Abstract
Objective
Converging evidence suggests shared genetic underpinnings of attention-deficit/hyperactivity disorder (ADHD) and autism spectrum disorder (ASD). Studies of infants at risk for ASD have proliferated over the past decade; the few studies that have followed these infants beyond age 3 report a range of difficulties facing a subset of these infants as they reach school-age, including elevated levels of attention problems and externalizing behavior. Given this, we aimed to identify early predictors of school-age ADHD outcomes in a sample of infant siblings at risk for ASD.
Method
This study reports on a sample of 59 infants at high and low risk for ASD who had been followed for over a decade, collecting data at regular intervals from 3 to 36 months and then determining diagnostic outcome at 8–10 years of age.
Results
Seventeen participants were diagnosed with DSM-5 ADHD at school-age (n = 14 high-risk, 3 low-risk). As infants, the ADHD outcome group demonstrated atypical longitudinal patterns of sustained visual attention. A significantly larger proportion of their parents reported behavior/temperament problems at 36 months of age, and examiners noted the presence of inattentive, hyperactive, and/or impulsive behaviors in this group by 18 months of age.
Conclusions
These data suggest that behavioral indicators of risk for later ADHD may be present early in development, which may improve earlier detection and treatment of the disorder.
Keywords: ADHD, autism spectrum disorder, infancy, early detection, high-risk
Attention-deficit/hyperactivity disorder (ADHD) is highly prevalent, affecting 5–8% of children (Faraone et al., 2005). It is characterized by developmentally atypical and extreme levels of inattention-disorganization and/or hyperactive-impulsive behavior and is diagnosed at an average age of 7 (Visser et al., 2014). Significant long-term, multi-domain impairments are common (for a review, see Shaw et al., 2012). Thus, earlier identification is critical, given growing interest in earlier intervention (Sonuga-Barke & Halperin, 2010). Only a handful of studies have examined infant manifestations of ADHD. Two found evidence of parent-reported temperament differences, including reduced inhibitory control and attention and increased activity level and negative affect, in infants of parents with elevated ADHD symptoms (Auerbach et al., 2008; Sullivan et al., 2015). Other studies of epidemiological or community samples have demonstrated that children with elevated parent-rated ADHD symptoms had lower infant motor and language scores, as well as reactivity/regulation-related temperament challenges (Arnett et al., 2013; Willoughby et al., 2016). However, none had diagnostic outcomes on participants, so it is not clear which infant behaviors predict a later diagnosis of ADHD versus which are endophenotypes.
ADHD and autism spectrum disorder (ASD) share overlapping genetic underpinnings (Rommelse et al., 2010) and there is evidence of shared familial transmission (Musser at al., 2014) and cross-aggregation of symptoms in families (Oerlemans et al., 2015). In recent years, studies of infant siblings of children with ASD have demonstrated high rates of ASD recurrence (up to 20%; Ozonoff et al., 2011). Siblings who do not develop ASD often display other difficulties, with nearly 30% demonstrating developmental concerns by age 3 (Ozonoff et al., 2014). Only a few studies have followed these samples beyond the preschool years, so relatively little is known about later-emerging problems.
The few studies that have followed infant sibling samples into middle childhood report substantial variability in outcomes, with a subgroup continuing to experience broad-based difficulties (Ben-Yizhak et al., 2011; Gamliel et al., 2009). The largest published school-age follow-up (Miller et al., 2015) assessed participants when they were 6–8 years old. Up to 43% of the non-ASD siblings of children with ASD had examiner-rated clinical concerns or scores in the clinical/impaired range across a variety of domains, similar to rates reported in other samples (Gamliel et al., 2009). Children classified with clinical concerns had elevated parent-reported psychopathology symptoms (including attention problems and externalizing symptoms), lower social responsiveness, and lower receptive/expressive language.
The more protracted emergence of clinical phenomena typically diagnosed later in childhood (e.g., ADHD) reduces feasibility of studies focused on early development of such conditions. The wide range of phenotypic variation and high rates of atypical outcomes characterizing high-risk infant sibling samples could provide an unprecedented opportunity to investigate the early emergence of a broader range of clinical outcomes beyond ASD. Given potential etiologic overlap between ASD and ADHD, as well as elevated rates of ADHD among siblings of children with ASD, studies of infant siblings may provide an opportunity to identify the earliest indicators of ADHD in well-characterized samples that have been prospectively evaluated in controlled laboratory settings from early in life. In this paper, we present data that provides the proof-of-principle for infant identification of risk for later ADHD in a small prospective sample of infants at high and low risk for ASD.
Method
Participants
The sample was drawn from a larger prospective study of younger siblings of children with ASD (high-risk group) or typical development (low-risk group). Children who received ASD diagnoses at any prior assessment point were excluded from the present follow-up data collection. Participants who were first diagnosed with ASD (n=2) or an ASD-related DSM-5 disorder (Social Communication Disorder [SCD]; n=1) at the present follow-up assessment were also excluded because of our focus on identifying early factors associated with ADHD. At time of initial recruitment, for the high-risk group, diagnoses of affected older siblings (probands) were confirmed with the Autism Diagnostic Observation Schedule (ADOS; Lord et al., 2000) and the Social Communication Questionnaire (SCQ; Rutter et al., 2003); exclusion criteria included birth before 32 weeks gestation or a known genetic disorder in the proband. Low-risk status was confirmed by an intake screener and proband SCQ scores below the ASD range; exclusion criteria included birth before 37 weeks gestation, developmental/learning/medical conditions in any older siblings, and ASD in first-, second-, or third-degree relatives. There was no exclusion for family history of common psychiatric disorders (ADHD, depression, anxiety).
Participants were enrolled before 18 months of age, with 79% of the sample having their first assessment by 12-months. Evaluations occurred at 3, 6, 12, 18, 24, and 36 months of age. When participants were 8–11 years old (mean=9.9 years, SD=0.96), they were invited back for a follow-up visit. Thirty-four high-risk infants (37% of original high-risk sample) and 23 low-risk infants (43% of original low-risk sample) were tested. The retained and non-retained samples did not differ in 36-month atypical clinical outcomes, psychopathology symptoms (Child Behavior Checklist), or ADOS scores (ps>.10). The retained group had higher 36-month nonverbal skills relative to the non-retained group (T-score=59.00 vs. 52.06, p<.01) but did not differ in verbal skills (T-score=53.80 vs. 50.72, p>.10).
At each stage, parents provided informed consent; children provided written assent for the school-age follow-up. Participants were assessed by masters- or doctoral-level examiners (licensed or closely-supervised by a licensed individual) with extensive experience in child psychopathology who were unaware of prior diagnostic outcomes or risk status. The university’s Institutional Review Board approved the study.
Measures
Infant predictors
Eye-tracking
Participants were administered a variety of eye-tracking tasks at 3, 6, 12, and 24 months of age using a Tobii ET-17 binocular infrared bright-pupil corneal reflection video oculographic eye-tracker. The construct of primary interest to the present study was sustained attention, defined as looking time to the whole screen regardless of stimuli/task content. We selected two tasks with the most longitudinal data, including a social preference task and an emotion-processing task.1 We then calculated percentage looking time to the whole screen for each and averaged resulting values across the two tasks at each age.
Parent concerns (Ozonoff et al., 2009)
At the end of the 6-, 12-, 18-, 24-, and 36-month assessments, parents were asked whether they had any developmental or behavioral concerns about their child. Parents’ verbal responses about current concerns were coded into pre-specified categories by examiners unaware of group membership or prior concerns: None, speech/language, social, stereotyped behavior, motor, medical/regulatory, behavior/temperament, unspecified autism, or general developmental concerns. Examiners were trained to 90% agreement on all categories. Here, we focused on the rate of behavior/temperament concerns, including any reports by parents of high activity level, poor attention, behavioral dysregulation (intensity of response, aggression, impulsivity, non-compliance), and difficulties in mood/general disposition. Concerns were dichotomized at each age (i.e., 1=≥1 concern, 0=no concerns).
Examiner-reported ADHD behaviors
Examiners provided written feedback reports to families after the 12-, 18-, 24-, and 36-month assessments. Through chart review and extraction, ADHD-related behaviors (inattention, hyperactivity, impulsivity) were identified by coders unaware of diagnostic outcome or risk group. Behaviors were collapsed across the three symptom domains and dichotomized at each age (i.e., 1=any report of inattention, hyperactivity, or impulsivity; 0=no report of these behaviors). Twenty-five percent of the files were randomly selected and double-coded for reliability purposes, resulting in a Cohen’s kappa of 0.95.
Missing data
Based on participants enrolled, missing data averages (ranges) across infant measures were: 26% at 3 months, 31% (24%–38%) at 6 months, 23% (3%–51%) at 12 months, 17% (4%–30%) at 18 months, 33% (17%–43%) at 24 months, and 27% (9%–45%) at 36 months.
School-age characterization measures
Child and Adolescent Symptom Inventory, 5th Edition (CASI-5; Gadow & Sprafkin, 2013)
This caregiver-report checklist provides measurements (DSM-5 symptom counts, T-scores) of a variety of childhood disorders. A symptom was considered present on the ADHD subscale if the parent endorsed it as occurring Often or Very Often.
NICHQ Vanderbilt Teacher Rating Scale (American Academy of Pediatrics, 2002)
This teacher-report checklist is commonly used to assess behavioral symptoms within the classroom context. Teachers of children suspected to have ADHD were asked to complete this form whenever possible to confirm diagnoses. A symptom was considered present if the teacher endorsed it as occurring Often or Very Often.
Kiddie-Schedule for Affective Disorders and Schizophrenia (K-SADS; Kaufman et al., 1997)
This semi-structured diagnostic interview for a range of childhood disorders was used in a confirmatory manner; if ADHD was suspected based on behavioral observations or parental endorsement of ≥6 ADHD symptoms in any one ADHD symptom category on the CASI-5, the ADHD section of K-SADS was administered.
Clinical best estimate diagnostic classification
At the end of the school-age assessment, examiners classified each child into one of eight outcome categories: ASD, SCD, BAP, ADHD, Speech-Language Problems, Learning Difficulties, Anxiety/Mood Problems, or Typically Developing (TD). ADHD group membership was determined by a licensed psychologist using DSM-5 criteria, based on parent-reported CASI-5 scores, behavioral observations, and, whenever possible, teacher ratings and K-SADS, and included children with inattentive and combined presentations. We utilized the “or” algorithm for integrating multi-informant data (Shemmassian & Lee, 2016), considering an ADHD symptom present if the parent or teacher or examiner endorsed it. Forty-two percent of the high-risk group (n=14/33) received a diagnosis of ADHD, versus 13% of the low-risk group (n=3/23). Of the 3 low-risk children with ADHD outcomes, two had older siblings with clinical diagnoses (one ADHD, one learning disability). Because of our interest in identifying early differences between infants with later ADHD versus TD outcomes, in addition to excluding the few children with ASD-related outcomes (described above), we excluded remaining participants with outcomes other than ADHD or TD (n=9)2 from further analysis. Thus, analyses focused only on children who received TD (n=30) or DSM-5 ADHD (n=17) outcomes at the school-age assessment.
Wechsler Abbreviated Scale of Intelligence, 2nd Edition (WASI-II; Wechsler, 2011)
The WASI-II is an abbreviated measure of intellectual functioning for ages 6–90 years. It has excellent psychometric properties (test-retest reliability >0.90, correlation with WISC-IV FSIQ=0.92).
Data Analytic Plan
Generalized Estimating Equations (GEE; Zeger et al., 1988) were used to analyze the data. This approach is appropriate for modeling normal (eye-tracking) and binary (parent- and examiner-reported behaviors) variables, and accounts for within-person correlation. It makes use of all available data for a child and allows for adjustment for effects of potential confounders. We used identity link and a normal variance function for eye-tracking data, and a logit link and binomial variance function for parent- and examiner-reported behavior data. To model within-child correlation, we used AR(1) for the normal variable and exchangeable log odds ratios for binary variables. Standard errors for regression coefficients were based on the empirical (robust) covariance matrix estimators and confidence intervals were computed using profile likelihood. All core models included terms for group (TD, ADHD), the linear effect of age (centered at baseline), and the group×age interaction. In secondary analyses, we added child sex as a covariate to the core models; this term was non-significant and did not alter results. Thus, results of core models are reported. Analyses were implemented using PROC GENMOD in SAS Version 9.4 (SAS Institute, Inc.). Hypothesis tests were two-sided; ps<.05 were considered statistically significant.
Results
Table 1 contains participant characteristics by outcome group (TD vs. ADHD).
Table 1.
Participant characteristics.
| TD Outcome (n=30) | ADHD Outcome (n=17) | p-valuea | |
|---|---|---|---|
| Male sex (n, %) | 13 (43%) | 10 (59%) | ns |
| High-risk (n, %) | 13 (43%) | 14 (82%) | p < .01 |
| Ethnicityb (n, % non-white) | 8 (27%) | 3 (18%) | ns |
| Household incomec (n, %) | ns | ||
| <$25,000 | 2 (7%) | 0 (0%) | |
| $25,000–$49,999 | 0 (0%) | 0 (0%) | |
| $50,000–$74,999 | 7 (25%) | 5 (29%) | |
| $75,000–$99,999 | 9 (32%) | 4 (24%) | |
| ≥$100,000 | 10 (36%) | 8 (47%) | |
| Age, years (M, SD) | 10.15 (1.02) | 9.53 (0.88) | ns |
| WASI-II FSIQ (M, SD) | 111.23 (9.15) | 111.24 (15.63) | ns |
| CASI-5 T-scores (M, SD) | |||
| Inattention | 52.13 (5.70) | 65.41 (12.02) | p < .001 |
| Hyperactivity-impulsivity | 51.87 (6.08) | 64.12 (12.97) | p < .01 |
| ADHD total | 51.67 (5.59) | 65.29 (12.79) | p < .001 |
Note. TD = Typically Developing; ADHD = Attention-Deficit/Hyperactivity Disorder; WASI-II = Wechsler Abbreviated Scale of Intelligence, Second Edition; FSIQ = Full Scale IQ; CASI-5 = Child and Adolescent Symptom Inventory, Fifth Edition.
Group differences assessed using χ2 tests for sex, risk group, and ethnicity; Fisher’s exact test for household income; and two-samples t-tests for remaining variables.
Frequency missing n = 1 in TD group.
Frequency missing n = 2 in TD group.
Infant Predictors of ADHD
Sustained visual attention
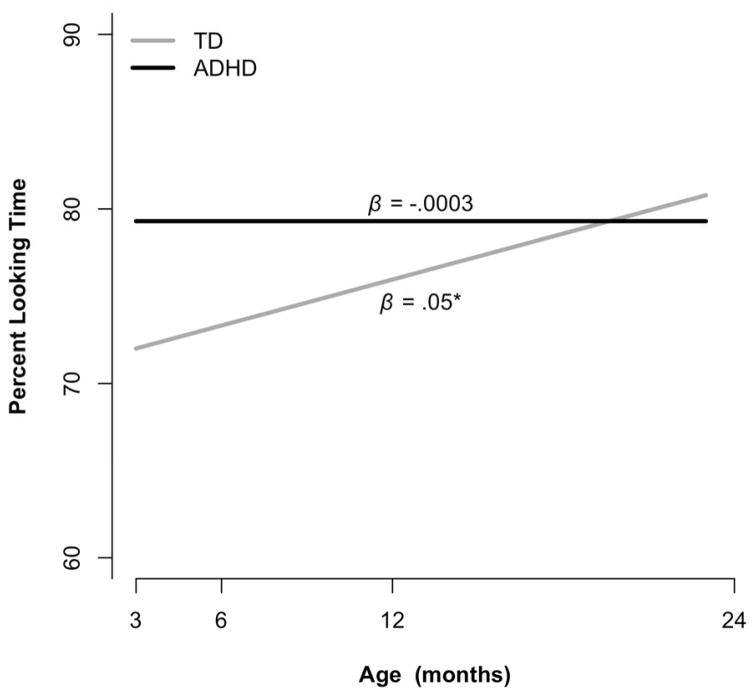
Table 2 and Figure 1 summarize results for percent looking time. Although the interaction between group and age did not reach statistical significance (p=.14), given the proof-of-principle nature of this study, we examined linear slopes of the two groups between 3–24 months of age. As shown in Figure 1, the TD group significantly increased in percent looking time between 3 and 24 months (β=0.05, p=.04) whereas the ADHD group exhibited no growth (β=−0.0003, p=.99). The groups differed at baseline (3 months), with the ADHD group having longer percent looking time relative to the TD group (estimated difference=7.3%, SE=3.7%, p<.05).
Table 2.
Parameter estimates (SE) for core models predicting sustained visual attention, parent concerns, and examiner-reported behaviors.
| Model term | Sustained visual attentiona | Parent-reported behavior/temperament concernsb | Examiner-reported ADHD behaviorsb |
|---|---|---|---|
| Estimated trajectory for TD group | |||
| Baseline | 0.72 (0.03)*** | −1.90 (0.49)*** | −1.32 (0.42)** |
| Linear age effect (year) | 0.05 (0.03)* | 0.09 (0.31) | −0.37 (0.29) |
| Estimated difference between ADHD and TD groups | |||
| Baseline | 0.07 (0.04)* | −0.63 (0.82) | 0.63 (0.56) |
| Linear age effect (year) | −0.05 (0.04) | 1.05 (0.50)* | 0.88 (0.40)* |
Note. Baseline is 3-months for sustained visual attention, 6-months for parent concerns, and 12-months for examiner reports. SE = Standard Error; TD = Typically Developing; ADHD = Attention-Deficit/Hyperactivity Disorder.
p < .001;
p < .01;
p < .05.
Estimated from Generalized Estimating Equations models for normal (a) and binary (b) data that included terms for group (ADHD, TD), age, and their interaction, and accounted for the within-child clustering due to repeated observations.
Figure 1.
Estimated trajectories of overall looking time during eye-tracking for each group. Slopes represent unit change per year.
*p < .05
Parent concerns
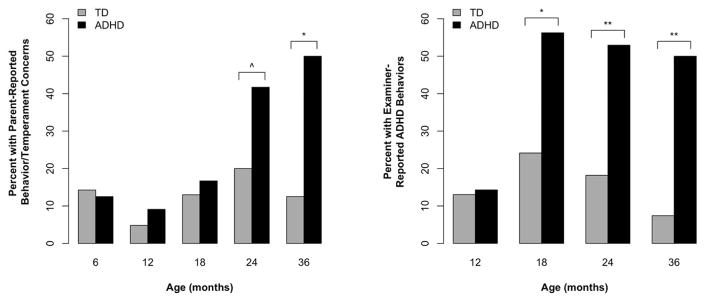
Table 2 and Figure 2 summarize results for parent-reported behavior/temperament concerns. The interaction between group and age was significant (p=.04). At 6 months, both groups had similar proportions of parent-reported behavior/temperament concerns; this proportion increased in the ADHD group between 6–36 months, while remaining stable and low in the TD group. At 24 months, there was a trend toward a higher proportion of parent-reported behavior/temperament concerns in the ADHD (42%) versus TD group (20%), OR=2.57, 95% CI=0.90–7.32, p=.08. At 36 months, the two groups significantly differed, with 50% of parents from the ADHD group reporting at least one behavior/temperament concern versus 13% of the TD group, OR=7.32, 95% CI=1.51–35.62, p<.02.
Figure 2.
Parent- and examiner-reported ADHD-related behaviors.
^p < .10; *p < .05; **p < .001
Examiner-reported ADHD-related behaviors
Table 2 and Figure 2 summarize the results for examiner-reported ADHD-related behaviors. The interaction between group and age was significant (p=.03). At 12 months, both groups had similarly low proportions of examiner-reported ADHD-related behaviors, which increased in the ADHD group between 12–36 months while remaining stable in the TD group. At 18 months, 56% of the ADHD group’s reports contained mention of these behaviors versus 24% of the TD group, which was significantly different, OR=2.90, 95% CI=1.17–7.18, p<.05. At 24 months, 53% of the reports from the ADHD group described such behaviors versus 18% of the TD group, OR=4.50, 95% CI=1.91–10.60, p<.001. This pattern continued at 36 months, with 50% of the ADHD group versus 7% of the TD group having these behaviors documented, OR=10.84, 95% CI=3.19–36.80, p<.001.
Discussion
We provide proof-of-principle data suggesting that earlier detection of ADHD risk may be possible. This is the first study to evaluate prospectively-collected infant predictors, from as early as 3 months of age, of verified DSM-5 diagnoses of ADHD. This is also the first ASD infant sibling study to leverage the vast amounts of longitudinal data collected in these study designs to identify early predictors of other forms of psychopathology.
With respect to the development of early sustained visual attention, the TD group showed a trajectory consistent with the typical developmental pattern of look duration during the first years of life (Colombo, 2002), with a significantly increasing slope between 3–24 months of age. Prior work has suggested that a shift toward endogenously-driven (executive) attention regulation occurs around 12 months of age (Colombo, 2002). The ADHD group appears to follow a different developmental course relative to the TD group; they did not exhibit the normative pattern of increasing look time, instead demonstrating a lack of growth between 3–24 months of age. The ADHD group had higher overall looking times early in infancy, at 3 months of age. A robust literature suggests that long-looking times early in infancy may be detrimental, reflecting slower cognitive processing; longer looking times early in infancy are longitudinally associated with poorer outcomes (e.g., Cuevas & Bell, 2014). However, it is difficult to determine whether this difference at 3-months might also reflect underlying genetic risk for ASD. These issues will be important to evaluate further as we follow larger numbers of infants into the school-age years.
We also systematically solicited parent concerns at every assessment, finding that the level of parent-reported behavior/temperament concerns distinguished children with and without ADHD outcomes by 36 months of age, and possibly even beginning by 24 months (although not statistically significant, 42% of parents of children with ADHD outcomes reported concerns at age 2 versus 20% of parents of TD children). The mean age of ADHD diagnosis is around age 7, so this is, in fact, an early age for parental identification of ADHD symptoms (Sonuga-Barke et al., 2011). This suggests that parents are able to identify early behavioral patterns indicative of later ADHD. Results were even stronger when based on examiner-reported ADHD-related behaviors acquired via chart review, with the suggestion that examiners can identify behaviors as early as 18 months that may be predictive of a later diagnosis of ADHD. Moreover, both parents and examiners reported increases in ADHD-related behaviors from infancy through toddlerhood in the ADHD group, versus relative stability or decreases in the TD group, reflecting the normative occurrence of ADHD-related behaviors in very young children, and the shift in this threshold over time. Examiners identified these behaviors earlier than parents (18 months vs. 24–36 months), potentially indicating differential thresholds for normative levels of ADHD-related behaviors between trained professionals and parents. Nearly all of the parents of children with ADHD outcomes in this sample also had a child with ASD, and thus, their threshold for early “concerning” behavior may differ.
Although we provide unique prospective data and intriguing leads related to the early emergence of ADHD, our approach has several limitations. First, our sample was small and overall retention rate low; although the retained sample did not differ from the non-retained sample in most domains evaluated, generalizability of our findings remains unclear. It is imperative to re-examine these findings in larger prospective studies. Relatedly, given the variation in enrollment ages and the nature of longitudinal studies, we had a range of missing data across measures and ages. We used robust variance estimators in GEE, which yield consistent estimates if data are missing completely at random. Plans to conduct follow-up assessments of the remainder of our original sample, as well as coordinated follow-up studies of the many existing infant sibling samples, will help to remedy this. Additionally, most of the children who received school-age ADHD diagnoses were from the group at risk for ASD. Whether ADHD in the context of genetic risk for ASD is comparable to ADHD in the absence of such risk is unknown3; we also cannot speak to specificity of these early differences to ADHD alone, since children with ASD outcomes were not included in this study. It will be critical to evaluate similar questions in samples at heightened familial risk for ADHD. Finally, because the original study was not focused on early prediction of ADHD, our measures may not be as sensitive to infant manifestations of ADHD as instruments selected specifically for this purpose. This, too, can be addressed by new studies focused, at the outset, on infants at familial risk for ADHD, which are currently underway in our laboratory.
While we cannot rule out the possibility that the high rates of ADHD in our sample are due to retention biases, the retained sample was not more impaired than the non-retained sample at 36 months of age on any measure evaluated, making such an explanation less likely. There have been few recurrence risk studies of ADHD, but those that have been conducted find high rates of two or more affected individuals with ADHD in a family: 25% among non-twin siblings, 40% in twins, and as high as 57% in children of parents with ADHD (Biederman et al., 1995; Langer et al., 2013). These studies are, however, plagued by stoppage effects (see Wood et al., 2015) and it is possible that recurrence risk among younger-born siblings of children with ADHD would be even higher. Moreover, the rate of ADHD in our low-risk group is similar to recent CDC estimates (11%; Visser et al., 2014), and two of the three low-risk infants who developed ADHD had family histories of ADHD or learning disorder, potentially increasing their risk for ADHD. Ultimately, given our small sample and ascertainment methods, we cannot yet draw firm conclusions regarding the prevalence of ADHD among younger-born siblings of children with ASD.
In conclusion, our data suggest that it may be possible to detect a signal for ADHD early in life and that many younger siblings of children with ASD meet criteria for ADHD by school-age, emphasizing that continued follow-up of infant sibling samples beyond age 3 is critical. Our findings also highlight the importance of taking a developmental approach to understanding the onset and course of ADHD, examining behavior over time to evaluate differences in developmental progressions or patterns. Further follow-up studies will provide a better understanding of the broad range of difficulties that may affect high-risk siblings as they grow, with implications for screening, monitoring, and intervention beyond age 3. These datasets, enriched with a wide range of phenotypic variation, can be leveraged far beyond their initial purpose–the early diagnosis of ASD–to better understand the emergence of other forms of psychopathology, such as ADHD, subsequently providing a foundation for future prospective studies of these disorders.
Acknowledgments
This study was supported by grants from the National Institute of Mental Health: R01MH068398 (Ozonoff), K99MH106642 (Miller); and the National Institute of Child Health and Human Development: Intellectual and Developmental Disabilities Research Center U54HD079125 (Abbeduto).
We gratefully acknowledge the families who have participated in our ongoing longitudinal investigation, along with the large number of students, staff, and research assistants who have contributed to this project.
Footnotes
The emotion-processing task involved infants passively viewing 5-second movies of expressive faces; the social preference task involved infants observing pairings of active social (continuous movie footage of an infant) and nonsocial (moving objects; e.g., a spinning chair) stimuli.
These outcomes included learning problems (n=4), broader autism phenotype (n=3), and anxiety/mood problems (n=2).
In an initial attempt to address this, we examined school-age ADOS and Social Responsiveness Scale (SRS) scores between the ADHD and TD groups. The two groups had comparable ADOS severity scores (TD mean=1.28, ADHD mean=1.59); none of the children had scores above the ASD cutoff. The ADHD group had higher SRS total T-scores (TD mean=41.45, ADHD mean=47.44) but scores were still well below the elevated/clinical range. Although the sample of low-risk children with ADHD outcomes was small, the high-risk ADHD outcome group was generally comparable to the low-risk ADHD outcome group in terms of ADOS (mean=1.57 vs. 1.67, respectively) and SRS (mean=48.92 vs. 41.00, respectively) scores, which, at a group level, were all in the non-clinical range. These comparisons suggest that (1) the children with ADHD outcomes in our sample (mostly high-risk), in general, do not exhibit atypical levels of ASD symptoms compared to the TD children, and (2) the low- and high-risk children with ADHD have generally comparable levels of ASD symptoms. Larger studies are needed to address the question of whether the manifestation of ADHD symptoms is similar between the low- and high-risk groups.
The authors report no conflicts of interest.
References
- American Academy of Pediatrics, National Initiative for Children’s Healthcare Quality. Caring for Children with ADHD: A Resource Toolkit for Clinicians. Chicago: Centers for Education and Research on Therapeutics; 2002. [Google Scholar]
- Arnett AB, MacDonald B, Pennington BF. Cognitive and behavioral indicators of ADHD symptoms prior to school age. Journal of Child Psychology & Psychiatry. 2013;54:1284–1294. doi: 10.1111/jcpp.12104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerbach JG, Berger A, Atzaba-Poria N, Arbelle S, Cypin N, Friedman A, Landau R. Temperament at 7, 12, and 25 months in children at familial risk for ADHD. Infant & Child Development. 2008;17:321–338. [Google Scholar]
- Ben-Yizhak N, Yirmiya N, Seidman I, Alon R, Lord C, Sigman M. Pragmatic language and school related linguistic abilities in siblings of children with autism. Journal of Autism & Developmental Disorders. 2011;41:750–760. doi: 10.1007/s10803-010-1096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biederman J, Faraone SV, Mick E, Spencer T, Wilens T, Kiely K, … Warburton R. High risk for attention deficit hyperactivity disorder among children of parents with childhood onset of the disorder: A pilot study. American Journal of Psychiatry. 1995;152:431–435. doi: 10.1176/ajp.152.3.431. [DOI] [PubMed] [Google Scholar]
- Colombo J. Infant attention grows up: The emergence of a developmental cognitive neuroscience perspective. Current Directions in Psychological Science. 2002;11:196–200. [Google Scholar]
- Cuevas K, Bell MA. Infant attention and early childhood executive function. Child Development. 2014;85:397–404. doi: 10.1111/cdev.12126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraone SV, Perlis RH, Doyle AE, Smoller JW, Goralnick JJ, Holmgren MA, Sklar P. Molecular genetics of attention-deficit/hyperactivity disorder. Biological Psychiatry. 2005;57:1313–1323. doi: 10.1016/j.biopsych.2004.11.024. [DOI] [PubMed] [Google Scholar]
- Gadow KD, Sprafkin J. Child & Adolescent Symptom Inventory-5 manual. Stony Brook, NY: Checkmate Plus; 2013. [Google Scholar]
- Gamliel I, Yirmiya N, Jaffe DH, Manor O, Sigman M. Developmental trajectories in siblings of children with autism: Cognition and language from 4 months to 7 years. Journal of Autism & Developmental Disorders. 2009;39:1131–1144. doi: 10.1007/s10803-009-0727-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, … Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children – Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. Journal of the American Academy of Child & Adolescent Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Langer I, Garbe E, Banaschewski T, Mikolajczyk RT. Twin and sibling studies using health insurance data: The example of Attention Deficit/Hyperactivity Disorder (ADHD) PLOS One. 2013;8:e62177. doi: 10.1371/journal.pone.0062177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Leventhal BL, DiLavore PC, … Rutter M. The Autism Diagnostic Observation Schedule – Generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism & Developmental Disorders. 2000;30:205–223. [PubMed] [Google Scholar]
- Miller M, Iosif A, Young GS, Hill M, Phelps-Hanzel E, Hutman T, … Ozonoff S. School-age outcomes of infants at risk for autism spectrum disorder. Autism Research. 2015;9:632–642. doi: 10.1002/aur.1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musser ED, Hawkey E, Kachan-Liu SS, Lees P, Roullet JB, Goddard K, … Nigg JT. Shared familial transmission of autism spectrum and attention- deficit/hyperactivity disorders. Journal of Child Psychology & Psychiatry. 2014:819–827. doi: 10.1111/jcpp.12201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oerlemans AM, Hartman CA, De Bruijn YGE, Van Steijn DJ, Franke B, Buitelaar JK, Rommelse NNJ. Simplex and multiplex stratification in ASD and ADHD families: A promising approach for identifying overlapping and unique underpinnings of ASD and ADHD? Journal of Autism & Developmental Disorders. 2015;45:645–657. doi: 10.1007/s10803-014-2220-9. [DOI] [PubMed] [Google Scholar]
- Ozonoff S, Young GS, Belding A, Hill M, Hutman T, Johnson S, … Iosif AM. The broader autism phenotype in infancy: When does it emerge? Journal of the American Academy of Child & Adolescent Psychiatry. 2014;53:398–407. doi: 10.1016/j.jaac.2013.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, Young GS, Carter A, Messinger D, Yirmiya N, Zwaigenbaum L, … Stone WL. Recurrence risk for autism spectrum disorders: A Baby Siblings Research Consortium study. Pediatrics. 2011;128:e1–e8. doi: 10.1542/peds.2010-2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, Young GS, Steinfeld MB, Hill MM, Cook I, Hutman T, … Sigman M. How early do parent concerns predict later autism diagnosis? Journal of Developmental & Behavioral Pediatrics. 2009;30:367–375. doi: 10.1097/dbp.0b013e3181ba0fcf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rommelse NN, Franke B, Geurts HM, Hartman CA, Buitelaar JK. Shared heritability of attention-deficit/hyperactivity disorder and autism spectrum disorder. European Child & Adolescent Psychiatry. 2010;19:281–295. doi: 10.1007/s00787-010-0092-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M, Bailey A, Lord C. Social communication questionnaire: Manual. Western Psychological Services; 2003. [Google Scholar]
- Shaw M, Hodgkins P, Caci H, Young S, Kahle J, Woods AG, Arnold LE. A systematic review and analysis of long-term outcomes in attention deficit hyperactivity disorder: Effects of treatment and non-treatment. BMC Medicine. 2012:10. doi: 10.1186/1741-7015-10-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shemmassian SK, Lee SS. Predictive utility of four methods of incorporating parent and teacher symptom ratings of ADHD for longitudinal outcomes. Journal of Clinical Child & Adolescent Psychology. 2016;45:176–187. doi: 10.1080/15374416.2014.971457. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJS, Halperin JM. Developmental phenotypes and pathways in attention deficit/hyperactivity disorder: Potential targets for intervention? Journal of Child Psychology & Psychiatry. 2010;51:368–389. doi: 10.1111/j.1469-7610.2009.02195.x. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJS, Koerting J, Smith E, McCann DC, Thompson M. Early detection and intervention for attention-deficit/hyperactivity disorder. Expert Review of Neurotherapeutics. 2011;11:557–563. doi: 10.1586/ern.11.39. [DOI] [PubMed] [Google Scholar]
- Sullivan EL, Holton KF, Nousen EK, Barling AN, Sullivan CA, Propper CB, Nigg JT. Early identification of ADHD risk via infant temperament and emotion regulation: A pilot study. Journal of Child Psychology & Psychiatry. 2015;56:949–957. doi: 10.1111/jcpp.12426. [DOI] [PubMed] [Google Scholar]
- Visser SN, Danielson ML, Bitsko RH, Holbrook JR, Kogan MD, Ghandour RM, … Blumberg SJ. Trends in the parent-report of health care provider-diagnosed and medicated attention-deficit/hyperactivity disorder in the United States, 2003–2011. Journal of the American Academy of Child & Adolescent Psychiatry. 2014;53:34–46. doi: 10.1016/j.jaac.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. 2. San Antonio, TX: Pearson; 2011. [Google Scholar]
- Willoughby MT, Gottfredson NC, Stifter CA The Family Life Project Investigators. Observed temperament from ages 6–36 months predicts parent- and teacher- reported attention-deficit/hyperactivity disorder symptoms in first grade. Development & Psychopathology. 2016 doi: 10.1017/S0954579415001236. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Wood CL, Warnell F, Johnson M, Hames A, Pearce MS, McConachie H, Parr JR. Evidence for ASD recurrence rates and reproductive stoppage from large UK ASD research family databases. Autism Research. 2015;8:73–81. doi: 10.1002/aur.1414. [DOI] [PubMed] [Google Scholar]
- Zeger SL, Liang K, Albert PS. Models for longitudinal data: A generalized estimating equation approach. Biometrics. 1988;44:1049–1060. [PubMed] [Google Scholar]