Abstract
BACKGROUND:
Sugar-sweetened beverages are associated with type 2 diabetes. To assess whether this association holds for the fructose-containing sugars they contain, we conducted a systematic review and meta-analysis of prospective cohort studies.
METHODS:
We searched MEDLINE, Embase, CINAHL and the Cochrane Library (through June 2016). We included prospective cohort studies that assessed the relation of fructose-containing sugars with incident type 2 diabetes. Two independent reviewers extracted relevant data and assessed risk of bias. We pooled risk ratios (RRs) using random effects meta-analyses. The overall quality of the evidence was assessed using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) system.
RESULTS:
Fifteen prospective cohort studies (251 261 unique participants, 16 416 cases) met the eligibility criteria, comparing the highest intake (median 137, 35.2 and 78 g/d) with the lowest intake (median 65, 9.7 and 25.8 g/d) of total sugars, fructose and sucrose, respectively. Although there was no association of total sugars (RR 0.91, 95% confidence interval [CI] 0.76–1.09) or fructose (RR 1.04, 95% CI 0.84–1.29) with type 2 diabetes, sucrose was associated with a decreased risk of type 2 diabetes (RR 0.89, 95% CI 0.80–0.98). Our confidence in the estimates was limited by evidence of serious inconsistency between studies for total sugars and fructose, and serious imprecision in the pooled estimates for all 3 sugar categories.
INTERPRETATION:
Current evidence does not allow us to conclude that fructose-containing sugars independent of food form are associated with increased risk of type 2 diabetes. Further research is likely to affect our estimates.
Trial registration:
ClinicalTrials.gov, no. NCT01608620
Sugars, particularly fructose-containing sugars, have been implicated as an important driver in the rise in incidence of type 2 diabetes.1,2 Sugar-sweetened beverages, which represent the greatest source of fructose-containing sugars in the diet,3 form most of the basis for this link.4,5 It remains unclear whether the association between beverages sweetened with sugars and type 2 diabetes can be explained by the fructose that these beverages contain. Several high-quality systematic reviews and meta-analyses have assessed the relation of sugar-sweetened beverages with incident type 2 diabetes. Our objective was to conduct a systematic review and meta-analysis of prospective cohort studies to determine the role of fructose-containing sugars independent of food form in the development of type 2 diabetes.
Methods
Our systematic review and meta-analysis followed the Cochrane Handbook for Systematic Reviews and Interventions,6 and reported results according to Meta-analysis of Observational Studies in Epidemiology (MOOSE) guideline7 and PRISMA guideline (www.prisma-statement.org). The study protocol is registered (ClinicalTrials.gov identifier, NCT01608620).
Data sources and searches
We searched MEDLINE, Embase, CINAHL and the Cochrane Library databases through June 2016. The search strategy is presented in supplementary Table 1 (Appendix 1, available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.160706/-/DC1). The search was restricted to human studies without language restrictions. Manual searches of the reference lists of included studies supplemented electronic searches.
Study selection
We included prospective cohort studies that assessed intake of fructose-containing sugars (total sugars, fructose, sucrose, high-fructose corn syrup or added sugars) and incident type 2 diabetes in participants who did not have diabetes.
Data extraction
Two reviewers (C.T. and R.T.) independently extracted relevant data from included studies. The main outcome was type 2 diabetes risk expressed as risk ratios (RRs) with 95% confidence intervals (CI). Authors were contacted for missing data.
Risk of bias
The Newcastle-Ottawa Scale (NOS) was used to assess the risk of bias in included studies, where up to 9 points were awarded based on cohort selection, comparability (adjustments) and ascertainment of the outcome.8 Owing to concerns regarding the use of cut-off scores,6 we did not use our prespecified cut-off score for NOS.9 Differences were reconciled by consensus.
Grading of the evidence
The quality and strength of the evidence was assessed using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) system.10–22 Included observational studies started at low-quality evidence by default and then were downgraded or upgraded based on prespecified criteria. Criteria to downgrade included study limitations (weight of studies showed risk of bias by NOS), inconsistency (substantial unexplained inter-study heterogeneity, I2 > 50% and p < 0.10), indirectness (presence of factors relating to the population, exposures and outcomes that limit generalizability), imprecision (95% CIs were wide or crossed a minimally important difference of 10% [RR 0.9–1.1]) and publication bias (significant evidence of small-study effects). Criteria to upgrade included a large size effect (RR > 2 or RR < 0.5 in the absence of plausible confounders), a dose–response gradient and attenuation by plausible confounding effects.
Statistical analysis
To obtain summary estimates, we natural log-transformed and pooled the RRs using the generic inverse variance method with random-effects models. We used RRs comparing extreme quantiles and scaled RRs per 100 g/d for total sugars, 50 g/d for fructose and 50 g/d for sucrose to standardize the doses based on estimated average intakes in Canada.23 Heterogeneity was assessed (Cochran Q statistic) and quantified (I2 statistic). If I2 was greater than or equal to 50%, we interpreted this as indicating substantial heterogeneity.6,16 We investigated possible sources of heterogeneity. To assess whether any single study exerted an undue influence on the summary estimates, we performed sensitivity analyses by systematically removing each study with recalculation of the summary estimates. We performed additional sensitivity analyses by restricting pooled analyses to studies using validated measures of sugars intake to assess any influence of how the exposures were assessed. Prespecified subgroup analyses were done by sex, follow-up, NOS and individual domains of NOS using meta-regression analyses. Linear and nonlinear dose–response analyses were assessed by using generalized least squares trend (GLST) estimation models and spline curve modelling (MKSPLINE procedure). If 10 or more cohort comparisons were available, we investigated publication bias by visual inspection of funnel plots and using the Begg and Egger tests. Data were analyzed using Review Manager (RevMan) version 5.2 (The Nordic Cochrane Centre) and Stata version 12 (StataCorp).
Results
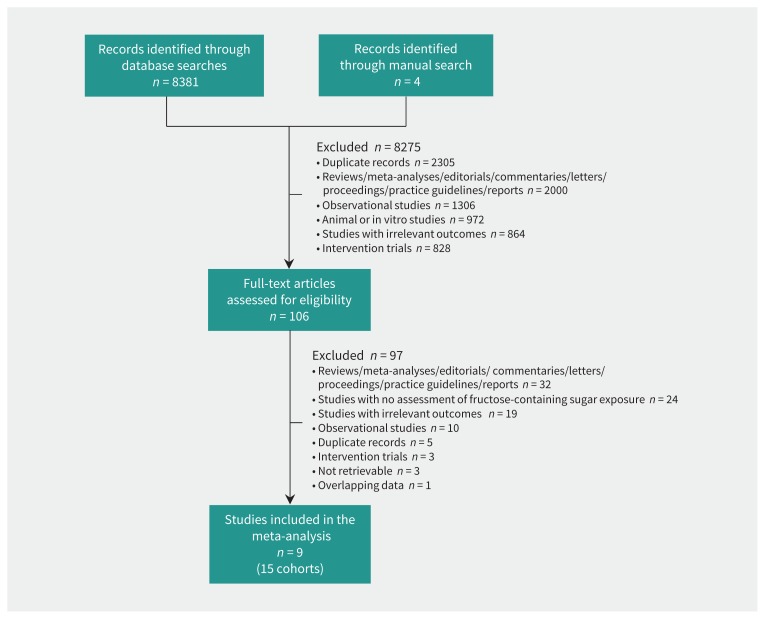
Figure 1 shows the flow of the literature search. Out of 8381 reports, we included 9 reports of 15 cohort studies involving 251 261 unique participants and 16 416 cases of type 2 diabetes:24–32 12 cohort comparisons (n = 105 846, 13 727 cases) for total sugars, 6 cohort comparisons (n = 107 972, 3833 cases) for fructose and 8 cohort comparisons (n = 192 332, 4535 cases) for sucrose. There were no cohort comparisons available for high-fructose corn syrup or added sugars.
Figure 1:
Summary of evidence search and selection.
Table 1 shows the characteristics of the included studies. Participants were from 11 countries and had a median age of 52.6 years (interquartile range [IQR] 20–79 yr). There were more female than male participants, with 4 large female cohorts and 11 smaller mixed cohorts. Median follow-up was 12 years (IQR 4–12 yr), 6.3 years (IQR 6–12 yr) and 6.2 years (IQR 6–12 yr) for total sugars, fructose and sucrose, respectively. Ascertainment of incident cases was done by medical record linkage (60%), self-report (27%) and physician diagnosis (13%).
Table 1:
Characteristics of prospective cohort studies investigating the dietary intake of total sugars, fructose and sucrose and incident type 2 diabetes
| Study, year | Cohort | Country | No. of participants | No. of incident cases | Age, yr | Duration of study, yr | Dietary intake assessment (at baseline) | Sugars exposure,† g/d | Method of outcome assessment | Funding source‡ |
|---|---|---|---|---|---|---|---|---|---|---|
| Colditz et al., 199224 | Nurses Health Study | United States | 84 360 (F) | 702 | 34–59 | 6 | Validated SFFQ | Sucrose exposure unknown | Self-report | Agency |
| Meyer et al., 200025 | Iowa Women’s Health Study | United States | 35 988 (F) | 1141 | 55–69 | 6 | Validated FFQ | Sucrose 25.8–57.7, fructose 12.5–35.5 | Self-report | Agency |
| Janket et al., 200326 | Women’s Health Study | United States | 38 480 (F) | 918 | 46–61 | 6 | Validated SFFQ | Sucrose 25.8–57.2, fructose 25.8–57.2, total sugars 25.8–57.2 | Self-report | Agency |
| Hodge et al., 200427 | Melbourne Collaborative Cohort Study | Australia | 31 641 | 365 | 27–75 | 4 | FFQ | Total sugars 63.6–194.4 | Physician diagnosis | Agency |
| Barclay et al., 200728 | Blue Mountains Eye Study | Australia | 1833 | 138 | ≥ 49 | 10 | Validated SFFQ | Total sugars 100 | Self-report | NA |
| Montonen et al., 200729 | Finnish Mobile Health Clinic Examination Survey | Finland | 4284 | 175 | 40–69 | 12 | Interview with questionnaire | Sucrose 28.5–79.5, fructose 6.0–28.8, total sugars 92.0–171 | Medical record linkage | NA |
| Schulze et al., 200830 | EPIC- Potsdam | Germany | 9702 (M), 15 365 (F) | 491 (M), 355 (F) | 35–65 | 7–11 | Validated SFFQ | Sucrose 22.5–102 (M), 28.2–83.4 (F); fructose 8.4–40.6 (M), 11–34.8 (F) | Physician diagnosis | Agency |
| Sluijs et al., 201331 | EPIC-InterAct Study | The Netherlands | 2290 | 828 | 20–70 | 12 | Quantitative Dietary Questionnaire | Total sugars 65–137 | Medical record linkage | Agency |
| Sluijs et al., 201331 | EPIC-InterAct Study | Denmark | 4037 | 2055 | 50–64 | 12 | Validated SFFQ | Total sugars 65–137 | Medical record linkage | Agency |
| Sluijs et al., 201331 | EPIC-InterAct Study | France | 867 (F) | 288 | 40–65 | 12 | Quantitative Dietary Questionnaire | Total sugars 65–137 | Medical record linkage | Agency |
| Sluijs et al., 201331 | EPIC-InterAct Study | Germany | 3578 | 1584 | 40–65 (M), 35–65 (F) | 12 | Quantitative Dietary Questionnaire | Total sugars 65–137 | Medical record linkage | Agency |
| Sluijs et al., 201331 | EPIC-InterAct Study | Italy | 3393 | 1437 | 35–74 | 12 | Quantitative Dietary Questionnaire; Naples, Validated SFFQ; Ragusa, Dietary Interview | Total sugars 65–137 | Medical record linkage | Agency |
| Sluijs et al., 201331 | EPIC-InterAct Study | Spain | 5889 | 2564 | 40–65 (M), 35–65 (F) | 12 | Quantitative Dietary Questionnaire, Dietary Interview | Total sugars 65–137 | Medical record linkage | Agency |
| Sluijs et al., 201331 | EPIC-InterAct Study | Sweden | 5401 | 2622 | 30–72 | 12 | Validated SFFQ, 14-d record | Total sugars 65–137 | Medical record linkage | Agency |
| Ahmadi- Abhari et al., 201432 | EPIC-Norfolk Study | England | 4153 | 753 | 40–79 | 6.3 | Validated SFFQ, 7-d food diary | Sucrose 25–76.5, fructose 8–32, total sugars 71.5–149.5 | Medical record linkage | Agency |
Note: F = females, FFQ = Food-Frequency Questionnaire, HR = hazard ratio, IQR = interquartile range, M = males, NA = not available, SD = standard deviation, SFFQ = Semiquantitative Food-Frequency Questionnaire.
Durations reported as mean ± SD, median (IQR) or as a range.
Sugars exposure reported as median (IQR) or as a range.
Agency funding is that from government, university or not-for-profit health agency sources.
Median intakes for total sugars, fructose and sucrose were 65 g/d (IQR 25.8–100 g/d), 9.7 g/d (IQR 6–25.8 g/d) and 25.8 g/d (IQR 22.5–28.5 g/d), respectively, in the lowest quantile of intake. In the highest quantile of intake, median intakes for total sugars, fructose and sucrose were 137 g/d (IQR 57.2–194.4 g/d), 35.2 g/d (IQR 28.8–57.2 g/d) and 78 g/d (IQR 57.2–102 g/d), respectively. Dietary intake was assessed by food frequency questionnaires, semiquantitative food frequency questionnaires (47%), quantitative dietary questionnaires (20%) or mixed methods (33%). No studies differentiated between added sugars and naturally occurring sugars.
Funding sources did not include industry funding. Thirteen studies reported funding from agency alone, whereas the other 2 studies did not report funding sources.
Supplementary Table 2 (Appendix 1) shows the statistical adjustments performed in the included studies. All studies adjusted for the prespecified primary confounding variable (age) and adjusted for at least 4 of 5 secondary confounding variables (markers of overweight/obesity, family history of diabetes, energy intake, physical activity, sex).
Supplementary Table 3 (Appendix 1) shows the NOS scores for the included studies. Although several studies lost points for selection and outcome assessment, there was no evidence of serious risk of bias across the included studies.
Visual inspection of funnel plots (Supplementary Figure 18, Appendix 1), and formal testing with the Begg (p = 0.7) and Egger tests (p = 0.4) did not show evidence of publication bias for total sugars. Publication bias was not assessed for fructose and sucrose because there were less than 10 cohort comparisons.
Supplementary Table 4 (Appendix 1) shows a summary of the GRADE assessments for the association of total sugars, fructose and sucrose intake with incident type 2 diabetes. The evidence for a lack of harm was rated as very low quality for total sugars and fructose because of downgrades for serious inconsistency and imprecision, and low quality for sucrose because of a downgrade for serious imprecision and an upgrade for a significant inverse dose–response gradient.
Intake of sugars and type 2 diabetes
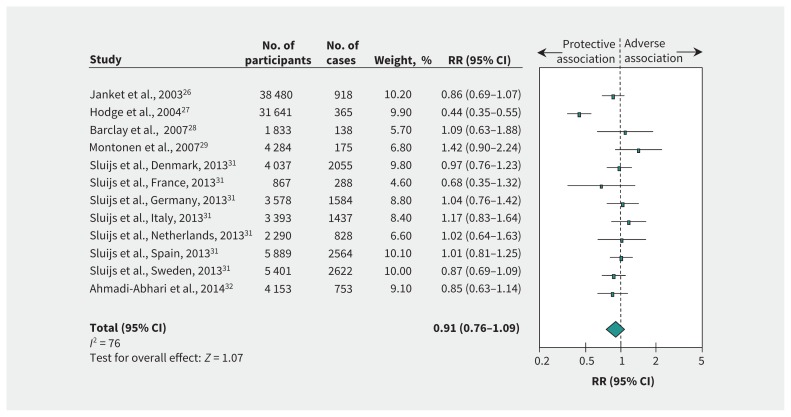
Figure 2 and supplementary Figure 1 (Appendix 1) show the relation between intake of total sugars and incident type 2 diabetes. There was no association (RR 0.91, 95% CI 0.76–1.09) with evidence of substantial heterogeneity (I2 = 76%, p < 0.001) when we compared the highest and the lowest levels of intake. Risk ratio per 100 g/d intake was 0.92 (95% CI 0.77–1.08), with evidence of substantial heterogeneity (I2 = 79%, p < 0.001).
Figure 2:
Relation between intake of total sugars and incident type 2 diabetes (highest v. lowest level of intake). Pooled risk estimate is represented by the blue diamond. Values of I2 ≥ 50% indicate substantial heterogeneity.6,16 Values greater than 1.0 indicate an adverse association. CI = confidence interval, RR = risk ratio.
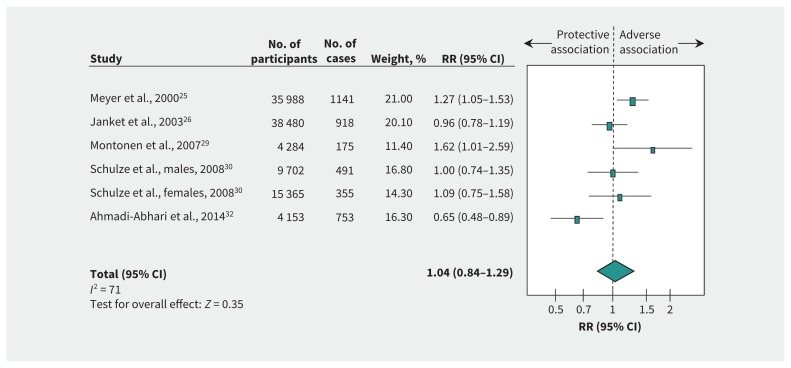
Figure 3 and supplementary Figure 2 (Appendix 1) show the relation between fructose intake and incident type 2 diabetes. We found no association (RR 1.04, 95% CI 0.84–1.29) with evidence of substantial heterogeneity among studies (I2 = 71%, p < 0.01) when we compared the highest and the lowest levels of intake. Risk ratio per 50 g/d intake was 1.09 (95% CI 0.73–1.63), with evidence of substantial heterogeneity (I2 = 75%, p < 0.01).
Figure 3:
Relation between intake of fructose and incident type 2 diabetes (highest v. lowest level of intake). Pooled risk estimate is represented by the blue diamond. Values of I2 ≥ 50% indicate substantial heterogeneity.6,16 Values greater than 1.0 indicate an adverse association. CI = confidence interval, RR = risk ratio.
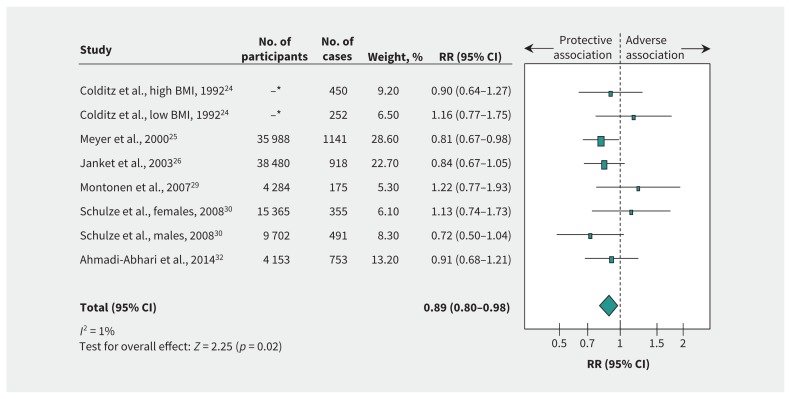
Figure 4 and supplementary Figure 3 (Appendix 1) show the relation between sucrose intake and incident type 2 diabetes. We found a significant protective association (RR 0.89, 95% CI 0.80–0.98) with no evidence of heterogeneity (I2 = 1%, p = 0.4) when we compared the highest and the lowest levels of intake. Risk ratio per 50 g/d intake was 0.91 (95% CI 0.83–1.00), with no evidence of heterogeneity (I2 = 12%, p = 0.34).
Figure 4:
Relation between intake of sucrose and incident type 2 diabetes (highest v. lowest level of intake). Pooled risk estimate is represented by the blue diamond. Values of I2 ≥ 50% indicate substantial heterogeneity.6,16 Values greater than 1.0 indicate an adverse association. BMI = body mass index, CI = confidence interval, RR = risk ratio. *For Colditz and colleagues,24 the total number of participants with high and low BMI was 84 360.
Additional analyses
The systematic removal of each study did not modify the lack of an association for total sugars or fructose. However, the removal of the study by Hodge and colleagues27 (I2 = 9%, p = 0.4) in the analysis of total sugars and the study by Ahmadi-Abhari and colleagues32 (I2 = 40%, p = 0.2) in the fructose analysis did explain away most of the evidence of heterogeneity. The inverse association for sucrose was lost through the systematic removal of the studies by Colditz and colleagues24 (high body mass index cohort comparison; RR 0.89, 95% CI 0.79–1.01), Meyer and colleagues25 (RR 0.92, 95% CI 0.81–1.04), Janket and colleagues26 (RR 0.91, 95% CI 0.80–1.03), Schulze and colleagues30 (cohort comparison of males; RR 0.90, 95% CI 0.81–1.01) or Ahmadi-Abhari and colleagues32 (RR 0.89, 95% CI 0.79–1.01). However, the recalculated 95% CI did not include evidence of clinically important harm.
Restricting analyses to studies in which sugars were assessed using validated measures neither modified the associations nor the evidence of heterogeneity for total sugars, fructose or sucrose (supplementary Figures 4–6, Appendix 1).
Supplementary Figures 7–12 (Appendix 1) provide the a priori subgroup analyses. There was no evidence of effect modification for the associations of total sugars, fructose and sucrose with incident type 2 diabetes in a priori subgroup analyses. Any evidence of significant interstudy heterogeneity was not explained by a priori subgroup analyses.
There was no evidence of a dose–response gradient for total sugars or fructose using GLST estimation (supplementary Figures 13 and 14, Appendix 1). No dose–response gradient or dose thresholds for fructose was seen using the MKSPLINE procedure (supplementary Figure 15, Appendix 1), whereas total sugars could not be modelled because of insufficient data. Results of GLST estimation for sucrose showed evidence of a significant inverse relationship with incident type 2 diabetes per 25 g/d intake (RR 0.92, 95% CI 0.85–0.99, p = 0.03) (supplementary Figure 16, Appendix 1), but this relation was not seen for results using the MKSPLINE procedure (supplementary Figure 17, Appendix 1).
Interpretation
We conducted a systematic review and meta-analysis of prospective cohort studies of the relation between intake of sugars and incident type 2 diabetes. Pooled analyses showed that intakes of total sugars and fructose were not associated with type 2 diabetes, whereas intake of sucrose was associated with an 11% decrease in type 2 diabetes.
Our results do not support a hypothesis that the positive association seen between sugar-sweetened beverages and diabetes is mediated by the fructose-containing sugars they contain. Systematic reviews and meta-analyses have shown that sugar-sweetened beverages are associated with an increase in the risk of type 2 diabetes.4,5,33 Our pooled analyses failed to show a similar increase despite the inclusion of data from mostly the same set of cohorts.
The lack of an adverse association is difficult to reconcile with the biological mechanisms and ecological observations linking fructose-containing sugars to type 2 diabetes.34–39 It is also difficult to reconcile with our earlier systematic review and meta-analysis showing a positive association between fructose intake and gout,9 given the emerging links between uric acid and diabetes.37 One possible explanation for the lack of agreement is residual confounding from reverse causality. People at high risk of type 2 diabetes may avoid sugars as a preventive strategy, which decreases the risk associated with intake of total sugars, fructose or sucrose. Another explanation may relate to the increased intake of healthier food sources of sugars other than sugar-sweetened beverages, which themselves show null or protective associations with type 2 diabetes.
Although sugar-sweetened beverages are sources of most fructose-containing sugars in Canadian and American diets, other sources contribute meaningfully to overall intake23,40 (e.g., grains and grain products, fruit and fruit products, and dairy and dairy products).23,40 Many of these other food sources, which tend to be sweetened with sucrose, have either shown no association (e.g., cakes and cookies,41 and sherbert42) or a protective association (e.g., whole-grain cereals, fruit, yogurt and even ice cream42–44) with type 2 diabetes (Appendix 2, available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.160706/-/DC1). An inverse dose–response gradient, similar to that for sucrose, has even been found for whole-grain cereals, fruit and yogurt.42–44 Taken together, lack of an adverse association between intakes of total sugars, fructose or sucrose and diabetes may reflect important contributions from these other food sources.
In the absence of a particular adverse association between fructose-containing sugars and incident diabetes, one must consider alternative explanations for the observed association between sugar-sweetened beverages and diabetes. One explanation may relate to uncompensated energy. Systematic reviews and meta-analyses of controlled feeding trials45–51 have shown that the adverse effect of sugars on cardiometabolic risk factors is mediated by excess energy, with a signal for harm largely restricted to comparisons in which sugars supplement background diets with excess energy. It is possible that the food form of sugar-sweetened beverages promotes the excess energy intake. Evidence from systematic reviews and meta-analyses of acute preload trials have shown that sugars in liquid form elicit a weaker satiety response and are less compensated by a decrease in energy intake at subsequent meals than sugars in solid form,52 a mechanism that might contribute to weight gain and type 2 diabetes.53–55 Another possibility is that sugar-sweetened beverages are a marker of an unhealthy lifestyle.56–59 High consumers of sugar-sweetened beverages consume more energy, take less physical activity and smoke more,60–62 all of which may be difficult to measure and adjust for in observational studies.63
Strengths and limitations
The strengths of our study are that we identified all available prospective cohorts through a systematic search strategy, performed quantitative syntheses and conducted an assessment of the quality and strength of the evidence by using the GRADE assessment.
Despite the inclusion of several large, high-quality cohorts, the inability to rule out residual confounding is a limitation inherent in all observational studies, and a reason that observational studies start at low quality by GRADE. Sources of residual confounding include reverse causality, the reliability of self-report intake64 and measurement of the exposure to sugars, measured and unmeasured confounders included in statistical models, and important collinearity effects from related dietary and lifestyle patterns. Another important limitation is inconsistency between studies. Although the evidence for heterogeneity was partially explained by the removal of several individual studies during sensitivity analyses, residual inconsistency could not be ruled out for total sugars and fructose. A final limitation is the imprecision in the estimates of pooled risk. The 95% CIs were wide and could not rule out clinically important benefit or harm for total sugars and fructose. In addition, there was some instability in the precision of the summary estimates for sucrose.
Balancing the strengths and weaknesses, the evidence was assessed as very low quality for total sugars and fructose, which was based on downgrades for inconsistency and imprecision, and low quality for sucrose, because of the combination of a downgrade for imprecision and an upgrade for an inverse dose–response gradient. In comparison, the evidence for the association between intake of sugar-sweetened beverages and type 2 diabetes4,5 would similarly be assessed by GRADE as low quality based on the combination of a downgrade for inconsistency and an upgrade for a positive dose–response gradient.
Conclusion
Our systematic review and meta-analysis of available prospective cohort studies does not support an adverse association between intake of fructose-containing sugars independent of food form and risk of type 2 diabetes. Our confidence in the evidence for this conclusion is generally weak. Sources of uncertainty include the risk of residual confounding in observational studies that prevent causal inferences from being drawn, serious inconsistency between studies and imprecision in estimates of pooled risk for total sugars and fructose, and serious imprecision in estimates of pooled risk for sucrose. Although our observation of a negative dose–response gradient between sucrose and incident diabetes might strengthen our confidence in the lack of harm associated with sucrose, more research is likely to have an important effect on our estimates. In the absence of a clear signal for harm, sugars alone do not appear to explain the relation between sugar-sweetened beverages and type 2 diabetes. More “food-based” research is needed to assess whether the same relation holds for other important food sources of sugars, such as grain and grain-based products, fruit and fruit products, and dairy and dairy products.
Acknowledgements
The authors thank Teruko Kishibe (information specialist with Scotiabank Health Sciences Library, St. Michael’s Hospital) for help with the development of the search terms used. Aspects of this work were presented at the Advances in Carbohydrates and Fibre in Nutrition Conference, Canadian Nutrition Society, 2014 Jan. 11, Toronto, Ont.; and the 32nd International Symposium on Diabetes and Nutrition, Diabetes and Nutrition Study Group of the European Association of the Study of Diabetes, 2014 June 25–27, Reykjavik, Iceland.
Footnotes
This article has been peer reviewed.
Contributors: All of the authors had full access to the data for this study and take full responsibility for the integrity of the data and the accuracy of the data analysis. Russell de Souza, Arash Mirrahimi, Lawrence Leiter, Joseph Beyene, Cyril Kendall, David Jenkins and John Sievenpiper conceived and designed the study. Christine Tsilas, Russell de Souza, Sonia Blanco Mejia, Arash Mirrahimi, Viranda Jayalath, Adrian Cozma, Vanessa Ha, Reem Tawfik, Marco Di Buono, Lawrence Leiter, Joseph Beyene, Tauseef Khan, Cyril Kendall, David Jenkins and John Sievenpiper analyzed and interpreted the data. Christine Tsilas, Sonia Blanco Mejia and John Sieven-piper drafted the article. All of the authors revised the article critically for important intellectual content, gave final approval of the version to be published and agreed to be accountable for all aspects of the work.
Competing interests: Russell de Souza has served as an external resource person to the World Health Organization (WHO) Nutrition Guidance Expert Advisory Group (NUGAG) Subgroup on Diet and Health (guidelines for trans fats and saturated fats), and received renumeration from WHO for travel and accommodation. He also received compensation for contract research conducted for the Institute of Nutrition, Metabolism, and Diabetes at the Canadian Institutes of Health Research (CIHR), Health Canada and WHO. He has received research grants from the Canadian Foundation for Dietetic Research and CIHR, and lecture fees from McMaster Children’s Hospital. Vanessa Ha has received research support from WHO. She has received a travel award and doctoral scholarship from CIHR. Alexandra Jenkins is part owner, Vice-President and Director of Research for Glycemic Index Laboratories, Toronto, Ont. She has received research support from the Canadian Diabetes Association (CDA). Thomas Wolever is part owner and President of Glycemic Index Laboratories. Cyril Kendall has received research support from the Advanced Foods and Materials Network, Agricultural Bioproducts Innovation Program through the Pulse Research Network, Agriculture and Agri-Food Canada, Almond Board of California, Barilla, Calorie Control Council, CIHR, Canola Council of Canada, The Coca-Cola Company, The International Tree Nut Council Nutrition Research & Education Foundation, Kellogg, Loblaw Companies Ltd., Pulse Canada, Saskatchewan Pulse Growers and Unilever. He has received consultant fees from American Pistachio Growers; speaker fees from American Peanut Council, Tate & Lyle and The WhiteWave Foods Company; and travel funding from Sabra Dipping Company, Tate & Lyle, International Tree Nut Council Research & Education Foundation, California Walnut Commission, Sun-Maid, The Peanut Institute, General Mills, Oldways Foundation and International Nut and Dried Fruit Council Foundation. He is a member of the Clinical Practice Guidelines Expert Committee for Nutrition Therapy of the European Association for the Study of Diabetes (EASD), the Diabetes and Nutrition Study Group of the EASD and the International Carbohydrate Quality Consortium, and is the Director for the Toronto 3D Knowledge Synthesis and Clinical Trials Foundation. David Jenkins has received research grants from Saskatchewan Pulse Growers, Agricultural Bioproducts Innovation Program through the Pulse Research Network, Advanced Foods and Materials Network, Loblaw Companies Ltd., Unilever, Barilla, Almond Board of California, Agriculture and Agri-Food Canada, Pulse Canada, Kellogg’s Canada, Quaker Oats Canada, Proctor & Gamble Technical Centres, Bayer Consumer Care (Springfield, New Jersey), PepsiCo/Quaker, International Nut and Dried Fruit Council Foundation, Soyfoods Association of North America, The Coca-Cola Company (investigator initiated, unrestricted grant), Solae, Hain Celestial, Sanitarium Company, Orafti, International Tree Nut Council Nutrition Research & Education Foundation, The Peanut Institute, Canola Council of Canada, Flax Council of Canada, Calorie Control Council, CIHR, Canada Foundation for Innovation (CFI) and Ontario Research Fund. He has been on the speaker’s panel, served on the scientific advisory board, and/or received travel support and/or honoraria from Almond Board of California, Canadian Agriculture Policy Institute, Loblaw Companies Ltd., Griffin Hospital (for the development of the NuVal scoring system), The Coca-Cola Company, EPICURE, Danone, Diet Quality Photo Navigation, Saskatchewan Pulse Growers, Sanitarium Company, Orafti, American Peanut Council, The International Tree Nut Council Nutrition Research & Education Foundation, The Peanut Institute, Herbalife International, Pacific Health Laboratories, Nutritional Fundamentals for Health, Barilla, Metagenics, Bayer Consumer Care, Unilever Canada and Netherlands, Solae, Kellogg’s, Quaker Oats, Procter & Gamble, Abbott Laboratories, Canola Council of Canada, Dean Foods, California Strawberry Commission, Hain Celestial, PepsiCo, Alpro Foundation, Pioneer Hi-Bred International, DuPont Nutrition and Health, Spherix Consulting, The WhiteWave Foods Company, Advanced Foods and Materials Network, Flax Council of Canada, Nutritional Fundamentals for Health, Agriculture and Agri-Food Canada, Canadian Agri-Food Policy Institute, Pulse Canada, Soyfoods Association of North America, Nutrition Foundation of Italy (NFI), Nutrasource Diagnostics, McDougall Program, Toronto Knowledge Translation Working Group (St. Michael’s Hospital), Canadian College of Naturopathic Medicine, The Hospital for Sick Children, Canadian Nutrition Society (CNS), American Society for Nutrition (ASN), Arizona State University, Paolo Sorbini Foundation and Institute of Nutrition, Metabolism and Diabetes. He received an honorarium from the US Department of Agriculture to present the 2013 W.O. Atwater Memorial Lecture. He received the 2013 Award for Excellence in Research from the International Nut and Dried Fruit Council Foundation. He received funding and travel support from The Canadian Society of Endocrinology and Metabolism to produce minicases for the CDA. His spouse (Alexandra Jenkins) is a director and partner of Glycemic Index Laboratories, and his sister (Caroline Brydson) received funding through a grant from the St. Michael’s Hospital Foundation to develop a cookbook for one of his studies. John Sievenpiper has received research support from CIHR, CDA, PSI Foundation, Banting & Best Diabetes Centre, CNS, ASN, Calorie Control Council, INC International Nut and Dried Fruit Council Foundation, National Dried Fruit Trade Association, The Coca-Cola Company (investigator initiated, unrestricted), Dr Pepper Snapple Group (investigator initiated, unrestricted), The Tate and Lyle Nutritional Research Fund at the University of Toronto, and The Glycemic Control and Cardiovascular Disease in Type 2 Diabetes Fund at the University of Toronto. He has received reimbursement of travel expenses, speaker fees and/or honoraria from CDA, CNS, University of Alabama at Birmingham, Oldways Preservaton Trust, NFI, Diabetes and Nutrition Study Group (DNSG) of the of the European Association for the Study of Diabetes (EASD), International Life Sciences Institute North America, Dr Pepper Snapple Group, Corn Refiners Association, World Sugar Research Organization, Dairy Farmers of Canada, Società Italiana di Nutrizione Umana (SINU), III World Congress of Public Health Nutrition, C3 Collaborating for Health, Sprim Brasil, The WhiteWave Foods Company, Rippe Lifestyle Institute, mdBriefCase Group, Federation of European Nutrition Societies (FENS), New York Academy of Sciences, International Diabetes Federation, American Heart Association (AHA), ASN, FoodMinds LLC, Memac Ogilvy & Mather LLC, Pulse Canada, PepsiCo, BCFN Foundation, The Ginger Network and Dietitians of Canada. He has ad hoc consulting arrangements with Winston & Strawn LLP, Perkins Coie LLP and Tate & Lyle. He is a member of the European Fruit Juice Association Scientific Expert Panel. He is on the Clinical Practice Guidelines Expert Committees of the CDA, EASD and Canadian Cardiovascular Society, as well as an expert writing panel of the ASN. He serves as an unpaid scientific advisor for the Food, Nutrition, and Safety Program, and the Technical Committee on Carbohydrates of the International Life Sciences Institute North America. He is a member of the International Carbohydrate Quality Consortium, Executive Board Member of the DNSG of the EASD, and Director of the Toronto 3D Knowledge Synthesis and Clinical Trials foundation. His spouse is an employee of Unilever Canada. Arash Mirrahimi has received a research grant from CIHR. No other competing interests were declared.
Funding: This work was funded by the Canadian Institutes of Health Research (CIHR, funding reference no. 129920) through the Canada-wide Human Nutrition Trialists’ Network and an unrestricted grant from the Calorie Control Council. The Diet, Digestive tract, and Disease (3D), funded through the Canada Foundation for Innovation (CFI) and the Ministry of Research and Innovation’s Ontario Research Fund (ORF), provided the infrastructure for the conduct of this project. Vanessa Ha was supported by an Ontario Graduate Scholarship. Adrian Cozma was funded by an Ontario Graduate Scholarship and by a CIHR–Frederick Banting and Charles Best Canada Graduate Scholarship, and Banting & Best Diabetes Centre–Novo Nordisk Studentship. David Jenkins was supported by the Government of Canada through the Canada Research Chair Endowment. John Sieven-piper was supported by a PSI Graham Farquharson Knowledge Translation Fellowship, Canadian Diabetes Association Clinician Scientist Award, CIHR INMD/CNS–New Investigator Partnership Prize, and Banting & Best Diabetes Centre – Sun Life Financial New Investigator Award. None of the sponsors had a role in any aspect of the present study, including design and conduct of the study; collection, management, analysis and interpretation of the data; and preparation, review, approval of the manuscript or decision to publish.
Data sharing: The data sets supporting the conclusions of this article are included within the article and its additional files.
References
- 1.Lustig RH, Schmidt LA, Brindis CD. Public health: the toxic truth about sugar. Nature 2012;482:27–9. [DOI] [PubMed] [Google Scholar]
- 2.Bray GA. Fructose: pure, white, and deadly? Fructose, by any other name, is a health hazard. J Diabetes Sci Technol 2010;4:1003–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marriott BP, Olsho L, Hadden L, et al. Intake of added sugars and selected nutrients in the United States, National Health and Nutrition Examination Survey (NHANES) 2003–2006. Crit Rev Food Sci Nutr 2010;50:228–58. [DOI] [PubMed] [Google Scholar]
- 4.Malik VS, Popkin BM, Bray GA, et al. Sugar-sweetened beverages and risk of metabolic syndrome and type 2 diabetes: a meta-analysis. Diabetes Care 2010; 33:2477–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Imamura F, O’Connor L, Ye Z, et al. Consumption of sugar sweetened beverages, artificially sweetened beverages, and fruit juice and incidence of type 2 diabetes: systematic review, meta-analysis, and estimation of population attributable fraction. BMJ 2015;351:h3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Higgins JPT, Green S, eds. Cochrane handbook for systematic reviews of interventions. Version 5.1.0. Oxford: Cochrane Collaboration; 2011. Available: www.handbook.cochrane.org (accessed 2014 Sept. 25). [Google Scholar]
- 7.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008–12. [DOI] [PubMed] [Google Scholar]
- 8.Wells GA, Shea B, O’Connell D, et al. The Newcastle–Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa: Ottawa Hospital Research Institute; 2014. Available: www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed 2017 Mar. 30). [Google Scholar]
- 9.Jamnik J, Rehman S, Blanco Mejia S, et al. Fructose intake and risk of gout and hyperuricemia: a systematic review and meta-analysis of prospective cohort studies. BMJ Open 2016;6:e013191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 2011; 64:383–94. [DOI] [PubMed] [Google Scholar]
- 11.Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines: 2. Framing the question and deciding on important outcomes. J Clin Epidemiol 2011;64:395–400. [DOI] [PubMed] [Google Scholar]
- 12.Balshem H, Helfand M, Schünemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol 2011;64:401–6. [DOI] [PubMed] [Google Scholar]
- 13.Guyatt GH, Oxman AD, Vist G, et al. GRADE guidelines: 4. Rating the quality of evidence — study limitations (risk of bias). J Clin Epidemiol 2011;64:407–15. [DOI] [PubMed] [Google Scholar]
- 14.Guyatt GH, Oxman AD, Montori V, et al. GRADE guidelines: 5. Rating the quality of evidence — publication bias. J Clin Epidemiol 2011;64:1277–82. [DOI] [PubMed] [Google Scholar]
- 15.Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines 6. Rating the quality of evidence — imprecision. J Clin Epidemiol 2011;64:1283–93. [DOI] [PubMed] [Google Scholar]
- 16.Guyatt GH, Oxman AD, Kunz R, et al. GRADE Working Group. GRADE guidelines: 7. Rating the quality of evidence — inconsistency. J Clin Epidemiol 2011; 64:1294–302. [DOI] [PubMed] [Google Scholar]
- 17.Guyatt GH, Oxman AD, Kunz R, et al. GRADE Working Group. GRADE guidelines: 8. Rating the quality of evidence — indirectness. J Clin Epidemiol 2011;64: 1303–10. [DOI] [PubMed] [Google Scholar]
- 18.Guyatt GH, Oxman AD, Sultan S, et al. GRADE Working Group. GRADE guidelines: 9. Rating up the quality of evidence. J Clin Epidemiol 2011;64:1311–6. [DOI] [PubMed] [Google Scholar]
- 19.Brunetti M, Shemilt I, Pregno S, et al. GRADE guidelines: 10. Considering resource use and rating the quality of economic evidence. J Clin Epidemiol 2013;66:140–50. [DOI] [PubMed] [Google Scholar]
- 20.Guyatt G, Oxman AD, Sultan S, et al. GRADE guidelines: 11. Making an overall rating of confidence in effect estimates for a single outcome and for all outcomes. J Clin Epidemiol 2013;66:151–7. [DOI] [PubMed] [Google Scholar]
- 21.Guyatt GH, Oxman AD, Santesso N, et al. GRADE guidelines: 12. Preparing summary of findings tables-binary outcomes. J Clin Epidemiol 2013;66:158–72. [DOI] [PubMed] [Google Scholar]
- 22.Guyatt GH, Thorlund K, Oxman AD, et al. GRADE guidelines: 13. Preparing summary of findings tables and evidence profiles-continuous outcomes. J Clin Epidemiol 2013;66:173–83. [DOI] [PubMed] [Google Scholar]
- 23.Brisbois TD, Marsden SL, Anderson GH, et al. Estimated intakes and sources of total and added sugars in the Canadian diet. Nutrients 2014;6:1899–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Colditz GA, Manson JE, Stampfer MJ, et al. Diet and risk of clinical diabetes in women. Am J Clin Nutr 1992;55:1018–23. [DOI] [PubMed] [Google Scholar]
- 25.Meyer KA, Kushi LH, Jacobs DR, Jr, et al. Carbohydrates, dietary fiber, and incident type 2 diabetes in older women. Am J Clin Nutr 2000;71:921–30. [DOI] [PubMed] [Google Scholar]
- 26.Janket SJ, Manson JE, Sesso H, et al. A prospective study of sugar intake and risk of type 2 diabetes in women. Diabetes Care 2003;26:1008–15. [DOI] [PubMed] [Google Scholar]
- 27.Hodge AM, English DR, O’Dea K, et al. Glycemic index and dietary fiber and the risk of type 2 diabetes. Diabetes Care 2004;27:2701–6. [DOI] [PubMed] [Google Scholar]
- 28.Barclay AW, Flood VM, Rochtchina E, et al. Glycemic index, dietary fiber, and risk of type 2 diabetes in a cohort of older Australians. Diabetes Care 2007; 30:2811–3. [DOI] [PubMed] [Google Scholar]
- 29.Montonen J, Jarvinen R, Knekt P, et al. Consumption of sweetened beverages and intakes of fructose and glucose predict type 2 diabetes occurrence. J Nutr 2007;137:1447–54. [DOI] [PubMed] [Google Scholar]
- 30.Schulze MB, Schulz M, Heidemann C, et al. Carbohydrate intake and incidence of type 2 diabetes in the European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Br J Nutr 2008;99:1107–16. [DOI] [PubMed] [Google Scholar]
- 31.Sluijs I, Beulens JW, van der Schouw YT, et al. InterAct consortium. Dietary glycemic index, glycemic load, and digestible carbohydrate intake are not associated with risk of type 2 diabetes in eight European countries. J Nutr 2013;143:93–9. [DOI] [PubMed] [Google Scholar]
- 32.Ahmadi-Abhari S, Luben RN, Powell N, et al. Dietary intake of carbohydrates and risk of type 2 diabetes: the European Prospective Investigation into Cancer–Norfolk study. Br J Nutr 2014;111:342–52. [DOI] [PubMed] [Google Scholar]
- 33.Xi B, Li S, Liu Z, et al. Intake of fruit juice and incidence of type 2 diabetes: a systematic review and meta-analysis. PLoS One 2014;9:e93471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Basu S, Yoffe P, Hills N, et al. The relationship of sugar to population-level diabetes prevalence: an econometric analysis of repeated cross-sectional data. PLoS One 2013;8:e57873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Elliott SS, Keim NL, Stern JS, et al. Fructose, weight gain, and the insulin resistance syndrome. Am J Clin Nutr 2002;76:911–22. [DOI] [PubMed] [Google Scholar]
- 36.Tappy L, Le KA. Metabolic effects of fructose and the worldwide increase in obesity. Physiol Rev 2010;90:23–46. [DOI] [PubMed] [Google Scholar]
- 37.Johnson RJ, Nakagawa T, Sanchez-Lozada LG, et al. Sugar, uric acid, and the etiology of diabetes and obesity. Diabetes 2013;62:3307–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Teff KL, Elliott SS, Tschöp M, et al. Dietary fructose reduces circulating insulin and leptin, attenuates postprandial suppression of ghrelin, and increases tri-glycerides in women. J Clin Endocrinol Metab 2004;89:2963–72. [DOI] [PubMed] [Google Scholar]
- 39.Teff KL, Grudziak J, Townsend RR, et al. Endocrine and metabolic effects of consuming fructose- and glucose-sweetened beverages with meals in obese men and women: influence of insulin resistance on plasma triglyceride responses. J Clin Endocrinol Metab 2009;94:1562–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marriott BP, Cole N, Lee E. National estimates of dietary fructose intake increased from 1977 to 2004 in the United States. J Nutr 2009;139:1228S–35S. [DOI] [PubMed] [Google Scholar]
- 41.Buijsse B, Boeing H, Drogan D, et al. InterAct Consortium. Consumption of fatty foods and incident type 2 diabetes in populations from eight European countries. Eur J Clin Nutr 2015;69:455–61. [DOI] [PubMed] [Google Scholar]
- 42.Aune D, Norat T, Romundstad P, et al. Dairy products and the risk of type 2 diabetes: a systematic review and dose-response meta-analysis of cohort studies. Am J Clin Nutr 2013;98:1066–83. [DOI] [PubMed] [Google Scholar]
- 43.Aune D, Norat T, Romundstad P, et al. Whole grain and refined grain consumption and the risk of type 2 diabetes: a systematic review and dose-response meta-analysis of cohort studies. Eur J Epidemiol 2013;28:845–58. [DOI] [PubMed] [Google Scholar]
- 44.Li M, Fan Y, Zhang X, et al. Fruit and vegetable intake and risk of type 2 diabetes mellitus: meta-analysis of prospective cohort studies. BMJ Open 2014;4:e005497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Te Morenga L, Mallard S, Mann J. Dietary sugars and body weight: systematic review and meta-analyses of randomised controlled trials and cohort studies. BMJ 2012;346:e7492. [DOI] [PubMed] [Google Scholar]
- 46.Kaiser KA, Shikany JM, Keating KD, et al. Will reducing sugar-sweetened beverage consumption reduce obesity? Evidence supporting conjecture is strong, but evidence when testing effect is weak. Obes Rev 2013;14:620–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Malik VS, Pan A, Willett WC, et al. Sugar-sweetened beverages and weight gain in children and adults: a systematic review and meta-analysis. Am J Clin Nutr 2013; 98:1084–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sievenpiper JL, de Souza RJ, Mirrahimi A, et al. Effect of fructose on body weight in controlled feeding trials: a systematic review and meta-analysis. Ann Intern Med 2012;156:291–304. [DOI] [PubMed] [Google Scholar]
- 49.Wang DD, Sievenpiper JL, de Souza RJ, et al. Effect of fructose on postprandial triglycerides: a systematic review and meta-analysis of controlled feeding trials. Atherosclerosis 2014;232:125–33. [DOI] [PubMed] [Google Scholar]
- 50.Chiu S, Sievenpiper JL, de Souza RJ, et al. Effect of fructose on markers of non-alcoholic fatty liver disease (NAFLD): a systematic review and meta-analysis of controlled feeding trials. Eur J Clin Nutr 2014;68:416–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chiavaroli L, de Souza RJ, Ha V, et al. Effect of fructose on established lipid targets: a systematic review and meta-analysis of controlled feeding trials. J Am Heart Assoc 2015;4:e001700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Almiron-Roig E, Palla L, Guest K, et al. Factors that determine energy compensation: a systematic review of preload studies. Nutr Rev 2013;71:458–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.De Castro JM. The effects of the spontaneous ingestion of particular foods or beverages on the meal pattern and overall nutrient intake of humans. Physiol Behav 1993;53:1133–44. [DOI] [PubMed] [Google Scholar]
- 54.DiMeglio DP, Mattes RD. Liquid versus solid carbohydrate: effects on food intake and body weight. Int J Obes Relat Metab Disord 2000;24:794–800. [DOI] [PubMed] [Google Scholar]
- 55.Rolls BJ, Kim S, Fedoroff IC. Effects of drinks sweetened with sucrose or aspartame on hunger, thirst and food intake in men. Physiol Behav 1990;48:19–26. [DOI] [PubMed] [Google Scholar]
- 56.van Dam RM, Rimm EB, Willett WC, et al. Dietary patterns and risk for type 2 diabetes mellitus in U.S. men. Ann Intern Med 2002;136:201–9. [DOI] [PubMed] [Google Scholar]
- 57.Fung TT, Schulze M, Manson JE, et al. Dietary patterns, meat intake, and the risk of type 2 diabetes in women. Arch Intern Med 2004;164:2235–40. [DOI] [PubMed] [Google Scholar]
- 58.Montonen J, Knekt P, Härkänen T, et al. Dietary patterns and the incidence of type 2 diabetes. Am J Epidemiol 2005;161:219–27. [DOI] [PubMed] [Google Scholar]
- 59.Schulze MB, Hoffmann K, Manson JE, et al. Dietary pattern, inflammation, and incidence of type 2 diabetes in women. Am J Clin Nutr 2005;82:675–84, quiz 714–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cohen L, Curhan G, Forman J. Association of sweetened beverage intake with incident hypertension. J Gen Intern Med 2012;27:1127–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.de Koning L, Malik VS, Kellogg MD, et al. Sweetened beverage consumption, incident coronary heart disease, and biomarkers of risk in men. Circulation 2012;125:1735–41, S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ambrosini GL, Oddy WH, Huang RC, et al. Prospective associations between sugar-sweetened beverage intakes and cardiometabolic risk factors in adolescents. Am J Clin Nutr 2013;98:327–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sievenpiper JL, de Souza RJ. Are sugar-sweetened beverages the whole story? Am J Clin Nutr 2013;98:261–3. [DOI] [PubMed] [Google Scholar]
- 64.Dhurandhar NV, Schoeller D, Brown AW, et al. Energy Balance Measurement Working Group. Energy balance measurement: when something is not better than nothing. Int J Obes (Lond) 2015;39:1109–13. [DOI] [PMC free article] [PubMed] [Google Scholar]