Abstract
Background
Patients with coronary microvascular dysfunction (CMD) often have diastolic dysfunction, representing an important therapeutic target. Ranolazine—a late sodium current inhibitor—improves diastolic function in animal models and subjects with obstructive coronary artery disease (CAD).
Hypothesis
We hypothesized that ranolazine would beneficially alter diastolic function in CMD.
Methods
To test this hypothesis, we performed retrospective tissue tracking analysis to evaluate systolic/diastolic strain, using cardiac magnetic resonance imaging cine images acquired in a recently completed, randomized, double‐blind, placebo‐controlled, crossover trial of short‐term ranolazine in subjects with CMD and from 43 healthy reference controls.
Results
Diastolic strain rate was impaired in CMD vs controls (circumferential diastolic strain rate: 99.9% ± 2.5%/s vs 120.1% ± 4.0%/s, P = 0.0003; radial diastolic strain rate: −199.5% ± 5.5%/s vs −243.1% ± 9.6%/s, P = 0.0008, case vs control). Moreover, peak systolic circumferential strain (CS) and radial strain (RS) were also impaired in cases vs controls (CS: −18.8% ± 0.3% vs −20.7% ± 0.3%; RS: 35.8% ± 0.7% vs 41.4% ± 0.9%; respectively; both P < 0.0001), despite similar and preserved ejection fraction. In contrast to our hypothesis, however, we observed no significant changes in left ventricular diastolic function in CMD cases after 2 weeks of ranolazine vs placebo.
Conclusions
The case‐control comparison both confirms and extends our prior observations of diastolic dysfunction in CMD. That CMD cases were also found to have subclinical systolic dysfunction is a novel finding, highlighting the utility of this retrospective approach. In contrast to previous studies in obstructive CAD, ranolazine did not improve diastolic function in CMD.
Keywords: Coronary microvascular dysfunction, microvascular ischemia, ranolazine, tissue tracking
1. INTRODUCTION
Presentation with signs and symptoms of myocardial ischemia, in the absence of obstructive coronary artery disease (CAD), is more frequent in women, is associated with increased risk of major cardiovascular events in follow‐up, and is a major burden to the healthcare system.1, 2, 3, 4, 5, 6 The exact mechanism responsible remains incompletely understood, and as a result, effective treatment remains elusive.
Sex‐specific research initiatives, including the National Heart, Lung, and Blood Institute–sponsored Women's Ischemic Syndrome Evaluation (WISE) study, have provided important insight into the pathophysiology of this debilitating disease. For example, data from the WISE study indicate that more than half of the women with signs and symptoms of ischemia, but no obstructive CAD, have coronary microvascular dysfunction (CMD).7, 8, 9, 10, 11 We also have observed greater than expected diastolic dysfunction in this cohort,12, 13 together with a higher incidence of heart failure hospitalizations1—most notably heart failure with preserved ejection fraction (HFpPEF).14 These data, and reports from others, have led to the hypothesis that CMD may be mechanistically linked with diastolic dysfunction in such subjects and may serve as a precursor to HFpPEF. Drug therapies specifically targeting these pathways are therefore of particular interest.
Late sodium current inhibition using ranolazine—a Food and Drug Administration–approved medication for treatment of chronic angina—has demonstrated both safety and efficacy as an anti‐anginal agent among subjects believed to have obstructive CAD.15, 16, 17, 18, 19, 20, 21 Despite a promising pilot trial, suggesting that late sodium channel inhibition may also improve angina symptoms and myocardial perfusion in women with CMD,22 results from our recent Phase III clinical trial suggest only a modest benefit.23 It is possible, however, that late sodium channel inhibition could improve other aspects of the CMD phenotype. In particular, improving intracellular sodium homeostasis should, in theory, limit calcium influx via the sodium–calcium exchanger, shorten the action potential duration, and improve left ventricular (LV) relaxation (ie, diastolic function). This has previously been demonstrated in preclinical rodent studies24, 25 and small clinical studies of obstructive CAD.26, 27 Whether late sodium current inhibition can similarly improve diastolic function in patients with CMD remains unknown and is the focus of this investigation. To address this question, we leveraged the cardiac magnetic resonance cine images acquired in our recently completed, randomized, double‐blind, placebo‐controlled, crossover trial of ranolazine (NCT01342029), and performed retrospective tissue tracking analysis to evaluate LV strain changes in response to short‐term ranolazine administration. To characterize strain abnormalities in our cases with CMD, we also performed a case‐control comparison in a cohort of healthy controls.
2. METHODS
2.1. Subject population
2.1.1. Cases
Subject data were collected as part of a double‐blind, placebo‐controlled, crossover trial, testing the efficacy of short‐term ranolazine (500 mg ranolazine/placebo for 1 week and increased to 1000 mg ranolazine/placebo [as tolerated] for 1 week, twice daily) in subjects with CMD. A detailed description of the methodology has previously been published.23 Institutional review boards at Cedars‐Sinai Medical Center, Los Angeles and the University of Florida, Gainesville, approved the study, and all subjects gave written informed consent prior to study participation. Briefly, both men and women with symptoms thought due to ischemia, no obstructive CAD (<50% epicardial coronary stenosis), and preserved LV ejection fraction, who had abnormal coronary reactivity testing (CRT), coronary flow reserve (CFR) <2.5, or no dilation (≤0% change) with acetylcholine, or stress cardiac magnetic resonance imaging (CMRI) myocardial perfusion reserve index <2.0, were enrolled. Angina frequency, nitroglycerin diary, and 36‐Item Short Form Health Survey were recorded as previously described.23
2.1.2. Reference controls
To compare LV strain in our CMD cohort to healthy reference controls, 43 women without symptoms, cardiac risk factors, or signs suggesting ischemic heart disease, who had a normal maximal Bruce‐protocol exercise treadmill stress test were recruited from the community between September 2007 and August 2015. All reference control studies were performed at Cedars‐Sinai Medical Center where institutional approval was obtained, and all participants provided written informed consent.
2.2. Study design
All CMRI studies were performed on a 1.5 Tesla magnet (Siemens Healthcare, Erlangen, Germany) with electrocardiogram (ECG) gating and a vendor‐provided cardiac coil using a highly standardized protocol. Cine function images were acquired in 10 to 12 short‐axis slices using a steady‐state free precession pulse sequence with ECG gating during breath hold at end‐expiration (flip angle = 80°; resolution: 1.4 × 1.4 mm2; slice thickness: 8 mm; matrix size: 256 × 208; receiver bandwidth = 930 Hz/pixel; Echo Time [TE] = 1.26 ms). The primary outcome was LV relaxation rate (ie, circumferential and radial diastolic strain rate), measured by myocardial tissue tracking. Secondary outcome variables included LV systolic strain, also by tissue tracking, along with global morphology and systolic function: LV mass and volume, left atrial volume,28 and LV ejection fraction.
To characterize the level of dysfunction present in our subject population, we first compared CMD cases (placebo visit) with our reference controls. Then, to test whether ranolazine improves diastolic function in CMD, we then compared the placebo arm with the treatment arm of the randomized, double‐blind, placebo‐controlled trial.
2.3. Myocardial strain analysis using CMRI tissue tracking
As illustrated in Figure 1, myocardial tissue tracking was performed offline using previously acquired steady‐state free precession cine images, and dedicated software (cvi42; Circle Cardiovascular Imaging Inc., Calgary, AB, Canada). Myocardial tissue tracking is closely related to reference‐standard myocardial tissue tagging (see Supporting Figure 1 in the online version of this article), as described in previous reports.29, 30, 31 For each subject and control, masked to patient data and treatment period, a series of short‐axis CMRI images were chosen, spanning the left ventricle (base to ‐apex). Care was taken to avoid (1) basal slices that included LV outflow tract and/or left atrium, and (2) apical slices without clear delineation of the LV lumen at end‐systole. As a general rule, the apical slice was chosen 1 to 2 cm proximal to luminal obliteration. LV endocardial and epicardial borders were manually delineated at end‐diastole. In case of insufficient tracking, defined as apparent deviations of the contours from the endocardial and epicardial borders, contours were manually corrected and the tissue tracking algorithm reapplied. All of the data were analyzed by a single observer, who was blinded to each subject's medical history and/or treatment order. Our in‐lab intrarater variability, expressed as a coefficient of variation, for each of the primary endpoints is as follows (mean ± standard deviation [SD]): circumferential strain, 1% ± 1%; radial strain, 3% ± 2%; circumferential diastolic strain rate, 6% ± 5%; and radial diastolic strain rate, 7% ± 5%.
Figure 1.
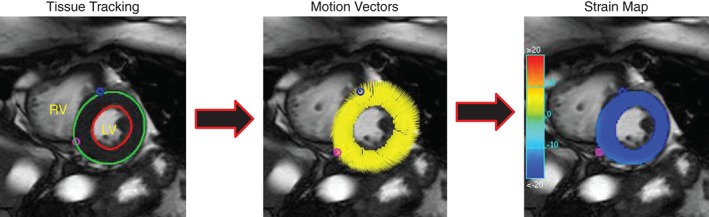
Myocardial tissue tracking in a representative midventricular short‐axis cine image. (Left) Contours are drawn on the endocardial and epicardial boarders at a single phase of the cardiac cycle. (Middle) Tissue‐tracking software propagates the contours automatically and follows the motion of the contour throughout the cardiac cycle, displayed as motion vectors across the ventricular wall. (Right) Strain (circumferential or radial) can then be displayed in the form of color maps throughout the cardiac cycle. In this case, circumferential strain is displayed. With these data derived, diastolic strain rate is simply calculated as the time derivative of either circumferential or radial strain. Abbreviations: LV, left ventricle; RV, right ventricle.
2.4. Statistical analysis
Data are expressed as mean ± standard error, or as absolute frequency and percentage for categorical data, unless otherwise specified. Differences between CMD cases and controls were compared using 2‐sample Student t test or Mann‐Whitney U test. Treatment comparisons were paired t tests on the treatment differences against the null hypothesis of 0 difference (ranolazine–placebo). Linear regression models were tested using treatment differences and outcomes. A stepwise procedure was used to choose the variables that were significantly associated with the outcomes. Statistical significance was set a priori at P < 0.05.
3. RESULTS
Pertinent case and control subject characteristics are summarized in Table 1. A total of 128 cases completed the trial, with complete and analyzable data from both treatment periods. Although the ages were similar, the controls were on average 4 years older than controls (55 ± 10 vs 51 ± 10 years, respectively, P = 0.007), with cases having a higher body mass index (BMI) (29.3 ± 7.6 vs 25.6 ± 3.7 kg/m2, P < 0.01). Heart rate did not differ in cases compared to controls (P = 0.140); however, systolic blood pressure was slightly higher in cases compared to controls (P = 0.002), and diastolic blood pressure was slightly lower (P = 0.04).
Table 1.
Baseline demographic and clinical variables
| Variables | Cases | Controls |
|---|---|---|
| No. | 128 | 43 |
| Female | 96% | 100% |
| Age, y | 55 ± 10 | 51 ± 10 1 |
| BMI, kg/m2 | 29.3 ± 7.6 | 25.6 ± 3.7 1 |
| Race, non‐Caucasian | 31 (24.2%) | 17 (39.5%) |
| Tobacco use | ||
| Current | 2 (1.6%) | 2 (4.7%) |
| Former | 38 (29.7%) | 13 (30.2%) |
| Never | 88 (68.8)% | 27 (62.8) |
| History of hypertension | 69 (53.9%) | 0 |
| History of diabetes | 23 (18.0%) | 0 |
| History of hyperlipidemia | 70 (54.7%) | 1 (2.3%) |
| Family history of premature coronary artery disease | 83 (64.8%) | 13 (30.2%) |
| LV ejection fraction, % | 68 ± 8 | 65 ± 6 1 |
| Heart rate, bpm | 67 ± 12 | 64 ± 11 |
| Systolic blood pressure, mm Hg | 128 ± 20 | 119 ± 14 1 |
| Diastolic blood pressure, mm Hg | 62 ± 13 | 66 ± 9 1 |
| Medications | ||
| β‐blockers | 54 (42.2%) | 0 |
| Calcium channel blockers | 29 (22.7%) | 0 |
| Angiotensin‐converting enzyme inhibitors | 13 (10.2%) | 0 |
| Nitrates | 50 (39.1%) | 0 |
| Statins | 74 (57.8%) | 0 |
| Hormone replacement therapy | 16 (12.5%) | 0 |
Abbreviations: BMI, body mass index; LV, left ventricular.
Date are presented as mean ± standard deviation or absolute frequency (%).
P < 0.05.
3.1. Case‐control comparison
Myocardial tissue tracking confirmed previous observations, demonstrating significant group differences in the rate of early ventricular relaxation in cases compared to controls (Figure 2). In particular, both circumferential strain rate and radial diastolic strain rate (CSRd and RSRd, respectively) were significantly reduced in cases compared to reference controls (CSRd: 99.9 ± 2.5 vs 120.1 ± 4.0, P = 0.0003; RSRd: −199.5 ± 5.5 vs ‐243.1 ± 9.6, P = 0.0008, cases vs controls), as shown in Figure 2A,B. Myocardial tissue tracking also revealed, for the first time, systolic strain abnormalities in cases compared to controls (circumferential strain [CS]: −18.8 ± 0.3 vs −20.7 ± 0.3; radial strain [RS]: 35.8 ± 0.7 vs 41.4 ± 0.9; respectively; both P < 0.0001), as shown in Figure 2C,D, despite similar and preserved LV ejection fractions (68% ± 8% vs 65% ± 6%, case vs control, P = 0.04). Importantly, these group differences remained after adjusting for age, BMI, and blood pressure. We observed no relationship between myocardial perfusion reserve index and LV diastolic or systolic function. Left atrial volume normalized to body surface area, a secondary measure of LV diastolic function, was not significantly different between cases and controls (39.2 ± 1.0 mL/m2 vs 41.9 ± 1.5 mL/m2, P = 0.104).
Figure 2.
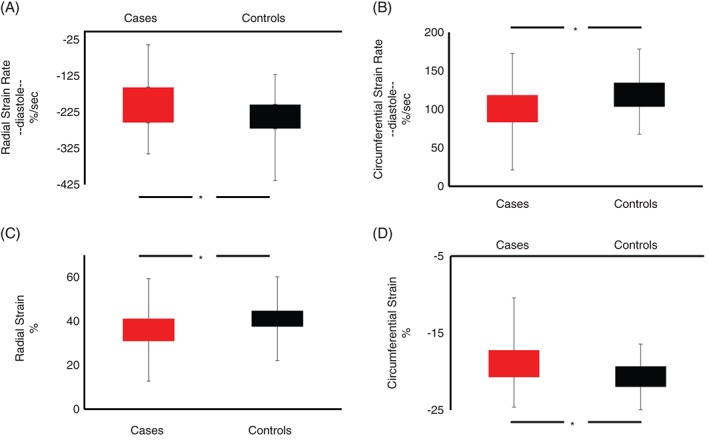
Group strain and strain rate data comparing cases with coronary microvascular dysfunction (red bars) with healthy matched controls (black bars). Top panels (A and B) illustrate group differences in radial diastolic strain rate (A) and circumferential diastolic strain rate (B). Bottom panels (C and D) illustrate group differences in peak radial (C) and circumferential (D) strain. *P < 0.05.
3.2. Influence of late sodium channel inhibition on systolic and diastolic function
In contrast to our hypothesis, short‐term late sodium channel inhibition with ranolazine did not improve either LV circumferential diastolic strain rate (ΔCSRd = 2.0 ± 1.7, P = 0.25) or radial diastolic strain rate (ΔRSRd = −2.6 ± 4.3, P = 0.54). Likewise, systolic CS and RS also remained unchanged (ΔCS = 0.09% ± 0.15%, P = 0.545; ΔRS = −0.13% ± 0.47%, P = 0.785), as did left atrial volume (Δ left atrial volume normalized to body surface area = 0.62 ± 0.67 mL/m2, P = 0.356) and LV ejection fraction (Δ ejection fraction −0.03 ± 3.33, P = 0.92). Moreover, we observed no relationship between the change in myocardial perfusion reserve index and the change in LV function with ranolazine.
Subanalysis of the subjects with clinically significant limited coronary flow reserve <2.5 and/or subjects with the lowest diastolic strain rate (ie, 1 and 2 SD below the mean), also did not reveal any major benefit in LV strain/strain rate with ranolazine. Modeling demonstrated that among subjects with the same change in angina frequency and change in reported depression, usage of nitrates was associated with a higher estimated change in radial diastolic strain rate of 23.2 units (P = 0.013). Use of nitrates also tended to predict the greatest change in radial diastolic strain rate (15.1%/s), compared to those not on nitrates (P = 0.08), independent of other modeling factors.
4. DISCUSSION
Women with signs and symptoms of ischemia, but no obstructive CAD, often have coronary microvascular dysfunction and are at increased risk for developing HFpPEF. We have previously shown, in 2 separate small proof‐of‐concept studies,12, 13 that the rate of LV relaxation is impaired in women with CMD. The data herein both confirm and extend those previous observations using novel, retrospective, myocardial tissue tracking in a much larger cohort of cases and controls. First, we confirm that LV relaxation rate is reduced in patients with CMD. Second, we extend our previous work by documenting subclinical LV systolic dysfunction for the first time in this subject population. With this foundation, we tested whether late sodium channel inhibition could rescue LV dysfunction in CMD. In contrast to previous work in obstructive CAD, however, we observed no detectable major benefit with ranolazine on LV relaxation or systolic strain.
CMRI is the clinical reference‐standard imaging tool for the evaluation of LV morphology and function. Notably, magnetic resonance cine imaging provides excellent intrinsic blood‐to‐tissue contrast and a high degree of reproducibility. As such, cine imaging is an intrinsic part of every cardiac magnetic resonance imaging (MRI) exam worldwide. CMRI is also the reference technique for measuring myocardial tissue deformation (ie, strain), which is increasingly recognized as a more sensitive measure for the detection of LV dysfunction than other, more traditional, cine‐derived global metrics of LV function.32, 33, 34 Conventional strain imaging has not been widely adopted throughout the magnetic resonance community, however, largely due to its associated technical challenges, including difficult and sometimes unavailable imaging sequences, longer and/or additional breath holds and scanner time, and lack of available postprocessing software. Myocardial tissue tracking with MRI, which utilizes standard cine images to assess myocardial deformation, has recently emerged as an exciting new tool for reliably measuring LV strain,29, 30, 31 independent of field strength.35 Importantly, this approach to strain measurement does not require any image acquisition beyond the standard routine CMRI procedure.
Application of myocardial tissue tracking to our existing clinical dataset reestablishes diastolic dysfunction as an important clinical phenotype in CMD, confirming observations from 2 separate small proof‐of‐concept studies from our laboratory.12, 13 Given the increased incidence of heart failure in this subject population,1 of whom the majority of cases have preserved ejection fraction,14 diastolic dysfunction may represent an important therapeutic target. We speculate that interventions aimed at alleviating diastolic dysfunction in patients with CMD may prevent future development of clinical HFpEF. That we also observe subclinical systolic dysfunction in cases compared to controls further strengthens the relationship between CMD and HFpEF. LV strain is also impaired in HFpEF,36, 37, 38 and associated with a higher risk of heart failure hospitalization, cardiovascular death, and aborted sudden death.36
Ranolazine has been shown to improve diastolic function in subjects with CAD.26, 27 To test whether ranolazine is equally effective in subjects with CMD who have no obstructive CAD, we leveraged the recently completed randomized, placebo‐controlled trial of late sodium current inhibition with ranolazine in CMD (NCT01342029).23 In line with a recent study in subjects with aortic stenosis,39 we observed no major differences in diastolic or systolic function between the placebo arm and the ranolazine arm. Although the reason for this negative finding remains unclear, we acknowledge that it may be related to the treatment regimen. For example, in our positive pilot study,22 we administered ranolazine for 4 weeks (2 weeks 500 mg twice daily, followed by 2 weeks of 1000 mg twice daily), resulting in better self‐administered questionnaire scores and a trend toward an improvement in myocardial perfusion reserve index (which was significant in women with CFR <3.0). In the present trial, however, subjects were treated for 2 weeks (1 week of 500 mg twice daily and 1 week of 1000 mg twice daily), which may have at least partially contributed to the present findings.
4.1. Limitations
Myocardial tissue tracking uses proprietary modeling algorithms to track the endocardial and epicardial boarders of the left ventricle, providing detailed information about global LV strain. Other more conventional strain imaging modalities derive their measurement of strain very differently. For example, speckle‐tracking echocardiography relies on tracking acoustic backscatter within the myocardium itself. Similarly, gold‐standard tissue tagging changes the tissue magnetization in such a way that the myocardium is divided into quantifiable square patterns. In both of these methods, regional changes in tissue deformation, spanning the epicardium, midwall, and endocardium, can be measured throughout the cardiac cycle. Thus, a major limitation of myocardial tissue tracking is its inability to differentiate and quantify regional deformation changes separately in the epicardium, midwall, and endocardium.
Myocardial tissue tracking of short‐axis cine images provides quantitative information about circumferential and radial tissue deformation (ie, strain and strain rate). Although LV strain has previously been shown to have important prognostic value,32, 33, 34 we acknowledge that the rate of circumferential or radial strain is not a conventional diastolic metric (like Dopple‐derived mitral inflow, annular tissue velocity, or pulmonary venous velocity). However, we believe the rate of LV tissue deformation more closely reflects changes in myocardial relaxation rate per se, rather than transmitral filling velocities, which can be drastically influenced by changes in filling pressure. This is exemplified in a previous report from our group in cases with CMD, where we observed diastolic strain abnormalities despite significantly elevated LV filling pressures.13
A fundamental assumption of this investigation is that CMD prolongs the late sodium current, even under resting conditions, such that inhibition of this current would improve intracellular calcium homeostasis. This is an important consideration when interpreting the present results. Future investigations are needed, evaluating the effects of late sodium channel inhibition on diastolic function during periods of physiological stress.
5. CONCLUSION
Impaired myocardial deformation, as represented by a reduction in LV circumferential and radial strain and strain rate, provides important insight into the pathophysiology of disease. Here, we used myocardial tissue tracking, a simple, noninvasive postprocessing tool used to measure myocardial deformation, to both confirm diastolic dysfunction in CMD and extend these observations to subclinical systolic dysfunction for the first time. Application of this technique also afforded the opportunity to test whether short‐term treatment with the late sodium channel inhibitor ranolazine could improve LV function in subjects with CMD. Our finding that ranolazine did not improve LV function in subjects with CMD may reflect the nonischemic state of the resting myocardium in these subjects, and warrants further investigation.
5.1. Conflicts of interest
M.D.N. reports receiving research support from Gilead Sciences. C.N.B.M. reports receiving research support from the Gilead Sciences and served on the grant review committee for Gilead. E.M.H. reports receiving research grants from Gilead Sciences. C.S. reports receiving research grants from Gilead Sciences. P.K.M. reports receiving research grants from Gilead Sciences. L.E.J.T. reports receiving research grants from Gilead Sciences. C.J.P. reports receiving research grants from Gilead Sciences.
Supporting information
eFigure 1. In‐house comparison between myocardial feature tracking and gold‐standard magnetic resonance tissue tagging showing a good relationship between the two techniques for circumferential diastolic strain rate (A) and circumferential strain (B).
Nelson MD, Sharif B, Shaw JL, Cook‐Wiens G, Wei J, Shufelt C, Mehta PK, Thomson LEJ, Berman DS, Thompson RB, Handberg EM, Pepine CJ, Li D and Bairey Merz CN. Myocardial tissue deformation is reduced in subjects with coronary microvascular dysfunction but not rescued by treatment with ranolazine. Clin Cardiol. 2017;40:300–306. 10.1002/clc.22660
Funding information Gilead; American Heart Association, Grant/Award number: 16SDG27260115; Harry S. Moss Heart Trust; National Heart, Lung and Blood Institute, Grant/Award numbers: N01‐HV‐68161, N01‐HV‐68162, N01‐HV‐68163, N01‐HV‐68164; General Clinical Research Center grant, Grant/Award number: MO1‐RR00425; National Center for Research Resources, Grant/Award number:UL1RR033176; National Institutes of Health/National Center for Advancing Translational Sciences; University of California Los Angeles Clinical and Translational Science Institute, Grant/Award number: UL1TR000124; University of Florida Clinical and Translational Science Institute, Grant/Award numbers:UL1TR001427, gR01 HL089765; Gustavus and Louis Pfeiffer Research Foundation, Denville, New Jersey; Women's Guild of Cedars‐Sinai Medical Center, Los Angeles, California;; Edythe L. Broad Women's Heart Research Fellowship; Cedars‐Sinai Medical Center, Los Angeles, California; Constance Austin Women's Heart Research Fellowship; Barbra Streisand Women's Cardiovascular Research and Education Program; Erika Glazer Women's Heart Health Project, Cedars‐Sinai Medical Center, Los Angeles, California.
REFERENCES
- 1. Gulati M, Cooper‐DeHoff RM, McClure C, et al. Adverse cardiovascular outcomes in women with nonobstructive coronary artery disease: a report from the Women's Ischemia Syndrome Evaluation Study and the St James Women Take Heart Project. Arch Intern Med. 2009;169:843–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jespersen L, Hvelplund A, Abildstrom SZ, et al. Stable angina pectoris with no obstructive coronary artery disease is associated with increased risks of major adverse cardiovascular events. Eur Heart J. 2012;33:734–744. [DOI] [PubMed] [Google Scholar]
- 3. Go AS, Mozaffarian D, Roger V, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics 2014 update: a report from the American Heart Association. Circulation. 2014;129:e28–e292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shaw LJ, Merz CNB, Pepine CJ, et al. The economic burden of angina in women with suspected ischemic heart disease: results from the National Institutes of Health—National Heart, Lung, and Blood Institute—sponsored Women's Ischemia Syndrome Evaluation. Circulation. 2006;114:894–904. [DOI] [PubMed] [Google Scholar]
- 5. Shaw LJ, Bugiardini R, Merz CNB. Women and ischemic heart disease: evolving knowledge. J Am Coll Cardiol. 2009;54:1561–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pepine CJ, Ferdinand KC, Shaw LJ, et al; ACC CVD in Women Committee. Emergence of nonobstructive coronary artery disease: a woman's problem and need for change in definition on angiography. J Am Coll Cardiol. 2015;66:1918–1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Marroquin OC, Kip KE, Mulukutla SR, et al. Inflammation, endothelial cell activation, and coronary microvascular dysfunction in women with chest pain and no obstructive coronary artery disease. Am Heart J. 2005;150:109–115. [DOI] [PubMed] [Google Scholar]
- 8. Pepine CJ, Anderson RD, Sharaf BL, et al. Coronary microvascular reactivity to adenosine predicts adverse outcome in women evaluated for suspected ischemia: results from the National Heart, Lung and Blood Institute WISE (Women's Ischemia Syndrome Evaluation) study. J Am Coll Cardiol. 2010;55:2825–2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pepine CJ, Petersen JW, Bairey Merz CN. A microvascular‐myocardial diastolic dysfunctional state and risk for mental stress ischemia: a revised concept of ischemia during daily life. JACC Cardiovasc Imaging. 2014;7:362–365. [DOI] [PubMed] [Google Scholar]
- 10. Thomson LEJ, Wei J, Agarwal M, et al. Cardiac magnetic resonance myocardial perfusion reserve index is reduced in women with coronary microvascular dysfunction: a National Heart, Lung, and Blood Institute‐sponsored study from the Women's Ischemia Syndrome Evaluation. Circ Cardiovasc Imaging. 2015;8:e002481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wei J, Mehta PK, Johnson BD, et al. Safety of coronary reactivity testing in women with no obstructive coronary artery disease: results from the NHLBI‐sponsored Women's Ischemia Syndrome Evaluation (WISE) study. JACC Cardiovasc Interv. 2012;5:646–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nelson MD, Szczepaniak LS, Wei J, et al. Diastolic dysfunction in women with signs and symptoms of ischemia in the absence of obstructive coronary artery disease: a hypothesis‐generating study. Circ Cardiovasc Imaging. 2014;7:510–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wei J, Nelson MD, Szczepaniak EW, et al. Myocardial steatosis as a possible mechanistic link between diastolic dysfunction and coronary microvascular dysfunction in women. Am J Physiol Heart Circ Physiol. 2016;310:H14–H19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bakir M, Nelson M, Jones E, et al. Heart failure hospitalization in women with signs and symptoms of ischemia: a report from the Women's Ischemia Syndrome Evaluation Study. Int J Cardiol. 2016;223:936–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Timmis AD, Chaitman BR, Crager M. Effects of ranolazine on exercise tolerance and HbA1c in patients with chronic angina and diabetes. Eur Heart J. 2005;27:42–48. [DOI] [PubMed] [Google Scholar]
- 16. Wenger NK, Chaitman B, Vetrovec GW. Gender comparison of efficacy and safety of ranolazine for chronic angina pectoris in four randomized clinical trials. Am J Cardiol. 2007;99:11–18. [DOI] [PubMed] [Google Scholar]
- 17. Rousseau MF, Pouleur H, Cocco G, Wolff AA. Comparative efficacy of ranolazine versus atenolol for chronic angina pectoris. Am J Cardiol. 2005;95:311–316. [DOI] [PubMed] [Google Scholar]
- 18. Stone PH, Gratsiansky NA, Blokhin A, Huang IZ, Meng L. Antianginal efficacy of ranolazine when added to treatment with amlodipine: the ERICA (Efficacy of Ranolazine in Chronic Angina) trial. J Am Coll Cardiol. 2006;48:566–575. [DOI] [PubMed] [Google Scholar]
- 19. Chaitman BR, Pepine CJ, Parker JO. Effects of ranolazine with atenolol, amlodipine, or diltiazem on exercise tolerance and angina frequency in patients with severe chronic angina: a randomized controlled trial. JAMA. 2004;291:309–316. [DOI] [PubMed] [Google Scholar]
- 20. Chaitman BR, Skettino SL, Parker JO, et al; MARISA Investigators . Anti‐ischemic effects and long‐term survival during ranolazine monotherapy in patients with chronic severe angina. J Am Coll Cardiol. 2004;43:1375–1382. [DOI] [PubMed] [Google Scholar]
- 21. Chaitman BR. Ranolazine for the treatment of chronic angina and potential use in other cardiovascular conditions. Circulation. 2006;113:2462–2472. [DOI] [PubMed] [Google Scholar]
- 22. Mehta PK, Goykhman P, Thomson LEJ, et al. Ranolazine improves angina in women with evidence of myocardial ischemia but no obstructive coronary artery disease. JACC: Cardiovasc Imaging. 2011;4:514–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bairey Merz CN, Handberg EM, Shufelt CL, et al. A randomized, placebo‐controlled trial of late Na current inhibition (ranolazine) in coronary microvascular dysfunction (CMD): impact on angina and myocardial perfusion reserve. Eur Heart J. 2016;37:1504–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Williams S, Pourrier M, McAfee D, Lin S, Fedida D. Ranolazine improves diastolic function in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2014;306:H867–H881. [DOI] [PubMed] [Google Scholar]
- 25. Lovelock JD, Monasky MM, Jeong EM, et al. Ranolazine improves cardiac diastolic dysfunction through modulation of myofilament calcium sensitivity. Circ Res. 2012;110:841–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Figueredo VM, Pressman GS, Romero‐Corral A, Murdock E, Holderbach P, Morris DL. Improvement in left ventricular systolic and diastolic performance during ranolazine treatment in patients with stable angina. J Cardiovasc Pharmacol Ther. 2011;16:168–172. [DOI] [PubMed] [Google Scholar]
- 27. Hayashida W, van Eyll C, Rousseau M, Pouleur H. Effects of ranolazine on left ventricular regional diastolic function in patients with ischemic heart disease. Cardiovasc Drug Ther. 1994;8:741–747. [DOI] [PubMed] [Google Scholar]
- 28. Maceira A, Cosin‐Sales J, Roughton M, Prasad S, Pennell D. Reference left atrial dimensions and volumes by steady state free precession cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2010;12:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schuster A, Paul M, Bettencourt N, et al. Cardiovascular magnetic resonance myocardial feature tracking for quantitative viability assessment in ischemic cardiomyopathy. Int J Cardiol. 2013;166:413–420. [DOI] [PubMed] [Google Scholar]
- 30. Hor KN, Gottliebson WM, Carson C, et al. Comparison of magnetic resonance feature tracking for strain calculation with harmonic phase imaging analysis. JACC: Cardiovasc Imaging. 2010;3:144–151. [DOI] [PubMed] [Google Scholar]
- 31. Augustine D, Lewandowski A, Lazdam M, et al. Global and regional left ventricular myocardial deformation measures by magnetic resonance feature tracking in healthy volunteers: comparison with tagging and relevance of gender. J Cardiovasc Magn Reson. 2013;15:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Motoki H, Borowski AG, Shrestha K, et al. Incremental prognostic value of assessing left ventricular myocardial mechanics in patients with chronic systolic heart failure. J Am Coll Cardiol. 2012;60:2074–2081. [DOI] [PubMed] [Google Scholar]
- 33. Buss SJ, Emami M, Mereles D, et al. Longitudinal left ventricular function for prediction of survival in systemic light‐chain amyloidosis: incremental value compared with clinical and biochemical markers. J Am Coll Cardiol. 2012;60:1067–1076. [DOI] [PubMed] [Google Scholar]
- 34. Choi EY, Rosen BD, Fernandes VR, et al. Prognostic value of myocardial circumferential strain for incident heart failure and cardiovascular events in asymptomatic individuals: the Multi‐Ethnic Study of Atherosclerosis. Eur Heart J. 2013;34:2354–2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schuster A, Morton G, Hussain ST, et al. The intra‐observer reproducibility of cardiovascular magnetic resonance myocardial feature tracking strain assessment is independent of field strength. Eur J Radiol. 2013;82:296–301. [DOI] [PubMed] [Google Scholar]
- 36. Shah AM, Claggett B, Sweitzer NK, et al. Prognostic importance of impaired systolic function in heart failure with preserved ejection fraction and the impact of spironolactone. Circulation. 2015;132:402–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hasselberg NE, Haugaa KH, Sarvari SI, et al. Left ventricular global longitudinal strain is associated with exercise capacity in failing hearts with preserved and reduced ejection fraction. Eur Heart J Cardiovasc Imaging. 2015;16:217–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Stampehl MR, Mann DL, Nguyen JS, Cota F, Colmenares C, Dokainish H. Speckle strain echocardiography predicts outcome in patients with heart failure with both depressed and preserved left ventricular ejection fraction. Echocardiography. 2015;32:71–78. [DOI] [PubMed] [Google Scholar]
- 39. Singh A, Steadman CD, Khan JN, Reggiardo G, McCann GP. Effect of late sodium current inhibition on MRI measured diastolic dysfunction in aortic stenosis: a pilot study. BMC Res Notes. 2016;9:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. In‐house comparison between myocardial feature tracking and gold‐standard magnetic resonance tissue tagging showing a good relationship between the two techniques for circumferential diastolic strain rate (A) and circumferential strain (B).


