Abstract
Background
The performance of severity-of-illness scores varies in different scenarios and must be validated prior of being used in a specific settings and geographic regions. Moreover, models’ calibration may deteriorate overtime and performance of such instruments should be reassessed regularly. Therefore, we aimed at to validate the SAPS 3 in a large contemporary cohort of patients admitted to Brazilian ICUs. In addition, we also compared the performance of the SAPS 3 with the MPM0-III.
Methods
This is a retrospective cohort study in which 48,816 (medical admissions = 67.9%) adult patients are admitted to 72 Brazilian ICUs during 2013. We evaluated models’ discrimination using the area under the receiver operating characteristic curve (AUROC). We applied the calibration belt to evaluate the agreement between observed and expected mortality rates (calibration).
Results
Mean SAPS 3 score was 44.3 ± 15.4 points. ICU and hospital mortality rates were 11.0 and 16.5%. We estimated predicted mortality using both standard (SE) and Central and South American (CSA) customized equations. Predicted mortality rates were 16.4 ± 19.3% (SAPS 3-SE), 21.7 ± 23.2% (SAPS 3-CSA) and 14.3 ± 14.0% (MPM0-III). Standardized mortality ratios (SMR) obtained for each model were: 1.00 (95% CI, 0.98–0.102) for the SAPS 3-SE, 0.75 (0.74–0.77) for the SAPS 3-CSA and 1.15 (1.13–1.18) for the MPM0-III. Discrimination was better for SAPS 3 models (AUROC = 0.85) than for MPM0-III (AUROC = 0.80) (p < 0.001). We applied the calibration belt to evaluate the agreement between observed and expected mortality rates (calibration): the SAPS 3-CSA overestimated mortality throughout all risk classes while the MPM0-III underestimated it uniformly. The SAPS 3-SE did not show relevant deviations from ideal calibration.
Conclusions
In a large contemporary database, the SAPS 3-SE was accurate in predicting outcomes, supporting its use for performance evaluation and benchmarking in Brazilian ICUs.
Electronic supplementary material
The online version of this article (doi:10.1186/s13613-017-0276-3) contains supplementary material, which is available to authorized users.
Keywords: Severity-of-illness scores, Validation, Intensive care units, Outcomes, Standardized mortality rate
Background
Severity-of-illness scores have broad applicability in intensive care setting. Although they should not be used on individual basis, they are useful to evaluate ICU performance, to monitor it overtime, to guide resource management and quality improvements, and for benchmarking purposes [1]. However, the performance of these models varies in different scenarios because of differences in case mix, clinical management patterns, admission policies as well as pre- and post-ICU care. Therefore, severity-of-illness scores must be validated prior to their use in a specific setting or geographic region.
The three most used severity-of-illness scores are the Acute Physiology and Chronic Health Evaluation (APACHE) [2], the Mortality Probability Models (MPM0-III) [3] and the Simplified Acute Physiology Score (SAPS 3) [4, 5]. Among them, the only score developed using data from patients and intensive care units (ICU) worldwide (307 ICUs in 35 countries) was the SAPS 3 score. Besides a general standard equation, investigators also developed seven regional equations to estimate hospital mortality, thus allowing comparisons among ICUs on a more common level.
In 2009, the Brazilian Association of Intensive Care (Associação de Medicina Intensiva Brasileira, AMIB) chose the SAPS 3 score as the severity-of-illness score recommended for performance evaluation and benchmarking in Brazilian ICUs [6]. However, to our knowledge, validation studies reported conflicting results and were mostly single centered, involving specific patient populations [7–13] and with relatively small sample sizes [14–16]. Moreover, as the calibration of severity-of-illness scores is expected to deteriorate overtime, the performance of such instruments should be reassessed on a regular basis [17]. Therefore, in the present study, we aimed at to validate the SAPS 3 in a large contemporary cohort of patients admitted to Brazilian ICUs. In addition, we also compared the performance of the SAPS 3 with the MPM0-III.
Methods
Design and setting
This was a secondary analysis of the ORCHESTRA study, a multicenter retrospective cohort study of critical care organization and outcomes in 59,693 patients admitted to 78 ICUs at 51 Brazilian hospitals during 2013 [18].
Selection of participants, data collection and definitions
Participating ICUs in the ORCHESTRA study were selected from the Brazilian Research in Intensive Care Network (BRICNet). For the purposes of the present study, we excluded ICUs exclusively admitting cardiac patients (n = 6) (Fig. 1) and a total of 72 ICUs at 50 hospitals were involved. We included all consecutive patients aged ≥16 years admitted to the participating ICUs during 2013. In the ORCHESTRA study, readmissions and patients with missing core data [age, location before ICU admission, main ICU admission diagnosis, SAPS 3 score, ICU and hospital length of stay (LOS) and vital status at hospital discharge] were excluded. In the present study, besides the patients admitted to cardiac units (n = 3951), we also excluded those who did not meet both the SAPS 3 and MPM0-III eligibility criteria [patients aged <18 years (n = 358), who underwent cardiac surgeries (n = 2971), with acute myocardial infarction (n = 3568) and burns (n = 29)]. Therefore, a total of 48,816 patients constituted the study population.
Fig. 1.
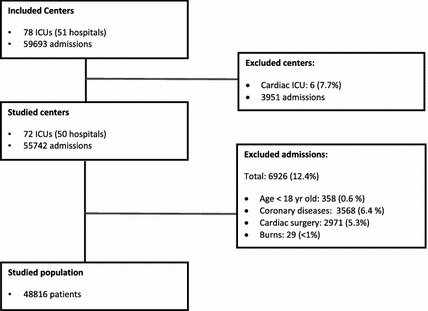
Study flowchart
We obtained de-identified patient data from the Epimed Monitor System®, (Epimed Solutions®, Rio de Janeiro, Brazil), a commercial cloud-based registry for quality improvement, performance evaluation and benchmarking purposes. ICUs using the Epimed Monitor System® prospectively collect data in a structured electronic case report form, most typically using a trained case manager. Key data elements included demographics, admission diagnosis, location before ICU admission, comorbidities based on the Charlson Comorbidity Index [19], functional status one week before hospital admission [20], scores including the SAPS 3 score, MPM0-III score and the Sequential Organ Failure Score (SOFA) [21], use of ICU support, ICU and hospital LOS and destination after hospital discharge. The SAPS 3 and MPM0-III scores were calculated using data from the ICU admission (±1 h). As recommended, missing values were coded as the reference or “normal” category for each variable. Estimated mortality rates using both the standard equation (SAPS 3-SE) and the one customized for Central and South American countries (SAPS 3-CSA) are provided in the system. In the present study, the primary outcome of interest was in-hospital mortality at the patient level.
Statistical analysis
We described ICU and patient characteristics using standard descriptive statistics and reported continuous variables as mean ± standard deviation or median (25–75% interquartile range, IQR), as appropriate. We reported categorical variables as absolute numbers (frequency percentages).
We assessed models’ discrimination (ability of each model to discriminate between patients who lived from those who died) by estimating the area under the receiver operating characteristic curve (AUROC). Comparisons between AUROCs by a pairwise evaluation of the three scores discrimination power were performed by Delong method [22]. We used the calibration belt, proposed by the GiViTI group [23, 24], to investigate the relationship between the observed and expected outcomes. Using this approach, a generalized polynomial logistic function between the outcome and the logit transformation of the predicted probability was fitted, with the respective 95 and 80% confidence intervals (CI) boundaries. A statistically significant deviation from the bisector (the line of perfect calibration) occurs when the 95% CI boundaries of the calibration belt do not include the bisector [23]. Calibration curves were constructed by plotting predicted mortality rates (x-axis) against observed mortality rates (y-axis). Standardized mortality rates (SMR) with respective 95% confidence intervals (CI) were calculated for each model by dividing observed by predicted mortality rates. A two-tailed p value <0.05 was considered statistically significant. We performed the statistical analyses using R (http://www.r-project.org) and SPSS 21 (IBM Corp., Armonk, NY).
Results
Our final sample consisted of 48,816 patients admitted by 72 ICUs in the study period (Fig. 1). Table 1 gives the main hospital and ICU characteristics. Most of ICUs were medical–surgical (n = 62, 86.1%) located at private hospitals (n = 45, 90.0%). Median number of patients per ICU was 517 (361–817).
Table 1.
Hospital and ICU characteristics
| Characteristics | |
|---|---|
| Hospitals | (n = 50) |
| Type of hospital | |
| Private, for profit | 31 (62.0%) |
| Private, philanthropic | 14 (28.0%) |
| Public | 5 (10.0%) |
| Hospital beds (n) | |
| <150 | 19 (38.0%) |
| 150–300 | 20 (40.0%) |
| ≥301 | 11 (22.0%) |
| Intermediate/step-down unit | |
| No | 25 (50.0%) |
| Yes | 25 (50.0%) |
| Training programs in critical care | |
| No | 28 (56.0%) |
| Yes | 22 (44.0%) |
| ICUs | (n = 72) |
|---|---|
| Medical–surgical ICU | |
| Yes | 62 (86.1%) |
| No | 10 (13.9%) |
| Active ICU beds (n) | 22 (11–34) |
| ≤10 | 23 (31.9%) |
| 10–20 | 27 (37.5%) |
| >20 | 22 (30.6%) |
| ICU bed occupancy rate (%) | 73 (62–83) |
Results for continuous variables are reported as mean ± SD and median (IQR)
ICU intensive care unit, IQR interquartile range, SD standard deviation
Table 2 reports the main patients’ characteristics. The main reasons for ICU admission were postoperative care (26.3%), followed by sepsis (22.3%), cardiovascular complications (11.3%) and neurological complications (11.4%). At ICU admission, invasive mechanical ventilation was used in 7550 (15.5%) and noninvasive ventilation was used in 4875 (10.0%) of patients. Vasopressors were required by 6158 (12.6%) and renal replacement therapy by 1578 (3.2%).
Table 2.
Main patients’ characteristics and outcomes
| Characteristics | |
|---|---|
| Patients (n) | 48,816 |
| Age (years) | 65 (48–79) |
| <45 | 10,354 (21.2%) |
| 45–64 | 13,555 (27.8%) |
| 65–74 | 8661 (17.7%) |
| 75–84 | 9666 (19.8%) |
| ≥85 | 6580 (13.5%) |
| Gender | |
| Female | 25,400 (52.0%) |
| Male | 23,416 (48.0%) |
| Health insurance coverage | |
| Public health insurance | 5779 (11.8%) |
| Private health insurance | 36,821 (75.4%) |
| Admission costs paid with patient’s own resources | 6216 (13.7%) |
| Comorbidities | |
| Diabetes mellitus | 11,037 (22.6%) |
| Cancer | 9647 (19.8%) |
| Chronic renal failure | 4469 (9.2%) |
| Coronary artery disease | 2993 (6.1%) |
| Cardiac failure | 2468 (5.1%) |
| Chronic pulmonary disease | 2551 (5.2%) |
| Charlson Comorbidity Index (points) | 1 (0–2) |
| Characteristics | n = 48,816 |
|---|---|
| Functional status before hospital admission | |
| Ambulant | 36,835 (75.5%) |
| Minor assistance | 8556 (17.5%) |
| Major assistance or bedridden | 3425 (7.0%) |
| Source of ICU admission | |
| Emergency department | 25,738 (52.7%) |
| Operating room | 14,979 (30.7%) |
| Ward/floor | 4212 (8.6%) |
| Transfer from other hospitals | 1876 (3.8%) |
| Other | 2011 (4.1%) |
| Hospital days prior to ICU admission (n) | 0 (0–1) |
| Admission diagnostic category | |
| Scheduled surgery | 12,825 (26.3%) |
| Emergency surgery | 2854 (5.8%) |
| Cardiovascular** | 5511 (11.3%) |
| Sepsis** | 10,876 (22.3%) |
| Neurological** | 5588 (11.4%) |
| Respiratory** | 2542 (5.2%) |
| Gastrointestinal** | 2263 (4.6%) |
| Other medical admissions** | 6357 (13.0%) |
| SAPS 3 (points) | 43 (34–53) |
| SOFA score on Day 1 (points) | 1 (0–4) |
| Support on Day 1 | |
| Mechanical ventilation | 7550 (15.5%) |
| Noninvasive ventilation | 4875 (10.0%) |
| Vasopressors | 6158 (12.6%) |
| Renal replacement therapy | 1578 (3.2%) |
| ICU LOS (days) | 3 (1–5) |
| Hospital LOS (days) | 8 (4–18) |
| ICU mortality | 5385 (11.0%) |
| Hospital mortality | 8031 (16.5%) |
| Destination at hospital discharge | |
| Home | 37,683 (77.2%) |
| Other hospital | 556 (1.1%) |
| Hospice/home care | 355 (0.7%) |
| Other/unknown | 2191 (4.5%) |
| Died | 8031 (16.5%) |
Results for continuous variables are reported as mean ± SD and median (IQR)
IQR interquartile range, ICU intensive care unit, SAPS Simplified Acute Physiology Score, SOFA Sequential Organ Failure Score, LOS length of stay, SD standard deviation
** These admission categories refer to medical diagnosis only
Median ICU and hospital LOS were 3 (1–5) and 8 (4–18) days, respectively. Mean SAPS 3 was 44.3 ± 15.4 points. A total of 5385 (11.0%) died in the ICUs; 2646 died in the hospital after the ICUs discharge and the hospital mortality rate was 16.5%. Table 2 reports the main patients’ characteristics and outcomes.
Predicted mortality rates were 16.4 ± 19.3% (SAPS 3-SE), 21.7 ± 23.2% (SAPS 3-CSA) and 14.3 ± 14.0% (MPM0-III). Table 3 gives the performance analyses for the studied scores. In summary, the SMR was appropriate using the SAPS 3-SE, while the SAPS 3-CSA overestimated and the MPM0-III underestimated the hospital mortality. Overall, discrimination was good, but higher for the SAPS 3 score (Table 3). Calibration was acceptable for the SAPS 3-SE only. In the calibration belt analysis, there was only minimal over- (below the first percentile) and underprediction (between the 8th and 14th percentiles) in the first two risk deciles. Conversely, the SAPS 3-CSA uniformly overestimated mortality in all risk range and the MPM0-III tended in general to underestimation (Figs. 2, 3).
Table 3.
Scores performances comparison
| Mortality | Discrimination | Calibration* | Precision | ||||
|---|---|---|---|---|---|---|---|
| Predicted mortality (±SD) | SMR (95% CI) | AUROC | 95% CI | Over the bisector 95% CI | Under the bisector 95% CI | Brier score | |
| SAPS 3-SE | 16.4 ± 19.3% | 1.00 (0.98–1.02) | 0.850 | 0.84–0.85 | 0.00–0.01 | 0.08–0.12 | 0.098 |
| SAPS 3-CSA | 21.7 ± 23.2% | 0.75 (0.74–0.77) | 0.850 | 0.84–0.85 | Never | Always | 0.103 |
| MPM0-III | 14.3 ± 14.0% | 1.15 (1.13–1.18) | 0.800 | 0.79–0.80 | 0.07–0.97 | 0.01–0.03 | 0.111 |
SAPS 3-SE Simplified Acute Physiology Score 3-Standard Equation, SAPS 3-CSA Simplified Acute Physiology Score 3-Customized equation for Central and South American Countries, MPM0-III Mortality Probability Models III, SMR Standardized mortality rates, AUROC area under the curve
* Calibration described as bisector deviation intervals, as proposed by GiViTI, (Italian Group for the Evaluation of Intervention in Intensive Care Medicine)
Fig. 2.
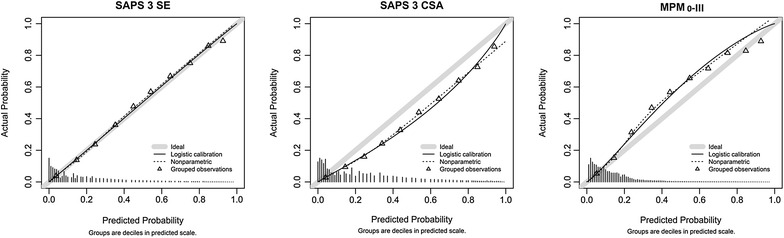
Calibration plots for SAPS 3-SE, SAPS 3-CSA and MPM0-III, with predicted mortality rates stratified by 10% intervals of mortality risk (x-axis) against observed mortality rates (y-axis)
Fig. 3.
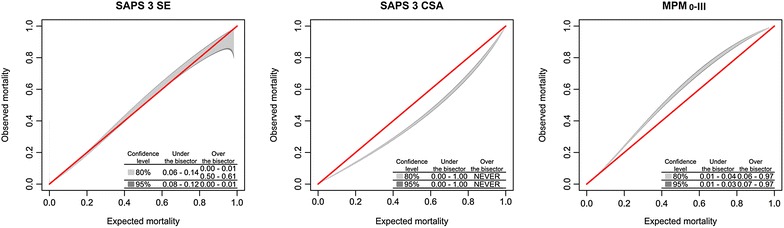
Calibration Belt for SAPS 3-SE, SAPS 3-CSA and MPM0-III, described as bisector deviation intervals, as proposed by GiViTI, (Italian Group for the Evaluation of Intervention in Intensive Care Medicine). The times the calibration belt significantly deviates from the bisector using 80 and 95% confidence levels are described in the lower right part of the plots
As most of the included ICUs were located at private hospitals, we performed subgroup analyses according to the type of hospital and specific subgroups of patients (Additional file 1: eTable 1 and eFigures 1–8). In patients admitted to private hospitals, we found results comparable to the ones observed for all the studied population and the SAPS 3-SE was the only model with a good performance. However, in patients admitted to public hospitals, none of the models was accurate in predicting hospital mortality. Finally, we performed additional analyses of the SAPS 3 performance in all patients (n = 55,742) fulfilling only the eligibility criteria reported the original publication of the model [4]. Models’ discrimination (AUROC = 0.855) for both the SAPS 3-SE and SAPS 3-CSA and calibration (Additional file 1: eFigure 3) were also appropriate. In Additional file 1: eTable 2, we provided information on patients’ characteristics and outcomes for our cohort of patients and the one reported in the SAPS 3 study.
Discussion
In the present study, we demonstrated that the SAPS 3-SE was able to accurately predict outcomes in a large contemporary cohort of Brazilian ICU patients. Conversely, the MPM0-III score had a relatively worse calibration and tended to significantly underestimate mortality, while the SAPS 3-CSA overestimated mortality despite a reasonable discrimination. Moreover, the SAPS 3-SE provided more precise estimations, resulting in a SMR closer to 1.0. In the calibration curves, the lines of observed mortality of the SAPS 3-SE were uniformly closer to the line of ideal prediction across all risk classes.
In the last years, mostly driven by official recommendations provided by AMIB, the SAPS 3 became the severity-of-illness score used in the vast majority of Brazilian ICUs to evaluate ICU performance as well as for benchmarking. However, validation studies of SAPS 3 were performed in specific subgroup of patients or in single-center studies involving a general ICU population [7–16]. In general, both the SAPS 3-SE and SAPS 3-CSA equations were evaluated in the studies. Overall, discrimination was usually good, but calibration results varied among the studies.
In these previous studies, the SAPS 3-SE had a poor calibration and tended usually to underestimate mortality [7–10, 12]. The SAPS 3-SE tended to overestimate mortality in only two studies (one of them comprising patients with acute coronary syndromes), both with a relatively low mortality rate [11, 16]. On the other hand, the SAPS 3-CSA accurately predicted mortality in five studies involving patients with cancer [8, 9], acute kidney injury [10, 12] and those who underwent surgical procedures [15]. Our results confirm that the MPM0-III, however, was inaccurate in predicting mortality. These results are in line with almost all previous studies performed in Brazil [9, 10, 12, 16].
There is a known phenomenon with traditional calibration statistics (such as Hosmer–Lemeshow goodness of fit) in prediction models validation/calibration studies with many thousands included subjects, in which often p values are highly significant despite visually good calibration curves, very small absolute errors, and acceptable calibration slope and intercept. This occurs because with a large sample size the power is big enough to detect, as statistically significant, irrelevant small differences. At the other extreme, one must be cautious in the interpretation of calibration results with small cohorts, because, even when the calibration curve, the calibration intercept and slope points to a miscalibration, the p values of traditional calibration statistics may not be significant, raising concern about the study low power [25]. Therefore, in small cohorts, the lack of correspondence between expected and observed probabilities can also result in misaligned calibration curves, when sample size cannot be enough to achieve statistical significance [26]. In addition, specific subgroups of patients were included in these studies, whose results may not be fully transposed to general populations of critical care patients in different scenarios.
It is a well-known phenomenon that the performance of prognostic scores (chiefly the calibration) tends to deteriorate overtime. Zimmerman et al. [2] when reporting the development of the APACHE IV elegantly demonstrated this. Soares et al. [8] also documented the temporal compromising of calibration studying the SAPS 3 score in a cohort of patients with cancer admitted to the ICU over a 3-year period in Brazil. This is why the performance of prognostic scores should be reassessed periodically.
The cohort composition could also interfere with the score performance. Comparing our cohort and original SAPS 3 development cohort, we had comparable median age, but clinical patients predominated (67.9 vs. 43.5% in the SAPS 3 cohort), with lower median SAPS 3 scores (43 points vs. 48 points) and lower hospital mortality (16.5 vs. 23.5%) (Additional file 1: eTable 2). Despite these case mix differences, currently the SAPS 3-SE model was well fitted to our population, which might reflect changes in the provision of health care resulting in lower risk-adjusted mortality. In this sense, our results have potential implications for ICU performance evaluation and more importantly for benchmarking purposes in Brazilian ICUs. On the one hand, we provide robust evidence that although the SAPS 3 remains useful in our country, the customized equation for Latin American countries should be no longer used.
Our study has many strengths including being, to our knowledge, the largest validation study of severity-of-illness scores in Brazil and using more contemporary data from several centers countrywide. Moreover, we consider there is a negligible potential for discharge bias, [27] once our percentage of patients discharged to other hospitals and hospice care facilities was minimal.
Our study has also several limitations that should be considered in the interpretation of our results. First, although we have evaluated a large number of Brazilian ICUs, we used a convenience sample, predominantly composed by private hospitals and they may not be representative of the entire country. Second, we have not audited data collection, as we used data recorded in a registry for performance evaluation and benchmarking. Therefore, we cannot estimate the effect of missing variables in the scores estimations. However, trained healthcare professionals that work as case managers register data in all ICUs. Third, we did not assess end-of-life decisions, as they are not regularly registered in the database, and therefore, we were unable to account for this factor in the analysis.
In conclusion, using a large contemporary database, we demonstrated that the SAPS 3-SE was accurate in predicting outcomes, supporting its use for performance evaluation and benchmarking in Brazilian ICUs.
Additional file
Additional file 1.Electronic Supplementary Material for External Validation of SAPS 3 and MPM0-III scores in 48,816 patients from 72 Brazilian ICUs.
Authors’ contributions
GMM participated in study conception, data analysis and interpretation and drafting of the manuscript. LSCFR participated in study conception, data acquisition, data analysis and interpretation and drafting of the manuscript. TCL participated in data acquisition, data interpretation and revising manuscript for important intellectual content. MFAL, RMH, FVCM, AA, JESSP, HBNA, GVR, ARS, GCF, GBAF, CLM, RARF and VPS participated in data acquisition and revising manuscript for important intellectual content. PEAAB participated in data analysis and interpretation and drafting of the manuscript. FAB participated in data acquisition, data interpretation and revising manuscript for important intellectual content. JIFS participated in study conception, data interpretation and drafting of the manuscript. MS participated in study conception, data analysis and interpretation and drafting of the manuscript. The complete list of investigators is reported in electronic supplementary material (ESM). All authors read and approved the final manuscript.
Competing interests
Dr. Soares and Dr. Salluh are founders and equity shareholders of Epimed Solutions®, which commercializes the Epimed Monitor System®, a cloud-based software for ICU management and benchmarking. The other authors declare that they have no competing interests.
Availability of data and materials
All relevant data are within the paper and ESM. Other information and dataset details are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Local Ethics Committee at the Instituto D’Or de Pesquisa (Parecer: 334.835) and the Brazilian National Ethics Committee (CAAE: 19687113.8.1001.5249) approved the study. Because the study used existing data from an ICU registry, the need for informed consent was waived.
Funding
This study was supported in part by the National Council for Scientific and Technological Development (CNPq) (Grant No. 304240/2014-1), Carlos Chagas Filho Foundation for Research Support of the State of Rio de Janeiro (FAPERJ) and departmental funds from the D’Or Institute for Research and Education.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- AMIB
Associação de Medicina Intensiva Brasileira
- APACHE
Acute Physiology and Chronic Health Evaluation
- AUROC
area under receiver operating characteristic
- BRICNet
Brazilian Research in Intensive Care Network
- CI
confidence interval
- ESM
electronic supplementary material
- ICU
intensive care unit
- LOS
length of stay
- MPM0-III
Mortality Probability Model III
- SAPS
Simplified Acute Physiology Score
- SAPS 3-CSA
SAPS 3, customized equation for Central and South American Countries
- SAPS 3-SE
SAPS 3, standard equation
- SMR
standardized mortality rates
- SOFA
sequential organ failure assessment
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1186/s13613-017-0276-3) contains supplementary material, which is available to authorized users.
Contributor Information
Giulliana Martines Moralez, Email: giulliana.moralez@gmail.com.
Ligia Sarmet Cunha Farah Rabello, Email: ligiarabello@yahoo.com.br.
Thiago Costa Lisboa, Email: tlisboa@hotmail.com.
Mariza da Fonte Andrade Lima, Email: marizafonte@gmail.com.
Rodrigo Marques Hatum, Email: rhatum@gmail.com.
Fernando Vinicius Cesar De Marco, Email: fmarco@vivalle.com.br.
Alessandra Alves, Email: ale76@terra.com.br.
Jorge Eduardo da Silva Soares Pinto, jorge-eduardo@email.com.br.
Hélia Beatriz Nunes de Araújo, Email: beatriznatfonseca@gmail.com.
Grazielle Viana Ramos, Email: grazielle_viana@hotmail.com.
Aline Reis Silva, Email: reissilva.aline@gmail.com.
Guilherme Côrtes Fernandes, Email: cortesfernandes@gmail.com.
Guilherme Brenande Alves Faria, Email: guibrenande@gmail.com.
Ciro Leite Mendes, Email: ciro.l.mendes@gmail.com.
Roberto Álvaro Ramos Filho, Email: robertoalvaroramos@yahoo.com.br.
Valdênia Pereira de Souza, Email: psouzaval@gmail.com.
Pedro Emmanuel Alvarenga Americano do Brasil, Email: emmanuel.brasil@gmail.com.
Fernando Augusto Bozza, Email: bozza.fernando@gmail.com.
Jorge Ibrain Figueira Salluh, Email: jorgesalluh@gmail.com.
Marcio Soares, Phone: +55 21 3883-6000, Email: marciosoaresms@gmail.com.
References
- 1.Salluh JIF, Soares M. ICU severity of illness scores: APACHE, SAPS and MPM. Curr Opin Crit Care. 2014;20(5):557–565. doi: 10.1097/MCC.0000000000000135. [DOI] [PubMed] [Google Scholar]
- 2.Zimmerman JE, Kramer AA, McNair DS, Malila FM. Acute Physiology and Chronic Health Evaluation (APACHE) IV: hospital mortality assessment for today’s critically ill patients. Crit Care Med. 2006;34(5):1297–1310. doi: 10.1097/01.CCM.0000215112.84523.F0. [DOI] [PubMed] [Google Scholar]
- 3.Higgins TL, Teres D, Copes WS, Nathanson BH, Stark M, Kramer AA. Assessing contemporary intensive care unit outcome: an updated Mortality Probability Admission Model (MPM0-III) Crit Care Med. 2007;35(3):827–835. doi: 10.1097/01.CCM.0000257337.63529.9F. [DOI] [PubMed] [Google Scholar]
- 4.Metnitz PGH, Moreno RP, Almeida E, Jordan B, Bauer P, Campos RA, et al. SAPS 3: from evaluation of the patient to evaluation of the intensive care unit. Part 1: objectives, methods and cohort description. Intensive Care Med. 2005;31(10):1336–1344. doi: 10.1007/s00134-005-2762-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moreno RP, Metnitz PGH, Almeida E, Jordan B, Bauer P, Campos RA, et al. SAPS 3: from evaluation of the patient to evaluation of the intensive care unit. Part 2: development of a prognostic model for hospital mortality at ICU admission. Intensive Care Med. 2005;31(10):1345–1355. doi: 10.1007/s00134-005-2763-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Associação Brasileira de Terapia Intensiva (AMIB). Regulamento técnico para funcionamento de unidades de Terapia Intensiva—AMIB. http://www.amib.org.br/fileadmin/RecomendacoesAMIB.pdf. 2009 [cited 2016 Jun 6].
- 7.de Oliveira VM, Brauner JS, Rodrigues Filho E, Susin RGA, Draghetti V, Bolzan ST, et al. Is SAPS 3 better than APACHE II at predicting mortality in critically ill transplant patients? Clin Sao Paulo Braz. 2013;68(2):153–158. doi: 10.6061/clinics/2013(02)OA06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soares M, Salluh JIF. Validation of the SAPS 3 admission prognostic model in patients with cancer in need of intensive care. Intensive Care Med. 2006;32(11):1839–1844. doi: 10.1007/s00134-006-0374-4. [DOI] [PubMed] [Google Scholar]
- 9.Soares M, Silva UVA, Teles JMM, Silva E, Caruso P, Lobo SMA, et al. Validation of four prognostic scores in patients with cancer admitted to Brazilian intensive care units: results from a prospective multicenter study. Intensive Care Med. 2010;36(7):1188–1195. doi: 10.1007/s00134-010-1807-7. [DOI] [PubMed] [Google Scholar]
- 10.Costa e Silva VT, de Castro I, Liano F, Muriel A, Rodriguez-Palomares JR, Yu L. Performance of the third-generation models of severity scoring systems (APACHE IV, SAPS 3 and MPM-III) in acute kidney injury critically ill patients. Nephrol Dial Transplant. 2011;26(12):3894–3901. doi: 10.1093/ndt/gfr201. [DOI] [PubMed] [Google Scholar]
- 11.Nassar Junior AP, Mocelin AO, Andrade FM, Brauer L, Giannini FP, Nunes ALB, et al. SAPS 3, APACHE IV or GRACE: which score to choose for acute coronary syndrome patients in intensive care units? Sao Paulo Med J Rev Paul Med. 2013;131(3):173–178. doi: 10.1590/1516-3180.2013.1313474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maccariello ER, Valente C, Nogueira L, Ismael M, Valenca RVR, Machado JES, et al. Performance of six prognostic scores in critically ILL patients receiving renal replacement therapy. Rev Bras Ter Intensiva. 2008;20(2):115–123. doi: 10.1590/S0103-507X2008000200001. [DOI] [PubMed] [Google Scholar]
- 13.Alves CJ, Franco GPP, Nakata CT, Costa GLG, Costa GLG, Genaro MS, et al. Evaluation of prognostic indicators for elderly patients admitted in intensive care units. Rev Bras Ter Intensiva. 2009;21(1):1–8. doi: 10.1590/S0103-507X2009000100001. [DOI] [PubMed] [Google Scholar]
- 14.Serpa Neto A, de Assuncao MSC, Pardini A, Silva E. Feasibility of transitioning from APACHE II to SAPS III as prognostic model in a Brazilian general intensive care unit. A retrospective study. Sao Paulo Med. J Rev Paul Med. 2015;133(3):199–205. doi: 10.1590/1516-3180.2013.8120014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silva Junior JM, Malbouisson LMS, Nuevo HL, Barbosa LGT, Marubayashi LY, Teixeira IC, et al. Applicability of the simplified acute physiology score (SAPS 3) in Brazilian hospitals. Rev Bras Anestesiol. 2010;60(1):20–31. doi: 10.1590/S0034-70942010000100003. [DOI] [PubMed] [Google Scholar]
- 16.Nassar APJ, Mocelin AO, Nunes ALB, Giannini FP, Brauer L, Andrade FM, et al. Caution when using prognostic models: a prospective comparison of 3 recent prognostic models. J Crit Care. 2012;27(4):423.e1–423.e7. doi: 10.1016/j.jcrc.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 17.Keegan MT, Gajic O, Afessa B. Severity of illness scoring systems in the intensive care unit. Crit Care Med. 2011;39(1):163–169. doi: 10.1097/CCM.0b013e3181f96f81. [DOI] [PubMed] [Google Scholar]
- 18.Soares M, Bozza FA, Angus DC, Japiassu AM, Viana WN, Costa R, et al. Organizational characteristics, outcomes, and resource use in 78 Brazilian intensive care units: the ORCHESTRA study. Intensive Care Med. 2015;41(12):2149–2160. doi: 10.1007/s00134-015-4076-7. [DOI] [PubMed] [Google Scholar]
- 19.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 20.Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5(6):649–655. doi: 10.1097/00000421-198212000-00014. [DOI] [PubMed] [Google Scholar]
- 21.Vincent JL, Moreno R, Takala J, Willatts S, De Mendonca A, Bruining H, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22(7):707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 22.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837–845. doi: 10.2307/2531595. [DOI] [PubMed] [Google Scholar]
- 23.Finazzi S, Poole D, Luciani D, Cogo PE, Bertolini G. Calibration belt for quality-of-care assessment based on dichotomous outcomes. PLoS ONE. 2011;6(2):e16110. doi: 10.1371/journal.pone.0016110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poole D, Rossi C, Latronico N, Rossi G, Finazzi S, Bertolini G. Comparison between SAPS II and SAPS 3 in predicting hospital mortality in a cohort of 103 Italian ICUs. Is new always better? Intensive Care Med. 2012;38(8):1280–1288. doi: 10.1007/s00134-012-2578-0. [DOI] [PubMed] [Google Scholar]
- 25.Kramer AA, Zimmerman JE. Assessing the calibration of mortality benchmarks in critical care: The Hosmer–Lemeshow test revisited. Crit Care Med. 2007;35(9):2052–2056. doi: 10.1097/01.CCM.0000275267.64078.B0. [DOI] [PubMed] [Google Scholar]
- 26.Peek N, Arts DGT, Bosman RJ, van der Voort PHJ, de Keizer NF. External validation of prognostic models for critically ill patients required substantial sample sizes. J Clin Epidemiol. 2007;60(5):491–501. doi: 10.1016/j.jclinepi.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 27.Reineck LA, Pike F, Le TQ, Cicero BD, Iwashyna TJ, Kahn JM. Hospital factors associated with discharge bias in ICU performance measurement. Crit Care Med. 2014;42(5):1055–1064. doi: 10.1097/CCM.0000000000000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1.Electronic Supplementary Material for External Validation of SAPS 3 and MPM0-III scores in 48,816 patients from 72 Brazilian ICUs.
Data Availability Statement
All relevant data are within the paper and ESM. Other information and dataset details are available from the corresponding author on reasonable request.


