Abstract
Unimpaired hand function is critical in almost all activities of daily living. Burn injury can result in hypertrophic scar formation that can lead to debilitating functional deficits and poor aesthetic outcomes. Initial algorithms of acute burn management involve early debridement and skin grafting and early mobilization to prevent formation of hypertrophic scarring and ultimately digit contractures. While non-operative modalities in the early phase of scar maturation are critical to minimize hypertrophic scar formation, surgical management is often indicated in order to restore hand function. The essential tenant of operative scar management is release of tension, which can often be achieved through local tissue rearrangement. Laser therapy has emerged as a central pillar of subsequent scar rehabilitation with several modalities that address scar texture, color, pruritis and thickness. These can be utilized in conjunction with local corticosteroid treatment and other emerging modalities to modulate the scar and achieve optimal hand function. These treatment tools provide an effective resource for the reconstructive surgeon to treat hypertrophic hand scars.
Keywords: Hand, burn, hypertrophic scar, laser
Introduction
According to the Burn Association Repository, approximately 500,000 burn victims seek medical treatment every year with 39% of these injuries involving the upper extremity and hand as observed in previous studies [1–3]. Although the hand comprises only 3–5% of the body surface area, it is highly susceptible to injury, both through its close proximity to the thermal source but also because it is commonly used as a shield to protect other parts of the body [4]. The hand is therefore prone to absorb a high amount of energy, which may result in severe injury. Injury patterns can vary between different burn etiologies and can have significant impact on the expected severity and management. While injuries from flame/fire are the predominant etiology during recreational and work-related activities in adults, scald burns and contact burns account for the majority of pediatric hand burn injuries [5]. Electrical injuries also pose a challenge in hand reconstruction, as these patients can require fasciotomies, which heal with hypertrophic scars [6].
Acute management of hand burns should be performed at specialized burn centers that are equipped to provide a multidisciplinary team approach.
Aggressive early treatment of hand burns is critical and involves a combination of debridement, autografting, edema prophylaxis, early mobilization, splinting and optimal hand rehabilitation [7, 8]. A thorough initial assessment of the type of burn mechanism and burn depth should be accomplished in order to guide the need for surgical therapy. One should also be mindful that the acute burn wound is a dynamic environment and may be influenced by factors including edema, local and systemic inflammation and bacterial contamination which can contribute to conversion of the burn depth [9]. While epidermal burns will likely go on to heal without scar formation, a delay in re-epithelialization of partial thickness burns beyond 2–3 weeks will result in hypertrophic scarring. Deep partial thickness as well as full-thickness burns are therefore best treated with early excision to viable depth and skin grafting in order to preserve hand function [10]. In general, the palmar skin of the hand should be allowed more time to heal by secondary intention as the properties of the glaborous skin are difficult to replace with a skin graft. Early grafting of the palm will place thin skin in an area that requires durability and can lead to severe contractures (Figure 1). While thin spilt-thickness grafts are sufficient to resurface other parts of the body, the hand is best treated with thick unmeshed autografts (0.012–0.018 inch thickness) or full thickness grafts in order to prevent secondary contracture and to optimize the final appearance. In general we use thick split thickness grafts in the acute period and full thickness grafts with subsequent contracture releases. Surgical management should be followed by hand splinting in anti-contracture positioning, commonly intrinsic plus, and elevation followed by early range of motion when the skin graft has appropriately healed [11].
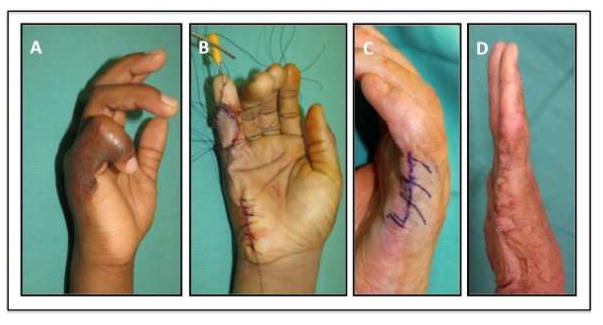
Figure 1.
58 year-old male with history of deep partial thickness burns to the right hand from natural gas explosion. (A) Initial presentation with scar band contractures involving the volar surfaces of the index, middle, ring and small fingers as well as wide scar contracture over the ulnar border of thenar eminence. (B) 2 weeks following z-plasty releases of digital contractures and full-thickness skin grafting from abdomen to palmar contracture. (C) 4 months following surgery.
Despite aggressive medical and surgical management in the acute phase of the burn injury, hypertrophic scar formation and contractures are complications resulting in substantial functional and aesthetic impairment. This has the potential to not only limit quality of life but can also impact the ability to perform a profession in which unimpaired hand function is required [12, 13]. Therefore, adequate management of hand burn hypertrophic scars and contractures is paramount in the rehabilitation of the burned hand. This will oftentimes present the surgeon with a challenging task. Here we aim to review common approaches to the prevention and management of hypertrophic scars and contractures of the burned hand.
Development of hypertrophic scarring in the burn wound
Several risk factors for hypertrophic scar formation have been identified and include young age, infection, skin stretch and anatomic location (i.e. axilla, neck) [14]. While superficial burn wounds tend to heal without complications, deeper partial and full thickness burns have a significantly increased risk to result in hypertrophic scar formation [1]. In epidermal burns, the dermis remains entirely intact and reepithelization occurs from preserved keratinocytes within the superficial dermis. Similarly, superficial partial thickness burns involve the epidermis and superficial dermis leading to blistering with complete regeneration occurring though migration of keratinocytes from preserved hair follicles and sweat glands. These superficial injuries may require careful monitoring alone. In contrast, in deep partial-thickness burns the density of skin adnexae is significantly decreased leading to prolonged time to reepithelization and scarring [10]. Early assessment of burn depth is therefore critical to administer optimal treatment and prevent hypertrophic scar formation.
Normal wound healing proceeds through a complex balance of the three phases of inflammation, proliferation and remodeling. The principles underlying burn wound healing are unique secondary to a strong systemic immune response with increased production of pro-inflammatory cytokines (TNF-a, IL-1, IL-6) and cell mobilization, which have a lasting impact on the local wound healing environment [15, 16]. Furthermore, increased and persistent contractile forces across a large wound area trigger a cascade during which mechanical forces are translated into increased inflammation [17]. A recent study has identified the focal adhesion kinase (FAK) as a critical regulator of this process, which has been shown to sense mechanical forces and activate inflammatory downstream effectors that drive increased fibrosis through deposition of aberrant extracellular matrix [18].
Hypertrophic scarring in the hand
While measures to counteract hypertrophic scar formation in the acute phase of wound healing is critical, late complications cannot always be avoided [19, 20]. The complex anatomy of the hand allows for an indispensable interaction with the environment through a balance of fine motor biomechanics and tactile sensation. Therefore, even small scar bands can result in significant functional impairment. The dorsum of the hand is especially susceptible to hypertrophic scar formation due to the thin and pliable nature of the skin in this area, which requires less thermal force to induce burn to the deeper dermal tissues and hypertrophic scarring. Dorsal scarring may not only inhibit passive flexion at the MCP joint but further result in hyperextension and subluxation of the joint in severe cases [21]. On the other hand, hypertrophic scar formation of the palm can ultimately result in a cupping deformity, which is characterized by convex warping of the longitudinal and horizontal palmar arches [19] (Figure 1).
Thermal injury involving opposing surfaces of border digits will often times result in scar band or web formation reducing the interdigital space and limiting abduction. Furthermore, if the first webspace is involved, thumb abduction is affected causing adduction contractures and significantly reducing grip strength and function.
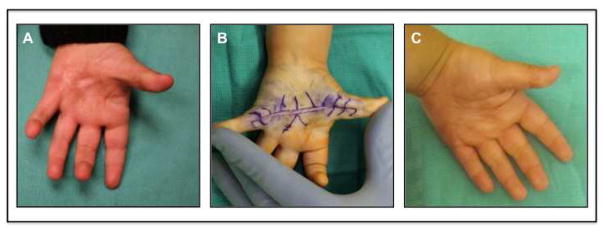
Flexion contracture of the small finger is one of the most difficult reconstructive challenges for surgeons as this leads to contracture of the collateral ligaments, flexor tendons and skin and results in shortening of the vessels and nerves (Figure 2). Additionally, these scars can lead to significant functional and aesthetic deformities with its most severe form of burn syndactyly [22]. The best treatment for these patients is prevention. Thus, aggressive range of motion and splinting should be employed in the acute period. If the flexion contracture continues to progress despite conservative measures, then temporary K-wire placement should be performed to counteract the contracture forces.
Figure 2.
6 year-old male with small finger contracture treated with hypothenar flap. (A) Pre operative photo with severe small finger contracture. (B) Intraoperative photograph showing hypothenar flap inset and digit fixation in extension with K-wire. (C) Intraoperative placement of series of z-plasties along hypertrophic scar band. (D) 1 month postoperative photograph with good range of motion.
Another common and stigmatic deformity of the burned hand includes the finger nail. Burn injury to the dorsum of the digit can result in retraction of the of the soft tissue proximal to the nail fold leading to exposure of the germinal matrix and alterations in nail growth [23].
Treatment of hypertrophic scars
Early scar management
During the initial phase of thermal injury the principles of acute management are directed towards reducing the extent of injury and minimizing or even preventing hypertrophic scar formation. Early surgical debridement and autografting when appropriate should be accomplished within the first 72 hours [8]. Debridement is performed with weck blades from 0.08–0.01 inch thickness to debride the necrotic tissue. Knowing the depth of debridement comes with experience and assessment of tissue moisture. Although pinpoint bleeding can be observed as an endpoint, the use of a tourniquet to minimize blood loss can require the surgeon to rely on tissue moisture. Grafts to the hand should be non-meshed sheet grafts when possible and should be harvested at a thickness of 0.012 inches or thicker (Figure 3). Meticulous hemostasis is needed, especially if sheet grafts are used. Dressings can be taken down after 48–72 hours and any hematomas under the sheet graft can be aspirated with an 18G needle. If large area burns occur that prevent autograft coverage, debridement and homograft should be performed as a temporary coverage until autograft is available.
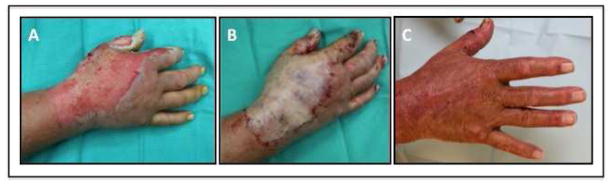
Figure 3.
Early debridement and sheet grafting to dorsal hand. (A) Preoperative photograph of burn injury. (B) Intraoperative placement of unmeshed thick STSG. (C) 2 month postoperative photopgraph with good range of motion.
The initial weeks after a burn injury are characterized by consistent remodeling of the affected tissue and several treatment modalities can be utilized to modulate hypertrophic scar formation in this critical period. In the early phase, the persistent presence of edema can prevent full range of motion in the finger joints and thus result in tightening of joint capsules as well as shortening of collateral ligaments leading to contractures. Hand elevation, as well as elastic edema wraps can be utilized to promote absorption of the edema fluid. Furthermore, early range of motion exercises in a patient who is able to participate once surgically appropriate, is imperative to prevent stiffening [24]. During the phase of operative recovery and in the unconscious patient, splinting of the hand in anti-deformity position during which the ligamentous structures are taut is critical in order to counteract the forces of wound bed contraction. For the hand, this can be commonly accomplished with the intrinsic plus position, which is characterized by wrist extension, MCP joint flexion and PIP/DIP joint flexion and thumb abduction [25, 26]. Two to three weeks after the operative phase, scar massage can be initiated, which has been shown to decrease pruritus and hypersensitivity associated with hypertrophic scarring in some studies [27], although a lasting effect on the scar height is controversial. While the scar continues to mature pressure therapy can be an effective therapeutic adjunct in reducing hypertrophic scar formation. Although high level evidence in the literature has been lacking, a recent meta-analysis concluded that pressure garments can result in a small decrease of hypertrophic scar height[28]. We typically begin compression therapy four weeks after grafting or once dressing changes are no longer necessary. In the recovering burn hand pressure on the palm, dorsum and fingers can be achieved with custom-made gloves. Furthermore, special inserts are oftentimes used in the interdigital space to reduce web space scarring.
Injectables
Intralesional injections of anti-inflammatory and antimitotic agents have been widely employed to treat and prevent the formation of hypertrophic scarring. Intralesional corticosteroids, most commonly triamcinolone, are a prominent therapy for hypertrophic scars and have been effective in improving pigmentation and texture of formed scars [29]. Treatment regimens, including dose, frequency, and duration of triamcinolone injections are variable and range from 10 to 40mg/ml every two to four weeks for a series of treatments until clinical improvement of the scar is observed. As a stand-alone treatment triamcinolone injection has demonstrated a response rate in 50–100% of patients, however initial success is tempered by high rates of recurrence and side-effects including hypo-pigmentation (more common in Fitzpatrick 3 and higher), ulceration, and itching at the site of administration [30]. As such, intralesional corticosteroid injections are often used in conjunction with other treatment modalities including surgery, and laser treatment to synergistically improve outcomes. Additionally, the surgeon must not inject too much corticosteroid at a single treatment (especially in children) to avoid adrenal insufficiency. Other injectable modalities include, anti-neoplastic agents such as 5- fluorouracil (5-FU) and Bleomycin, as well as antibiotics such as Mitomycin C and have been employed to similar effect [31]. Similar to triamcinolone, these anti-mitotic agents have been demonstrated to reduce angiogenesis and diminish the proliferation of fibroblasts in the wound bed. 5-FU is a pyrimidine analogue anti-metabolite, and was initially shown to reduce the proliferation of fibroblasts in culture [32]. Clinically, the drug has been used most often in combination with corticosteroids and other treatment modalities including pulsed-dye-laser therapy. Combination therapy demonstrates significant resolution of hypertrophic scarring, and furthermore, treatment with 5-FU mitigates the adverse effects induced by steroid injection alone.
Fat Grafting
Autologous fat grafting entails the harvest, enrichment, and injection of adipose tissue to improve scar resolution, fill contour deformity, and improve wound healing. The use of fat grafting has gained increasing favor in secondary burn reconstruction, and has been employed in multiple settings including relief of breast capsular contraction and treatment of post-radiation skin changes to improve overlying skin quality [33, 34]. The frequency of treatment required to achieve an optimal response remains uncertain, and many of the studies conducted to assess the utility of lipofilling remain limited by small sample sizes and subjective metrics. Nevertheless, several reports indicate improved scar outcomes following fat grafting. In hand reconstruction, Byrne et. al., demonstrated significant improvement of active motion, regression of scar tissue, and self-reported satisfaction of surgical outcome in patients having undergone a single fat grafting procedure [35]. Fat grafting in burn regions can be challenging as there is a constricted tissue envelope to allow for injection of the fat into multiple layers. Resolution of scar tissue following fat grafting remains incompletely understood, but the responsible mechanisms most likely include a multitude of biochemical and mechanical factors. Further studies are needed to validate the proposed mechanisms of improvement. Proponents claim that lipofilling replaces subdermal tissue, releasing the debilitating intrinsic and extrinsic tension caused by scar tissue. Caution not to inject excess adipose tissue must be taken to avoid a distorted appearance.
Laser therapy
Recent advances in laser technology have paved the way for new techniques in hypertrophic scar management [16, 36]. Besides functional disability, hypertrophic burn scars can result in significant discomfort due to the restrictive nature of the scar, erythematous discoloration, pruritus, pain and abnormal appearance. Over the past decade, different modalities of laser therapy have become a versatile and integral part of scar management. Utilizing selective photothermolysis and fractional ablation to target hyperthrophic scar tissue provides a novel method to modulate the composition of the scar and may even result in reduction of scar restriction and contracture release [37]. Overall, laser therapy is associated with a low complication risk profile [38]. Laser therapy is usually initiated around 6–12 months.
While the precise biologic mechanism accounting for the effect of scar improvement with laser treatment is a subject of ongoing investigation, its clinical effectiveness has been established in several clinical studies. A recently conducted prospective cohort study examining the effects of PDL and ablative carbon dioxide laser treatment on hypertrophic burn scars demonstrated significant improvement in scar texture, coloration, pain and pruritus while other clinical reports have successfully utilized laser therapy in the treatment of contractures in the adult and pediatric hand burn population [39].
Pulse Dye Laser
The pulse dye laser (PDL) has been shown to be particularly effective in the treatment of erythematous and pruritic scars, although some reports suggest an additional positive effect on scar pliability and height. It has a wavelength of 585 or 595 nm and has been widely utilized in the treatment of vascular malformations. The PDL laser demonstrates selective photothermolysis of microvessels within the hypertrophic scar and reduction of the inflammatory response. Thus PDL is especially helpful in erythematous scars. Its use in non-erythematous scars, however, is minimal and therefore its use in Fitzpatrick 5 and higher is limited. The side effect profile is limited and can include transient erythema and changes in pigmentation and therefore be used cautiously in patients with darker skin tone. Serial treatments, which are commonly spaced 2–3 months apart are oftentimes required to achieve a stable result (Figure 4). Settings vary based on different systems, however, we usually begin treatment with a spot size of 7 mm and a fluence of 12 Joule/cm2. If no blistering is seen, we will oftentimes increase the fluence at the second treatment whereas if blistering occurs, fluence will be decreased.
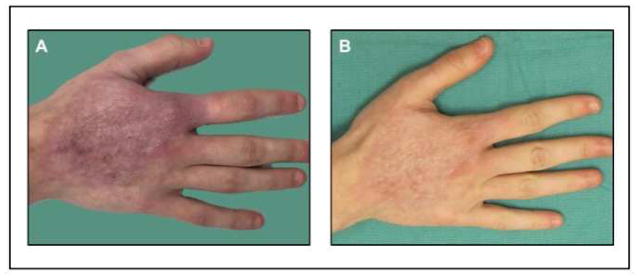
Figure 4.
23 year-old female with history of right hand burn resulting in erythematous dorsal hand scar. (A) Initial appearance prior to treatment. (B) Following one session of pulse dye laser treatment.
Fractional CO2 laser
The fractional CO2 laser is particularly useful in the treatment of raised hypertrophic scars and uneven scars as commonly seen after meshed skin graft. Several reports also indicate substantial improvement with other associated symptoms including pruritus, erythema and pain [40]. Mechanistically, due to its high wave length of 10,600 nm the CO2 laser targets water which is stored in all layers of the skin, but specifically in collagen of the dermis. While the exact mechanistic principles are yet to be established, the fractional CO2 laser generates columns of microperforations that penetrate into the dermis and disrupt the disorganized collagen bundles of the hypertrophic scar and break up the disorganized collagen structure initially established after the burn. Each individual wound is separated from the next with a treatment density of less than 10% of the skin. This generates an immediate mechanical release of tension in some restrictive scars and allows for adjacent, non-injured tissue to restore the ablated tissue.
Furthermore, this treatment breaks down the disorganized collagen organization and allows the microscopic wound to heal in a much more controlled low-inflammation state (several months after the inflammation from the burn injury). Histologic analysis revealed remodeling of the extracellular matrix with significant decrease in type I collagen and an increase in type III collagen with the dermal architecture closer resembling normal uninjured skin [41]. Similarly to PDL laser, fractional CO2 photothermolysis requires multiple sessions to achieve a final result.
This treatment can be combined with corticosteroids and laser assisted triamcinolone delivery has recently been reported to be effective in the treatment of hypertrophic scars (Figure 5). Settings again differ based on laser systems. In general we believe that deeper scar penetration with high energy (up to 150MJ) is beneficial for thick burn scars. When using deep penetration settings, however, it is crucial to use a low density to avoid new thermal injuries. We typically use a density of 3% when using high energy (100–150mJ). For less thick scars or when trying to improve the appearance of meshed grafts, we will utilize lower settings usually around 30MJ and increase the density to 5–10%. Additionally, for surface irregularities such as those used from previous meshed grafts, we will use a superficial CO2 handpiece or Computerized Pattern Generator (CPG). With this laser we will use an energy of 80–100 MJ and 75–200Hz. This handpiece, however, can cause hypopigmentation so caution must be used in patients with Fitzpatrick grade III or above. If treating a scar that is thick and red, we will only use the deep laser and superficial PDL (Figure 5). We do not use the PDL at the same time as the CPG to avoid superficial skin injury.
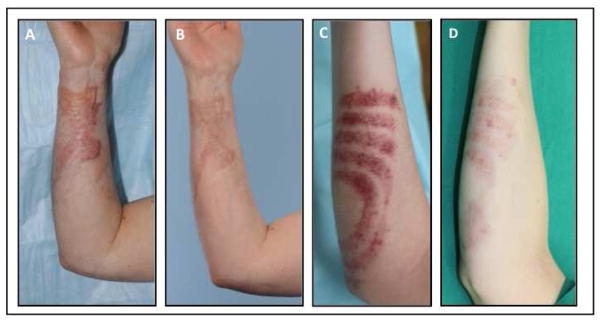
Figure 5.
43 year-old female with history of deep partial thickness grease burns to the right volar wrist resulting in a hypertrophic and erythematous scar. (A) Preoperative presentation prior to treatment. (B) Following two sessions of fractional CO2 laser photothermolysis with combined triamcinolone injection. (C) 22 year-old female after debridement and grafting of stove burn with thick, red, hypertrophic scars. (C) Preoperative presentation. (D) Following two sessions of fractional CO2 photothermolysis combined with pulse dye laser and triamcinolone injection.
Alexandrite Laser
This laser can be used for laser hair ablation. This is particularly useful in patients who had full thickness grafts from hair bearing regions grafted to regions where hair is not desired (palm, face). When this arises, this laser modality can be used to ablate the hair by targeting the melanin in the hair follicles. Like PDL and CO2, several treatments may be necessary to achieve an optimal result. Care must be taken not to use energy that is too high to avoid thermal injury. Additionally, this laser has improved efficacy in patients with dark hair and light skin (Fitzpatrick I–II).
Operative management
Scar management in the acute phase of the thermal injury and during initial scar maturation can significantly ameliorate hypertrophic scar formation and prevent sequale leading to scar banding. However, after non-invasive treatment options are exhausted, operative techniques need to be considered for the treatment of persistent hand scars that cause functional or aesthetic impairment. Timing of the operative procedure should be carefully chosen allowing enough time for complete scar maturation since premature intervention can result in increased inflammation and additional scarring. We usually begin reconstructive procedures starting at 6 months after injury. While a comprehensive review of operative techniques involved in burn hand reconstruction is beyond the scope of this paper several well-established techniques critical for scar management are discussed below.
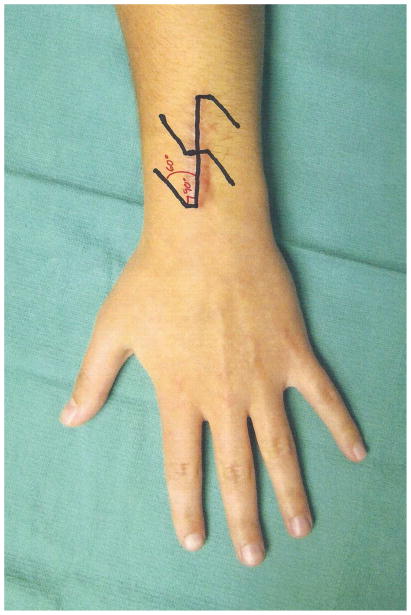
Local skin flaps are commonly employed to correct mild and moderate hypertrophic scar contractures thus avoiding the need for more complex procedures [42]. Simple linear scar bands, which can oftentimes occur across joints are best addressed with a scar lengthening z-plasty. The classic design of a z-plasty is oriented with its central limb along the hypertrophic scar band and with the lateral limbs at a 60 degree angle. Making the corner 90 degrees prior to extending the Z-plasty to 60 degrees helps improve perfusion to the tip of the z-plasty [43] (Figure 6). The flaps can safely be raised in scar tissue if maintained thick and incorporating underlying adipose tissue thus achieving active lengthening of 75%. Adjustments of the angle to 90 degrees results in increased lengthening of 125%, however, involves larger limbs and thus increased sacrifice of adjacent width. This approach can be modified by including a series of smaller z-plasties along a scar thus effectively achieving similar lengthening but avoiding donor site morbidity with larger flaps (Figure 7). We tend to use smaller flaps over the digits and palm whereas we use larger flaps for axillary contractures.
Figure 6.
Z-plasty design. Making the corner 90 degrees prior to extending the Z-plasty to 60 degrees helps improve perfusion to the tip of the z-plasty.
Figure 7.
4 year-old boy with history of left hand palm burn from a stove resulting in hypertrophic scar band extending from thumb, midpalm and to small finger. (A) Preoperative presentation. (B) Intraoperative design of z-plasty releases. (C) 6 months follow-up after scar release.
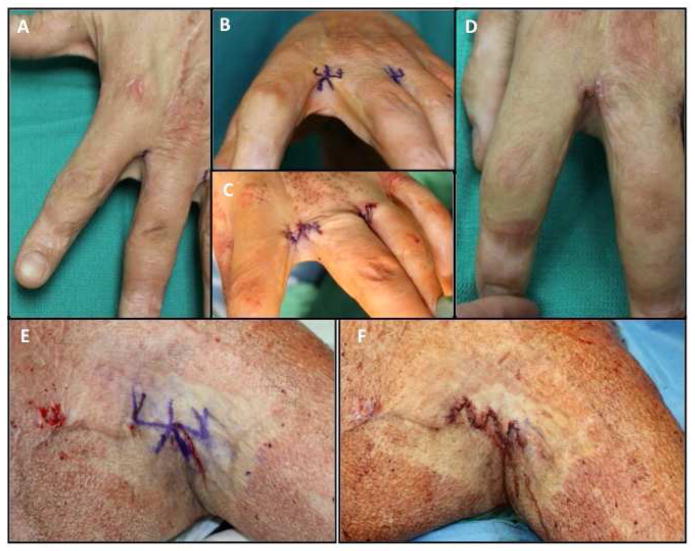
Webspace contractures are commonly addressed with modifications of z-plasties and a variety of local flaps have been devised [44, 45]. The 5-flap z-plasty is frequently utilized and is very effective in creating a concavity and lengthening within the webspace due to its geometric design (Figure 8). The Y to V region of this flap should be placed in the region of desired deepening. Other options include V-Y advancement flaps, which utilize the supple dorsal tissue that is advanced into the web space. These flaps which can be further be combined with forms of z-plasties [46].
Figure 8.
Webspace contracture addressed with 5-flap jumping man z-plasty. (A) Pre operative webbing between index and middle fingers. (B) Intraoperative marking of 5-flap jumping man z-plasty. (C) Intraoperative release and rearrangement. (D) One month postoperative appearance. (E) 65 year-old male with deep partial thickness burns to trunk and upper arms with resulting axillary scar contracture. Design of jumping man 5-flap z-plasty. (F) Immediate appearance after z-plasty transposition.
Axillary scar contractures are the second most common contractures behind neck contractures and are difficult to improve. Small linear bands can be released with Z plasties. (Figure 8) Larger more restrictive contractures can be treated with release and thick STSG or FTSG. Others have described treatment of severe contractures with pedicled flaps or with regional and free tissue transfer [47].
Palmar burn scars commonly involve a large surface and can therefore result in tight contractures. Mild forms can be addressed with a sequence of z-plasties (Figure 7). If the contracture is severe, release of the scar may be required leaving a large defect. This defect can be filled with a full thickness skin graft if no crucial structures are exposed [48]. Full-thickness skin grafts are preferred over split-thickness graft due to the decreased effect of secondary contraction in order to minimize scarring (Figure 3). If the contracture release leads to exposed tendon or bone, local or distant flaps may be required [49]. The extensor tendons dorsally often benefit from a fascia only reverse radial forearm flap with a skin graft for coverage or a reverse dorsal interosseous flap. Alternatives include free fascia only or fasciocutaneous flaps though bulky flaps should be avoided if possible [50].
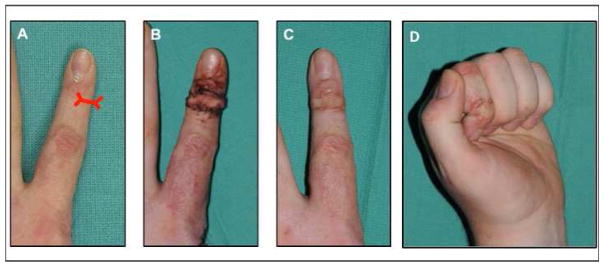
A common consequence of dorsal hand burns is contracture of the eponychial fold. This is caused by excess tension across the dorsal digit, which often restricts DIP flexion. To address the contracture and eponychial fold deformity, we use a technique of release and full thickness graft proximal to the DIP. This involves a distally based bipedicled flap, which is designed to release the proximal nail fold contracture. This is accompanied by an extensive release of the dorsal soft tissues and resurfacing of the resulting defect with a full-thickness skin graft. Restoration of normal anatomy can thus be achieved and results in normalized nail growth [23] (Figure 9).
Figure 9.
25 year-old male with left index finger nail bed deformity from flame burn injury. (A) Presentation prior to treatment. (B) 2 weeks following nail bed release and full-thickness skin grafting. (C) 6-week postoperative follow-up with improved DIP flexion. (D) Postoperative range of motion of the DIP joint.
Another common and difficult reconstructive challenge as discussed above is small finger scar contracture. For these patients, pre op x-ray and analysis of the level of contracture of the joint is crucial to a successful outcome. If severe joint contracture is present or the patient has a boutonnière deformity from previous debridement or injury to the extensor tendon will not be improved by an operation addressing the skin contracture alone. The surgeon should also avoid the urge to straighten the small finger in one operation as this will often lead to venous congestion or ischemia due to the shortening of the vessels from the contracture. If the skin is the only area causing the contracture then a z-plasty or series of z-plasties can be used. In more severe contractures, a release and full thickness hypothenar flap can be used (Figure 2). Finally, in those cases with joint and skin contracture, a Joint Jack can be used for slow distraction and stretching of the contracture. This device places two K-wires in the middle phalanx and has a series of bands that connect to the wrist slowly stretching the contracture [51].
Summary
Hypertrophic scarring following burn injury of the hand can result in detrimental functional deficits as well as impaired appearance. While early management of the acute burn injury is critical to minimize the sequalae of scar formation, hypertrophic scarring cannot always be avoided. A variety of non-invasive and invasive therapeutic modalities are available to the reconstructive hand surgeon to treat and modulate a formed scar with the goal to restore hand function. Local tissue rearrangement techniques form a central pillar to address deforming scar tissue, while adjunct therapies including laser treatment and injectables have demonstrated excellent results in scar rehabilitation.
Key Points.
Early management of acute hand burns with debridement, grafting and mobilization is critical to minimize development of hypertrophic scarring.
Once hypertrophic scars have formed, surgical scar release can be achieved with a variety of procedures reaching from local tissue rearrangement to free flaps in order to restore hand function.
Different modalities including laser therapy have become the standard of care in the rehabilitation of hypertrophic scars and can ameliorate scar texture, thickness, color and pruritus.
Acknowledgments
BL Supported by funding from NIH/National Institute of General Medical Sciences Grant K08GM109105-0, Plastic Surgery Foundation, the Association for Academic Surgery Roslyn Award, American Association for the Surgery of Trauma Research & Education Foundation Scholarship, American Association of Plastic Surgery Academic Scholarship, American College of Surgeons Clowes Award, AAPS/PSF Pilot Award, International FOP Association. Dr B. Levi collaborates on a project unrelated to this review with Boehringer Ingelheim.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McKee DM. Acute management of burn injuries to the hand and upper extremity. J Hand Surg Am. 2010;35(9):1542–4. doi: 10.1016/j.jhsa.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 2.Ng D, et al. Work-related burns: a 6-year retrospective study. Burns. 1991;17(2):151–4. doi: 10.1016/0305-4179(91)90140-c. [DOI] [PubMed] [Google Scholar]
- 3.Sheridan RL, et al. The acutely burned hand: management and outcome based on a ten-year experience with 1047 acute hand burns. J Trauma. 1995;38(3):406–11. doi: 10.1097/00005373-199503000-00022. [DOI] [PubMed] [Google Scholar]
- 4.Richards WT, et al. Acute surgical management of hand burns. J Hand Surg Am. 2014;39(10):2075–2085 e2. doi: 10.1016/j.jhsa.2014.07.032. [DOI] [PubMed] [Google Scholar]
- 5.McCauley RL. Reconstruction of the pediatric burned hand. Hand Clin. 2009;25(4):543–50. doi: 10.1016/j.hcl.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 6.Arnoldo BD, Purdue GF. The diagnosis and management of electrical injuries. Hand Clin. 2009;25(4):469–79. doi: 10.1016/j.hcl.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 7.Pan BS, Vu AT, Yakuboff KP. Management of the Acutely Burned Hand. J Hand Surg Am. 2015;40(7):1477–84. doi: 10.1016/j.jhsa.2015.02.033. quiz 1485. [DOI] [PubMed] [Google Scholar]
- 8.Mohammadi AA, et al. Early excision and skin grafting versus delayed skin grafting in deep hand burns (a randomised clinical controlled trial) Burns. 2011;37(1):36–41. doi: 10.1016/j.burns.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 9.Ong YS, Samuel M, Song C. Meta-analysis of early excision of burns. Burns. 2006;32(2):145–50. doi: 10.1016/j.burns.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 10.Monstrey S, et al. Assessment of burn depth and burn wound healing potential. Burns. 2008;34(6):761–9. doi: 10.1016/j.burns.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 11.Dewey WS, Richard RL, Parry IS. Positioning, splinting, and contracture management. Phys Med Rehabil Clin N Am. 2011;22(2):229–47. v. doi: 10.1016/j.pmr.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 12.Fufa DT, Chuang SS, Yang JY. Postburn contractures of the hand. J Hand Surg Am. 2014;39(9):1869–76. doi: 10.1016/j.jhsa.2014.03.018. [DOI] [PubMed] [Google Scholar]
- 13.Hegge T, et al. Scar contractures of the hand. Clin Plast Surg. 2011;38(4):591–606. doi: 10.1016/j.cps.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 14.Butzelaar L, et al. Currently known risk factors for hypertrophic skin scarring: A review. J Plast Reconstr Aesthet Surg. 2016;69(2):163–9. doi: 10.1016/j.bjps.2015.11.015. [DOI] [PubMed] [Google Scholar]
- 15.Kwan P, et al. Scar and contracture: biological principles. Hand Clin. 2009;25(4):511–28. doi: 10.1016/j.hcl.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 16.Tredget EE, Levi B, Donelan MB. Biology and principles of scar management and burn reconstruction. Surg Clin North Am. 2014;94(4):793–815. doi: 10.1016/j.suc.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wong VW, et al. Mechanical force prolongs acute inflammation via T-cell-dependent pathways during scar formation. FASEB J. 2011;25(12):4498– 510. doi: 10.1096/fj.10-178087. [DOI] [PubMed] [Google Scholar]
- 18.Wong VW, et al. Focal adhesion kinase links mechanical force to skin fibrosis via inflammatory signaling. Nat Med. 2012;18(1):148–52. doi: 10.1038/nm.2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cartotto R, et al. Common postburn deformities and their management. Surg Clin North Am. 2014;94(4):817–37. doi: 10.1016/j.suc.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 20.Kreymerman PA, et al. Reconstruction of the burned hand. Plast Reconstr Surg. 2011;127(2):752–9. doi: 10.1097/PRS.0b013e3181fed7c1. [DOI] [PubMed] [Google Scholar]
- 21.Graham TJ, Stern PJ, True MS. Classification and treatment of postburn metacarpophalangeal joint extension contractures in children. J Hand Surg Am. 1990;15(3):450–6. doi: 10.1016/0363-5023(90)90058-y. [DOI] [PubMed] [Google Scholar]
- 22.Grishkevich VM. Flexion contractures of fingers: contracture elimination with trapeze-flap plasty. Burns. 2011;37(1):126–33. doi: 10.1016/j.burns.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 23.Donelan MB, Garcia JA. Nailfold reconstruction for correction of burn fingernail deformity. Plast Reconstr Surg. 2006;117(7):2303–8. doi: 10.1097/01.prs.0000218709.03848.a5. discussion 2309. [DOI] [PubMed] [Google Scholar]
- 24.Moore ML, Dewey WS, Richard RL. Rehabilitation of the burned hand. Hand Clin. 2009;25(4):529–41. doi: 10.1016/j.hcl.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 25.Rrecaj S, et al. Outcome of Physical Therapy and Splinting in Hand Burns Injury. Our Last Four Years’ Experience. Mater Sociomed. 2015;27(6):380– 2. doi: 10.5455/msm.2015.27.380-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schouten HJ, Nieuwenhuis MK, van Zuijlen PP. A review on static splinting therapy to prevent burn scar contracture: do clinical and experimental data warrant its clinical application? Burns. 2012;38(1):19– 25. doi: 10.1016/j.burns.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 27.Richard R, et al. Burn rehabilitation and research: proceedings of a consensus summit. J Burn Care Res. 2009;30(4):543–73. doi: 10.1097/BCR.0b013e3181adcd93. [DOI] [PubMed] [Google Scholar]
- 28.Anzarut A, et al. The effectiveness of pressure garment therapy for the prevention of abnormal scarring after burn injury: a meta-analysis. J Plast Reconstr Aesthet Surg. 2009;62(1):77–84. doi: 10.1016/j.bjps.2007.10.052. [DOI] [PubMed] [Google Scholar]
- 29.Maguire HC., Jr Treatment of Keloids with Triamcinolone Acetonide Injected Intralesionally. JAMA. 1965;192:325–6. doi: 10.1001/jama.1965.03080170053019. [DOI] [PubMed] [Google Scholar]
- 30.Tang YW. Intra- and postoperative steroid injections for keloids and hypertrophic scars. Br J Plast Surg. 1992;45(5):371–3. doi: 10.1016/0007-1226(92)90007-k. [DOI] [PubMed] [Google Scholar]
- 31.Wang XQ, et al. A review of the effectiveness of antimitotic drug injections for hypertrophic scars and keloids. Ann Plast Surg. 2009;63(6):688–92. doi: 10.1097/SAP.0b013e3181978753. [DOI] [PubMed] [Google Scholar]
- 32.Jemec B, et al. The effect of 5-fluorouracil on Dupuytren fibroblast proliferation and differentiation. Chir Main. 2000;19(1):15–22. doi: 10.1016/s1297-3203(00)73455-x. [DOI] [PubMed] [Google Scholar]
- 33.Klinger M, et al. Autologous fat graft in scar treatment. J Craniofac Surg. 2013;24(5):1610–5. doi: 10.1097/SCS.0b013e3182a24548. [DOI] [PubMed] [Google Scholar]
- 34.Brongo S, et al. Use of lipofilling for the treatment of severe burn outcomes. Plast Reconstr Surg. 2012;130(2):374e–376e. doi: 10.1097/PRS.0b013e3182590387. [DOI] [PubMed] [Google Scholar]
- 35.Byrne M, et al. Early experience with fat grafting as an adjunct for secondary burn reconstruction in the hand: Technique, hand function assessment and aesthetic outcomes. Burns. 2016;42(2):356–65. doi: 10.1016/j.burns.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 36.Hultman CS, et al. Laser resurfacing and remodeling of hypertrophic burn scars: the results of a large, prospective, before-after cohort study, with long-term follow-up. Ann Surg. 2014;260(3):519–29. doi: 10.1097/SLA.0000000000000893. discussion 529–32. [DOI] [PubMed] [Google Scholar]
- 37.Taudorf EH, et al. Non-ablative fractional laser provides long-term improvement of mature burn scars--a randomized controlled trial with histological assessment. Lasers Surg Med. 2015;47(2):141–7. doi: 10.1002/lsm.22289. [DOI] [PubMed] [Google Scholar]
- 38.Clayton JL, et al. Incidence and management of adverse events after the use of laser therapies for the treatment of hypertrophic burn scars. Ann Plast Surg. 2013;70(5):500–5. doi: 10.1097/SAP.0b013e31827eac79. [DOI] [PubMed] [Google Scholar]
- 39.Krakowski AC, et al. Ablative fractional laser resurfacing helps treat restrictive pediatric scar contractures. Pediatrics. 2014;134(6):e1700–5. doi: 10.1542/peds.2014-1586. [DOI] [PubMed] [Google Scholar]
- 40.Levi B, et al. The Use of CO2 Fractional Photothermolysis for the Treatment of Burn Scars. J Burn Care Res. 2016;37(2):106–14. doi: 10.1097/BCR.0000000000000285. [DOI] [PubMed] [Google Scholar]
- 41.Ozog DM, et al. Evaluation of clinical results, histological architecture, and collagen expression following treatment of mature burn scars with a fractional carbon dioxide laser. JAMA Dermatol. 2013;149(1):50–7. doi: 10.1001/2013.jamadermatol.668. [DOI] [PubMed] [Google Scholar]
- 42.Hop MJ, et al. Reconstructive surgery after burns: a 10-year follow-up study. Burns. 2014;40(8):1544–51. doi: 10.1016/j.burns.2014.04.014. [DOI] [PubMed] [Google Scholar]
- 43.Rohrich RJ, Zbar RI. A simplified algorithm for the use of Z-plasty. Plast Reconstr Surg. 1999;103(5):1513–7. quiz 1518. [PubMed] [Google Scholar]
- 44.Sari E, et al. Combination of rhomboid flap and double Z-plasty technique for reconstruction of palmar and dorsal web space burn contractures. Burns. 2015;41(2):408–12. doi: 10.1016/j.burns.2014.07.017. [DOI] [PubMed] [Google Scholar]
- 45.Grishkevich VM. First web space post-burn contracture types: contracture elimination methods. Burns. 2011;37(2):338–47. doi: 10.1016/j.burns.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 46.Peker F, Celebiler O. Y-V advancement with Z-plasty: an effective combined model for the release of post-burn flexion contractures of the fingers. Burns. 2003;29(5):479–82. doi: 10.1016/s0305-4179(03)00048-2. [DOI] [PubMed] [Google Scholar]
- 47.Ogawa R, et al. Reconstruction of axillary scar contractures--retrospective study of 124 cases over 25 years. Br J Plast Surg. 2003;56(2):100–5. doi: 10.1016/s0007-1226(03)00035-3. [DOI] [PubMed] [Google Scholar]
- 48.Yotsuyanagi T, et al. Double combined Z-plasty for wide-scar contracture release. J Plast Reconstr Aesthet Surg. 2013;66(5):629–33. doi: 10.1016/j.bjps.2013.01.027. [DOI] [PubMed] [Google Scholar]
- 49.Karanas YL, Buntic RF. Microsurgical reconstruction of the burned hand. Hand Clin. 2009;25(4):551–6. doi: 10.1016/j.hcl.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 50.Jones NF, Jarrahy R, Kaufman MR. Pedicled and free radial forearm flaps for reconstruction of the elbow, wrist, and hand. Plast Reconstr Surg. 2008;121(3):887–98. doi: 10.1097/01.prs.0000299924.69019.57. [DOI] [PubMed] [Google Scholar]
- 51.Houshian S, Gynning B, Schroder HA. Chronic flexion contracture of proximal interphalangeal joint treated with the compass hinge external fixator. A consecutive series of 27 cases. J Hand Surg Br. 2002;27(4):356–8. doi: 10.1054/jhsb.2002.0796. [DOI] [PubMed] [Google Scholar]