Abstract
Purpose
Fewer than 1 in 5 patients with cirrhosis receive hepatocellular carcinoma (HCC) surveillance; however, most studies were performed in select patient populations, which may not be informative of practice patterns in population-based community practices. Further, few reported guideline-concordant consistent surveillance rates.
Goals
Characterize guideline-concordant HCC surveillance rates and patient-level factors associated with surveillance among a population-based cohort of patients with cirrhosis.
Study
We retrospectively characterized HCC surveillance among cirrhosis patients followed between January 2010 and December 2012 at an integrated healthcare delivery system in Washington state. Consistent surveillance was defined as an ultrasound every 6 months, and inconsistent surveillance was defined as ≥1 ultrasound during the two-year follow-up period. Univariate and multivariate analyses were conducted to identify correlates of HCC surveillance receipt.
Results
Of 1137 patients with cirrhosis, 22 (2%) underwent consistent surveillance, 371 (33%) had inconsistent surveillance, and 744 (65%) received no surveillance during follow-up. Correlates of HCC surveillance receipt in multivariate analysis included Gastroenterology/Hepatology subspecialty care (OR 1.88, 95%CI 1.44 – 2.46), Child Pugh B/C cirrhosis (OR 1.61, 95%CI 1.07 – 2.43), elevated AST (OR 1.63, 95%CI 1.13 – 2.35), and etiology of liver disease. Compared to hepatitis C-infected patients, patients with hepatitis B infection were more likely to undergo surveillance ((OR 2.72, 95%CI 1.28 – 5.81), while patients with alcohol-related cirrhosis (OR 0.63, 95%CI 0.42 – 0.93) and nonalcoholic steatohepatitis (OR 0.39, 95%CI 0.28 – 0.56) were less likely to undergo surveillance.
Conclusions
Although one-third of patients undergo inconsistent HCC surveillance, less than 2% of patients receive guideline-concordant biannual HCC surveillance.
Keywords: Liver cancer, screening, cirrhosis, early detection, ultrasound
INTRODUCTION
Hepatocellular carcinoma (HCC) is the third leading cause of cancer-related death worldwide and one of the leading causes of death in patients with cirrhosis(1). HCC incidence in the United States (US) has more than doubled over the past two decades and is anticipated to continue increasing over the next 20 years. By 2030, HCC is projected to surpass breast and colorectal cancer to become the third leading cause of cancer-related death in the United States(2).
HCC surveillance has the potential to improve early tumor detection and overall survival in patients with cirrhosis, who are at high risk for developing HCC(3). Patients with early stage HCC achieve 5-year survival rates near 70% with curative treatment including resection or transplantation, which is in stark contrast to a median survival of one year for those with advanced HCC who are only eligible for palliative treatments(4). Several cohort studies have demonstrated patients undergoing surveillance have earlier stages of disease as well as improved survival than those who had not undergone surveillance(5). However, two systematic reviews evaluating HCC surveillance in patients with cirrhosis highlighted the lack of Level I data and concluded available data from cohort studies have notable limitations, including selection bias, lead time bias, and length time bias. Based on current evidence, several societies including the National Comprehensive Cancer Network (NCCN), Veterans Affairs (VA), and American Association for Study of Liver Diseases (AASLD) recommend HCC surveillance with an ultrasound every 6 months in patients with cirrhosis(6, 7).
Whereas colorectal and breast cancer screening rates exceed 50%(8), fewer than 20% of patients with cirrhosis undergo HCC surveillance(9). Patients receiving subspecialty care have higher surveillance rates, but only 20-40% of patients with cirrhosis are followed by gastroenterologists nationally and the majority of patients with cirrhosis continue to receive their liver-related care, including HCC surveillance, through primary care providers (PCP)(10-12). Most studies only assessed inconsistent surveillance rates, such as receipt of one ultrasound or alpha-fetoprotein in a two-year period, and few distinguished different patterns of surveillance including consistent surveillance every 6 months as recommended by guidelines(9).
Further, most studies examining HCC surveillance uptake have been conducted in academic or safety-net hospital settings, which may not reflect HCC surveillance delivery in other community practices(13, 14). The multi-center studies examining this issue were similarly conducted in selected populations, using the SEER-Medicare(15) and VA databases(16), and may be less representative than data from large integrated health care systems, such as those within the National Cancer Institute (NCI)-funded Cancer Research Network (CRN). Differences in organizational factors have led to variations in colon, breast, and cervical cancer surveillance rates, highlighting the importance of characterizing HCC surveillance practices in these diverse practice settings(17). For example, cancer surveillance rates are negatively correlated with higher clinic patient volumes but are higher in organizations with increased surveillance facility availability(18). Similarly, organizational processes, such as provider reminder and/or audit-feedback systems, can impact provider recommendation and cancer screening process completion rates(19). Finally, organizations can differ in the perceived importance of subspecialty society recommendations that are not otherwise endorsed by US Preventive Services Task Force (USPSTF) other major guideline committee.
Therefore, the aims of our study were to 1) characterize patterns of HCC surveillance testing and 2) patient-level factors associated with surveillance receipt among a population-based cohort of patients with cirrhosis followed in a large integrated healthcare delivery system.
MATERIALS AND METHODS
Study Population
We conducted a retrospective cohort study of patients with cirrhosis who were enrolled at Group Health, an integrated healthcare delivery system in Washington state. Group Health is involved in several multi-site efforts to charactertize the cancer screening processes, including through the CRN, Population-Based Reseach Optimizing Screening through Personalized Regimens (PROSPR), and the Breast Cancer Surveillance Consortium(19, 20). Patients were initially identified by a set of ICD-9 codes, which are highly sensitive and specific for cirrhosis (456.0, 456.1, 456.2, 456.21, 567.23, 571.2, 571.5, 572.2, 572.3, and 572.4). Cirrhosis was defined by the presence of any of the above cirrhosis-related ICD-9 codes, as this algorithm was found to be sufficiently accurate and has been used in other studies evaluating cirrhosis-related care(14, 21-22). Patients were required to have at least one outpatient primary care provider (PCP) clinic visit between January 1, 2010 and December 31, 2010 and at least one year of prior continuous enrollment to obtain baseline data. The first visit during the January 2010 – December 2010 time frame was defined as a patient's index visit. Patients were excluded if they were not Group Health members, had a history of HCC or liver transplantation prior to his/her index visit, or were followed for less than 6 months after index date. This study was approved by the Institutional Review Boards of UT Southwestern Medical Center and Group Health.
Data Collection
Patient demographics, clinical history, laboratory data, and imaging results were electronically extracted from Group Health computerized clinical and administrative databases.
Hepatocellular Carcinoma Surveillance Outcomes
Dates of all abdominal ultrasounds were extracted for the two years following the index visit. We characterized patients based on receipt of HCC surveillance (i.e. abdominal ultrasound), our primary outcome of interest, during the follow-up period for each patient. Patient follow-up was censored at the first of HCC diagnosis, liver transplantation, death, disenrollment from the health plan, or two-year date after the patient's index visit. Receipt of surveillance during follow-up was categorized as consistent, inconsistent, or no surveillance. Consistent surveillance was defined as the receipt of abdominal ultrasound every 6 months, inconsistent surveillance defined as the receipt of at least one abdominal ultrasound over the study period but less than consistent surveillance, and no surveillance defined as not undergoing any abdominal ultrasounds during the time frame. Only abdominal ultrasound imaging was considered surveillance imaging given CT and MRI are not routinely used for HCC surveillance purposes at Group Health. Ultrasounds performed in the emergency room and inpatient exams were not included in our primary outcome given the low likelihood of surveillance intent; however, they were included for our secondary outcome capturing ultrasound receipt for any indication.
Covariates
Clinical variables of interest included liver disease etiology, degree of liver dysfunction, comorbidity status, and alcohol and/or drug abuse in the year preceding index visit. We classified patients according to etiology of liver disease, including hepatitis C virus (HCV), hepatitis B virus (HBV), alcohol-related liver disease, nonalcoholic steatohepatitis (NASH), and other based on laboratory data and ICD-9 codes (Supplemental Table). NASH cirrhosis was defined in patients who had evidence of the metabolic syndrome in the absence of HCV infection, HBV infection, or alcohol-related cirrhosis. Child Pugh score, a marker of liver dysfunction, was calculated for each patient based on presence of ascites, encephalopathy, and laboratory data (bilirubin, albumin, and INR) in the year preceding index visit(23). For patients with multiple available lab values, those closest to index visit were selected. The presence of hepatic decompensation, including ascites and encephalopathy, was determined using a combination of ICD-9 codes and pharmacy codes for spironolactone, furosemide, lactulose and/or rifaximin. Comorbidity status in the twelve months prior to the index visit was assessed using the Charlson comorbidity index, as has been done in prior studies using data from Group Health(19, 24). The presence of alcohol and drug abuse was determined using ICD-9 codes.
We captured length of enrollment at Group Health prior to the index visit, allowing up to a 3-month gap in coverage. We recorded primary care visits and receipt of subspecialty gastroenterology care in the year preceding index visit. For provider-level variables, we captured professional degree (MD, DO, PA) and age for the patient's PCP at time of index visit. Finally, zip-code level variables of interest included census-percent of persons with high school education, socioeconomic quintile, percent of households on public assistance, and travel time to nearest clinic at the time of the index visit.
Statistical Analysis
In univariate analysis, Fisher exact and Mann Whitney rank-sum tests were performed to identify baseline patient-factors associated with receipt of HCC surveillance. The multivariate logistic regression model included variables of a priori clinical importance (i.e., baseline Child Pugh class and receipt of Gastroenterology/Hepatology care) and any factors significant on univariate analysis. Receipt of HCC surveillance was categorized as any (consistent or inconsistent) vs. no surveillance during the follow-up period given the small number of patients with consistent surveillance. Predictor variables with p<0.10 in univariate analysis were included in multivariate models to minimize type II error. Statistical significance was defined as p< 0.05 for multivariate analyses. All data analysis was performed using Stata 11 (StataCorp, College Station, TX) and SAS 9.4 (SAS Institute, NC).
RESULTS
Patient Characteristics
We initially identified 1397 patients who met inclusion criteria. Patients were excluded if they had a history of liver transplantation (n=130), history of HCC (n=48), refused to participate in retrospective analyses (n=12), or were followed for less than 6 months after index visit (n=70). After applying these exclusion criteria, 1137 patients with cirrhosis remained for analyses. Patient characteristics are detailed in Table 1. The median age of patients was 60 (interquartile range (IQR) 54–70) years, and 583 (51.3%) were men. Our population consisted of 76.1% non-Hispanic Caucasians, 7.8% Asians, 5.3% African Americans, and 4.8% Hispanic Caucasians. The most common etiologies of cirrhosis were HCV infection (28.9%), NASH (28.7%), and alcohol-induced liver disease (16.2%). Nearly one-fourth of patients (n=262) had cirrhosis without documented evidence of HCV, HBV, alcohol abuse or NASH. The median Child-Pugh score at diagnosis was 5 (IQR 5-6), with 89.1% of patients having Child-Pugh A cirrhosis. Patients had a median of 15.3 years (IQR 8.03–22.0 years) Group Health enrollment before their index visit. Patients had a median of 7 (IQR 4-11) PCP visits, and 422 (37.1%) had at least one Gastroenterology/Hepatology visit in the year preceding their index visit.
Table 1.
Baseline Patient Characteristics Stratified by Receipt of HCC Surveillance
| Variable | All Patients (n=1137) | Surveillance (n=393) | No Surveillance (n=744) | p-value |
|---|---|---|---|---|
| Age | 60 [54, 70] | 59 [54, 65] | 61 [54, 72] | 0.004 |
| Gender (% male) | 583 (51.3) | 204 (51.9) | 379 (50.9) | 0.80 |
| Race/ethnicity* | 0.25 | |||
| White | 865 (76.1) | 299 (76.1) | 566 (76.1) | |
| Black | 60 (5.3) | 23 (5.9) | 37 (5.0) | |
| Hispanic | 56 (4.8) | 17 (4.3) | 39 (5.2) | |
| Asian | 89 (7.8) | 38 (9.7) | 51 (6.9) | |
| Etiology of Liver Disease* | < 0.001 | |||
| Hepatitis C | 329 (28.9) | 157 (39.9) | 172 (23.1) | |
| Hepatitis B | 36 (3.2%) | 25 (6.4) | 11 (14.8) | |
| Alcohol-related | 184 (16.2) | 66 (16.8) | 118 (15.9) | |
| NASH | 326 (28.7) | 79 (20.1) | 247 (33.2) | |
| Other | 262 (23.0) | 66 (16.8) | 196 (26.3) | |
| Child Pugh Class (% Child A) | 1013 (89.1) | 332 (84.5) | 681 (91.5) | <0.001 |
| Presence of ascites | 873 (76.8) | 282 (71.8) | 591 (79.4) | 0.004 |
| Presence of hepatic encephalopathy | 1061 (93.3) | 359 (91.3) | 702 (94.4) | 0.06 |
| Platelet count (*103/μL) | 175 [113,240] | 141 [95, 202] | 203 [130, 257] | <0.001 |
| AST (U/L) | 35 [25, 57] | 40 [28, 68] | 31 [23, 50] | <0.001 |
| Albumin (g/dL) | 4.2 [3.8, 4.4] | 4.2 [3.8, 4.4] | 4.2 [3.9, 4.4] | 0.35 |
| Bilirubin (mg/dL) | 0.6 [0.4, 1.0] | 0.6 [0.4, 1.1] | 0.5 [0.4, 0.8] | <0.001 |
| INR | 1.1 [1.0, 1.3] | 1.1 [1.0, 1.3] | 1.1 [1.0, 1.4] | 0.48 |
| Charlson comorbidity index | 1 [0, 3] | 1 [0, 3] | 1 [0, 3] | 0.18 |
| Number primary care visits in year prior to index visit | 7 [4, 11] | 7 [3, 12] | 7 [4, 11] | 0.99 |
| Gastroenterology/Hepatology visits in year prior to index visit | 0 [0, 1] | 0 [0, 2] | 0 [0, 1] | <0.001 |
| Gastroenterology/Hepatology care in year prior to index visit | 422 (37.1) | 190 (48.4) | 232 (31.2) | < 0.001 |
| Neighborhood-level Income quartile | 3 [2, 3] | 3 [1, 3] | 3 [2, 4] | 0.80 |
| Neighborhood-level Education quartile | 2 [1, 4] | 2 [1, 3] | 3 [2, 4] | 0.14 |
| Distance to nearest clinic (miles) | 4 [2, 7] | 4 [2, 7] | 4 [2, 7] | 0.90 |
Note: All values are reported as median (interquartile range) or n (%) unless otherwise specified.
Data regarding race/ethnicity were missing in 60 patients
AST – aspartate aminotransferase; HCC – hepatocellular carcinoma; INR – international normalized ratio; NASH – nonalcoholic steatohepatitis
Receipt of Surveillance
Surveillance was performed at least once during follow-up in 393 (34.6%) patients. An additional 53 patients received an ultrasound in the emergency room or as an inpatient, presumably for non-surveillance purposes, and were classified as having no surveillance. Of the 1053 with at least 12 months of follow-up, only 22 had consistent surveillance (Figure 1). Consistent surveillance was performed in 1.7% of the 58 patients followed for 12 – <18 months and 2.1% of the 995 patients followed for 18-24 months.
Figure 1.
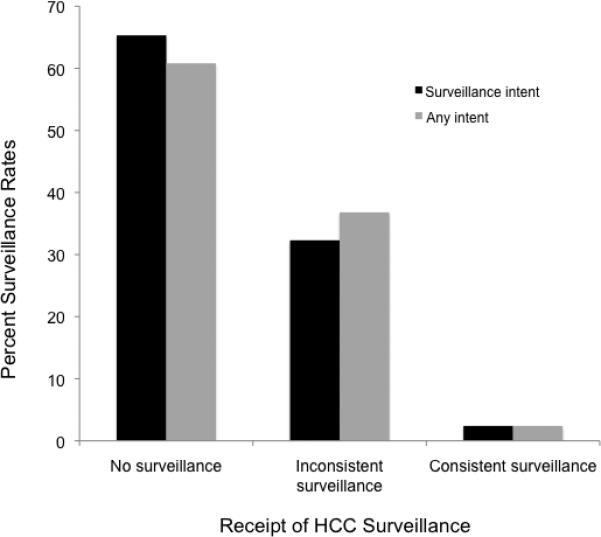
Hepatocellular Carcinoma Surveillance Rates
Of included patients with at least 12 months of follow-up, 2.4% received consistent surveillance, 32.3% inconsistent surveillance, and 65.3% received no surveillance. Data were similar when including ultrasounds performed for any intent.
Inconsistent surveillance occurred in 27.4% of 84 patients followed for 6 – <12 months, 27.6% of 58 patients followed for 12 – <18 months, and 33.6% of 995 patients followed for 18-24 months (data now shown). Receipt of inconsistent surveillance was not significantly different according to length of follow-up (p=0.48). Of those followed for 18 – <24 months with inconsistent surveillance, <5 patients had two surveillance ultrasound exams and 17 had only one surveillance ultrasound; of those followed for 24 months, 35 patients had 3 surveillance exams, 86 had 2 surveillance exams, and 185 had only one surveillance ultrasound. Alpha fetoprotein (AFP) had been performed at least once during follow-up in 240 (61.1%) patients with inconsistent surveillance and 77 (10.4%) of those without surveillance.
In univariate analysis, receipt of any surveillance was associated with age, liver disease etiology, Child Pugh class, Gastroenterology/Hepatology subspecialty care in year prior to index date, thrombocytopenia, and elevated AST. The presence of ascites, hepatic encephalopathy, and bilirubin were also significant on univariate analysis but not included in multivariate analysis given they are components of Child Pugh class. We found no association between receipt of surveillance and gender, race/ethnicity, Charlson comorbidity index, neighborhood-level income or education, or number of primary care visits in the year preceding index visit. In multivariate analysis, surveillance was associated with Gastroenterology/Hepatology subspecialty care (OR 1.88, 95%CI 1.44 – 2.46), baseline AST >40 U/L (OR 1.63, 95%CI 1.13 – 2.35), Child Pugh B/C cirrhosis (OR 1.61, 95%CI 1.07 – 2.43) (Table 2), and liver disease etiology. Compared to hepatitis C-infected patients, patients with hepatitis B infection were more likely to undergo surveillance ((OR 2.72, 95%CI 1.28 – 5.81), while patients with alcohol-related cirrhosis (OR 0.63, 95%CI 0.42 – 0.93) and nonalcoholic steatohepatitis (OR 0.39, 95%CI 0.28 – 0.56) were less likely to undergo surveillance (Figure 2). These risk factors demonstrated acceptable discrimination between the presence and absence of inconsistent surveillance (c-statistic of 0.69).
Table 2.
Correlates of Hepatocellular Carcinoma Surveillance (N=1137)
| Variable | Multivariate Analysis * |
Percent receiving HCC surveillance during follow-up | |
|---|---|---|---|
| OR | 95% CI | ||
| Etiology of Liver Disease | |||
| Hepatitis C virus | Reference | Reference | 47.7% |
| Hepatitis B virus | 2.72 | 1.28 – 5.81 | 69.4% |
| Alcohol-related | 0.63 | 0.42 – 0.93 | 35.9% |
| NASH | 0.39 | 0.28 – 0.56 | 24.2% |
| Other | 0.45 | 0.31 – 0.64 | 25.2% |
| Child Pugh B or C cirrhosis at baseline | 1.61 | 1.07 – 2.43 | 49.2% vs. 32.8% |
| Receipt of Hepatology care during year prior to index visit | 1.88 | 1.44 – 2.46 | 45.0% vs. 28.4% |
| Baseline AST > 40 U/L | 1.63 | 1.13 – 2.35 | 51.2% vs. 31.8% |
Adjusted for age and thrombocytopenia, which were significant on univariate analysis
AST – aspartate aminotransferase; HCC – hepatocellular carcinoma; NASH – nonalcoholic steatohepatitis
Figure 2.
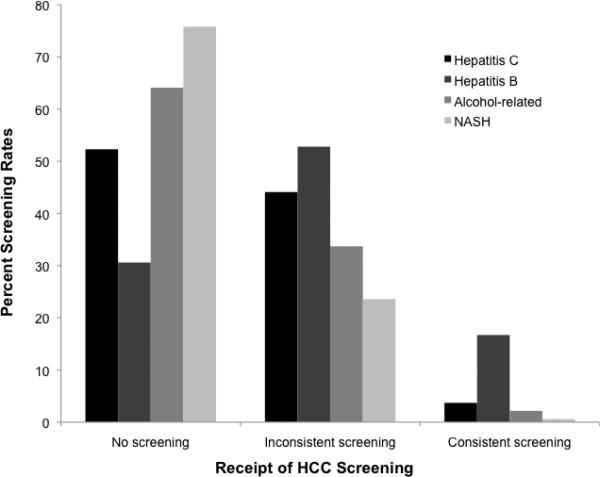
Hepatocellular Carcinoma Surveillance Rates Stratified by Cirrhosis Etiology
DISCUSSION
Although a meta-analysis found fewer than 20% of patients in the United States undergo HCC surveillance, most studies were conducted in selected populations, e.g. Medicare or VA patients, which may not be representative of HCC surveillance delivery in community practices(9). Furthermore, few studies reported guideline-concordant surveillance rates. To the best of our knowledge, our study is one of the first to report surveillance rates among a large cohort of patients followed in a non-academic population-based integrated health system outside of the VA. Although one-third of patients underwent HCC surveillance at least once during the 2-year study period, fewer than 2% underwent consistent guideline-concordant HCC surveillance. These data are consistent with another recent report from a commercial health insurance database, in which the mean and median proportion of time up-to-date with surveillance was only 0.34 and 0.31, respectively (11).
Although surveillance among HBV patients is supported by a large randomized controlled trial, there is not Level I evidence supporting this practice among patients with cirrhosis(25). Furthermore, two systematic reviews evaluating HCC surveillance in patients with cirrhosis concluded available data have notable limitations, precluding firm conclusions about the risk-benefit ratio and/or adoption by several guideline societies. Few health care organizations, including Group Health, have policies or programs to encourage HCC surveillance (e.g., system-level invitations or audit/feedback to providers regarding HCC surveillance rates); so, decisions and recommendations for HCC surveillance are highly dependent on individual provider attitudes and beliefs(26, 27). This likely explains why most patients in this study who underwent surveillance received inconsistent surveillance once or twice over the two-year study period.
It is not surprising that one of the strongest correlates for receipt of HCC surveillance was Gastroenterology/Hepatology subspecialty care. This association is likely mediated by increased awareness of American Association for the Study of Liver Diseases (AASLD) guidelines and beliefs that HCC surveillance improves mortality. However, primary care providers, not hepatologists, follow most patients with cirrhosis nationally, particularly in areas with limited subspecialty availability (e.g. rural areas and urban safety-net hospitals). Although most primary care providers are aware of subspecialty society (e.g. AASLD and NCCN) recommendations and believe HCC surveillance may improve HCC early detection, only half believe surveillance results in a survival benefit and only one-third believe failure to perform surveillance poses any legal concern (28). Further, many are dissuaded from performing surveillance given the lack of US Preventive Services Task Force (USPSTF) recommendation(28). A meta-analysis of HCC surveillance studies identified several deficiencies in current literature, including the need for high-quality studies assessing effects of surveillance on health outcomes, harms of surveillance, and patient acceptance(29).
Receipt of HCC surveillance was also strongly associated with etiology of liver disease, with significantly lower rates among patients with alcohol- and NASH-related cirrhosis. Prior studies have shown alcohol- and NASH-related HCC are less likely to be detected via surveillance given under-recognition of the at-risk population (25); however, all patients in our study were recognized as having cirrhosis. This association could be related to multiple factors including clinic time constraints if these subgroups had more active issues, provider beliefs regarding less benefits of surveillance in these subgroups, or provider beliefs regarding lower likelihood of adherence. Alternatively, these patients may have lower levels of engagement with clinical care or personal reasons for deciding not to receive HCC surveillance testing (26).
Our study has several limitations. Our analysis focused on patients with cirrhosis at a single integrated healthcare delivery system and may not be generalized to other practice settings. As an evidence-based healthcare organization, Group Health requires high levels of evidence before issuing guidelines for clinical health services and adoption of HCC surveillance may be lower than other health systems. Second, we identified patients using ICD-9 codes that are sensitive and specific for cirrhosis, although its positive predictive value is imperfect so some patients in our cohort may not have had cirrhosis. Third, we did not exclude patients with Child C cirrhosis or severe comorbidity who may not benefit from HCC surveillance so it is possible our study underestimated rates of appropriate surveillance; however, we did not find association between comorbidity index and receipt of HCC surveillance. Fourth, we could not determine indication for ultrasound exams, although we excluded those performed in the emergency room or inpatient hospitalization given low likelihood of surveillance intent. Fifth, given its retrospective nature, our study was limited by measurement bias (e.g. degree of liver dysfunction), unmeasured confounders, and missing data. Finally, our study evaluated HCC surveillance utilization but did not link this process measure to downstream outcomes of treatment receipt and/or survival. However, the likelihood of missing data for our outcome variable, i.e. HCC surveillance, is low given Group Health is an integrated healthcare delivery system and patients typically get their non-emergent outpatient medical care through Group Health.
Overall, we found 1 in 3 patients underwent any HCC surveillance but fewer than 2% had consistent guideline-concordant surveillance. Widespread implementation of HCC surveillance in large integrated health systems, such as Group Health, is likely contingent on availability of higher quality data evaluating the benefits and harms of HCC surveillance in patients with cirrhosis. HCC surveillance decisions are currently left to individual providers, resulting in variable practice patterns, including higher rates among those receiving Gastroenterology/Hepatology subspecialty care. Although a randomized controlled trial evaluating HCC surveillance may not be feasible, high quality cohort studies may provide sufficient rationale for promoting HCC surveillance and should be pursued(5). For example, colonoscopy is widely accepted for colorectal cancer screening without randomized data, based on well-conducted cohort and case-control studies(30). In the absence of better quality data, we are likely to continue seeing low rates of HCC surveillance in clinical practice.
Supplementary Material
Acknowledgements
We would like to acknowledge Sarah McDonald, who served as project manager, and Douglas Kane, who helped with data collection
Financial support: This work was conducted with support from the National Cancer Institute (1U24CA171524, PI: Kushi), AHRQ Center for Patient-Centered Outcomes Research (R24HS022418), PI: Halm, and the American Cancer Society and Simmons Cancer Center grant ACS-IRG-02-196 awarded to Dr. Singal. The collection of cancer incidence data used in this study was supported, in part, by the Cancer Surveillance System of the Fred Hutchinson Cancer Research Center, which is funded the Surveillance, Epidemiology and End Results (SEER) Program of the National Cancer Institute (contract numbers N01-CN-67009 and N01-PC-35142) with additional support from the Fred Hutchinson Cancer Research Center and the State of Washington. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the American Cancer Society.
Footnotes
Conflicts of Interest: None
REFERENCES
- 1.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–76. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 2.Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–21. doi: 10.1158/0008-5472.CAN-14-0155. [DOI] [PubMed] [Google Scholar]
- 3.Singal A, Volk ML, Waljee A, et al. Meta-analysis: surveillance with ultrasound for early-stage hepatocellular carcinoma in patients with cirrhosis. Aliment Pharmacol Ther. 2009;30:37–47. doi: 10.1111/j.1365-2036.2009.04014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Llovet JM, Bustamante J, Castells A, et al. Natural history of untreated nonsurgical hepatocellular carcinoma: rationale for the design and evaluation of therapeutic trials. Hepatology. 1999;29:62–7. doi: 10.1002/hep.510290145. [DOI] [PubMed] [Google Scholar]
- 5.Singal AG, Pillai A, Tiro J. Early Detection, Curative Treatment, and Survival Rates for Hepatocellular Carcinoma Surveillance in Patients with Cirrhosis: A Meta-analysis. PLoS Med. 2014;11:e1001624. doi: 10.1371/journal.pmed.1001624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–36. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 7.Bruix J, Sherman M, Llovet JM, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421–30. doi: 10.1016/s0168-8278(01)00130-1. [DOI] [PubMed] [Google Scholar]
- 8.American Cancer Society . Colorectal Cancer Facts & Figures 2011-2013. American Cancer Society; Atlanta: 2011. [Google Scholar]
- 9.Singal AG, Yopp A, C SS, Packer M, Lee WM, Tiro JA. Utilization of hepatocellular carcinoma surveillance among American patients: a systematic review. J Gen Intern Med. 2012;27:861–7. doi: 10.1007/s11606-011-1952-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sanyal A, Poklepovic A, Moyneur E, Barghout V. Population-based risk factors and resource utilization for HCC: US perspective. Curr Med Res Opin. 2010;26:2183–91. doi: 10.1185/03007995.2010.506375. [DOI] [PubMed] [Google Scholar]
- 11.Goldberg DS, Valderrama A, Kamalakar R, Sansgiry SS, Babajanyan S, Lewis JD. Hepatocellular Carcinoma Surveillance Among Cirrhotic Patients With Commercial Health Insurance. J Clin Gastroenterol. 2016;50:258–65. doi: 10.1097/MCG.0000000000000411. [DOI] [PubMed] [Google Scholar]
- 12.Serper M, Choi G, Forde KA, Kaplan DE. Care delivery and outcomes among US veterans with hepatitis B: A national cohort study. Hepatology. 2016;63:1774–82. doi: 10.1002/hep.28340. [DOI] [PubMed] [Google Scholar]
- 13.Singal AG, Marrero JA, Yopp A. Screening process failures for hepatocellular carcinoma. J Natl Compr Canc Netw. 2014;12:375–82. doi: 10.6004/jnccn.2014.0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singal AG, X. L, Tiro J, et al. Racial, Social, and Clinical Determinants of Hepatocellular Carcinoma Surveillance. Am J Med. 2014 doi: 10.1016/j.amjmed.2014.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davila JA, Morgan RO, Richardson PA, Du XL, McGlynn KA, El-Serag HB. Use of surveillance for hepatocellular carcinoma among patients with cirrhosis in the United States. Hepatology. 2010;52:132–41. doi: 10.1002/hep.23615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davila JA, Henderson L, Kramer JR, et al. Utilization of surveillance for hepatocellular carcinoma among hepatitis C virus-infected veterans in the United States. Ann Intern Med. 2011;154:85–93. doi: 10.7326/0003-4819-154-2-201101180-00006. [DOI] [PubMed] [Google Scholar]
- 17.Price RA. Organizational factors and the cancer screening process. J Natl Cancer Inst. 2010;40:38–57. doi: 10.1093/jncimonographs/lgq008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yano EM, Soban LM, Parkerton PH, Etzioni DA. Primary care practice organization influences colorectal cancer screening performance. Health Serv Res. 2007;42:1130–49. doi: 10.1111/j.1475-6773.2006.00643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chubak J, Garcia MP, Burnett-Hartman AN, et al. Time to Colonoscopy after Positive Fecal Blood Test in Four U.S. Health Care Systems. Cancer Epidemiol Biomarkers Prev. 2016;25:344–50. doi: 10.1158/1055-9965.EPI-15-0470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chubak J, Bowles EJ, Yu O, Buist DS, Fujii M, Boudreau DM. Breast cancer recurrence in relation to antidepressant use. Cancer Causes Control. 2016;27:125–36. doi: 10.1007/s10552-015-0689-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nehra MS, Ma Y, Clark C, Amarasingham R, Rockey DC, Singal AG. Use of administrative claims data for identifying patients with cirrhosis. J Clin Gastroenterol. 2013;47:e50–4. doi: 10.1097/MCG.0b013e3182688d2f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gopal P, Yopp AC, Waljee AK, et al. Factors That Affect Accuracy of alpha-Fetoprotein Test in Detection of Hepatocellular Carcinoma in Patients With Cirrhosis. Clin Gastroenterol Hepatol. 2013 doi: 10.1016/j.cgh.2013.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646–9. doi: 10.1002/bjs.1800600817. [DOI] [PubMed] [Google Scholar]
- 24.Bowles EJ, Buist DS, Chubak J, et al. Endocrine therapy initiation from 2001 to 2008 varies by age at breast cancer diagnosis and tumor size. J Oncol Pract 2012. 8:113–20. doi: 10.1200/JOP.2011.000417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang BH, Yang BH, Tang ZY. Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol. 2004;130:417–22. doi: 10.1007/s00432-004-0552-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singal AG, Nehra M, Adams-Huet B, et al. Detection of hepatocellular carcinoma at advanced stages among patients in the HALT-C trial: where did surveillance fail? Am J Gastroenterol. 2013;108:425–32. doi: 10.1038/ajg.2012.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singal AG, Tiro JA, Gupta S. Improving hepatocellular carcinoma screening: applying lessons from colorectal cancer screening. Clin Gastroenterol Hepatol. 2013;11:472–7. doi: 10.1016/j.cgh.2012.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dalton-Fitzgerald E, Tiro J, Kandunoori P, Halm EA, Yopp A, Singal AG. Practice Patterns and Attitudes of Primary Care Providers and Barriers to Surveillance of Hepatocellular Carcinoma in Patients with Cirrhosis. Clin Gastroenterol Hepatol. 2014 doi: 10.1016/j.cgh.2014.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kansagara D, Papak J, Pasha AS, et al. Screening for hepatocellular carcinoma in chronic liver disease: a systematic review. Ann Intern Med. 2014;161:261–9. doi: 10.7326/M14-0558. [DOI] [PubMed] [Google Scholar]
- 30.Elmunzer BJ, Singal AG, Sussman JB, et al. Comparing the effectiveness of competing tests for reducing colorectal cancer mortality: a network meta-analysis. Gastrointest Endosc. 2015;81:700–9. e3. doi: 10.1016/j.gie.2014.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


