Abstract
BACKGROUND
It has been observed that trauma patients often display elevated procoagulant activity that could be caused, in part, by tissue factor (TF). We previously observed that trauma patients with thermal, blunt and penetrating injuries have active FIXa and FXIa in their plasma. In the current study we evaluated the effect of injury severity, with or without accompanying shock, on the frequency and concentration of TF, FIXa and FXIa in plasma from trauma patients.
METHODS
Eighty trauma patients were enrolled and divided equally into 4 groups based on their injury severity score (ISS) and base deficit (BD):
Group 1: Non-severe injury, no shock
Group 2: Non-severe injury, with shock
Group 3: Severe injury, no shock
Group 4: Severe injury, with shock
Blood was collected at a 0 time-point (first blood draw upon arrival at hospital) and citrate plasma was prepared, frozen and stored at −80C. FXIa, FIXa and TF activity assays were based on a response of thrombin generation to corresponding monoclonal inhibitory antibodies.
RESULTS
The frequency and median concentrations of TF were relatively low in non-severe injury groups (17.5 % and 0 pM respectively) but were higher in those with severe injury (65% and 0.5 pM, respectively). While FXIa was observed in 91% of samples and was high across all 4 groups, median concentrations were highest (by approximately 4-fold) in groups with shock. FIXa was observed in 80% of plasma samples and concentrations varied in a relatively narrow range between all 4 groups. No endogenous activity was observed in plasma from healthy individuals.
CONCLUSIONS
1) Frequency and concentration of TF is higher in patients with a higher trauma severity; 2) Concentration of FXIa is higher in patients with shock; 3) For the first time reported, the vast majority of plasma samples from trauma patients contain active FIXa and FXIa.
LEVEL of EVIDENCE
Diagnostic Tests or Criteria, level I. Diagnostic Tests.
Keywords: Trauma, shock, FXIa, TF, thrombin generation
INTRODUCTION
Trauma is the leading cause of death worldwide among young adults,1 in which uncontrolled hemorrhage accounts for approximately 60% of deaths in patients whose injuries were potentially treatable.2 Survival of the patient depends on the management of two conditions: early phase bleeding and late phase thrombosis.3 The protocol for early phase bleeding involves massive transfusions and, as more information comes to light regarding trauma-induced coagulopathy (TIC), more alterations are being made in the management of bleeding following severe trauma.4 Early detection of coagulopathy is critical as it is indicative of injury severity and is a prognostic factor for transfusions.5 A multitude of factors contribute to TIC, including anemia, hemodilution, hypothermia, acidosis (defined as shock in terms of base deficit (BD)), hemorrhagic shock and the trauma itself.3 On the other hand, severe acidosis (≤ pH 7.1) has been shown to inhibit the propagation phase of thrombin generation and accelerate fibrin degradation,6 thus contributing to overall hemostatic changes in trauma patients.
The elevated procoagulant activity of trauma patients has been hypothesized to be caused, in part, by tissue factor (TF) located on the subendothelium, blood cells,7 microparticles8 and more recently suggested on platelets,9 although this last claim is disputed.10, 11 TF is a membrane-bound glycoprotein that initiates coagulation when exposed to FVIIa circulating in blood, subsequently forming the extrinsic factor (F)Xase (FVIIa:TF) complex which activates FIX and FX and ultimately results in thrombin generation. Robust thrombin generation leads to cleavage of fibrinogen and activation of FXIII, allowing the formation of a solid fibrin clot.12 Elevated levels of TF antigen have been observed in a multitude of illnesses associated with tissue damage13, 14 and hematologic disorders.15, 16
In addition to TF, we have previously observed detectable and active FXIa and FIXa corresponding to a worse prognosis in the plasma of cardiovascular13, 16, 17 and trauma patients, often present for several days to weeks.18, 19 While TF initiates the extrinsic pathway of coagulation, FXI(a) plays a role in both the intrinsic (contact) and extrinsic pathways and is directly upstream of FIX. FXIa subsequently activates FIX to FIXa which complexes with FVIIIa and leads to FX activation – the so-called merging point of the two pathways. FXI can be activated via the contact pathway by FXIIa on artificial surfaces or under more physiologic conditions in a feedback loop by thrombin via the extrinsic pathway.12 DNA and RNA released from stimulated and dying cells20, 21 along with polyphosphates released from activated platelets22, 23 have also been shown to lead to FXIa generation by FXIIa. Elevated levels of FXIa have been detected and implicated in a number of prothrombotic states13, 17, 24 and deficiencies have been shown to protect against ischemic stroke25.
Our previous studies showed that the only 3 proteins endogenously present in trauma patient plasma capable of initiating thrombin generation and, consequentially coagulation, are active TF, FXIa and FIXa.18, 19 It is not known how the release of TF, FXIa and FIXa is effected by injury type and severity, therefore in our current study we examined plasma from trauma patients with the hypothesis that the severity of an injury with or without accompanying shock would correlate with higher frequencies and concentrations of these 3 proteins.
METHODS
Materials
Pooled citrate platelet-poor plasma (PPP) was prepared in-house using 10 healthy donors.13 Trypsin inhibitor from corn (CTI; prevents contact pathway initiation of coagulation) was prepared as previously described.26, 27 Phospholipid vesicles (PCPS) composed of 25% dioleoyl-sn-glycero-3-phospho-L-serine and 75% of 1,2-dioleoyl-sn-glycero-3-phosphocholine (both from Avanti Polar Lipids, Inc; Alabaster, AL) were prepared as previously described.28 Inhibitory monoclonal anti-TF (αTF-5; prevents binding of TF to FVIIa), anti-FXIa (αFXI-2; inhibits FIX activation by FXIa) and anti-FIXa (αFIX-91; inhibits FX activation by FIXa) antibodies were produced and characterized in-house.29, 30 The fluorogenic substrate used was benzyloxycarbonyl-Gly-Gly-Arg-7-amido-4methylcoumarin• HCl (Z-GGR-AMC) (Bachem, Torrance, CA). TF, FIXa and FXIa activity in plasma was calculated from calibration curves developed with human FIXa, human FXIa and relipidated TF1–26326 (all from Haematologic Technologies, Inc., Essex Junction, VT). Human thrombin was produced in-house.31 HBS buffer was prepared using N-[2-hydroxyethyl]piperazine-NN-[2-ethanesulfonic acid] (HEPES) and NaCl (Fisher Scientific, Waltham, MA) (HBS; 20 mM HEPES, 0.15 M NaCl, pH 7.4). CaCl2 used was from Fisher Scientific, Waltham, MA. The thrombin generation assay was performed in untreated, polystyrene 96-well plates (Costar, Lowell, MA). The plate reader used was the BioTek Synergy 4 and analysis was performed using the Gen5 plate reader software (BioTek, Winooski, VT).
Patients
Eighty trauma patients from a major urban level 1 trauma center were selected based on presence or absence of severe injury (ISS > 15) and presence or absence of shock (BD ≤ −6 mmol/L) (Table 1). Overall, 47 patients arrived with blunt trauma and 33 with penetrating trauma. This was a randomly selected cohort separated into 4 injury groups from injured trauma patients requiring the highest level trauma activation (transfers excluded) between 2005 and 2013. Sixty-two patients were male and 18 female. The age of patients varied between 18 and 90 years (average 42.6±19.2 years). Transport times were collected for 61 of 80 patients (13 from group 1, 14 from group 2 and 17 from both groups 3 and 4), with median total time to draw of 37 min and a range of 18–259 min.
Table 1.
Characteristics of Patient Groups
| Group 1 | ISS ≤ 15 and BD > −6 (non-severe injury, no shock) |
| Group 2 | ISS ≤ 15 and BD ≤ −6 (non-severe injury with shock) |
| Group 3 | ISS > 15 and BD > −6 (severe injury, no shock) |
| Group 4 | ISS > 15 and BD ≤ −6 (severe injury with shock) |
Blood Sample Collection and Citrate Plasma Preparation
The study was approved by the University of California, San Francisco Committee on Human Research. The mechanism of consent consisted of an initial waiver of informed consent allowing blood draws prior to getting patient or surrogate consent. As soon as possible, full written informed consent was obtained from the patient or family (depending on condition). If surrogate consent was obtained and the patient status improved, then a follow up for patient written consent occurred. The consent mechanism also consisted of waivers of consent that had the approval to be applied in appropriate circumstances, such as the patient’s expiration, the patient’s inability to regain capacity for consent (followed for at least 30 days), the absence of family, etc. If unable to obtain consent and the appropriate waiver could not be applied, the patient was excluded from the study and the collected blood sample(s) were disposed of. All patients in this study are either consented (written, by family and/or by patient) into the study or had an IRB-approved waiver applied.
Blood samples were collected at each patients initial 0 hour draw and citrate plasma was prepared, frozen and stored at −80°C. No additional freeze/thaw cycles were involved prior to FXIa, FIXa and TF activity assays.
Thrombin Generation Assay (TGA) for FXIa, FIXa and TF
The assay is a modification of previously published assays32, 33 and is based on the response of the lag phase of thrombin generation in contact-pathway inhibited (CTI) plasma to the addition of inhibitory monoclonal antibodies to FXIa, FIXa and TF. Citrated plasma samples were thawed at 37ºC for 3 min and 5 mg/mL CTI was immediately added to achieve a 0.1 mg/mL final concentration. Eighty μL of each plasma sample was added to a 96-well plate and inhibitory monoclonal antibodies (anti-TF, anti-XI and anti-IX) were added (when desired) to achieve a 0.1 mg/mL final concentration. Twenty μL of a 2.5 mM Z-GGR-AMC/90 mM CaCl2 solution in HBS was added to plasma samples to achieve final concentrations of 417 μM/15 mM, respectively, followed by a 3 minute incubation period at 37ºC to allow recalcification of the plasma. Twenty μL of a 120 μM PCPS solution in HBS was then added to plasma samples to achieve a final concentration of 20 μM, thus initiating thrombin generation. Fluorescence readings began immediately and hydrolysis of the AMC substrate (at 370 nm excitation and 460 nm emission wavelengths) was followed over a 3600 s period. Changes in fluorescence were converted to thrombin concentration using a calibration curve built by sequential dilutions of human thrombin. Concentrations of TF, FXIa and FIXa were calculated from corresponding calibration curves constructed by titrating purified proteins into multi-donor pooled plasma from healthy individuals.
Quantitation of endogenous FXIa, FIXa and TF activity in TGA
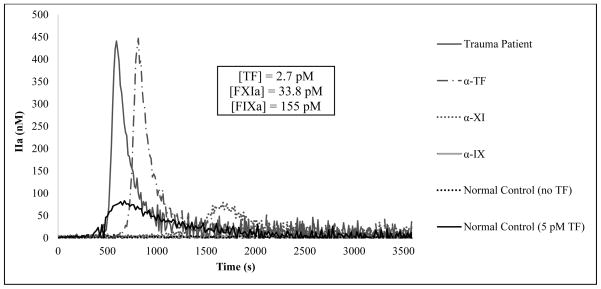
In the absence of an exogenous initiator, recalcified citrate plasma containing CTI from healthy individuals (multi-donor or individual donors) does not generate any detectable level of thrombin during the 60 minute duration of the assay. In contrast, the majority of trauma patients with a wide range of both injury type and severity do generate relatively high levels of thrombin under the same experimental conditions. This relatively robust thrombin generation is exemplified in Fig. 1 as it shows thrombin production over time in a trauma patient plasma sample without the addition of an exogenous initiator yielding a lag phase of 514 seconds, a peak thrombin value of 441 nM and a maximum rate of generation of 7.1 nM/s. To compare, healthy plasma initiated with 5 pM relipidated TF yields a lag phase of 501 s, a peak thrombin value of 83 nM and a maximum rate of generation of 1.6 nM/s.
Figure 1.
Thrombin generation over time in re-calcified citrate plasma from a trauma patient and from healthy individuals. No exogenous activator was added to trauma patient plasma and contact pathway was prevented by CTI with either no inhibitory antibodies added (———) or in the presence of inhibitory antibodies against TF (—— · —), FXIa (•••••), and FIX (‗‗‗‗). As a control, pooled plasma from healthy individuals was evaluated in the absence of an exogenous initiator (•••••) or in the presence of 5 pM TF (———).
To quantitate endogenous concentrations of the 3 proteins in a given plasma sample, inhibitory monoclonal antibodies against each protein were individually added to separate sample aliquots and the prolongation in the lag phase was determined. Previously performed titrations of TF, FXIa and FIXa into multi-donor plasma under the same experimental conditions showed a linear response of the lag phase to the amount of exogenous protein added. Based on such calibration curves, the prolongation in the lag phase from the endogenous potential to that when αTF is added (Fig. 1) (from 514 s to 696 s) corresponds to a TF concentration of 2.7 pM. Had the addition of αTF led to virtually no difference in thrombin generation profiles, it would be concluded that the plasma sample contained no active TF. A similar quantitation was done to obtain FXIa levels by measuring the prolongation of the lag phase with the addition of an αFXI antibody. In Fig. 1, the prolongation from 514 s to 1424 s corresponds to a FXIa concentration of 33.8 pM. Furthermore, a prolongation of the lag phase from 1424 s to >3,600 s, i.e. the complete abolishment of thrombin generation over the duration of the assay, with the addition of an αFIX antibody corresponds to a FIXa concentration of 155 pM.
Statistical Analysis
Comparison of TF, FXIa and FIXa frequency on the basis of injury severity or shock was conducted using Fisher’s exact test with 95% confidence intervals and corresponding p-values, with a statistical significance limit of p < 0.0025 to control for pair-wise comparisons within each group. For analysis of injury severity, groups 1 and 2 were combined (non-severe injury) and groups 3 and 4 were combined (severe injury). Similarly, for the analysis of shock groups 1 and 3 were combined (no shock) and also groups 2 and 4 (with shock).
RESULTS
Patients
Across all 4 groups, the ISS varied between 1 and 75 (average 19.3±17.2). For group 1, there was 10% mortality at discharge, a median of 3.5 days spent in the hospital and a range of ISS scores from 1–14 with 2 patients (10%) sustaining TBI. Group 2 had a similar percent mortality at discharge and median hospital stay (10% and 2.5 days, respectively) along with a range of ISS scores from 1–13 with no patients sustaining TBI. Unlike groups 1 and 2, group 3 had both a greater percent mortality and median hospital stay (45% and 14 days, respectively) along with a wider range of ISS scores and percent TBI (17–59 and 75%, respectively). Similarly, group 4 had a 45% mortality at discharge, a median stay of 8 days in the hospital and a range of ISS scores from 16–75 with 60% of patients sustaining TBI. According to available data, 1 patient from group 2 received 4 units of packed red blood cells (pRBC) and 1 L plasmalyte prior to their draw along 3 patients from group 4 who each received 1 unit pRBC prior to their draws. All patients who received blood product transfusions fell within the range of TF, FXIa and FIXa levels in their respective groups.
Tissue Factor
TF activity was observed in 33 of 80 patient samples (41.3%) (Table 2). The frequency of TF in patients with non-severe injuries from groups 1 and 2 was relatively low (20% and 15%, respectively) and increased by at least 3-fold in patients with severe injury from groups 3 and 4 (60% and 70%, respectively). Groups were combined on the basis of severe vs non-severe injury and the relative risk (RR) for the presence of TF was calculated to be 3.71 (95% confidence interval (CI) = 1.83 – 7.56), indicating that individuals with severe injuries are upwards of 4 times more likely to have detectable levels of TF in this thrombin generation assay than individuals with non-severe injuries. When groups were combined based on the presence or absence of shock (groups 2 and 4 and groups 1 and 3, respectively) the RR calculated was approximately 1 (RR = 1.06; 95% CI = 0.63 – 1.79)), indicating no correlation between detectable TF and the presence of shock.
Table 2.
Protein Frequency and Concentrations in Patient Groups
| Patient Group | TF | FXIa | FIXa | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Frequency | Concentration (pM) | Frequency | Concentration (pM) | Frequency | Concentration (pM) | ||||
| Range | Median | Range | Median | Range | Median | ||||
| 1 | 4/20 (20%) | 0 – >6.4 | 0 | 18/20 (90%) | 0 – >64 | 5.7 | 9/20 (45%) | 0 – >1000 | 0 |
| 2 | 3/20 (15%) | 0 – >6.4 | 0 | 19/20 (95%) | 0 – >64 | 23.2 | 18/20 (90%) | 0 – >1000 | 250 |
| 3 | 12/20 (60%) | 0 – >6.4 | 0.4 | 17/20 (85%) | 0 – >64 | 6.8 | 18/20 (90%) | 0 – >1000 | 197.5 |
| 4 | 14/20 (70%) | 0 – >6.4 | 0.6 | 19/20 (95%) | 0 – >64 | 25.2 | 19/20 (95%) | 0 –>1000 | 197.5 |
| Overall | 33/80 (41%) | 0 – >6.4 | 0 | 73/80 (91%) | 0 – >64 | 12.7 | 64/80 (80%) | 0 –>1000 | 142.5 |
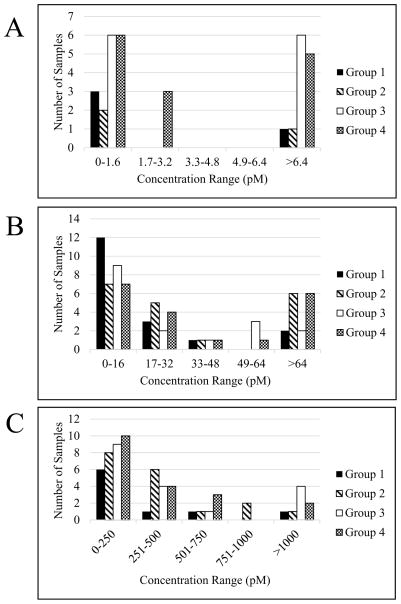
The concentration of TF varied in a wide range in all 4 groups, but was the lowest in samples from patients with non-severe injuries (groups 1 and 2, both with median values of 0 pM) and the highest in those from patients with severe injuries (groups 3 and 4 with median values 0.4 pM and 0.6 pM, respectively). The entire range of TF concentrations across all 4 groups (0 pM - >6.4 pM) was divided into 5 sub-ranges and the prevalence in each was established for each group (Fig. 2A), again illustrating that most often the highest concentrations of TF are found in patients with severe injury from groups 3 and 4.
Figure 2.
Distribution of TF (A), FXIa (B) and FIXa (C) concentrations in trauma patient groups (see Table 1).
Factor XIa
Overall, FXIa was observed in 73 out of 80 patient samples (91%) and the frequency was similar across all 4 groups of patients, regardless of injury severity and/or shock (90%, 95%, 85% and 95% for groups 1, 2, 3 and 4, respectively). It was calculated that individuals with severe injury were just as likely to have detectable levels of FXIa as individuals with non-severe injuries (RR = 0.97; 95% CI = 0.85 – 1.12) and, additionally, the presence of shock was shown to have just as much of an effect on FXIa frequency as the absence of shock (RR = 1.09; 95% CI = 0.95 – 1.25).
Similar to TF, the concentration of FXIa varied in a wide range in the entire study population (0 pM - >64 pM). Unlike TF, however, FXIa was present at higher concentrations in patients with shock from groups 2 and 4 (median values of 23.2 pM and 25.2 pM, respectively) and did not correlate with the severity of injury in patients from groups 1 and 3 (median values of 5.7 pM and 6.8 pM, respectively). As with TF, the entire range of FXIa concentrations across all 4 groups was divided into 5 sub-ranges and the prevalence in each was established for each group (Fig. 2B). As expected, high FXIa concentrations were observed at a higher frequency in groups 2 and 4 (with shock) than in groups 1 and 2 (without shock).
Factor IXa
Active FIXa was observed in 64 out of 80 patients (80%). From group 1 plasma samples, only 9 out of 20 (45%) had detectable FIXa, whereas in groups 2–4 only 1 or 2 plasma samples had no FIXa activity (90% – 95% frequency). It was calculated that individuals with severe injury are slightly more likely to have detectable levels of FIXa than individuals with non-severe injury (RR = 1.37; 95% CI = 1.09 – 1.73), along with the presence of shock vs. no shock (RR = 1.37; 95% CI = 1.09 – 1.73).
As with TF and FXIa, there was a very wide range of FIXa concentrations across all 4 groups (0 pM - >1000 pM) and also within all groups (Table 2). Unlike TF and FXIa, the median concentration of FIXa varied in a relatively narrow range between groups 2, 3 and 4 (250 pM, 197.5 pM and 197.5 pM, respectively), and the lowest was observed in group 1 (0 pM). The range of concentrations was divided into 5 sub-ranges and the prevalence was established in each group (Fig. 2C), illustrating the lack of correlation between FIXa levels and injury severity (groups 3 and 4) and/or presence of shock (groups 2 and 4).
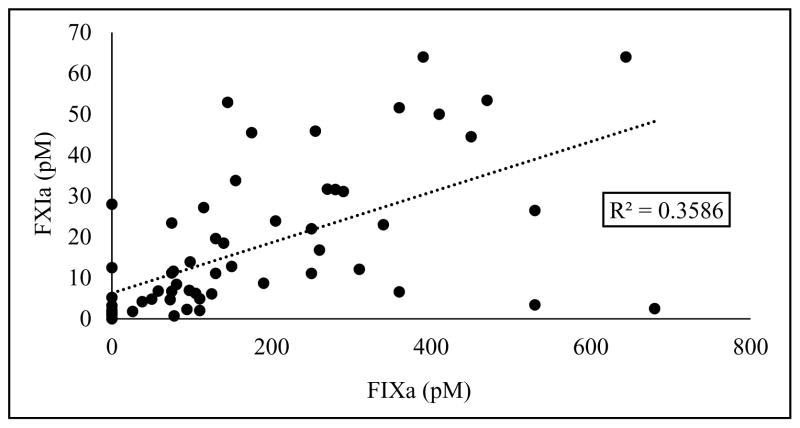
For patients with quantifiable amounts of the 3 proteins within the entire cohort, there was a good correlation between FIXa and FXIa concentrations (R2 = 0.36; Fig 3), but no correlation between TF and FXIa or FIXa was observed (data not shown).
Figure 3.
Correlation between FXIa and FIXa in patients with quantifiable concentrations of corresponding proteins.
DISCUSSION
In this study, we illustrate the prevalence and wide range of TF, FXIa and FIXa concentrations in plasma from trauma patients with various ISS and BD scores that correspond to the extent of injury and level of shock, respectively. The levels and frequency of these 3 proteins are shown to correlate with either trauma severity or shock or both.
TF, an integral membrane protein found in many types of cells, is an important and highly studied initiator of the blood coagulation process. Multiple studies have shown the expression of TF activity on blood cells such as monocytes and neutrophils upon stimulation with agonists such as inflammation-related cytokines7 and also on microparticles8 shed from such blood cells. More recently, the presence of TF in platelet α granules has been suggested,9 although there is no common agreement with regard to the presence of TF activity and antigen in platelets.10, 11 In whole blood from healthy individuals, levels of active TF do not exceed 20 fM, if at all present,7, 34 whereas increased levels of TF antigen have been previously observed in patients suffering from a multitude of illnesses associated with tissue damage that are independent of shock, including cardiovascular diseases,13 trauma and subsequent sepsis.14 Along with those disorders associated with tissue damage, increased TF levels have been detected in hematologic disorders such as sickle-cell disease (SCD)15 and cerebrovascular events.16
Although there is debate as to the origin and prevalence of TF in the blood of both healthy and non-healthy individuals, our data regarding TF being of the highest frequencies and concentrations in the cohorts with the highest trauma severity is consistent with the knowledge that TF, located on the subendothelium of all blood vessels larger than capillaries,35 is positioned in such a way as to trigger the coagulation cascade following prominent vessel damage and subsequent intravascular exposure.36 This is also consistent with studies that have shown that TF activity becomes enhanced by endotoxin stimulation and inflammation7 which may occur following the tissue disruption during severe trauma. Since shock, in terms of BD, does not directly reflect tissue damage but more so metabolic alterations, it is perhaps somewhat expected that BD had less effect on TF presence/activity in trauma patients than injury severity.
As TF plays a critical role in the initiation of the extrinsic pathway of coagulation, FXI(a) maintains a role in both the intrinsic (contact) and extrinsic pathway and can be activated via either route. In TF-triggered hemostasis, FXIa is generated in a feedback loop by thrombin, thus bypassing the contact pathway,12 however under certain conditions FXIa can be generated via activation by FXII. Such conditions include exposure of blood to artificial surfaces (classically stated as negatively charged surfaces but also occurring on neutral or positively charged surfaces),37 polyphosphate release from stimulated or dying cells (DNA and RNA)20, 21 and inorganic polyphosphate release from activated platelets.22, 23 Activated FXI subsequently activates FIX which, in complex with FVIIIa, activates FX to FXa and ultimately leads to thrombin generation. FXIa can also enhance thrombin generation by activating FV and FVIII38 and by proteolysis of tissue factor pathway inhibitor.39 Increased levels of FXIa have been shown to correlate with a higher risk of venous thrombosis40 and, on the other end of the spectrum, FXI deficiencies have been shown to protect against ischemic stroke.25 There is mounting evidence that illustrates the importance of the intrinsic pathway in thrombosis41 and suggests contact pathway proteins such as FXII and FXI as prime targets for antithrombotic strategies.42 Increased thrombotic risk known to confer with high plasma concentrations of FXI may be explained by an increase in endogenous thrombin potential43 and/or impaired fibrinolysis and increased clot stability thus lessening the elimination of thrombi within blood vessels.44
While FXIa is not observed in the plasma of healthy individuals,13 it has been detected and implicated in prothrombotic states observed in acute coronary syndrome13 and stable angina17 along with hypertensive individuals45 and those with chronic obstructive pulmonary disease.46 The presence of FXIa in patients with ischemic cerebrovascular events has been shown to correspond with worse prognosis47 which could suggest the use FXIa as a novel thrombotic marker for worse outcome in such a population. Active FXIa is frequently observed in the aforementioned populations despite the immense amount of physiologic serine protease inhibitors (serpins) because none of them can efficiently inhibit FXIa,48 thus allowing 80–90% of FXIa to survive in blood for at least 30 min during citrate plasma preparation.13
From the 4 designated cohorts based on injury severity and/or shock, we found that, although the frequency was relatively consistent across all cohorts, the highest concentrations of FXIa occurred in the cohorts with shock and was seemingly independent of injury extent. We also observed a correlation between the concentrations of FXIa and FIXa (R2 = 0.36) throughout the entire cohort of this study, whereas no correlations between the concentrations of TF and FXIa or FIXa were observed. These data suggest that the contact pathway is primarily responsible for FXIa generation in trauma patient blood, although the contribution from the TF-driven extrinsic pathway cannot be dismissed as TF is a membrane protein and plasma levels may not represent those in vivo. Our preliminary data (not shown) support this hypothesis and are consistent with previously published observations.49 Although we are suggesting a pathway of FXIa generation driven by shock and independent of the TF-driven pathway, the mechanism of shock-triggered activation of the contact pathway remains unclear.
Downstream of FXI(a) is FIX(a), which can be activated by either FXIa of the contact pathway and/or the extrinsic tenase complex (FVIIa/TF) of the TF-pathway. FIXa forms the intrinsic FXase complex with FVIIIa, which, in addition to the extrinsic FXase complex, activates FX and subsequently promotes thrombin generation. Published studies have showed that activation of FX by the intrinsic FXase complex as compared to that by the extrinsic FXase complex is approximately 50-fold more efficient, thus making FIXa a potential marker for hypercoagulability. Due to FIXa’s incompletely formed active site in the absence of FVIIIa, inactivation of FXIa by plasma inhibitors is relatively inefficient, thus allowing FIXa to efficiently diffuse from TF-bearing cells to platelets and serve as a link between the initiation and propagations phases of coagulation.12 Increased levels of FIXa have been observed in the prothrombotic states of acute coronary syndromes,24 consistent with the prevalence observed in this study across all 4 cohorts. The absence of a clearly defined relationship or correspondence of FIXa to either injury severity and/or shock is consistent with a previous study that suggested the mechanism of regulating FIXa generation and longevity differs from those observed for TF and FXIa.50
Limitations of this study include; 1) Analysis was limited to a single time-point (admission). It is likely that, given time and exacerbation, concentrations of TF, FXIa and FIXa would alter. Additionally, complete data compilation and long term follow-up would be necessary to evaluate predictive value of TF, FXIa and FIXa levels in patients falling under the designated cohorts with and without blood product transfusions. 2) Analysis was limited to the quantitation of TF, FXIa and FIXa and excluded any other related biomarkers that could prove informative to TIC. 3) The entire population size was quite small. This study was intended as an initial investigation of the effect of trauma and injury severity with or without shock on the concentrations of these 3 proteins as primary mediators of the contact and extrinsic coagulation cascades. Correlating the findings of a larger study to clinical outcomes could provide important prognostic information and a better understanding of the phenotype of coagulopathy induced by varying concentrations of these proteins and should be investigated next.
In conclusion, the current study demonstrates the presence of quantifiable TF, and for the first time reported in manuscript format, FXIa and FIXa activity in the majority of trauma patients. The frequency and concentration of TF is higher in patients with a higher trauma severity and is independent of shock, whereas FXIa is of the highest concentrations in patients with shock and does not appear to be significantly affected by trauma severity. FIXa correlates only slightly with both severe injury and the presence of shock. Taken together, these data suggest separate pathways of TF and FXIa generation based on injury severity and shock, respectively. This study is part of a larger work in progress and we ultimately believe that measuring these 3 proteins could help in the stratifying of trauma patients and, as a consequence, lead to an improved treatment.
Acknowledgments
We would like to thank Dr. Kenneth G. Mann for the opportunity to use his laboratory resources and also Dr. Peter Callas for his assistance with the statistical analysis. This work was supported by UM1 HL120877 grant from NIH and Systems Biology grant ARO-W911NF-10-1-0376 from DoD.
Footnotes
CONFLICT OF INTEREST
Authors declare no conflict of interest
MEETING PRESENTATION
Portions of this work were presented at the 57th ASH Annual Meeting, Orlando, FL, December 5–8, 2015.
AUTHORSHIP
S.M.P. analyzed plasma samples, participated in data analysis and wrote the manuscript; M.J.C. participated in the design of the study, recruited patients and edited the manuscript; A.S.C., M.F.N., L.Z.K. and B.M.H. took care about patients, collected plasma samples, provided clinical data and edited the manuscript; S.B. designed the study, participated in data analysis and edited the manuscript.
CONFLICTS OF INTEREST
Authors declare no conflict of interest.
References
- 1.Sauaia A, Moore FA, Moore EE, Moser KS, Brennan R, Read RA, Pons PT. Epidemiology of trauma deaths: a reassessment. J Trauma. 1995;38(2):185–93. doi: 10.1097/00005373-199502000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Teixeira PG, Inaba K, Hadjizacharia P, Brown C, Salim A, Rhee P, Browder T, Noguchi TT, Demetriades D. Preventable or potentially preventable mortality at a mature trauma center. J Trauma. 2007;63(6):1338–46. doi: 10.1097/TA.0b013e31815078ae. discussion 46–7. [DOI] [PubMed] [Google Scholar]
- 3.Gando S, Sawamura A, Hayakawa M. Trauma, shock, and disseminated intravascular coagulation: lessons from the classical literature. Ann Surg. 2011;254(1):10–9. doi: 10.1097/SLA.0b013e31821221b1. [DOI] [PubMed] [Google Scholar]
- 4.Spahn DR, Bouillon B, Cerny V, Coats TJ, Duranteau J, Fernandez-Mondejar E, Filipescu D, Hunt BJ, Komadina R, Nardi G, et al. Management of bleeding and coagulopathy following major trauma: an updated European guideline. Crit Care. 2013;17(2):R76. doi: 10.1186/cc12685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brohi K, Singh J, Heron M, Coats T. Acute traumatic coagulopathy. J Trauma. 2003;54(6):1127–30. doi: 10.1097/01.TA.0000069184.82147.06. [DOI] [PubMed] [Google Scholar]
- 6.Martini WZ. Coagulopathy by hypothermia and acidosis: mechanisms of thrombin generation and fibrinogen availability. J Trauma. 2009;67(1):202–8. doi: 10.1097/TA.0b013e3181a602a7. discussion 8–9. [DOI] [PubMed] [Google Scholar]
- 7.Butenas S, Bouchard BA, Brummel-Ziedins KE, Parhami-Seren B, Mann KG. Tissue factor activity in whole blood. Blood. 2005;105(7):2764–70. doi: 10.1182/blood-2004-09-3567. [DOI] [PubMed] [Google Scholar]
- 8.Del Conde I, Shrimpton CN, Thiagarajan P, Lopez JA. Tissue-factor-bearing microvesicles arise from lipid rafts and fuse with activated platelets to initiate coagulation. Blood. 2005;106(5):1604–11. doi: 10.1182/blood-2004-03-1095. [DOI] [PubMed] [Google Scholar]
- 9.Panes O, Matus V, Saez CG, Quiroga T, Pereira J, Mezzano D. Human platelets synthesize and express functional tissue factor. Blood. 2007;109(12):5242–50. doi: 10.1182/blood-2006-06-030619. [DOI] [PubMed] [Google Scholar]
- 10.Bouchard BA, Mann KG, Butenas S. No evidence for tissue factor on platelets. Blood. 2010;116(5):854–5. doi: 10.1182/blood-2010-05-285627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Osterud B, Olsen JO. Human platelets do not express tissue factor. Thromb Res. 2013;132(1):112–5. doi: 10.1016/j.thromres.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 12.Mann KG, Brummel-Ziedins K, Orfeo T, Butenas S. Models of blood coagulation. Blood Cells Mol Dis. 2006;36(2):108–17. doi: 10.1016/j.bcmd.2005.12.034. [DOI] [PubMed] [Google Scholar]
- 13.Butenas S, Undas A, Gissel MT, Szuldrzynski K, Zmudka K, Mann KG. Factor XIa and tissue factor activity in patients with coronary artery disease. Thromb Haemost. 2008;99(1):142–9. doi: 10.1160/TH07-08-0499. [DOI] [PubMed] [Google Scholar]
- 14.Gando S, Nanzaki S, Sasaki S, Kemmotsu O. Significant correlations between tissue factor and thrombin markers in trauma and septic patients with disseminated intravascular coagulation. Thromb Haemost. 1998;79(6):1111–5. [PubMed] [Google Scholar]
- 15.Key NS, Slungaard A, Dandelet L, Nelson SC, Moertel C, Styles LA, Kuypers FA, Bach RR. Whole blood tissue factor procoagulant activity is elevated in patients with sickle cell disease. Blood. 1998;91(11):4216–23. [PubMed] [Google Scholar]
- 16.Undas A, Slowik A, Gissel M, Mann KG, Butenas S. Circulating activated factor XI and active tissue factor as predictors of worse prognosis in patients following ischemic cerebrovascular events. Thromb Res. 2011;128(5):e62–6. doi: 10.1016/j.thromres.2011.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zabczyk M, Butenas S, Plicner D, Fijorek K, Sadowski J, Undas A. Factors associated with the presence of circulating active tissue factor and activated factor XI in stable angina patients. Blood Coagul Fibrinolysis. 2012;23(3):189–94. doi: 10.1097/MBC.0b013e32834ee194. [DOI] [PubMed] [Google Scholar]
- 18.Butenas S, Gissel MT, Park MS. Factor XIa and tissue factor activity in trauma patients. J Thromb Haemost. 2015;13(Suppl 2):211. [Google Scholar]
- 19.Butenas S, Manning C, Freeman K. Factor XIa, factor IXa and tissue factor activity in blunt trauma patients. J Thromb Haemost. 2015;13(Suppl 2):569. [Google Scholar]
- 20.Gansler J, Jaax M, Leiting S, Appel B, Greinacher A, Fischer S, Preissner KT. Structural requirements for the procoagulant activity of nucleic acids. PLoS One. 2012;7(11):e50399. doi: 10.1371/journal.pone.0050399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kannemeier C, Shibamiya A, Nakazawa F, Trusheim H, Ruppert C, Markart P, Song Y, Tzima E, Kennerknecht E, Niepmann M, et al. Extracellular RNA constitutes a natural procoagulant cofactor in blood coagulation. Proc Natl Acad Sci U S A. 2007;104(15):6388–93. doi: 10.1073/pnas.0608647104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muller F, Mutch NJ, Schenk WA, Smith SA, Esterl L, Spronk HM, Schmidbauer S, Gahl WA, Morrissey JH, Renne T. Platelet polyphosphates are proinflammatory and procoagulant mediators in vivo. Cell. 2009;139(6):1143–56. doi: 10.1016/j.cell.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith SA, Morrissey JH. Polyphosphate: a new player in the field of hemostasis. Curr Opin Hematol. 2014;21(5):388–94. doi: 10.1097/MOH.0000000000000069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Minnema MC, Peters RJ, de Winter R, Lubbers YP, Barzegar S, Bauer KA, Rosenberg RD, Hack CE, ten Cate H. Activation of clotting factors XI and IX in patients with acute myocardial infarction. Arterioscler Thromb Vasc Biol. 2000;20(11):2489–93. doi: 10.1161/01.atv.20.11.2489. [DOI] [PubMed] [Google Scholar]
- 25.Salomon O, Steinberg DM, Koren-Morag N, Tanne D, Seligsohn U. Reduced incidence of ischemic stroke in patients with severe factor XI deficiency. Blood. 2008;111(8):4113–7. doi: 10.1182/blood-2007-10-120139. [DOI] [PubMed] [Google Scholar]
- 26.Cawthern KM, van't Veer C, Lock JB, DiLorenzo ME, Branda RF, Mann KG. Blood coagulation in hemophilia A and hemophilia C. Blood. 1998;91(12):4581–92. [PubMed] [Google Scholar]
- 27.Hojima Y, Pierce JV, Pisano JJ. Hageman factor fragment inhibitor in corn seeds: purification and characterization. Thromb Res. 1980;20(2):149–62. doi: 10.1016/0049-3848(80)90381-3. [DOI] [PubMed] [Google Scholar]
- 28.Higgins DL, Mann KG. The interaction of bovine factor V and factor V-derived peptides with phospholipid vesicles. J Biol Chem. 1983;258(10):6503–8. [PubMed] [Google Scholar]
- 29.Butenas S, Dee JD, Mann KG. The function of factor XI in tissue factor-initiated thrombin generation. J Thromb Haemost. 2003;1(10):2103–11. doi: 10.1046/j.1538-7836.2003.00431.x. [DOI] [PubMed] [Google Scholar]
- 30.Parhami-Seren B, Butenas S, Krudysz-Amblo J, Mann KG. Immunologic quantitation of tissue factors. J Thromb Haemost. 2006;4(8):1747–55. doi: 10.1111/j.1538-7836.2006.02000.x. [DOI] [PubMed] [Google Scholar]
- 31.Lundblad RL, Kingdon HS, Mann KG. Thrombin. Methods Enzymol. 1976;45:156–76. doi: 10.1016/s0076-6879(76)45017-6. [DOI] [PubMed] [Google Scholar]
- 32.Mann KG, Whelihan MF, Butenas S, Orfeo T. Citrate anticoagulation and the dynamics of thrombin generation. J Thromb Haemost. 2007;5(10):2055–61. doi: 10.1111/j.1538-7836.2007.02710.x. [DOI] [PubMed] [Google Scholar]
- 33.Hemker HC, Giesen P, Al Dieri R, Regnault V, de Smedt E, Wagenvoord R, Lecompte T, Beguin S. Calibrated automated thrombin generation measurement in clotting plasma. Pathophysiol Haemost Thromb. 2003;33(1):4–15. doi: 10.1159/000071636. [DOI] [PubMed] [Google Scholar]
- 34.Santucci RA, Erlich J, Labriola J, Wilson M, Kao KJ, Kickler TS, Spillert C, Mackman N. Measurement of tissue factor activity in whole blood. Thromb Haemost. 2000;83(3):445–54. [PubMed] [Google Scholar]
- 35.Fleck RA, Rao LV, Rapaport SI, Varki N. Localization of human tissue factor antigen by immunostaining with monospecific, polyclonal anti-human tissue factor antibody. Thromb Res. 1990;59(2):421–37. doi: 10.1016/0049-3848(90)90148-6. [DOI] [PubMed] [Google Scholar]
- 36.Morrissey JH. Tissue factor: in at the start.. and the finish? J Thromb Haemost. 2003;1(5):878–80. doi: 10.1046/j.1538-7836.2003.00219.x. [DOI] [PubMed] [Google Scholar]
- 37.Macfarlane RG. An enzyme cascade in the blood clotting mechanism, and its function as a biochemical amplifier. Nature. 1964;202:498–9. doi: 10.1038/202498a0. [DOI] [PubMed] [Google Scholar]
- 38.Whelihan MF, Orfeo T, Gissel MT, Mann KG. Coagulation procofactor activation by factor XIa. J Thromb Haemost. 2010;8(7):1532–9. doi: 10.1111/j.1538-7836.2010.03899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Puy C, Tucker EI, Matafonov A, Cheng Q, Zientek KD, Gailani D, Gruber A, McCarty OJ. Activated factor XI increases the procoagulant activity of the extrinsic pathway by inactivating tissue factor pathway inhibitor. Blood. 2015;125(9):1488–96. doi: 10.1182/blood-2014-10-604587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cushman M, O'Meara ES, Folsom AR, Heckbert SR. Coagulation factors IX through XIII and the risk of future venous thrombosis: the Longitudinal Investigation of Thromboembolism Etiology. Blood. 2009;114(14):2878–83. doi: 10.1182/blood-2009-05-219915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Long AT, Kenne E, Jung R, Fuchs TA, Renne T. Contact system revisited: an interface between inflammation, coagulation, and innate immunity. J Thromb Haemost. 2016;14(3):427–37. doi: 10.1111/jth.13235. [DOI] [PubMed] [Google Scholar]
- 42.Gailani D, Bane CE, Gruber A. Factor XI and contact activation as targets for antithrombotic therapy. J Thromb Haemost. 2015;13(8):1383–95. doi: 10.1111/jth.13005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Siegemund A, Petros S, Siegemund T, Scholz U, Seyfarth HJ, Engelmann L. The endogenous thrombin potential and high levels of coagulation factor VIII, factor IX and factor XI. Blood Coagul Fibrinolysis. 2004;15(3):241–4. doi: 10.1097/00001721-200404000-00007. [DOI] [PubMed] [Google Scholar]
- 44.von dem Borne PA, Cox LM, Bouma BN. Factor XI enhances fibrin generation and inhibits fibrinolysis in a coagulation model initiated by surface-coated tissue factor. Blood Coagul Fibrinolysis. 2006;17(4):251–7. doi: 10.1097/01.mbc.0000224843.33216.5f. [DOI] [PubMed] [Google Scholar]
- 45.Agorasti A, Mourvati E, Trivellas T, Papadopoulos V, Bazntiara I, Christoforidou A, Passadakis P. Changes in haemostatic and platelet activation markers in non-dipper hypertensive patients. Int Urol Nephrol. 2012;44(2):523–33. doi: 10.1007/s11255-011-9926-9. [DOI] [PubMed] [Google Scholar]
- 46.Jankowski M, Undas A, Kaczmarek P, Butenas S. Activated factor XI and tissue factor in chronic obstructive pulmonary disease: links with inflammation and thrombin generation. Thromb Res. 2011;127(3):242–6. doi: 10.1016/j.thromres.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Suri MF, Yamagishi K, Aleksic N, Hannan PJ, Folsom AR. Novel hemostatic factor levels and risk of ischemic stroke: the Atherosclerosis Risk in Communities (ARIC) Study. Cerebrovasc Dis. 2010;29(5):497–502. doi: 10.1159/000297966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wuillemin WA, Minnema M, Meijers JC, Roem D, Eerenberg AJ, Nuijens JH, ten Cate H, Hack CE. Inactivation of factor XIa in human plasma assessed by measuring factor XIa-protease inhibitor complexes: major role for C1-inhibitor. Blood. 1995;85(6):1517–26. [PubMed] [Google Scholar]
- 49.Pedicord DL, Seiffert D, Blat Y. Feedback activation of factor XI by thrombin does not occur in plasma. Proc Natl Acad Sci U S A. 2007;104(31):12855–60. doi: 10.1073/pnas.0705566104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kusak PCD, Gissel M, Plens K, Butenas S, Undas A. Activated factor IX, factor XI and tissue factor identify patients with permanent atrial fibrillation treated with warfarin who are at risk of ischemic stroke. Arch Med Sci. doi: 10.5114/aoms.2015.54791. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]