Abstract
Research indicates that alcohol misuse is associated with behavioral disinhibition, but the neurophysiological mechanisms governing this relationship remain largely unknown. Recent work suggests that successful inhibition and cognitive control involves electrophysiological theta-band dynamics, including medial frontal cortex (MFC) power enhancement and functional connectivity between the MFC and dorsal prefrontal cortex (dPFC) regions, which may be disrupted by alcohol misuse. In addition, research suggests that, compared to men, women are at heightened risk of experiencing the negative physical and neurocognitive correlates of drinking. The present study tested the hypothesis that alcohol misuse has a deleterious effect on theta-band response inhibition EEG dynamics in a sample of 300 24-year-old same-sex twins. A cotwin control (CTC) design was used to disentangle premorbid risk for alcohol use from the causal effects of alcohol exposure. Drinking was negatively associated with theta-band MFC power and MFC-dPFC connectivity during response inhibition, and this effect was stronger among women. The CTC analysis suggested that, for women, reduced nogo-related theta-band MFC power and MFC-dPFC connectivity were both consistent with the potential deleterious causal effects of alcohol exposure. These findings suggest that diminished theta-band MFC power and MFC-dPFC connectivity may be neurophysiological mechanisms underlying alcohol-related disinhibition. Although preliminary, these results suggest that normative levels of alcohol use during emerging adulthood have potential sex-specific causal effects on response inhibition EEG dynamics, and thus have potentially significant public health implications.
Keywords: Alcohol, Cotwin Control, Functional Connectivity, Inhibitory Control, Sex Differences, Theta
Introduction
Impairments in inhibitory control processes are strongly associated with alcohol misuse, and suggest the presence of alcohol-related neurocognitive dysfunction (Dick et al., 2010; Smith et al., 2014). Research using functional magnetic resonance imaging (fMRI) implicates the medial frontal cortex (MFC) and dorsal medial/lateral prefrontal cortex (dPFC) regions as crucial components of a cortical network related to successful inhibition (Simmonds et al., 2008). Recent theoretical and empirical work suggests that inhibitory and cognitive control processes are associated with theta-band (4-8 Hz) electrophysiological signals, including both local midfrontal cortex/MFC activity (Huster et al., 2013) and interregional MFC-dPFC communication through oscillatory synchronization (Clayton et al., 2015; Fries, 2005). Therefore, disruptions in theta-band MFC power or MFC-dPFC communication may be neurophysiological mechanisms associated with alcohol-related disinhibition. It is often difficult to disentangle the causal effects of alcohol from genetic/shared environmental risk towards alcohol misuse, and various lines of research support both the strong heritability of alcohol-related brain dysfunction (Iacono et al., 2008) and the potential neurotoxic effects of alcohol (Jacobus and Tapert, 2013). Because of this, previous research on response inhibition-related brain dynamics and drinking (Kamarajan et al., 2004) is ambiguous regarding any potential etiology explaining the association. Since purely observational/correlational research cannot establish causal mechanisms, data obtained from quasi-experimental designs, such as comparing members of a twin pair who vary in their degree of drinking (i.e., cotwin control design), is needed to test etiological hypotheses (Vaidyanathan et al., 2015).
In the last decade, research has begun to identify the electroencephalographic features of response inhibition (see Huster et al., 2013, for a review). The most prominent and consistently replicated component during demands of response inhibition or conflict is an increase in theta power over the MFC (Harper et al., 2014; Lavallee et al., 2014; Nigbur et al., 2011; Yamanaka and Yamamoto, 2010). Recent accounts of cognitive control processes implicate MFC theta in monitoring the environment and behavior for situations of conflict/uncertainty (e.g., determining whether to withhold a prepotent response) and signaling medial/lateral prefrontal regions to exert cognitive control and adapt/regulate behavior (Clayton et al., 2015).
Empirical evidence suggests that the communication between MFC and prefrontal regions is biophysically realized through interregional coordinated theta-band rhythmic activity (i.e., phase synchronized oscillations), which forms a dynamic functional network for information transfer among distal cortical regions to execute cognitive/inhibitory control processes (Clayton et al., 2015; Fries, 2005). While this theta-band MFC–prefrontal cortex functional network has been observed across a variety of cognitively demanding events (for a discussion, see Cavanagh and Frank, 2014), less is known about the network dynamics during inhibitory control. Meta-analyses of fMRI studies investigating the functional neural correlates of response inhibition have revealed co-activation in MFC and dorsolateral/dorsal medial prefrontal cortex regions (Simmonds et al., 2008; Swick et al., 2011), and thus these two regions may form a functional network through theta-band oscillatory synchrony during situations requiring inhibitory control.
Given the importance of theta dynamics during inhibition and cognitive control, and findings suggesting a link between drinking and reduced prefrontal cortex grey and white matter integrity (for a review, see Welch et al., 2013), reduced theta-band MFC power and/or interregional MFC-dPFC functional integration may be potential candidate neurophysiological mechanisms of alcohol-related disinhibition and suboptimal inhibitory control.
It is difficult to make strong inferences of etiological mechanisms regarding any observed association between alcohol use and some outcome (e.g., brain function) given the multitude of causal interpretations that can be made in purely observational research. For example, the finding that drinkers show poorer white matter integrity than matched controls does not offer evidence of etiology, since the association could be reflective of a causal effect of alcohol, a premorbid vulnerability towards alcohol misuse, or a combination of both factors. Quasi-experimental research designs, such as the cotwin control (CTC) method, can help disentangle causal environmental exposure effects from premorbid genetic/shared environmental characteristics and strengthen etiological inferences (Vaidyanathan et al., 2015). The CTC design takes advantage of the genetic and rearing environment similarity among twins discordant for their degree of exposure to some environmental agent (e.g., alcohol; McGue et al., 2010). Since monozygotic (MZ) twins share 100% of their genes and rearing environment, comparisons within an MZ pair fully account for all genetic/shared environmental familial factors, whether measured or not, that may influence the propensity for an individual to drink and potentially confound any nonshared causal effect of drinking on an outcome (e.g., brain connectivity). Comparisons within dizygotic (DZ) twin pairs, who share 50% of their genotype, control fully for shared environmental effects and partially for genetic effects. When investigating the potential causal effects of alcohol on the brain within the CTC method, the neural dynamics of the lesser-drinking twin are an estimate of what the brain dynamics of the heavier-drinking twin would have been had he or she drank less. If alcohol exposure has a potential deleterious causal effect on inhibitory control-related theta dynamics, the twin who consumed more alcohol should exhibit less activity/connectivity than the twin who drank less. Conversely, if genetic/shared environmental risk factors underlie poor brain network functioning, both the heavier- and lesser-drinking twins would evidence the same degree of diminished theta dynamics.
The current study sought to evaluate the potential causal effect of normative levels of drinking on EEG correlates of response inhibition in a population-based sample of 24-year-old same-sex twins. These twins were assessed at the end of emerging adulthood (age 18-24), which is an important developmental period during which peak levels of alcohol misuse (Vrieze et al., 2012) occur concomitantly with continuing neural development throughout adulthood (Bennett and Baird, 2006; Lebel et al., 2012). Heavy drinking during this period may have deleterious effects on the still-developing young adult brain, and a comprehensive set of drinking behaviors (e.g., frequency/quantity of alcohol use, maximum consumption, number of times intoxicated) were assessed to cover this developmental period. Thus, this genetically informed dataset is well suited to identify potential causal effects of alcohol exposure on inhibition-related theta-band EEG dynamics.
First, we predicted increased theta-band MFC power and MFC-dPFC functional connectivity following stimuli signaling the need for inhibition. Second, we tested the hypothesis that heavier alcohol use would be associated with decreased inhibition-related theta power and MFC-dPFC connectivity. Third, in light of research suggesting a heightened vulnerability for women towards developing alcohol-related neurocognitive deficits (Nolen-Hoeksema, 2004) and brain atrophy (Mann et al., 2005; Welch et al., 2013), we expected that the impact of drinking on response inhibition-related brain dynamics would be greater among women. Fourth, significant drinking effects were followed up with the CTC method to test etiological hypotheses. We hypothesized that the relationship between reduced inhibition-related MFC theta would be reflective of both a premorbid characteristic shared by members of a twin pair consuming more alcohol than lesser-using pairs (familial effect; Kamarajan et al., 2006), and causal effects of alcohol exposure. Given that functional connectivity is a sensitive and transient process (Fries, 2005), and evidence that alcohol exposure is related to poor prefrontal white matter integrity (Jacobus and Tapert, 2013; Welch et al., 2013), we hypothesized that alcohol exposure would have a deleterious causal effect on MFC-dPFC connectivity beyond any genetic/shared environmental risk effect.
Methods
Participants
Participants consisted of 309 young adult MZ and DZ twins drawn from the Minnesota Twin Family Study Enrichment Sample, who were originally assessed at age 11 and then reassessed approximately every three years (Keyes et al., 2009). The current report uses data from the 24-year-old assessment (age: mean±SD, 24.8±0.7). Nine individuals were dropped because of excessive EEG artifacts. The final sample comprised 300 twins (185 women), with 182 MZ (86 complete pairs) and 118 DZ twins (51 complete pairs).
Self-report and diagnostic assessment
Alcohol use history was obtained with the Substance Abuse Module (SAM) of the Composite International Diagnostic Interview (Robins et al., 1987). Twins were assessed simultaneously in separate rooms by different trained interviewers. The SAM was modified to include questions regarding the frequency and quantity of drinking and Alcohol Use Disorder (AUD) diagnostic criteria from the DSM-5. A composite drinking index was derived by summing responses from four self-report SAM items: (1) total number of times intoxicated from alcohol (0 = never to 6 = 150 times or more), (2) frequency of drinking in the past 12 months (0 = never to 5 = two or more times every day), (3) typical number of drinks consumed in one session in the past 12 months (0 = none to 6 = 30 or more), and (4) the maximum number of drinks consumed in a 24-hr period since last assessment (0 = none to 6 = 30 or more). Possible scores ranged from 0 to 23 (see Table S1 in Supplement for descriptive statistics). These four measures tap different aspects of alcohol use/exposure and demonstrated significant item-level correlations (Table S2 in Supplement). Combining them into a single composite index decreases the risk of observing false-positive effects, which would be inflated if analyses were conducted for each measure separately, and produces a measure that arguably has greater construct validity than any of them individually. Previous research provides strong evidence for the construct and psychometric validity of this index. A longitudinal study using this index reported strong internal consistency and parent-offspring correlation when assessed across multiple time points spanning adolescence through emerging adulthood (McGue et al., 2014). In addition, two reports suggest that higher scores on this index are related to reduced prefrontal gray matter volume and neurocognitive functioning (Malone et al., 2014; Wilson et al., 2015). DSM-5 AUD symptoms were available for a subset (n = 253) of twins, and were coded, by consensus, by two individuals with advanced clinical training. 8.5% (14/165) of females and 25.0% (22/88) of males met criteria for DSM-5 AUD. These rates are comparable to those reported in similar samples (Vrieze et al., 2012).
Go/Nogo task
Behavioral and EEG data were collected during a complex 1-back go/nogo task. Two white letters were alternately centrally presented on a black background, and participants were instructed to make a button press whenever a letter followed a different letter (go; e.g., second letter in the sequence X-Y), but withhold their response when the letter was identical to the preceding letter (nogo; e.g., third letter in the sequence X-Y-Y). The task consisted of three blocks of 144 pseudorandomized trials (108 go/36 nogo), with different letters used for each block (XY/DU/OP). The stimulus duration was 300ms, response window was 1150ms, and intertrial interval was 900ms. 20 practice trials were administered before EEG recording.
EEG recording and signal processing
EEG data were acquired at 1024 Hz with an analog DC to 205 Hz bandpass filter from 61 scalp electrodes using a BioSemi ActiveTwo system (Biosemi, Amsterdam, Netherlands) arranged according to the international 10/10 system. Vertical and horizontal electrooculogram (EOG) data were acquired from electrodes placed above and below the right eye and bilaterally on the temples, respectively. Electrodes were placed on both earlobes to serve as an offline average reference.
Signals were processed in MATLAB (version 7.1, Mathworks, Inc.) and EEGLAB (Delorme and Makeig, 2004). Continuous signals were downsampled to 256 Hz, highpass filtered at 0.1 Hz (firfilt plugin; Kaiser window of order 1286), and rereferenced to averaged earlobe signals. An automated procedure was used to detect signal artifacts and inter-electrode electrolyte bridging (Tenke and Kayser, 2001). Descriptive statistics (e.g., absolute temporal variance) were calculated for each electrode and 1s time-range in the continuous data. Data that exceeded ±4 of the normalized median absolute deviation (Rousseeuw and Croux, 1993) in 25% or 75% of a given time range or electrode, respectively, were removed from the data. Signals were then decomposed with Infomax independent component (IC) analysis, and the time course and inverse weights (topography) of each IC were correlated with the time course of a criterion channel (bipolar vertical or horizontal EOG) and a prototypical inverse weight (topography) of a blink or saccade IC, respectively. ICs were subtracted from the data if the squared correlation coefficient exceeded an empirically derived threshold (expectation maximization algorithm; Mognon et al., 2011).
Epochs of ±2s centered on stimulus onset were taken, and screened for the artifacts detailed above. Rejected channels within epochs containing ≥75% uncontaminated channels were interpolated via spherical spline method (Perrin et al., 1989); otherwise, the trial/epoch was discarded. Commission errors on nogo trials and omission errors on go trials were excluded. Epochs were baseline corrected by subtracting the mean prestimulus interval (fixation dot) activity from −200 to −1 ms and downsampled to 128 Hz. Signals were scalp Laplacian transformed (Lagrange order = 50; m = 4; λ = 10−5) using a spherical spline surface (Kayser and Tenke, 2006; Perrin et al., 1989). This is a reference-free spatial filter recommended for connectivity analyses (Cohen, 2015) that sharpens topographic localization and attenuates spatially-broad volume-conducted activity (single source activity projected to multiple electrodes) that obscures long-range connectivity (Winter et al., 2007).
Time-frequency analysis
Single-trial signals were transformed into time-frequency representations via wavelet convolution (Cohen, 2014) by multiplying the power spectrum of the EEG (obtained via fast Fourier transform [FFT]) by the power spectrum of complex Morlet wavelets [ei2πft e−t2/(2σ2))], where f is frequency, which ranged from 2 to 40 Hz in 25 logarithmic steps, t is time, and σ defines the width of each frequency band, set according to c/(2πf), where c is the number of wavelet cycles, which increased from 3.5 to 8 in 25 logarithmic steps to obtain comparable time/frequency precision, and then taking the inverse FFT. The resulting complex signals were down-sampled in time to 64 time bins/second. Because unequal trial numbers between conditions may bias power and connectivity estimates, for each subject, a random sample (without replacement) of go trials was selected to match the number of nogo trials (M = 89.83, SD = 15.93) before calculating power and interelectrode connectivity. This was performed 25 times, and the results were averaged.
From the resulting complex signal Zt, an estimate of the frequency-specific power at each time point was calculated as [real(Zt) + imag(Zt)]2. Trial-averaged power was decibel transformed [10 × log10(power/baseline)], where baseline power was calculated as the condition-averaged prestimulus power from −250 to −50ms.
Interelectrode functional connectivity was calculated with the weighted phase-lag index (wPLI; for the mathematical definition, see Vinck et al., 2011). The wPLI is defined as the absolute value of the average sign of phase differences (cross-spectrum imaginary component) between two electrodes, weighted by the average distance of the phase differences from the real axis (magnitude of the cross-spectrum imaginary component). Since the projection of electrical activity from a common source to the scalp is instantaneous (has no phase lag), this measure ignores 0/180 phase lag differences, and is insensitive to spurious connectivity due to single source volume conduction (Vinck et al., 2011). The wPLI values can range from 0 (no connectivity, or 0/180 phase lag due to volume conduction) to 1 (perfect phase-lagged connectivity).
Selection of electrodes and time-frequency regions of interest
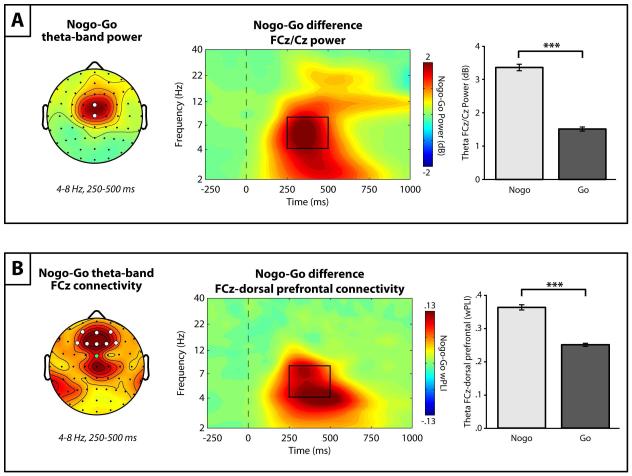
Theta power values were quantified as the mean power over a region of interest (ROI) spanning 4-8 Hz and 250-500 ms post-stimulus for each condition based on the grand average time-frequency representation (Figure 1A). A topographic map of theta activity (Figure 1A) revealed a focal power increase around electrodes FCz/Cz, and for each subject and condition the pooled activity across these electrodes was used to quantify theta power (referred to as midfrontal/MFC theta for convenience) for statistical analysis.
Figure 1.
The grand averaged stimulus-locked theta-band EEG dynamics. (A) Left: The grand averaged topographic distribution of theta-band (4-8 Hz) power between 250-500ms post-stimulus onset, illustrating increased midfrontal theta power over FCz and Cz (white electrodes). Right: The grand averaged time-frequency plot of stimulus-locked (time = 0, dashed line) power pooled across FCz/Cz electrodes, illustrating that increased post-stimulus power occurs primarily in the theta-band. (B) Left: The grand averaged topographic distribution of theta-band FCz–seeded (green electrode) functional connectivity, as measured by the weighted phase lag index (wPLI), between 250-500ms post-stimulus, revealing strong connectivity between FCz and dorsal medial and dorsolateral prefrontal channels (dPFC; white electrodes). Right: The grand averaged time-frequency plot of FCz–dPFC connectivity (pooled across dPFC electrodes), demonstrating increased theta-band connectivity between 250-500 after stimulus onset. The black outline boxes denote the region of interest used for statistical analyses.
For functional connectivity, the pairwise wPLI values between a midfrontal electrode (FCz) and all other channels were computed. FCz was chosen as the seed based on the power results and previous work investigating MFC-based connectivity during cognitive control and response conflict using surface EEG (e.g., Nigbur et al., 2012). Given a priori hypotheses regarding theta-band interregional functional connectivity during response inhibition, a topographic map of grand averaged connectivity between 4-8Hz and 250-500ms post-stimulus was plotted (Figure 1B). This revealed an increase in connectivity between MFC electrode FCz and dorsomedial/dorsolateral prefrontal cortex regions (electrodes Fz/F1/F2/F3/F4/AFz/AF3/AF4). Although other areas of interregional connectivity were observed, in particular just posterior to FCz over the central/centroparietal cortex, the choice to focus on dPFC connectivity was guided by a priori hypotheses regarding MFC-dPFC connectivity during inhibition and cognitive control (Clayton et al., 2015; Simmonds et al., 2008). The grand averaged time-frequency connectivity plot between FCz and the pooled dPFC electrodes (referred to as MFC/FCz–dPFC connectivity for convenience) confirmed strong connectivity in the theta-band (4-8 Hz) between 250-500 ms (Figure 1B), and the mean wPLI across this ROI was calculated and then pooled across dorsal prefrontal electrodes for each subject/condition.
Statistical analyses
Statistical analyses were conducted with linear mixed models (LMMs) in R (R Core Team, 2015) using lmer from the lme4 package (Bates et al., 2015) with denominator degrees of freedom adjusted via Kenward-Roger approximation from the lmerTest package (Kuznetsova et al., 2014). Random intercepts at the individual and twin pair level accounted for within-individual and -pair correlations, respectively. The effect of stimulus type (go, nogo) was evaluated with a one-level LMM (effect coded for nogo). To investigate the effect of alcohol use and the moderating effect of sex on theta-band MFC power and MFC-dPFC connectivity, separate two-level LMMs were fit to nogo or go data with fixed effects for sex (center effect coded for women), drinking index scores, and their interaction. Similar models were separately fit to the go or nogo behavioral error rates, which were square root transformed to reduce skew (untransformed values are reported for descriptive purposes). Standard biometric models were fit in the OpenMX package for R (Boker et al., 2011) to estimate the amount of variance attributed to additive genetic (A), shared environment (C), and nonshared environment (E) for drinking index scores and theta-band measures.
Significant drinking effects were followed up with a cotwin control analysis, which decomposes alcohol effects into within-pair and twin-pair mean (between-pair or -family) effects (Begg and Parides, 2003; McGue et al., 2010). The within-pair effect represents each individual twin’s deviation from their twin-pair mean, and reflects the nonshared environmental effects of alcohol exposure unconfounded by shared genetic or environmental influences. A significant within-pair effect for theta dynamics would be consistent with the hypothesis that alcohol exposure has a causal effect on EEG components. Observing an effect at the individual level in the absence of a significant within-pair effect indicates that the individual-level effect is due primarily to factors shared by twins that are confounded with exposure and theta dynamics. The twin-pair mean drinking index captures such confounding factors, accounting for all shared genetic and environmental factors, whether measured or not, that may influence use. In this design, comparisons within MZ and DZ pairs provide a full shared environmental control, and MZ pairs provide full genetic control and DZ pairs provide partial genetic control (McGue et al., 2010). An appropriate estimate of the magnitude of family-level effects -- the effect of twin- and family-level characteristics independent of the characteristics of each individual twin -- is given by the difference between the estimated twin-pair mean effect and the within-pair effect (Begg and Parides, 2003; see also Bryk and Raudenbush, 1992, pp. 121-123, for a discussion of such contextual effects). A significant difference score effect for theta dynamics would be consistent with the hypothesis that the association reflects a preexisting vulnerability towards drinking. The standard error of this difference can be estimated by means of the delta method (Oehlert, 1992). We consider such between-pair effects as significant if the difference score is at least twice its standard error, which corresponds to a t-statistic ≥ 2. LMMs were fit with fixed effects for the between- and within-pair effects and a random intercept at the twin-pair level. Because prior research (Kamarajan et al., 2006; Kamarajan et al., 2004) justifies a directional hypothesis for the negative relationship between drinking and theta-band dynamics, one-tailed significance testing was use for the CTC.
Results
Behavioral results
The mean accuracy rates were 98.58% (SD = 2.16) for go and 87.14% (SD = 11.24) for nogo conditions. The mean reaction time on correct go trials was 464.41 ms (SD = 89.96), while incorrect responses to nogo stimuli (false alarms) had a mean reaction time of 482.34 ms (SD = 134.30). More errors were committed on nogo trials than go trials [t(299) = 21.76, p < .001]. Drinking was not related to error rates in either condition (ps ≥ 0.13, for all main/interaction effects).
Table 1 presents descriptive statistics, intraclass correlations, and biometric heritability for MFC theta power, MFC-dPFC theta-band connectivity, and drinking index scores. MZ twin correlations exceeded those of DZ twins for all measures, and each variable demonstrated moderate additive genetic heritability and nonshared environmental effects. Women had significantly lower drinking index scores than men [t(159) = −6.79, p < .001].
Table 1.
Means (SD), twin intraclass correlations, and estimated biometric heritability
| Mean (SD) |
Intraclass Correlations |
AE Components |
||||
|---|---|---|---|---|---|---|
| Males | Females | MZ | DZ | A | E | |
| Drinking Index Score |
12.64 (3.80) |
9.27 (3.47) |
0.64 | 0.19 | .59 (.45–.70) |
.41 (.30–.55) |
| Nogo midfrontal theta power |
3.12 (1.67) |
3.50 (1.67) |
0.55 | 0.38 | .57 (.42–.68) |
.43 (.32–.58) |
| Go midfrontal theta power |
1.39 (1.02) |
1.59 (1.09) |
0.59 | 0.23 | .59 (.44–.70) |
.41 (.30–.56) |
| Nogo FCz-dorsal prefrontal connectivity |
0.36 (0.14) |
0.37 (0.14) |
0.45 | 0.28 | .47 (.30–.61) |
.53 (.39–.70) |
| Go FCz-dorsal prefrontal connectivity |
0.24 (0.08) |
0.26 (0.08) |
0.43 | 0.03 | .39 (.21–.55) |
.61 (.45–.79) |
Notes: Values for power are in decibels, and theta-band connectivity is in weighted phase lag index units, which range from 0 (no connectivity) to 1 (perfect connectivity). Sample sizes for descriptive statistics were 115 males and 185 females. Sample sizes for intraclass correlations and biometric ACE models were 182 monozygotic (MZ) twins and 118 dizygotic (DZ) twins. Biometric heritability was estimated from standard biometric factor models, where the proportion of variance in each phenotypic measure is decomposed into additive genetic heritability (A), shared/common environmental (C), and nonshared/unique environmental (E) components (95% confidence intervals are provided in parentheses). Different ACE (e.g., AE, CE) models were compared using Akaike’s information criterion (AIC = χ2 – 2df), which is a combined metric of goodness-of-fit and parsimony of a given model. The model with the lowest AIC (i.e., most negative) was considered the best fit, which was an AE model for all measures.
Effects of response inhibition on theta-band components
As depicted in the nogo minus go difference plots in Figure 2, nogo trials were associated with greater MFC theta power (Figure 2A) than go trials [t(299) = 27.46, p < .001]. In addition, nogo trials were associated with greater theta-band connectivity between electrode FCz (MFC) and dorsal prefrontal regions (Figure 2B) than go trials [t(299) = 17.06, p < .001].
Figure 2.
The effect of response inhibition (nogo condition minus go condition) on theta-band dynamics. (A) Left: The topography of the nogo minus go difference for theta-band (4-8 Hz) power over the 250-500ms time window, which shows a strong theta power enhancement over FCz/Cz (white electrodes) for nogo trials. Middle: The time-frequency plot of the nogo minus go condition difference for power pooled across FCz and Cz electrodes. Right: Bar chart of FCz/Cz theta power between 250-500ms for each condition, confirming greater theta power during the nogo condition (*** p < 0.001). (B) Left: The topographic map of the nogo-go difference for FCz–seeded (green electrode) connectivity plotted for the theta-band over a 250-500ms time window, displaying a robust increase in connectivity between FCz and dorsal prefrontal channels (dPFC; white electrodes) during demands of response inhibition (nogo). Middle: The time-frequency plot of the nogo minus go difference for FCz–dPFC connectivity (pooled across dPFC electrodes). Right: Bar chart of FCz–dPFC theta-band connectivity between 250-500ms for each condition, confirming that FCz–dPFC connectivity is increased during the nogo condition (*** p < 0.001).
Drinking and theta-band EEG dynamics
MFC theta power
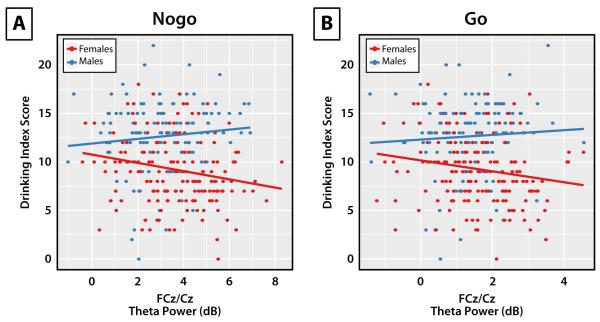
Results from the MFC theta power LMMs fit separately for nogo and go conditions are reported in Table 2. For nogo theta power, a main effect of sex suggested that women exhibited greater power than men. This was qualified by a significant interaction between drinking index scores and sex, which suggested that the negative association between alcohol use and nogo theta power was greater for women. Separate LMMs for women and men examining the association between drinking and nogo MFC theta confirmed this effect [women: t(176) = −2.83, p = 0.005, r = −.21; men: t(112) = 1.33, p = 0.185, r = .13]. No significant effects were found for go-related theta. Figure 3 shows the association between drinking and theta power by condition, which illustrates that, for women, greater alcohol use is associated with reduced nogo MFC theta.
Table 2.
Relationship between midfrontal theta-band power and drinking
|
Nogo
|
Go
|
|||
|---|---|---|---|---|
| Parameter | t-statistic (df) | p-value | t-statistic (df) | p-value |
| Drinking Index | −1.38 (294) | 0.170 | −1.62 (295) | 0.107 |
| Sex (Women/Men) | 2.96 (290) | 0.003 | 1.79 (287) | 0.074 |
| Drinking × Sex Interaction | −2.83 (296) | 0.005 | −1.66 (296) | 0.097 |
Notes: Results of the linear mixed models of the relationship between drink index scores and sex on midfrontal theta-band power (dB) separately for nogo and go conditions. Drinking was negatively associated with nogo midfrontal theta power only in women. P-values were calculated via Kenward-Roger approximation. Significant effects are bolded.
Figure 3.
The association between alcohol use and midfrontal (FCz/Cz) theta-band power as a function of sex. (A) The relationship between midfrontal theta power during the nogo condition and scores on the drinking index, plotted separately for females (red) and males (blue). (B) Same as in panel A, but for midfrontal theta power during the go condition. Lines represent the lines of best fit for each sex. These plots illustrate that alcohol consumption is selectively related to diminished midfrontal theta power during response inhibition (nogo) only for women.
MFC-dPFC theta-band connectivity
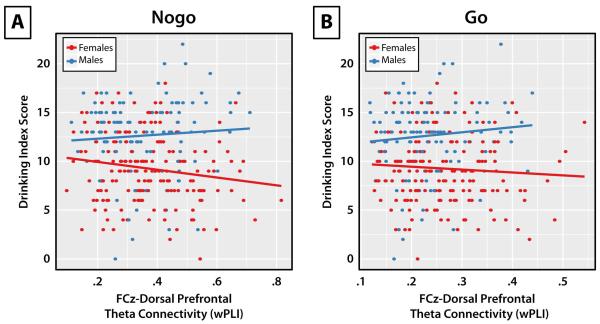
Table 3 details the results of LMMs fit separately for theta-band connectivity between electrode FCz (MFC) and the dPFC region in the nogo and go conditions. For nogo connectivity, a significant interaction between drinking and sex suggested that greater alcohol use was associated with reduced connectivity during nogo trials, and that this effect was stronger for women than men. Separate LMMs for men and women of drinking index scores predicting nogo connectivity confirmed this effect [women: t(180) = −2.37, p = 0.019, r = −0.17; men: t(106) = 0.84, p = 0.40, r = −0.08]. For go connectivity, a main effect of sex suggested that women exhibited greater connectivity than men during go trials. Drinking was not significantly related to go connectivity. Figure 4 displays the association between alcohol use and theta-band connectivity for nogo and go conditions, which illustrates the negative association between drinking and diminished nogo-related connectivity for women.
Table 3.
Relationship between theta-band FCz-dorsal prefrontal connectivity and drinking
|
Nogo
|
Go
|
|||
|---|---|---|---|---|
| Parameter | t-statistic (df) | p-value | t-statistic (df) | p-value |
| Drinking Index | −1.36 (296) | 0.176 | −0.54 (292) | 0.593 |
| Sex (Women/Men) | 1.92 (282) | 0.056 | 2.09 (269) | 0.038 |
| Drinking × Sex Interaction | −2.16 (295) | 0.032 | −1.54 (286) | 0.124 |
Notes: Results of the linear mixed models of the relationship between drink index scores and sex on theta-band FCz-dorsal prefrontal functional connectivity (weighted phase-lag index) separately for nogo and go conditions. Drinking was negatively associated with nogo theta-band FCz-dorsal prefrontal connectivity only in women. P-values were calculated via Kenward-Roger approximation. Significant effects are bolded.
Figure 4.
The association between alcohol use and FCz–dorsal prefrontal cortex (dPFC) theta-band connectivity as a function of sex. (A) The relationship between FCz–dPFC connectivity during the nogo condition and scores on the drinking index, plotted separately for females (red) and males (blue). (B) Same as in panel A, but for FCz–dPFC connectivity during the go condition. Lines represent the lines of best fit for each sex. These scatterplots illustrate that drinking is selectively related to reduced connectivity during demands of response inhibition in the nogo condition only for women.
Cotwin control analysis
The cotwin control analysis was conducted with 85 complete pairs of female twins (52 MZ pairs) to test whether the negative relationship between alcohol (mis)use and nogo-related theta-band MFC power or MFC-dPFC connectivity is better explained by the causal nonshared environmental effects of alcohol exposure on the brain or a preexisting genetic/shared environmental risk towards drinking.
For nogo theta power, the within-pair difference effect was significant [t(84) = −2.04, p = 0.022]. The twin-pair mean estimate was also significant [t(83) = −1.97, p = 0.026]. However, the difference between the twin-pair coefficient and the within-pair coefficient, which is a more appropriate estimate of the between-pair effect, was not [t = −0.32]. The within-pair effect did not differ between MZ and DZ twins; adding zygosity and zygosity by within-pair effect terms did not significantly improve model fit [Δχ2(2) = 1.332, p = 0.514]; thus, these terms were removed from the final model. This pattern suggests that reduced MFC theta power during response inhibition is consistent with a potential causal effect of alcohol exposure.
Results from the CTC model for nogo theta-band connectivity were consistent with a significant causal nonshared effect of alcohol exposure [within-pair effect: t(84) = −1.84, p = 0.035], but not a significant premorbid vulnerability [twin-pair mean effect: t(83) = −1.12, p = 0.133; difference between twin-pair and within-pair coefficients effect: t = 0.33]. Again the within-pair effect did not significantly differ between MZ and DZ twins; the zygosity and zygosity by within-pair effect terms did not improve model fit [Δχ2(2) = 3.42, p = 0.181] and were removed from the final model. These results are consistent with a potential deleterious causal effect of alcohol exposure on theta-band MFC-dPFC connectivity during inhibition.
Discussion
The present study evaluated the association between alcohol consumption and EEG components of response inhibition/cognitive control in a large population-based twin sample whose drinking behaviors were comparable to normative levels observed in the general population (Substance Abuse and Mental Health Services Administration, 2014). Results indicated that heavier alcohol consumption was associated with diminished midfrontal theta power and MFC-dPFC theta-band functional connectivity during demands of response inhibition. This association was moderated by sex, such that the relationship between drinking and theta-band dynamics was greater for women, which suggests that women are at higher risk to experience alcohol-related brain dysfunction. A cotwin control analysis of within-twin-pair differences in alcohol consumption revealed that, for women, reduced inhibition-related theta power and connectivity were consistent with the potential deleterious causal effects of alcohol exposure. The current report provides, to the best of our knowledge, the first evidence of a potential causal link between alcohol exposure and inhibition-related theta-band EEG dynamics, which may in turn underlie alcohol-related disinhibition.
Alcohol (mis)use was negatively associated with diminished theta-band MFC power and connectivity between MFC and dPFC regions during nogo, but not go, trials. This pattern of effects suggests that alcohol use is selectively related to theta-band EEG dynamics associated with response inhibition and conflict monitoring (Clayton et al., 2015; Huster et al., 2013). The consequence of this may be a reduced ability to detect when a change in behavior (e.g., withholding a prepotent response) is necessary, or the inability to ignore irrelevant information and suppress an inappropriate competing response. No association between drinking and behavioral performance was found, which may suggest that brain-based correlates of inhibition are more sensitive to the effects of drinking than overt behavioral measures.
As hypothesized, the effects of drinking on nogo-related theta dynamics were stronger among women than among men, suggesting that women may be more susceptible to experiencing the negative effects of alcohol on the brain. This is consistent with research suggesting that alcohol-related grey and white matter damage (Hommer, 2003; Hommer et al., 2001; Welch et al., 2013) and neurocognitive decline (Nolen-Hoeksema, 2004) progress more rapidly and at lower levels of misuse among women (Mann et al., 2005), and can occur as early as young adulthood (Jacobus and Tapert, 2013; Welch et al., 2013). While the mechanisms of this heightened vulnerability are currently unclear, equal doses of alcohol have been shown to produce higher blood ethanol levels in women than men, which may potentially cause physical/neurocognitive damage at lower levels or durations of misuse (Nolen-Hoeksema, 2004). Future studies should examine the moderating effects of sex on alcohol-related brain deficits to further understand sex differences in the consequences of alcohol consumption.
Analysis of within-pair differences in drinking using the CTC design indicated that, for women, the potential causal effect of drinking (within-pair effect) was significantly related to both nogo-related theta-band MFC power and MFC-dPFC connectivity. This pattern of results suggests that, after accounting for all shared environmental and genetic effects, the heavier drinking twin exhibited significantly less MFC power and MFC-dPFC connectivity during demands of response inhibition than her lesser drinking cotwin. Since the brain of the lesser-using twin provides an approximation to what the brain of her heavier-using cotwin would have looked like if she had not drank heavily, the results of the CTC strongly suggest that that alcohol exposure may have a deleterious causal link to diminished response inhibition-related prefrontal theta-band dynamics.
The current study is not without limitations. Despite the relatively large sample size, the failure to find a zygosity effect may be due to a lack of power. The experimental go/nogo task was complex, combining response inhibition, 1-back working memory, and oddball effects, so it is possible that the elicited MFC theta dynamics are not pure correlates of inhibition (Huster et al., 2013). However, a fMRI meta-analysis reported substantial overlapping cortical activation (including MFC) during simple (static stimulus mapping) and complex (context-dependent stimulus mapping) go/nogo tasks (Simmonds et al., 2008). We used a complex task to elicit dPFC connectivity because in past research, dPFC activation was only observed in complex conditions (Simmonds et al., 2008). While the CTC design provides a strong methodology for identifying cause and effect, it cannot account for factors not shared by twins that are potentially confounded with drinking, such as other substance exposure or psychiatric history of disinhibitory disorders (Iacono et al., 2008), which may influence brain functioning. However, the likelihood that such factors were in play is diminished by the fact that they would have had quite selective effects, influencing only the inhibition-related theta dynamics in women. While the current results support a causal effect of alcohol exposure, they do not necessarily identify specific causal mechanisms. Alcohol exposure may have neurotoxic effects on frontal cortex regions (Jacobus and Tapert, 2013), or impact the developmental trajectory of cognitive control processes (Malone et al., 2014), or increase the likelihood of exposure to another nonshared effect (e.g., illicit substances, head trauma). Future studies using magnetic resonance imaging can help disentangle potential neurodegenerative effects of alcohol exposure from purely neurocognitive changes.
Although preliminary, the finding that normative alcohol use may have sex-specific causal effects on midfrontal theta brain dynamics during response inhibition has potentially significant public health implications. If drinking during early adulthood truly has a deleterious causal effect on brain processes of response inhibition that is specific for women, interventions should focus on both the toxic effects of alcohol on the still-developing young adult brain and the heightened susceptibility among women for developing alcohol-induced brain dysfunction.
Supplementary Material
Acknowledgements
This work was supported by the National Institutes of Health grant R01 DA036216. J.H. was supported by the National Science Foundation Graduate Research Fellowship under Grant No. 00039202.
Footnotes
Author Contributions
J.H., S.M.M., and W.G.I conceptualized the project. W.G.I and S.M.M designed the study and protocol, which is part of the Enrichment Study of the Minnesota Twin Family Study (Keyes et al., 2009). J.H. processed and analyzed the EEG data and conducted statistical analyses. All authors contributed to the design of statistical analyses and interpretation of results. J.H. managed the literature search, drafted the manuscript, and prepared the figures. All authors edited, revised, and approved the final manuscript.
References
- Bates D, Maechler M, Bolker B, Walker S, Christensen RHB, Singmann H, Dai B, Eigen C, Rcpp L. Package ‘lme4’. 2015.
- Begg MD, Parides MK. Separation of individual-level and cluster-level covariate effects in regression analysis of correlated data. Statistics in Medicine. 2003;22:2591–2602. doi: 10.1002/sim.1524. [DOI] [PubMed] [Google Scholar]
- Bennett CM, Baird AA. Anatomical changes in the emerging adult brain: a voxel-based morphometry study. Human brain mapping. 2006;27:766–777. doi: 10.1002/hbm.20218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boker S, Neale M, Maes H, Wilde M, Spiegel M, Brick T, Spies J, Estabrook R, Kenny S, Bates T, Mehta P, Fox J. OpenMx: An Open Source Extended Structural Equation Modeling Framework. Psychometrika. 2011;76:306–317. doi: 10.1007/s11336-010-9200-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryk AS, Raudenbush SW. Hierarchical linear models: Applications and data analysis methods. 1st ed Sage; Newbury Park, CA: 1992. [Google Scholar]
- Cavanagh JF, Frank MJ. Frontal theta as a mechanism for cognitive control. Trends in Cognitive Sciences. 2014;18:414–421. doi: 10.1016/j.tics.2014.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton MS, Yeung N, Kadosh RC. The roles of cortical oscillations in sustained attention. Trends in Cognitive Sciences. 2015;19:188–195. doi: 10.1016/j.tics.2015.02.004. [DOI] [PubMed] [Google Scholar]
- Cohen MX. Analyzing Neural Time Series Data: Theory and Practice. Analyzing Neural Time Series Data: Theory and Practice. 2014:1–578. [Google Scholar]
- Cohen MX. Comparison of different spatial transformations applied to EEG data: A case study of error processing. International Journal of Psychophysiology. 2015;97:245–257. doi: 10.1016/j.ijpsycho.2014.09.013. [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Dick DM, Smith G, Olausson P, Mitchell SH, Leeman RF, O'Malley SS, Sher K. Understanding the construct of impulsivity and its relationship to alcohol use disorders. Addict Biol. 2010;15:217–226. doi: 10.1111/j.1369-1600.2009.00190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries P. A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. Trends in Cognitive Sciences. 2005;9:474–480. doi: 10.1016/j.tics.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Harper J, Malone SM, Bernat EM. Theta and delta band activity explain N2 and P3 ERP component activity in a go/no-go task. Clin Neurophysiol. 2014;125:124–132. doi: 10.1016/j.clinph.2013.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hommer DW. Male and female sensitivity to alcohol-induced brain damage. Alcohol Research & Health. 2003;27:181–185. [PMC free article] [PubMed] [Google Scholar]
- Hommer DW, Momenan R, Kaiser E, Rawlings RR. Evidence for a gender-related effect of alcoholism on brain volumes. American Journal of Psychiatry. 2001;158:198–204. doi: 10.1176/appi.ajp.158.2.198. [DOI] [PubMed] [Google Scholar]
- Huster RJ, Enriquez-Geppert S, Lavallee CF, Falkenstein M, Herrmann CS. Electroencephalography of response inhibition tasks: Functional networks and cognitive contributions. International Journal of Psychophysiology. 2013;87:217–233. doi: 10.1016/j.ijpsycho.2012.08.001. [DOI] [PubMed] [Google Scholar]
- Iacono WG, Malone SM, McGue M. Behavioral disinhibition and the development of early-onset addiction: common and specific influences. Annu Rev Clin Psychol. 2008;4:325–348. doi: 10.1146/annurev.clinpsy.4.022007.141157. [DOI] [PubMed] [Google Scholar]
- Jacobus J, Tapert SF. Neurotoxic Effects of Alcohol in Adolescence. Annual Review of Clinical Psychology. 2013;9:703–721. doi: 10.1146/annurev-clinpsy-050212-185610. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamarajan C, Porjesz B, Jones K, Chorlian D, Padmanabhapillai A, Rangaswamy M, Stimus A, Begleiter H. Event-related oscillations in offspring of alcoholics: neurocognitive disinhibition as a risk for alcoholism. Biol Psychiatry. 2006;59:625–634. doi: 10.1016/j.biopsych.2005.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamarajan C, Porjesz B, Jones KA, Choi K, Chorlian DB, Padmanabhapillai A, Rangaswamy M, Stimus AT, Begleiter H. The role of brain oscillations as functional correlates of cognitive systems: a study of frontal inhibitory control in alcoholism. International Journal of Psychophysiology. 2004;51:155–180. doi: 10.1016/j.ijpsycho.2003.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayser J, Tenke CE. Principal components analysis of Laplacian waveforms as a generic method for identifying ERP generator patterns: I. Evaluation with auditory oddball tasks. Clin Neurophysiol. 2006;117:348–368. doi: 10.1016/j.clinph.2005.08.034. [DOI] [PubMed] [Google Scholar]
- Keyes MA, Malone SM, Elkins IJ, Legrand LN, McGue M, Iacono WG. The Enrichment Study of the Minnesota Twin Family Study: Increasing the Yield of Twin Families at High Risk for Externalizing Psychopathology. Twin Research and Human Genetics. 2009;12:489–501. doi: 10.1375/twin.12.5.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznetsova A, Brockhoff P, Christensen R. lmerTest: tests in linear mixed effects models. 2014. R package version 2.0-20.
- Lavallee CF, Meemken MT, Herrmann CS, Huster RJ. When holding your horses meets the deer in the headlights: time-frequency characteristics of global and selective stopping under conditions of proactive and reactive control. Frontiers in human neuroscience. 2014;8:994–994. doi: 10.3389/fnhum.2014.00994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, Gee M, Camicioli R, Wieler M, Martin W, Beaulieu C. Diffusion tensor imaging of white matter tract evolution over the lifespan. NeuroImage. 2012;60:340–352. doi: 10.1016/j.neuroimage.2011.11.094. [DOI] [PubMed] [Google Scholar]
- Malone SM, Luciana M, Wilson S, Sparks JC, Hunt RH, Thomas KM, Iacono WG. Adolescent Drinking and Motivated Decision-Making: A Cotwin-Control Investigation with Monozygotic Twins. Behavior Genetics. 2014;44:407–418. doi: 10.1007/s10519-014-9651-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann K, Ackermann K, Croissant B, Mundle G, Nakovics H, Diehl A. Neuroimaging of gender differences in alcohol dependence: Are women more vulnerable? Alcoholism-Clinical and Experimental Research. 2005;29:896–901. doi: 10.1097/01.alc.0000164376.69978.6b. [DOI] [PubMed] [Google Scholar]
- McGue M, Malone S, Keyes M, Iacono WG. Parent-Offspring Similarity for Drinking: A Longitudinal Adoption Study. Behavior Genetics. 2014;44:620–628. doi: 10.1007/s10519-014-9672-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGue M, Osler M, Christensen K. Causal Inference and Observational Research: The Utility of Twins. Perspectives on Psychological Science. 2010;5:546–556. doi: 10.1177/1745691610383511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mognon A, Jovicich J, Bruzzone L, Buiatti M. ADJUST: An automatic EEG artifact detector based on the joint use of spatial and temporal features. Psychophysiology. 2011;48:229–240. doi: 10.1111/j.1469-8986.2010.01061.x. [DOI] [PubMed] [Google Scholar]
- Nigbur R, Cohen MX, Ridderinkhof KR, Stuermer B. Theta Dynamics Reveal Domain-specific Control over Stimulus and Response Conflict. Journal of Cognitive Neuroscience. 2012;24:1264–1274. doi: 10.1162/jocn_a_00128. [DOI] [PubMed] [Google Scholar]
- Nigbur R, Ivanova G, Stuermer B. Theta power as a marker for cognitive interference. Clinical Neurophysiology. 2011;122:2185–2194. doi: 10.1016/j.clinph.2011.03.030. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S. Gender differences in risk factors and consequences for alcohol use and problems. Clinical Psychology Review. 2004;24:981–1010. doi: 10.1016/j.cpr.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Oehlert GW. A note on the delta method. American Statistician. 1992;46:27–29. [Google Scholar]
- Perrin F, Pernier J, Bertrand O, Echallier JF. Spherical splines for scalp potential and current density mapping. Electroencephalogr Clin Neurophysiol. 1989;72:184–187. doi: 10.1016/0013-4694(89)90180-6. [DOI] [PubMed] [Google Scholar]
- R Core Team . R: A language and environment for statistical computing. Vienna, Austria: 2015. 2014. URL http://www R-project org. [Google Scholar]
- Robins L, Babor T, Cottler L. Composite international diagnostic interview: expanded substance abuse module. Authors; St Louis: 1987. [Google Scholar]
- Rousseeuw PJ, Croux C. Alternatives to the median absolute deviation. Journal of the American Statistical Association. 1993;88:1273–1283. [Google Scholar]
- Simmonds DJ, Pekar JJ, Mostofsky SH. Meta-analysis of Go/No-go tasks demonstrating that fMRI activation associated with response inhibition is task-dependent. Neuropsychologia. 2008;46:224–232. doi: 10.1016/j.neuropsychologia.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JL, Mattick RP, Jamadar SD, Iredale JM. Deficits in behavioural inhibition in substance abuse and addiction: A meta-analysis. Drug and Alcohol Dependence. 2014;145:1–33. doi: 10.1016/j.drugalcdep.2014.08.009. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration . Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings (NSDUH Series H-48, HHS Publication No.(SMA) 14-4863) Substance Abuse and Mental Health Services Administration; Rockville, MD: 2014. [Google Scholar]
- Swick D, Ashley V, Turken U. Are the neural correlates of stopping and not going identical? Quantitative meta-analysis of two response inhibition tasks. Neuroimage. 2011;56:1655–1665. doi: 10.1016/j.neuroimage.2011.02.070. [DOI] [PubMed] [Google Scholar]
- Tenke CE, Kayser J. A convenient method for detecting electrolyte bridges in multichannel electroencephalogram and event-related potential recordings. Clin Neurophysiol. 2001;112:545–550. doi: 10.1016/s1388-2457(00)00553-8. [DOI] [PubMed] [Google Scholar]
- Vaidyanathan U, Vrieze SI, Iacono WG. The Power of Theory, Research Design, and Transdisciplinary Integration in Moving Psychopathology Forward. Psychological Inquiry. 2015;26:209–230. doi: 10.1080/1047840X.2015.1015367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinck M, Oostenveld R, van Wingerden M, Battaglia F, Pennartz CMA. An improved index of phase-synchronization for electrophysiological data in the presence of volume-conduction, noise and sample-size bias. Neuroimage. 2011;55:1548–1565. doi: 10.1016/j.neuroimage.2011.01.055. [DOI] [PubMed] [Google Scholar]
- Vrieze SI, Hicks BM, Iacono WG, McGue M. Decline in Genetic Influence on the Co-Occurrence of Alcohol, Marijuana, and Nicotine Dependence Symptoms From Age 14 to 29. American Journal of Psychiatry. 2012;169:1073–1081. doi: 10.1176/appi.ajp.2012.11081268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch KA, Carson A, Lawrie SM. Brain Structure in Adolescents and Young Adults with Alcohol Problems: Systematic Review of Imaging Studies. Alcohol and Alcoholism. 2013;48:433–444. doi: 10.1093/alcalc/agt037. [DOI] [PubMed] [Google Scholar]
- Wilson S, Malone SM, Thomas KM, Iacono WG. Adolescent drinking and brain morphometry: A co-twin control analysis. Developmental cognitive neuroscience. 2015;16:130–138. doi: 10.1016/j.dcn.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter WR, Nunez PL, Ding J, Srinivasan R. Comparison of the effect of volume conduction on EEG coherence with the effect of field spread on MEG coherence. Statistics in Medicine. 2007;26:3946–3957. doi: 10.1002/sim.2978. [DOI] [PubMed] [Google Scholar]
- Yamanaka K, Yamamoto Y. Single-trial EEG Power and Phase Dynamics Associated with Voluntary Response Inhibition. Journal of Cognitive Neuroscience. 2010;22:714–727. doi: 10.1162/jocn.2009.21258. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.