Abstract
In selected centers, colonic manometry with non-high resolution catheters is used to document colonic motor dysfunction in chronic constipation. Recently, high resolution manometry (HRM) catheters, with more closely spaced sensors have been used for this purpose. Corestti et al. assessed colonic pressures with HRM in 17 healthy people and 10 constipated patients. The main finding was pan-colonic pressurizations, which occurred frequently, increased after eating and cholinergic stimulation, were associated with the desire to pass flatus, and were less frequent in slow transit constipation. These events resemble esophageal common cavity pressure waves. Further studies are necessary to understand the pathogenesis, functional consequences, and clinical utility of pan-colonic pressurizations.
Delayed colonic transit is widely used to identify colonic motor dysfunction in patients with chronic constipation.(1) Selected centers also use colonic manometry, which can be integrated with a barostat, to document colonic motor dysfunction.(2–4) While manometry sensors are located on a catheter, the barostat records colonic wall motion with a balloon opposed to the colonic mucosa.(3) In one study, a barostat and manometry disclosed normal colonic motor function in 53% of patients with slow transit constipation.(3) Hence, colonic barostat and/or manometry testing should be strongly considered prior to subtotal colectomy in patients with medically-refractory slow transit constipation. A colonic barostat can also guide pharmacological therapy in constipation,(5) and by contrast to colonic transit and manometry, also diagnose megacolon.(6) For example, the acute effects of intravenous neostigmine on colonic tone predicted the long term effects of pyridostigmine on colonic transit.(5)
Despite these observations, colonic manometry is infrequently used in clinical practice, perhaps because the procedure is invasive, requires expertise that is not widely available, and is only useful in a small subset of constipated patients. Colonic manometry studies, either stationary or ambulatory, evaluate motor activity under fasting conditions and after physiological (e.g., meals) and pharmacological (eg, bisacodyl and neostigmine) stimuli. Pressure events are non propagated or propagated either in an anterograde or retrograde manner. Propagated sequences are of low or high amplitude (i.e., high amplitude propagated sequences [HAPS]), which are also known as high amplitude propagated contractions.(4) HAPS have attracted considerable interest because they can transfer colonic contents over long distances, are induced by pharmacological agents (eg, bisacodyl, glycerol, and neostigmine), and often precede defecation. HAPS that reach the rectum are associated with relaxation of the internal anal sphincter and often followed by defecation. While HAPS occur infrequently, an average of 4–5 times daily in healthy people, they can be instantly recognized against a background of irregular and highly variable colonic pressure activity.(7) Colonic motor dysfunction is identified by fewer high amplitude propagated contractions and/or impaired responses to physiological and pharmacological stimuli.(2, 3, 8) However, because some healthy people have no colonic HAPS over 24 hours, the absence of HAPS may not be abnormal.
Earlier studies used non-high resolution colonic manometry catheters, in which adjacent water perfused or solid state sensors were separated by 10–15 cm. More recently, research studies have used high resolution manometry catheters, in which the sensors are more closely spaced, i.e., 2.5 cm in this study (9) and 1 cm in other studies.(10, 11) Compared to non-high resolution catheters, high resolution catheters more accurately detect the frequency, morphology, and directionality of colonic propagating sequences.(10) All pressure sensors – whether high resolution or not – are subject to the limitation that they generally do not detect contractions which do not occlude the lumen, particularly when the lumen is not filled with a viscous fluid.(12) Also, extra colonic events, (e.g., cough-induced artifact) may result in pressure changes. Hence, pressure changes recorded with manometry are more accurately labeled as pressurizations, pressure events, or pressure activity, as in this study, rather than contractions.
In an elegant study in this issue of the journal, Corsetti and colleagues used HRM to highlight another motor pattern, namely pan colonic pressurizations.(9) Because the proximal location of the motility catheter varied among subjects, it is unclear if these pressure events were recorded in the entire colon in all subjects. These pan colonic pressurizations occurred more frequently after eating and cholinergic stimulation, were associated with the desire to pass flatus, and were less frequent in slow transit constipation. Previous studies using non-high resolution (7, 13–17) and HRM catheters (10, 11) had observed simultaneous colonic pressure events, but not pan colonic pressurizations. While some of those studies did not record pressures throughout the colon, this discrepancy is puzzling, because Corsetti observed that pan colonic pressurizations occurred frequently, at an average of 85 ± 38 (Mean ± SD) times over 7 hours, or approximately every 5 minutes.(9) Studies with other HRM catheters have shown that when the space between sensors is reduced from 2 to 1 cm, twice as many propagated sequences were identified.(10) Therefore, it is conceivable that some rapidly propagated pressure events might have been misidentified as simultaneous events in this study, where adjacent sensors were separated by 2.5 cm.(9, 10)
The pathogenesis of pan colonic pressurizations is unclear. Conceptually, events inside and/or outside the colon may be responsible. In this study, the configuration of pressure waves during straining and pan colonic pressurizations was different. Abdominal wall electromyographic activity increased during straining but not during pan colonic pressurizations. Prostigmine increased the frequency of pan colonic pressurizations. Taken together, these observations suggest that pan colonic pressurizations are, at a minimum, not exclusively due to abdominal wall straining. Because they are seemingly simultaneous and have an identical configuration in all sensors, they resemble esophageal common cavity waves, which are defined as a sudden increase in basal intraesophageal pressure that equilibrates with gastric pressure and mirrors its respiratory pattern.(18, 19) In the esophagus, common cavity waves, which are observed during burping and gastroesophageal reflux, include three components that are related to the initiation of acid reflux or belching (phase 1), a sustained rise in pressure (phase 2), and a contractile response (phase 3).(Figure 1) Hence, the pressure changes during esophageal common cavity waves not only reflect viscus contraction, but also the movement of luminal contents. The contractile response, either primary, secondary or tertiary peristalsis, clears the esophageal reflux and returns basal pressure to normal.(Figure 1) Some patients sense these esophageal common cavity waves and respond with a Valsalva maneuver at the onset of phase 1, triggering another episode of reflux, i.e., both visceral and somatic mechanisms contribute to the esophageal common cavity.(18, 19)
Figure 1. Schematized Representation of Esophageal Pressure Profile During a Common Cavity Event.
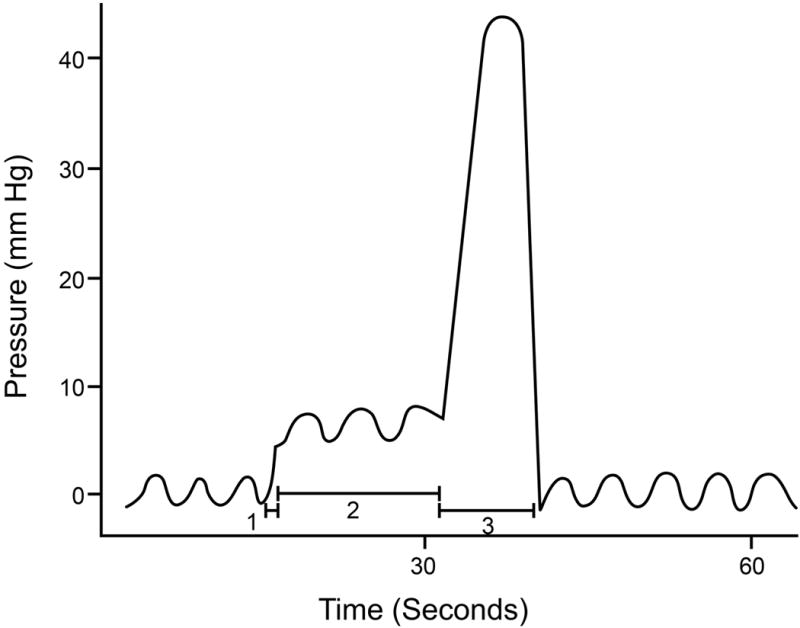
Observe 3 components, i.e., (1), a sudden increase in esophageal pressure, that (2) is sustained until (3) an esophageal contraction returns pressure toward baseline. Reproduced with permission from Shay SS, Eggli D, Oliver G, et al. Cycling, a manometric phenomenon due to repetitive episodes of gastroesophageal reflux and clearance. Digestive Diseases & Sciences 1989;34:1340–8.
Likewise, it is conceivable that pancolonic pressurizations are initiated by colonic movement of gas or fluid, perhaps a bolus spewing from the ileum to the cecum, which triggers colonic contraction and/or voluntary contraction of the abdominal wall that leads to pancolonic pressurization. Prostigmine increased the frequency of pancolonic pressurizations, suggesting a visceral component to at least some pancolonic pressurizations. However, if the colonic contraction were too strong, the colon would be compartmentalized into segments, precluding the formation of a common cavity. Indeed, the observed mean amplitude of these pressure events was relatively low, i.e., 15 mmHg.(9) Perhaps increased rectal pressure during these events is perceived as the sensation of the desire to pass flatus, induces involuntary relaxation of the internal anal sphincter, and voluntary passage of flatus. To emphasize, the internal anal sphincter relaxed 5 seconds after pressure increased in the proximal colon, suggesting that it was triggered by rectal distention. By contrast, during HAPS, the anal sphincter relaxes with the initiation of the pressure event in the proximal colon.
What is the functional significance and clinical utility of pan colonic pressurizations? Perhaps, some of these events serve as clearance mechanisms. Healthy people pass flatus an average of 10 times daily,(20) which is (fortunately) much lower than the average frequency of pancolonic pressurizations (i.e., every 5 minutes) in this study.(9) While constipated patients had fewer events, further studies are necessary to clarify the diagnostic utility of counting pancolonic pressurizations. Because some healthy people did not have any pancolonic pressurizations over a 7 hour period, even the absence of pancolonic pressurizations is not necessarily abnormal. Dissecting the contribution of visceral (i.e., colonic contraction and fluid motion) and somatic (e.g., abdominal wall, diaphragm, and pelvic floor) elements to these events will require innovative approaches, such as integrating manometry with MRI, which can evaluate these mechanisms.(21–23) In the meantime, Corsetti and colleagues have reminded us of the challenges of studying colonic motor function in health and disease, demonstrated how systematic study and meticulous observation can uncover new findings, and highlighted the complexity of the factors contributing to intraluminal pressure activity in humans.
Acknowledgments
The author is grateful to Drs. Joseph Murray and Phil Dinning for their helpful comments.
Financial support: This work was supported by grant DK78924 from the National Institutes of Health (NIH), US Public Health Service.
Footnotes
Guarantor of the article: Adil E. Bharucha
Specific author contributions: AEB conceived and drafted the manuscript and has approved the final draft.
Potential competing interests: None.
References
- 1.Bharucha AE, Pemberton JH, Locke GR., 3rd American gastroenterological association technical review on constipation. Gastroenterology. 2013;144:218–38. doi: 10.1053/j.gastro.2012.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rao SSC, Sadeghi P, Beaty J, et al. Ambulatory 24-hour colonic manometry in slow-transit constipation. American Journal of Gastroenterology. 2004;99:2405–16. doi: 10.1111/j.1572-0241.2004.40453.x. [DOI] [PubMed] [Google Scholar]
- 3.Ravi K, Bharucha AE, Camilleri M, et al. Phenotypic Variation Of Colonic Motor Functions In Chronic Constipation. Gastroenterology. 2010;138:89–97. doi: 10.1053/j.gastro.2009.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bharucha AE. High amplitude propagated contractions. Neurogastroenterology & Motility. 2012;24:977–82. doi: 10.1111/nmo.12019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bharucha AE, Low PA, Camilleri M, et al. Pilot study of pyridostigmine in constipated patients with autonomic neuropathy. Clinical Autonomic Research. 2008;18:194–202. doi: 10.1007/s10286-008-0476-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O’Dwyer RH, Acosta A, Camilleri M, et al. Clinical Features and Colonic Motor Disturbances in Chronic Megacolon in Adults. Digestive diseases and sciences. 2015;60:2398–407. doi: 10.1007/s10620-015-3645-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rao SS, Sadeghi P, Beaty J, et al. Ambulatory 24-h colonic manometry in healthy humans. American Journal of Physiology Gastrointestinal & Liver Physiology. 2001;280:629–639. doi: 10.1152/ajpgi.2001.280.4.G629. [DOI] [PubMed] [Google Scholar]
- 8.Bassotti G. If it is inert, why does it move? [comment] Neurogastroenterology & Motility. 2004;16:395–6. doi: 10.1111/j.1365-2982.2004.00512.x. [DOI] [PubMed] [Google Scholar]
- 9.Corsetti M, Pagliaro G, Demedts I, et al. Pan-Colonic Pressurizations Associated With Relaxation of the Anal Sphincter in Health and Disease: A New Colonic Motor Pattern Identifi ed Using High-Resolution Manometry. American Journal of Gastroenterology. 2016 doi: 10.1038/ajg.2016.341. In press. [DOI] [PubMed] [Google Scholar]
- 10.Dinning PG, Wiklendt L, Gibbins I, et al. Low-resolution colonic manometry leads to a gross misinterpretation of the frequency and polarity of propagating sequences: Initial results from fiber-optic high-resolution manometry studies. Neurogastroenterology & Motility. 2013;25:e640–9. doi: 10.1111/nmo.12170. [DOI] [PubMed] [Google Scholar]
- 11.Dinning PG, Wiklendt L, Maslen L, et al. Colonic motor abnormalities in slow transit constipation defined by high resolution, fibre-optic manometry. Neurogastroenterology & Motility. 2015;27:379–88. doi: 10.1111/nmo.12502. [DOI] [PubMed] [Google Scholar]
- 12.Arkwright JW, Dickson A, Maunder SA, et al. The effect of luminal content and rate of occlusion on the interpretation of colonic manometry. Neurogastroenterology & Motility. 2013;25:e52–9. doi: 10.1111/nmo.12051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Narducci F, Bassotti G, Gaburri M, et al. Twenty four hour manometric recording of colonic motor activity in healthy man. Gut. 1987;28:17–25. doi: 10.1136/gut.28.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moreno-Osset E, Bazzocchi G, Lo S, et al. Association between postprandial changes in colonic intraluminal pressure and transit. Gastroenterology. 1989;96:1265–73. doi: 10.1016/s0016-5085(89)80013-7. [DOI] [PubMed] [Google Scholar]
- 15.De Schryver AM, Samsom M, Smout AI. Effects of a meal and bisacodyl on colonic motility in healthy volunteers and patients with slow-transit constipation. Digestive Diseases & Sciences. 2003;48:1206–12. doi: 10.1023/a:1024178303076. [DOI] [PubMed] [Google Scholar]
- 16.Dinning PG, Szczesniak MM, Cook IJ. Twenty-four hour spatiotemporal mapping of colonic propagating sequences provides pathophysiological insight into constipation. Neurogastroenterology & Motility. 2008;20:1017–21. doi: 10.1111/j.1365-2982.2008.01147.x. [DOI] [PubMed] [Google Scholar]
- 17.Dinning PG, Szczesniak MM, Cook IJ. Spatio-temporal analysis reveals aberrant linkage among sequential propagating pressure wave sequences in patients with symptomatically defined obstructed defecation. Neurogastroenterology & Motility. 2009;21:945–e75. doi: 10.1111/j.1365-2982.2009.01323.x. [DOI] [PubMed] [Google Scholar]
- 18.Shay SS, Eggli D, Oliver G, et al. Cycling, a manometric phenomenon due to repetitive episodes of gastroesophageal reflux and clearance. Digestive Diseases & Sciences. 1989;34:1340–8. doi: 10.1007/BF01538066. [DOI] [PubMed] [Google Scholar]
- 19.Aanen MC, Bredenoord AJ, Samsom M, et al. The gastro-oesophageal common cavity revisited. Neurogastroenterology & Motility. 2006;18:1056–61. doi: 10.1111/j.1365-2982.2006.00831.x. [DOI] [PubMed] [Google Scholar]
- 20.Furne JK, Levitt MD. Factors influencing frequency of flatus emission by healthy subjects. Digestive Diseases & Sciences. 1996;41:1631–5. doi: 10.1007/BF02087912. [DOI] [PubMed] [Google Scholar]
- 21.Bharucha AE, Fidler JL, Huprich JE, et al. A prospective randomized controlled study of erythromycin on gastric and small intestinal distention: Implications for MR enterography. European Journal of Radiology. 2014;83:2001–2006. doi: 10.1016/j.ejrad.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pritchard SE, Marciani L, Garsed KC, et al. Fasting and postprandial volumes of the undisturbed colon: normal values and changes in diarrhea-predominant irritable bowel syndrome measured using serial MRI. Neurogastroenterology & Motility. 2014;26:124–30. doi: 10.1111/nmo.12243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoad CL, Menys A, Garsed K, et al. Colon wall motility: comparison of novel quantitative semi-automatic measurements using cine MRI. Neurogastroenterology & Motility. 2016;28:327–35. doi: 10.1111/nmo.12727. [DOI] [PubMed] [Google Scholar]


