Abstract
A growing body of research has indicated that acute stress can critically impact memory. However, there are a number of inconsistencies in the literature, and important questions remain regarding the conditions under which stress effects emerge as well as basic questions about how stress impacts different phases of memory. In this meta-analysis, we examined 113 independent studies in humans with 6,216 participants that explored effects of stress on encoding, post-encoding, retrieval, or post-reactivation phases of episodic memory. The results indicated that when stress occurred prior to or during encoding it impaired memory, unless both the delay between the stressor and encoding was very short and the study materials were directly related to the stressor, in which case stress improved encoding. In contrast, post-encoding stress improved memory unless the stressor occurred in a different physical context than the study materials. When stress occurred just prior to or during retrieval, memory was impaired, and these effects were larger for emotionally valenced materials than neutral materials. Although stress consistently increased cortisol, the magnitude of the cortisol response was not related to the effects of stress on memory. Nonetheless, the effects of stress on memory were generally reduced in magnitude for women taking hormonal contraceptives. These analyses indicate that stress disrupts some episodic memory processes while enhancing others, and that the effects of stress are modulated by a number of critical factors. These results provide important constraints on current theories of stress and memory, and point to new questions for future research.
Stress can have pronounced effects on our ability to remember past events. For example, as most students are aware, the acute stress brought about by taking an exam can often make it difficult to retrieve information that might otherwise be available. Indeed, a number of laboratory studies have now verified that acute social and/or physical stress can significantly impair memory retrieval (e.g., Kuhlmann, Piel, & Wolf, 2005; Roozendaal, 2002; Schwabe et al., 2009; Schwabe, Wolf, & Oitzl, 2010; Smeets, Otgaar, Candel, & Wolf, 2008). However, there is growing evidence that when acute stress (hereafter used interchangeably with stress, for brevity) is encountered shortly after information is learned (i.e., post-encoding stress), stress can have beneficial effects on memory and can effectively rescue memories from the effects of forgetting (e.g., Andreano & Cahill, 2006; Beckner, Tucker, Delville, & Mohr, 2006; Cahill, Gorski, & Le, 2003; Roozendaal, 2002; Smeets et al., 2008). Because we rely on memory in almost every aspect of daily life—such as in recognizing our friends and colleagues, remembering our grocery lists, and remembering to take daily medications—and many people experience stressful situations frequently, understanding how and when stress enhances or impairs memory has important implications for all of us.
The scientific literature on acute stress and memory has grown rapidly over the past 10 years, but there are a number of inconsistencies in the emerging literature (for earlier reviews, see for example de Kloet, Oitzl, & Joëls, 1999; Gagnon & Wagner, 2016; Joëls, Pu, Wiegert, Oitzl, & Krugers, 2006; Schwabe, Joëls, Roozendaal, Wolf, & Oitzl, 2012). For example, stress effects have been found to preferentially impact emotional memories in some studies (Cahill et al., 2003), but to have similar or even more pronounced effects on neutral memories in others (McCullough & Yonelinas, 2013). In addition, how stress impacts the process of encoding information into memory is particularly controversial, as there are some studies showing that stress impairs encoding (e.g., Maheu, Collicutt, Kornik, Moszkowski, & Lupien, 2005; Payne et al., 2007), but others showing that stress enhances encoding (e.g., Payne et al., 2007; Smeets, Giesbrecht, Jelicic, & Merckelbach, 2007). Because the experimental methods often differ considerably across these studies, it has been difficult to determine the factors that are responsible for the reported discrepancies. However, the large number of studies that have now been published affords us the opportunity to use meta-analytic methods to determine the conditions under which stress improves or impairs memory and to identify the factors that moderate those effects.
How Does Stress Influence the Neural Substrates of Memory?
Stress influences multiple neural pathways and brain systems that are critical for episodic memory. For example, stress first exerts rapid effects in the brain by producing a surge in both dopaminergic and noradrenergic activity within the prefrontal cortex (Arnsten, 2009; Shansky & Lipps, 2013). Stress then acts through the sympathetic-adrenal-medullary (SAM) axis to upregulate peripheral adrenaline and noradrenaline (Allen, Kennedy, Cryan, Dinan, & Clarke, 2014; Joëls, Fernandez, & Roozendaal, 2011; Schwabe et al., 2012; Thoma, Kirschbaum, Wolf, & Rohleder, 2012). These hormones then stimulate afferents of the vagus nerve and ultimately influence the hippocampus, amygdala, and prefrontal cortex, among other regions (de Quervain, Aerni, & Roozendaal, 2007; Roozendaal, Okuda, de Quervain, & McGaugh, 2006; Schwabe et al., 2012; Schwabe & Wolf, 2011). On a slightly longer timescale of about 15 to 60 minutes, stress acts through the hypothalamic-pituitary-adrenal (HPA) axis, which upregulates production of glucocorticoids (cortisol in humans), among other hormones (Allen et al., 2014; Dickerson & Kemeny, 2004; Joëls et al., 2011; Kudielka, Schommer, Hellhammer, & Kirschbaum, 2004; Schwabe et al., 2012). After upregulation from the adrenal glands, cortisol makes its way through circulation, freely crosses the blood brain barrier, and can directly influence neural activity in the hippocampus, amygdala, and prefrontal cortex by binding to receptors located on neurons in those regions (Butts, Weinberg, Young, & Phillips, 2011; de Quervain, Roozendaal, & McGaugh, 1998; de Quervain, Roozendaal, Nitsch, McGaugh, & Hock, 2000; Patel, Katz, Karssen, & Lyons, 2008; Roozendaal, 2002; Yuen et al., 2009). On an even longer timescale, stress also acts to upregulate immune system production of inflammatory proteins (known as proinflammatory cytokines) through noradrenergic stimulation of immune system cells (Bierhaus et al., 2003; Slavich & Irwin, 2014). Proinflammatory cytokines can directly influence neural activity by binding to their receptors on neurons, or they can indirectly influence neural activity through stimulation of the vagus nerve (Dantzer, O’Connor, Freund, Johnson, & Kelley, 2008; Raison, Capuron, & Miller, 2006). In either case, proinflammatory cytokines alter activity in the hippocampus, amygdala, and prefrontal cortex, among other regions (Audet, Jacobson-Pick, Wann, & Anisman, 2011; Harrison, Cercignani, Voon, & Critchley, 2015; T. K. Inagaki, Muscatell, Irwin, Cole, & Eisenberger, 2012; Zalcman et al., 1994).
These effects of stress on the brain do not occur in isolation. For example, the HPA axis and immune system regulate each other with feedback loops (Sapolsky, Rivier, Yamamoto, Plotsky, & Vale, 1987; Silverman & Sternberg, 2012). More importantly for this paper, glucocorticoids and noradrenaline critically interact to modulate activity within brain regions supporting memory, such as the hippocampus (de Quervain et al., 2007; Joëls et al., 2011, 2006; Roozendaal, Okuda, de Quervain, et al., 2006; Schwabe et al., 2012). Blocking noradrenergic activity, for example, blocks the effects of glucocorticoids on memory (de Quervain et al., 2007; Joëls et al., 2011; Schwabe et al., 2012).
Stress also exerts effects at the synaptic and molecular levels in the brain (Conrad, 2010; Conrad, Lupien, & McEwen, 1999; Diamond, Campbell, Park, Halonen, & Zoladz, 2007; Joëls et al., 2011, 2006; Roozendaal, 2002; Sandi & Pinelo-Nava, 2007). Stress prior to learning, for example, impairs long-term potentiation (LTP; thought to be critical for memory) in the hippocampus and elsewhere (Diamond et al., 2006; Maroun & Richter-Levin, 2003). In contrast, stress during learning enhances LTP (Conboy & Sandi, 2010). This time-dependent effect on hippocampal LTP is due at least in part to a biphasic effect of activity in the basolateral amygdala—a highly stress-responsive brain region (Schwabe et al., 2012)—on hippocampal plasticity (Akirav & Richter-Levin, 1999, 2002; Diamond et al., 2007). Importantly, this biphasic effect is mediated at least in part by noradrenaline and glucocorticoids (Akirav & Richter-Levin, 1999, 2002). That is, there are well-described pathways linking the biological effects of stress to synaptic and molecular changes in neurons related to memory.
Thus, stress impacts brain regions in several neural systems that are thought to be involved in memory (see below), including the amygdala, the hippocampus, and the prefrontal cortex, through a wide variety of routes. Moreover, some of these effects can be expected to occur within seconds after the stressor occurs, whereas other effects are expected to unfold over longer periods, up to hours after the stressor has passed.
Which Memory Processes Are Influenced by Stress?
In the current paper we focus on examining the effects of stress on episodic memory, which is the ability to remember past events as measured on tests such as recognition and free recall (for the effects of stress on other forms of human memory such as implicit memory and working memory, see for example (Luethi, Meier, & Sandi, 2008; Shields, Sazma, & Yonelinas, 2016)). In the discussion we will consider how these results in humans compare to results obtained in various animal learning tasks. In studies of episodic memory, stress can have different effects on memory depending on the phase of memory processing that the stressor impacts. For example, stress can impact the encoding of the initial event, the retention of the stored information (i.e., the post-encoding period), and the retrieval of previously encoded information. In addition, recent studies have suggested that stress may impact memory if it occurs when memories are re-activated at some time between the initial encoding and the final retrieval phase. Note that although the different phases of memory may sometimes engage overlapping cognitive processes, these different phases need not all be influenced by stress in similar ways, and so it is important to separately examine studies focused on these different memory phases.
Episodic memory is critically dependent on a variety of separable memory processes supported by a network of brain regions, many of which are influenced by stress. Most critical is the hippocampus, which is essential for “binding” or associating the different features that make up an event (e.g., Eichenbaum, Otto, & Cohen, 1992; Scoville & Milner, 1957; Yonelinas, 2013; Zola-Morgan, Squire, & Amaral, 1986). The hippocampus is generally thought to support memory encoding by binding together object information it receives from the ventral “what” stream with the contextual information that it receives from the dorsal “where” stream, as well as supporting the subsequent retrieval of those associations in tests of recognition and recall (Davachi, 2006; Diana, Yonelinas, & Ranganath, 2007; H Eichenbaum, Yonelinas, & Ranganath, 2007; Mayes, Montaldi, & Migo, 2007; Norman & O’Reilly, 2003). In addition, the amygdala—which plays a key role in processing emotion—supports episodic memory for emotional events, and is either thought to form bindings between objects and emotions (Yonelinas & Ritchey, 2015) or to modulate the hippocampal binding of that information (McGaugh, 2004). Relatedly, the prefrontal cortex, in conjunction with other brain regions, is thought to be involved in supporting executive control processes that are important for encoding and retrieval. For example, memory encoding benefits from semantic elaboration as well as selective attention during encoding, both of which depend on the prefrontal cortex (Blumenfeld & Ranganath, 2007; Fletcher, Shallice, Frith, Frackowiak, & Dolan, 1998; Gershberg & Shimamura, 1995; Iidaka, Anderson, Kapur, Cabeza, & Craik, 2000; Mangels, 1997; Parkin, 1997). In addition, memory retrieval benefits from prefrontal-dependent executive control processes that support organized memory search as well as memory monitoring (Dobbins, Foley, Schacter, & Wagner, 2002; Gershberg & Shimamura, 1995; Henson, Rugg, Shallice, & Dolan, 2000; Levy & Anderson, 2002; Rocchetta & Milner, 1993). Thus, stress can influence episodic memory through its impact on the neural bases of binding, emotion, and/or executive function.
Current Theories of Stress and Memory
Several theories have been proposed to account for the effects of stress on memory including “consolidation”, “dual-mode”, “executive control” and “reconsolidation” accounts. One broad class of theories that has been useful in understanding the effects of stress on memory are consolidation theories (Cahill & McGaugh, 1998; Joëls et al., 2011, 2006, McGaugh, 2000, 2004, 2015). The main idea behind these theories is that recently encoded events are likely to be forgotten unless there is an active process of consolidation whereby the initial fragile memory traces formed by the encoding event are “stabilized” or “solidified” into long-term memories. This process is thought to be dependent on the medial temporal lobes and is assumed to be facilitated by stress.1 If stress is experienced shortly after encoding it will aid in consolidating memory for recent information, resulting in slowed forgetting relative to conditions in which stress is not experienced. The process of consolidation is thought to be enhanced by the conjunctive effects of the noradrenergic and glucocorticoid responses to stress, specifically in the amygdala and the hippocampus (Joëls et al., 2011, 2006, McGaugh, 2000, 2015).
The most direct prediction about episodic memory from consolidation theory is that post-encoding stress should facilitate consolidation of recently encoded events, and so it should slow forgetting. In addition, because of the role of the amygdala in supporting emotional memory, and its sensitivity to both the noradrenergic and corticosteroid responses to stress, it can also be expected that stress should have its greatest effects on memory for emotional or arousing materials (Cahill & McGaugh, 1998; Joëls et al., 2011; McGaugh, 2000, 2004, 2015). Thus, a second prediction from consolidation theory is that post-encoding stress should preferentially benefit memory for arousing materials.
Although initial consolidation-based explanations of the effects of stress on memory focused on the effects of stress during the post-encoding phase, a “dual-mode” model has been proposed in which the same consolidation processes that enhance memory retention also impact both memory encoding and memory retrieval (de Kloet et al., 1999; Diamond et al., 2007; Joëls et al., 2006; Schwabe et al., 2012). That is, there is assumed to be a fast-acting “memory formation mode” that can last up to 30 minutes after stress onset, followed by a slower “memory storage mode” that can last hours. During the initial period, fast-acting stress hormones (e.g., noradrenaline and “nongenomic” effects of cortisol) alter processing in the hippocampus, amygdala, and prefrontal cortex, which increases attention to and encoding of stress-relevant materials. This memory formation mode is assumed to compete with or suppress the retrieval of unrelated information (Cadle & Zoladz, 2015; Schwabe et al., 2012). In contrast, after a longer delay, glucocorticoids begin to exert slower, genomic effects (i.e., effects on and through changes in gene expression), initiating a “memory storage mode”. These genomic glucocorticoid effects are thought to facilitate the consolidation of recently encoded memories and impair the ability to encode new information, thus reducing interference from novel information and further benefitting recently encoded memories.
The dual-mode theory predicts that post-encoding stress should enhance memory because the encoded items will benefit from both the fast memory formation mode and the slower memory storage mode. In contrast, when stress occurs during or prior to retrieval, it should impair memory because both the initial memory formation mode and the slower memory storage mode inhibit retrieval. Moreover, this model predicts that stress will also impact encoding, but these effects will depend on the time delay between the stressor and the onset of the encoding phase. That is, if stress occurs immediately prior to or during encoding it should enhance memory because the study event occurs during the fast memory formation mode, and the slower memory storage mode will further consolidate those memories after they have been encoded. However, if the stressor precedes the study event by more than 20–30 minutes, then memory encoding should be impaired because the memory formation mode would have ended and been replaced by the memory storage mode, which inhibits new encoding. So, this theory predicts that stress immediately prior to or during encoding will enhance memory, particularly for materials related to the stressor, but as the delay between stress and encoding increases, the effects of stress should reverse, such that stress begins to impair memory.
Another account of how stress impacts memory assumes that stress impacts executive functions that are involved in memory encoding and retrieval (Gagnon & Wagner, 2016; Mather & Sutherland, 2011). We will refer to this as the “executive control” theory of stress and memory. Executive functions are known to support both effective memory encoding (Blumenfeld & Ranganath, 2007; Simons & Spiers, 2003; Spaniol et al., 2009) and successful memory retrieval (Dobbins et al., 2002; Gagnon & Wagner, 2016; Levy & Anderson, 2002; Simons & Spiers, 2003; Spaniol et al., 2009). Moreover, stress appears to impair various executive functions such as working memory, selective attention, and cognitive flexibility (Alexander, Hillier, Smith, Tivarus, & Beversdorf, 2007; Sänger, Bechtold, Schoofs, Blaszkewicz, & Wascher, 2014; Schoofs, Wolf, & Smeets, 2009; Shields, Bonner, & Moons, 2015; Shields, Sazma, et al., 2016), presumably through the catecholamine (e.g., noradrenaline) and glucocorticoid disruption of frontal lobe function (Arnsten, 2009; Shansky & Lipps, 2013). Together, these lines of evidence support the notion that stress may impact memory in part by impairing executive functions that influence encoding and retrieval (Gagnon & Wagner, 2016; Joëls et al., 2006; Mather & Sutherland, 2011; Schwabe et al., 2012).
Thus, by the executive control account, stress should generally impair memory when stress impacts the encoding phase or the retrieval phase by limiting the executive process necessary for effective encoding and retrieval. However, an important exception to this rule is that because stress is generally expected to shift attention toward threat-related stimuli while attenuating the processing of unrelated materials (Gagnon & Wagner, 2016; Mather & Sutherland, 2011), stress that occurs during or prior to encoding is expected to facilitate encoding of stress-related information at the cost of information that is unrelated to the stressor.
One additional account of stress and memory is “reconsolidation theory” (e.g., Schwabe, Nader, & Pruessner, 2014) which proposes that if an old memory is re-activated after it has already gone through an initial consolidation process, the reactivation will make that memory labile once again (similar to the initial post-encoding period), and it will become susceptible to modification. Thus, if subjects are stressed shortly after reactivation (i.e., post-reactivation) this should also benefit future memory for those items by allowing for another round of consolidation and enhancing that consolidation through stress. Although the mechanisms for this process are still debated (Besnard, Caboche, & Laroche, 2012; Schwabe et al., 2014), several studies have now examined the effects of post-reactivation stress on memory.
Most theories of stress and memory assume that the effects of stress on memory are driven by independent and/or interactive effects of glucocorticoid stress hormones—cortisol in humans, corticosterone in rodents—and noradrenaline (Gagnon & Wagner, 2016; Het, Ramlow, & Wolf, 2005; Joëls et al., 2011; McGaugh, 2000; Schwabe et al., 2012). The consolidation theories emphasize the interactive effects of these hormones in the hippocampus and amygdala, whereas the executive control theories emphasize their role in the prefrontal cortex and other regions primarily underpinning executive functions, but which are also crucial to memory-related processing. Thus, from each of these perspectives one could expect that there might be a close relationship between the magnitude of the cortisol response to stress and the magnitude of the effect on memory, though the strength of this relationship may be reduced depending on the extent to which cortisol interacts with other stress hormones such as noradrenaline.
Understanding Discrepancies in Stress Effects on Memory
The above theories, despite their impressive breadth and clarity, may or may not be able to account for inconsistencies observed in the stress and memory literature. To understand these inconsistencies, it is important to examine the empirical literature to identify what factors have been proposed as moderators of stress effects on memory. This examination will thus help us conduct a meta-analysis that is sensitive to both theoretical predictions and predictions derived from empirical literature. For ease of understanding, we categorize factors that have been proposed to account for heterogeneity and inconsistency in effects of stress on memory as either participant/sample variables or study design variables in our review.
The participant/sample variables with the strongest evidence for moderating effects in the stress and memory literature are the following. First, a number of studies have indicated that stress can influence memory differently depending on the sex of the participants (Andreano & Cahill, 2006, 2009; McCullough & Yonelinas, 2013). In addition, there is evidence suggesting that menstrual phase (Andreano, Arjomandi, & Cahill, 2008), the use of hormonal contraceptives (Andreano & Cahill, 2009), and sex hormones (Barros, Tufik, & Andersen, 2015; T. Inagaki, Gautreaux, & Luine, 2010) influence memory or the effects of stress on memory. Similarly, multiple studies have suggested that effects of stress on memory differ as a function of age (Hidalgo et al., 2015; Hidalgo, Almela, Villada, & Salvador, 2014; Pulopulos et al., 2013). Finally, although not directly studied within the context of stress and memory, there is also reason to examine effects of whether a study excluded participants who smoked, used psychoactive medications, reported current illnesses, or had a BMI over 30, given literature indicating that these variables may alter stress-responsive physiological systems (Allen et al., 2014; Childs & De Wit, 2009; O’Connor et al., 2009).
The study design variables with the strongest evidence for moderating effects of stress include the following. One obvious design factor that varies across studies is the stressor type, (i.e., the manipulation used to induce stress). For example, common stressors include the Trier Social Stress Task (TSST), where participants give a speech and perform complex arithmetic in front of a stern panel of evaluators, the cold-pressor task (CPT), where participants immerse their non-dominant arms in ice water, and the Socially-Evaluated Cold Pressor Task (SECPT), which is a hybrid task involving both ice water and social evaluation. There is some evidence that different stress induction procedures elicit reliably different physiological stress responses (Dickerson & Kemeny, 2004) and some indication that the TSST may produce a larger cortisol increase than the other methods (Skoluda et al., 2015).
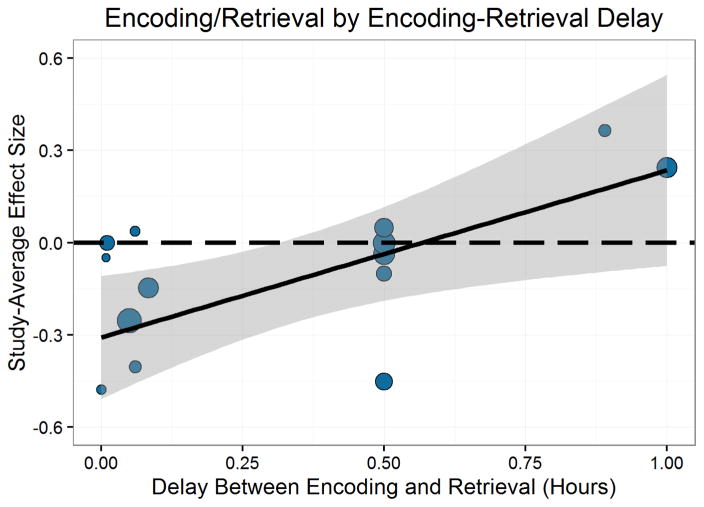
In addition, the delay between the stressor and the specific memory phase varies widely between studies, and there is empirical evidence that suggests that the timing of stress in relation to learning or retrieval may be an important determinant of the effects of stress on memory (Schwabe & Wolf, 2014; Zoladz et al., 2011). For example, if stress acts in part through actions of cortisol, which is not expected to reach peak levels until approximately 20 minutes after stress is initiated (Dickerson & Kemeny, 2004), then the effects of stress on memory may depend upon the delay after the stressor. Moreover, because of the different time-dependent effects of cortisol described above (Joëls et al., 2011; Schwabe et al., 2012; Shields et al., 2015), the effects of stress may be quite different at different delay periods (Schwabe et al., 2012).
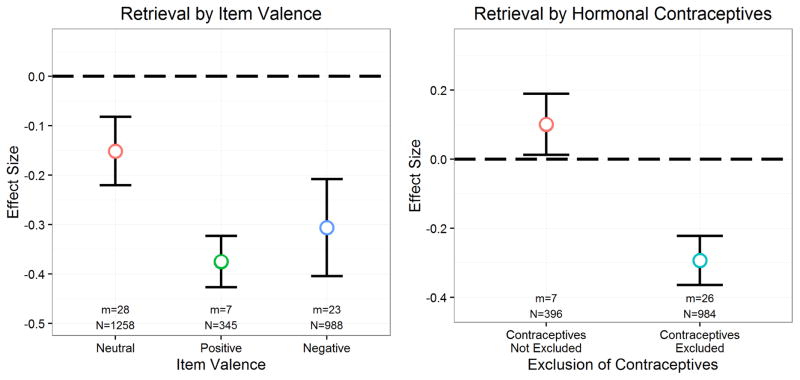
There is also evidence that the valence of the learned materials can impact the effects of stress on memory (Cahill et al., 2003). That is, post-encoding benefits of stress are sometimes found to be restricted to emotional materials (Cahill et al., 2003), other times to impact emotional and neutral materials (McCullough & Yonelinas, 2013), and yet in other cases to preferentially impact neutral materials (Yonelinas, Parks, Koen, Jorgenson, & Mendoza, 2011). In addition, there is evidence that stress may impair retrieval of negative information more so than neutral information (Gagnon & Wagner, 2016; Kuhlmann et al., 2005).
The relevance of the learned materials to the stressor may be another critical factor in accounting for heterogeneity in stress effects on memory. That is, some studies have found that stress enhances memory for information related to the experienced stressor, but not for unrelated information learned at the same time as the stress-relevant information (e.g., Smeets et al., 2007; Wiemers, Sauvage, Schoofs, Hamacher-Dang, & Wolf, 2013).
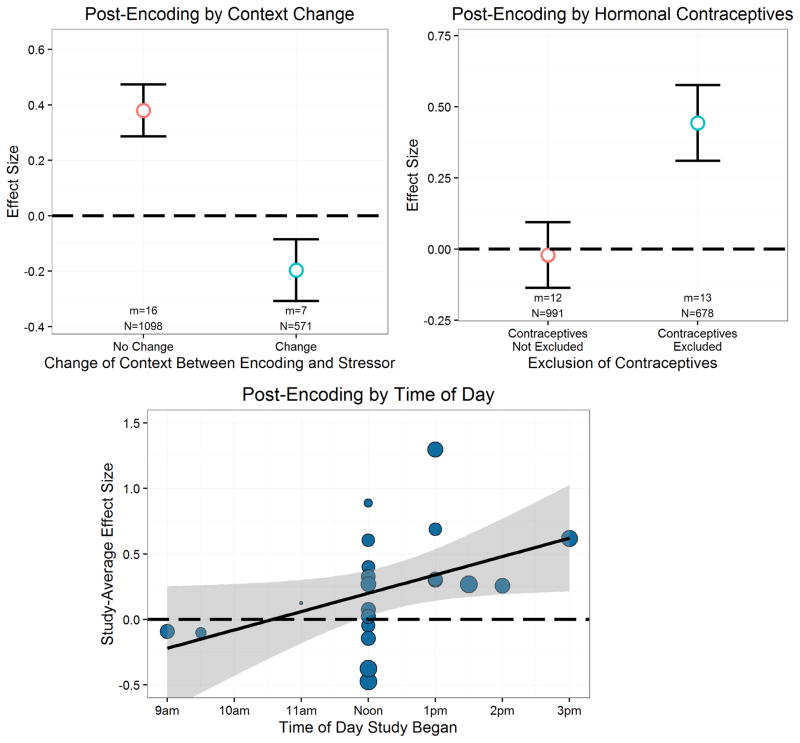
In addition, there is recent evidence to suggest that a change in spatial context between encoding and stress can impact the effects of stress. For example, Trammell and Clore (2014) found an impairing effect of post-encoding stress on memory, which is in contrast to the typically-observed enhancing effect. The primary methodological difference they proposed to explain the discrepant findings between their studies and others that found enhancements was that their participants changed contexts between learning and stress, whereas participants in most other studies experience stress in the same context as learning.
Further, the type of memory test (i.e., free recall, cued recall, or recognition) may influence the effects of stress on memory. Many studies have found effects of stress using recall tasks (Andreano & Cahill, 2006; Cahill et al., 2003), whereas others failed to observe effects on recall but observed enhanced recognition performance (McCullough & Yonelinas, 2013). One may also expect that different types of recognition processes, such as recollection and familiarity (Yonelinas, 2002), may be differentially impacted by stress, however, only a very small number of studies have included these measures (e.g., McCullough, Ritchey, Ranganath, & Yonelinas, 2015; McCullough & Yonelinas, 2013; Wiemers et al., 2013).
Similarly, the use of an immediate recall task may modulate effects of stress on encoding. One study experimentally manipulated the use of an immediate recall task and found that stress effects on encoding were only seen when an immediate recall task was not used (Wolf, 2012). Thus, there is reason to consider the use of an immediate recall task as a moderator of stress effects on encoding.
We also considered time of day as a potentially important moderator of stress effects on memory for two reasons. First, a meta-analysis of cortisol administration studies found that cortisol administration enhanced encoding when cortisol was administered in the afternoon, whereas cortisol administration impaired encoding when cortisol was administered in the morning (Het et al., 2005). Second, there is one empirical study showing that stress prior to encoding impaired subsequent memory when stress (and encoding) occurred in the morning but stress had no effect on encoding when experienced in the afternoon (Maheu et al., 2005).
There were several other variables that had not been directly implicated in previous studies, but that we felt might have some impact of the magnitude of stress effects on memory. These included variables related to learning, such as the sensory modality of stimulus presentation (i.e., visual/verbal/both), material type (pictures, words, narrative/slideshow, autobiographical), the study list length, the duration of the encoding phase, and incidental vs intentional encoding. Other potentially relevant variables included the duration of the stressor, delay between encoding and retrieval, whether there was a context change between encoding and retrieval, the number of novel items in the recognition test, and whether stress was manipulated between or within subjects.
How do stress-induced changes in cortisol relate to memory?
Cortisol is an important component of the stress response, and there is considerable evidence that cortisol responses influence memory, and a number of models have proposed that the cortisol response is critically involved in producing the observed memory effects (Gagnon & Wagner, 2016; Joëls et al., 2011; Schwabe et al., 2012). These claims are based on the fact that stress produces increases in glucocorticoids via activation of the HPA axis (Allen et al., 2014; McEwen, 2007) coupled with animal work showing glucocorticoids can exert causal influences on memory (de Quervain et al., 1998; Joëls et al., 2011; Roozendaal, 2002). In addition, some studies have found that the magnitude of a person’s cortisol response to post-encoding stress is related to their memory performance (Andreano & Cahill, 2006; McCullough et al., 2015). Moreover, cortisol administration independently influences memory encoding and retrieval in ways that can parallel purported effects of stress (Het et al., 2005).
The role of cortisol in potentially mediating stress effects on memory has been used to explain why stress effects on memory are sometimes not observed in certain conditions. For example, males exhibit more robust cortisol responses to common laboratory stressors (Kirschbaum, Wüst, & Hellhammer, 1992), and they have sometimes been found to exhibit more pronounced stress effects on memory (e.g., Andreano & Cahill, 2006), presumably because of their larger cortisol responses. Moreover, some studies suggest that use of hormonal contraceptives dampens the stress-induced cortisol response (Kirschbaum, Kudielka, Gaab, Schommer, & Hellhammer, 1999; Marinari, Leshner, & Doyle, 1976), and women taking hormonal contraceptives show altered stress effects on memory (Nielsen, Segal, Worden, Yim, & Cahill, 2013). These results suggest that the reduced sensitivity of women to the stress effects on memory may arise because of relatively smaller cortisol responses.
However, the relationship between cortisol and the observed memory effects of stress has not been systematically assessed, and there are reasons to suspect that it may reflect only part of the story, with other components of the stress response also playing critical roles. For example, stress effects on memory may also be driven by effects on hormones other than cortisol such as progesterone, estradiol, or DHEA (Barros et al., 2015; T. Inagaki et al., 2010; Sripada et al., 2013) or by immune system responses (Harrison, Doeller, Voon, Burgess, & Critchley, 2014; Reichenberg et al., 2001). Although there were too few studies reporting these other biological measures to support an analysis of these biomarkers, the cortisol analysis is useful in assessing the claim that cortisol plays an important role in mediating the effects of stress on memory, and it may provide insights into the neural mechanisms supporting those effects. We also note that neuroimaging studies are also useful in assessing the neural mechanisms, but at this point the number of such studies is also rather limited (e.g., Henckens, Hermans, Pu, Joëls, & Fernández, 2009; Qin, Hermans, van Marle, & Fernández, 2012), and so these imaging results will not be considered in our analyses.
Current Research
In the current paper, we conducted a meta-analysis of the human studies that have examined the effects of acute stress on episodic memory. To our knowledge such a meta-analysis has never been conducted. Such an analysis is important not only for understanding when stress will impact memory, but also to assess current theories of memory and stress. We addressed this gap by conducting a meta-analytic review, by systematically examining stress effects on each separate memory phase (e.g., encoding, post-encoding, post-reactivation and retrieval), as well as studies examining effects of stress on more than one memory phase. In addition, we attempted to elucidate potentially important moderators—outlined above—of stress effects on phases of memory using a meta-regression approach. Finally, by examining the relationship between cortisol, stress, and memory we attempted to determine the role of cortisol in moderating the stress effects on memory.
Method
Study Selection and Inclusion Criteria
Literature review
To obtain studies for use in the meta-analysis, we performed an exhaustive search of the databases PsycINFO, PubMed, and Web of Science using the following search string:
((memory) AND (emotion OR positive OR negative OR neutral OR emotional) AND (encoding OR retrieval OR consolidation OR pre-encoding OR post-encoding OR storage OR reconsolidation) AND (Recognition OR Recall) AND (Stress OR Stressful OR Stressor)).2
We concluded this search in October 2015. In this search, PubMed returned 267 results, PsycINFO returned 223 results, and Web of Science returned 469 results. References from relevant articles were reviewed, and studies that were potentially relevant were examined from those references. For all articles considered, we followed Dickerson and Kemeny (2004) in reviewing abstracts and examining full texts whenever an article had the potential to include a relevant effect (e.g., if a study incorporated or could have incorporated an acute stressor, given our search string, the full-text of the article was reviewed). The first three authors reviewed all articles that were selected to have their full text reviewed, and a decision that one of these articles did not meet our inclusion criteria and should be excluded from analyses was made by mutual agreement of the first three authors. Figure 1 depicts a flow diagram illustrating our review and inclusion process.
Figure 1.
Flow diagram illustrating the process of our review, screening, and article selections.
Inclusion criteria
Our nine inclusion criteria for this study were as follows: studies had to (1) experimentally manipulate (2) acute stress and assess effects on (3) human participants (4) without a known psychological/psychiatric disorder (5) who encoded, consolidated, reactivated, and/or retrieved memories within temporal proximity to the stressor or control task. (6) To ensure that acute stress was the primary manipulation rather than arousal, the stressor task used had to either be a previously validated stressor or include a biological measure of stress validation (e.g., cortisol, cytokine reactivity) that is not also sensitive to the effects of acute arousal without stress. (7) Because stress hormones exert genomic effects on neural processes for hours after cessation of stress, the control condition could not have been subjected to a laboratory stressor on the same day as a memory procedure. This entails that if a study used a within-subjects, crossover design, the counterbalance of stress and control had to be separated by at least one day. (8) To separate effects of stress on long-term memory from stress effects on working memory, if encoding and retrieval were on the same day, a brief delay or interfering task had to separate memory encoding and retrieval. (9) Finally, because we were interested in assessing effects on memory accuracy rather than potential effects of stress on response bias, we only included recognition data if a study reported some bias-corrected measure (i.e., d’) or if a bias-corrected measure was unavailable, if no differences existed between groups in false alarms. To control for potential differences in learning between groups, if a study reported both proportion of total items recalled and proportion of immediate recall, we used proportion of immediate recall in analyses.3 We chose these inclusion criteria to best isolate the relationship between acute stress and memory processes. Our inclusion criteria for studies given above implicitly defines what we designated a “study.” Our final designation of a study was any independent (i.e., completely orthogonal) set of an experimental group and a control group that met the above inclusion criteria.
Definitions of stress in relation to memory phases
Because memory involves multiple phases (e.g., encoding, retention, and retrieval), and stress is thought to potentially influence these phases in different ways, we categorized studies in terms of the phase stress was expected to impact:
-
1
Encoding studies were those in which the stressor occurred prior to or during encoding.
-
2
Post-Encoding (Retention) studies were those in which the stressor occurred shortly after the encoding phase was competed.
-
3
Retrieval studies were those in which the stressor occurred shortly prior to or during retrieval.
Other studies however, used short enough retention intervals that stress (which can have effects that last for hours) was expected to impact more than one memory phase, and so were categorized as such:
-
4
Post-Encoding/Retrieval studies were those in which the stressor occurred shortly after encoding and there was a short enough interval between encoding and retrieval (<90minutes) that the stressor likely impacted both the retention and retrieval phases.
-
5
Encoding/Retention/Retrieval studies (hereafter encoding/retrieval studies for brevity) were those in which the stressor occurred prior to or during encoding, and there was a short enough interval between encoding and retrieval (<90minutes) that stress likely impacted encoding, retention, and retrieval.
Finally, a number of studies examined memory for information initially learned on one day, and subsequently reactivated on a later day with stress following the memory reactivation, and examined retrieval on a later day. These studies were categorized as such:
-
6
Post-Reactivation studies were those in which stressor onset occurred prior to or following memory reactivation, and neither encoding nor eventual retrieval of reactivated memories occurred on the same day as memory reactivation.
Selected studies
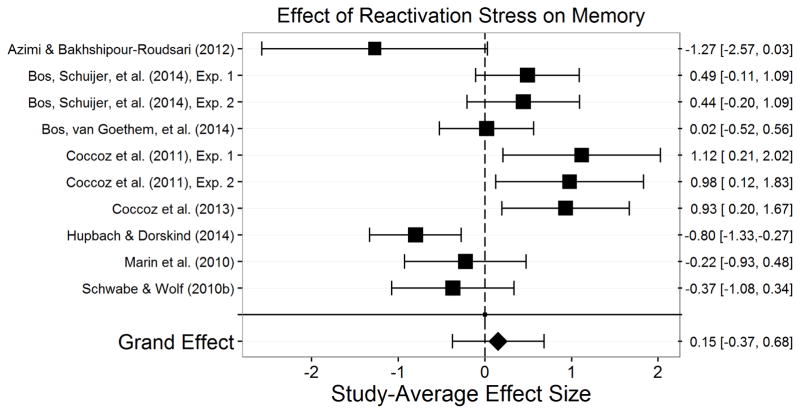
Our search and study inclusion criteria led to the incorporation of 113 studies, 108 of which were published in 88 peer-reviewed papers, and 5 of which were unpublished or reported in unpublished theses or dissertations. We chose to cite the published paper if a study was presented in both a thesis/dissertation and a published paper. Of these 113 studies, 33 assessed effects of stress at encoding, 23 assessed effects of stress post-encoding, 31 assessed effects on retrieval, 9 assessed effects on post-encoding/retrieval, 15 assessed effects on encoding/retrieval, and 10 assessed effects of stress post-reactivation.
Coding of Covariates and Moderators
We coded for a number of potential moderators of the effects of stress on memory, most of which were assessed because there was empirical or theoretical reason to believe the moderating effect would be significant. Two raters coded each study, and the agreement between raters was very good (89%). All discrepancies in study coding between raters were discussed and resolved. See Table 1 for a complete list of coded moderators.
Table 1.
List of Moderators Considered In Analyses
| Moderator | Variable Type | Reference |
|---|---|---|
| Incidental or Intentional Encoding | Categorical (dummy-coded) | Incidental Encoding |
| Exclusion of Smokers | Categorical (dummy-coded) | Inclusion |
| Exclusion of Women During Menstrual Period | Categorical (dummy-coded) | Inclusion |
| Exclusion Hormonal Contraceptives Usage | Categorical (dummy-coded) | Inclusion |
| Exclusion of All Illnesses | Categorical (dummy-coded) | Inclusion |
| Exclusion of All Psychoactive Medication | Categorical (dummy-coded) | Inclusion |
| Exclusion of BMI Greater Than 30 | Categorical (dummy-coded) | Inclusion |
| Use of an Immediate Recall Task Post-Encoding | Categorical (dummy-coded) | No Immediate Recall |
| Stressor Relevance of Items (Integral/Nonintegral) | Categorical (dummy-coded) | Nonintegral |
| Context Change Between Learning and Stress | Categorical (dummy-coded) | No Change |
| Context Change Between Learning and Retrieval | Categorical (dummy-coded) | No Change |
| Stress Manipulated Between or Within Groups | Categorical (dummy-coded) | Between Groups |
| Item Valence | Categorical (dummy-coded) | Neutral Items |
| Study Material Type | Categorical (contrast-coded) | |
| Sensory Modality of Study Material Presentation | Categorical (contrast-coded) | |
| Memory Task Type | Categorical (contrast-coded) | |
| Stressor Type | Categorical (contrast-coded) | |
| Participant Age | Continuous | |
| Percent Male Participants | Continuous | |
| Time of Day Study Began | Continuous | |
| Study Item List Length | Continuous | |
| Number of Novel Items in a Recognition Task | Continuous | |
| Delay (hours) Between Item Encoding and Retrieval | Continuous | |
| Delay (min) Between Stress Onset and Encoding | Continuous | |
| Delay (min) Between Encoding and Stress Onset | Continuous | |
| Delay (min) Between Stress Onset and Retrieval | Continuous | |
| Delay (min) Between Reactivation and Stress Onset | Continuous | |
| Stressor Duration (min) | Continuous | |
| Stress-Induced Δ-Cortisol (nmol/L) | Continuous | |
| Length of Encoding Phase (min) | Continuous | |
| Participant Homogeneity | Continuous |
Whenever possible, we incorporated the following information on moderators from explicit statements within the manuscript. If the manuscript did not explicitly state that information regarding a moderator but it could be inferred from their study protocol, we coded the moderator as it could be inferred (e.g., the manuscript did not state that participants either did or did not change contexts, but the study’s stressor was one that required a room change—such as the Trier Social Stress Test without modifications—we coded the context as changed). Finally, when the information was not directly available in the manuscript or inferable from the protocol used, we emailed the corresponding authors of studies for that information. If that information was not obtainable, we did not include that study within a given moderator analysis.
Stressor type was coded as follows. Stressors were coded as “social” stressors if they included social evaluation but did not include pain (e.g., the Trier Social Stress Test). Stressors were coded as “pain” stressors if they included pain but did not include social evaluation (e.g., the Cold Pressor Task). Stressors were coded as “hybrid” stressors if they included both social evaluation and pain (e.g., the Socially Evaluated Cold Pressor Task). Stressors were coded as “other” if they included none of these characteristics (e.g., skydiving, mock prisoner of war stressor, threat of shock coupled with gruesome pictures).
Item type was coded as “words” if the items were presented as words or lists of words without accompanying pictures or other details to be remembered, “pictures”, “narrative/slideshow” if items were presented as a narrative accompanied by pictures, or “self-related” if the items were autobiographical memories or personal questions. Finally, studied items were coded as “other” if the items were not any of the above.
The memory task was coded “integral” to the stressor if items studied were highly related to the stressor (e.g., personality words studied after a speech on one’s personality to a critical evaluator panel) or if the stressor and memory task were indistinguishable to participants (e.g., face recognition for faces of the evaluators in the Trier Social Stress Test) and “nonintegral” otherwise. Sensory modality of item presentation was coded as “auditory” if the items were presented auditorily but not visually, “visual” if the items were presented visually but not auditorily, and “both” if the items were simultaneously presented auditorily and visually. Item valence was coded as “neutral”, “positive”, “negative” or “multiple” if more than one preceding valence type was included (notably, most studies with valenced materials will have positive, negative, and neutral item valence effects). We also considered criteria commonly reported by studies as a reason to exclude participants. All six of these study exclusion criteria we considered (i.e., excluded smokers, all illnesses, BMI over 30, women currently menstruating, hormonal contraceptive use, psychoactive medication use) were coded “excluded” if the study explicitly excluded the potential participants in question and coded “unreported or included” otherwise.4 The “participant homogeneity” moderator represents the sum of the exclusion criteria moderators and is thus a variable ranging from 0–6 (with 6 having the strictest criteria and excluding the most participants).
Studied item valence was dummy-coded using neutral items as a reference group in order to examine potential differences in stress effects on positive items, negative items, or multiple valences relative to stress effects on neutral items. Stressor type, memory task type, studied item type, and sensory modality of item presentation were all contrast-coded in order to examine potential differences in stress effects without using one group of effects as a reference. All other categorical variables were dummy-coded with reference groups listed in Table 1.
Continuous variables considered as moderators were centered for analyses at the lowest obtained for each phase of memory and stress to make interpretation of the intercept (i.e., the effect size) equal to the effect of stress on memory at that lowest value of the covariate. Despite centering for analyses, graphs present uncentered data for ease of interpretability. If the average participant age was not given in the article, the median participant age was used if it was reported; if neither of these statistics were listed, the midpoint of the reported participant age range was used.
To assess stress effects on cortisol, we calculated the pretest-posttest-control group effect size (Morris, 2008) and converted from d to g using the correct transformation. We used the baseline samples as the pretest values and the peak reactivity samples (whichever value was the greatest in the stress group and the corresponding sample from the control group at this time) as the posttest values. This effect size provides an unbiased index of the effect of stress on the change in cortisol relative to the change in a control group, thus representing the effect size closest to how cortisol is analyzed in most studies.
The pretest-posttest correlation is required to calculate the variance of the pretest-posttest-control group effect size, and this correlation was unknown to us given that no study reported this. As such, we set the pretest-posttest correlation at .3. Sensitivity analyses from .0 to .8 indicated no differences in stress effects on cortisol with high or low correlations used to derive the variance of the effects.
Because we were able to analyze stress effects on cortisol across all studies (e.g., stress at encoding, stress at retrieval, etc.), we chose to use the pretest-posttest-control group effect size, gppc+. Because this effect size examines the difference from baseline to post-manipulation between groups (i.e., how change in cortisol over time differed between groups), it represents the best measure of effect size for determining stress effects on cortisol. However, because many studies did not provide enough information to derive gppc+ (e.g., they only reported Δ-cortisol), and reduced power greatly impacted our ability to detect cortisol effects on different memory phases (e.g., encoding, retrieval), we converted all cortisol values to nmol/L and used Δ-cortisol (posttest-pretest for the stress group) to examine cortisol effects on memory.5 Out of all studies considered in this meta-analysis, 78 provided enough information for us to extract gppc+ for cortisol, whereas 95 provided enough information for us to extract Δ-cortisol.6
Analytic Strategy
The effect size measure of interest was the standardized mean difference between stress and control groups. We used Hedges’ g rather than Cohen’s d as the effect size for analysis, given that the former is a relatively unbiased estimate of the population standardized mean difference effect size while the latter is a biased estimate. Whenever possible, we calculated Hedges’ g from the means, standard deviations, and sample sizes presented in the article. If means and standard deviations were not reported and the design was between-studies, we used t or one-way F statistics—or p values resulting from tests of those two statistics—to calculate the effect size. If none of these statistics were reported, we emailed corresponding authors for these statistics. If we were unable to obtain the necessary statistics for a study from the corresponding author, that study was excluded from analysis. For within-studies designs, we converted effect size estimates and their variances into the between-study effect size metric following Morris and DeShon (2002).7
Given the multifaceted nature of memory, most studies often report more than one outcome (e.g., effects of stress on positive, negative, or neutral items; effects of stress on recall, cued recall, or recognition; etc.). Multiple outcomes are a problem for conventional meta-analytic methods, as averaging effect sizes within studies without accounting for their correlations can alter or obscure true effect size estimates (Borenstein, Hedges, Higgins, & Rothstein, 2009; Scammacca, Roberts, & Stuebing, 2014). Thus, we employed the meta-analytic technique of robust variance estimation, a random-effects meta-regression that can account for dependence between effect size estimates (Hedges, Tipton, & Johnson, 2010; Tanner-Smith & Tipton, 2014). This technique robustly estimates effect size weights and standard errors for the given effects, allowing for multiple outcomes within studies (Hedges et al., 2010). We employed the robu() function of the robumeta package in R, version 3.2.2, to conduct our analyses of stress effects on memory, using the correlated weights given by Hedges et al. (2010) and using the small sample corrections suggested by Tipton (2014). We did not use small-sample corrections in our analyses of stress effects on cortisol because we were able to examine effects across 78 studies. To account for dependency, ρ was set to the recommended .80 (Tanner-Smith & Tipton, 2014).8 Because we were more interested in understanding factors that influence the effects of stress on memory than we were interested in understanding factors that contribute to heterogeneity in analyses, continuous moderator analyses do not separate continuous moderators into within- and between-study continuous moderators.
Degrees of freedom for all primary analyses were estimated using the Satterwaite approximation, where df=2/cv2 and cv represents the coefficient of variation, as simulation studies have indicated that this method of estimating degrees of freedom is most analytically valid with study set sizes under 40 using the RVE meta-analytic technique (Tipton, 2014). Because of how the degrees of freedom are estimated, if the degrees of freedom are less than four, there is a heightened risk of a Type I error and the analysis results cannot be trusted to represent population values (Tipton, 2014). However, because this estimation of degrees of freedom is extremely sensitive to outliers given a study set size such as in this meta-analysis (since degrees of freedom are divided by the coefficient of variation), one can be relatively confident that when degrees of freedom are greater than four, outlying studies are not driving observed significant effects.
In presenting our results, we discuss each effect sequentially and examine concurrent effects in a final model at the end of each section. We make exceptions to this rule when, by our examination of the data, two effects appear to be largely conflated and merit further attention before proceeding. For the forward stepwise regressions presented at the end of each subsection of our primary analyses, we chose a one-tailed test for these analyses a priori to ensure we had included all contributing moderators. We did not include methodological potential moderators with no a priori hypothesized effect or direction (e.g., study item list length) within these forward stepwise regression analyses. All of the effects considered in these stepwise regressions were hypothesized a priori to have an effect in an expected direction, justifying the use of a one-tailed test.
For all of the following analyses, a positive effect size indicates that stress enhanced memory relative to a control condition, whereas a negative effect size indicates that stress impaired memory relative to a control condition. In addition, because the outcome in these analyses is the standardized mean difference between groups (the effect size), a significant continuous moderator means that the effect size estimate depends upon levels of that continuous variable. In other words, if the coefficient for a continuous moderator is significant, it means that as the continuous variable increases or decreases, the effect of stress on memory relative to a control condition increases or decreases.
Results
Effects of Stress on Cortisol
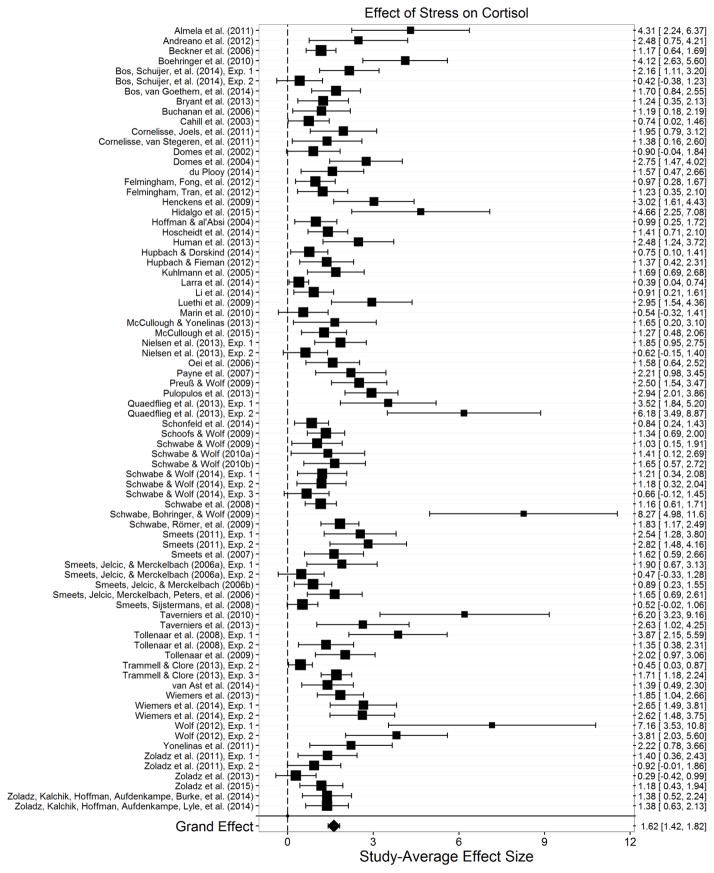
Of the studies examining stress effects on memory, 78 studies included enough information to allow us to accurately derive the pretest-postest control group effect size for cortisol. This effect size allows us to best determine the stress-induced increase in cortisol relative to a control group. These cortisol analyses included 4,238 participants.
The overall effect of stress on increases in cortisol relative to a control group was strong and significant, gppc+=1.62, t(77)=16.0, p<.001, 95% CIg [1.42, 1.82] (Figure 2). There was some heterogeneity in these effects, however, τ2=0.53, indicating that this effect likely differed as a function of moderators. To assess publication bias, we conducted Egger’s test for funnel plot asymmetry (Egger, Davey Smith, Schneider, & Minder, 1997); interestingly, there was strong evidence for publication bias in these effects, t(76)=5.55, p<.001, with estimates indicating that positive effects greater in magnitude were more likely to be published than effects weaker in magnitude. To address this concern, we conducted a trim and fill analysis (Duval, 2005; Duval & Tweedie, 2000a, 2000b) to estimate the number of missing studies and the correct effect size estimate. This analysis indicated that while the actual effect may be weaker than what was estimated, with 16 studies estimated to be missing (SE=5.81) with effects less than the average reported effect, the estimated effect size including those estimated 16 studies was still strong and highly significant, g+=1.319, p<.001. Thus, because our analysis focused on studies examining stress effects on memory, we took evidence for publication bias in stress effects on cortisol to imply that researchers often simply chose not to report the secondary analysis of stress effects on cortisol if it did not strengthen their papers, rather than a lack of a true effect.
Figure 2.
Effect of stress on cortisol. Size of the square indicates the relative weight assigned to that study in the analysis. Error bars represent the 95% confidence interval of the effect. This meta-analysis indicates that stress significantly increased cortisol from baseline to post-manipulation relative to a control condition.
We next examined potential moderators of stress effects on cortisol. As expected, age, B=.036, t(73)=2.73, p=.008, percent male participants, B=.010, t(76)=4.41, p<.001, and time of day, B=.001, t(73)=2.66, p=.010, emerged as significant moderators of the effect of stress on cortisol, with stress effects on cortisol increasing as each of these variables increased. Interestingly, a significant quadratic effect emerged for time of day, Blinear= −.002, Bquadratic<.001, t(72)=2.60, p=.011, with a relatively consistent effect of stress on cortisol before 1pm that dramatically accelerated to large effects of stress on cortisol in the afternoon. We did not find any evidence for quadratic effects of age or percent male in moderating the effect of stress on cortisol, ps>.242. Although effects were all in the enhancing direction, we did not find significant evidence that excluding participants who smoked regularly, took psychoactive medications, were currently sick, or had a BMI greater than 30 moderated the effects of stress on cortisol, ps>.079. In contrast, as expected, excluding women taking hormonal contraceptives, B=.892, t(76)=5.48, p<.001, or excluding contraceptive-free women during their menstrual period, B=1.02, t(76)=6.31, p<.001, increased the effects of stress on cortisol. Thus, the relatively greater effect of stress on cortisol in men compared to women may be even greater when women taking hormonal contraceptives or during their menstrual period are included in analyses.
Finally, we examined stressor type as a moderator of stress effects on cortisol, as currently no meta-analysis has examined differences in cortisol responses to standardized laboratory stressors used in memory studies (Dickerson & Kemeny, 2004 examined cortisol responses to laboratory stressors but excluded the now common cold-pressor task). Thus, we examined how stress-induced cortisol increases in the Trier Social Stress Test (TSST), Cold-Pressor Task (CPT), Socially Evaluated Cold-Pressor Task (SECPT), Maastricht Acute Stress Test (MAST), and Fear Factor Stress Test (FFST) compared to each other. In our dataset for studies with complete cortisol data, 31 studies used the TSST, 19 used the CPT, 16 used the SECPT, 3 used the MAST, 2 used the FFST, and 7 used an unstandardized stressor. Because so few studies used the MAST and FFST, we do not present analyses of these stressors here. As expected, the TSST produced a greater cortisol increase (gppc+=1.931, p<.001) than the CPT, B=.830, t(72)=3.94, p<.001, and a marginally greater increase than the SECPT, B=.532, t(72)=1.98, p=.051. The effect of the SECPT on cortisol (gppc+=1.399, p<.001), however, did not significantly differ from the CPT (gppc+=1.101, p<.001), B=.299, t(72)=1.28, p=.204. Thus, the TSST reliably produced a larger increase in cortisol than did the SECPT or CPT, and no significant differences emerged between the SECPT and CPT.
Effects of Stress on Memory: Preliminary Analyses
Study characteristics
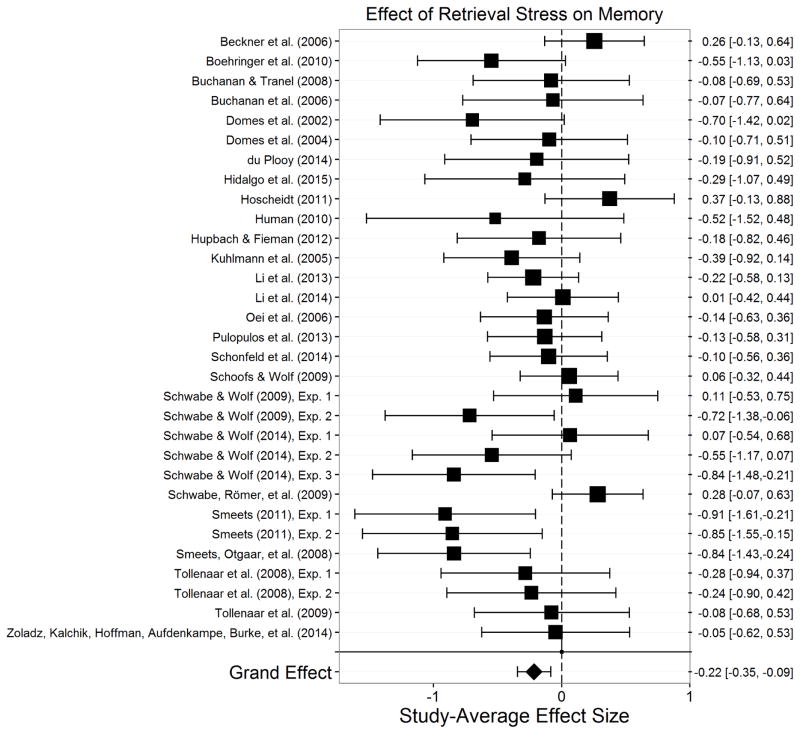
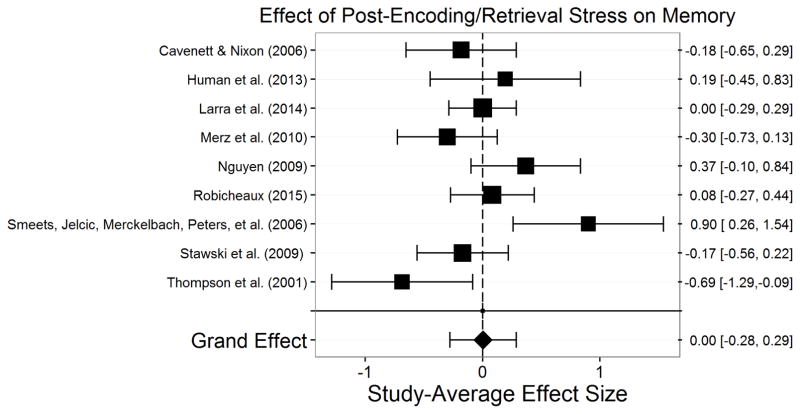
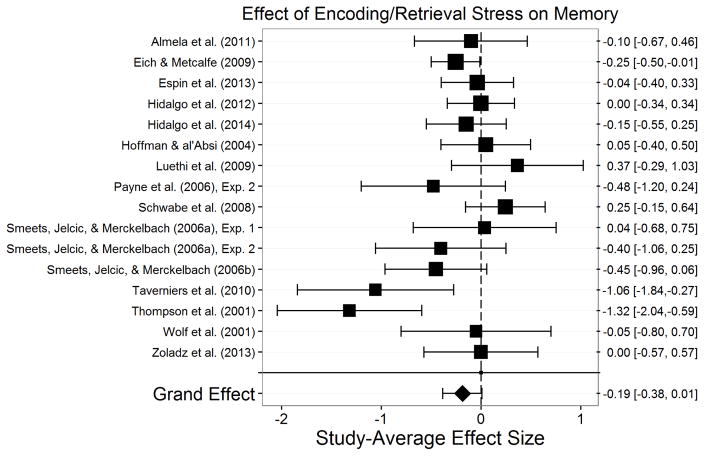
The final sample consisted of 113 studies—each of which is represented by m—assessing stress effects on memory in 6,216 participants. Appendix A presents a summary of each of these studies. There were 399 total effect sizes, each of which is represented by k. The number of effect sizes per study we obtained is relatively common in social science research (Scammacca et al., 2014) and is similar to the number of effect sizes per study seen in similar meta-analyses (Shields et al., 2015). Encoding stress effects were examined in 33 studies (k=131) with 1,607 participants. Post-encoding stress effects were examined in 23 studies (k=83) with 1,668 participants. Retrieval stress effects were examined in 31 studies (k=102) with 1,410 participants. Post-encoding/retrieval stress effects were examined in 9 studies (k=15) with 697 participants. Encoding/retrieval stress effects on were examined in 16 studies (k=48) with 1,148 participants. Finally, post-reactivation stress effects were examined in 10 studies (k=20) with 344 participants.
Assessment of publication bias
To assess publication bias, we conducted Egger’s test for funnel plot asymmetry (Egger et al., 1997) in stress effects on each memory phase (see Supplementary Figure 1). Egger’s test returned nonsignificant for the overall study set, t(111)= −0.20, p=.845, post-encoding, t(21)=1.05, p=.307, post-encoding/retrieval, t(7)=0.31, p=.765, encoding/retrieval, t(14)= −0.22, p=.827, and post-reactivation, t(8)=0.79, p=.450 indicating a lack of evidence for publication bias in these effects. There was, however, evidence for publication bias in stress effects on encoding, t(31)= −2.46, p=.020, and in stress effects on retrieval, t(29)= −3.27, p=.003. Estimates indicate that impairing effects of stress on both encoding and retrieval were published disproportionately more than null or enhancing effects of stress.
The significant evidence for publication bias in stress effects on retrieval prompts a concern that if more null effects of stress on retrieval would have been published these studies might have reduced the effect to a trivial or negligible size. To examine this, we conducted trim and fill analyses (Duval, 2005; Duval & Tweedie, 2000a, 2000b) for stress effects on encoding and retrieval. The trim and fill analysis for stress effects on encoding did not estimate any missing studies (estimated missing = 0; SE = 3.49), indicating that Egger’s test for publication bias may have overestimated publication bias for stress effects on encoding. The trim and fill analysis for stress effects on retrieval estimated that one unpublished study was missing from analyses of stress effects on retrieval (estimated missing = 1; SE = 3.51). Although the actual effect may be weaker than what was estimated, the estimated effect of stress on retrieval including the estimated one missing study was still significant, p=.002. Thus, despite some evidence for publication bias, the trim and fill analyses indicate that we can be confident that the effects of stress on encoding and retrieval are true effects. Moreover, the lack of evidence for publication bias in stress effects on most memory phases provides confidence that the observed effects of stress on memory processes are true effects.
Achieved power analysis
To ensure that we had appropriate power to detect effects, we conducted power analyses for our random effects meta-analyses (Valentine, Pigott, & Rothstein, 2010).9 As shown in Table 2, our analyses were extremely well powered, with almost all analyses obtaining approximately .90 power to detect even small effects (i.e., |g| = .20) and all analyses obtaining .90 power or greater to detect medium effects (i.e., |g| = .50). Thus, nearly all of our analyses had sufficient power to detect even subtle effects of stress on memory.
Table 2.
Power Analyses Describing Achieved Power to Detect Effects of Stress on Different Phases of Memory in a Two-Tailed Test, Rounded to Two Decimals
|
|
|||
|---|---|---|---|
| Achieved Power to Detect a | |||
|
| |||
| Effect of Stress on | Small Effect (i.e., |g| = .20) | Medium Effect (i.e., |g| = .50) | Large Effect (i.e., |g| = .80) |
| Encoding | .95 | 1 | 1 |
| Post-Encoding | .95 | 1 | 1 |
| Retrieval | .93 | 1 | 1 |
| Post-Encoding/Retrieval | .70 | 1 | 1 |
| Encoding/Retrieval | .85 | 1 | 1 |
| Reactivation | .26 | .90 | 1 |
Primary Analyses
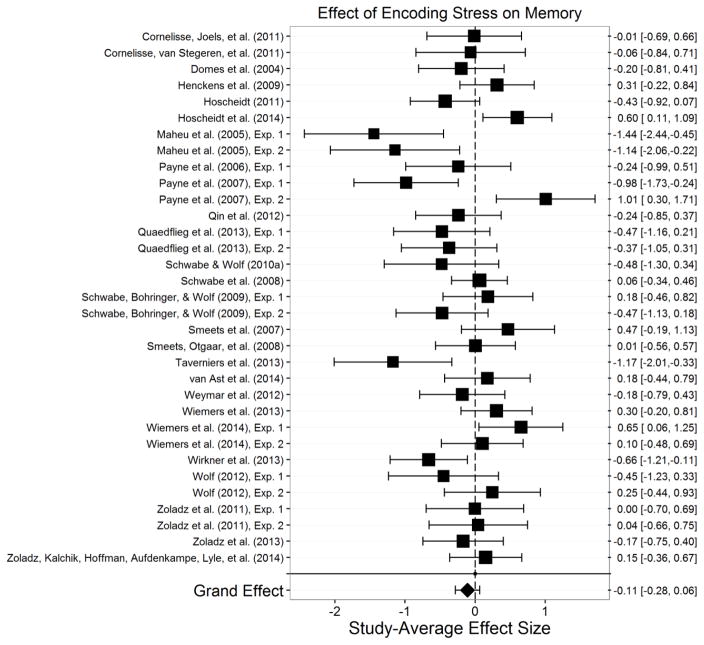
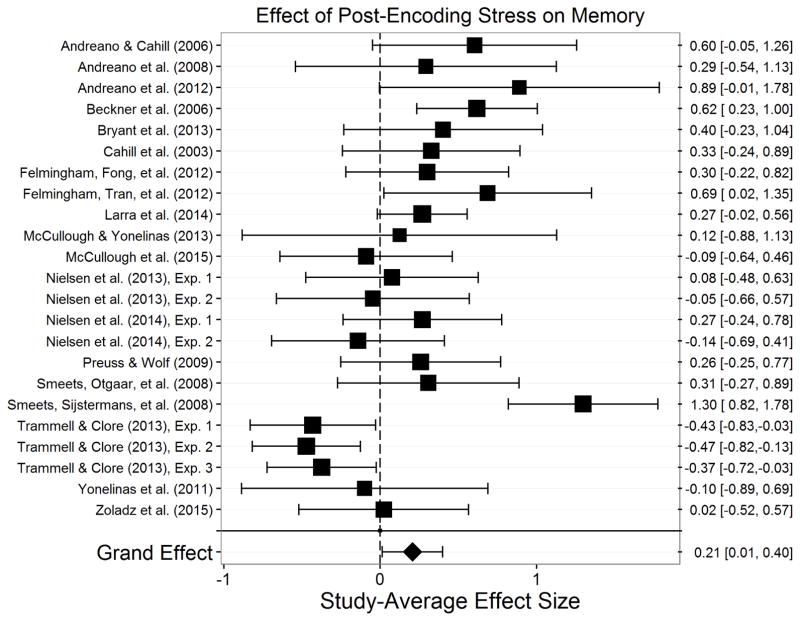
Encoding.10
The overall effect of stress on encoding (m=33, k=131, N=1,607) was not significant, g+= −.109, t(31.5)= −1.28, p=.211, 95% CIg [−.282, .065] (Figure 3). There was, however, some heterogeneity in these effects, τ2=0.21, indicating that this null effect likely differed as a function of moderators. Thus, we explored the effects of moderators expected a priori to play an important role in the effects of stress on encoding. In the interest of assisting future researchers with study design, the effects of all potential moderators of stress effects on encoding are displayed in Table 3.
Figure 3.
Effect of stress on encoding. Size of the square indicates the relative weight assigned to that study in the analysis. Error bars represent the 95% confidence interval of the effect. Points to the left of zero indicate a study-average impairment of encoding, and points to the right of zero indicate a study-average enhancement of encoding. This meta-analysis indicated that stress did not significantly influence encoding across all studies and paradigms.
Table 3.
Potential Moderators of Encoding Stress Effects on Memory
| Moderator | B | df | p |
|---|---|---|---|
| Incidental or Intentional Encoding | .094 | 22.9 | .570 |
| Exclusion of Smokers | .166 | 23.5 | .318 |
| Exclusion of Women During Menstrual Period | −.141 | 29.5 | .422 |
| Exclusion Hormonal Contraceptives Usage | −.144 | 22.9 | .453 |
| Exclusion of All Illnesses | −.022 | 27.4 | .902 |
| Exclusion of All Psychoactive Medication | .052 | 14.1 | .804 |
| Exclusion of BMI Greater Than 30 | −.077 | 17.1 | .676 |
| Use of an Immediate Recall Task Post-Encoding | −.056 | 11.6 | .778 |
| Stressor Relevance of Items (Integral/Nonintegral) | .514 | 6.5 | .034 |
| Context Change Between Stress and Learning | .055 | 26.1 | .727 |
| Context Change Between Learning and Retrieval | .056 | 11.2 | .806 |
| Stress Manipulated Between or Within Groups | NA | ||
| Item Valence (Compared to Neutral) | |||
| Negative | .040 | 26.7 | .840 |
| Positive | .008 | 13.0 | .957 |
| Study Material Type | |||
| Pictures | −.063 | 19.8 | .646 |
| Words | .119 | 18.6 | .382 |
| Narrative/Slideshow | −.217 | 8.6 | .430 |
| Autobiographical | NA | ||
| Other | .156 | 3.9 | .595 |
| Sensory Modality of Study Material Presentation | |||
| Verbal | .108 | 2.4 | .601 |
| Visual | .015 | 6.6 | .908 |
| Verbal + Visual | −.123 | 7.7 | .418 |
| Memory Task Type | |||
| Free Recall | −.196 | 22.3 | .078 |
| Cued Recall | .140 | 6.8 | .219 |
| Recognition | .056 | 21.6 | .554 |
| Stressor Type | |||
| Socio-Evaluative | .087 | 7.2 | .580 |
| Pain | .195 | 1.3 | .320 |
| Hybrid (Socio-Evaluative & Pain) | −.108 | 8.4 | .417 |
| Other | −.174 | 2.7 | .614 |
| Participant Age | −.032 | 10.8 | .391 |
| Percent Male Participants | −.004 | 20.1 | .061 |
| Time of Day Study Began | < −.001 | 5.6 | .762 |
| Study Item List Length | < .001 | 5.1 | .718 |
| Number of Novel Items in a Recognition Task | < .001 | 1.5 | .999 |
| Delay (hours) Between Item Encoding and Retrieval | −.002 | 4.1 | .093 |
| Delay (min) Between Stress Onset and Encoding | −.017 | 7.5 | .017 |
| Stressor Duration (min) | −.002 | 4.6 | .836 |
| Stress-Induced Δ-Cortisol (nmol/L) | −.022 | 4.5 | .404 |
| Length of Encoding Phase (min) | .033 | 8.5 | .182 |
| Participant Homogeneity | −.013 | 15.2 | .828 |
Note: Significant (p<.05) moderators are shown in boldface font. B represents the change in the effect size for every one-unit change in the moderator. For dummy-coded categorical variables, B represents the difference between estimated effects for each group; for contrast-coded categorical variables, B represents the difference between the group in question and the average estimated effect. If df < 4, there is a twofold greater risk of making a Type I error. The listed p value represents the significance of the moderator in question. When there were not enough studies to estimate an effect, NA is listed in the column for B.
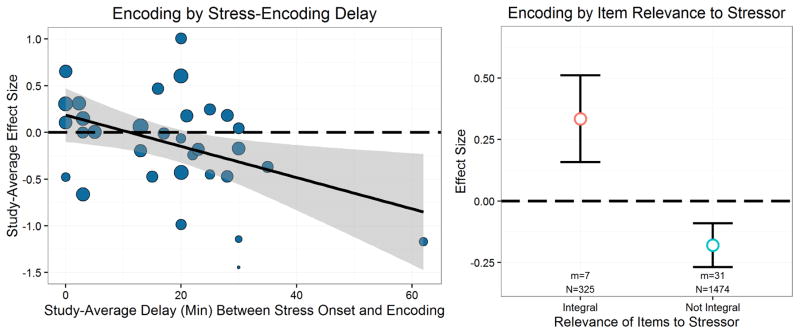
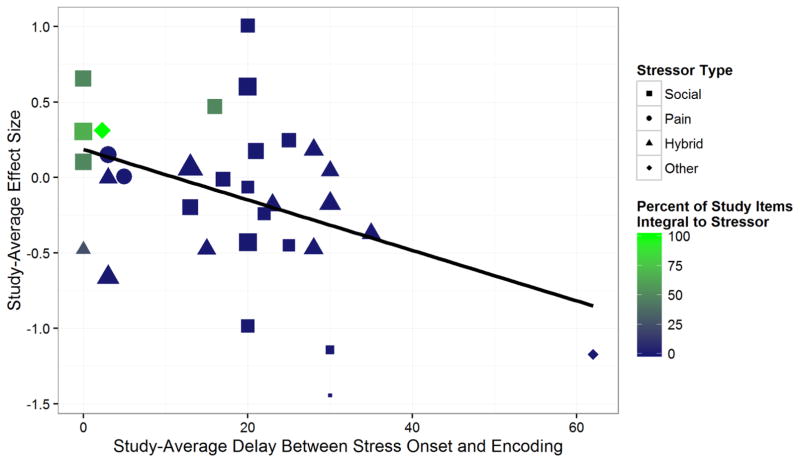
We first examined whether the delay between stress onset and encoding (hereafter stress-encoding delay) moderated the effect of stress on encoding, given a strong theoretical reason to expect this effect (Schwabe et al., 2012). As hypothesized, the stress-encoding delay moderated the effects of stress on encoding, B= −.0167, t(7.5)= −3.06, p=.017 (Figure 4). This slope estimate represents the effect of each minute of the stress-encoding delay, and the intercept represents the effect of stress on encoding when there is no stress-encoding delay. This analysis thus indicates that with no delay between stress onset and the encoding task, stress nonsignificantly enhances encoding, g+=.186, p=.143. As the delay increases, however, the effect of stress on encoding becomes progressively more negative, and at just over 11min post-stressor onset, the effect of stress on encoding begins to impair memory, rather than enhance it. Further, the effect of stress on encoding becomes a significant impairing effect with a stress-encoding delay of approximately 22min.
Figure 4.
Significant moderators of stress effects on encoding. Size of circles in the continuous plot indicates the relative weight given to that study in the analysis. Effects of stress on encoding were moderated by the stress-encoding delay as well as the relevance of the learned items to the stressor.
We next examined whether the relevance of encoding material to the stressor (i.e., integral or nonintegral) moderated the effect of stress on encoding, given prior work suggesting that stress at encoding may enhance, rather than impair, memory when the stimuli are relevant to the stressor (e.g., Wiemers, Sauvage, Schoofs, Hamacher-Dang, & Wolf, 2013). As expected, the relevance of the encoded material to the stressor moderated the effect of stress on encoding, t(6.5)=2.68, p=.034 (Figure 4). When the materials encoded were integral to the stressor, stress tended to enhance encoding, g+=.334, t(4.8)=1.89, p=.119, but when the materials encoded were nonintegral (i.e., not relevant) to the stressor, stress tended to impair encoding, g+= −.180, t(28.3)= −2.02, p=.053.
By examining our data, however, we noted that it is very difficult to disentangle the effects of item relevance from the stress-encoding delay (see Figure 5). That is, an examination of Figure 5 shows that almost all studies that included stressor-relevant items included only very short delays between stress and encoding. Thus, at a study-average level, it is unclear whether the beneficial effects of stress that were observed were due to a short stress-encoding delay or due to the relevance of the items to the stressor. However, although we graphically depict everything at the study-average level for ease of visual interpretation, our statistics operate at the level of individual effects and suggest that both the stress-encoding delay and the relevance of the learned items to the stressor independently moderate stress effects on encoding. We return to this issue below when discussing how to maximize stress effects on encoding.
Figure 5.
Depiction of the interrelations of stressor type, item relevance, and stress-encoding delay. Size of the dots indicates the relative weight given to each study in the analysis. This graph illustrates that at a study-average level it is difficult to disentangle item relevance to the stressor from delay, especially once stressor type is also taken into account.
We also examined whether the time of day the study was conducted moderated the effect of stress on encoding, given previous work suggesting that the effect of cortisol administration on memory encoding depended upon the time of day the study began (Het et al., 2005). Surprisingly, time of day did not moderate stress effects on encoding, either by a linear, B < −.001, t(5.6)= −0.32, p=.762, or quadratic function, Bquadratic< −.001, t(2.4)= −2.41, p=.117. Coding time of day as a categorical variable (i.e., morning and afternoon) rather than as a continuous variable did not alter these results (p=.652).
Additionally, we examined whether the stress-induced increase in cortisol moderated the effect of stress on encoding (cf. Het et al., 2005). Contrary to our expectations, the stress-induced cortisol increase did not moderate stress effects on encoding, B= −.022, t(4.6)= −0.92, p=.404. Because Het et al. found that the effects of cortisol depended upon the time of day that cortisol was administered, we controlled for time of day as well as examined a potential interaction with time of day. Neither of these changes revealed any association between stress-induced cortisol increases and the effect of stress on encoding. There was marginal evidence for an inverted-U moderating effect of stress-induced cortisol increases on stress effects on encoding, Blinear=.066, Bquadratic= −.004, t(3.6)= −2.72, p=.059, with small and large stress-induced cortisol increases tending to impair encoding more than moderate increases. However, closer examination of these data revealed this effect was driven by an outlier, as reflected in the low df—because df are sensitive to variability, they are less than four in this case, and because the df for the quadratic effect are less than 4, there is a twofold increase in the likelihood of making a Type I error. The quadratic effect was no longer close to significance after removing this single outlier (p=.443).
We further examined whether the use of an immediate recall task moderated the effect of stress on encoding (cf. Wolf, 2012). Surprisingly, the use of an immediate recall task did not moderate the effects of stress at encoding, t(11.6)= −0.29, p=.778. Because Wolf (2012) found evidence for this effect in recall with pre-encoding stress, we next restricted the analysis to studies in which stress occurred prior to encoding (rather than including stress-during-encoding studies in the analysis) and focused on only studies with recall as the retrieval task. However, even in this restricted analysis, the use of an immediate recall task did not moderate the effect of stress on encoding, t(9.8)=0.43, p=.679.
We examined potential effects of age, gender, item valence, stressor type, delay between encoding and retrieval, and memory task type across all stress effects on encoding due to the literature’s consistent consideration of these variables as methodologically relevant. Of these potential moderators, none emerged as significant moderators of stress effects on encoding (ps>.05).
Because of the differential effects of stress on encoding as a function of the stress-encoding delay, we examined whether any other potential moderators altered the relation between the stress-encoding delay and the effect of stress on memory. In these analyses, we found that as the percent of male participants increased, the effect of the stress-encoding delay increased in magnitude, t(9.3)= −2.35, p=.043 (i.e., the effect of stress on encoding become more negative as the delay between stress and encoding became longer). This result was also obtained by contrasting studies that excluded women taking hormonal contraceptives (p=.035; see Supplementary Figure 2) or studies that excluded women during their menstrual period (p=.017) with studies that included those participants. Moreover, hybrid pain/socio-evaluative stressors moderated the relation between the stress-encoding delay and stress effects on encoding, t(9.3)=3.57, p=.006—though we should note that there were not enough studies using a pain-based stressor without a social-evaluative component to examine the effects of pain-only stressors on the relation between the stress-encoding delay and stress effects on encoding. If the stressor was a hybrid (pain and social evaluation) stressor such as the SECPT, stress during encoding did not produce an encoding enhancement at short delays but produced a general impairment (g+= −.341), t(6.3)= −3.49, p=.012—unlike a nonhybrid stressor, which enhanced encoding at no delay (g+=.383, t(7.5)=4.25, p=.003). In addition, for nonhybrid stressors, each minute increase in the stress-encoding delay significantly alters the estimated effect of stress on encoding by B= −.026, p=.007. However, if the stressor was a hybrid stressor, the stress-encoding delay was not a significant moderator of the effects of stress on encoding, B=.006, p=.437, and the difference between the slopes for hybrid and non-hybrid stressors was significant (p=.006).
For the benefit of future research, we will attempt to highlight the conditions necessary to produce the biggest stress effects on encoding. We used a forward stepwise regression approach to determine all simultaneously significant moderators (p < .05, one-tailed) in one model, including the largest effect at each step. In this model, we found that the significant moderators were the stress-encoding delay by stressor type interaction and the relevance of studied items to the stressor.
Considering all simultaneously significant moderators controllable by the experimenter in one model, the biggest reliably-obtained enhancing effect of stress on encoding should be obtained when encoding happens during a non-hybrid stressor and the items encoded are relevant to the stressor. The estimated effect size with these moderators at their specified conditions is moderate and significant, g+=0.592, t(4.0)=4.60, p=.010, 95% CIg [0.233, 0.951]. A sample size of 88 (44 stress, 44 control) is necessary to achieve 80% power to detect this effect in a two-tailed test. Similarly, the largest reliable impairing effect of stress on encoding will be obtained by manipulating those same variables within the ranges observed in our study.11 That is, using a non-hybrid stressor, a stress-encoding delay of approximately 35min, and studied items that were unrelated to the stressor, the estimated stress-induced impairment in encoding is moderate and significant, g+= −0.473, t(4.0)= −2.82, p=.048, 95% CIg [−0.941, −0.005]. A sample size of 134 (67 stress, 67 control) is necessary to achieve 80% power to detect this effect in a two-tailed test.
In sum, stress prior to encoding tended to decrease memory unless the delay between stress and encoding was very short or if the materials were stressor-relevant, in which case stress appeared to improve performance. One exception to this pattern was seen with the hybrid socio-evaluative/pain stressor—with these stressor paradigms, stress impaired encoding even at a short delay. In addition, the effect of the stress-encoding delay was reduced in magnitude in studies that included females taking hormonal contraceptives or females that were tested during their menstrual period.
Post-encoding
The overall effect of post-encoding stress on memory (m=23, k=83, N=1,668) was significant, g+=.206, t(21.5)=2.22, p=.037, 95% CIg [.013, .399] (Figure 6), such that post-encoding stress generally enhanced memory. There was some heterogeneity in these effects, τ2=0.26, indicating that this enhancing effect likely differed as a function of moderators. The effects of each potential moderator on post-encoding stress effects are displayed in Table 4.
Figure 6.
Effect of post-encoding stress on memory. Size of the square indicates the relative weight assigned to that study in the analysis. Error bars represent the 95% confidence interval of the effect. Points to the left of zero indicate a study-average impairment in memory, and points to the right of zero indicate a study-average enhancement in memory. This meta-analysis indicated that post-encoding stress significantly enhanced memory across all studies and paradigms.
Table 4.
Potential Moderators of Post-Encoding Stress Effects on Memory
| Moderator | B | df | p |
|---|---|---|---|
| Incidental or Intentional Encoding | .281 | 4.3 | .241 |
| Exclusion of Smokers | −.107 | 2.6 | .528 |
| Exclusion of Women During Menstrual Period | .131 | 15.3 | .541 |
| Exclusion Hormonal Contraceptives Usage | .464 | 20.7 | .018 |
| Exclusion of All Illnesses | .297 | 1.2 | .320 |
| Exclusion of All Psychoactive Medication | .361 | 11.7 | .105 |
| Exclusion of BMI Greater Than 30 | .088 | 1.2 | .533 |
| Use of an Immediate Recall Task Post-Encoding | .078 | 7.9 | .672 |
| Context Change Between Learning and Stress | −.576 | 11.0 | .002 |
| Context Change Between Learning and Retrieval | NA | ||
| Stress Manipulated Between or Within Groups | NA | ||
| Item Valence (Compared to Neutral) | |||
| Negative | .089 | 17.4 | .618 |
| Positive | −.141 | 3.8 | .601 |
| Study Material Type | |||
| Pictures | −.209 | 14.5 | .153 |
| Words | −.333 | 2.9 | .191 |
| Narrative/Slideshow | .100 | 7.9 | .554 |
| Autobiographical | NA | ||
| Other | .442 | 2.5 | .241 |
| Sensory Modality of Study Material Presentation | |||
| Verbal | .297 | 2.8 | .083 |
| Visual | −.119 | 8.2 | .276 |
| Verbal + Visual | −.179 | 5.1 | .110 |
| Memory Task Type | |||
| Free Recall | −.098 | 6.1 | .544 |
| Cued Recall | NA | ||
| Recognition | .098 | 6.1 | .544 |
| Stressor Type | |||
| Socio-Evaluative | .003 | 1.2 | .985 |
| Pain | −.063 | 1.9 | .694 |
| Hybrid (Socio-Evaluative & Pain) | NA | ||
| Other | .060 | 1.1 | .841 |
| Participant Age | .040 | 2.1 | .088 |
| Percent Male Participants | −.001 | 10.6 | .639 |
| Time of Day Study Began | .001 | 4.0 | .014 |
| Study Item List Length | −.002 | 1.6 | .194 |
| Number of Novel Items in a Recognition Task | .001 | 3.0 | .800 |
| Delay (hours) Between Item Encoding and Retrieval | < .001 | 1.1 | .863 |
| Delay (min) Between Encoding and Stress Onset | −.007 | 1.9 | .299 |
| Stressor Duration (min) | −.011 | 2.1 | .479 |
| Stress-Induced Δ-Cortisol (nmol/L) | −.005 | 3.4 | .855 |
| Length of Encoding Phase (min) | −.016 | 1.6 | .387 |
| Participant Homogeneity | .142 | 6.7 | .083 |
Note: Significant (p<.05) moderators are shown in boldface font. B represents the change in the effect size for every one-unit change in the moderator. For dummy-coded categorical variables, B represents the difference between estimated effects for each group; for contrast-coded categorical variables, B represents the difference between the group in question and the average estimated effect. If df < 4, there is a twofold greater risk of making a Type I error. The listed p value represents the significance of the moderator in question. When there were not enough studies to estimate an effect, NA is listed in the column for B.
We first examined whether participant sex would moderate post-encoding stress effects, given previous reports of sex differences in post-encoding stress effects (e.g., McCullough & Yonelinas, 2013). Although the percentage of male participants did not moderate post-encoding stress effects, B= −.001, t(10.6)= −0.48, p=.639, excluding hormonal contraceptive use did moderate post-encoding stress effects on memory, t(20.6)=2.58, p=.018 (Figure 7; see also Supplementary Figure 3 for more detail). Studies that excluded hormonal contraceptive use showed a significant memory enhancing effect of post-encoding stress, g+=.444, t(11.1)=3.34, p=.007, whereas studies that did not exclude hormonal contraceptive use did not show a significant memory enhancing effect of post-encoding stress, g+= −.021, t(10.1)= −0.18, p=.863. These effects did not extend to whether the study excluded women during their menstrual period, p=.541, or—as noted above—to the percentage of male participants in the study. Thus, it appears hormonal contraceptive use in females is a critical factor that negates the memory-enhancing effects of post-encoding stress. We then examined whether the valence of items moderated effects of post-encoding stress on memory, given prior studies suggesting that post-encoding stress enhances memory for negatively-valenced information to a greater extent than neutral information (e.g., Cahill, Gorski, & Le, 2003). Surprisingly, although we found that negatively-valenced materials predicted a slightly greater effect of post-encoding stress than neutral materials, B=.089, this difference was not significant, t(17.4)=0.51, p=.618. Similarly, stress effects on memory for positively-valenced items did not differ from neutral items, t(3.8)= −0.57, p=.601. Thus, post-encoding stress enhanced memory regardless of emotional valence.
Figure 7.
Significant moderators of post-encoding stress effects. Size of circles in the continuous plot indicates the relative weight given to that study in the analysis. Effects of post-encoding stress were moderated by whether the encoding task and stressor were conducted in the same physical context, the inclusion of hormonal contraceptives, and the time of day the study began.
We next examined whether a context change between encoding and the stressor would moderate post-encoding stress effects (cf. Trammell & Clore, 2014). As expected, a change of context from encoding to the stressor moderated post-encoding stress effects, t(11.0)= −3.95, p=.002 (Figure 7). If the context between learning and stress remained constant, the memory-enhancing effect of post-encoding stress was significant, g+=.380, t(14.6)=4.04, p=.001, whereas with a change of context, the effect of post-encoding stress on memory was nonsignificant and tended towards an impairment, g+= −.196, t(5.6)= −1.76, p=.131. Thus, the current results indicate that a change of context between encoding and stress onset can dramatically reduce—and may even reverse—the memory-enhancing effects of post-encoding stress.12
Additionally, we examined whether the stress-induced increase in cortisol was related to the effects of post-encoding stress on memory. Contrary to our expectations, the stress-induced cortisol increase did not moderate post-encoding stress effects in a linear, B= −.005, t(3.4)= −0.20, p=.855 or quadratic function, Blinear= −.086, Bquadratic=.006, t(5.2)=1.51, p=.189.
We also examined potential effects of age, stressor type, memory task type, and time of day the study began because of the literature’s consistent consideration of these variables as methodologically relevant. Of these moderators, only time of day emerged as a significant moderator of post-encoding stress effects, B=.001, t(4.0)=4.18, p=.014 (all other ps>.088). These results indicate that the memory-enhancing effect of post-encoding stress increases as the time of day the study begins is later, and there was no evidence for a quadratic effect of time of day, Blinear=.001, Bquadratic<.001, t(3.2)=0.48, p=.663—although it should be noted the latest any study started analysis was 3 pm (see Figure 7). Thus, post-encoding stress enhances memory more in the afternoon than in the morning.
For the benefit of future research, we will attempt to highlight the conditions necessary to produce the biggest post-encoding stress effect. We used a forward stepwise regression approach to determine all simultaneously significant moderators (p < .05, one-tailed) in one model, including the largest effect at each step. Considering all moderators simultaneously significant together, our analyses indicate that the biggest reliably obtained effect of post-encoding stress would be obtained if a study began at 1pm, excluded women taking hormonal contraceptives, and kept the context constant (i.e., no change of rooms, odors, etc.) between encoding and the stressor. The estimated effect size with these conditions is strong and significant, g+=0.689, t(9.5)=4.85, p<.001, 95% CIg [0.370, 1.009]. A sample size of 70 (35 stress, 35 control) is necessary to achieve 80% power to detect this effect in a two-tailed test. Thus, to obtain a stress-induced enhancement of post-encoding processes, future researchers should begin a study in the afternoon, exclude females using hormonal contraceptives, and keep the context constant between the learning phase and the stressor.
In sum, post-encoding stress generally enhances memory. The enhancing effect of post-encoding stress is stronger if the stressor and encoding task occur later in the day, and if analyses are restricted to only men or to women not using hormonal contraceptives. The enhancing effect of post-encoding stress can be significantly reduced, and was effectively eliminated, with a change in context between encoding and the stressor.
Retrieval
The overall effect of stress on retrieval (m=31, k=102, N=1,410) was significant, g+= −.215, t(28.8)= −3.39, p=.002, 95% CIg [−.346, −.085] (Figure 8), such that stress impaired memory retrieval. There was low heterogeneity across these effects, τ2=0.11, indicating that the impairing effect of stress on retrieval are relatively consistent across various conditions. Nonetheless, we explored the effects of moderators expected a priori to play an important role in the effects of stress on retrieval. The effects of all potential moderators on stress effects on retrieval are displayed in Table 5.
Figure 8.
Effect of retrieval stress on memory. Size of the square indicates the relative weight assigned to that study in the analysis. Error bars represent the 95% confidence interval of the effect. Points to the left of zero indicate a study-average impairment in retrieval, and points to the right of zero indicate a study-average enhancement in retrieval. This meta-analysis indicated that stress significantly impaired retrieval across all studies and paradigms.
Table 5.
Potential Moderators of Retrieval Stress Effects on Memory
| Moderator | B | df | p |
|---|---|---|---|
| Incidental or Intentional Encoding | −.035 | 11.2 | .810 |
| Exclusion of Smokers | −.238 | 26.8 | .075 |
| Exclusion of Women During Menstrual Period | −.070 | 27.0 | .623 |
| Exclusion Hormonal Contraceptives Usage | −.395 | 8.1 | .009 |
| Exclusion of All Illnesses | −.023 | 27.9 | .861 |
| Exclusion of All Psychoactive Medication | −.150 | 9.1 | .352 |
| Exclusion of BMI Greater Than 30 | .091 | 13.3 | .532 |
| Use of an Immediate Recall Task Post-Encoding | −.108 | 22.2 | .379 |
| Context Change Between Learning and Retrieval | .243 | 1.1 | .261 |
| Stress Manipulated Between or Within Groups | .216 | 8.5 | .114 |
| Item Valence (Compared to Neutral) | |||
| Negative | −.166 | 24.8 | .068 |
| Positive | −.248 | 7.1 | .024 |
| Study Material Type | |||
| Pictures | −.059 | 3.5 | .411 |
| Words | −.214 | 20.0 | .025 |
| Narrative/Slideshow | .243 | 2.6 | .192 |
| Autobiographical | .039 | 7.7 | .427 |
| Other | −.008 | 8.7 | .922 |
| Sensory Modality of Study Material Presentation | |||
| Verbal | −.002 | 3.9 | .993 |
| Visual | −.101 | 2.8 | .593 |
| Verbal + Visual | .102 | 1.2 | .790 |
| Memory Task Type | |||
| Free Recall | .023 | 18.7 | .792 |
| Cued Recall | −.052 | 6.0 | .684 |
| Recognition | .028 | 15.4 | .789 |
| Stressor Type | |||
| Socio-Evaluative | −.030 | 18.8 | .704 |
| Pain | −.152 | 3.4 | .386 |
| Hybrid (Socio-Evaluative & Pain) | −.213 | 11.3 | .152 |
| Other | NA | ||
| Participant Age | −.002 | 1.4 | .817 |
| Percent Male Participants | < .001 | 15.4 | .966 |
| Time of Day Study Began | < .001 | 8.8 | .537 |
| Study Item List Length | −.002 | 3.6 | .451 |
| Number of Novel Items in a Recognition Task | < .001 | 7.7 | .984 |
| Delay (hours) Between Item Encoding and Retrieval | < .001 | 1.1 | .875 |
| Delay (min) Between Stress Onset and Retrieval | −.003 | 3.5 | .646 |
| Stressor Duration (min) | .014 | 21.0 | .142 |
| Stress-Induced Δ-Cortisol (nmol/L) | −.001 | 6.4 | .968 |
| Length of Encoding Phase (min) | −.018 | 1.9 | .784 |
| Participant Homogeneity | −.053 | 12.5 | .238 |
Note: Significant (p<.05) moderators are shown in boldface font. B represents the change in the effect size for every one-unit change in the moderator. For dummy-coded categorical variables, B represents the difference between estimated effects for each group; for contrast-coded categorical variables, B represents the difference between the group in question and the average estimated effect. If df < 4, there is a twofold greater risk of making a Type I error. The listed p value represents the significance of the moderator in question. When there were not enough studies to estimate an effect, NA is listed in the column for B.
We first examined whether item valence moderated the effects of stress on retrieval, given previous experimental evidence (Kuhlmann et al., 2005). As expected, stress impaired retrieval of negatively-valenced items (g+= −.303, df=20.5, p=.005) to a marginally greater degree than neutral items (g+= −.136, df=21.3, p=.0499), t(24.8)= −1.91, p=.068 (Figure 9). Similarly, retrieval stress effects on positive items (g+= −.385, df=5.7, p<.001) were significantly more impairing than neutral items, t(7.1)= −2.87, p=.024 (Figure 9). Thus, stress impaired retrieval of emotionally-valenced items more than neutral items.
Figure 9.
Significant moderators of stress effects on retrieval. Effects of stress on retrieval were greater for negative and positive items than for neutral items. In addition, effects of stress on retrieval were greater when women using hormonal contraceptives were excluded from the study.
We next examined whether the delay between stress onset and retrieval (i.e., the stress-retrieval delay) moderated the effect of stress on retrieval, given empirical evidence suggesting this delay as a moderator (Schwabe & Wolf, 2014). Unexpectedly, the stress-retrieval delay did not moderate the effects of stress on retrieval, B= −.003, t(3.5)= −0.50, p=.646. Coding this analysis as the delay between stress offset—rather than onset—and retrieval (cf. Schwabe & Wolf 2014) did not alter this result; that is, the stress offset to retrieval delay did not moderate effects of stress on retrieval either, p=.311.
We also examined whether the stress-induced cortisol increase moderated the effects of stress on retrieval. Contrary to our expectations, the stress-induced cortisol increase did not moderate the effects of stress on retrieval, B= −.001, t(6.4)= −0.04, p=.968, nor was there any evidence for a quadratic moderating effect, Blinear= −.047, Bquadratic=.003, t(6.0)=0.98, p=.363. Controlling for all variables—individually or concurrently—that influenced the cortisol response to stress in our data (see section 4.1) did not alter the lack of effect of stress-induced cortisol increases on stress effects on retrieval.
We examined potential effects of age, sex, exclusion of hormonal contraceptives, exclusion of women during their menstrual period, stressor type, memory task, the delay between encoding and retrieval, and time of day the study began, because of the literature’s consistent consideration of these variables as methodologically relevant. We did not have enough studies with a different context at encoding and retrieval to examine potential moderating effects of this context change on stress effects on retrieval. Of these variables, only exclusion of contraceptives emerged as a moderator of stress effects on retrieval (all other ps>.185), t(8.1)= −3.44, p=.009 (Figure 9; see Supplementary Figure 4 for more detail). If a study examining stress effects on retrieval did not exclude women taking hormonal contraceptives, the effect of stress on retrieval was negligible, g+=.101, t(5.2)=1.15, p=.302. If, however, a study examining stress effects on retrieval excluded women taking hormonal contraceptives, the impairing effect of stress on retrieval was significant, g+= −.294, t(23.2)= −4.14, p<.001.
To identify the conditions necessary to produce the biggest stress effect on retrieval we used a forward stepwise regression to determine all simultaneously significant moderators (p < .05, one-tailed). Considering all simultaneously significant moderators together, we found that the biggest reliably obtained effect of stress on retrieval would occur if a study examined effects on positively- or negatively-valenced items and excluded women taking hormonal contraceptives. The estimated effect size with these conditions is moderate and significant, g+= −0.388, t(19.7)= −4.51, p<.001, 95% CIg [−0.567, −0.208]. A sample size of 202 (101 stress, 101 control) is necessary to achieve 80% power to detect this effect in a two-tailed test. The effect of stress on retrieval of neutral items with these conditions (g+= −0.229) is significantly smaller t(25.0)= 2.24, p=.034. We should also note that all studies of stress effects on retrieval with negative items included a mix of neutral items in the study list, and all studies with positive items included a mix of negative and neutral items in the study list. As such, our data cannot address whether valence itself moderates stress effects on retrieval, or if emotional valence coupled with neutral items moderates stress effects on retrieval. Thus, to obtain a stress-induced impairment on retrieval, future researchers should use a study list with a mixture of emotional and neutral items, examine effects on emotionally-valenced items, and exclude women taking hormonal contraceptives.
In sum, stress prior to retrieval generally led to a decrease in memory. These effects were larger for negative and positive materials than neutral materials, and these effects were reduced in magnitude if women who were taking contraceptives were included in the study.
Post-encoding/retrieval
We considered stress to affect both post-encoding and retrieval processes (i.e., post-encoding/retrieval) if stress onset occurred within 60min post-encoding and stress offset occurred within 90min of retrieval. For all studies fitting these criteria, stress onset occurred within 20min of encoding, and retrieval occurred within 35min of stress offset. The overall effect of stress on post-encoding/retrieval (m=9, k=15, N=697) was not significant, g+=.004, t(7.7)=0.03, p=.974, 95% CIg [−.279, .287] (Figure 10). This null effect is not surprising, given the prior analyses revealing that post-encoding stress generally enhances memory whereas retrieval stress impairs memory. There was low heterogeneity in these effects, τ2=0.09, indicating that this nonsignificant effect likely did not differ as a function of moderators. Nonetheless, we explored the effects of moderators expected a priori to play an important role in post-encoding or retrieval stress effects as well as potential methodologically-relevant moderators. In the interest of assisting future researchers with study design, the effects of all potential moderators of stress effects on post-encoding/retrieval are displayed in Table 6.
Figure 10.
Effects of post-encoding/retrieval stress on memory. Size of the square indicates the relative weight assigned to that study in the analysis. Error bars represent the 95% confidence interval of the effect. Points to the left of zero indicate a study-average impairment in memory, and points to the right of zero indicate a study-average enhancement in memory. This meta-analysis indicated that stress that impacted both the post-encoding and retrieval phases of memory did not influence memory across all studies and paradigms.
Table 6.
Potential Moderators of Post-Encoding/Retrieval Stress Effects on Memory
| Moderator | B | df | p |
|---|---|---|---|
| Incidental or Intentional Encoding | −.435 | 4.3 | .146 |
| Exclusion of Smokers | NA | ||
| Exclusion of Women During Menstrual Period | .627 | 3.3 | .048 |
| Exclusion Hormonal Contraceptives Usage | .644 | 1.4 | .274 |
| Exclusion of All Illnesses | NA | ||
| Exclusion of All Psychoactive Medication | NA | ||
| Exclusion of BMI Greater Than 30 | NA | ||
| Use of an Immediate Recall Task Post-Encoding | .158 | 3.9 | .557 |
| Context Change Between Learning and Stress | −.046 | 1.8 | .823 |
| Context Change Between Learning and Retrieval | −.507 | 1.5 | .248 |
| Stress Manipulated Between or Within Groups | −.578 | 1.6 | .155 |
| Item Valence (Compared to Neutral) | |||
| Negative | −.018 | 1.6 | .938 |
| Positive | NA | ||
| Study Material Type | |||
| Pictures | NA | ||
| Words | −.195 | 5.1 | .452 |
| Narrative/Slideshow | NA | ||
| Autobiographical | .030 | 3.7 | .895 |
| Other | .165 | 1.6 | .731 |
| Sensory Modality of Study Material Presentation | |||
| Verbal | −.348 | 4.3 | .267 |
| Visual | .348 | 4.3 | .267 |
| Verbal + Visual | NA | ||
| Memory Task Type | |||
| Free Recall | −.216 | 5.8 | .450 |
| Cued Recall | NA | ||
| Recognition | .216 | 5.8 | .450 |
| Stressor Type | |||
| Socio-Evaluative | .152 | 4.7 | .479 |
| Pain | .049 | 1.4 | .669 |
| Hybrid (Socio-Evaluative & Pain) | NA | ||
| Other | −.403 | 1.3 | .246 |
| Participant Age | −.098 | 3.4 | .203 |
| Percent Male Participants | .003 | 4.1 | .697 |
| Time of Day Study Began | −.002 | 1.6 | .175 |
| Study Item List Length | .004 | 2.1 | .371 |
| Number of Novel Items in a Recognition Task | .026 | 1.0 | .569 |
| Delay (hours) Between Item Encoding and Retrieval | −.031 | 3.6 | .961 |
| Delay (min) Between Encoding and Stress Onset | −.021 | 1.9 | .459 |
| Delay (min) Between Stress Onset and Retrieval | .001 | 1.7 | .975 |
| Stressor Duration (min) | .008 | 4.8 | .584 |
| Stress-Induced Δ-Cortisol (nmol/L) | .100 | 2.4 | .222 |
| Length of Encoding Phase (min) | −.010 | 1.4 | .271 |
| Participant Homogeneity | .302 | 2.9 | .063 |
Note: Significant (p<.05) moderators are shown in boldface font. B represents the change in the effect size for every one-unit change in the moderator. For dummy-coded categorical variables, B represents the difference between estimated effects for each group; for contrast-coded categorical variables, B represents the difference between the group in question and the average estimated effect. If df < 4, there is a twofold greater risk of making a Type I error. The listed p value represents the significance of the moderator in question. When there were not enough studies to estimate an effect, NA is listed in the column for B.
We examined whether the delay between stress onset and retrieval, sex (including hormonal contraceptive use), age, memory task type, stress-induced cortisol increase, study-item valence, a context change between learning and stress, stressor type, delay between encoding and retrieval, and the time of day the study began moderated the effect of stress on post-encoding/retrieval. None of these potential moderators, however, influenced effects of stress on post-encoding/retrieval, ps>.174, although we should note that with only nine studies examining stress effects on post-encoding/retrieval, we likely lacked the power necessary to detect any subtle moderating effects.
In sum, we did not find any reliable effect of stress on memory when the stressor impacted both the post-encoding period and the retrieval period. This is broadly consistent with the results described above, in that when stress selectively impacts the post-encoding period it enhances memory, whereas when stress selectively impacts retrieval it impairs memory; thus, stress has no overall effect when it influences both post-encoding and retrieval processes.
Encoding/retrieval
We considered stress to affect encoding, post-encoding, and retrieval processes (i.e., encoding/retrieval) when stress offset occurred prior to or during encoding and within 90min of retrieval. For all such studies, stress offset ranged from 35min before encoding to during encoding, and retrieval occurred with 65 min of stress offset (with encoding and retrieval being separated by 60 minutes at most). The overall effect of stress on encoding/retrieval (m=16, k=48, N=1,148) was marginally significant and negative, g+= −.185, t(14.2)= −2.02, p=.062, 95% CIg [−.382, .011] (Figure 11). There was some heterogeneity in these effects, τ2=0.12, indicating that this impairing effect might differ as a function of moderators. The effects of all potential moderators on stress effects on encoding/retrieval are displayed in Table 7.
Figure 11.
Effect encoding/retrieval stress on memory. Size of the square indicates the relative weight assigned to that study in the analysis. Error bars represent the 95% confidence interval of the effect. Points to the left of zero indicate a study-average impairment in memory, and points to the right of zero indicate a study-average enhancement in memory. This meta-analysis indicated that stress that impacted encoding, post-encoding, and retrieval phases of memory marginally impaired memory across all studies and paradigms.
Table 7.
Potential Moderators of Encoding/Retrieval Stress Effects on Memory
| Moderator | B | df | p |
|---|---|---|---|
| Incidental or Intentional Encoding | −.076 | 3.0 | .764 |
| Exclusion of Smokers | .269 | 5.3 | .130 |
| Exclusion of Women During Menstrual Period | .038 | 13.7 | .848 |
| Exclusion Hormonal Contraceptives Usage | −.025 | 12.6 | .897 |
| Exclusion of All Illnesses | .074 | 5.0 | .775 |
| Exclusion of All Psychoactive Medication | .328 | 12.1 | .085 |
| Exclusion of BMI Greater Than 30 | −.060 | 1.3 | .938 |
| Use of an Immediate Recall Task Post-Encoding | .029 | 13.3 | .888 |
| Context Change Between Stress and Learning | −.181 | 4.2 | .381 |
| Context Change Between Learning and Retrieval | NA | ||
| Stress Manipulated Between or Within Groups | −.068 | 8.0 | .771 |
| Item Valence (Compared to Neutral) | |||
| Negative | .066 | 3.6 | .611 |
| Positive | .213 | 2.3 | .180 |
| Study Material Type | |||
| Pictures | NA | ||
| Words | NA | ||
| Narrative/Slideshow | NA | ||
| Autobiographical | NA | ||
| Other | NA | ||
| Sensory Modality of Study Material Presentation | |||
| Verbal | .017 | 8.7 | .898 |
| Visual | −.192 | 2.7 | .389 |
| Verbal + Visual | .175 | 5.0 | .274 |
| Memory Task Type | |||
| Free Recall | .062 | 6.0 | .554 |
| Cued Recall | NA | ||
| Recognition | −.043 | 3.1 | .774 |
| Stressor Type | |||
| Socio-Evaluative | .126 | 4.7 | .422 |
| Pain | NA | ||
| Hybrid (Socio-Evaluative & Pain) | .368 | 1.5 | .181 |
| Other | −.494 | 2.9 | .159 |
| Participant Age | −.005 | 2.0 | .489 |
| Percent Male Participants | −.003 | 8.8 | .377 |
| Time of Day Study Began | −.001 | 5.2 | .210 |
| Study Item List Length | −.017 | 2.3 | .024 |
| Number of Novel Items in a Recognition Task | −.009 | 1.5 | .644 |
| Delay (hours) Between Item Encoding and Retrieval | .554 | 5.2 | .015 |
| Delay (min) Between Stress Onset and Encoding | −.001 | 1.3 | .556 |
| Delay (min) Between Stress Onset and Retrieval | <−.001 | 1.4 | .999 |
| Stressor Duration (min) | −.001 | 1.3 | .748 |
| Stress-Induced Δ-Cortisol (nmol/L) | −.026 | 2.1 | .475 |
| Length of Encoding Phase (min) | −.014 | 1.4 | .907 |
| Participant Homogeneity | .051 | 10.2 | .476 |
Note: Significant (p<.05) moderators are shown in boldface font. B represents the change in the effect size for every one-unit change in the moderator. For dummy-coded categorical variables, B represents the difference between estimated effects for each group; for contrast-coded categorical variables, B represents the difference between the group in question and the average estimated effect. If df < 4, there is a twofold greater risk of making a Type I error. The listed p value represents the significance of the moderator in question. When there were not enough studies to estimate an effect, NA is listed in the column for B.
The only variable found to modulate the negative effect of stress on encoding/retrieval with df > 4 was the delay in hours between encoding and retrieval, B=.554, t(5.2)=3.59, p=.015, indicating that the impairing effect of stress became smaller as the delay between encoding and retrieval increased (Figure 12). Additional analyses indicated that the biggest stress-induced impairment of encoding/retrieval would be obtained if there was a zero-minute delay (e.g., only a very brief interfering task) between stress and retrieval, g+= −0.312, t(7.8)= −3.39, p=.010, 95% CIg [−0.525, −0.098]. A sample of 314 participants (157 stress, 157 control) is necessary to achieve 80% power to detect this effect in a two-tailed test.
Figure 12.
Delay between encoding and retrieval moderated effects of stress on memory when the stressor occurred within a timeframe to influence encoding, post-encoding, and retrieval phases. With less time between encoding and retrieval, stress at encoding/retrieval impaired memory to a greater extent than with more time.
In sum, stress on average impairs memory when the stressor impacts the encoding, post-encoding and retrieval periods. This impairment is greatest at a short delay between stress offset and retrieval and attenuates as that delay increases. The results are broadly consistent with the earlier results, in the sense that stress during retrieval and during encoding sometimes impaired memory, whereas post-encoding stress usually enhanced memory.
Post-reactivation
We considered stress to affect post-reactivation processes when stress followed reactivation of a memory no more than 60min post-reactivation, and when learning, reactivation, and each retrieval phases took place on separate days. The overall effect of post-reactivation stress on memory (m=10, k=20, N=344) was not significant, g+=.154, t(8.9)= 0.66, p=.526, 95% CIg [−0.375, 0.683] (Figure 13). There was approximately moderate heterogeneity in these effects, τ2=0.82, indicating that this nonsignificant effect likely differed as a function of moderators. We should note, however, that with only 10 post-reactivation studies we did not have the power to fully test these moderating effects. The effects of all potential moderators on post-reactivation stress effects on memory are displayed in Table 8.
Figure 13.
Effects of reactivation stress on memory. Size of the square indicates the relative weight assigned to that study in the analysis. Error bars represent the 95% confidence interval of the effect. Points to the left of zero indicate a study-average impairment in memory, and points to the right of zero indicate a study-average enhancement in memory. This meta-analysis indicated that post-reactivation stress did not influence memory across all studies and paradigms.
Table 8.
Potential Moderators of Reactivation Stress Effects on Memory
| Moderator | B | df | p |
|---|---|---|---|
| Incidental or Intentional Encoding | .051 | 6.7 | .923 |
| Exclusion of Smokers | .629 | 7.9 | .205 |
| Exclusion of Women During Menstrual Period | −.145 | 1.6 | .893 |
| Exclusion Hormonal Contraceptives Usage | −.030 | 1.7 | .977 |
| Exclusion of All Illnesses | .128 | 4.0 | .769 |
| Exclusion of All Psychoactive Medication | .951 | 1.6 | .125 |
| Exclusion of BMI Greater Than 30 | NA | ||
| Use of an Immediate Recall Task Post-Encoding | .567 | 1.6 | .224 |
| Stressor Relevance of Items (Integral/Nonintegral) | NA | ||
| Context Change Between Reactivation and Stress | 1.196 | 3.8 | .007 |
| Context Change Between Learning and Retrieval | .421 | 1.4 | .604 |
| Stress Manipulated Between or Within Groups | NA | ||
| Item Valence (Compared to Neutral) | |||
| Negative | .346 | 6.3 | .554 |
| Positive | .535 | 4.0 | .360 |
| Study Material Type | |||
| Pictures | NA | ||
| Words | .289 | 3.8 | .298 |
| Narrative/Slideshow | NA | ||
| Autobiographical | −.786 | 1.6 | .183 |
| Other | .497 | 5.1 | .220 |
| Sensory Modality of Study Material Presentation | |||
| Verbal | NA | ||
| Visual | .162 | 4.1 | .502 |
| Verbal + Visual | .338 | 1.2 | .182 |
| Memory Task Type | |||
| Free Recall | .129 | 6.8 | .598 |
| Cued Recall | −.156 | 6.8 | .526 |
| Recognition | NA | ||
| Stressor Type | |||
| Socio-Evaluative | NA | ||
| Pain | .296 | 6.5 | .324 |
| Hybrid (Socio-Evaluative & Pain) | −.296 | 6.5 | .324 |
| Other | NA | ||
| Participant Age | .370 | 3.8 | .176 |
| Percent Male Participants | .006 | 2.3 | .718 |
| Time of Day Study Began | .002 | 1.8 | .202 |
| Study Item List Length | .007 | 2.1 | .247 |
| Number of Novel Items in a Recognition Task | NA | ||
| Delay (hours) Between Item Encoding and Retrieval | −.006 | 3.5 | .083 |
| Delay (min) Between Reactivation and Stress Onset | −.109 | 1.5 | .207 |
| Stressor Duration (min) | −.037 | 1.7 | .224 |
| Stress-Induced Δ-Cortisol (nmol/L) | −.001 | 2.8 | .994 |
| Length of Encoding Phase (min) | −.014 | 1.4 | .655 |
| Participant Homogeneity | .379 | 2.5 | .124 |
Note: The context change between reactivation and stress should not be trusted; see the main text for an explanation. Significant (p<.05) moderators are shown in boldface font. B represents the change in the effect size for every one-unit change in the moderator. For dummy-coded categorical variables, B represents the difference between estimated effects for each group; for contrast-coded categorical variables, B represents the difference between the group in question and the average estimated effect. If df < 4, there is a twofold greater risk of making a Type I error. The listed p value represents the significance of the moderator in question. When there were not enough studies to estimate an effect, NA is listed in the column for B.
We first examined whether stress-induced cortisol increases moderated the effect of post-reactivation stress on memory. We found a marginal quadratic relation between stress-induced cortisol increases and post-reactivation stress effects on memory, Blinear= −.887, t(2.4)= −3.63, p=.053, Bquadratic= .095, t(2.1)= 4.08, p=.052, with small and large post-reactivation stress-induced cortisol increases tending to enhance memory, but moderate cortisol increases tending to impair memory. However, because the df are less than 4 in the above analyses, there is a twofold greater risk of making a Type I error.
We next examined whether a change in context between memory reactivation and stress moderated the effect of post-reactivation stress on memory, and found that a change in context between memory reactivation and stress moderated the effect of post-reactivation stress on memory, t(3.8)=5.28, p=.007, such that a change in context between reactivation and stress onset was associated with an enhancement of memory, g+=1.01, t(2.0)=18.3, p=.003, whereas a constant context was associated with a nonsignificant impairment in memory, g+= −.191, t(5.9)= −0.87, p=.419. However, because df are less than four in the above contrast, there is a twofold greater risk of making a Type I error. In addition, it is important to note that all studies that employed a context change between reactivation and stress without a change of context between encoding and reactivation were conducted in the same laboratory using the same paradigm, thus holding numerous factors constant, whereas none of the studies with a constant context between reactivation and stress used the same paradigm. Thus, it is unclear whether a change in context between reactivation and stress actually produced an enhancing effect of post-reactivation stress on memory or if another methodological factor was responsible.
We also examined the effects of sex, the exclusion of women taking hormonal contraceptives, and the exclusion of women during their menstrual period, and found that none of these variables influenced the effects of post-reactivation stress on memory, ps>.717. Finally, we examined the delay between reactivation and stress onset, the delay between encoding and retrieval, participant age, time of day, item valence, memory task type, and stressor type, given the tendency in the stress and memory literature to treat these variables as methodologically relevant. None of these variables were found to be significant moderators, ps>.175.
In sum, we did not find meta-analytic evidence for an overall post-reactivation stress effect on memory or any reliable moderators of this potential stress effect. However, given the small number of reactivation studies that have been published, and the notable heterogeneity in observed effects (cf. Table 2 demonstrating the low power of these analyses), we hesitate to draw any strong conclusions about post-reactivation stress effects (or lack thereof) until more research has been conducted.
Discussion
In this meta-analysis, we examined the effects of acute social and physical stressors on episodic memory. We assessed the results of 113 studies, which included a total of 6,216 participants, that examined the effects of stress on encoding, post-encoding, retrieval, and post-reactivation phases. We explored the effects of several variables that previous studies indicated might be critical, as well as several methodological factors that were available in most studies. In general, we found that whether stress enhanced or impaired memory critically depended upon whether stress occurred during the encoding, retention, or retrieval phase. Moreover, there was evidence that the timing of the stressor, the context in which the stressor was experienced, and the nature of the studied materials played critical roles in determining how stress impacted the different memory phases. There was little evidence that the effects of stress on memory were modulated by the overall cortisol changes produced by stress in individual studies, but the effects of stress on memory were generally reduced in women taking hormonal contraceptives. We first discuss the effects of stress on cortisol, and we then describe the effects of stress on each phase of memory. We then consider those results in light of current theories of stress and memory, and highlight questions for further research.
Stress and Cortisol
Given that cortisol is thought to provide an index of stress and has been proposed to moderate the effects of stress on memory, we first assessed the ability of acute laboratory stressors to elicit cortisol responses. We found that stress significantly increased cortisol, and the TSST elicited a larger cortisol increase than either the CPT or SECPT—which is consistent with individual studies that have compared these stressors (Giles, Mahoney, Brunyé, Taylor, & Kanarek, 2014; Schwabe, Haddad, & Schachinger, 2008; Skoluda et al., 2015). Moreover, studies that performed the stress manipulation in the afternoon showed a significantly larger cortisol increase than studies that began in the morning—consistent with an earlier meta-analysis (Dickerson & Kemeny, 2004).
Additionally, our analyses indicated that stress elicited larger cortisol responses as the percentage of males included in the study increased, and revealed larger cortisol responses in studies that excluded women who were taking hormonal contraceptives or women in their menstrual phase. This is consistent with previous work showing that men exhibit consistently higher cortisol responses to laboratory stressors than women (Kirschbaum et al., 1992) and that both hormonal contraceptives and menstrual cycle phase blunt or modulate cortisol responses to stress (Kirschbaum et al., 1999; Marinari et al., 1976). The finding that excluding women during their menstrual period increased effects of stress on cortisol may seem surprising because of the suppressive effects of estrogen on cortisol responses (Ycaza Herrera & Mather, 2015) and because estrogen is low during the menstrual period. One possible explanation for this effect is that progesterone increases stress responses (Sakaki & Mather, 2012) and excluding women in the menstrual phase may yield a larger proportion of women in the luteal—high progesterone—phase of their cycle.
In sum, to the extent that cortisol provides an index of the stress response, it appears that the stress induction methods used in studies of stress and memory consistently produce stress responses. These analyses therefore showed that acute stress inductions in the stress and memory literature are successful, and thus put us in a position to make valid claims about effects of acute stress on memory.
Encoding
Overall, there was no significant main effect of stress on encoding, but several factors significantly influenced whether stress enhanced or impaired memory encoding. Two of the strongest moderators of stress effects on encoding were the delay between stress and encoding (i.e., the stress-encoding delay) and the relevance of the learned information to the stressor (i.e., stressor relevance). In general, the analyses indicated that stress impaired encoding unless the items learned were related to the stressor and the stress-encoding delay was very short, in which case stress appeared to enhance encoding.
To our knowledge, only two human studies have directly manipulated the stress-encoding delay to examine time-dependence effects of pre-encoding stress. The first of these (Zoladz et al., 2011) found weak support for the idea that the stress-encoding delay plays an important role. Zoladz et al. found a memory enhancement for only positive items (and no effect for negative or neutral items) in a no delay condition and an impairment in only negative items in a 30 minute delay condition. The second study (Quaedflieg, Schwabe, Meyer, & Smeets, 2013), however, failed to find any behavioral differences in stress effects on encoding between the immediate and 30 minute delay conditions. Importantly, however, both of these studies employed a hybrid socio-evaluative/pain stressor, and our results indicate that this stressor type—or any pain-based stressor, see below—may have prevented actual effects of the stress-encoding delay from emerging. Future studies testing this possibility are needed.
We also found evidence that the stressor type appeared to moderate the stress-encoding delay effect. That is, in general, as the delay between stress and encoding increased, the effects of stress became more negative. However, this pattern was not observed in studies that used the hybrid socio-evaluative/pain stressors (i.e., the SECPT), where stress appeared to lead to a general impairment in encoding, regardless of delay. When interpreting this finding it is important to note that we did not have enough power to determine whether this effect was specific to hybrid stressors, or if stressors that only included pain, such as the CPT, also showed this effect, as only two studies of stress at encoding used a stressor involving pain without social evaluation. Thus, one possibility is that pain-based stressors might be unique. For example, they may require continuous response inhibition (i.e., not retracting one’s arm from painful ice water), and this inhibition may have a general impairing effect on memory encoding regardless of delay (Chiu & Egner, 2015). Alternatively, it is possible that the combination of the social and physical stress itself is what is producing disruptive effects on memory even at short stress-encoding delays. Future research contrasting the delay effects of different stressors will be important in resolving this issue.
The significant moderating effect of stressor relevance is consistent with several previous studies that directly examined this factor. For example, Wiemers et al. (2013) and Smeets, Giesbrecht, Jelicic, and Merckelbach (2007) both found that stress prior to or during encoding enhanced memory only for information relevant to the stressor. These results indicate that stressor relevance is important in determining the effects of stress on encoding.
Sex and exclusion of hormonal contraceptives significantly moderated stress-encoding delay effects, although sex was no longer a significant moderator once the exclusion of hormonal contraceptives was controlled (data not shown). These results are consistent with prior research indicating that sex hormones modulate memory encoding processes; namely, that testosterone (Ackermann et al., 2012; van Wingen, Mattern, Verkes, Buitelaar, & Fernández, 2008) and estradiol (Kramár, Babayan, Gall, & Lynch, 2013; Srivastava et al., 2011) enhance memory encoding. Because hormonal contraceptive use decreases both estradiol and testosterone (Graham & Milad, 2013; Liening, Stanton, Saini, & Schultheiss, 2010), it is not surprising that hormonal contraceptives might blunt stress effects on encoding.
Surprisingly, stress-induced cortisol increases did not significantly moderate stress effects on encoding, nor did we find any evidence for the effects of time-of-day. This contrasts with an earlier meta-analysis that found a direct effect of cortisol administration and time-of-day on memory encoding (Het et al., 2005). In our analyses, controlling for the time of day only weakened the nonsignificant effect of cortisol further (data not shown). We expound upon the lack of observed cortisol effects in the Theoretical Integration section below. In addition, we did not observe a moderating effect of using an immediate recall task in stress effects on encoding, as was expected from a prior study (Wolf, 2012).
The valence of study items (i.e., positive, negative, neutral) is commonly assessed as a potential moderator of stress effects on encoding (e.g., van Ast, Cornelisse, Meeter, & Kindt, 2014; Wolf, 2012; Zoladz et al., 2011), but in this meta-analysis we failed to find any evidence that stress influenced encoding items of one valence more than another. One explanation for this lack of effect might be that oftentimes studies present participants with a mixed list of neutral and emotional items together. It is possible that mixing item valences diminishes any interaction between stress and item valence, obscuring effects. To assess this possibility further, we controlled for the valences included in the study list (data not shown) and found that it did not alter the results, nor did the study list valences interact with memory for specific item valence in producing stress effects on encoding.
In sum, stress prior to encoding led to a decrease in memory unless the delay between the stressor and encoding was very short and the materials were relevant to the stressor. In addition, the effect of the stress-encoding delay was reduced in women on hormonal contraceptives and in women who were menstruating, highlighting the potential importance of sex hormones in the stress effects on encoding. Surprisingly, we found no moderating effects of cortisol reactivity or time of day (despite our finding that time of day significantly influenced cortisol reactivity). In addition, we found no moderating effects of item valence, or the use of an immediate recall task, despite prior work suggesting that these variables may moderate stress effects on encoding. This suggests that the effects of these moderators may be relatively specific to precise experimental conditions, rather than generalizing across studies of stress.
Post-Encoding Stress
Post-encoding stress led to a general increase in episodic memory, but there were several important moderators of this effect. Most notably, when the stress manipulation was administered in the same context as the initial encoding phase, stress led to an increase in memory, whereas in studies in which the context was changed between encoding and stress (e.g., moving to another room) stress did not improve memory. This is consistent with a recent report that found that post-encoding stress did not benefit memory across a series of experiments, each of which involved a context change between the encoding phase and the stressor (Trammell & Clore, 2014; see also McCullough et al., 2015). Most other studies in the literature that found an enhancing effect of post-encoding stress on memory did not have a context change between encoding and stress. No published studies have directly examined the effects of changing the context of the post-encoding stressor, so studies that directly examine the effects of changing context within a single experiment will be important in verifying the importance of context changes on stress and memory.
Another significant moderator of the post-encoding effects was time of day, which indicated that studies that began in the afternoon showed a larger effect than studies that began in the morning. As described earlier, time of day can affect the cortisol response; what is interesting here is that time of day did not interact with cortisol change, so in these post-encoding stress studies, time of day seems to have some other effect on memory in addition to or independent of any effect it may have on the cortisol response. In addition, contrary to our expectations, cortisol change in response to stress did not moderate the effects of post-encoding stress on memory (either linearly or quadratically).
Despite no association of post-encoding stress effects with effects of stress on cortisol, we found that contraceptive use was a significant moderator of the stress effects on memory. If a study excluded women taking hormonal contraceptives, memory was significantly better for the post-encoding stress group, whereas studies that did not exclude women taking hormonal contraceptives did not find beneficial effects of stress on memory. Participant sex was not found to moderate post-encoding stress effects on memory, so contraceptive use appears to be a critical factor for whether studies find an enhancing effect of post-encoding stress on memory or not, rather than participant sex. Contraceptive use also did not interact with cortisol change, suggesting that dampening the cortisol response in itself (see analyses of stress effects on cortisol) does not fully explain the contraceptive effects. Instead, this suggests that contraceptives may act through pathways aside from cortisol to dampen the stress effects on memory. One means by which this might happen is via effects of estradiol, which both increases as a result of stress (Lennartsson, Kushnir, Bergquist, Billig, & Jonsdottir, 2012) and enhances memory retention (T. Inagaki et al., 2010). Because hormonal contraceptives reduce ovarian estradiol output, leading to decreased levels of estradiol (Graham & Milad, 2013), hormonal contraceptives may blunt effects of post-encoding stress because of their effects on estradiol (see also Barros et al., 2015). Conversely, estradiol exerts opposing effects on cortisol (Ycaza Herrera & Mather, 2015), which may be a mechanism through which hormonal contraceptives modulate effects of stress on memory.
Contrary to expectations, effects of post-encoding stress on memory did not differ for negative, positive, or neutral materials. Thus, although it is possible additional experimental conditions moderate the extent to which valence plays a role, we did not find evidence for valence being an important moderator of post-encoding stress effects on memory when considered across experiments.
In sum, post-encoding stress generally improved memory. However, this effect appeared to be eliminated when the context between encoding and stress was changed, indicating that post-encoding stress may be limited to improving memory for items that were encoded in the same spatial context as the stressor. Although the effects of post-encoding stress on memory were not related to cortisol reactivity or sex, the beneficial effects of stress were reduced when women taking hormonal contraceptives were included, and when the study was conducted in the morning rather than the afternoon, suggesting some hormonal or immune system sensitivity of the stress effects. Finally, contrary to expectations, material valence did not emerge as a significant moderator of post-encoding stress effects on memory.
Retrieval
Stress generally impaired memory retrieval, and these effects were largest for emotional materials and in studies in which women taking oral contraceptives were excluded. The finding that stress impaired retrieval of negative and positive items more so than neutral items is consistent with a recent review that highlighted the relatively greater impairing effect of stress on retrieving emotional material (Gagnon & Wagner, 2016).
Similar to effects observed for encoding and post-encoding stress, studies that excluded women taking oral contraceptives showed a greater effect of stress on memory retrieval. Specifically, when a study did not exclude women taking hormonal contraceptives from participating, there was no significant impairing effect of stress on retrieval. In contrast, if a study excluded women taking hormonal contraceptives, stress significantly impaired retrieval. Like results discussed in previous sections, this result implies that sex hormones may be more important for stress effects on retrieval than previously thought, highlighting the importance of considering these hormones when examining stress effects on memory.
We found that the delay between stress onset and retrieval (i.e., the stress-retrieval delay) did not moderate stress effects on retrieval. This result did not differ when we examined the delay between stress offset, rather than onset, and retrieval (data not shown). This contrasts with a recent study that found that the negative effects of stress on retrieval were greatest at 90min post-stressor (Schwabe & Wolf, 2014). However, we note that all the other studies considered in this meta-analysis tested retrieval within 40min of stressor offset; thus, more research with longer delays between stress and retrieval would be important before making strong claims about this potential moderator.
In sum, stress exerted a relatively consistent impairing effect on memory retrieval. Although this effect did not seem to be related to stress-induced cortisol responses, the impairing effect of stress on retrieval was greater when information to be retrieved was emotionally charged, as well as when considering only men, or women not taking hormonal contraceptives.
Post-Encoding/Retrieval
When the stressor was expected to influence both the post-encoding period and the retrieval phase, we found no significant effect of stress, and there was no indication that the effects were moderated by any other variables. Although the lack of effects could be due to the relatively smaller number of studies of this type (nine studies), the results are consistent with the main conclusions described above in that post-encoding stress generally increased memory whereas retrieval stress generally reduced memory. Thus, it makes sense that there would be no overall effect of stress in studies in which the stressor impacts both post-encoding and retrieval phases.
Encoding/Retrieval
Although we did not have strong a priori hypotheses for stress effects on memory when a single stressor was expected to impact the encoding, post-encoding, and retrieval phases of memory, 16 such studies were reported and stress tended to impair memory. However, the impairing effects of stress were found to reverse in studies with longer delays between encoding and retrieval. The detrimental effects of stress that were observed are consistent with the fact that stress generally impairs retrieval and can impair encoding processes, both of which would be impacted in these designs. Why the detrimental effects decreased in magnitude with longer encoding-retrieval delays is not entirely clear, but it could be that using shorter delays effectively reduced any positive effects produced by post-encoding stress.
Post-Reactivation
We found no overall significant effects of post-reactivation stress on memory. Although we found some preliminary evidence for potentially important moderators of post-reactivation stress effects, our lack of power limited our ability to detect small effects in these analyses (see Table 2). Thus, future research should continue to examine what effects, if any, post-reactivation stress has on memory.
Relating the Current Results to Studies of Nonhuman Animals
The effects of stress have been studied quite extensively in rats and mice, and these studies have provided a rich body of knowledge about the neuromodulatory mechanisms that are influenced by stress (for thorough reviews see (Conrad, 2010; Diamond et al., 2007; Finsterwald & Alberini, 2014; Joëls et al., 2011; Roozendaal, 2002; Sandi & Pinelo-Nava, 2007)). Relating those results to human episodic memory, however, is made somewhat difficult because of the inherent differences between tasks typically used to assess memory in animal and human studies Nonetheless, it is worth considering how the current findings relate to those generally reported in the animal literature.
At the broadest level, the animal literature is consistent in showing that post-encoding stress or coadministration of glucocorticoids and a noradrenergic agonist generally benefits memory, whereas stress or a similar pharmacological manipulation during retrieval impairs memory (Finsterwald & Alberini, 2014; Roozendaal, 2002; Roozendaal, Okuda, de Quervain, et al., 2006). Moreover, this literature has also found stress during encoding may benefit memory—especially for stress-related information (e.g., the location of an escape from a threatening situation)—if stress and learning occur in the same context (e.g., within the same space and close proximity within time) (Conboy & Sandi, 2010; Joëls et al., 2006). As far as we are aware, there have not been studies that have examined the effects of varying context between the study materials and the post-encoding stressor, although in general stress or glucocorticoid administration often occurs outside of the learning apparatus (e.g., Roozendaal, Okuda, Van der Zee, & McGaugh, 2006). Given that the post-encoding effects in humans appear to only occur when the study event occurs in the same spatial context as the stressor, it would seem important to determine whether the same holds in nonhuman animal post-encoding stress paradigms as well.
One factor that has been examined at some length in the human literature, but that to our knowledge has not been directly manipulated in the same way within the animal literature, is the impact of the emotional valence of the encoding materials. Based largely on animal studies pointing to a role of the amygdala in producing stress effects (e.g., Akirav & Richter-Levin, 1999, 2002; Diamond et al., 2007; Joëls et al., 2011; Roozendaal & McGaugh, 1997; Roozendaal, Okuda, de Quervain, et al., 2006; Schwabe et al., 2012), it was expected that stress effects on memory would be larger for emotional then neutral materials, but this expectation was not strongly supported by the human literature. The only emotion effect that was consistently observed was that the impairing effects of stress at retrieval were larger for positive and marginally larger for negative materials than for neutral materials. Future studies in nonhuman animals aiming to determine if stress has comparable effects on memory for emotionally salient compared to neutral materials will be important.
The animal results also somewhat parallel the current human results in the sense that stress effects are often larger in male than female rodents (Andreano & Cahill, 2009). This seems important, suggesting that sex or sex hormone effects in stress and memory may be quite general. Notably, we found that effects of hormonal contraceptives were consistently seen in studies examining effects of stress during the encoding, post-encoding, and retrieval phases of memory, with the use of hormonal contraceptives dampening effects of stress on each of these phases. Thus, consistent with animal literature (Barros et al., 2015; Graham & Milad, 2013; Harburger, Pechenino, Saadi, & Frick, 2008; Kramár et al., 2013), our results suggest that sex hormones may be important modulators of memory and stress effects on memory.
In sum, despite differences in paradigms and species, there are a number of behavioral similarities observed in the human and nonhuman studies of stress and memory. However, there are several findings emerging in the human literature that have not yet been directly examined in the animal literature and these seem to be important targets of future research.
General Discussion and Theoretical Integration
The effects of stress on memory are complex, and it is clear that whether stress impairs or enhances memory depends on a number of factors. Although the results of this meta-analysis provide support for a number of the predictions of existing theories of stress and memory, no single theory seems capable of accounting for all these results without extension or modification. For example, consolidation theory (Cahill & McGaugh, 1998; McGaugh, 2000, 2015) is in good agreement with some aspects of the post-encoding stress studies, but it fails to account for other important aspects of those studies, and it fails to provide accounts for impairing effects of stress on encoding or retrieval. The primary prediction of the consolidation account is that post-encoding stress will enhance memory (McGaugh, 2000), and this prediction was strongly supported. In addition, although the consolidation account does not make explicit predictions about the effect of stress on encoding processes per se, to the extent that stress occurred during or immediately prior to encoding one may expect increased cortisol levels to persist into the post-encoding period, and thus to result in enhancements in memory. Thus, the consolidation model is broadly consistent with the finding that encoding stress can improve memory if it occurs immediately prior to encoding. However, it is not clear from this account why encoding stress leads to a decrease in memory when it occurs much earlier than the encoding phase. In addition, one of the core predictions of this approach is that stress should enhance memory for emotional materials more so than neutral memories (Cahill & McGaugh, 1998; Joëls et al., 2011; McGaugh, 2000, 2004, 2015). Overall, there was no effect of emotionality on post-encoding stress, with some studies showing an advantage for emotional materials, others showing the opposite, and still others showing no difference. One possibility, however, is that the manipulations of stress and emotion conducted in the studies of human memory were not sufficiently powerful to lead to consolidation. Thus, it is possible that the consolidation process does accurately account for traumatic memory formation for emotional events that precede stress, but it does not account for the types of memory typically examined in laboratory studies of human memory.
Consolidation theory also fails to provide an explanation for why post-encoding stress would only impact memory when the stressor occurred in the same spatial context as the study materials. This theory, which is tied to neurobiological mechanisms, predicts that stress should benefit memory when it occurs shortly after the encoding phase, as stress and the associated hormonal changes facilitate long-term potentiation of recently encoded events (McGaugh, 2000), and thus there is no clear reason why a change in context would negate those neurobiological effects.
Another limitation of the consolidation account is that it says little about the impairing effects of stress on memory that were observed when stress occurred prior to encoding or retrieval (McGaugh, 2000), as few clear predictions regarding stress effects on encoding and retrieval processes are provided by consolidation theory alone. Given the consistency of those results, this shortcoming seems critical. One could argue that the consolidation model was not intended to account for the effects of encoding or retrieval stress. In fact, it has been most directly tested in animal studies of memory, and only recently applied to understand human episodic memory in post-encoding stress effects (e.g., Cahill et al., 2003). The consolidation model has been quite successful in accounting for a variety of results from the animal literature (Joëls et al., 2011; McGaugh, 2000; Roozendaal, 2002), so the problems that it has accounting for human episodic memory should not necessarily be seen as undermining the utility of that model to account for the animal literature.
The executive control theory of stress and memory (Gagnon & Wagner, 2016) can account naturally for a number of the results from studies examining the effects of stress on encoding and retrieval, but it runs into other problems with those studies, and it fails to account for the effects of post-encoding stress. That is, if stress generally limits executive control process and draws attention toward the stress-related materials (Mather & Sutherland, 2011), stress should generally reduce both encoding of information not relevant to the stressor and memory retrieval, as was generally observed. Moreover, at time of encoding, to the extent that stress draws attention toward stress-relevant materials, stress should enhance memory encoding for stress relevant materials, as was also observed. In addition, the executive control account can also explain why effects of stress on encoding become more impairing with a longer delay between stress and encoding, because stress effects on executive functions such as working memory become more impairing with a longer delay as well (Shields, Sazma, et al., 2016).
The executive control theory does not make strong a priori predictions about the effects of the emotionality of the materials, but it could account for the finding that stress at time of retrieval seems to impair memory for emotional materials more so than neutral materials. That is, the effects of stress on executive processes, such as monitoring and retrieval orientation, may be more critical in the case of emotionally-charged items than in the case of neutral items. For example, emotional stimuli require more executive control to ignore than do neutral items (e.g., Shields, Kuchenbecker, Pressman, Sumida, & Slavich, 2016), and divided attention manipulations at test can reduce recognition memory for emotional more than neutral materials, suggesting that executive processes are more critical for emotional than neutral materials during retrieval (Maddox, Naveh-Benjamin, Old, & Kilb, 2012). Nonetheless, further work aimed that determining if the effects of stress on retrieval reflect alteration in executive processes would be useful.
One potential problem for the executive control account is that to the extent that recall is more heavily dependent on executive control than is recognition (Gagnon & Wagner, 2016), the encoding and retrieval deficits should have been larger for recall than recognition, and there was little evidence for this. However, it should be acknowledged that most studies that have directly contrasted recall and recognition had their recognition tests follow the free recall tests, and any carry-over effects may be expected to make the recognition and recall results more similar. Thus, additional studies that directly contrast the magnitude of the recall and recognition effects will be important in testing the executive control theories. In addition, only a small number of studies have examined the differential effects of stress on the processes that contribute to recall and recognition such as recollection and familiarity, thus future studies examining whether stress differentially impacts these different forms of episodic memory will also be important (McCullough et al., 2015; McCullough & Yonelinas, 2013). Regardless of the recall/recognition results, the executive function theories are limited in that they do not provide an explanation for why post-encoding stress would improve memory. Given the consistency of those results, they point to an important limitation of the executive control approach.
The dual-mode consolidation approach (Cadle & Zoladz, 2015; Diamond et al., 2007; Joëls et al., 2011; Schwabe et al., 2012) can account for a number of the encoding, post-encoding and retrieval effects of stress. Indeed, this model incorporates aspects of the traditional consolidation model and the executive control approaches. For example, because both the memory formation mode and memory storage mode are expected to enhance memory for recently encoded information (Schwabe et al., 2012), post-encoding stress should improve memory, as was found. Moreover, because both modes lead to an inhibition of memory retrieval processes (Cadle & Zoladz, 2015; Schwabe et al., 2012), stress before or during retrieval should impair memory, as was also observed. In addition, in line with our current findings, this model predicts that stress prior to encoding will impair encoding unless the stress-encoding delay is very short, in which case the memory formation mode is expected to lead to an improvement in memory for stress-relevant materials (Schwabe et al., 2012).
However, as with the traditional consolidation models, the dual-mode approach predicts that the encoding and post-encoding effects of stress should be greatest for emotional materials (Schwabe et al., 2012), and there was little direct support for this prediction in either the encoding or post-encoding stress studies. Moreover, how the dual-mode theory would account for the finding that stress only improved memory when it occurred in the same spatial context as the study materials is also not clear. We note that Joëls et al. (2006) suggested that context changes may play an important role in determining the effects of stress on encoding. That is, they argued that in order for stress to enhance memory encoding, it may be necessary for the study materials to be presented while both noradrenergic activity and glucocorticoid activity is high. Thus, if subjects are stressed in one context then moved to another context to encode materials into memory, stress will no longer facilitate encoding, as noradrenergic activity will have presumably returned to baseline. Although it is not obvious how such an account could explain the fact that post-encoding benefits of stress are eliminated when the study-stress context changes (as the delay between encoding and stress is the same in these two types of studies), it is possible that the model could be modified to account for these results.
Another somewhat troubling aspect of the results for the dual-mode models is that stress at retrieval was found to impair negative and positive materials more than neutral materials. One potential account is to argue that the inhibition of retrieval processes produced by the “memory formation” mode may impair the ability to simultaneously retrieve information, especially information closely related to current circumstances (Cadle & Zoladz, 2015; Schwabe et al., 2012). However, this explanation does not account for why stress impairs retrieval of positive information more than neutral information.
These inconsistencies notwithstanding, the dual-mode model is notable in that it successfully predicted many of the effects we observed within the meta-analysis (Schwabe et al., 2012). Whether the dual-mode model can be extended to account for the context-dependent effect of post-encoding stress or the effects of valence has yet to be seen, but this model’s unusual a priori predictions, such as a significant effect of the stress-encoding delay (Diamond et al., 2007; Joëls et al., 2011; Schwabe et al., 2012), were supported by our data. We also note that the model has received support from various animal studies as well (Akirav & Richter-Levin, 1999, 2002; Diamond et al., 2007).
Perhaps one of the more surprising and theoretically challenging findings of the current review was that post-encoding stress improved memory only when the stressor occurred in the same context as the study event. Although as suggested above there may be ways in which existing models (e.g., Joëls et al., 2006) can be modified to account for these results, another possibility is that the post-encoding stress effects may not rely on consolidation mechanisms per se, but rather may reflect changes in post-encoding interference, or shifts in experimental context (Sazma, McCullough, & Yonelinas, 2016). For example, post-encoding stress may benefit memory for the study list because it reduces the episodic encoding of information occurring after the study list, and so it reduces interference. Such a reduction in interference would be expected to be particularly important when the stressor and the study event shared the same context. That is, because episodic memory tasks like recall and recognition require the retrieval of item-context bindings (i.e., subjects must indicate if an item was encountered in the specified study context), increasing the number of items encoded in that context should increase the amount of interference. However, if the stress/control manipulation occurs in a different context than the study event, then stress should have much less of an impact on interference, as the current results seem to suggest. This contextual binding account is also consistent with the finding that post-encoding stress benefits memory for both emotional and neutral materials, as long as they occur in the same context as the stressor, which was another finding that was problematic for the consolidation accounts. A related possibility is that stress may act to produce a shift in mental context, and this shift acts to isolate the earlier study list from the interfering effects of information encoded after the stressor. In this way, changing physical context by shifting rooms, or changing mental context by inducing stress may act to reduce retroactive interference, and effectively slow forgetting. We acknowledge that these accounts are entirely speculative, and so future studies that aim to contrast these different explanations of post-encoding stress will be critical, particularly if existing models (e.g., Joëls et al., 2006) cannot be extended to account for this context-dependent post-encoding stress effect.
An unexpected finding was the lack of association of cortisol with stress effects on memory. Stress-induced increases in cortisol were not associated with stress effects on memory when stress occurred prior to or during encoding, shortly after encoding, or before retrieval. This result is at odds with prior work that has found that pharmacological manipulations of glucocorticoids produce effects on memory similar to those of stress (Het et al., 2005; Joëls et al., 2011; Roozendaal, 2002; Roozendaal & McGaugh, 1997; Schwabe et al., 2012), or that that blocking actions of glucocorticoids can block effects of stress on memory (de Quervain et al., 1998; Roozendaal, 2002; Schwabe et al., 2012; Vogel, Fernández, Joëls, & Schwabe, 2016). There are a number of potential reasons that may explain why cortisol was not found to be related to memory in the current review. For example, nonlinearities in the cortisol-memory relationship may have obscured any noticeable effects in the meta-analysis. That is, there is evidence from animal and human studies that stress-induced cortisol increases may have inverted U-shape effects on memory performance, and so averaging across subjects may mask such a relationship. Similarly, the cortisol-memory relationship may be obscured because of interactions with other factors, such as arousal. That is, glucocorticoids critically interact with noradrenaline to contribute to stress effects on memory (Joëls et al., 2011; Roozendaal & McGaugh, 1997; Schwabe et al., 2012; van Stegeren, Roozendaal, Kindt, Wolf, & Joëls, 2010). For example, administration of a noradrenergic antagonist blocks the enhancing memory-enhancing effects of post-learning glucocorticoid administration (Roozendaal & McGaugh, 1997). It is possible that various paradigms influence noradrenaline and cortisol to varying degrees, and these differences would be held constant within a study but not across studies, which could mask associations of cortisol with memory—since cortisol interacts with noradrenaline to contribute to effects of stress on memory. Thus, by not concurrently examining noradrenergic activity across studies, correlations of stress effects on cortisol with stress effects on memory may fail to return significant despite cortisol playing a role in effects of stress on memory.
Our data thus suggest that for understanding the biological basis of stress effects on memory, it may be important to look beyond manipulations or measures of cortisol alone and instead to measuring or simultaneously manipulating multiple hormones and immune system processes influenced by stress. Indeed, our data suggest that sex hormones may play an important role in modulating stress effects on memory, and there is evidence to suggest that other stress-responsive hormones, such as DHEA (Sripada, Welsh, Marx, & Liberzon, 2014; Yabuki et al., 2015), and immune system processes (Harrison et al., 2014; Reichenberg et al., 2001) may influence memory. Thus, we suggest future stress and memory research consider factors other than cortisol alone when considering the biological level of analysis of stress effects on memory.
Conclusion
In this meta-analysis, we examined the results of studies assessing effects of acute stress on memory. Because encoding, post-encoding, retrieval, and post-reactivation phases of memory all differ, many of the effects of stress were selective to specific phases of memory. For example, effects on encoding were strongly moderated by item relevance to the stressor and by the delay between stress and encoding, but these variables did not moderate stress effects on other memory phases. Nonetheless, some general trends did emerge. Overall, post-encoding stress tended to enhance memory, whereas stress at retrieval impaired memory, and stress at encoding could enhance or impair memory depending upon key moderators. Similarly, hormonal contraceptive use blunted effects of stress during the encoding, post-encoding period, retrieval phases, indicating that sex hormones play an important role in stress effects on memory. Surprisingly, effects of stress on cortisol did not predict effects of stress on memory during any memory phase, indicating that stress may act through pathways in addition to cortisol to influence memory.
It is our hope that by synthesizing the wealth of stress and memory data that has been collected to date, we can aid future stress and cognition researchers in designing effective studies that test critical theories, while minimizing the noise from additional factors. By quantitatively elucidating factors that modulate stress effects on encoding, post-encoding, retrieval, and post-reactivation phases of memory, we hope that our meta-analysis will be useful in helping researchers achieve this goal.
Supplementary Material
Acknowledgments
This research was supported by NIH MH059352 and NEI EY025999 to Andrew Yonelinas. The authors wish to thank Tony Buchanan, Almut Hupbach, Shawn Elizabeth Nielsen, Tim Robicheaux, Pia Schönfeld, Mathias Weymar, and Phillip Zoladz for providing us with requested unpublished or additional statistics from their work.
Appendix A
| Study | Phase of Memory Studied | k | Study Design | N | Study- Average g |
Age | % Male Participants |
|---|---|---|---|---|---|---|---|
| Almela et al. (2011) | Encoding/Retrieval | 2 | Within- Subjects |
30 | −0.1 | 62.09 | 50 |
| Andreano & Cahill (2006) | Post-Encoding | 2 | Between- Subjects |
82 | 0.604 | 21.5 | 50 |
| Andreano et al. (2008) | Post-Encoding | 3 | Between- Subjects |
64 | 0.292 | 24 | 0 |
| Andreano et al. (2012) | Post-Encoding | 2 | Between- Subjects |
20 | 0.89 | 39.9 | 0 |
| Azimi & Bakhshipour-Roudsari (2012) | Reactivation | 3 | Between- Subjects |
20 | −1.271 | 20.4 | 0 |
| Beckner et al. (2006) | Post-Encoding Retrieval | 4 | Between- Subjects |
157 | Post-Encoding: 0.618 Retrieval: 0.255 |
18.77 | 35.6 |
| Boehringer et al. (2010) | Retrieval | 3 | Between- Subjects |
51 | −0.549 | 24.57 | 100 |
| Bos, Schuijer, et al. (2014), Exp. 1 | Reactivation | 3 | Between- Subjects |
43 | 0.491 | 21.3 | 48.83 |
| Bos, Schuijer, et al. (2014), Exp. 2 | Reactivation | 3 | Between- Subjects |
36 | 0.444 | 21.51 | 50 |
| Bos, van Goethem, et al. (2014) | Reactivation | 2 | Between- Subjects |
51 | 0.02 | 21.84 | 45.1 |
| Bryant et al. (2013) | Post-Encoding | 4 | Between- Subjects |
78 | 0.402 | 19.78 | 50 |
| Buchanan & Tranel (2008) | Retrieval | 2 | Between- Subjects |
40 | −0.082 | 20 | 50 |
| Buchanan et al. (2006) | Retrieval | 4 | Between- Subjects |
30 | −0.069 | 18.9 | 53.33 |
| Cahill et al. (2003) | Post-Encoding | 4 | Between- Subjects |
48 | 0.327 | 20.1 | 29.17 |
| Cavenett & Nixon (2006) | Post-Encoding/Retrieval | 4 | Between- Subjects |
70 | −0.184 | 26.47 | 64.29 |
| Coccoz et al. (2011), Exp. 1 | Reactivation | 1 | Between- Subjects |
20 | 1.116 | 23 | 42.5 |
| Coccoz et al. (2011), Exp. 2 | Reactivation | 1 | Between- Subjects |
22 | 0.977 | 23 | 100 |
| Coccoz et al. (2013) | Reactivation | 1 | Between- Subjects |
30 | 0.931 | 24 | |
| Cornelisse, Joels, et al. (2011) | Encoding | 1 | Between- Subjects |
32 | −0.013 | 21.75 | 100 |
| Cornelisse, van Stegeren, et al. (2011) | Encoding | 4 | Between- Subjects |
77 | −0.065 | 20.44 | 50 |
| Domes et al. (2002) | Retrieval Encoding | 1 | Between- Subjects |
32 | −0.697 | 47.3 | 0 |
| Domes et al. (2004) | Retrieval | 12 | Between- Subjects |
60 | Encoding: −0.197 Retrieval: −0.097 |
27.1 | 100 |
| du Plooy (2014) | Retrieval | 8 | Between- Subjects |
60 | −0.195 | 19.97 | 50 |
| Eich & Metcalfe (2009) | Encoding/Retrieval | 1 | Between- Subjects |
261 | −0.254 | 36.37 | 54.02 |
| Espin et al. (2013) | Encoding/Retrieval | 2 | Between- Subjects |
119 | −0.036 | 19.33 | 26.89 |
| Felmingham, Fong, et al. (2012) | Post-Encoding | 2 | Between- Subjects |
56 | 0.301 | 0 | |
| Felmingham, Tran, et al. (2012) | Post-Encoding | 4 | Between- Subjects |
80 | 0.688 | 29 | 50 |
| Henckens et al. (2009) | Encoding | 1 | Within- Subjects |
18 | 0.312 | 22 | 100 |
| Hidalgo et al. (2012) | Encoding/Retrieval | 1 | Within- Subjects |
46 | 0 | 21.56 | 37 |
| Hidalgo et al. (2014) | Encoding/Retrieval | 2 | Within- Subjects |
67 | −0.147 | 41.6 | 50.71 |
| Hidalgo et al. (2015) see note | Retrieval | 4 | Between- Subjects |
50 | −0.287 | 22.5 | 50 |
| Hoffman & al’Absi (2004) | Encoding/Retrieval | 2 | Within- Subjects |
25 | 0.048 | 24.8 | 40 |
| Hoscheidt (2011) | Encoding Retrieval | 4 | Between- Subjects |
90 | Encoding: −0.429 Retrieval: 0.375 |
19 | 44.44 |
| Hoscheidt et al. (2014) | Encoding | 1 | Between- Subjects |
68 | 0.602 | 19 | 55.88 |
| Human (2010) | Retrieval | 2 | Between- Subjects |
18 | −0.519 | 20.22 | 50 |
| Human et al. (2013) | Post-Encoding/Retrieval | 1 | Between- Subjects |
36 | 0.193 | 20.5 | 100 |
| Hupbach & Dorskind (2014) | Reactivation | 1 | Between- Subjects |
58 | −0.801 | 100 | |
| Hupbach & Fieman (2012) | Retrieval | 2 | Between- Subjects |
75 | −0.177 | 27.5 | 50 |
| Kuhlmann et al. (2005) | Retrieval | 5 | Within- Subjects |
19 | −0.39 | 24.58 | 100 |
| Larra et al. (2014) | Post-Encoding and Post-Encoding/Retrieval |
2 | Between- Subjects |
206 | Post-Encoding: 0.269 Post-Encoding/Retrieval: ~ 0 |
23 | 48.54 |
| Li et al. (2013) | Retrieval | 2 | Within- Subjects |
42 | −0.222 | 23.63 | 100 |
| Li et al. (2014) | Retrieval | 2 | Within- Subjects |
27 | 0.01 | 24.25 | 100 |
| Luethi et al. (2009) | Encoding/Retrieval | 2 | Between- Subjects |
35 | 0.366 | 23.4 | 100 |
| Maheu et al. (2005), Exp. 1 | Encoding | 2 | Between- Subjects |
19 | −1.444 | 22.5 | 100 |
| Maheu et al. (2005), Exp. 2 | Encoding | 2 | Between- Subjects |
20 | −1.143 | 22.5 | 100 |
| Marin et al. (2010) | Reactivation | 2 | Between- Subjects |
32 | −0.225 | 22.09 | 50 |
| McCullough & Yonelinas (2013) | Post-Encoding | 16 | Between- Subjects |
38 | 0.124 | 19.35 | 50 |
| McCullough et al. (2015) | Post-Encoding | 4 | Between- Subjects |
49 | −0.09 | 24.2 | 100 |
| Merz et al. (2010) | Post-Encoding/Retrieval | 3 | Within- Subjects |
29 | −0.301 | 23.17 | 48.2 |
| Nguyen (2009) | Post-Encoding/Retrieval | 1 | Between- Subjects |
72 | 0.368 | 20.86 | 39 |
| Nielsen et al. (2013), Exp. 1 | Post-Encoding | 3 | Between- Subjects |
49 | 0.076 | 20.37 | 0 |
| Nielsen et al. (2013), Exp. 2 | Post-Encoding | 3 | Between- Subjects |
41 | −0.047 | 20.37 | 0 |
| Nielsen et al. (2014), Exp. 1 | Post-Encoding | 2 | Between- Subjects |
60 | 0.27 | 20.1 | 0 |
| Nielsen et al. (2014), Exp. 2 | Post-Encoding | 2 | Between- Subjects |
49 | −0.142 | 20.3 | 0 |
| Oei et al. (2006) | Retrieval | 2 | Within- Subjects |
20 | −0.135 | 21.86 | 100 |
| Payne et al. (2006), Exp. 1 | Encoding | 8 | Between- Subjects |
56 | −0.24 | 50 | |
| Payne et al. (2006), Exp. 2 | Encoding/Retrieval | 8 | Between- Subjects |
61 | −0.477 | 50 | |
| Payne et al. (2007), Exp. 1 | Encoding | 3 | Between- Subjects |
31 | −0.984 | 42.11 | |
| Payne et al. (2007), Exp. 2 | Encoding | 3 | Between- Subjects |
34 | 1.006 | 42.11 | |
| Preuβ & Wolf (2009) | Post-Encoding | 3 | Between- Subjects |
58 | 0.259 | 23.6 | 51.72 |
| Pulopulos et al. (2013) | Retrieval | 8 | Between- Subjects |
76 | −0.132 | 64.63 | 50 |
| Qin et al. (2012) | Encoding | 1 | Between- Subjects |
40 | −0.239 | 22.18 | 100 |
| Quaedflieg et al. (2013), Exp. 1 | Encoding | 4 | Between- Subjects |
32 | −0.475 | 21.25 | 100 |
| Quaedflieg et al. (2013), Exp. 2 | Encoding | 4 | Between- Subjects |
32 | −0.371 | 21.25 | 100 |
| Robicheaux (2015) | Post-Encoding/Retrieval | 2 | Between- Subjects |
128 | 0.084 | 28.91 | |
| Schönfeld et al. (2014) | Retrieval | 4 | Between- Subjects |
72 | −0.101 | 23.2 | 50 |
| Schoofs & Wolf (2009) | Retrieval | 3 | Within- Subjects |
36 | 0.058 | 24.47 | 0 |
| Schwabe & Wolf (2009), Exp. 1 | Retrieval | 1 | Between- Subjects |
36 | 0.11 | 25.1 | 50 |
| Schwabe & Wolf (2009), Exp. 2 | Retrieval | 1 | Between- Subjects |
36 | −0.718 | 25.1 | 50 |
| Schwabe & Wolf (2010a) | Encoding | 16 | Between- Subjects |
48 | −0.478 | 23.6 | 50 |
| Schwabe & Wolf (2010b) | Reactivation | 3 | Between- Subjects |
32 | −0.369 | 23.3 | 50 |
| Schwabe & Wolf (2014), Exp. 1 | Retrieval | 2 | Between- Subjects |
40 | 0.066 | 23.61 | 50 |
| Schwabe & Wolf (2014), Exp. 2 | Retrieval | 2 | Between- Subjects |
40 | −0.546 | 23.61 | 50 |
| Schwabe & Wolf (2014), Exp. 3 | Retrieval | 2 | Between- Subjects |
40 | −0.84 | 23.61 | 50 |
| Schwabe et al. (2008) | Encoding Encoding/Retrieval |
9 | Between- Subjects |
96 | Encoding: 0.061 Encoding/Retrieval: 0.245 |
23.3 | 50 |
| Schwabe, Bohringer, & Wolf (2009), Exp. 1 | Encoding | 3 | Between- Subjects |
36 | 0.183 | 25 | 50 |
| Schwabe, Bohringer, & Wolf (2009), Exp. 2 | Encoding | 3 | Between- Subjects |
36 | −0.471 | 25 | 50 |
| Schwabe, Römer, et al. (2009) | Retrieval | 2 | Within- Subjects |
44 | 0.282 | 23.7 | 100 |
| Smeets (2011), Exp. 1 | Retrieval | 2 | Between- Subjects |
38 | −0.91 | 19.9 | 44.73 |
| Smeets (2011), Exp. 2 | Retrieval | 2 | Between- Subjects |
38 | −0.852 | 19.9 | 44.73 |
| Smeets et al. (2007) | Encoding | 4 | Between- Subjects |
52 | 0.469 | 23.08 | 25 |
| Smeets, Jelcic, & Merckelbach (2006a), Exp. 1 | Encoding/Retrieval | 4 | Between- Subjects |
58 | 0.037 | 19.91 | 50 |
| Smeets, Jelcic, & Merckelbach (2006a), Exp. 2 | Encoding/Retrieval | 4 | Between- Subjects |
92 | −0.403 | 19.74 | 50 |
| Smeets, Jelcic, & Merckelbach (2006b) | Encoding/Retrieval | 6 | Between- Subjects |
60 | −0.452 | 19.65 | 50 |
| Smeets, Jelcic, Merckelbach, Peters, et al. (2006) | Post-Encoding/Retrieval | 1 | Between- Subjects |
40 | 0.901 | 19.2 | 100 |
| Smeets, Otgaar, et al. (2008) | Encoding Post-Encoding Retrieval |
6 | Between- Subjects |
90 | Encoding: 0.005 Post-Encoding: 0.308 Retrieval: −0.839 |
20.6 | 5.8 |
| Smeets, Sijstermans, et al. (2008) | Post-Encoding | 1 | Between- Subjects |
80 | 1.297 | 20.3 | 40 |
| Stawski et al. (2009) | Post-Encoding/Retrieval | 1 | Between- Subjects |
100 | −0.17 | 18.94 | 26 |
| Taverniers et al. (2010) | Encoding/Retrieval | 1 | Between- Subjects |
27 | −1.057 | 27.4 | 100 |
| Taverniers et al. (2013) | Encoding | 1 | Between- Subjects |
24 | −1.171 | 27.04 | 100 |
| Thompson et al. (2001) | Post-Encoding/Retrieval Encoding/Retrieval |
3 | Within- Subjects |
16 | Post-Encoding/Retrieval: −.7 Encoding/Retrieval: −1.32 |
26 | 87.5 |
| Tollenaar et al. (2008), Exp. 1 | Retrieval | 4 | Between- Subjects |
35 | −0.284 | 21.34 | 100 |
| Tollenaar et al. (2008), Exp. 2 | Retrieval | 4 | Between- Subjects |
35 | −0.236 | 21.34 | 100 |
| Tollenaar et al. (2009) | Retrieval | 4 | Between- Subjects |
40 | −0.081 | 21.7 | 100 |
| Trammell & Clore (2013), Exp. 1 | Post-Encoding | 1 | Between- Subjects |
97 | −0.431 | 18.97 | 39.18 |
| Trammell & Clore (2013), Exp. 2 | Post-Encoding | 1 | Between- Subjects |
131 | −0.472 | 18.47 | 47.3 |
| Trammell & Clore (2013), Exp. 3 | Post-Encoding | 1 | Between- Subjects |
127 | −0.374 | 18.88 | 45.67 |
| van Ast et al. (2014) | Encoding | 8 | Between- Subjects |
40 | 0.175 | 22 | 100 |
| Weymar et al. (2012) | Encoding | 2 | Between- Subjects |
40 | −0.183 | 24.5 | 100 |
| Wiemers et al. (2013) | Encoding | 3 | Between- Subjects |
60 | 0.305 | 23.87 | 50 |
| Wiemers et al. (2014), Exp. 1 | Encoding | 2 | Between- Subjects |
44 | 0.654 | 24.12 | 47.7 |
| Wiemers et al. (2014), Exp. 2 | Encoding | 2 | Between- Subjects |
45 | 0.103 | 24.12 | 48.9 |
| Wirkner et al. (2013) | Encoding | 3 | Between- Subjects |
52 | −0.662 | 23 | 55.77 |
| Wolf (2012), Exp. 1 | Encoding | 3 | Between- Subjects |
24 | −0.45 | 24 | 100 |
| Wolf (2012), Exp. 2 | Encoding | 3 | Between- Subjects |
32 | 0.246 | 24.84 | 100 |
| Wolf et al. (2001) | Encoding/Retrieval | 2 | Between- Subjects |
58 | −0.05 | 24.09 | 50 |
| Yonelinas et al. (2011) | Post-Encoding | 8 | Between- Subjects |
50 | −0.099 | 25.2 | 50 |
| Zoladz et al. (2011), Exp. 1 | Encoding | 6 | Between- Subjects |
31 | −0.003 | 19.68 | 27.78 |
| Zoladz et al. (2011), Exp. 2 | Encoding | 6 | Between- Subjects |
31 | 0.044 | 19.68 | 27.78 |
| Zoladz et al. (2013) | Encoding Encoding/Retrieval |
18 | Between- Subjects |
97 | Encoding: −0.172 Encoding/Retrieval: ~ 0 |
19.18 | 50 |
| Zoladz et al. (2015) | Post-Encoding | 12 | Between- Subjects |
52 | 0.023 | 20.3 | 51.92 |
| Zoladz, Kalchik, Hoffman, Aufdenkampe, Burke, et al. (2014) | Retrieval | 12 | Between- Subjects |
93 | −0.047 | 19.45 | 50 |
| Zoladz, Kalchik, Hoffman, Aufdenkampe, Lyle, et al. (2014) | Encoding | 4 | Between- Subjects |
120 | 0.151 | 19.7 | 50 |
Note: The data for older participants in Hidalgo et al. (2015) were originally presented in another study included in our analyses. As such, only younger participants from this study were included in our meta-analyses.
Footnotes
Note that stress- related consolidation is sometimes referred to as cellular or synaptic consolidation, which occurs within a few hours of encoding, and is distinct from “systems consolidation”, which is said to involve the transfer of hippocampal memory traces to the cortex, which can occur gradually over many years (Dudai, 2004; Squire & Alvarez, 1995).
We included this search term rather as an “AND” rather than an “OR” based upon pilot searching. Including this term as an “OR” term along with “memory” quadrupled our results in each database (e.g., PubMed went from 267 articles to 1,106), and by browsing the first few pages of each returned search we determined that almost if not all of these additional articles were irrelevant to our analyses (e.g., they were studies of stress and other cognitive processes that simply referenced stress effects on memory within the text). We found numerous articles that did not highlight or vary the timing of the stressor that nonetheless came up due to the description of the memory task within the methodology section (e.g., “Encoding took place…”). In short, we believe that this search string represented an efficient way of returning all relevant memory studies without returning irrelevant ones. Additionally, to ensure that our review was comprehensive, we conducted numerous nonexhaustive searches of Google Scholar using simple strings such as (stress AND memory) and similar variations. Further, we browsed references of numerous literature reviews of stress and memory in order to ensure we had not missed any studies referenced by prior reviews—we had not.
Numerous studies reported the proportion of studied items remembered at delayed recall to the number of studied items remembered at immediate recall. These scores thus entail that a score of 1.0 would imply that a participant remembered at the delayed recall test all of the items they remembered at the immediate recall test, thus controlling for individual differences in initial learning of the items.
Studies that only included males were assigned a value of “excluded” for the variable assessing exclusion of women during their menstrual period and the variable assessing exclusion of the use of hormonal contraceptives, even though the studies did not explicitly report these exclusions.
Using gppc+ for analyses relating memory to cortisol did not alter any of the results.
Three papers only reported effects of stress on cortisol averaged across their two stress and two control groups per paper. As such, to avoid giving extra weight to these studies, considered them as single studies in analyses of stress effects on cortisol, leaving 78 studies for analyses of stress effects on cortisol.
This conversion requires the correlation between performance in the stress condition with performance in the control condition if the stress/control condition is a within-subjects manipulation. None of the studies in this meta-analysis reported this correlation, so we set the correlation between these conditions at r=.30 (a moderate correlation) to account for measurement error and expected differences between the stress and control condition. Because so few of our studies used a within-subjects design (11.4%), sensitivity analyses setting the correlation between .00 and .80 and running the resultant meta-analysis showed that setting this correlation at .30 did not alter any of the reported results.
Sensitivity analyses with values of ρ ranging from 0 to 1.0 evidenced no change in any effect size estimate greater in absolute value than 0.0005 across all effects of stress on memory phases, a change which was inconsequential for all analyses.
We used the average sample size for the stress and control groups as the “typical” sample size per group as well as the observed heterogeneity (τ2) in order to demonstrate the actual power of our analyses.
In the main analyses of ‘encoding’ we included studies in which the stressor occurred during encoding as well as studies in which stress occurred just prior to encoding. The difference between these studies was assessed by examining the effect of the time delay between stress and encoding. In addition, a secondary analysis was conducted in which we analyzed these two types of studiers separately (see Supplementary Material). Those results were found to be consistent with the main analysis, with the exception that the significance levels of the effects were generally reduced given the reduced samples.
The effects of a hybrid stressor on encoding do not differ as a function of any moderator. Thus, the use of a non-hybrid can produce a reliably bigger impairment in encoding with a long stress-encoding delay.
Even though the delay between learning and the stressor did not moderate the effects of post-encoding stress on memory (see Table 4), it could be possible that changing contexts reduced the effects of stress on memory because it introduced a longer delay between study and the stressor. However, when we controlled for the encoding-stressor delay, context change remained a significant moderator of post-encoding stress effects on memory, B= −.635, t(6.2)= −4.29, p=.005, whereas the encoding-stressor delay was not a significant moderator, B=.006, t(2.6)=1.56, p=.229, indicating that the context effects were not due to increased delays. One other potential concern was that because the reported experimental methods often did not explicitly mention whether the rooms were change between the encoding phase and the stressor we had to infer whether such changed occurred (i.e., the standard Trier Social Stress Test requires a room change, but this was often not stated explicitly in the methods sections). To address this, we conducted a secondary analysis that included only studies that explicitly stated that either they changed contexts (4 studies) between learning and stress or they did not (7 studies)—11 studies in total. Context change was a significant moderator of post-encoding stress effects, t(5.4)= −4.92, p=.004, such that studies with a constant context between encoding and stress significantly enhanced memory (g+=.529, p=.018), whereas studies with a changed context between encoding and stress did not, g+= −.217, p=.107).
Studies Included in Meta-Analysis
- Almela M, Hidalgo V, Villada C, Espín L, Gómez-Amor J, Salvador A. The impact of cortisol reactivity to acute stress on memory: Sex differences in middle-aged people. Stress. 2011;14:117–127. doi: 10.3109/10253890.2010.514671. http://dx.doi.org/10.3109/10253890.2010.514671. [DOI] [PubMed] [Google Scholar]
- Andreano JM, Arjomandi H, Cahill L. Menstrual cycle modulation of the relationship between cortisol and long-term memory. Psychoneuroendocrinology. 2008;33:874–882. doi: 10.1016/j.psyneuen.2008.03.009. http://dx.doi.org/10.1016/j.psyneuen.2008.03.009. [DOI] [PubMed] [Google Scholar]
- Andreano JM, Cahill L. Glucocorticoid release and memory consolidation in men and women. Psychological Science. 2006;17:466–470. doi: 10.1111/j.1467-9280.2006.01729.x. http://dx.doi.org/10.1111/j.1467-9280.2006.01729.x. [DOI] [PubMed] [Google Scholar]
- Andreano J, Waisman J, Donley L, Cahill L. Effects of breast cancer treatment on the hormonal and cognitive consequences of acute stress. Psycho-Oncology. 2012;21:1091–1098. doi: 10.1002/pon.2006. http://dx.doi.org/10.1002/pon.2006. [DOI] [PubMed] [Google Scholar]
- Azimi Z, Bakhshipour-Roudsari A. The impairing role of stress on autobiographical memory reconsolidation. Zahedan Journal of Research in Medical Sciences. 2012;14:51–55. Retrieved from http://www.zjrms.ir/article-1-2007-en.html. [Google Scholar]
- Beckner VE, Tucker DM, Delville Y, Mohr DC. Stress facilitates consolidation of verbal memory for a film but does not affect retrieval. Behavioral Neuroscience. 2006;120:518–527. doi: 10.1037/0735-7044.120.3.518. http://dx.doi.org/10.1037/0735-7044.120.3.518. [DOI] [PubMed] [Google Scholar]
- Boehringer A, Schwabe L, Schachinger H. A combination of high stress-induced tense and energetic arousal compensates for impairing effects of stress on memory retrieval in men. Stress. 2010;13:444–453. doi: 10.3109/10253891003725256. http://dx.doi.org/10.3109/10253891003725256. [DOI] [PubMed] [Google Scholar]
- Bos MGN, Jacobs van Goethem TH, Beckers T, Kindt M. Cortisol response mediates the effect of post-reactivation stress exposure on contextualization of emotional memories. Psychoneuroendocrinology. 2014;50:72–84. doi: 10.1016/j.psyneuen.2014.07.030. http://dx.doi.org/10.1016/j.psyneuen.2014.07.030. [DOI] [PubMed] [Google Scholar]
- Bos MGN, Schuijer J, Lodestijn F, Beckers T, Kindt M. Stress enhances reconsolidation of declarative memory. Psychoneuroendocrinology. 2014;46:102–113. doi: 10.1016/j.psyneuen.2014.04.011. http://dx.doi.org/10.1016/j.psyneuen.2014.04.011. [DOI] [PubMed] [Google Scholar]
- Bryant RA, McGrath C, Felmingham KL, Brewin C, Gregory J, Lipton M, … Schachinger H. The roles of noradrenergic and glucocorticoid activation in the development of intrusive memories. PLoS ONE. 2013;8(4):e62675. doi: 10.1371/journal.pone.0062675. http://dx.doi.org/10.1371/journal.pone.0062675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan TW, Tranel D. Stress and emotional memory retrieval: Effects of sex and cortisol response. Neurobiology of Learning and Memory. 2008;89:134–141. doi: 10.1016/j.nlm.2007.07.003. http://dx.doi.org/10.1016/j.nlm.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan TW, Tranel D, Adolphs R. Impaired memory retrieval correlates with individual differences in cortisol response but not autonomic response. Learning & Memory. 2006;13:382–387. doi: 10.1101/lm.206306. http://dx.doi.org/10.1101/lm.206306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill L, Gorski L, Le K. Enhanced human memory consolidation with post-learning stress: Interaction with the degree of arousal at encoding. Learning & Memory. 2003;10:270–274. doi: 10.1101/lm.62403. http://dx.doi.org/10.1101/lm.62403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavenett T, Nixon RDV. The effect of arousal on memory for emotionally-relevant information: A study of skydivers. Behaviour Research and Therapy. 2006;44:1461–1469. doi: 10.1016/j.brat.2005.11.002. http://dx.doi.org/10.1016/j.brat.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Coccoz V, Maldonado H, Delorenzi A. The enhancement of reconsolidation with a naturalistic mild stressor improves the expression of a declarative memory in humans. Neuroscience. 2011;185:61–72. doi: 10.1016/j.neuroscience.2011.04.023. http://dx.doi.org/10.1016/j.neuroscience.2011.04.023. [DOI] [PubMed] [Google Scholar]
- Coccoz V, Sandoval AV, Stehberg J, Delorenzi A. The temporal dynamics of enhancing a human declarative memory during reconsolidation. Neuroscience. 2013;246:397–408. doi: 10.1016/j.neuroscience.2013.04.033. http://dx.doi.org/10.1016/j.neuroscience.2013.04.033. [DOI] [PubMed] [Google Scholar]
- Cornelisse S, Joëls M, Smeets T. A randomized trial on mineralocorticoid receptor blockade in men: Effects on stress responses, selective attention, and memory. Neuropsychopharmacology. 2011;36:2720–2728. doi: 10.1038/npp.2011.162. http://dx.doi.org/10.1038/npp.2011.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelisse S, van Stegeren AH, Joels M. Implications of psychosocial stress on memory formation in a typical male versus female student sample. Psychoneuroendocrinology. 2011;36:569–578. doi: 10.1016/j.psyneuen.2010.09.002. http://dx.doi.org/10.1016/j.psyneuen.2010.09.002. [DOI] [PubMed] [Google Scholar]
- Domes G, Heinrichs M, Reichwald U, Hautzinger M. Hypothalamic-pituitary-adrenal axis reactivity to psychological stress and memory in middle-aged women: High responders exhibit enhanced declarative memory performance. Psychoneuroendocrinology. 2002;27:843–853. doi: 10.1016/s0306-4530(01)00085-3. http://dx.doi.org/10.1016/S0306-4530(01)00085-3. [DOI] [PubMed] [Google Scholar]
- Domes G, Heinrichs M, Rimmele U, Reichwald U, Hautzinger M. Acute stress impairs recognition for positive words--association with stress-induced cortisol secretion. Stress. 2004;7:173–181. doi: 10.1080/10253890412331273213. http://dx.doi.org/10.1080/10253890412331273213. [DOI] [PubMed] [Google Scholar]
- du Plooy CP. The Effects of acute stress on retrieval of visual and spatial material. University of Cape Town; 2014. Retrieved from http://www.bme.uct.ac.za/sites/default/files/image_tool/images/150/THESIS_DuPlooy_2015_PhD.pdf. [Google Scholar]
- Eich TS, Metcalfe J. Effects of the stress of marathon running on implicit and explicit memory. Psychonomic Bulletin & Review. 2009;16:475–479. doi: 10.3758/PBR.16.3.475. http://dx.doi.org/10.3758/PBR.16.3.475. [DOI] [PubMed] [Google Scholar]
- Espin L, Almela M, Hidalgo V, Villada C, Salvador A, Gomez-Amor J. Acute pre-learning stress and declarative memory: Impact of sex, cortisol response and menstrual cycle phase. Hormones and Behavior. 2013;63:759–765. doi: 10.1016/j.yhbeh.2013.03.013. http://dx.doi.org/10.1016/j.yhbeh.2013.03.013. [DOI] [PubMed] [Google Scholar]
- Felmingham KL, Fong WC, Bryant RA. The impact of progesterone on memory consolidation of threatening images in women. Psychoneuroendocrinology. 2012;37:1896–1900. doi: 10.1016/j.psyneuen.2012.03.026. http://dx.doi.org/10.1016/j.psyneuen.2012.03.026. [DOI] [PubMed] [Google Scholar]
- Felmingham KL, Tran TP, Fong WC, Bryant RA. Sex differences in emotional memory consolidation: The effect of stress-induced salivary alpha-amylase and cortisol. Biological Psychology. 2012;89:539–544. doi: 10.1016/j.biopsycho.2011.12.006. http://dx.doi.org/10.1016/j.biopsycho.2011.12.006. [DOI] [PubMed] [Google Scholar]
- Henckens MJAG, Hermans EJ, Pu Z, Joëls M, Fernández G. Stressed memories: How acute stress affects memory formation in humans. The Journal of Neuroscience. 2009;29:10111–10119. doi: 10.1523/JNEUROSCI.1184-09.2009. http://dx.doi.org/10.1523/JNEUROSCI.1184-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidalgo V, Almela M, Villada C, Salvador A. Acute stress impairs recall after interference in older people, but not in young people. Hormones and Behavior. 2014;65:264–272. doi: 10.1016/j.yhbeh.2013.12.017. http://dx.doi.org/10.1016/j.yhbeh.2013.12.017. [DOI] [PubMed] [Google Scholar]
- Hidalgo V, Pulopulos MM, Puig-Perez S, Espin L, Gomez-Amor J, Salvador A. Acute stress affects free recall and recognition of pictures differently depending on age and sex. Behavioural Brain Research. 2015;292:393–402. doi: 10.1016/j.bbr.2015.07.011. http://dx.doi.org/10.1016/j.bbr.2015.07.011. [DOI] [PubMed] [Google Scholar]
- Hidalgo V, Villada C, Almela M, Espín L, Gómez-Amor J, Salvador A. Enhancing effects of acute psychosocial stress on priming of non-declarative memory in healthy young adults. Stress. 2012;15:329–338. doi: 10.3109/10253890.2011.624224. http://dx.doi.org/10.3109/10253890.2011.624224. [DOI] [PubMed] [Google Scholar]
- Hoffman R, Al’Absi M. The effect of acute stress on subsequent neuropsychological test performance. Archives of Clinical Neuropsychology. 2004;19:497–506. doi: 10.1016/j.acn.2003.07.005. http://dx.doi.org/10.1016/j.acn.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Hoscheidt SM. Impairing and enhancing effects of psychosocial stress on episodic memory and eyewitness report. University of Arizona; 2011. Retrieved from http://hdl.handle.net/10150/202762. [Google Scholar]
- Hoscheidt SM, LaBar KS, Ryan L, Jacobs WJ, Nadel L. Encoding negative events under stress: High subjective arousal is related to accurate emotional memory despite misinformation exposure. Neurobiology of Learning and Memory. 2014;112:237–247. doi: 10.1016/j.nlm.2013.09.008. http://dx.doi.org/10.1016/j.nlm.2013.09.008. [DOI] [PubMed] [Google Scholar]
- Human R. The effects of acute psychological stress on working memory and verbal declarative memory. University of Cape Town; 2010. Retrieved from http://www.dpru.uct.ac.za/sites/default/files/image_tool/images/117/Robyn.Human_.pdf. [Google Scholar]
- Human R, Thomas KG, Dreyer A, Amod aR, Wolf PS, Jacobs WJ. Acute psychosocial stress enhances visuospatial memory in healthy males. South African Journal of Psychology. 2013;43:300–313. http://dx.doi.org/10.1177/0081246313496913. [Google Scholar]
- Hupbach A, Dorskind JM. Stress selectively affects the reactivated components of a declarative memory. Behavioral Neuroscience. 2014;128:614–620. doi: 10.1037/bne0000006. http://dx.doi.org/10.1037/bne0000006. [DOI] [PubMed] [Google Scholar]
- Hupbach A, Fieman R. Moderate stress enhances immediate and delayed retrieval of educationally relevant material in healthy young men. Behavioral Neuroscience. 2012;126:819–825. doi: 10.1037/a0030489. http://dx.doi.org/10.1037/a0030489. [DOI] [PubMed] [Google Scholar]
- Larra MF, Schulz A, Schilling TM, Ferreira de Sá DS, Best D, Kozik B, Schächinger H. Heart rate response to post-learning stress predicts memory consolidation. Neurobiology of Learning and Memory. 2014;109:74–81. doi: 10.1016/j.nlm.2013.12.004. http://dx.doi.org/10.1016/j.nlm.2013.12.004. [DOI] [PubMed] [Google Scholar]
- Li S, Weerda R, Guenzel F, Wolf OT, Thiel CM. ADRA2B genotype modulates effects of acute psychosocial stress on emotional memory retrieval in healthy young men. Neurobiology of Learning and Memory. 2013;103:11–18. doi: 10.1016/j.nlm.2013.03.006. http://dx.doi.org/10.1016/j.nlm.2013.03.006. [DOI] [PubMed] [Google Scholar]
- Li S, Weerda R, Milde C, Wolf OT, Thiel CM. Effects of acute psychosocial stress on neural activity to emotional and neutral faces in a face recognition memory paradigm. Brain Imaging and Behavior. 2014;8:598–610. doi: 10.1007/s11682-013-9287-3. http://dx.doi.org/10.1007/s11682-013-9287-3. [DOI] [PubMed] [Google Scholar]
- Luethi M, Meier B, Sandi C. Stress effects on working memory, explicit memory, and implicit memory for neutral and emotional stimuli in healthy men. Frontiers in Behavioral Neuroscience. 2008;2:5. doi: 10.3389/neuro.08.005.2008. http://dx.doi.org/10.3389/neuro.08.005.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maheu FS, Collicutt P, Kornik R, Moszkowski R, Lupien SJ. The perfect time to be stressed: A differential modulation of human memory by stress applied in the morning or in the afternoon. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2005;29:1281–1288. doi: 10.1016/j.pnpbp.2005.08.012. http://dx.doi.org/10.1016/j.pnpbp.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Marin MF, Pilgrim K, Lupien SJ. Modulatory effects of stress on reactivated emotional memories. Psychoneuroendocrinology. 2010;35:1388–1396. doi: 10.1016/j.psyneuen.2010.04.002. http://dx.doi.org/10.1016/j.psyneuen.2010.04.002. [DOI] [PubMed] [Google Scholar]
- McCullough AM, Ritchey M, Ranganath C, Yonelinas A. Differential effects of stress-induced cortisol responses on recollection and familiarity-based recognition memory. Neurobiology of Learning and Memory. 2015;123:1–10. doi: 10.1016/j.nlm.2015.04.007. http://dx.doi.org/10.1016/j.nlm.2015.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough AM, Yonelinas AP. Cold-pressor stress after learning enhances familiarity-based recognition memory in men. Neurobiology of Learning and Memory. 2013;106:11–17. doi: 10.1016/j.nlm.2013.06.011. http://dx.doi.org/10.1016/j.nlm.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merz CJ, Wolf OT, Hennig J. Stress impairs retrieval of socially relevant information. Behavioral Neuroscience. 2010;124:288–293. doi: 10.1037/a0018942. http://dx.doi.org/10.1037/a0018942. [DOI] [PubMed] [Google Scholar]
- Nguyen DTH. The effects of psychosocial stress and social support on memory. San Jose State University; 2009. Retrieved from http://scholarworks.sjsu.edu/etd_theses. [Google Scholar]
- Nielsen SE, Ahmed I, Cahill L. Postlearning stress differentially affects memory for emotional gist and detail in naturally cycling women and women on hormonal contraceptives. Behavioral Neuroscience. 2014;128:482–493. doi: 10.1037/a0036687. http://dx.doi.org/10.1037/a0036687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen SE, Segal SK, Worden IV, Yim IS, Cahill L. Hormonal contraception use alters stress responses and emotional memory. Biological Psychology. 2013;92:257–266. doi: 10.1016/j.biopsycho.2012.10.007. http://dx.doi.org/10.1016/j.biopsycho.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oei NYL, Everaerd WTAM, Elzinga BM, van Well S, Bermond B. Psychosocial stress impairs working memory at high loads: An association with cortisol levels and memory retrieval. Stress. 2006;9:133–141. doi: 10.1080/10253890600965773. http://dx.doi.org/10.1080/10253890600965773. [DOI] [PubMed] [Google Scholar]
- Payne JD, Jackson ED, Hoscheidt S, Ryan L, Jacobs WJ, Nadel L. Stress administered prior to encoding impairs neutral but enhances emotional long-term episodic memories. Learning & Memory. 2007;14:861–868. doi: 10.1101/lm.743507. http://dx.doi.org/10.1101/lm.743507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne JD, Jackson ED, Ryan L, Hoscheidt S, Jacobs JW, Nadel L. The impact of stress on neutral and emotional aspects of episodic memory. Memory. 2006;14:1–16. doi: 10.1080/09658210500139176. http://dx.doi.org/10.1080/09658210500139176. [DOI] [PubMed] [Google Scholar]
- Preuβ D, Wolf OT. Post-learning psychosocial stress enhances consolidation of neutral stimuli. Neurobiology of Learning and Memory. 2009;92:318–326. doi: 10.1016/j.nlm.2009.03.009. http://dx.doi.org/10.1016/j.nlm.2009.03.009. [DOI] [PubMed] [Google Scholar]
- Pulopulos MM, Almela M, Hidalgo V, Villada C, Puig-Perez S, Salvador A. Acute stress does not impair long-term memory retrieval in older people. Neurobiology of Learning and Memory. 2013;104:16–24. doi: 10.1016/j.nlm.2013.04.010. http://dx.doi.org/10.1016/j.nlm.2013.04.010. [DOI] [PubMed] [Google Scholar]
- Qin S, Hermans EJ, van Marle HJF, Fernández G. Understanding low reliability of memories for neutral information encoded under stress: Alterations in memory-related activation in the hippocampus and midbrain. The Journal of Neuroscience. 2012;32:4032–4041. doi: 10.1523/JNEUROSCI.3101-11.2012. http://dx.doi.org/10.1523/JNEUROSCI.3101-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaedflieg CWEM, Schwabe L, Meyer T, Smeets T. Time dependent effects of stress prior to encoding on event-related potentials and 24h delayed retrieval. Psychoneuroendocrinology. 2013;38(12):3057–3069. doi: 10.1016/j.psyneuen.2013.09.002. http://dx.doi.org/10.1016/j.psyneuen.2013.09.002. [DOI] [PubMed] [Google Scholar]
- Robicheaux TR. Stress and eyewitness memory: Timing of stressor and association with cortisol stress responding. University of Nebraska; 2015. Retrieved from http://digitalcommons.unl.edu/psychdiss. [Google Scholar]
- Schönfeld P, Ackermann K, Schwabe L. Remembering under stress: Different roles of autonomic arousal and glucocorticoids in memory retrieval. Psychoneuroendocrinology. 2014;39:249–256. doi: 10.1016/j.psyneuen.2013.09.020. http://dx.doi.org/10.1016/j.psyneuen.2013.09.020. [DOI] [PubMed] [Google Scholar]
- Schoofs D, Wolf OT. Stress and memory retrieval in women: No strong impairing effect during the luteal phase. Behavioral Neuroscience. 2009;123:547–554. doi: 10.1037/a0015625. http://dx.doi.org/10.1037/a0015625. [DOI] [PubMed] [Google Scholar]
- Schwabe L, Bohringer A, Chatterjee M, Schachinger H. Effects of pre-learning stress on memory for neutral, positive and negative words: Different roles of cortisol and autonomic arousal. Neurobiology of Learning and Memory. 2008;90:44–53. doi: 10.1016/j.nlm.2008.02.002. http://dx.doi.org/10.1016/j.nlm.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Schwabe L, Wolf OT. The context counts: Congruent learning and testing environments prevent memory retrieval impairment following stress. Cognitive, Affective & Behavioral Neuroscience. 2009;9:229–236. doi: 10.3758/CABN.9.3.229. http://dx.doi.org/10.3758/CABN.9.3.229. [DOI] [PubMed] [Google Scholar]
- Schwabe L, Wolf OT. Learning under stress impairs memory formation. Neurobiology of Learning and Memory. 2010a;93:183–188. doi: 10.1016/j.nlm.2009.09.009. http://dx.doi.org/10.1016/j.nlm.2009.09.009. [DOI] [PubMed] [Google Scholar]
- Schwabe L, Wolf OT. Stress impairs the reconsolidation of autobiographical memories. Neurobiology of Learning and Memory. 2010b;94:153–157. doi: 10.1016/j.nlm.2010.05.001. http://dx.doi.org/10.1016/j.nlm.2010.05.001. [DOI] [PubMed] [Google Scholar]
- Schwabe L, Wolf OT. Timing matters: Temporal dynamics of stress effects on memory retrieval. Cognitive, Affective, & Behavioral Neuroscience. 2014;14:1041–1048. doi: 10.3758/s13415-014-0256-0. http://dx.doi.org/10.3758/s13415-014-0256-0. [DOI] [PubMed] [Google Scholar]
- Smeets T. Acute stress impairs memory retrieval independent of time of day. Psychoneuroendocrinology. 2011;36:495–501. doi: 10.1016/j.psyneuen.2010.08.001. http://dx.doi.org/10.1016/j.psyneuen.2010.08.001. [DOI] [PubMed] [Google Scholar]
- Smeets T, Giesbrecht T, Jelicic M, Merckelbach H. Context-dependent enhancement of declarative memory performance following acute psychosocial stress. Biological Psychology. 2007;76:116–123. doi: 10.1016/j.biopsycho.2007.07.001. http://dx.doi.org/10.1016/j.biopsycho.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Smeets T, Jelicic M, Merckelbach H. Stress-induced cortisol responses, sex differences, and false recollections in a DRM paradigm. Biological Psychology. 2006a;72:164–172. doi: 10.1016/j.biopsycho.2005.09.004. http://dx.doi.org/10.1016/j.biopsycho.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Smeets T, Jelicic M, Merckelbach H. The effect of acute stress on memory depends on word valence. International Journal of Psychophysiology. 2006b;62:30–37. doi: 10.1016/j.ijpsycho.2005.11.007. http://dx.doi.org/10.1016/j.ijpsycho.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Smeets T, Jelicic M, Merckelbach H, Peters M, Fett A, Taverniers J, … Dautzenberg J. Enhanced memory performance on an internal-internal source monitoring test following acute psychosocial stress. Behavioral Neuroscience. 2006;120:1204–1210. doi: 10.1037/0735-7044.120.6.1204. http://dx.doi.org/10.1037/0735-7044.120.6.1204. [DOI] [PubMed] [Google Scholar]
- Smeets T, Otgaar H, Candel I, Wolf OT. True or false? Memory is differentially affected by stress-induced cortisol elevations and sympathetic activity at consolidation and retrieval. Psychoneuroendocrinology. 2008;33:1378–1386. doi: 10.1016/j.psyneuen.2008.07.009. http://dx.doi.org/10.1016/j.psyneuen.2008.07.009. [DOI] [PubMed] [Google Scholar]
- Smeets T, Sijstermans K, Gijsen C, Peters M, Jelicic M, Merckelbach H. Acute consolidation stress enhances reality monitoring in healthy young adults. Stress: The International Journal on the Biology of Stress. 2008;11:235–245. doi: 10.1080/10253890701754076. http://dx.doi.org/10.1080/10253890701754076. [DOI] [PubMed] [Google Scholar]
- Stawski RS, Sliwinski MJ, Smyth JM. The effects of an acute psychosocial stressor on episodic memory. European Journal of Cognitive Psychology. 2009;21(6):897–918. doi: 10.1080/09541440802333042. http://dx.doi.org/10.1080/09541440802333042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taverniers J, Taylor MK, Smeets T. Delayed memory effects after intense stress in Special Forces candidates: Exploring path processes between cortisol secretion and memory recall. Stress. 2013;16:311–320. doi: 10.3109/10253890.2012.721824. http://dx.doi.org/http://dx.doi.org/10.3109/10253890.2012.721824. [DOI] [PubMed] [Google Scholar]
- Taverniers J, Van Ruysseveldt J, Smeets T, von Grumbkow J. High-intensity stress elicits robust cortisol increases, and impairs working memory and visuo-spatial declarative memory in Special Forces candidates: A field experiment. Stress. 2010;13:323–333. doi: 10.3109/10253891003642394. http://dx.doi.org/10.3109/10253891003642394. [DOI] [PubMed] [Google Scholar]
- Thompson LA, Williams KL, L’Esperance PR, Cornelius J. Context-dependent memory under stressful conditions: The case of skydiving. Human Factors. 2001;43:611–619. doi: 10.1518/001872001775870377. http://dx.doi.org/10.1518/001872001775870377. [DOI] [PubMed] [Google Scholar]
- Tollenaar MS, Elzinga BM, Spinhoven P, Everaerd W. Autobiographical memory after acute stress in healthy young men. Memory. 2009;17:301–310. doi: 10.1080/09658210802665845. http://dx.doi.org/10.1080/09658210802665845. [DOI] [PubMed] [Google Scholar]
- Tollenaar MS, Elzinga BM, Spinhoven P, Everaerd WAM. The effects of cortisol increase on long-term memory retrieval during and after acute psychosocial stress. Acta Psychologica. 2008;127:542–552. doi: 10.1016/j.actpsy.2007.10.007. http://dx.doi.org/10.1016/j.actpsy.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Trammell JP, Clore GL. Does stress enhance or impair memory consolidation? Cognition & Emotion. 2014;28(2):361–74. doi: 10.1080/02699931.2013.822346. http://dx.doi.org/10.1080/02699931.2013.822346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ast VA, Cornelisse S, Meeter M, Kindt M. Cortisol mediates the effects of stress on the contextual dependency of memories. Psychoneuroendocrinology. 2014;41:97–110. doi: 10.1016/j.psyneuen.2013.12.007. http://dx.doi.org/10.1016/j.psyneuen.2013.12.007. [DOI] [PubMed] [Google Scholar]
- Weymar M, Schwabe L, Löw A, Hamm AO. Stress sensitizes the brain: Increased processing of unpleasant pictures after exposure to acute stress. Journal of Cognitive Neuroscience. 2012;24:1511–1518. doi: 10.1162/jocn_a_00174. http://dx.doi.org/10.1162/jocn_a_00174. [DOI] [PubMed] [Google Scholar]
- Wiemers US, Sauvage MM, Schoofs D, Hamacher-Dang TC, Wolf OT. What we remember from a stressful episode. Psychoneuroendocrinology. 2013;38:2268–2277. doi: 10.1016/j.psyneuen.2013.04.015. http://dx.doi.org/10.1016/j.psyneuen.2013.04.015. [DOI] [PubMed] [Google Scholar]
- Wiemers US, Sauvage MM, Wolf OT. Odors as effective retrieval cues for stressful episodes. Neurobiology of Learning and Memory. 2014;112:230–236. doi: 10.1016/j.nlm.2013.10.004. http://dx.doi.org/10.1016/j.nlm.2013.10.004. [DOI] [PubMed] [Google Scholar]
- Wirkner J, Weymar M, Löw A, Hamm AO. Effects of pre-encoding stress on brain correlates associated with the long-term memory for emotional scenes. PLoS ONE. 2013;8(9) doi: 10.1371/journal.pone.0068212. http://dx.doi.org/10.1371/journal.pone.0068212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf OT. Immediate recall influences the effects of pre-encoding stress on emotional episodic long-term memory consolidation in healthy young men. Stress. 2012;15:272–280. doi: 10.3109/10253890.2011.622012. http://dx.doi.org/10.3109/10253890.2011.622012. [DOI] [PubMed] [Google Scholar]
- Wolf OT, Schommer NC, Hellhammer DH, McEwen BS, Kirschbaum C. The relationship between stress induced cortisol levels and memory differs between men and women. Psychoneuroendocrinology. 2001;26:711–720. doi: 10.1016/s0306-4530(01)00025-7. http://dx.doi.org/10.1016/S0306-4530(01)00025-7. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP, Parks CM, Koen JD, Jorgenson J, Mendoza SP. The effects of post-encoding stress on recognition memory: Examining the impact of skydiving in young men and women. Stress. 2011;14:136–144. doi: 10.3109/10253890.2010.520376. http://dx.doi.org/10.3109/10253890.2010.520376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoladz PR, Clark B, Warnecke A, Smith L, Tabar J, Talbot JN. Pre-learning stress differentially affects long-term memory for emotional words, depending on temporal proximity to the learning experience. Physiology & Behavior. 2011;103:467–476. doi: 10.1016/j.physbeh.2011.01.016. http://dx.doi.org/10.1016/j.physbeh.2011.01.016. [DOI] [PubMed] [Google Scholar]
- Zoladz PR, Kalchik AE, Hoffman MM, Aufdenkampe RL, Burke HM, Woelke SA, … Talbot JN. Brief, pre-retrieval stress differentially influences long-term memory depending on sex and corticosteroid response. Brain and Cognition. 2014;85:277–285. doi: 10.1016/j.bandc.2014.01.010. http://dx.doi.org/10.1016/j.bandc.2014.01.010. [DOI] [PubMed] [Google Scholar]
- Zoladz PR, Kalchik AE, Hoffman MM, Aufdenkampe RL, Lyle SM, Peters DM, … Rorabaugh BR. ADRA2B deletion variant selectively predicts stress-induced enhancement of long-term memory in females. Psychoneuroendocrinology. 2014;48:111–122. doi: 10.1016/j.psyneuen.2014.06.012. http://dx.doi.org/10.1016/j.psyneuen.2014.06.012. [DOI] [PubMed] [Google Scholar]
- Zoladz PR, Peters DM, Cadle CE, Kalchik AE, Aufdenkampe RL, Dailey AM, … Rorabaugh BR. Post-learning stress enhances long-term memory and differentially influences memory in females depending on menstrual stage. Acta Psychologica. 2015;160:127–133. doi: 10.1016/j.actpsy.2015.07.008. http://dx.doi.org/10.1016/j.actpsy.2015.07.008. [DOI] [PubMed] [Google Scholar]
- Zoladz PR, Warnecke AJ, Woelke SA, Burke HM, Frigo RM, Pisansky JM, … Talbot JN. Pre-learning stress that is temporally removed from acquisition exerts sex-specific effects on long-term memory. Neurobiology of Learning and Memory. 2013;100:77–87. doi: 10.1016/j.nlm.2012.12.012. http://dx.doi.org/10.1016/j.nlm.2012.12.012. [DOI] [PubMed] [Google Scholar]
References
- Ackermann S, Spalek K, Rasch B, Gschwind L, Coynel D, Fastenrath M, … de Quervain DJ-F. Testosterone levels in healthy men are related to amygdala reactivity and memory performance. Psychoneuroendocrinology. 2012;37:1417–1424. doi: 10.1016/j.psyneuen.2012.01.008. http://dx.doi.org/10.1016/j.psyneuen.2012.01.008. [DOI] [PubMed] [Google Scholar]
- Akirav I, Richter-Levin G. Biphasic modulation of hippocampal plasticity by behavioral stress and basolateral amygdala stimulation in the rat. The Journal of Neuroscience. 1999;19:10530–10535. doi: 10.1523/JNEUROSCI.19-23-10530.1999. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10575049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akirav I, Richter-Levin G. Mechanisms of amygdala modulation of hippocampal plasticity. The Journal of Neuroscience. 2002;22:9912–9921. doi: 10.1523/JNEUROSCI.22-22-09912.2002. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12427848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander JK, Hillier A, Smith RM, Tivarus ME, Beversdorf DQ. Beta-adrenergic modulation of cognitive flexibility during stress. Journal of Cognitive Neuroscience. 2007;19:468–478. doi: 10.1162/jocn.2007.19.3.468. http://dx.doi.org/10.1162/jocn.2007.19.3.468. [DOI] [PubMed] [Google Scholar]
- Allen AP, Kennedy PJ, Cryan JF, Dinan TG, Clarke G. Biological and psychological markers of stress in humans: Focus on the Trier Social Stress Test. Neuroscience and Biobehavioral Reviews. 2014;38:94–124. doi: 10.1016/j.neubiorev.2013.11.005. http://dx.doi.org/10.1016/j.neubiorev.2013.11.005. [DOI] [PubMed] [Google Scholar]
- Andreano JM, Arjomandi H, Cahill L. Menstrual cycle modulation of the relationship between cortisol and long-term memory. Psychoneuroendocrinology. 2008;33:874–882. doi: 10.1016/j.psyneuen.2008.03.009. http://dx.doi.org/10.1016/j.psyneuen.2008.03.009. [DOI] [PubMed] [Google Scholar]
- Andreano JM, Cahill L. Glucocorticoid release and memory consolidation in men and women. Psychological Science. 2006;17:466–470. doi: 10.1111/j.1467-9280.2006.01729.x. http://dx.doi.org/10.1111/j.1467-9280.2006.01729.x. [DOI] [PubMed] [Google Scholar]
- Andreano JM, Cahill L. Sex influences on the neurobiology of learning and memory. Learning & Memory. 2009;16:248–266. doi: 10.1101/lm.918309. http://dx.doi.org/10.1101/lm.918309. [DOI] [PubMed] [Google Scholar]
- Arnsten AFT. Stress signalling pathways that impair prefrontal cortex structure and function. Nature Reviews Neuroscience. 2009;10:410–422. doi: 10.1038/nrn2648. http://dx.doi.org/10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audet MC, Jacobson-Pick S, Wann BP, Anisman H. Social defeat promotes specific cytokine variations within the prefrontal cortex upon subsequent aggressive or endotoxin challenges. Brain, Behavior, and Immunity. 2011;25:1197–1205. doi: 10.1016/j.bbi.2011.03.010. http://dx.doi.org/10.1016/j.bbi.2011.03.010. [DOI] [PubMed] [Google Scholar]
- Barros LA, Tufik S, Andersen ML. The role of progesterone in memory: An overview of three decades. Neuroscience and Biobehavioral Reviews. 2015;49:193–204. doi: 10.1016/j.neubiorev.2014.11.015. http://dx.doi.org/10.1016/j.neubiorev.2014.11.015. [DOI] [PubMed] [Google Scholar]
- Beckner VE, Tucker DM, Delville Y, Mohr DC. Stress facilitates consolidation of verbal memory for a film but does not affect retrieval. Behavioral Neuroscience. 2006;120:518–527. doi: 10.1037/0735-7044.120.3.518. http://dx.doi.org/10.1037/0735-7044.120.3.518. [DOI] [PubMed] [Google Scholar]
- Besnard A, Caboche J, Laroche S. Reconsolidation of memory: A decade of debate. Progress in Neurobiology. 2012;99:61–80. doi: 10.1016/j.pneurobio.2012.07.002. http://dx.doi.org/10.1016/j.pneurobio.2012.07.002. [DOI] [PubMed] [Google Scholar]
- Bierhaus A, Wolf J, Andrassy M, Rohleder N, Humpert PM, Petrov D, … Nawroth PP. A mechanism converting psychosocial stress into mononuclear cell activation. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:1920–1925. doi: 10.1073/pnas.0438019100. http://dx.doi.org/10.1073/pnas.0438019100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenfeld RS, Ranganath C. Prefrontal cortex and long-term memory encoding: An integrative review of findings from neuropsychology and neuroimaging. The Neuroscientist. 2007;13:280–291. doi: 10.1177/1073858407299290. http://dx.doi.org/10.1177/1073858407299290. [DOI] [PubMed] [Google Scholar]
- Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to Meta-Analysis. Chichester, UK: John Wiley & Sons, Ltd; 2009. http://dx.doi.org/10.1002/9780470743386. [Google Scholar]
- Butts KA, Weinberg J, Young AH, Phillips AG. Glucocorticoid receptors in the prefrontal cortex regulate stress-evoked dopamine efflux and aspects of executive function. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:18459–18464. doi: 10.1073/pnas.1111746108. http://dx.doi.org/10.1073/pnas.1111746108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadle CE, Zoladz PR. Stress time-dependently influences the acquisition and retrieval of unrelated information by producing a memory of its own. Frontiers in Psychology. 2015;6:910. doi: 10.3389/fpsyg.2015.00910. http://dx.doi.org/10.3389/fpsyg.2015.00910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill L, Gorski L, Le K. Enhanced human memory consolidation with post-learning stress: Interaction with the degree of arousal at encoding. Learning & Memory. 2003;10:270–274. doi: 10.1101/lm.62403. http://dx.doi.org/10.1101/lm.62403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill L, McGaugh JL. Mechanisms of emotional arousal and lasting declarative memory. Trends in Neurosciences. 1998 doi: 10.1016/s0166-2236(97)01214-9. http://dx.doi.org/10.1016/S0166-2236(97)01214-9. [DOI] [PubMed]
- Childs E, De Wit H. Hormonal, cardiovascular, and subjective responses to acute stress in smokers. Psychopharmacology. 2009;203:1–12. doi: 10.1007/s00213-008-1359-5. http://dx.doi.org/10.1007/s00213-008-1359-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu YC, Egner T. Inhibition-induced forgetting: When more control leads to less memory. Psychological Science. 2015;26:27–38. doi: 10.1177/0956797614553945. http://dx.doi.org/10.1177/0956797614553945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conboy L, Sandi C. Stress at learning facilitates memory formation by regulating AMPA receptor trafficking through a glucocorticoid action. Neuropsychopharmacology. 2010;35:674–685. doi: 10.1038/npp.2009.172. http://dx.doi.org/10.1038/npp.2009.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad CD. A critical review of chronic stress effects on spatial learning and memory. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2010;34:742–755. doi: 10.1016/j.pnpbp.2009.11.003. http://dx.doi.org/10.1016/j.pnpbp.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Conrad CD, Lupien SJ, McEwen BS. Support for a bimodal role for type II adrenal steroid receptors in spatial memory. Neurobiology of Learning and Memory. 1999;72:39–46. doi: 10.1006/nlme.1998.3898. http://dx.doi.org/10.1006/nlme.1998.3898. [DOI] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: When the immune system subjugates the brain. Nature Reviews Neuroscience. 2008;9:46–56. doi: 10.1038/nrn2297. http://dx.doi.org/10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davachi L. Item, context and relational episodic encoding in humans. Current Opinion in Neurobiology. 2006;16:693–700. doi: 10.1016/j.conb.2006.10.012. http://dx.doi.org/10.1016/j.conb.2006.10.012. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Oitzl MS, Joëls M. Stress and cognition: Are corticosteroids good or bad guys? Trends in Neurosciences. 1999;22:422–426. doi: 10.1016/s0166-2236(99)01438-1. http://dx.doi.org/10.1016/S0166-2236(99)01438-1. [DOI] [PubMed] [Google Scholar]
- de Quervain DJF, Aerni A, Roozendaal B. Preventive effect of beta-adrenoceptor blockade on glucocorticoid-induced memory retrieval deficits. The American Journal of Psychiatry. 2007;164:967–969. doi: 10.1176/ajp.2007.164.6.967. http://dx.doi.org/164/6/967[pii]\n10.1176/appi.ajp.164.6.967. [DOI] [PubMed] [Google Scholar]
- de Quervain DJF, Roozendaal B, McGaugh JL. Stress and glucocorticoids impair retrieval of long-term spatial memory. Nature. 1998;394:787–790. doi: 10.1038/29542. http://dx.doi.org/10.1038/29542. [DOI] [PubMed] [Google Scholar]
- de Quervain DJF, Roozendaal B, Nitsch RM, McGaugh JL, Hock C. Acute cortisone administration impairs retrieval of long-term declarative memory in humans. Nature Neuroscience. 2000;3:313–314. doi: 10.1038/73873. http://dx.doi.org/10.1038/73873. [DOI] [PubMed] [Google Scholar]
- Diamond DM, Campbell AM, Park CR, Halonen J, Zoladz PR. The temporal dynamics model of emotional memory processing: A synthesis on the neurobiological basis of stress-induced amnesia, flashbulb and traumatic memories, and the Yerkes-Dodson law. Neural Plasticity. 2007;2007(60803):1–33. doi: 10.1155/2007/60803. http://dx.doi.org/10.1155/2007/60803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond DM, Campbell AM, Park CR, Woodson JC, Conrad CD, Bachstetter AD, Mervis RF. Influence of predator stress on the consolidation versus retrieval of long-term spatial memory and hippocampal spinogenesis. Hippocampus. 2006;16:571–576. doi: 10.1002/hipo.20188. http://dx.doi.org/10.1002/hipo.20188. [DOI] [PubMed] [Google Scholar]
- Diana RA, Yonelinas AP, Ranganath C. Imaging recollection and familiarity in the medial temporal lobe: a three-component model. Trends in Cognitive Sciences. 2007;11:379–386. doi: 10.1016/j.tics.2007.08.001. http://dx.doi.org/10.1016/j.tics.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychological Bulletin. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. http://dx.doi.org/10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Dobbins IG, Foley H, Schacter DL, Wagner AD. Executive control during episodic retrieval: Multiple prefrontal processes subserve source memory. Neuron. 2002;35:989–996. doi: 10.1016/s0896-6273(02)00858-9. http://dx.doi.org/10.1016/S0896-6273(02)00858-9. [DOI] [PubMed] [Google Scholar]
- Dudai Y. The neurobiology of consolidations, or, how stable is the engram? Annual Review of Psychology. 2004;55:51–86. doi: 10.1146/annurev.psych.55.090902.142050. http://dx.doi.org/10.1146/annurev.psych.55.090902.142050. [DOI] [PubMed] [Google Scholar]
- Duval S. The trim and fill method. Rothstein HR, Sutton AJ, Borenstein M, editors. Publication Bias in Meta-Analysis: Prevention, Assessment and Adjustments. 2005:127–144. http://dx.doi.org/10.1002/0470870168.ch8.
- Duval S, Tweedie R. A nonparametric “trim and fill” method of accounting for publication bias in meta-analysis. Journal of the American Statistical Society. 2000a;95(449):89–98. http://dx.doi.org/10.2307/2669529. [Google Scholar]
- Duval S, Tweedie R. Trim and fill: A simple funnel-plot–based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000b;56:455–463. doi: 10.1111/j.0006-341x.2000.00455.x. http://dx.doi.org/10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. http://dx.doi.org/10.1136/bmj.316.7129.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Otto T, Cohen NJ. The hippocampus—what does it do? Behavioral and Neural Biology. 1992;57(1):2–36. doi: 10.1016/0163-1047(92)90724-i. http://dx.doi.org/10.1016/0163-1047(92)90724-I. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annual Review of Neuroscience. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. http://dx.doi.org/10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finsterwald C, Alberini CM. Stress and glucocorticoid receptor-dependent mechanisms in long-term memory: From adaptive responses to psychopathologies. Neurobiology of Learning and Memory. 2014;112:17–29. doi: 10.1016/j.nlm.2013.09.017. http://dx.doi.org/10.1016/j.nlm.2013.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher PC, Shallice T, Frith CD, Frackowiak RSJ, Dolan RJ. The functional roles of prefrontal cortex in episodic memory. II. Retrieval. Brain. 1998;121:1249–1256. doi: 10.1093/brain/121.7.1249. http://dx.doi.org/10.1093/brain/121.7.1249. [DOI] [PubMed] [Google Scholar]
- Gagnon SA, Wagner AD. Acute stress and episodic memory retrieval: Neurobiological mechanisms and behavioral consequences. Annals of the New York Academy of Sciences. 2016 doi: 10.1111/nyas.12996. http://dx.doi.org/10.1111/nyas.12996. [DOI] [PubMed]
- Gershberg FB, Shimamura AP. Impaired use of organizational strategies in free recall following frontal lobe damage. Neuropsychologia. 1995;33:1305–1333. doi: 10.1016/0028-3932(95)00103-a. http://dx.doi.org/10.1016/0028-3932(95)00103-A. [DOI] [PubMed] [Google Scholar]
- Giles GE, Mahoney CR, Brunyé TT, Taylor HA, Kanarek RB. Stress effects on mood, HPA axis, and autonomic response: comparison of three psychosocial stress paradigms. PloS One. 2014;9(12):e113618. doi: 10.1371/journal.pone.0113618. http://dx.doi.org/10.1371/journal.pone.0113618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham BM, Milad MR. Blockade of estrogen by hormonal contraceptives impairs fear extinction in female rats and women. Biological Psychiatry. 2013;73:371–378. doi: 10.1016/j.biopsych.2012.09.018. http://dx.doi.org/10.1016/j.biopsych.2012.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harburger LL, Pechenino AS, Saadi A, Frick KM. Post-training progesterone dose-dependently enhances object, but not spatial, memory consolidation. Behavioural Brain Research. 2008;194:174–180. doi: 10.1016/j.bbr.2008.07.014. http://dx.doi.org/10.1016/j.bbr.2008.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison NA, Cercignani M, Voon V, Critchley HD. Effects of inflammation on hippocampus and substantia nigra responses to novelty in healthy human participants. Neuropsychopharmacology. 2015;40:831–838. doi: 10.1038/npp.2014.222. http://dx.doi.org/10.1038/npp.2014.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison NA, Doeller CF, Voon V, Burgess N, Critchley HD. Peripheral inflammation acutely impairs human spatial memory via actions on medial temporal lobe glucose metabolism. Biological Psychiatry. 2014 doi: 10.1016/j.biopsych.2014.01.005. http://dx.doi.org/10.1016/j.biopsych.2014.01.005. [DOI] [PMC free article] [PubMed]
- Hedges LV, Tipton E, Johnson MC. Robust variance estimation in meta-regression with dependent effect size estimates. Research Synthesis Methods. 2010;1:39–65. doi: 10.1002/jrsm.5. http://dx.doi.org/10.1002/jrsm.5. [DOI] [PubMed] [Google Scholar]
- Henckens MJAG, Hermans EJ, Pu Z, Joëls M, Fernández G. Stressed memories: How acute stress affects memory formation in humans. The Journal of Neuroscience. 2009;29:10111–10119. doi: 10.1523/JNEUROSCI.1184-09.2009. http://dx.doi.org/10.1523/JNEUROSCI.1184-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson RNA, Rugg MD, Shallice T, Dolan RJ. Confidence in recognition memory for words: Dissociating right prefrontal roles in episodic retrieval. Journal of Cognitive Neuroscience. 2000;12:913–923. doi: 10.1162/08989290051137468. http://dx.doi.org/10.1162/08989290051137468. [DOI] [PubMed] [Google Scholar]
- Het S, Ramlow G, Wolf OT. A meta-analytic review of the effects of acute cortisol administration on human memory. Psychoneuroendocrinology. 2005;30:771–784. doi: 10.1016/j.psyneuen.2005.03.005. http://dx.doi.org/10.1016/j.psyneuen.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Hidalgo V, Almela M, Villada C, Salvador A. Acute stress impairs recall after interference in older people, but not in young people. Hormones and Behavior. 2014;65:264–272. doi: 10.1016/j.yhbeh.2013.12.017. http://dx.doi.org/10.1016/j.yhbeh.2013.12.017. [DOI] [PubMed] [Google Scholar]
- Hidalgo V, Pulopulos MM, Puig-Perez S, Espin L, Gomez-Amor J, Salvador A. Acute stress affects free recall and recognition of pictures differently depending on age and sex. Behavioural Brain Research. 2015;292:393–402. doi: 10.1016/j.bbr.2015.07.011. http://dx.doi.org/10.1016/j.bbr.2015.07.011. [DOI] [PubMed] [Google Scholar]
- Iidaka T, Anderson ND, Kapur S, Cabeza R, Craik FI. The effect of divided attention on encoding and retrieval in episodic memory revealed by positron emission tomography. Journal of Cognitive Neuroscience. 2000;12:267–280. doi: 10.1162/089892900562093. http://dx.doi.org/10.1162/089892900562093. [DOI] [PubMed] [Google Scholar]
- Inagaki T, Gautreaux C, Luine V. Acute estrogen treatment facilitates recognition memory consolidation and alters monoamine levels in memory-related brain areas. Hormones and Behavior. 2010;58:415–426. doi: 10.1016/j.yhbeh.2010.05.013. http://dx.doi.org/10.1016/j.yhbeh.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki TK, Muscatell KA, Irwin MR, Cole SW, Eisenberger NI. Inflammation selectively enhances amygdala activity to socially threatening images. NeuroImage. 2012;59(4):3222–3226. doi: 10.1016/j.neuroimage.2011.10.090. http://dx.doi.org/10.1016/j.neuroimage.2011.10.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joëls M, Fernandez G, Roozendaal B. Stress and emotional memory: A matter of timing. Trends in Cognitive Sciences. 2011;15:280–288. doi: 10.1016/j.tics.2011.04.004. http://dx.doi.org/10.1016/j.tics.2011.04.004. [DOI] [PubMed] [Google Scholar]
- Joëls M, Pu Z, Wiegert O, Oitzl MS, Krugers HJ. Learning under stress: How does it work? Trends in Cognitive Sciences. 2006;10:152–158. doi: 10.1016/j.tics.2006.02.002. http://dx.doi.org/10.1016/j.tics.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, Hellhammer DH. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosomatic Medicine. 1999;61:154–162. doi: 10.1097/00006842-199903000-00006. http://dx.doi.org/0033-3174/99/6102-0154. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Wüst S, Hellhammer DH. Consistent sex differences in cortisol responses to psychological stress. Psychosomatic Medicine. 1992;54:648–657. doi: 10.1097/00006842-199211000-00004. http://dx.doi.org/10.1097/00006842-199211000-00004. [DOI] [PubMed] [Google Scholar]
- Kramár EA, Babayan AH, Gall CM, Lynch G. Estrogen promotes learning-related plasticity by modifying the synaptic cytoskeleton. Neuroscience. 2013;239:3–16. doi: 10.1016/j.neuroscience.2012.10.038. http://dx.doi.org/10.1016/j.neuroscience.2012.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudielka BM, Schommer NC, Hellhammer DH, Kirschbaum C. Acute HPA axis responses, heart rate, and mood changes to psychosocial stress (TSST) in humans at different times of day. Psychoneuroendocrinology. 2004;29:983–992. doi: 10.1016/j.psyneuen.2003.08.009. http://dx.doi.org/10.1016/j.psyneuen.2003.08.009. [DOI] [PubMed] [Google Scholar]
- Kuhlmann S, Piel M, Wolf OT. Impaired memory retrieval after psychosocial stress in healthy young men. The Journal of Neuroscience. 2005;25:2977–2982. doi: 10.1523/JNEUROSCI.5139-04.2005. http://dx.doi.org/10.1523/JNEUROSCI.5139-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennartsson AK, Kushnir MM, Bergquist J, Billig H, Jonsdottir IH. Sex steroid levels temporarily increase in response to acute psychosocial stress in healthy men and women. International Journal of Psychophysiology. 2012;84:246–253. doi: 10.1016/j.ijpsycho.2012.03.001. http://dx.doi.org/10.1016/j.ijpsycho.2012.03.001. [DOI] [PubMed] [Google Scholar]
- Levy BJ, Anderson MC. Inhibitory processes and the control of memory retrieval. Trends in Cognitive Sciences. 2002;6:299–305. doi: 10.1016/s1364-6613(02)01923-x. http://dx.doi.org/10.1016/S1364-6613(02)01923-X. [DOI] [PubMed] [Google Scholar]
- Liening SH, Stanton SJ, Saini EK, Schultheiss OC. Salivary testosterone, cortisol, and progesterone: Two-week stability, interhormone correlations, and effects of time of day, menstrual cycle, and oral contraceptive use on steroid hormone levels. Physiology & Behavior. 2010;99:8–16. doi: 10.1016/j.physbeh.2009.10.001. http://dx.doi.org/10.1016/j.physbeh.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Luethi M, Meier B, Sandi C. Stress effects on working memory, explicit memory, and implicit memory for neutral and emotional stimuli in healthy men. Frontiers in Behavioral Neuroscience. 2008;2:5. doi: 10.3389/neuro.08.005.2008. http://dx.doi.org/10.3389/neuro.08.005.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddox GB, Naveh-Benjamin M, Old S, Kilb A. The role of attention in the associative binding of emotionally arousing words. Psychonomic Bulletin & Review. 2012;19:1128–1134. doi: 10.3758/s13423-012-0315-x. http://dx.doi.org/10.3758/s13423-012-0315-x. [DOI] [PubMed] [Google Scholar]
- Maheu FS, Collicutt P, Kornik R, Moszkowski R, Lupien SJ. The perfect time to be stressed: A differential modulation of human memory by stress applied in the morning or in the afternoon. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2005;29:1281–1288. doi: 10.1016/j.pnpbp.2005.08.012. http://dx.doi.org/10.1016/j.pnpbp.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Mangels JA. Strategic processing and memory for temporal order in patients with frontal lobe lesions. Neuropsychology. 1997;11:207–221. doi: 10.1037//0894-4105.11.2.207. [DOI] [PubMed] [Google Scholar]
- Marinari KT, Leshner AI, Doyle MP. Menstrual cycle status and adrenocortical reactivity to pyschological stress. Psychoneuroendocrinology. 1976;1:213–218. doi: 10.1016/0306-4530(76)90011-1. http://dx.doi.org/10.1016/0306-4530(76)90011-1. [DOI] [PubMed] [Google Scholar]
- Maroun M, Richter-Levin G. Exposure to acute stress blocks the induction of long-term potentiation of the amygdala-prefrontal cortex pathway in vivo. The Journal of Neuroscience. 2003;23:4406–4409. doi: 10.1523/JNEUROSCI.23-11-04406.2003. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12805280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather M, Sutherland MR. Arousal-biased competition in perception and memory. Perspectives on Psychological Science. 2011;6:114–133. doi: 10.1177/1745691611400234. http://dx.doi.org/10.1177/1745691611400234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayes A, Montaldi D, Migo E. Associative memory and the medial temporal lobes. Trends in Cognitive Sciences. 2007 doi: 10.1016/j.tics.2006.12.003. http://dx.doi.org/10.1016/j.tics.2006.12.003. [DOI] [PubMed]
- McCullough AM, Ritchey M, Ranganath C, Yonelinas A. Differential effects of stress-induced cortisol responses on recollection and familiarity-based recognition memory. Neurobiology of Learning and Memory. 2015;123:1–10. doi: 10.1016/j.nlm.2015.04.007. http://dx.doi.org/10.1016/j.nlm.2015.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough AM, Yonelinas AP. Cold-pressor stress after learning enhances familiarity-based recognition memory in men. Neurobiology of Learning and Memory. 2013;106:11–17. doi: 10.1016/j.nlm.2013.06.011. http://dx.doi.org/10.1016/j.nlm.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Physiology and neurobiology of stress and adaptation: Central role of the brain. Physiological Reviews. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. http://dx.doi.org/10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. Memory--A century of consolidation. Science. 2000;287:248–251. doi: 10.1126/science.287.5451.248. http://dx.doi.org/10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annual Review of Neuroscience. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. http://dx.doi.org/10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. Consolidating memories. Annual Review of Psychology. 2015;66:1–24. doi: 10.1146/annurev-psych-010814-014954. http://dx.doi.org/10.1146/annurev-psych-010814-014954. [DOI] [PubMed] [Google Scholar]
- Morris SB. Estimating effect sizes from pretest-posttest-control group designs. Organizational Research Methods. 2008;11:364–386. http://dx.doi.org/10.1177/1094428106291059. [Google Scholar]
- Morris SB, DeShon RP. Combining effect size estimates in meta-analysis with repeated measures and independent-groups designs. Psychological Methods. 2002;7:105–125. doi: 10.1037/1082-989x.7.1.105. http://dx.doi.org/10.1037/1082-989X.7.1.105. [DOI] [PubMed] [Google Scholar]
- Nielsen SE, Segal SK, Worden IV, Yim IS, Cahill L. Hormonal contraception use alters stress responses and emotional memory. Biological Psychology. 2013;92:257–266. doi: 10.1016/j.biopsycho.2012.10.007. http://dx.doi.org/10.1016/j.biopsycho.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman KA, O’Reilly RC. Modeling hippocampal and neocortical contributions to recognition memory: A complementary-learning-systems approach. Psychological Review. 2003;110:611–646. doi: 10.1037/0033-295X.110.4.611. http://dx.doi.org/10.1037/0033-295X.110.4.611. [DOI] [PubMed] [Google Scholar]
- O’Connor MF, Bower JE, Cho HJ, Creswell JD, Dimitrov S, Hamby ME, … Irwin MR. To assess, to control, to exclude: Effects of biobehavioral factors on circulating inflammatory markers. Brain, Behavior, and Immunity. 2009;23:887–897. doi: 10.1016/j.bbi.2009.04.005. http://dx.doi.org/10.1016/j.bbi.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkin AJ. Normal age-related memory loss and its relation to frontal lobe dysfunction. In: Rabbitt P, editor. Methodology Of Frontal And Executive Function. Psychology Press; 1997. pp. 171–194. [Google Scholar]
- Patel PD, Katz M, Karssen AM, Lyons DM. Stress-induced changes in corticosteroid receptor expression in primate hippocampus and prefrontal cortex. Psychoneuroendocrinology. 2008;33:360–367. doi: 10.1016/j.psyneuen.2007.12.003. http://dx.doi.org/10.1016/j.psyneuen.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne JD, Jackson ED, Hoscheidt S, Ryan L, Jacobs WJ, Nadel L. Stress administered prior to encoding impairs neutral but enhances emotional long-term episodic memories. Learning & Memory. 2007;14:861–868. doi: 10.1101/lm.743507. http://dx.doi.org/10.1101/lm.743507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulopulos MM, Almela M, Hidalgo V, Villada C, Puig-Perez S, Salvador A. Acute stress does not impair long-term memory retrieval in older people. Neurobiology of Learning and Memory. 2013;104:16–24. doi: 10.1016/j.nlm.2013.04.010. http://dx.doi.org/10.1016/j.nlm.2013.04.010. [DOI] [PubMed] [Google Scholar]
- Qin S, Hermans EJ, van Marle HJF, Fernández G. Understanding low reliability of memories for neutral information encoded under stress: Alterations in memory-related activation in the hippocampus and midbrain. The Journal of Neuroscience. 2012;32:4032–4041. doi: 10.1523/JNEUROSCI.3101-11.2012. http://dx.doi.org/10.1523/JNEUROSCI.3101-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaedflieg CWEM, Schwabe L, Meyer T, Smeets T. Time dependent effects of stress prior to encoding on event-related potentials and 24h delayed retrieval. Psychoneuroendocrinology. 2013;38:3057–3069. doi: 10.1016/j.psyneuen.2013.09.002. http://dx.doi.org/10.1016/j.psyneuen.2013.09.002. [DOI] [PubMed] [Google Scholar]
- Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends in Immunology. 2006;27(1):24–31. doi: 10.1016/j.it.2005.11.006. http://dx.doi.org/10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichenberg A, Yirmiya R, Schuld A, Kraus T, Haack M, Morag A, Pollmächer T. Cytokine-associated emotional and cognitive disturbances in humans. Archives of General Psychiatry. 2001;58:445–452. doi: 10.1001/archpsyc.58.5.445. http://dx.doi.org/10.1001/archpsyc.58.5.445. [DOI] [PubMed] [Google Scholar]
- della Rocchetta AI, Milner B. Strategic search and retrieval inhibition: The role of the frontal lobes. Neuropsychologia. 1993;31:503–524. doi: 10.1016/0028-3932(93)90049-6. http://dx.doi.org/10.1016/0028-3932(93)90049-6. [DOI] [PubMed] [Google Scholar]
- Roozendaal B. Stress and memory: Opposing effects of glucocorticoids on memory consolidation and memory retrieval. Neurobiology of Learning and Memory. 2002;78:578–595. doi: 10.1006/nlme.2002.4080. http://dx.doi.org/10.1006/nlme.2002.4080. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, McGaugh JL. Basolateral amygdala lesions block the memory-enhancing effect of glucocorticoid administration in the dorsal hippocampus of rats. European Journal of Neuroscience. 1997;9:76–83. doi: 10.1111/j.1460-9568.1997.tb01355.x. http://dx.doi.org/10.1111/j.1460-9568.1997.tb01355.x. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Okuda S, de Quervain DJF, McGaugh JL. Glucocorticoids interact with emotion-induced noradrenergic activation in influencing different memory functions. Neuroscience. 2006;138:901–910. doi: 10.1016/j.neuroscience.2005.07.049. http://dx.doi.org/10.1016/j.neuroscience.2005.07.049. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Okuda S, Van der Zee EA, McGaugh JL. Glucocorticoid enhancement of memory requires arousal-induced noradrenergic activation in the basolateral amygdala. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:6741–6746. doi: 10.1073/pnas.0601874103. http://dx.doi.org/10.1073/pnas.0601874103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaki M, Mather M. How reward and emotional stimuli induce different reactions across the menstrual cycle. Social and Personality Psychology Compass. 2012;6:1–17. doi: 10.1111/j.1751-9004.2011.00415.x. http://dx.doi.org/10.1111/j.1751-9004.2011.00415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandi C, Pinelo-Nava MT. Stress and memory: Behavioral effects and neurobiological mechanisms. Neural Plasticity. 2007;2007(78970):1–20. doi: 10.1155/2007/78970. http://dx.doi.org/10.1155/2007/78970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sänger J, Bechtold L, Schoofs D, Blaszkewicz M, Wascher E. The influence of acute stress on attention mechanisms and its electrophysiological correlates. Frontiers in Behavioral Neuroscienceehavioral Neuroscience. 2014;8:353. doi: 10.3389/fnbeh.2014.00353. http://dx.doi.org/10.3389/fnbeh.2014.00353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky R, Rivier C, Yamamoto G, Plotsky P, Vale W. Interleukin-1 stimulates the secretion of hypothalamic corticotropin-releasing factor. Science. 1987;238(4826):522–524. doi: 10.1126/science.2821621. http://dx.doi.org/10.1126/science.2821621. [DOI] [PubMed] [Google Scholar]
- Sazma MA, McCullough AM, Yonelinas AP. The importance of context for post-encoding stress effects on memory: An alternative to consolidation theory. Poster Presented at CNS; New York, NY. 2016. Poster. [Google Scholar]
- Scammacca N, Roberts G, Stuebing KK. Meta-analysis with complex research designs: Dealing with dependence from multiple neasures and multiple group comparisons. Review of Educational Research. 2014;84:328–364. doi: 10.3102/0034654313500826. http://dx.doi.org/10.3102/0034654313500826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoofs D, Wolf OT, Smeets T. Cold pressor stress impairs performance on working memory tasks requiring executive functions in healthy young men. Behavioral Neuroscience. 2009;123:1066–1075. doi: 10.1037/a0016980. http://dx.doi.org/10.1037/a0016980. [DOI] [PubMed] [Google Scholar]
- Schwabe L, Haddad L, Schachinger H. HPA axis activation by a socially evaluated cold-pressor test. Psychoneuroendocrinology. 2008;33:890–895. doi: 10.1016/j.psyneuen.2008.03.001. http://dx.doi.org/10.1016/j.psyneuen.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Schwabe L, Joëls M, Roozendaal B, Wolf OT, Oitzl MS. Stress effects on memory: An update and integration. Neuroscience and Biobehavioral Reviews. 2012;36:1740–1749. doi: 10.1016/j.neubiorev.2011.07.002. http://dx.doi.org/10.1016/j.neubiorev.2011.07.002. [DOI] [PubMed] [Google Scholar]
- Schwabe L, Nader K, Pruessner JC. Reconsolidation of human memory: Brain mechanisms and clinical relevance. Biological Psychiatry. 2014 doi: 10.1016/j.biopsych.2014.03.008. http://dx.doi.org/10.1016/j.biopsych.2014.03.008. [DOI] [PubMed]
- Schwabe L, Römer S, Richter S, Dockendorf S, Bilak B, Schächinger H. Stress effects on declarative memory retrieval are blocked by a β-adrenoceptor antagonist in humans. Psychoneuroendocrinology. 2009;34:446–454. doi: 10.1016/j.psyneuen.2008.10.009. http://dx.doi.org/10.1016/j.psyneuen.2008.10.009. [DOI] [PubMed] [Google Scholar]
- Schwabe L, Wolf OT. Stress-induced modulation of instrumental behavior: From goal-directed to habitual control of action. Behavioural Brain Research. 2011;219:321–328. doi: 10.1016/j.bbr.2010.12.038. http://dx.doi.org/10.1016/j.bbr.2010.12.038. [DOI] [PubMed] [Google Scholar]
- Schwabe L, Wolf OT. Timing matters: Temporal dynamics of stress effects on memory retrieval. Cognitive, Affective, & Behavioral Neuroscience. 2014;14:1041–1048. doi: 10.3758/s13415-014-0256-0. http://dx.doi.org/10.3758/s13415-014-0256-0. [DOI] [PubMed] [Google Scholar]
- Schwabe L, Wolf OT, Oitzl MS. Memory formation under stress: Quantity and quality. Neuroscience and Biobehavioral Reviews. 2010;34:584–591. doi: 10.1016/j.neubiorev.2009.11.015. http://dx.doi.org/10.1016/j.neubiorev.2009.11.015. [DOI] [PubMed] [Google Scholar]
- Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. Journal of Neurology, Neurosurgery, and Psychiatry. 1957;20:11–21. doi: 10.1136/jnnp.20.1.11. Retrieved from http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=497229&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shansky RM, Lipps J. Stress-induced cognitive dysfunction: Hormone-neurotransmitter interactions in the prefrontal cortex. Frontiers in Human Neuroscience. 2013;7:123. doi: 10.3389/fnhum.2013.00123. http://dx.doi.org/10.3389/fnhum.2013.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields GS, Bonner JC, Moons WG. Does cortisol influence core executive functions? A meta-analysis of acute cortisol administration effects on working memory, inhibition, and set-shifting. Psychoneuroendocrinology. 2015;58:91–103. doi: 10.1016/j.psyneuen.2015.04.017. http://dx.doi.org/10.1016/j.psyneuen.2015.04.017. [DOI] [PubMed] [Google Scholar]
- Shields GS, Kuchenbecker SY, Pressman SD, Sumida KD, Slavich GM. Better cognitive control of emotional information is associated with reduced pro-inflammatory cytokine reactivity to emotional stress. Stress. 2016;19:63–68. doi: 10.3109/10253890.2015.1121983. http://dx.doi.org/10.3109/10253890.2015.1121983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields GS, Sazma MA, Yonelinas AP. The effects of acute stress on core executive functions: A meta-analysis and comparison with effects of cortisol. Neuroscience & Biobehavioral Reviews. 2016;68:651–688. doi: 10.1016/j.neubiorev.2016.06.038. http://dx.doi.org/10.1016/j.neubiorev.2016.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman MN, Sternberg EM. Glucocorticoid regulation of inflammation and its functional correlates: From HPA axis to glucocorticoid receptor dysfunction. Annals of the New York Academy of Sciences. 2012;1261:55–63. doi: 10.1111/j.1749-6632.2012.06633.x. http://dx.doi.org/10.1111/j.1749-6632.2012.06633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons JS, Spiers HJ. Prefrontal and medial temporal lobe interactions in long-term memory. Nature Reviews Neuroscience. 2003;4:637–648. doi: 10.1038/nrn1178. http://dx.doi.org/10.1038/nrn1178. [DOI] [PubMed] [Google Scholar]
- Skoluda N, Strahler J, Schlotz W, Niederberger L, Marques S, Fischer S, … Nater UM. Intra-individual psychological and physiological responses to acute laboratory stressors of different intensity. Psychoneuroendocrinology. 2015;51:227–236. doi: 10.1016/j.psyneuen.2014.10.002. http://dx.doi.org/10.1016/j.psyneuen.2014.10.002. [DOI] [PubMed] [Google Scholar]
- Slavich GM, Irwin MR. From stress to inflammation and major depressive disorder: A social signal transduction theory of depression. Psychological Bulletin. 2014;140:774–815. doi: 10.1037/a0035302. http://dx.doi.org/10.1037/a0035302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeets T, Giesbrecht T, Jelicic M, Merckelbach H. Context-dependent enhancement of declarative memory performance following acute psychosocial stress. Biological Psychology. 2007;76:116–123. doi: 10.1016/j.biopsycho.2007.07.001. http://dx.doi.org/10.1016/j.biopsycho.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Smeets T, Otgaar H, Candel I, Wolf OT. True or false? Memory is differentially affected by stress-induced cortisol elevations and sympathetic activity at consolidation and retrieval. Psychoneuroendocrinology. 2008;33:1378–1386. doi: 10.1016/j.psyneuen.2008.07.009. http://dx.doi.org/10.1016/j.psyneuen.2008.07.009. [DOI] [PubMed] [Google Scholar]
- Spaniol J, Davidson PSR, Kim ASN, Han H, Moscovitch M, Grady CL. Event-related fMRI studies of episodic encoding and retrieval: Meta-analyses using activation likelihood estimation. Neuropsychologia. 2009;47:1765–1779. doi: 10.1016/j.neuropsychologia.2009.02.028. http://dx.doi.org/10.1016/j.neuropsychologia.2009.02.028. [DOI] [PubMed] [Google Scholar]
- Squire LR, Alvarez P. Retrograde amnesia and memory consolidation: A neurobiological perspective. Current Opinion in Neurobiology. 1995;5:169–177. doi: 10.1016/0959-4388(95)80023-9. http://dx.doi.org/10.1016/0959-4388(95)80023-9. [DOI] [PubMed] [Google Scholar]
- Sripada RK, Marx CE, King AP, Rajaram N, Garfinkel SN, Abelson JL, Liberzon I. DHEA enhances emotion regulation neurocircuits and modulates memory for emotional stimuli. Neuropsychopharmacology. 2013;38(9):1798–1807. doi: 10.1038/npp.2013.79. http://dx.doi.org/10.1038/npp.2013.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripada RK, Welsh RC, Marx CE, Liberzon I. The neurosteroids allopregnanolone and dehydroepiandrosterone modulate resting-state amygdala connectivity. Human Brain Mapping. 2014;35:3249–3261. doi: 10.1002/hbm.22399. http://dx.doi.org/10.1002/hbm.22399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava DP, Waters EM, Mermelstein PG, Kramár EA, Shors TJ, Liu F. Rapid estrogen signaling in the brain: Implications for the fine-tuning of neuronal circuitry. The Journal of Neuroscience. 2011;31:16056–16063. doi: 10.1523/JNEUROSCI.4097-11.2011. http://dx.doi.org/10.1523/JNEUROSCI.4097-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner-Smith EE, Tipton E. Robust variance estimation with dependent effect sizes: Practical considerations including a software tutorial in Stata and SPSS. Research Synthesis Methods. 2014;5:13–30. doi: 10.1002/jrsm.1091. http://dx.doi.org/10.1002/jrsm.1091. [DOI] [PubMed] [Google Scholar]
- Thoma MV, Kirschbaum C, Wolf JM, Rohleder N. Acute stress responses in salivary alpha-amylase predict increases of plasma norepinephrine. Biological Psychology. 2012;91:342–348. doi: 10.1016/j.biopsycho.2012.07.008. http://dx.doi.org/10.1016/j.biopsycho.2012.07.008. [DOI] [PubMed] [Google Scholar]
- Tipton E. Small sample adjustments for robust variance estimation with meta-regression. Psychological Methods. 2014;20:375–393. doi: 10.1037/met0000011. http://dx.doi.org/10.1037/met0000011. [DOI] [PubMed] [Google Scholar]
- Trammell JP, Clore GL. Does stress enhance or impair memory consolidation? Cognition & Emotion. 2014;28:361–374. doi: 10.1080/02699931.2013.822346. http://dx.doi.org/10.1080/02699931.2013.822346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentine JC, Pigott TD, Rothstein HR. How many studies do you need? A primer on statistical power for meta-analysis. Journal of Educational and Behavioral Statistics. 2010;35:215–247. http://dx.doi.org/10.3102/1076998609346961. [Google Scholar]
- van Ast VA, Cornelisse S, Meeter M, Kindt M. Cortisol mediates the effects of stress on the contextual dependency of memories. Psychoneuroendocrinology. 2014;41:97–110. doi: 10.1016/j.psyneuen.2013.12.007. http://dx.doi.org/10.1016/j.psyneuen.2013.12.007. [DOI] [PubMed] [Google Scholar]
- van Stegeren AH, Roozendaal B, Kindt M, Wolf OT, Joëls M. Interacting noradrenergic and corticosteroid systems shift human brain activation patterns during encoding. Neurobiology of Learning and Memory. 2010;93:56–65. doi: 10.1016/j.nlm.2009.08.004. http://dx.doi.org/10.1016/j.nlm.2009.08.004. [DOI] [PubMed] [Google Scholar]
- van Wingen G, Mattern C, Verkes RJ, Buitelaar J, Fernández G. Testosterone biases automatic memory processes in women towards potential mates. NeuroImage. 2008;43:114–120. doi: 10.1016/j.neuroimage.2008.07.002. http://dx.doi.org/10.1016/j.neuroimage.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Vogel S, Fernández G, Joëls M, Schwabe L. Cognitive adaptation under stress: A case for the mineralocorticoid receptor. Trends in Cognitive Sciences. 2016;20:192–203. doi: 10.1016/j.tics.2015.12.003. http://dx.doi.org/10.1016/j.tics.2015.12.003. [DOI] [PubMed] [Google Scholar]
- Wiemers US, Sauvage MM, Schoofs D, Hamacher-Dang TC, Wolf OT. What we remember from a stressful episode. Psychoneuroendocrinology. 2013;38:2268–2277. doi: 10.1016/j.psyneuen.2013.04.015. http://dx.doi.org/10.1016/j.psyneuen.2013.04.015. [DOI] [PubMed] [Google Scholar]
- Wolf OT. Immediate recall influences the effects of pre-encoding stress on emotional episodic long-term memory consolidation in healthy young men. Stress. 2012;15:272–280. doi: 10.3109/10253890.2011.622012. http://dx.doi.org/10.3109/10253890.2011.622012. [DOI] [PubMed] [Google Scholar]
- Yabuki Y, Shinoda Y, Izumi H, Ikuno T, Shioda N, Fukunaga K. Dehydroepiandrosterone administration improves memory deficits following transient brain ischemia through sigma-1 receptor stimulation. Brain Research. 2015;1622:102–113. doi: 10.1016/j.brainres.2015.05.006. http://dx.doi.org/10.1016/j.brainres.2015.05.006. [DOI] [PubMed] [Google Scholar]
- Ycaza Herrera A, Mather M. Actions and interactions of estradiol and glucocorticoids in cognition and the brain: Implications for aging women. Neuroscience & Biobehavioral Reviews. 2015;55:36–52. doi: 10.1016/j.neubiorev.2015.04.005. http://dx.doi.org/10.1016/j.neubiorev.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonelinas AP. The Nature of Recollection and Familiarity: A Review of 30 Years of Research. Journal of Memory and Language. 2002;46:441–517. http://dx.doi.org/10.1006/jmla.2002.2864. [Google Scholar]
- Yonelinas AP. The hippocampus supports high-resolution binding in the service of perception, working memory and long-term memory. Behavioural Brain Research. 2013;254:34–44. doi: 10.1016/j.bbr.2013.05.030. http://dx.doi.org/10.1016/j.bbr.2013.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonelinas AP, Parks CM, Koen JD, Jorgenson J, Mendoza SP. The effects of post-encoding stress on recognition memory: Examining the impact of skydiving in young men and women. Stress. 2011;14:136–144. doi: 10.3109/10253890.2010.520376. http://dx.doi.org/10.3109/10253890.2010.520376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonelinas AP, Ritchey M. The slow forgetting of emotional episodic memories: An emotional binding account. Trends in Cognitive Sciences. 2015;19:259–267. doi: 10.1016/j.tics.2015.02.009. http://dx.doi.org/10.1016/j.tics.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen EY, Liu W, Karatsoreos IN, Feng J, McEwen BS, Yan Z. Acute stress enhances glutamatergic transmission in prefrontal cortex and facilitates working memory. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:14075–14079. doi: 10.1073/pnas.0906791106. http://dx.doi.org/10.1073/pnas.0906791106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalcman SS, Green-Johnson JM, Murray L, Nance DM, Dyck D, Anisman H, Greenberg AH. Cytokine-specific central monoamine alterations induced by interleukin-1, -2 and -6. Brain Research. 1994;643(1):40–49. doi: 10.1016/0006-8993(94)90006-x. http://dx.doi.org/10.1016/0006-8993(94)90006-X. [DOI] [PubMed] [Google Scholar]
- Zoladz PR, Clark B, Warnecke A, Smith L, Tabar J, Talbot JN. Pre-learning stress differentially affects long-term memory for emotional words, depending on temporal proximity to the learning experience. Physiology & Behavior. 2011;103:467–476. doi: 10.1016/j.physbeh.2011.01.016. http://dx.doi.org/10.1016/j.physbeh.2011.01.016. [DOI] [PubMed] [Google Scholar]
- Zola-Morgan S, Squire L, Amaral D. Human amnesia and the medial temporal region: Enduring memory impairment following a bilateral lesion limited to field CA1 of the hippocampus. The Journal of Neuroscience. 1986;6:2950–2967. doi: 10.1523/JNEUROSCI.06-10-02950.1986. Retrieved from http://www.jneurosci.org/content/6/10/2950.short. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.