
A path diagram portraying this best fitting Cholesky model is displayed in Figure 1. The principal genetic features of the model are explained as follows. Firstly, the common additive genetic factors accounting for 56% (95% CI: 53–60%) of the variance in migraine also account for 5.5% (95% CI: 3.6–7.8%) of the variance in depression. Secondly, specific additive genetic factors account for 37% (95% CI: 32–41%) of the variance in broad depression. Individual (non-shared) environmental factors explain the remaining variance in liability to migraine and depression.
Figure 1.
Path diagrams of the bivariate Cholesky models for broad diagnoses. The square frame represents the observed trait and the circle frame represents the latent variable. AC and EC stand for the additive genetic variable and unique environmental variable common to migraine and depression; AS and ES stand for the additive genetic variable and unique environmental variable specific to depression.
Results from narrow diagnoses analyses
Univariate analyses
To improve the sensitivity of true positive cases and specificity of true negative controls for migraine and depression, we next performed SEM analyses for the more narrow clinically-accepted diagnoses of IHS MO/MA and MDD. As for the broad diagnoses, no differences in threshold liability distributions were observed within twin pairs and across zygosity groups for IHS MO/MA and MDD and single set of liability thresholds could be utilised for males with a displacement to account for the observed higher prevalence in females. The heritability of IHS MO/MA was estimated after excluding individuals with the broader ID migraine and self-report definitions, by selecting only individuals who provided responses to the ICHD-3 based questionnaire for MO/MA. Although the number of twins analysed reduced from 5319 to 2793 (Table 4), the best fitting ACE model data (see Table S4) estimated a heritability of 59% (95% CI: 43–76%) for IHS MO/MA. Similarly, the heritability of MDD was calculated based on the subset of individuals who were diagnosed with non-depressed or MDD (i.e., removing the cases of MiDD; sample size decreased from 5319 to 4534 twin pairs). For the best fitting AE model (Table S4), the heritability was estimated at 49% (43–54%) for MDD. For both IHS MO/MA and MDD, the non-scalar sex-limitation model after removing the specific additive genetic variable for males provided the best fit to our data, with an estimated male-female genetic correlation of 1 (95% CI: 0.31–1.0) for IHS migraine and 0.64 (95% CI: 0.23–1.0) for MDD. Although heritability estimates were slightly higher in females compared to males, sex-limitation models indicated no significant heritability differences between females and males.
Table 4.
Univariate analyses for IHS MO/MA and MDD with and without sex-limitation
| Disorders | Total sample | Sex-limitation | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Female | Male | ||||||||
|
| |||||||||
| A | C | E | A | C | E | A | C | E | |
| IHS MO/MA | 0.59 (0.43–0.76) | 0.25 (0.090–0.39) | 0.16 (0.12–0.21) | 0.65 (0.46–0.82) | 0.19 (0.044–0.36) | 0.16 (0.11–0.22) | 0.43 (0.083–0.75) | 0.32 (0.051–0.62) | 0.25 (0.15–0.39) |
| MDD | 0.49 (0.43–0.54) | – | 0.51 (0.46–0.57) | 0.51 (0.44–0.57) | – | 0.49 (0.43–0.56) | 0.46 (0.36–0.56) | – | 0.54 (0.44–0.64) |
A: additive genetic factors; C: non-unique environmental factors E: unique environmental factors.
Bivariate analyses
The variance components shared between IHS MO/MA and MDD were next estimated using one-threshold bivariate Cholesky SEMs. As for the univariate SEMs, the ACE/AE bivariate Cholesky model provided the best overall fit for the combined IHS MO/MA and MDD sample (N=2406, see Table S5, section 3). Similar to the univariate results, the trait-specific heritabilities from the bivariate model were increased relative to the broad diagnoses, being estimated at 53% (95% CI: 35–72%) for IHS MO/MA and 52% (95% CI: 44–59%) for MDD (Table 5). Importantly, we observed a substantially increased additive genetic correlation (rG=0.51, 95% CI: 0.37–0.69) and bivariate heritability (h2=13.3%, 95% CI: 7.0–14.5%) between IHS MO/MA and MDD, compared to the results of broad migraine and depression. Sex-limitation models indicated no significant difference in rG and h2 between females and males.
Table 5.
Bivariate analyses for IHS MO/MA and MDD with and without sex-limitation
| Disorders | Total sample | Sex-limitation | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Female | Male | ||||||||
|
| |||||||||
| A | C | E | A | C | E | A | C | E | |
| IHS MO/MA | 0.53 (0.35–0.72) | 0.29 (0.13–0.44) | 0.18 (0.13–0.24) | 0.60 (0.39–0.80) | 0.23 (0.054–0.41) | 0.16 (0.11–0.23) | 0.32 (0.040–0.70) | 0.36 (0.056–0.62) | 0.32 (0.19–0.48) |
| MDD | 0.52 (0.44–0.59) | – | 0.48 (0.41–0.56) | 0.56 (0.46–0.65) | – | 0.44 (0.35–0.50) | 0.42 (0.27–0.56) | – | 0.58 (0.44–0.73) |
| Bivariate variance | 0.13 (0.070–0.25) | – | 2.0×10−4 (8.6×10−11–0.018) | 0.094 (0.038–0.20) | – | 0.016 (5.7×10−7–0.074) | 0.13 (0.020–0.52) | – | 0.047 (1.6×10−6–0.18) |
| Correlation | 0.51 (0.37–0.69) | – | −0.020 (−0.19–0.15) | 0.41 (0.26–0.60) | – | −0.19 (−0.41–0.036) | 0.56 (0.19–1) | – | 0.29 (−8.5×10−4–0.56) |
A: additive genetic factors; C: non-unique environmental factors E: unique environmental factors.
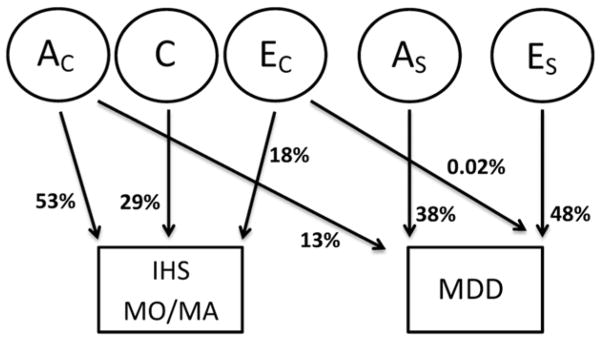
The best fitting bivariate Cholesky model for IHS MO/MA and MDD is portrayed in the path diagram illustrated in Figure 2. The principal genetic features of this model are as follows. Firstly, the common additive genetic factors accounting for 53% (95% CI: 35–72%) of the variance in IHS MO/MA also account for 13% (95% CI: 7–24%) of the variance in MDD. Secondly, there are specific additive genetic factors accounting for 38% (95% CI: 26–48%) of the variance in MDD. Individual (non-shared) environmental factors explain the remaining variance in liability to IHS MO/MA and MDD.
Figure 2.
Path diagrams of the bivariate Cholesky models for narrow diagnoses. The square frame represents the observed trait and the circle frame represents the latent variable. AC and EC stand for the additive genetic variable and unique environmental variable common to IHS MO/MA and MDD; AS and ES stand for the additive genetic variable and unique environmental variable specific to MDD. C stands for the non-unique environmental variable specific to IHS MO/MA.
Shared etiology or direct causation?
For both broad and narrow diagnoses, we found that the comorbidity between migraine and depression is best explained by a shared etiology (including shared genetic factors) rather than a causal relationship. That is, the bivariate analyses found a significant genetic correlation and non-significant environmental correlation between migraine and depression. Furthermore, there was no significant association between the MZ intrapair differences for migraine and depression (p-value=0.67 for the two-category broad migraine/depression status; p-value=0.66 for the three-category broad migraine/depression status; and p-value=0.73 for IHS MO/MA and MDD).
Discussion
This is the largest twin study to date on evaluating the genetic architecture of migraine and depression as well as their potential shared genetic components. Several findings are worth noting.
Firstly, the heritability of broad migraine and depression was estimated at 56% and 42%, respectively, consistent with previous European population-based studies (Bierut et al., 1999; Duncan et al., 2014; Honkasalo et al., 1995; Mulder et al., 2003; Nes et al., 2013; Svensson et al., 2003). This consistency extends to our finding of no significant non-additive genetic (D) and non-unique environmental (C) components. Also in line with previous findings (Nyholt et al., 2004), our results support the multiple threshold model, and indicate that different migraine classifications (self-report, ID migraine and IHS MO/MA) can be conceptualised as different levels of severity on a single dimension of liability. Analogously, MiDD and MDD exist as different severity levels on a single dimension of liability. These findings are nicely reflected by the three-category (two-threshold) model capturing more of the genetic contribution to both migraine and depression compared to the two-category (one-threshold) model. Therefore, broadening the migraine and depression phenotype in genetic studies by including individuals with sub-IHS diagnoses, such as self-reported and ID migraine, and sub-MDD diagnoses such as MiDD, should facilitate the identification of genetic risk factors due to improved power via increased sample size. That said, for the two-category definitions, the enhanced diagnostic sensitivity and specificity of narrow IHS MO/MA and MDD produced higher heritability estimates compared to the results from the one-threshold broad broad migraine and depression analyses, with heritability increasing from 48% to 59% for IHS MO/MA and from 40% to 49% for MDD, respectively. These latter results suggest that it might be important to model different diagnostic categories (e.g., via multinomial or ordinal logit models) to maximize power in genetic association studies.
Secondly, and also in line with three previous twin and family-based studies (Ligthart et al., 2010; Schur et al., 2009; Stam et al., 2010), both additive genetic correlation and bivariate heritability between broad migraine and depression (rG=0.36, 95% CI: 0.29–0.43; h2=5.5%, 95% CI: 3.6–7.8%) were significantly detected, indicating the presence of shared genetic components between the two disorders. Not surprisingly, both rG and h2 substantially increased between IHS MO/MA and MDD (rG=0.51, 95% CI: 0.37–0.69; h2=13.3%, 95% CI: 7.0–24.5%). It is also worth noting that rG and h2 increase (see Table S5, section 4) between broad migraine and MDD (N=4534, rG=0.39, 95% CI: 0.30–0.47; h2=7.3%, 95% CI: 4.5–10.9%) and between IHS MO/MA and broad depression (N=2793, rG=0.46, 95% CI: 0.33–0.62; h2=9.7%, 95% CI: 5.0–17.4%). These findings indicate that the shared genetic components between the two disorders can also be conceptualised on a single dimension of liability and exist regardless of the specific migraine and depression definition studies. Our finding of a slightly smaller bivariate heritability compared with the previous study of (Schur et al., 2009), is most likely due to differences in sample characteristics (e.g., the previous study only used female twins) and the diagnostic approaches and definitions of migraine and depression.
Thirdly, our results indicate that the co-occurrence of migraine and depression is most likely due to a shared etiology comprising shared genetic factors that influence both disorders rather than one primary disorder causing the other secondary disorder. Contrastingly, a Dutch twin study by Ligthart et al., (Ligthart et al., 2010) suggested a causal bi-directional relationship between migraine and anxious depression. This study reported a significant environmental correlation between MZ intrapair differences for LCA-derived migraine and anxious depression using only MZ twin pairs discordant for both disorders. However, when only utilising discordant MZ twin pairs in our study, the correlation between MZ intrapair differences for both broad and narrow migraine and depression was still non-significant (p-value=0.93 for the two-category broad migraine/depression status; p-value=0.51 for the three-category broad migraine/depression status; and p-value=0.19 for IHS MO/MA and MDD). Comparison with the current findings is complicated due to the use of different diagnostic measures. Firstly, the Dutch twin study coded migraine status by utilising an empirically derived migrainous headache classification based on LCA clustering of reported IHS MO/MA symptoms, and the survey questionnaire did not include the questions corresponding to the MO-related symptom “unilateral location” and MA-related aura characteristic symptoms, compared to the questionnaire used in our study (see Table S1). Additionally, the Dutch study analysed the role of anxious depression which consisted of a factor score based on several measures of anxiety, depression, and neuroticism. Further research will be required to determine whether anxiety and/or neuroticism play a confounding role in co-occurrence of migraine and depression.
Our study also has some limitations. Firstly, both migraine and depression status were diagnosed using self-reported questionnaire data, as opposed to the gold-standard of clinical-based interviews by neurologists or psychologists. Although our approach may result in some misclassification of migraine and depression status, it is not feasible to perform clinic-based interviews in samples larger enough to provide sufficient power for such familial aggregation studies. Moreover, our approach enabled narrow diagnoses of migraine and depression that satisfy clinically accepted criteria. Furthermore, our estimated lifetime prevalence of IHS MO/MA and DSM-III-R-based MDD are in a good agreement with published estimates (Arroyo-Quiroz et al., 2014; Bierut et al., 1999; Buse et al., 2012; Kendler et al., 1992). In addition to the clinically accepted definitions of IHS MO/MA and DSM MDD, our study utilised other diagnostic measurements or criteria of migraine (i.e., self-report migraine and ID migraine) and depression (i.e., MiDD) to both increase study power and examine the influence of different phenotypic distributions on genetic modelling of migraine and depression. Lastly, although this study did not analyse MO and MA separately, considering MO is the most common IHS MO/MA subtype and our results supporting the multiple threshold model and single liability of dimension, our findings do not corroborate the previous observation of genetic overlap between depression and MA and not MO (Stam et al., 2010).
Several conclusions can be drawn from our study. Firstly, genetic factors contribute significantly to the susceptibly to both migraine and depression, with no significant gender differences in the magnitude (sex-limitation) and no sex-specific effects (i.e., effects expressed in one sex but not the other) on risk for migraine and depression. Secondly, both univariate and bivariate heritability of migraine and depression increase with increased severity of the two disorders. Lastly, our results indicate that for both broad and narrow definitions, the observed comorbidity between migraine and depression can be explained by a shared etiology (rather than a causal relationship) that almost entirely comprises shared underlying genetically determined disease mechanisms. The identification of shared underlying genetic factors will improve our understanding of the relationship between migraine and co-occurring depression and facilitate the detection of novel pathways and thus identify new targets for drug therapy.
Supplementary Material
Acknowledgments
Huiying Zhao was supported by a National Health and Medical Research Council (NHMRC) Early Career Fellowship (APP1091816). Dale R. Nyholt was supported by an NHMRC Research Fellowship (APP0613674). This work was supported by an NHMRC project grant (APP1075175), the European Union’s Seventh Framework program (2007–2013) under grant agreement no. 602633 (EUROHEADPAIN) and the US National Institutes of Health (AA07535, AA011998, AA017688, AA10249, AA13320, AA13321, AA13326, AA14041, MH66206, DA12854, DA019951).
References
- Almasy L, Blangero J. Variance component methods for analysis of complex phenotypes. Cold Spring Harb Protoc. 2010;2010(5) doi: 10.1101/pdb.top77. pdb top77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arroyo-Quiroz C, Kurth T, Cantu-Brito C, Lopez-Ridaura R, Romieu I, Lajous M. Lifetime prevalence and underdiagnosis of migraine in a population sample of Mexican women. Cephalalgia. 2014;34(13):1088–1092. doi: 10.1177/0333102414529196. [DOI] [PubMed] [Google Scholar]
- Bierut LJ, Heath AC, Bucholz KK, Dinwiddie SH, Madden PA, Statham DJ, … Martin NG. Major depressive disorder in a community-based twin sample: are there different genetic and environmental contributions for men and women? Arch Gen Psychiatry. 1999;56(6):557–563. doi: 10.1001/archpsyc.56.6.557. [DOI] [PubMed] [Google Scholar]
- Boker S, Neale M, Maes H, Wilde M, Spiegel M, Brick T, … Fox J. OpenMx: An Open Source Extended Structural Equation Modeling Framework. Psychometrika. 2011;76(2):306–317. doi: 10.1007/s11336-010-9200-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boomsma D, Busjahn A, Peltonen L. Classical twin studies and beyond. Nat Rev Genet. 2002;3(11):872–882. doi: 10.1038/nrg932. [DOI] [PubMed] [Google Scholar]
- Breslau N, Davis GC, Schultz LR, Peterson EL. Joint 1994 Wolff Award Presentation. Migraine and major depression: a longitudinal study. Headache. 1994;34(7):387–393. doi: 10.1111/j.1526-4610.1994.hed3407387.x. [DOI] [PubMed] [Google Scholar]
- Breslau N, Lipton RB, Stewart WF, Schultz LR, Welch KM. Comorbidity of migraine and depression: investigating potential etiology and prognosis. Neurology. 2003;60(8):1308–1312. doi: 10.1212/01.wnl.0000058907.41080.54. [DOI] [PubMed] [Google Scholar]
- Breslau N, Schultz LR, Stewart WF, Lipton RB, Lucia VC, Welch KM. Headache and major depression: is the association specific to migraine? Neurology. 2000;54(2):308–313. doi: 10.1212/wnl.54.2.308. [DOI] [PubMed] [Google Scholar]
- Buse DC, Manack AN, Fanning KM, Serrano D, Reed ML, Turkel CC, Lipton RB. Chronic migraine prevalence, disability, and sociodemographic factors: results from the American Migraine Prevalence and Prevention Study. Headache. 2012;52(10):1456–1470. doi: 10.1111/j.1526-4610.2012.02223.x. [DOI] [PubMed] [Google Scholar]
- De Moor MH, Boomsma DI, Stubbe JH, Willemsen G, de Geus EJ. Testing causality in the association between regular exercise and symptoms of anxiety and depression. Arch Gen Psychiatry. 2008;65(8):897–905. doi: 10.1001/archpsyc.65.8.897. [DOI] [PubMed] [Google Scholar]
- Diagnostic and Statistical Manual of Mental Disorders Fourth Edition. The American Psychiatric Association; 1994. [Google Scholar]
- Duncan AE, Munn-Chernoff MA, Hudson DL, Eschenbacher MA, Agrawal A, Grant JD, … Heath AC. Genetic and environmental risk for major depression in African-American and European-American women. Twin Res Hum Genet. 2014;17(4):244–253. doi: 10.1017/thg.2014.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Headache Classification Committee of the International Headache S. The International Classification of Headache Disorders, 3rd edition (beta version) Cephalalgia. 2013;33(9):629–808. doi: 10.1177/0333102413485658. [DOI] [PubMed] [Google Scholar]
- Heath AC, Howells W, Kirk KM, Madden PA, Bucholz KK, Nelson EC, … Martin NG. Predictors of non-response to a questionnaire survey of a volunteer twin panel: findings from the Australian 1989 twin cohort. Twin Res. 2001;4(2):73–80. doi: 10.1375/1369052012182. [DOI] [PubMed] [Google Scholar]
- Honkasalo ML, Kaprio J, Winter T, Heikkila K, Sillanpaa M, Koskenvuo M. Migraine and concomitant symptoms among 8167 adult twin pairs. Headache. 1995;35(2):70–78. doi: 10.1111/j.1526-4610.1995.hed3502070.x. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Neale MC, Kessler RC, Heath AC, Eaves LJ. A population-based twin study of major depression in women. The impact of varying definitions of illness. Arch Gen Psychiatry. 1992;49(4):257–266. doi: 10.1001/archpsyc.1992.01820040009001. [DOI] [PubMed] [Google Scholar]
- Larsson B, Bille B, Pedersen NL. Genetic influence in headaches: a Swedish twin study. Headache. 1995;35(9):513–519. doi: 10.1111/j.1526-4610.1995.hed3509513.x. [DOI] [PubMed] [Google Scholar]
- Launer LJ, Terwindt GM, Ferrari MD. The prevalence and characteristics of migraine in a population-based cohort: the GEM study. Neurology. 1999;53(3):537–542. doi: 10.1212/wnl.53.3.537. [DOI] [PubMed] [Google Scholar]
- Ligthart L, Hottenga JJ, Lewis CM, Farmer AE, Craig IW, Breen G, … Nyholt DR. Genetic risk score analysis indicates migraine with and without comorbid depression are genetically different disorders. Hum Genet. 2014;133(2):173–186. doi: 10.1007/s00439-013-1370-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligthart L, Nyholt DR, Penninx BW, Boomsma DI. The shared genetics of migraine and anxious depression. Headache. 2010;50(10):1549–1560. doi: 10.1111/j.1526-4610.2010.01705.x. [DOI] [PubMed] [Google Scholar]
- Lipton RB, Dodick D, Sadovsky R, Kolodner K, Endicott J, Hettiarachchi J … study, I. D. M. v. A self-administered screener for migraine in primary care: The ID Migraine validation study. Neurology. 2003;61(3):375–382. doi: 10.1212/01.wnl.0000078940.53438.83. [DOI] [PubMed] [Google Scholar]
- Meanderthal S. Cholesky decomposition of variance-covariance matrices in the classic twin study. 2012 Jun 28; from https://sciencemeanderthal.wordpress.com/2012/06/28/cholesky-decomposition-of-variance-covariance-matrices-in-the-classic-twin-study/
- Modgill G, Jette N, Wang JL, Becker WJ, Patten SB. A population-based longitudinal community study of major depression and migraine. Headache. 2012;52(3):422–432. doi: 10.1111/j.1526-4610.2011.02036.x. [DOI] [PubMed] [Google Scholar]
- Mongini F, Keller R, Deregibus A, Raviola F, Mongini T, Sancarlo M. Personality traits, depression and migraine in women: a longitudinal study. Cephalalgia. 2003;23(3):186–192. doi: 10.1046/j.1468-2982.2003.00519.x. [DOI] [PubMed] [Google Scholar]
- Mulder EJ, Van Baal C, Gaist D, Kallela M, Kaprio J, Svensson DA, … Palotie A. Genetic and environmental influences on migraine: a twin study across six countries. Twin Res. 2003;6(5):422–431. doi: 10.1375/136905203770326420. [DOI] [PubMed] [Google Scholar]
- Neale MC, Roysamb E, Jacobson K. Multivariate genetic analysis of sex limitation and G x E interaction. Twin Res Hum Genet. 2006;9(4):481–489. doi: 10.1375/183242706778024937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nes RB, Czajkowski NO, Roysamb E, Orstavik RE, Tambs K, Reichborn-Kjennerud T. Major depression and life satisfaction: a population-based twin study. J Affect Disord. 2013;144(1–2):51–58. doi: 10.1016/j.jad.2012.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyholt DR, Gillespie NG, Heath AC, Merikangas KR, Duffy DL, Martin NG. Latent class and genetic analysis does not support migraine with aura and migraine without aura as separate entities. Genet Epidemiol. 2004;26(3):231–244. doi: 10.1002/gepi.10311. [DOI] [PubMed] [Google Scholar]
- Rijsdijk FV, Sham PC. Analytic approaches to twin data using structural equation models. Brief Bioinform. 2002;3(2):119–133. doi: 10.1093/bib/3.2.119. [DOI] [PubMed] [Google Scholar]
- RStudio Team. RStudio: integrated development for R. RStudio, Inc; Boston, MA: 2014. http://www.RStudio.com/ide. [Google Scholar]
- Saraceno B. The WHO World Health Report 2001 on mental health. Epidemiol Psichiatr Soc. 2002;11(2):83–87. doi: 10.1017/s1121189x00005546. [DOI] [PubMed] [Google Scholar]
- Schur EA, Noonan C, Buchwald D, Goldberg J, Afari N. A twin study of depression and migraine: evidence for a shared genetic vulnerability. Headache. 2009;49(10):1493–1502. doi: 10.1111/j.1526-4610.2009.01425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stam AH, de Vries B, Janssens AC, Vanmolkot KR, Aulchenko YS, Henneman P, … Terwindt GM. Shared genetic factors in migraine and depression: evidence from a genetic isolate. Neurology. 2010;74(4):288–294. doi: 10.1212/WNL.0b013e3181cbcd19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan PF, Neale MC, Kendler KS. Genetic epidemiology of major depression: review and meta-analysis. Am J Psychiatry. 2000;157(10):1552–1562. doi: 10.1176/appi.ajp.157.10.1552. [DOI] [PubMed] [Google Scholar]
- Svensson DA, Larsson B, Waldenlind E, Pedersen NL. Shared rearing environment in migraine: results from twins reared apart and twins reared together. Headache. 2003;43(3):235–244. doi: 10.1046/j.1526-4610.2003.03047.x. [DOI] [PubMed] [Google Scholar]
- Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, … Memish ZA. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2163–2196. doi: 10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright MJ, Martin NG. Brisbane Adolescent Twin Study: Outline of study methods and research projects. Australian Journal of Psychology. 2004;56(2):65–78. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.