Abstract
Prostate cancer (PCa) is a common cancer in men. Although current treatments effectively palliate symptoms and prolong life, the metastatic PCa remains incurable. It is important to find biomarkers and targets to improve metastatic PCa diagnosis and treatment. Here, we report a novel correlation between karyopherin α4 (KPNA4) and PCa pathological stages. KPNA4 mediates the cytoplasm-to-nucleus translocation of transcription factors including NF-κB while its role in PCa was largely unknown. We find that knockdown of KPNA4 reduces cell migration in multiple PCa cell lines, suggesting a role of KPNA4 in PCa progression. Indeed, stable knockdown of KPNA4 significantly reduces PCa invasion and distant metastasis in mouse models. Functionally, KPNA4 alters tumor microenvironment in terms of macrophage polarization and osteoclastogenesis by modulating TNF-α and -β. Further, KPNA4 is proved as a direct target of miR-708, a tumor-suppressive miRNA. We disclose the role of miR-708-KPNA4-TNF axes in PCa metastasis and KPNA4’s potential as a novel biomarker for PCa metastasis.
Keywords: KPNA4, prostate cancer, TNF-α, TNF-β, metastasis
Introduction
One hallmark of malignancy is the metastasis of cancer cells to distant organs, which may occur even after the removal of the primary tumor and years of disease-free survival (1). Accumulating evidence indicates that the immune system plays a critical role in controlling cancer progress (2). Tumor necrosis factor (TNF), one of the most intensively studied cytokines, plays a central role within the complicated cytokines network as well as in the immune response (3). Triggering TNF signaling may initiate an immune-suppressive environment (4), which facilitates tumor progress directly and contributes to tumor cell invasion and metastasis via the activation of the NF-κB pathway (5). Accordingly, blocking TNF-α has been demonstrated to be an effective strategy for preventing carcinogenesis (6, 7). Recent reports revealed that TNF-α expounds the critical role of tumor-stromal cell crosstalk (8) and promotes tumor-associated microphage (TAM) infiltration support the role of TNF-α in remodeling the tumor microenvironment (TME) (9).
TAMs are involved in cancer initiation and progression via regulation of the complex interplay between the immune system and cancer cells (10). A study conducted in 2006 revealed that deletion of TAMs in a mouse model effectively suppressed tumor growth and metastasis (11). Moreover, in circulation, TAM-like cells were found to interact with circulating tumor cells to promote the formation of a metastatic microenvironment (12). Specifically, co-culturing of immortalized prostate epithelial cells with macrophages can induce prostate carcinogenesis independent of any other carcinogens (13), suggesting that macrophage infiltration is the key to inflammation during tumorigenesis. Infiltration of TAMs is accompanied by the M1 to M2 polarization induced by the TME (14). A human specimens study revealed that M2 macrophages infiltration is associated with the release of cytokines derived from prostate cancer (PCa) cells (15). Furthermore, accumulating reports have shown that the declining M2 to M1 ratio of macrophages is an alternative approach in cancer treatment rather than a global deletion (16, 17).
Nevertheless, targeting TNF or TAMs may be effective at inhibiting cancer metastasis. It is more important to identify the intrinsic alterations inside the primary tumor that promote its metastasis. In the searching for such alterations, we noted that Karyopherin (KPNA), a group of importins that mediate nuclear translocation of multiple transcription factors, have the potential to be the initiating factors. KPNA4 is one of the main isoforms that mediate TNF-α-induced NF-κB p50/p65 nuclear import (18, 19). NF-κB pathway activation is evident in prostate cancer progression (20), and the NF-κB gene signature has been found to be associated with PCa progression (21). KPNA4 is also required for the nuclear localization of Notch intracellular domain as well as the activation of Notch signaling (22). Activation of Notch signaling is associated with tumor recurrence (23) as well as tumor-initiating and growth (24). Therefore, KPNA4 is a potential target for cancer therapy at least through its regulation of NF-κB and Notch signaling. A link between the KPNA family and gastric angiogenesis has been demonstrated in a model of gastric mucosal endothelial cells in vitro, in which the expressions of KPNA2 and KPNA4 were associated with the production of VEGF (25). In addition, MiR-181s have been identified as negative regulators of mesenchymal transition in glioblastoma by targeting KPNA4 (26), and miR-181a regulates prostate cell migration and invasion (27), indicating a potential link between KPNA4 and cancer metastasis. However, the role of KPNA4 in PCa progression and its detailed mechanisms of action have yet to be investigated in depth.
Furthermore, the commitment of miRNA dysregulation to cell transformation has been widely reported (28). We recently confirmed a pro-apoptosis role of miR-708 in PCa via the engagement of Endoplasmic reticulum (ER) stress (29). MicroRNAs (miRNAs) are important gene regulators that are responsible for the transcription and stabilization of mRNA (30). MiR-708 is a tumor-suppressive miRNA that blocks tumor initiation and metastasis by targeting CD44 (31) and neuronatin (32). It is intriguing that KPNA4 is a predicted target of miR-708. Here, our data reveal a novel signaling pathway mediated by miR-708 down-regulation of KPNA4 and TNF-α, which lead to the inhibition of tumor cell invasion and “re-education” of TAMs. These findings provide a potential biomarker and therapeutic target to improve PCa diagnosis and treatment.
Results
KPNA4 expression correlates with human PCa progression positively
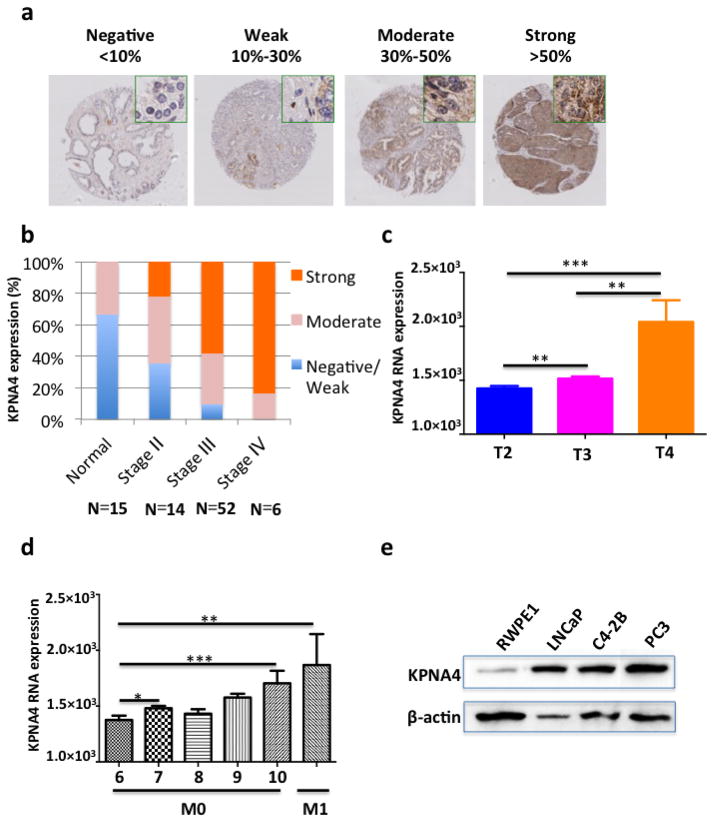
We observed a strong correlation between KPNA4 expression in PCa tissues and the clinical stages from early to late using immunohistochemistry-stained PCa tissue microarray slides (Figs. 1a,b), which shows that KPNA4 locates in both of the cytoplasm and nuclear of PCa cells. A The Cancer Genome Atlas dataset containing 498 PCa patient samples confirmed that KPNA is positively correlated with the clinical stages and Gleason scores of PCa, and the expression of KPNA4 is greater in metastatic site (M1) than the primary tumor (M0) (Figs. 1c,d). Furthermore, the KPNA4 protein levels are distinctly higher in human PCa cells, PC3, C4-2b and LNCaP cells than normal human prostate epithelial RWPE-1 cells (Fig. 1e), indicating a pro-malignance role of KPNA4.
Figure 1. KPNA4 expression is positively correlated with human prostate cancer progression.
(a). Immunohistochemical staining of KPNA4 in paraffin embedded human prostate cancer tissue microarray slides. (b) Statistics analysis of the relationship between KPNA4 expression and PCa stages as indicated. Analysis of the differential KPNA4 RNA expression level of PCa patient cases of the TCGA dataset that is classified by either (c) the pathologic T or (d) the Gleason score. *p<0.05, **p<0.01, ***p<0.001 (e) Endogenous KPNA4 protein expression level in normal and malignant prostate cell lines were determined by Western blotting, β-actin was used as loading control.
KPNA4 silencing suppresses PCa cell migration
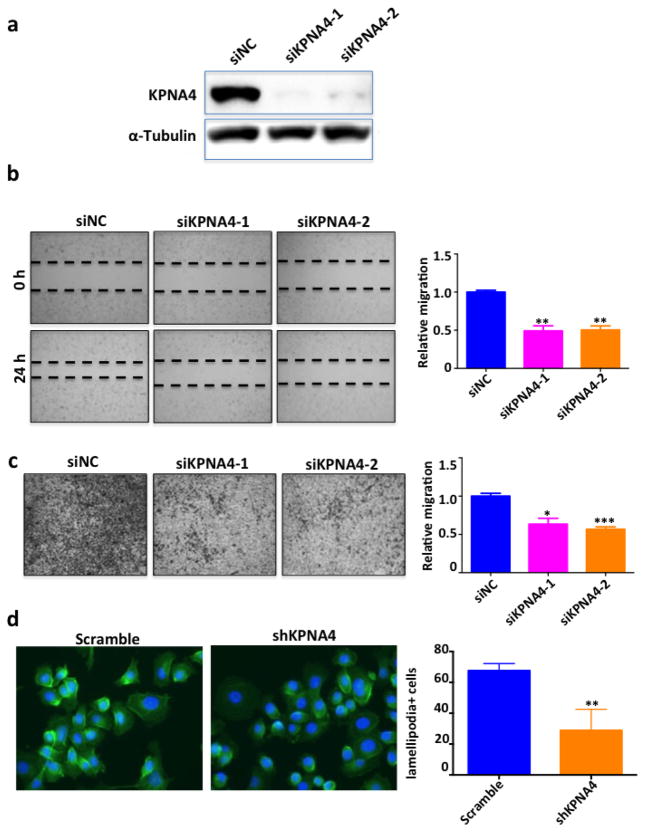
Subsequently, a transient transfection using siRNAs was performed to knockdown KPNA4 in PC3 cell line (Fig. 2a). It was found that cell mobility was significantly attenuated (Figs. 2b,c) after transfection. We verified similar effects of KPNA4 on migration in C4-2B and LNCaP cells (Supplementary Figs. 1a–d). Later, we established two stable PC3 cell lines with scrambled shRNA (scramble control) or shKPNA4 (PC3shKPNA4) using a retrovirus-mediated shRNA system. We measured lower KPNA4 levels in PC3shKPNA4 cells using Western blotting with both cytoplasm and nucleus extractions (Supplementary Fig. 2a). The reduction of KPNA4 in PC3shKPNA4 cells was accompanied by significant declines in cell migration and invasion (Supplementary Figs. 2b and c). The PC3shKPNA4 cells also exhibited decreases in lamellipodia-positive cells, indicating that less F-actin activation and cytoskeleton re-arrangement which is consistent with the impaired cell mobility (Fig. 2d). Importantly, KPNA4 regulated PCa cell migration without affecting proliferation (Supplementary Fig. 3).
Figure 2. Silencing of KPNA4 suppresses the migration ability of PCa cells.
(a) Knockdown efficiency of KPNA4 siRNAs were determined by Western blotting, α-tubulin was used as loading control. (b) Wound healing analysis and (c) Transwell assay was performed to determine the cell migration and invasion of PC3 cells which are transfected with either KPNA4 siRNA or control siRNA. *p<0.05, **p<0.01, ***p<0.001 (d) F-actin staining of PC3-shKPNA4 or scramble control, lamellipodia positive cells were counted as the invasive cells. *p<0.05.
miR-708 targets KPNA4 and inhibits PCa migration
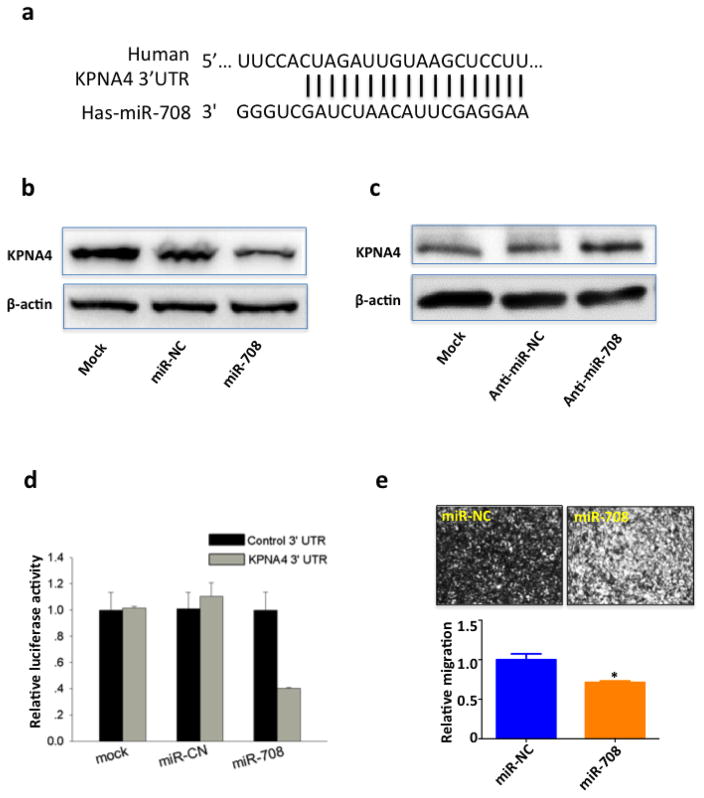
Interestingly, in contrast to significantly elevated KPNA4 expressions in protein levels in malignant PCa cells, KPNA4 mRNA levels remained similar in PCa and normal prostate epithelial cells (Supplementary Fig. 4a). This finding suggests that post-transcriptional regulation may be involved in the abnormal KPNA4 protein levels in PCa cells. miRNAs are important post-transcriptional regulators that are involved in mRNA stabilization and translation (30). Therefore, we adopted a bioinformatics approach and found an 18-nt perfect match between miR-708 and the 3′ untranslated region (UTR) of KPNA4 mRNA (Fig. 3a), supporting the assertion that KPNA4 functions as a target of miR-708. In fact, the miR-708 mimic indeed downregulated and the miR-708 inhibitor upregulated the expression of KPNA4 in the PC3 cell line (Figs. 3b,c). A dual-luciferase reporter further confirmed that miR-708 was bond to the 3′UTR of KPNA4 (Fig. 3d). More importantly, miR-708 level was strikingly lower in PCa cell lines than in the non-malignant control (Supplementary Fig. 4b), explaining the aberrantly high expression of KPNA4 in protein levels but not mRNA levels in PCa cells. Consistent with the efficacy of KPNA4 siRNA, transient transfection of the miR-708 mimic attenuated PC3 cell migration (Fig. 3e). Furthermore, miR-708 significantly inhibits cell migration in PC3scramble cells but not in PC3shKPNA4 cells (Supplementary Fig 5). These data suggest that KPNA4 is a key regulator mediating the inhibitory effect of miR-708 on cell invasion. In other words, the inhibitory effects of miR-708 on cell invasion functions through KPNA4. These data functionally support a regulation between miR-708 and KPNA4 in regulating cell migration. This result is consistent with recent reports on the anti-tumor role of miR-708 in PCa (29) and other types of cancer (32, 33).
Figure 3. miR-708 targets KPNA4 and inhibits PCa migration.
(a) Sequence alignment of miR-708 and KPNA4 3′UTR. KPNA4 expression in PC3 cells transfected with either (b) miR-708 mimic/negative control or (c) miR-708 inhibitor/negative control was determined by Western blotting, β-actin was used as loading control for total cell lysate. (d) HEK293T cells were co-transfected with miR-708 mimic or negative control mimic and dual-luciferase reporter plasmid inserted with KPNA4 3′UTR/control 3′UTR. Firefly luciferase was detected to determine the binding between miR-708 and KPNA4 3′UTR, renilla luciferase was used as internal control. (e) Transwell assay was performed to determine the cell invasion of PC3 cells that were transfected with either miR-708 mimic or control mimic for 48 hours.
KPNA4 knockdown attenuates primary tumor invasion and bone metastasis
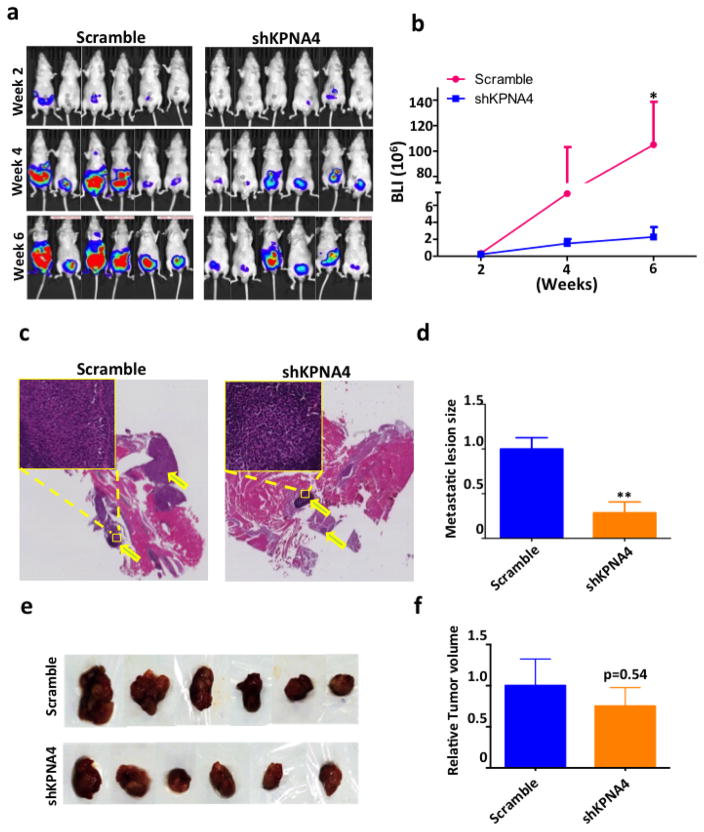
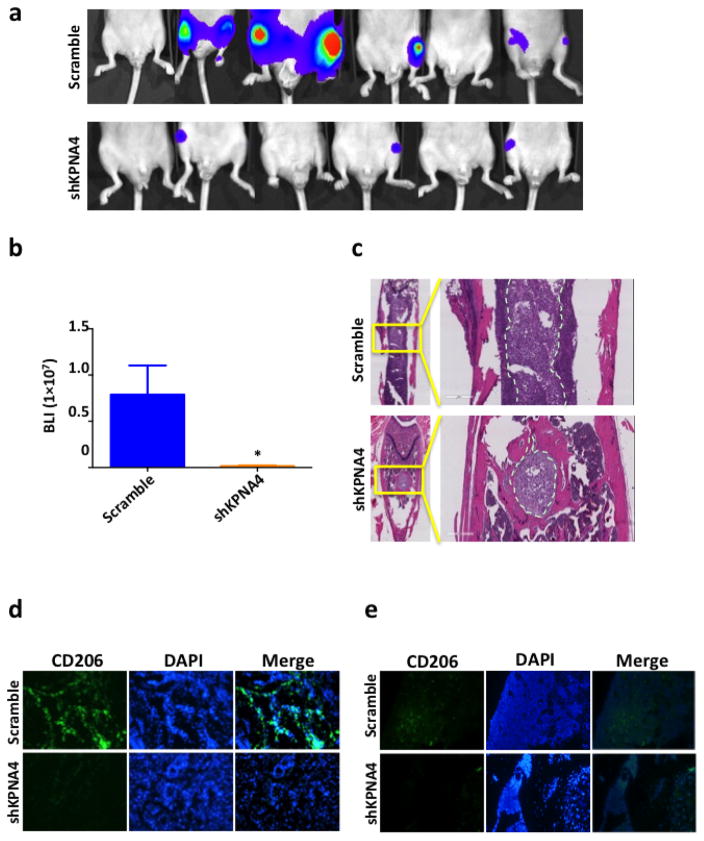
The biological significance of KPNA4 in PCa cells was determined in orthotopic and skeletal metastasis models in vivo. The PC3 cells labeled with luciferase allowed us to track tumor growth and metastasis in a real-time manner; we performed the luciferase activity assays to guarantee a robust correlation between cell number and bioluminescence signal in both PC3shKPNA4 and scramble control cells (Supplementary Fig. 6). Six weeks after orthotopic injection, the PC3shKPNA4 group exhibited considerably fewer proximal muscle invasions than the scramble control (Figs. 4a–d), which was consistent with the transwell assay in vitro. Primary tumor masses were similar in size between these two groups (Figs. 4e,f). Meanwhile, we evaluated skeletal metastasis in an intra-cardiac injection model in which shKPNA4 or scramble control PC3 cells were inoculated into the circulatory system through the left ventricle, and we detected metastatic lesions weekly using bioluminescent imaging. Both luciferase activity (Figs. 5a,b) and X-ray images (Supplementary Fig. 7) revealed that the rates of incidence and the sizes of the metastatic lesions were significantly reduced in the hind limbs of mice inoculated with PC3shKPNA4 cells. Hematoxylin and eosin staining also demonstrated a dramatic reduction in the metastatic lesion size in the PC3shKPNA4 group (Fig. 5c).
Figure 4. KPNA4 knockdown attenuates prostate tumor invasion.
(a) Luciferase-labeled PC3 sh-scramble or shKPNA4 cells (2×105) were orthotopically injected into prostate of nude mice. Bioluminescence was detected biweekly for 6 weeks to determine the primary tumor growth. (b) Tumor growth curve was generated according the luciferase activity. *p<0.05. (c) H&E staining of the invasive tumor tissue in the proximal muscle. (d) Size of invasive tumor tissue is calculated by image J. **p<0.01. (e) Samples of primary tumor tissues that were harvested in 6 weeks post the intra-prostatic injection. (f) Tumor volumes are measured immediately after harvest.
Figure 5. KPNA4 knockdown blocks bone metastasis of prostate cancer.
(a–b) Luciferase-labeled PC3 sh-scramble or shKPNA4 cells (1×106) were intracardiacly injected into the left ventrical of nude mice, bioluminescence imaging of the bone metastatic lesion was taken after 4 weeks post injection, *p<0.05. (c) H&E staining showing the metastatic lesions in the hind limbs. CD206 positive M2 TAMs infiltration in (d) primary tumor tissue or (e) bone marrow was determined by immunofluorenscence staining. DAPI was used as an indicator of nucleus.
Activation of NF-κB is associated with the metastatic phenotype and PCa progression to castration-resistant PCa (21, 34–37). NF-κB also acts as a key regulator in immune response through cytokine release (38). KPNA4 may alter cytokine expression via NF-κB activation to promote PCa progression. Indeed, PC3shKPNA4 cells exhibited reductions in TNF-α and -β in cytokine array analysis (Supplementary Fig. 8a) and was verified by Western blotting (Supplementary Fig. 8b). Meanwhile, NF-κB specific inhibitor (QNZ) abolished the regulatory effect of KPNA4 on TNF-α and –β expression (Supplementary Fig. 8c), indicating that KNPA4 need active NF-κB signaling to regulate TNF-α and –β expression. TNFs are the primary cytokines involved in the immune system(3), and their activation may facilitate tumor metastasis by promoting infiltration of TAMs (9). As expected, the infiltration of M2-polarized TAMs, CD206-positive macrophages, was significantly decreased in PC3shKPNA4 tumors compared with PC3-scrambled control tumors in the orthotopic model (Fig. 5d). Similarly, the skeletal metastatic lesions from the mice that received intra-cardiac injections of PC3shKPNA4 cells exhibited reduced M2 populations than mice receiving PC3-scrambled control cells (Fig. 5e). Hence, KPNA4 reduction in PCa cells decreases M2 TAMs in the tumor environment in vivo.
TNF-α and β mediates the KPNA4 induced PCa migration
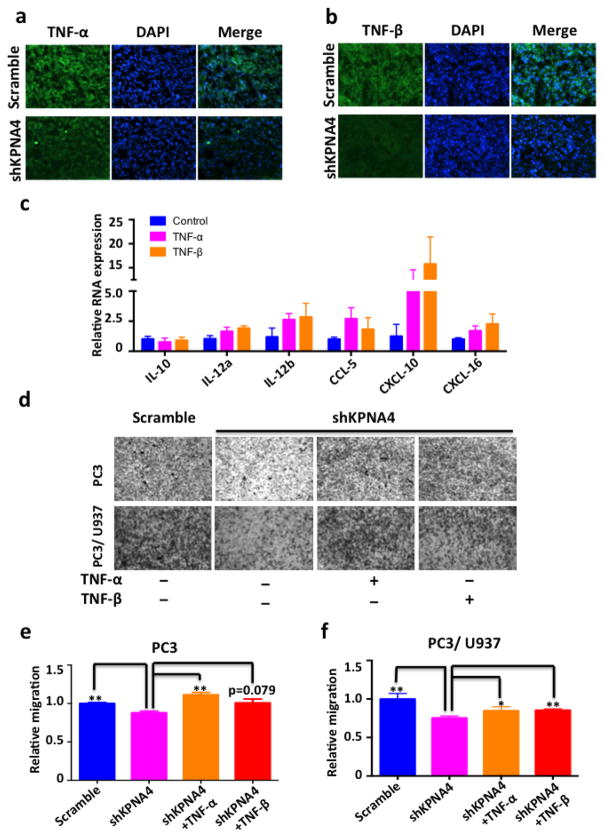
We next evaluated the expression of TNF-α and -β in primary tumor masses using immunofluorescence staining and confirmed their attenuation in orthotopic PC3shKPNA4 tumors (Figs. 6a,b). Meanwhile, we found that either recombinant TNF-α or TNF-β at low concentrations in murine primary macrophages drastically induce the expression of cytokines related to the M2 phenotype (Fig. 6c). In addition, TNF-α directly induces osteoclastogenesis via stimulation of macrophages in both RANKL-dependent and RANKL-independent manners (39, 40) and we confirmed that both TNF-α and -β were capable of promoting osteoclastogenesis in vitro (Supplementary Figs. 9a,b). Considering that the increases in osteoclastogenesis and osteoclast activity favors PCa skeletal metastasis (41, 42), we examined the capability of PC3shKPNA4 and PC3-scrambled control cells in inducing osteoclastogenesis and found that in comparison to PC3-scrambled control cells, conditioned medium from PC3shKPNA4 reduced osteoclast differentiation in RAW264.7 cells. More importantly, TNF-α rescued the reduction of osteoclast differentiation from PC3shKPNA4 cell-conditioned medium to the same level as the conditioned medium from PC3-scrambled control cells (Supplementary Figs. 9c, d). Skeletal metastasis derived from PC3shKPNA4 cells demonstrate reduced osteoclast formation in vivo (Supplementary Fig. 9e). Therefore, the dysregulation of KPNA4 in advanced PCa may not only directly stimulate cancer cell mobility but also play a multifaceted role in regulating cytokine crosstalk between cancer cells and cells residing in the tumor environment to affect PCa progression and skeletal metastasis.
Figure 6. TNF-α and β mediates the KPNA4 induced prostate cancer migration.
(a–b) TNF-α and TNF-β expression was evaluated in the primary tumor tissue by immunofluorenscence staining. (c) M2-phenotype associated cytokines of primary murine macrophages that were subjected to TNF-α or TNF-β stimulation (5ng/mL) was determined by real-time PCR. (d) Transwell assay of PC3-shKPNA4 or scramble control cells lines in the absence or presence of U937 cells to determine the cell invasion, PC3-shKPNA4 cells were stimulated with or without recombinant TNF-α or β cytokines (5ng/mL). (e–f) Invasive cells were quantitated by crystal violet staining assay. *p<0.05, **p<0.01.
Of note, cytokines had been proved as ideal targets for cancer (43). PCa cells may regulate the macrophages in tumor environment via pro-inflammatory factor expressions. At the very least, PC3shKPNA4 cells demonstrated an overall profound inhibition of pro-inflammatory cytokine expression in comparison with the scramble control cells (Supplementary Figs. 10a,b), which further supports the idea that KPNA4 may promote PCa progression via altering the TME. TNF promotes cell mobility via the activation of the NF-κB pathway in cancer cells (5). Therefore, TNF-α and β may directly enhance cell mobility in PCa cells. In fact, both TNF-α and β rescued the invasive ability of PC3shKPNA4 cells in the absence and presence of macrophages according to transwell assays (Figs. 6d–f).
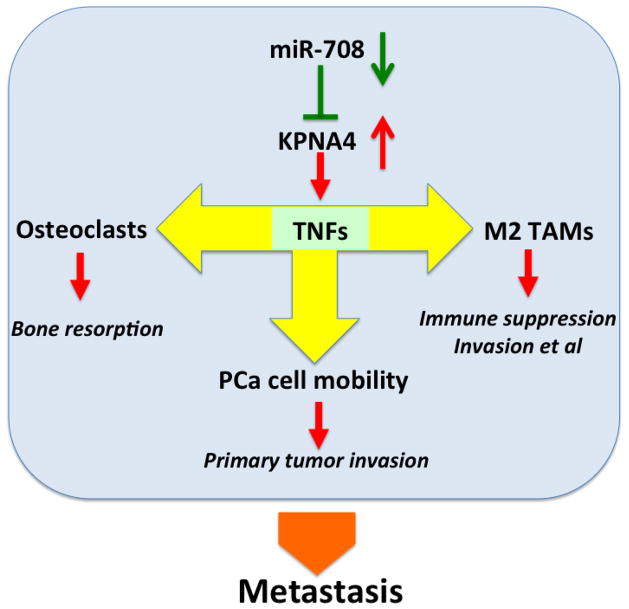
Collectively, we identified the novel role of KPNA4 in PCa metastasis. The dysregulation of the miR-708-KPNA4-TNF axis can facilitate the skeletal metastasis of PCa by promoting cancer cell mobility, polarizing TAMs and inducing osteoclastogenesis (Fig 7).
Figure 7. Schematic model of miR-708/KPNA4/TNF-α and β signaling pathway that regulates PCa microenvironment and bone metastasis.
In primary prostate tumor, miR-708 decrease in the cancer cells causes abnormal high expression of KPNA4, which will subsequently increase the TNF-α and β expression. Enrichment of TNF-α and β in tumor microenvironment can promote both of cancer cell mobility and M2 polarization of TAMs. On the other hand, increased TNF-α and β can enhance the osteoclastogenesis in bone environment, and eventually accelerate prostate cancer bone metastasis.
Discussion
Prostate cancer is the most frequent type of cancer based on the incidence of new cases for men in United States; it is also the second-leading cause of death among cancers (44). Specifically, metastatic castration-resistant PCa (mCRPC) is currently a lethal disease, and the majority of treatment approaches for this disease are only to extend patient survival by a few months (45). Here, we have demonstrated that inhibition of KPNA4 may effectively block the bone metastasis of PCa, including the mCRPC cell line, in a mouse model. Indeed, consistent with reports of the significance role of other KPNA members in multiple cancer types (46–49), KPNA4 is overexpressed in PCa cell lines. Importantly, our data show that its expression is strongly associated with pathological stages and Gleason scores in human PCa samples, indicating the pro-malignance role of this nuclear importer. In support of this observation, recent genomic profiling screening also demonstrated the importance of KPNA4 during the process of prostate carcinoma (50).
Our in vitro assay verified that transient knockdown of KPNA4 in PCa cell lines attenuates migration ability, which provides direct proof of KPNA4 promoting the progression of PCa. As a matter of fact, KPNA4 is a well-known importer for NF-κB nuclear localization (18, 51). The deletion of KPNA4 inhibits cell migration at least partially via the regulation of the NF-κB pathway. Further investigation revealed that only the protein level of KPNA4 was over-expressed in transformed prostate cell lines, but not the RNA level. This inconsistency between protein and RNA indicates that the dysregulation of KPNA4 in PCa cells is induced via a post-transcriptional pathway, which piques our interest in the miRNAs that act as important post-transcriptional regulators in various cell processes. Accumulating evidence has suggested that cancer patients have a unique signature in miRNA expression profile comparing to healthy controls (52). Some miRNAs such as miR-708 may function as tumor suppressors. miR-708 represses metastasis of breast (32), ovarian (33) and PCa (31) by targeting multiple genes mRNA. Our data clearly demonstrate that miR-708 is a negative regulator of KPNA4 expression in PCa. Furthermore, miR-708 was found to be de-regulated in PCa cell lines compared with normal prostate cell lines, which explains the abnormally high expression of KPNA4 protein (but not the RNA). In addition, KPNA4 exhibits a positive correlation with the stage of malignancy in human PCa samples on both the protein and RNA levels. This result implies that KPNA4 may be no longer controlled by miR-708 in malignant cells to increase the aggressiveness, a fact that is likely due to the extremely low expression level of miR-708 (i.e., too low to affect the expression of KPNA4 in malignant prostate cells).
Bone metastasis is the primary cause of mortality in patients with advanced PCa (53). Here, we used the aggressive PCa cell line, PC3, to evaluate how KPNA4 regulates PCa progression in vivo. Consistent with the in vitro assay, we found that KPNA4 knockdown can inhibit the invasion ability of primary tumors. Importantly, the metastatic model showed that the suppression of KPNA4 significantly reduced both the incidence and size of lesions in bone. We accordingly concluded that inhibition of KPNA4 represented an effective approach to preventing skeletal metastasis. As described earlier, KPNA4 is one of the primary importers that mediate the NF-κB nuclear translocation. KPNA4 deletion might lead to an impaired activation of NF-κB-regulated pathways, including the cytokines network. Using a cytokines array, we identified that TNF-α and -β were both downregulated in PC3shKPNA4 cells. Although TNF was initially recognized as a cytotoxic factor that can induce cell apoptosis via the caspase cascade, tumor cell-produced TNF has been shown to promote malignance in multiple cancer types (54–56). In this study, the induction of recombinant TNF-α and -β can rescue the KPNA4-deletion-induced impairment of PCa cell migration, which indicates that TNFs are the effectors of KPNA4 in PCa progression. On the other hand, TNF plays an important role in the interaction between tumor cells and macrophages, and the generation of the TAM phenotype (57, 58). In the PC3shKPNA4 primary tumor tissues, we detected decreased TNF-α and -β production and attenuated M2-phenotype macrophages. This finding further supports our assertion that TNF acts as a KPNA4 effector, which not only facilitates PCa cell mobility but also promotes the generation of TAMs. In fact, TNF-α and -β can solely induce the production of M2-phenotype-related cytokines in primary mouse monocytes. Therefore, by regulating TNF production, KPNA4 can modulate the TME via M2-polarized macrophages, which abet the tumor cells to bypass the surveillance of the immune system. Nevertheless, in the TME, KPNA4 may act as a switch of the cytokine crosstalk network and also as a regulator of TNFs. Tumor mass is characterized as a chronic inflammation environment (59) in which macrophages play a key role in the response to microenvironment signals (60). Here, using stimulations of the conditioned medium of PC3 cell lines, we explored the expression of inflammatory cytokines and receptors of raw264.7 macrophages using a polymerase chain reaction (PCR) array kit. Differentiated gene expression analysis (shKPNA4 VS scramble) revealed that the numbers of up-regulated and de-regulated genes were approximately the same; the de-regulated genes exhibited a much more significant fold change, which implied that PC3shKPNA4 induces a suppressed inflammatory environment. Cytokines were enriched in the TME. In particular, TNF-α plays a critical role in the inflammatory response (61). We believe that by regulating TNF expression, KPNA4 is also capable of enhancing the inflammatory response to promote PCa progression.
Bone-resorbing osteoclasts significantly contribute to skeletal metastasis by changing the dynamics of the osteoclast-osteoblast balance (62). Osteoclast-mediated bone resorption can facilitate PCa cell growth and survival via the release of factors such as TGF-β(63). In turn, PCa cells can also promote osteoclast development and function by inducing RANKL production (64). In this study, PC3shKPNA4-derived conditioned medium impaired osteoclast formation compared with the scramble control. Furthermore, TNF-α completely rescued the reduction of osteoclast differentiation in RAW264.7 cells treated with PC3shKPNA4 conditioned medium suggesting that TNF-α is the major mediator of KPNA4 in the regulation of osteoclastogenesis. These data support a molecular mechanism via which KPNA4-derived cytokines promote the formation of PCa metastatic lesions in the bone environment. Therefore, KPNA4 has a bi-faceted role in promoting PCa metastasis through regulating tumor cell mobility and TME. Hence, targeting KPNA4 could be an effective strategy to inhibit PCa metastasis via invoking anti-mobility in primary tumors and anti-osteoclastogenesis in the bone marrow environment.
Taken together, mechanistic evidence of KPNA4 in PCa metastasis warrants further validation in larger clinical samples; it is also necessary to evaluate the potential of KPNA4 as a biomarker for metastatic PCa diagnoses. From the perspective of treatment, the fact that KPNA4 deletion does not affect the proliferation of PCa cells suggests that a combined therapy of targeting KPNA4 paired with an anti-proliferation agent could be effective in inhibiting PCa progression and metastasis.
Materials and methods
1. Cell lines and RNAi
Human prostate cancer cell line PC3 was purchased from American Type Culture Collection (ATCC). Professor Laurie McCauley (University of Michigan) provided the human prostate cancer cell lines C4-2B, and Professor Peng Lee (New York University) provided the LNCaP, as well as human prostate epithelial cell line RWPE-1. All cell lines were authenticated and verified mycoplasma free. All PCa cell lines were cultured in RPMI 1640 medium (Invitrogen, Carlsbad, CA, USA) supplemented with 10% FBS, 100 U/mL penicillin and 100 μg/mL streptomycin. RWPE-1 was cultured in Keratinocyte Serum Free Medium (K-SFM) supplemented with 0.05 mg/mL bovine pituitary extract (BPE) and 5 ng/mL human recombinant epidermal growth factor (EGF). The cells were incubated in a 37°C, 5% (v/v) CO2 growth chamber.
To mediate transient RNA interference, KPNA4 siRNA (Invitrogen) were transfected by Lipofectamine-3000 (Invitrogen) at a final concentration of 50 nM for 48 hours. To establish the stable KPNA4 knockdown cell line, a retroviral system (Phoenix helper-free retrovirus producer lines, Nolan lab, Stanford University) was applied to package the virus following a standard procedure. We next cultured PC3 cells with the retrovirus for 24 hours, and the positively infected cells were selected using puromycin at 3 ng/mL for 72 hours.
2. miRNA and dual-luciferase assay
We seeded the cells in a 6-well plate 24 hours prior to the transfection. The miRNA-708 mimic or inhibitor as well as the negative controls (Invitrogen) were transfected with Lipofectamine-3000 at a final concentration of 50 nM for 48 hours. For dual-luciferase assay, the KPNA4 3′UTR reporter construct or control construct (GeneCopoeia, Rockville, MD, USA) were co-transfected with the miRNA-708 mimic or control mimic into the 293T cells which were seeded in 96-well plate (1×104 cells/well). After 48 hours of transfection, we measured firefly and renilla luciferase activities using the Dual Luciferase Reporter Assay System (Promega, Medison, MI, USA) according to the manufacturer’s protocol.
3. Western blotting and qPCR
Total protein was extracted using a RIPA lysis buffer (Thermo Scientific, Waltham, MA, USA), and nuclear protein was isolated using an EpiQuik Nuclear Extraction Kit (Epigentek, Farmingdale, NY, USA). Equal amounts of protein were denatured in a SDS sample buffer (2% SDS, 62.5 mM Tris-base [pH 6.8], 10% glycerol, 5% β-mercaptoethanol and 0.005% bromophenol blue) and loaded into a 10% SDS-PAGE gel (Invitrogen). The gel was preceded according to a standard PVDF membrane transfer and visualization protocol. The anti-KPNA4 and anti-CD206 primary antibody was purchased from Novus Biologicals (Bio-Techne, Minneapolis, MN, USA). The anti-β-actin, anti-Histone H4 primary antibodies, anti-mouse, anti-rabbit and anti-goat secondary antibodies were purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA), and the anti-TNF-α, anti-TNF-β and anti- α-tubulin primary antibodies were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA).
Total RNA was purified using Trizol reagent (Invitrogen). We synthesized cDNA using a Taqman reverse transcription kit (Applied Biosystems, Carlsbad, CA, USA). Taqman reverse transcription kit (Applied Biosystems) was applied for cDNA synthesis. SYBR green super mix (Applied Biosystems) was used for real-time PCR in a CFX384 Touch qPCR System (Bio-Rad, Hercules, CA, USA).
4. Mouse model
The male athymic nu/nu nude mice at the age of 6-week were purchased from Charles River Laboratories International, Inc. (Kinston, NY, USA). 6 mice were distributed to each group by random.
The PC3shKPNA4 and scramble control cells were harvested and hand re-suspended in HBSS solution at 1×107 cells/mL. For the metastatic model, we conducted an intra-cardiac injection by injecting 100 μL of cell suspension (1×106 cells) into the left ventricle of the mice (65). Bioluminescence images were obtained 24 hours after injection to ensure proper circulation distribution of cells, and images were retaken weekly for 4 weeks to track metastasis using an IVIS Lumina XR system (Caliper Life Sciences, Waltham, MA, USA). For the orthotopic model, the prostates of anesthetized nude mice were exposed via proper surgery, and 20 μL of cell suspension (2×105 cells) was orthotopically injected into the prostate (66). The primary tumor was allowed to grow for 6 weeks. Bioluminescence images were acquired weekly or bi-weekly for 4 or 6 weeks as indicated. After the mice were sacrificed, the hind limbs from the intra-cardiac-injected mice and the primary tumors from the intra-prostatic-injected mice were collected for X-ray, immunochemistry and immunofluorescence imaging. The investigators were not blinded to the group allocation during the experiment.
5. In vitro migration assay and cytoskeleton staining
A wound-healing assay was performed using 2.5×105 cells seeded into a 24-well plate. The cells were cultured with serum-reduced medium (1% FBS). After 24 hours, the wells were scraped with 200-μL tips. We recorded the wound widths immediately using a microscope and again after 48 hours for C4-2B and LNCaP, and 24 hours for PC3. Transwell assays were performed by seeding 5×104 cells into transwell chambers (Costar, Cambridge, MA, USA) with 5-μm-pore polycarbonate filters. The cells were cultured using serum-reduced medium (1% FBS) for 48 hours, and the cells attached to the chamber membrane were fixed in formalin and stained with 0.05% crystal violet (Invitrogen). Cells on the top of cylindrical chambers were removed using cotton swabs. Invasive cells were determined by detecting the O.D. 540 in a plate reader. For cytoskeleton staining, 1×104 cells were seeded in 96-well plate, fixed with 3.7% formaldehyde for 5 minutes, permeabilized with 0.1% TRITON-X-100 and then stained with a 50 μg/mL fluorescent phalloidin conjugate (Sigma, St. Louis, MO, USA) for 40 minutes at room temperature. Lamellipodia positive cells were imaged microscopically.
6. Immunochemistry and immunofluorescence
We purchased human PCa microarray slides from US Biomax Inc. (Rockville, MD, USA). Immunochemistry staining of the KPNA4 was performed in the histology core of the New York University Medical Center (tissue microarray data have been uploaded to figshare). For immunofluorescence staining, the primary tumor was embedded in O.C.T. compound (Sakura, Alphen aan den Rijn, The Netherlands), frozen and sectioned. Frozen sections were fixed with 10% formaldehyde for 15 minutes at room temperature and washed with PBS for three times. The fixed slides were blocked in blocking buffer (PBS/5% normal serum/0.3% Triton X-100) for 1 hour and incubated with anti-CD206, anti-TNF-α and anti-TNF-β primary antibodies at 1:100 dilutions at 4°C overnight. After being rinsed three times with PBS, the slides were incubated with the Alexa Fluor 488 conjugated secondary antibody (Cell Signaling Technology, Inc.) at room temperature for 2 hours. The specimens were rinsed with PBS after incubation and were then mounted for microscopic examination.
7. Statistical analysis
An analysis of the relationship between KPNA4 levels and clinical PCa stages or Gleason scores was carried out using the nonparametric Mann-Whitney-Wilcoxon (MWW) test. The results revealed statistically significant differences in KPNA4 expression in PCa with different Gleason scores (Kruskal-Wallis x2(4) = 19.98; p < 0.0005). We also examined the extent to which the TNM scores were associated with KPNA4 expression using the nonparametric MWW test. Our results revealed significant differences in KPNA4 expression as a function of TNM (Kruskal-Wallis x2(2)=18.85; p < 0.0001). We used GraphPad Prism (GraphPad Software, La Jolla, CA, USA) software or Microsoft Excel (Microsoft, Redmond, WA, USA) to statistically analyze the remaining experimental outcomes. We expressed the data as means ± SEMs of at least three independent determinations. Statistical significance was determined using the unpaired t-test. Results with p values less than 0.05(*), 0.01(**), or 0.001(***) are considered to be statistically significant.
8. Study approval
All of the animal experiments were performed according to the protocols approved by the Institutional Animal Care and Use Committee (IACUC) of New York University Medical Center following the guidelines for the proper care and use of animals for research purpose.
Supplementary Material
Acknowledgments
This work was supported by NIH grants R01CA180277 and R03CA172894 to Xin Li. We thank Dr. Deepak Saxena (New York University, New York, NY) for the proofreading.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Lu X, Mu E, Wei Y, Riethdorf S, Yang Q, Yuan M, et al. VCAM-1 promotes osteolytic expansion of indolent bone micrometastasis of breast cancer by engaging alpha4beta1-positive osteoclast progenitors. Cancer cell. 2011;20(6):701–14. doi: 10.1016/j.ccr.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zitvogel L, Kepp O, Galluzzi L, Kroemer G. Inflammasomes in carcinogenesis and anticancer immune responses. Nature immunology. 2012;13(4):343–51. doi: 10.1038/ni.2224. [DOI] [PubMed] [Google Scholar]
- 3.Brenner D, Blaser H, Mak TW. Regulation of tumour necrosis factor signalling: live or let die. Nature reviews Immunology. 2015;15(6):362–74. doi: 10.1038/nri3834. [DOI] [PubMed] [Google Scholar]
- 4.Hu X, Li B, Li X, Zhao X, Wan L, Lin G, et al. Transmembrane TNF-alpha promotes suppressive activities of myeloid-derived suppressor cells via TNFR2. Journal of immunology. 2014;192(3):1320–31. doi: 10.4049/jimmunol.1203195. [DOI] [PubMed] [Google Scholar]
- 5.Wu Y, Zhou BP. TNF-alpha/NF-kappaB/Snail pathway in cancer cell migration and invasion. British journal of cancer. 2010;102(4):639–44. doi: 10.1038/sj.bjc.6605530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bertrand F, Rochotte J, Colacios C, Montfort A, Tilkin-Mariame AF, Touriol C, et al. Blocking Tumor Necrosis Factor alpha enhances CD8 T cell-dependent immunity in experimental melanoma. Cancer research. 2015 doi: 10.1158/0008-5472.CAN-14-2524. [DOI] [PubMed] [Google Scholar]
- 7.Popivanova BK, Kitamura K, Wu Y, Kondo T, Kagaya T, Kaneko S, et al. Blocking TNF-alpha in mice reduces colorectal carcinogenesis associated with chronic colitis. The Journal of clinical investigation. 2008;118(2):560–70. doi: 10.1172/JCI32453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Finisguerra V, Di Conza G, Di Matteo M, Serneels J, Costa S, Thompson AA, et al. MET is required for the recruitment of anti-tumoural neutrophils. Nature. 2015;522(7556):349–53. doi: 10.1038/nature14407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hsu DS, Wang HJ, Tai SK, Chou CH, Hsieh CH, Chiu PH, et al. Acetylation of snail modulates the cytokinome of cancer cells to enhance the recruitment of macrophages. Cancer cell. 2014;26(4):534–48. doi: 10.1016/j.ccell.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 10.Ostuni R, Kratochvill F, Murray PJ, Natoli G. Macrophages and cancer: from mechanisms to therapeutic implications. Trends in immunology. 2015;36(4):229–39. doi: 10.1016/j.it.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 11.Luo Y, Zhou H, Krueger J, Kaplan C, Lee SH, Dolman C, et al. Targeting tumor-associated macrophages as a novel strategy against breast cancer. The Journal of clinical investigation. 2006;116(8):2132–41. doi: 10.1172/JCI27648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adams DL, Martin SS, Alpaugh RK, Charpentier M, Tsai S, Bergan RC, et al. Circulating giant macrophages as a potential biomarker of solid tumors. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(9):3514–9. doi: 10.1073/pnas.1320198111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fang LY, Izumi K, Lai KP, Liang L, Li L, Miyamoto H, et al. Infiltrating macrophages promote prostate tumorigenesis via modulating androgen receptor-mediated CCL4-STAT3 signaling. Cancer research. 2013;73(18):5633–46. doi: 10.1158/0008-5472.CAN-12-3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nature immunology. 2010;11(10):889–96. doi: 10.1038/ni.1937. [DOI] [PubMed] [Google Scholar]
- 15.Chen PC, Cheng HC, Wang J, Wang SW, Tai HC, Lin CW, et al. Prostate cancer-derived CCN3 induces M2 macrophage infiltration and contributes to angiogenesis in prostate cancer microenvironment. Oncotarget. 2014;5(6):1595–608. doi: 10.18632/oncotarget.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang X, Tian W, Cai X, Wang X, Dang W, Tang H, et al. Hydrazinocurcumin Encapsuled nanoparticles “re-educate” tumor-associated macrophages and exhibit anti-tumor effects on breast cancer following STAT3 suppression. PloS one. 2013;8(6):e65896. doi: 10.1371/journal.pone.0065896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang J, Zhang Z, Chen C, Liu Y, Si Q, Chuang TH, et al. MicroRNA-19a-3p inhibits breast cancer progression and metastasis by inducing macrophage polarization through downregulated expression of Fra-1 proto-oncogene. Oncogene. 2014;33(23):3014–23. doi: 10.1038/onc.2013.258. [DOI] [PubMed] [Google Scholar]
- 18.Fagerlund R, Kinnunen L, Kohler M, Julkunen I, Melen K. NF-{kappa}B is transported into the nucleus by importin {alpha}3 and importin {alpha}4. The Journal of biological chemistry. 2005;280(16):15942–51. doi: 10.1074/jbc.M500814200. [DOI] [PubMed] [Google Scholar]
- 19.Agrawal T, Gupta GK, Agrawal DK. Calcitriol decreases expression of importin alpha3 and attenuates RelA translocation in human bronchial smooth muscle cells. Journal of clinical immunology. 2012;32(5):1093–103. doi: 10.1007/s10875-012-9696-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mak P, Li J, Samanta S, Mercurio AM. ERbeta regulation of NF-kB activation in prostate cancer is mediated by HIF-1. Oncotarget. 2015;6(37):40247–54. doi: 10.18632/oncotarget.5377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jin R, Yi Y, Yull FE, Blackwell TS, Clark PE, Koyama T, et al. NF-kappaB gene signature predicts prostate cancer progression. Cancer research. 2014;74(10):2763–72. doi: 10.1158/0008-5472.CAN-13-2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sachan N, Mishra AK, Mutsuddi M, Mukherjee A. The Drosophila importin-alpha3 is required for nuclear import of notch in vivo and it displays synergistic effects with notch receptor on cell proliferation. PloS one. 2013;8(7):e68247. doi: 10.1371/journal.pone.0068247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abravanel DL, Belka GK, Pan TC, Pant DK, Collins MA, Sterner CJ, et al. Notch promotes recurrence of dormant tumor cells following HER2/neu-targeted therapy. The Journal of clinical investigation. 2015;125(6):2484–96. doi: 10.1172/JCI74883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yen WC, Fischer MM, Axelrod F, Bond C, Cain J, Cancilla B, et al. Targeting notch signaling with a notch2/notch3 antagonist (tarextumab) inhibits tumor growth and decreases tumor-initiating cell frequency. Clinical cancer research: an official journal of the American Association for Cancer Research. 2015;21(9):2084–95. doi: 10.1158/1078-0432.CCR-14-2808. [DOI] [PubMed] [Google Scholar]
- 25.Ahluwalia A, Jones MK, Tarnawski AS. Key role of endothelial importin-alpha in VEGF expression and gastric angiogenesis: novel insight into aging gastropathy. American journal of physiology Gastrointestinal and liver physiology. 2014;306(4):G338–45. doi: 10.1152/ajpgi.00382.2013. [DOI] [PubMed] [Google Scholar]
- 26.Wang H, Tao T, Yan W, Feng Y, Wang Y, Cai J, et al. Upregulation of miR-181s reverses mesenchymal transition by targeting KPNA4 in glioblastoma. Scientific reports. 2015;5:13072. doi: 10.1038/srep13072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liang J, Li X, Li Y, Wei J, Daniels G, Zhong X, et al. LEF1 targeting EMT in prostate cancer invasion is mediated by miR-181a. American journal of cancer research. 2015;5(3):1124–32. [PMC free article] [PubMed] [Google Scholar]
- 28.Lin S, Gregory RI. MicroRNA biogenesis pathways in cancer. Nature reviews Cancer. 2015;15(6):321–33. doi: 10.1038/nrc3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang J, Wei J, Wu Y, Wang Z, Guo Y, Lee P, et al. Metformin induces ER stress-dependent apoptosis through miR-708–5p/NNAT pathway in prostate cancer. Oncogenesis. 2015;4:e158. doi: 10.1038/oncsis.2015.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kasinski AL, Slack FJ. Epigenetics and genetics. MicroRNAs en route to the clinic: progress in validating and targeting microRNAs for cancer therapy. Nature reviews Cancer. 2011;11(12):849–64. doi: 10.1038/nrc3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saini S, Majid S, Shahryari V, Arora S, Yamamura S, Chang I, et al. miRNA-708 control of CD44(+) prostate cancer-initiating cells. Cancer research. 2012;72(14):3618–30. doi: 10.1158/0008-5472.CAN-12-0540. [DOI] [PubMed] [Google Scholar]
- 32.Ryu S, McDonnell K, Choi H, Gao D, Hahn M, Joshi N, et al. Suppression of miRNA-708 by polycomb group promotes metastases by calcium-induced cell migration. Cancer cell. 2013;23(1):63–76. doi: 10.1016/j.ccr.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 33.Lin KT, Yeh YM, Chuang CM, Yang SY, Chang JW, Sun SP, et al. Glucocorticoids mediate induction of microRNA-708 to suppress ovarian cancer metastasis through targeting Rap1B. Nat Commun. 2015;6:5917. doi: 10.1038/ncomms6917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jin R, Yamashita H, Yu X, Wang J, Franco OE, Wang Y, et al. Inhibition of NF-kappa B signaling restores responsiveness of castrate-resistant prostate cancer cells to anti-androgen treatment by decreasing androgen receptor-variant expression. Oncogene. 2015;34(28):3700–10. doi: 10.1038/onc.2014.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lamb LE, Zarif JC, Miranti CK. The androgen receptor induces integrin alpha6beta1 to promote prostate tumor cell survival via NF-kappaB and Bcl-xL Independently of PI3K signaling. Cancer research. 2011;71(7):2739–49. doi: 10.1158/0008-5472.CAN-10-2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nadiminty N, Lou W, Sun M, Chen J, Yue J, Kung HJ, et al. Aberrant activation of the androgen receptor by NF-kappaB2/p52 in prostate cancer cells. Cancer research. 2010;70(8):3309–19. doi: 10.1158/0008-5472.CAN-09-3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ammirante M, Luo JL, Grivennikov S, Nedospasov S, Karin M. B-cell-derived lymphotoxin promotes castration-resistant prostate cancer. Nature. 2010;464(7286):302–5. doi: 10.1038/nature08782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gerondakis S, Fulford TS, Messina NL, Grumont RJ. NF-kappaB control of T cell development. Nature immunology. 2014;15(1):15–25. doi: 10.1038/ni.2785. [DOI] [PubMed] [Google Scholar]
- 39.Lam J, Takeshita S, Barker JE, Kanagawa O, Ross FP, Teitelbaum SL. TNF-alpha induces osteoclastogenesis by direct stimulation of macrophages exposed to permissive levels of RANK ligand. The Journal of clinical investigation. 2000;106(12):1481–8. doi: 10.1172/JCI11176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kobayashi K, Takahashi N, Jimi E, Udagawa N, Takami M, Kotake S, et al. Tumor necrosis factor alpha stimulates osteoclast differentiation by a mechanism independent of the ODF/RANKL-RANK interaction. The Journal of experimental medicine. 2000;191(2):275–86. doi: 10.1084/jem.191.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li X, Loberg R, Liao J, Ying C, Snyder LA, Pienta KJ, et al. A destructive cascade mediated by CCL2 facilitates prostate cancer growth in bone. Cancer research. 2009;69(4):1685–92. doi: 10.1158/0008-5472.CAN-08-2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schneider A, Kalikin LM, Mattos AC, Keller ET, Allen MJ, Pienta KJ, et al. Bone turnover mediates preferential localization of prostate cancer in the skeleton. Endocrinology. 2005;146(4):1727–36. doi: 10.1210/en.2004-1211. [DOI] [PubMed] [Google Scholar]
- 43.Li X, Yao W, Yuan Y, Chen P, Li B, Li J, et al. Targeting of tumour-infiltrating macrophages via CCL2/CCR2 signalling as a therapeutic strategy against hepatocellular carcinoma. Gut. 2015 doi: 10.1136/gutjnl-2015-310514. [DOI] [PubMed] [Google Scholar]
- 44.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA: a cancer journal for clinicians. 2016 doi: 10.3322/caac.21332. [DOI] [PubMed]
- 45.Feng FY, Kothari V. Driven to metastasize: Kinases as potential therapeutic targets in prostate cancer. Proceedings of the National Academy of Sciences of the United States of America. 2016 doi: 10.1073/pnas.1522938113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mortezavi A, Hermanns T, Seifert HH, Baumgartner MK, Provenzano M, Sulser T, et al. KPNA2 expression is an independent adverse predictor of biochemical recurrence after radical prostatectomy. Clinical cancer research: an official journal of the American Association for Cancer Research. 2011;17(5):1111–21. doi: 10.1158/1078-0432.CCR-10-0081. [DOI] [PubMed] [Google Scholar]
- 47.Ikenberg K, Valtcheva N, Brandt S, Zhong Q, Wong CE, Noske A, et al. KPNA2 is overexpressed in human and mouse endometrial cancers and promotes cellular proliferation. The Journal of pathology. 2014;234(2):239–52. doi: 10.1002/path.4390. [DOI] [PubMed] [Google Scholar]
- 48.Rachidi SM, Qin T, Sun S, Zheng WJ, Li Z. Molecular profiling of multiple human cancers defines an inflammatory cancer-associated molecular pattern and uncovers KPNA2 as a uniform poor prognostic cancer marker. PloS one. 2013;8(3):e57911. doi: 10.1371/journal.pone.0057911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sakai M, Sohda M, Miyazaki T, Suzuki S, Sano A, Tanaka N, et al. Significance of karyopherin-{alpha} 2 (KPNA2) expression in esophageal squamous cell carcinoma. Anticancer research. 2010;30(3):851–6. [PubMed] [Google Scholar]
- 50.Xu A, Sun S. Genomic profiling screens small molecules of metastatic prostate carcinoma. Oncology letters. 2015;10(3):1402–8. doi: 10.3892/ol.2015.3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Theiss AL, Jenkins AK, Okoro NI, Klapproth JM, Merlin D, Sitaraman SV. Prohibitin inhibits tumor necrosis factor alpha-induced nuclear factor-kappa B nuclear translocation via the novel mechanism of decreasing importin alpha3 expression. Molecular biology of the cell. 2009;20(20):4412–23. doi: 10.1091/mbc.E09-05-0361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zadran S, Remacle F, Levine RD. miRNA and mRNA cancer signatures determined by analysis of expression levels in large cohorts of patients. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(47):19160–5. doi: 10.1073/pnas.1316991110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fournier PG, Juarez P, Jiang G, Clines GA, Niewolna M, Kim HS, et al. The TGF-beta Signaling Regulator PMEPA1 Suppresses Prostate Cancer Metastases to Bone. Cancer cell. 2015;27(6):809–21. doi: 10.1016/j.ccell.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kulbe H, Thompson R, Wilson JL, Robinson S, Hagemann T, Fatah R, et al. The inflammatory cytokine tumor necrosis factor-alpha generates an autocrine tumor-promoting network in epithelial ovarian cancer cells. Cancer research. 2007;67(2):585–92. doi: 10.1158/0008-5472.CAN-06-2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stathopoulos GT, Kollintza A, Moschos C, Psallidas I, Sherrill TP, Pitsinos EN, et al. Tumor necrosis factor-alpha promotes malignant pleural effusion. Cancer research. 2007;67(20):9825–34. doi: 10.1158/0008-5472.CAN-07-1064. [DOI] [PubMed] [Google Scholar]
- 56.Zins K, Abraham D, Sioud M, Aharinejad S. Colon cancer cell-derived tumor necrosis factor-alpha mediates the tumor growth-promoting response in macrophages by up-regulating the colony-stimulating factor-1 pathway. Cancer research. 2007;67(3):1038–45. doi: 10.1158/0008-5472.CAN-06-2295. [DOI] [PubMed] [Google Scholar]
- 57.Hagemann T, Robinson SC, Schulz M, Trumper L, Balkwill FR, Binder C. Enhanced invasiveness of breast cancer cell lines upon co-cultivation with macrophages is due to TNF-alpha dependent up-regulation of matrix metalloproteases. Carcinogenesis. 2004;25(8):1543–9. doi: 10.1093/carcin/bgh146. [DOI] [PubMed] [Google Scholar]
- 58.Hagemann T, Wilson J, Burke F, Kulbe H, Li NF, Pluddemann A, et al. Ovarian cancer cells polarize macrophages toward a tumor-associated phenotype. Journal of immunology. 2006;176(8):5023–32. doi: 10.4049/jimmunol.176.8.5023. [DOI] [PubMed] [Google Scholar]
- 59.Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer cell. 2005;7(3):211–7. doi: 10.1016/j.ccr.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 60.Gordon S. Alternative activation of macrophages. Nature reviews Immunology. 2003;3(1):23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 61.Balkwill F. Tumor necrosis factor or tumor promoting factor? Cytokine & growth factor reviews. 2002;13(2):135–41. doi: 10.1016/s1359-6101(01)00020-x. [DOI] [PubMed] [Google Scholar]
- 62.Krzeszinski JY, Wei W, Huynh H, Jin Z, Wang X, Chang TC, et al. miR-34a blocks osteoporosis and bone metastasis by inhibiting osteoclastogenesis and Tgif2. Nature. 2014;512(7515):431–5. doi: 10.1038/nature13375. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 63.Gartrell BA, Saad F. Managing bone metastases and reducing skeletal related events in prostate cancer. Nature reviews Clinical oncology. 2014;11(6):335–45. doi: 10.1038/nrclinonc.2014.70. [DOI] [PubMed] [Google Scholar]
- 64.Guise TA. Molecular mechanisms of osteolytic bone metastases. Cancer. 2000;88(12 Suppl):2892–8. doi: 10.1002/1097-0142(20000615)88:12+<2892::aid-cncr2>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 65.Jin JK, Tien PC, Cheng CJ, Song JH, Huang C, Lin SH, et al. Talin1 phosphorylation activates beta1 integrins: a novel mechanism to promote prostate cancer bone metastasis. Oncogene. 2015;34(14):1811–21. doi: 10.1038/onc.2014.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee YC, Jin JK, Cheng CJ, Huang CF, Song JH, Huang M, et al. Targeting constitutively activated beta1 integrins inhibits prostate cancer metastasis. Molecular cancer research: MCR. 2013;11(4):405–17. doi: 10.1158/1541-7786.MCR-12-0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.