Abstract
Numerous studies have found that congenitally blind individuals have better verbal memory than their normally sighted counterparts. However, it is not known whether this reflects superiority of verbal or memory abilities. In order to distinguish between these possibilities, we tested congenitally blind participants and normally sighted control participants, matched for age and education, on a range of verbal and spatial tasks. Congenitally blind participants were significantly better than sighted controls on all the verbal tasks but the groups did not differ significantly on the spatial tasks. Thus, the congenitally blind appear to have superior verbal, but not spatial, abilities. This may reflect greater reliance on verbal information and the involvement of visual cortex in language processing in the congenitally blind.
Keywords: digit span, spatial, memory, imagery
1 Introduction
A number of studies have shown that congenitally blind people perform better than normally sighted controls on a range of verbal memory tasks, including tests of short- and long-term recall (Amedi et al., 2003; Pasqualotto et al., 2013a), recognition (Amedi et al., 2003; Röder et al., 2001), serial word order (Raz et al., 2007), and working memory as indexed by digit span tasks (Tillman & Bashaw, 1968; Smits & Mommers, 1976; Hull & Mason, 1995; Withagen et al., 2013). However, superior verbal memory performance in the congenitally blind could reflect either enhanced verbal abilities in the absence of vision, regardless of the kind of verbal task used, or enhanced memory abilities that might generalize across different cognitive domains.
Previous studies of these tasks separately do not provide a clear answer to this question. For example, the evidence for a working memory advantage in the blind is primarily from studies of children (Tillman & Bashaw, 1968; Smits & Mommers, 1976; Hull & Mason, 1995; Withagen et al., 2013). However, although studies of adults show no significant differences between the blind and sighted (Castronovo & Delvenne, 2013; Pigeon & Marin-Lamellet, 2015) this was likely due to ceiling effects (and see Swanson & Luxenberg, 2009, for equivocal results in children). Similarly, some studies show that the congenitally blind and the sighted perform equally well on spatial memory tasks (Cornoldi et al., 1991; Ruggiero & Iachini, 2010). Other studies suggest that whether or not the blind and sighted differ in spatial memory ability depends on the specific aspect to be recalled, either the final location or the entire spatial pattern (Cornoldi et al., 2009) or whether more than one spatial pattern has to be maintained in memory at a time (Vecchi et al., 2004). Furthermore, the blind and the sighted may differ in the use of allocentric and egocentric reference frames (Pasqualotto et al., 2013b; Pasqualotto & Proulx, 2012). Importantly, however, none of these prior studies tested verbal and non-verbal tasks in the same participants.
We therefore compared congenitally blind adults with sighted control participants, matched for age and education, on a range of verbal and non-verbal (i.e. spatial) tasks. The tasks included tests of verbal and spatial memory to assess whether congenital blindness confers superiority only for verbal memory, or also for a non-verbal memory domain. The verbal memory tasks included a working memory test. To assess whether any superiority of the congenitally blind in each of these memory domains is restricted to the sphere of memory, or generalizes to non-memory spheres, we also included tests of verbal fluency and a test of spatial imagery. A preliminary version of the findings reported here was presented earlier as a conference report (Occelli et al., 2016).
2 Material & Methods
2.1 Participants
Twelve congenitally blind (6 male, 6 female) and 13 sighted (4 male, 9 female) individuals took part in the study. Congenitally blind and sighted control participants were matched for age (mean age (±s.d.) blind: 43.6 ± 15.2; sighted: 38.5 ± 16.0 years, t23 = .8, p = .4) and years of education (blind: 17.1 ± 1.8 years; sighted: 16.5 ± 3.0 years, t23 = .5, p = .6). Blindness resulted from a variety of ocular causes (Table 1); 5 blind participants had some residual light perception while the remainder had no light perception and none had shape perception. Both blind and sighted participants were recruited and tested either at Emory University (‘Emory’) or the Massachusetts Eye and Ear Infirmary (‘MEEI’). All participants spoke American English as their main language and reported normal hearing. None reported a history of neurological or psychiatric illness and all participants were completely or preferentially right-handed, based on the validated subset of the Edinburgh Handedness Inventory (Raczkowski et al., 1974). All procedures were conducted in accordance with the Declaration of Helsinki and approved by the Emory and MEEI Institutional Review Boards. All participants gave their informed consent prior to the study and were compensated for their time. Braille versions of the consent documents were provided for the blind participants.
Table 1. Demographic and clinical data for the congenitally blind participants.
| Age | Etiology | Light perception | Shape perception | |
|---|---|---|---|---|
| M | 61 | Retinopathy of prematurity | No | No |
| F | 62 | Retinopathy of prematurity & optic nerve atrophy | No* | No |
| F | 23 | Optic nerve hypoplasia | No | No |
| M | 35 | Retinopathy of prematurity | Minimal | No |
| F | 26 | Leber's congenital amaurosis | Minimal | No |
| F | 60 | Retinopathy of prematurity | No | No |
| M | 48 | Leber-like amaurosis (no genetic test) | Minimal | No |
| F | 30 | Optic nerve hypoplasia | No | No |
| F | 36 | Congenital anophthalmia | No | No |
| M | 36 | Congenital retinitis pigmentosa | Minimal | No |
| M | 40 | Juvenile macular degeneration/glaucoma | Yes | No |
| M | 66 | Retinopathy of prematurity | No | No |
Had some light perception until age 18 (when eyes were enucleated)
2.2 Procedures
The tests described below were administered in three sessions. In the first, participants performed a verbal memory task with immediate testing of recall (timepoint = T1), two verbal fluency tasks, and a spatial imagery task. In the second session, 24 - 48 hours later, participants were tested for delayed recall on the verbal memory task (T2), and also performed spatial memory and backwards digit span tasks. These sessions took place in a quiet room without external noise or distractions. Approximately one week after the second session, we contacted participants by phone to test further delayed recall on the verbal memory task (T3).
2.3 Verbal tasks
2.3.1 Verbal memory
We selected 20 concrete and 20 abstract words (18 abstract words were from Amedi et al. (2003) and we added two more with low imageability and concreteness ratings). We used the MRC Psycholinguistic Database 2.0 (Coltheart, 1981; Wilson, 1988), cross-checking to published ratings (Toglia & Battig, 1978; Kucera & Francis, 1967), to obtain word frequencies and ratings of imageability and concreteness; where there was no published rating, we obtained our own using instructions similar to those in Toglia & Battig (1978). The abstract and concrete sets of words differed significantly in concreteness (t38 = -24.5; p < .001) and imageability (t38= -13.8; p < .001) but were matched on length (number of syllables; t38 = .84; p = .4) and frequency (t38 = .15; p = .9). We then created two lists, each containing 10 abstract and 10 concrete words that were matched for length, frequency, imageability, and concreteness (all t38 < .4, all p > .4). These lists were recorded by one author (CS), speaking at the rate of one word per second, for auditory presentation.
Each list was presented twice in counterbalanced order. Participants were asked to listen carefully and to repeat each word after they heard it in order to ensure attention and facilitate encoding. After 20-30 minutes (T1), they were asked to recall as many words as possible from both lists, in any order and with no time limit; they were similarly instructed at T2 and T3. We recorded the percentage of concrete and abstract words correctly recalled at each timepoint.
2.3.2 Verbal fluency
Verbal fluency tests are widely used in neuropsychological assessment (Benton, 1994) and require participants to list as many words as they can that begin with a particular letter (phonemic fluency) or items belonging to a particular category (semantic fluency) within a time limit (Thurstone, 1938). Fluency tests draw on both memory (Ruff et al., 1997; Fagundo et al., 2008; Kraan et al., 2013; Mueller et al., 2015) and language (Ruff et al., 1997; Kraan et al., 2013; Stolwyk et al., 2015; Whiteside et al., 2016) processing, although in this case the type of memory involved is rapid retrieval from long-term storage of well-learned information in the language domain.
For the phonemic fluency task, participants were asked to generate as many items as possible starting with the letters ‘F’, ‘A’, and ‘S’ and for the semantic fluency task, as many items as possible belonging to the categories ‘animals’, ‘fruits’, and ‘musical instruments’. There was a 60-second time limit for each letter and category. Participants' verbal responses were digitally recorded for transcription and analysis. Performance was measured as the total number of items reported (excluding repetitions, proper names, and words with the same stem but different endings, e.g., ‘eat’ and ‘eating’) for each task.
2.3.3 Verbal working memory
As a test of verbal working memory, we used the Verbal Backwards Digit Span sub-test of the Wechsler Adult Intelligence Scale-Revised (WAIS-R: Wechsler, 1981). In this test, the experimenter read sequences of numbers of increasing length and participants had to repeat each sequence in reverse order. There were two sequences at each level and participants scored a point for each correctly reversed sequence but the test terminated if both sequences for a particular level were incorrect. The original test had seven levels of difficulty with spans increasing from 2 to 8 digits; we added a 9-digit level in order to counter potential ceiling effects (Castronovo & Delvenne, 2013; Pigeon & Marin-Lamellet, 2015).
2.4 Spatial tasks
2.4.1 Spatial memory
Neuropsychological testing uses the Corsi block test to assess spatial memory (Kessels et al., 2000). We used a haptic version of this test that could be completed by both blind and sighted participants, similar to that in previous studies (e.g., Cornoldi et al. 2009; Cattaneo et al., 2010). This comprised a custom-built 5 × 5 matrix of removable plastic cubes (4 cm/side) with one face of each cube covered with sandpaper to facilitate haptic discrimination (Figure 1). On each trial, 4 target cubes were arranged with the sandpapered side facing up and participants were allowed 10 s to haptically explore the matrix with both hands and memorize the locations of the target cubes. The experimenter then turned the target cubes over so that all cubes presented the same smooth surface. 20 s after the haptic encoding phase, the participant was asked to point to the locations of the memorized target cubes on the now blank matrix. Each participant completed five training trials and ten experimental trials. During encoding and testing, the matrix was located behind a curtain so that the task was based on purely haptic cues for both blind and sighted. Performance was measured as the percentage of individual locations correctly recalled.
Figure 1.

Custom-built 5 × 5 matrix used in the spatial memory task and for training in the spatial imagery task. The matrix was made of removable plastic cubes (4 cm/side), each with one face covered with sandpaper.
2.4.2 Spatial imagery
Visual imagery can be sub-divided into ‘pictorial’ object imagery and more ‘abstract’ spatial imagery (Kozhevnikov et al., 2005; Blazhenkova & Kozhevnikov, 2009). Individual preferences for object and spatial imagery are found not only in the visual, but also in the haptic modality (Lacey et al., 2011). Here, we tested spatial imagery, which emphasizes spatial relationships, using a task modified from an earlier study (Lacey et al., 2014). The task used here required participants to imagine cells numbered 1 to 25 in a 5 × 5 matrix (Figure 2) and, in response to auditorily presented four-digit strings, to imagine the shapes that would result from filling those four cells. Participants first underwent a training session using the haptic matrix described above, located behind a curtain so that training was based on purely haptic cues for both groups. This enabled participants to learn the numbered matrix and to construct a mental image of it. After training, participants completed 24 trials of the spatial imagery task. On each trial, they heard two four-digit strings, and had to decide whether the resulting two shapes were the same or different. In order to avoid participants performing the task by simply comparing the digit-strings, on ‘same’ trials, the shapes were represented by different sequences of numbers and participants were instructed to base their decision on the shapes they constructed, ignoring their location within the matrix (see Figure 2 for examples of ‘same’ and ‘different’ trials). Performance was measured as the percentage of correct responses. Scores were analyzed with analysis of covariance (ANCOVA) with the between-group factor of visual status; age, years of education, and gender were treated as covariates. The reported ANCOVA results reflect effects after filtering out covariate influences; the effects of the covariates are also reported.
Figure 2.
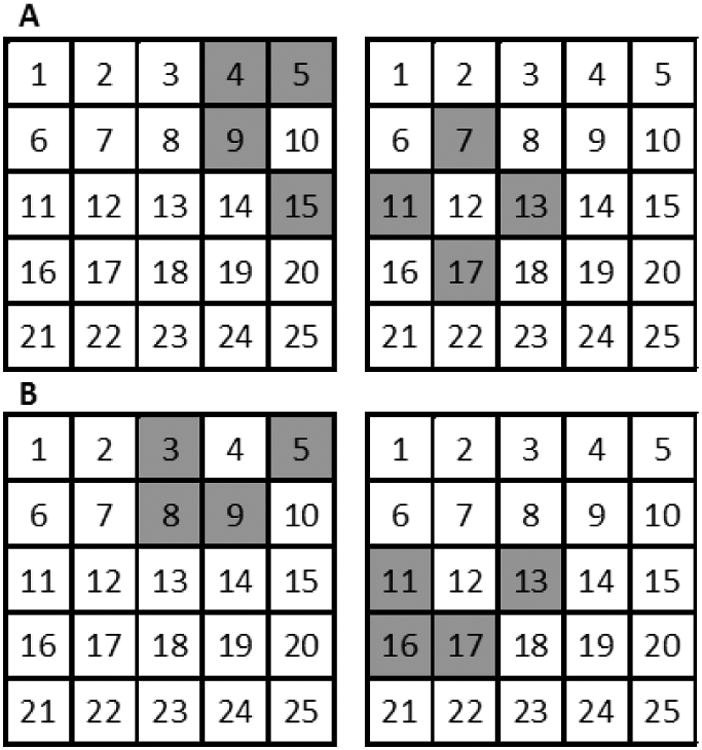
Examples of shapes to be formed from 4-digit strings in the spatial imagery task with representative (A) “different” and (B) “same” shape trials.
3 Results
The results are reported separately for each task. Effect sizes for significant differences and covariates are reported in terms of Cohen's d.
3.1 Verbal tasks
Repeated-measures (RM) ANCOVA of the verbal memory task (within group factors: timepoint and word-type) showed that the blind (mean ± sem: 30.8 ± 3.8%) performed better than the sighted (21.1 ± 2.4%: F1,20 = 15.7, p = .001, d = 1.65; Figure 3A) even after factoring out the covariates. Age was significantly related to performance (F1,20 = 8.7, p = .008, d = 1.23) in that recall declined with age (see correlation results below), but the number of years of education was not (F1,20 < .001, p = .9). Gender was also a significant covariate (F1,20 = 8.8, p = .007, d = 1.24) in that females (29.8 ± 3%) recalled more items than males (19.6 ± 3.1%). There were no other significant effects or second-order interactions; in particular, although recall declined over time for both blind and sighted, and for both concrete and abstract words, there was neither a main effect of timepoint (F2,40 = .05, p = .9), nor a main effect of word-type (F1,20 = 1.1, p = .3).
Figure 3.
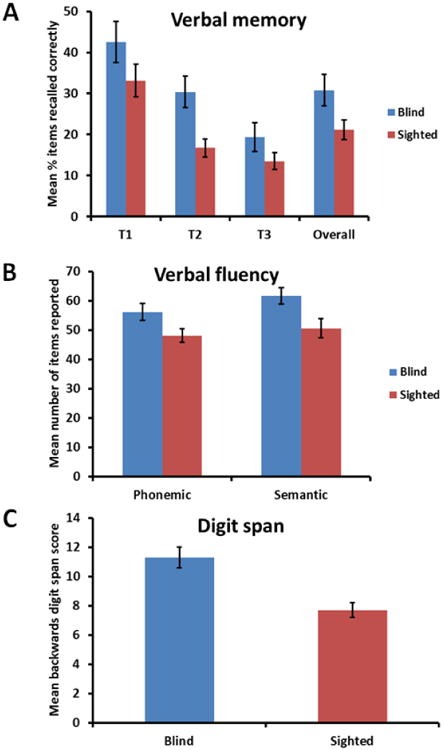
(A) The congenitally blind were significantly better overall at the verbal memory task than the sighted controls; (B) congenitally blind participants reported significantly more items than the sighted in both the semantic and phonemic fluency tests; (C) the congenitally blind had significantly higher backwards digit span scores than the sighted (maximum score = 16). Error bars in all graphs = standard error of the mean.
RM-ANCOVA of the fluency tasks (within-group factors: phonemic and semantic fluency) showed that the blind (phonemic: 56.2 ± 2.9; semantic: 61.6 ± 2.8) produced significantly more items than the sighted (phonemic: 48.1 ± 2.3; semantic: 50.6 ± 3.3: F1,20 = 8.6, p = .008, d = 1.22; Figure 3B) even after factoring out the covariates. Here, the number of years of education was a significant covariate (F1,20 = 5.8, p = .03, d = 1.01) in that the number of items produced increased with the number of years of education (see correlation results below), but age (F1,20 = 3.6, p = .07) and gender (F1,20 < .001, p = .9) were not. There were no other significant effects or interactions.
The blind also outperformed the sighted on the backwards digit span task (11.3 ± .7 vs. 7.7 ± .5 respectively: F1,20 = 19.8, p < .001, d = 1.86; Figure 3C) after factoring out the covariates; but none of the covariates was significantly related to performance (all F < 2.6, all p > .1).
3.2 Spatial tasks
One blind and one sighted participant were unable to perform the spatial imagery task because they found keeping the two sets of numbers in mind too difficult and they were therefore excluded from analysis. ANCOVA showed no significant effect of visual status on spatial imagery performance after filtering out the covariates (blind: 87.5 ± 3.6%; sighted: 79.2 ± 3.2%; F1,18 = 2.4, p = .14; Figure 4A) and no significant effect for any of the covariates.
Figure 4.
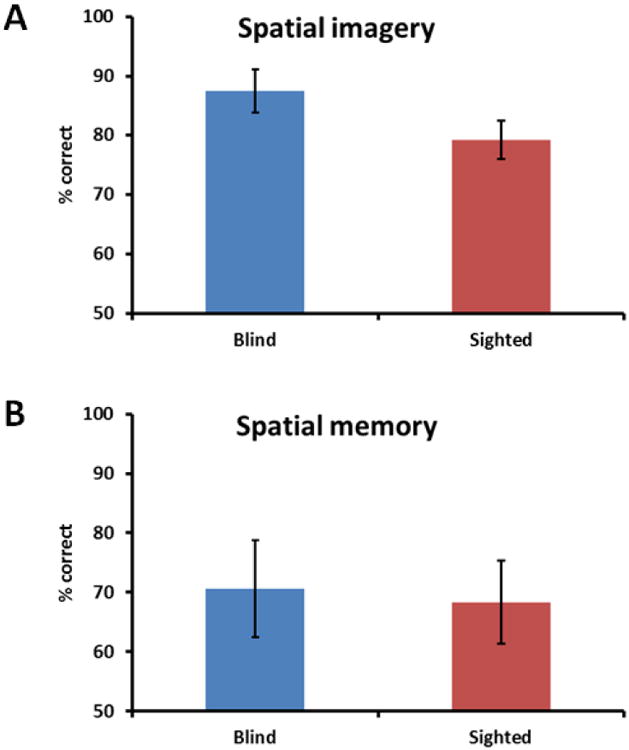
The congenitally blind did not differ significantly from the sighted on either the spatial imagery task (A) or the spatial memory task (B). Error bars in all graphs = standard error of the mean.
There was similarly no significant difference on ANCOVA between the blind and the sighted on the spatial memory task after factoring out the covariates (blind: 70.6 ± 8.2%; sighted: 68.3 ± 7.0%; F1,20 = .1, p = .7; Figure 4B); although performance declined with age (see correlation results below: F1,20 = 7.7, p = .01, d = 1.16), the education and gender covariates had no significant effect.
3.3 Correlational analyses
In order to further investigate the covariate effects reported above, we ran correlational analyses for the entire participant group. These showed that verbal memory tended to decline with age, significantly so at T3 (r = -.44, p = .03), as did spatial memory performance at the single timepoint that was tested (r = -.44, p = .03). Across all participants, there was a marginal trend for more years of education to be associated with reporting more items in both phonemic (r = .38, p = .06) and semantic (r = .36, p = .07) fluency tasks.
We also conducted these correlational analyses separately for the congenitally blind (Table 2A) and sighted (Table 2B) groups. These showed that the age-related decline in verbal memory was significant at T3 for the congenitally blind but not the sighted. However, verbal memory performance at each timepoint was strongly positively correlated with performance at each succeeding timepoint (i.e., T1 with T2 and T2 with T3); these correlations were significant in both the congenitally blind and the sighted groups (all r values > .66, all p values < .015). Phonemic and semantic fluency were positively correlated in both the congenitally blind (r = .58, p = .047) and sighted (r = .55, p = .049) groups. Semantic, but not verbal, fluency significantly declined with age in the sighted group (r = -.75, p = .003), but not the congenitally blind group. Better spatial memory was associated with better spatial imagery performance in the congenitally blind group (r = .77, p = .005) but not the sighted group. There was a positive correlation between digit span and phonemic fluency in the congenitally blind group (r = .58, p = .048) that was absent in the sighted group. Finally, in the sighted group, but not the congenitally blind group, there were positive correlations between spatial memory performance and both phonemic and semantic fluency.
Table 2.
Correlation analyses for (A) the congenitally blind, and (B) sighted controls. Significant correlations in bold.
| A | Age | Education | Verbal memory T1 | Verbal memory T2 | Verbal memory T3 | Phonemic fluency | Semantic fluency | Digit span | Spatial memory | Spatial imagery |
|---|---|---|---|---|---|---|---|---|---|---|
| Age | .65* | -.35 | -.57 | -.65* | .06 | .16 | -.4 | -.41 | -.31 | |
| Education | -.18 | -.32 | -.48 | .39 | .35 | .16 | -.46 | -.42 | ||
| Verbal memory T1 | .82** | .69* | -.24 | -.03 | .08 | -.13 | -.19 | |||
| Verbal memory T2 | .84** | -.11 | .14 | .14 | -.1 | -.2 | ||||
| Verbal memory T3 | -.02 | .03 | .13 | .19 | -.67 | |||||
| Phonemic fluency | .58* | .58* | .4 | .14 | ||||||
| Semantic fluency | .26 | .12 | -.43 | |||||||
| Digit span | .51 | .27 | ||||||||
| Spatial memory | .77** | |||||||||
| Spatial imagery | ||||||||||
|
| ||||||||||
| * p < .05 | ||||||||||
| ** p < .01 | ||||||||||
| B | Age | Education | Verbal memory T1 | Verbal memory T2 | Verbal memory T3 | Phonemic fluency | Semantic fluency | Digit span | Spatial memory | Spatial imagery |
|---|---|---|---|---|---|---|---|---|---|---|
| Age | -.07 | -.48 | -.44 | -.37 | -.29 | -.75** | .02 | -.42 | -.49 | |
| Education | -.05 | -.07 | -.15 | .39 | .36 | .43 | .52 | .21 | ||
| Verbal memory T1 | .66* | .42 | -.18 | .11 | -.06 | -.08 | .24 | |||
| Verbal memory T2 | .75** | .16 | .37 | .12 | .38 | .28 | ||||
| Verbal memory T3 | .33 | .46 | .11 | .45 | .47 | |||||
| Phonemic fluency | .55* | .26 | .63* | .57 | ||||||
| Semantic fluency | -.06 | .75** | .42 | |||||||
| Digit span | .5 | .05 | ||||||||
| Spatial memory | .43 | |||||||||
| Spatial imagery |
p < .05
p < .01
4 Discussion
Congenitally blind participants performed significantly better than sighted control participants on all the verbal tasks, but they did not differ significantly from the sighted on either the spatial memory or spatial imagery tasks. Although the gender, age, and education covariates affected performance on some tasks, covarying their effects out did not affect the overall effects of visual status. Gender differences have been reported in favor of females for verbal memory (e.g., Kimura & Clarke, 2002) and males for spatial imagery (e.g., Robert & Chevrier, 2003). Gender was a significant factor in the verbal memory task, with females outperforming males, but the effect of visual status remained significant after covarying the gender effect out even though there were more females than males overall because of the imbalance in the sighted group. Gender was unrelated to either verbal fluency or verbal working memory, and was not a significant covariate for either of the spatial measures used here, again despite the fact that there were more females than males overall. This may be because response time was unrestricted in the spatial tasks, a condition under which the male advantage either decreases or disappears (see Robert & Chevrier, 2003, for a brief discussion).There was some evidence for an age-related decline in both long-term verbal and spatial memory, and for verbal fluency to increase with higher levels of education, but otherwise age and years of education were unrelated to performance. Overall, these results favor the hypothesis that congenital blindness results in a specific superiority of verbal abilities, rather than a general enhancement of memory ability.
Congenitally blind participants were better than the sighted controls on all aspects of verbal memory, recalling more items, for both concrete and abstract words, at each timepoint, up to a week later. Consistent with prior studies (Amedi et al., 2003; Pasqualotto et al., 2013a; Röder et al., 2001; Raz et al., 2007), they exhibited a general superiority of verbal memory abilities compared to normally sighted people. A previous study has shown that the congenitally blind not only have better verbal recall than the sighted, but also reduced false memories for a semantically related lure (Pasqualotto et al., 2013a), suggesting that semantic categorization is more accurate in the congenitally blind. This may be related to the congenitally blind outperforming the sighted on both phonemic and semantic verbal fluency tasks and would fit with the notion that congenital blindness confers a specifically verbal advantage, as opposed to a general memory enhancement. There was also a significant positive correlation between digit span and phonemic fluency in the congenitally blind that was absent in the sighted. As pointed out in the Methods (2.3.2), the verbal fluency tasks we used likely draw on both memory and language abilities, albeit long-term memory for highly overlearned information. Therefore, the benefits of congenital blindness are not apparently limited to verbal memory for relatively new information, but extend to the rapid retrieval of well-learned information from long-term storage. However, the fact that this verbal working memory/fluency correlation was present only in the congenitally blind, and not the sighted, may also suggest a specifically verbal advantage for the blind.
In contrast, blindness from birth did not seem to confer any advantage in terms of spatial skills, either for spatial memory or for spatial imagery: the congenitally blind and sighted performed equally well on both tasks. This contrasts with our earlier study of later-onset blindness (after age 6), in which performance on a spatial imagery task, similar to the present one except in using a 4 × 4 lettered matrix, was impaired in the late-blind compared to the sighted, independently of the age at which form vision was lost (Occelli et al., 2014). That the congenitally blind were not significantly worse than the sighted suggests that visual experience may not be necessary for the development of proficient spatial imagery or spatial memory. It is possible that different cognitive strategies adopted by the congenitally blind could result in comparable performance via different mechanisms (Cattaneo et al., 2008; Renzi et al., 2013). For example, although the late blind and blindfolded sighted tend to employ an object-based, allocentric, reference frame while the congenitally blind prefer a self-based, egocentric frame (Pasqualotto et al., 2013b Pasqualotto & Proulx, 2012), these might result in equally effective task performance. The lack of performance differences on spatial tasks between sighted and congenitally blind people might also reflect the functional equivalence of spatial representations derived from different sensory inputs (Loomis et al., 2013). The relative differences between verbal and spatial domains assume importance in the context of rehabilitative approaches attempting to more fully integrate individuals with congenital blindness into society. Inter-individual differences, which were not assessed here, would be critical to consider for such approaches.
Finally, there were positive correlations between spatial memory performance and both phonemic and semantic fluency in the sighted but not the blind. These are hard to interpret and we can only speculate that since semantic categorization is predominantly a left hemisphere activity (e.g., Binney et al., 2016; Malone et al., 2016; Carota et al., 2017) and the spatial memory task could potentially be solved by using a categorical spatial reference frame (i.e., up, down, left, right: e.g., Kosslyn, 1987; Jager & Postma, 2003; Baumann et al., 2012), these two tasks may well have drawn on some of the same neural resources, at least in sighted subjects.
The mechanism by which the superiority of verbal abilities occurs in congenital blindness is currently unknown. Certainly, in the absence of vision from birth, verbal inputs and cues become more important in everyday experience. For instance, when a sighted person asks for directions, he or she can take advantage of cues offered by pointing or other gestures, in addition to the verbal information provided. In contrast, the blind person must encode and later recall the verbal material conveyed without reference to gestural cues. Thus, it seems reasonable to suggest that congenital blindness induces stronger reliance on verbal information, and that such reliance leads to better verbal memory, and potentially other verbal skills, through practice.
The neural basis for this may be related to the involvement of visual cortical areas in various aspects of language in the congenitally blind. For example, covert verb generation in response to nouns presented via Braille (Burton et al., 2002a) or auditory input (Burton et al., 2002b) recruits activity in early visual cortex, and transcranial magnetic stimulation over the occipital pole results in semantic errors during verb generation (Amedi et al., 2004). During covert recall of a previously learned word-list (a task similar to the verbal memory task of the present study), the magnitude of visual cortical activity was found to correlate with verbal memory performance (Amedi et al., 2003). Further, syntactic processing is associated with activity in various parts of visual cortex (Lane et al., 2015). Visual cortical areas in congenitally blind people also show stronger resting-state connectivity, compared to the sighted, with language areas in inferior frontal cortex (Liu et al., 2007).
Overall, we suggest that the superior verbal memory performance in the congenitally blind reported in previous studies (Amedi et al., 2003; Pasqualotto et al., 2013a; Röder et al., 2001; Raz et al., 2007) arises from an advantage specific to verbal abilities, rather than memory abilities. Further questions remain, however: for example, whether the verbal memory superiority of the congenitally blind is specific to retrieval or reflects better encoding. A finding of more efficient perceptual encoding of speech in the congenitally blind (Hugdahl et al., 2004) is relevant to this possibility. A specific limitation of the present study is that we tested a limited range of tasks and therefore we cannot say whether the congenitally blind have superior language skills across the board, or whether superiority might be limited to certain aspects of language. It would be informative, for example, to use tasks involving increasing syntactic complexity or semantic difficulty. In addition, claims of superior performance in the blind on a range of tasks including verbal (Amedi et al., 2003), semantic (Pasqualotto et al., 2013a), and serial memory (Raz et al., 2007), as well as numerical ability (Castronovo & Delvenne, 2013) and auditory memory (Roder et al., 2001; Rokem & Ahissar, 2009) rest primarily on studies of the congenitally blind. Thus, studies comparing congenitally, early, and late blind groups to the sighted are required in order to determine whether superior performance on these tasks is exclusive to the congenitally blind or, if not, how it emerges, e.g., as a function of vision loss at different ages.
Acknowledgments
This work was supported by NIH grant R01 EY012440 to KS and NIH/NEI grant R01 EY019924 to LBM. Support to CS and KS from the Veterans Administration is also gratefully acknowledged. We especially thank our participants for their involvement in our studies. We are also grateful to Amir Amedi for kindly providing an abstract word list; the Center for the Visually Impaired in Atlanta, Georgia, the Georgia chapter of the National Federation of the Blind and the Georgia Radio Reading Service for assistance in recruiting; Dr. Valerie Biousse for neuro-ophthalmologic assessment; and Gianpaolo Occelli for technical assistance.
References
- Amedi A, Floel A, Knecht S, Zohary E, Cohen LG. Transcranial magnetic stimulation of the occipital pole interferes with verbal processing in blind subjects. Nature Neurosci. 2004;7:1266–1270. doi: 10.1038/nn1328. [DOI] [PubMed] [Google Scholar]
- Amedi A, Raz N, Pianka P, Malach R, Zohary E. Early ‘visual’ cortex activation correlates with superior verbal memory performance in the blind. Nature Neurosci. 2003;6:758–766. doi: 10.1038/nn1072. [DOI] [PubMed] [Google Scholar]
- Baumann O, Chan E, Mattingley JB. Distinct neural networks underlie encoding of categorical versus coordinate spatial relations during active navigation. NeuroImage. 2012;60:1630–1637. doi: 10.1016/j.neuroimage.2012.01.089. [DOI] [PubMed] [Google Scholar]
- Benton AL. Neuropsychological assessment. Annu Rev Psychol. 1994;45:1–23. doi: 10.1146/annurev.ps.45.020194.000245. [DOI] [PubMed] [Google Scholar]
- Binney RJ, Hoffman P, Lambon Ralph MA. Mapping the multiple graded contributions of the anterior temporal lobe representational hub to abstract and social concepts: evidence from distortion-corrected fMRI. Cereb Cortex. 2016;26:4227–4241. doi: 10.1093/cercor/bhw260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazhenkova O, Kozhevnikov M. The new object-spatial-verbal cognitive style model: theory and measurement. Appl Cognitive Psych. 2009;23:638–663. [Google Scholar]
- Burton H, Snyder AZ, Conturo TE, Akbudak E, Ollinger JM, Raichle ME. Adaptive changes in early and late blind: a fMRI study of Braille reading. J Neurophysiol. 2002a;87:589–607. doi: 10.1152/jn.00285.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton H, Snyder AZ, Diamond JB, Raichle ME. Adaptive changes in early and late blind: a fMRI study of verb generation to heard nouns. J Neurophysiol. 2002b;88:3359–3371. doi: 10.1152/jn.00129.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carota F, Kriegeskorte N, Nili H, Pulvermüller F. Representational similarity mapping of distributional semantics in left inferior frontal, middle temporal, and motor cortex. Cereb Cortex. 2017 doi: 10.1093/cercor/bhw379. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castronovo J, Delvenne JF. Superior numerical abilities following early visual deprivation. Cortex. 2013;49:1435–1440. doi: 10.1016/j.cortex.2012.12.018. [DOI] [PubMed] [Google Scholar]
- Cattaneo Z, Fantino M, Silvanto J, Tinti C, Pascual-Leone A, Vecchi T. Symmetry perception in the blind. Acta Psychol. 2010;134:398–402. doi: 10.1016/j.actpsy.2010.04.002. [DOI] [PubMed] [Google Scholar]
- Cattaneo Z, Vecchi T, Cornoldi C, Mammarella I, Bonino D, Ricciardi E, Pietrini P. Imagery and spatial processes in blindness and visual impairment. Neurosci Biobehav Rev. 2008;32:1346–60. doi: 10.1016/j.neubiorev.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Coltheart M. The MRC Psycholinguistic Database. Q J Exp Psychol. 1981;33A:497–505. [Google Scholar]
- Cornoldi C, Cortesi A, Preti D. Individual differences in the capacity limitations of visuospatial short-term memory: research on sighted and totally congenitally blind people. Mem Cognition. 1991;19:459–468. doi: 10.3758/bf03199569. [DOI] [PubMed] [Google Scholar]
- Cornoldi C, Tinti C, Mammarella IC, Re AM, Varotto D. Memory for an imagined pathway and strategy effects in sighted and in totally congenitally blind individuals. Acta Psychol. 2009;130:11–16. doi: 10.1016/j.actpsy.2008.09.012. [DOI] [PubMed] [Google Scholar]
- Fagundo AB, López S, Romero M, Guarch J, Marcos T, Salamero M. Clustering and switching in semantic fluency: predictors of the development of Alzheimer's disease. Int J Geriatr Psych. 2008;23:1007–1013. doi: 10.1002/gps.2025. [DOI] [PubMed] [Google Scholar]
- Hugdahl K, Ek M, Takio F, Rintee T, Tuomainen J, Haarala C, Hämäläinen H. Blind individuals show enhanced perceptual and attentional sensitivity for identification of speech sounds. Brain Res. 2004;19:28–32. doi: 10.1016/j.cogbrainres.2003.10.015. [DOI] [PubMed] [Google Scholar]
- Hull T, Mason H. Performance of blind children on digit span tests. J Visual Impair Blin. 1995;89:166–169. [Google Scholar]
- Jager G, Postma A. On the hemispheric specialization for categorical and coordinate spatial relations: a review of the current evidence. Neuropsychologia. 2003;41:504–515. doi: 10.1016/s0028-3932(02)00086-6. [DOI] [PubMed] [Google Scholar]
- Kessels RPC, van Zandvoort MJE, Postma A, Kapelle LJ, de Haan EHF. The Corsi block-tapping task: standardization and normative data. Appl Neuropsychol. 2000;7:252–258. doi: 10.1207/S15324826AN0704_8. [DOI] [PubMed] [Google Scholar]
- Kimura D, Clarke PG. Women's advantage on verbal memory is not restricted to concrete words. Psychol Rep. 2002;91:1137–1142. doi: 10.2466/pr0.2002.91.3f.1137. [DOI] [PubMed] [Google Scholar]
- Kosslyn SM. Seeing and imagining in the cerebral hemispheres: a computational analysis. Psychol Rev. 1987;94:148–175. [PubMed] [Google Scholar]
- Kozhevnikov M, Kosslyn SM, Shephard J. Spatial versus object visualisers: a new characterisation of cognitive style. Mem Cognition. 2005;33:710–726. doi: 10.3758/bf03195337. [DOI] [PubMed] [Google Scholar]
- Kraan C, Stolwyk RJ, Testa RR. The abilities associated with verbal fluency performance in a young, healthy population are multifactorial and differ across fluency variants. Appl Neuropsychol. 2013;20:159–168. doi: 10.1080/09084282.2012.670157. [DOI] [PubMed] [Google Scholar]
- Kucera H, Francis WN. Computational Analysis of Present-day American English. Brown University Press; Providence RI, USA: 1967. [Google Scholar]
- Lacey S, Lin JB, Sathian K. Object and spatial imagery dimensions in visuo-haptic representations. Exp Brain Res. 2011;213:267–273. doi: 10.1007/s00221-011-2623-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey S, Stilla R, Sreenivasan K, Deshpande G, Sathian K. Spatial imagery in haptic shape perception. Neuropsychologia. 2014;60:144–158. doi: 10.1016/j.neuropsychologia.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane C, Kanjila S, Omaki A, Bedny M. Visual cortex of congenitally blind adults responds to syntactic movement. J Neurosci. 2015;35:12859–12868. doi: 10.1523/JNEUROSCI.1256-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Yu C, Liang M, Li J, Tian L, Zhou Y, Qin W, Li K, Jiang T. Whole brain functional connectivity in the early blind. Brain. 2007;130:2085–2096. doi: 10.1093/brain/awm121. [DOI] [PubMed] [Google Scholar]
- Loomis J, Klatzky RL, Giudice NA. Representing 3D space in working memory: spatial images from vision, hearing, touch, and language. In: Lacey S, Lawson R, editors. Multisensory Imagery. Springer LLC; New York USA: 2013. pp. 131–155. [Google Scholar]
- Malone PS, Glezer LS, Kim J, Jiang X, Riesenhuber M. Multivariate pattern analysis reveals category-related organization of semantic representations in anterior temporal cortex. J Neurosci. 2016;36:10089–10096. doi: 10.1523/JNEUROSCI.1599-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller KD, Koscik RL, LaRue A, Clark LR, Hermann B, Johnson SC, Sager MA. Verbal fluency and early memory decline: results from the Wisconsin Registry for Alzheimer's prevention. Arch Clin Neuropsych. 2015;30:448–57. doi: 10.1093/arclin/acv030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Occelli V, Lacey S, Stephens C, Sathian K. Superior verbal abilities in congenital blindness. Proceedings of Human Vision & Electronic Imaging. 2016;2016:094.1. [Google Scholar]
- Occelli V, Lin JB, Lacey S, Sathian K. Loss of form vision impairs spatial imagery. Front Hum Neurosci. 2014;8:159. doi: 10.3389/fnhum.2014.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasqualotto A, Proulx M. The role of visual experience for the neural basis of spatial cognition. Neurosci Biobehav R. 2012;36:1179–1187. doi: 10.1016/j.neubiorev.2012.01.008. [DOI] [PubMed] [Google Scholar]
- Pasqualotto A, Lam JS, Proulx MJ. Congenital blindness improves semantic and episodic memory. Behav Brain Res. 2013a;244:162–165. doi: 10.1016/j.bbr.2013.02.005. [DOI] [PubMed] [Google Scholar]
- Pasqualotto A, Spiller MJ, Jansari AS, Proulx M. Visual experience facilitates allocentric spatial representation. Behav Brain Res. 2013b;236:175–179. doi: 10.1016/j.bbr.2012.08.042. [DOI] [PubMed] [Google Scholar]
- Pigeon C, Marin-Lamellet C. Evaluation of the attentional capacities and working memory of early and late blind persons. Acta Psychol. 2015;155:1–7. doi: 10.1016/j.actpsy.2014.11.010. [DOI] [PubMed] [Google Scholar]
- Raczkowski D, Kalat JW, Nebes R. Reliability and validity of some handedness questionnaire items. Neuropsychologia. 1974;12:43–47. doi: 10.1016/0028-3932(74)90025-6. [DOI] [PubMed] [Google Scholar]
- Raz N, Striem E, Pundak G, Orlov T, Zohary E. Superior serial memory in the blind: a case of cognitive compensatory adjustment. Curr Biol. 2007;17:1129–1133. doi: 10.1016/j.cub.2007.05.060. [DOI] [PubMed] [Google Scholar]
- Renzi C, Cattaneo Z, Vecchi T, Cornoldi C. Mental imagery and blindness. In: Lacey S, Lawson R, editors. Multisensory Imagery. Springer LLC; New York USA: 2013. pp. 115–130. [Google Scholar]
- Robert M, Chevrier E. Does men's advantage in mental rotation persist when real three-dimensional objects are either felt or seen? Mem Cognition. 2003;31:1136–1145. doi: 10.3758/bf03196134. [DOI] [PubMed] [Google Scholar]
- Röder B, Rösler F, Neville HJ. Auditory memory in congenitally blind adults: a behavioral-electrophysiological investigation. Brain Res. 2001;11:289–303. doi: 10.1016/s0926-6410(01)00002-7. [DOI] [PubMed] [Google Scholar]
- Rokem A, Ahissar M. Interactions of cognitive and auditory abilities in congenitally blind individuals. Neuropsychologia. 2009;47:843–848. doi: 10.1016/j.neuropsychologia.2008.12.017. [DOI] [PubMed] [Google Scholar]
- Ruff RM, Lights RH, Parker SB, Levin HS. The psychological construct of word fluency. Brain Lang. 1997;57:394–405. doi: 10.1006/brln.1997.1755. [DOI] [PubMed] [Google Scholar]
- Ruggiero G, Iachini T. The role of vision in the Corsi block-tapping: evidence from blind and sighted people. Neuropsychology. 2010;24:674–679. doi: 10.1037/a0019594. [DOI] [PubMed] [Google Scholar]
- Smits BWGM, Mommers MJC. Differences between blind and sighted children on WISC verbal subtests. New Outlook for the Blind. 1976;70:240–246. [Google Scholar]
- Stolwyk R, Bannirchelvam B, Kraan C, Simpson K. The cognitive abilities associated with verbal fluency task performance differ across fluency variants and age groups in healthy young and old adults. J Clin Exp Neuropsyc. 2015;37:70–83. doi: 10.1080/13803395.2014.988125. [DOI] [PubMed] [Google Scholar]
- Swanson HL, Luxenberg D. Short-term memory and working memory in children with blindness: support for a domain general or domain specific system? Child Neuropsychol. 2009;15:280–294. doi: 10.1080/09297040802524206. [DOI] [PubMed] [Google Scholar]
- Thurstone LL. Primary Mental Abilities. University of Chicago Press; Chicago USA: 1938. [Google Scholar]
- Tillman HM, Bashaw WL. Multivariate analysis of the WISC scales for blind and sighted children. Psychol Rep. 1968;23:523–526. doi: 10.2466/pr0.1968.23.2.523. [DOI] [PubMed] [Google Scholar]
- Toglia MP, Battig WF. Handbook of Semantic Word Norms. Lawrence Erlbaum Associates; Hillsdale NJ, USA: 1978. [Google Scholar]
- Vecchi T, Tinti C, Cornoldi C. Spatial memory and integration processes in congenital blindness. Neuroreport. 2004;15:2787–2790. [PubMed] [Google Scholar]
- Wechsler D. Manual for the Wechsler Adult Intelligence Scale-Revised. The Psychological Corporation; New York USA: 1981. [Google Scholar]
- Whiteside DM, Kealey T, Semla M, Luu H, Rice L, Basso MR, Roper B. Verbal fluency: Language or executive function measure? Appl Neuropsychol Adult. 2015;23:29–34. doi: 10.1080/23279095.2015.1004574. [DOI] [PubMed] [Google Scholar]
- Wilson MD. The MRC Psycholinguistic Database: Machine Readable Dictionary, Version 2. Behav Res Meth Ins C. 1988;20:6–10. [Google Scholar]
- Withagen A, Kappers AM, Vervloed MP, Knoors H, Verhoeven L. Short term memory and working memory in blind versus sighted children. Res Dev Disabil. 2013;34:2161–2172. doi: 10.1016/j.ridd.2013.03.028. [DOI] [PubMed] [Google Scholar]


