Abstract
Twelve Holstein bulls were used in a 4 × 4 Latin square design to investigate the effects of using whole-crop wheat hay (WCWH) as a substitute for Leymus chinensis (LC) on apparent digestibility, plasma parameters, ruminal fermentation, and microbial communities. Experimental treatments were four proportions of WCWH, 0, 33, 67, and 100%, as a substitute for LC (WCWH0, WCWH33, WCWH67, and WCWH100, respectively). The WCWH100 group showed a higher nutritional intake of crude protein (CP) and higher apparent digestibility of organic matter (OM), CP, and ether extract (EE) than the WCWH0 group (P < 0.05). Urea N, NH3-N, isobutyrate and isovalerate levels were higher (P < 0.05) in the WCWH100 group than in the WCWH0 group. 16S rRNA high-throughput sequencing analysis revealed similarities in the community composition, species diversity and relative abundance of dominant bacteria at the phylum and genus levels among the four groups. Collectively, our data indicated that WCWH can be used to replace LC in the diet of finishing dairy bulls without having a negative impact on apparent digestibility, plasma parameters, and ruminal bacteria composition. These results offer the first deep insight into the effects of replacing LC with WCWH on the performance parameters and rumen microbiota in Holstein bulls, and may aid in ruminant farming.
Introduction
Leymus chinensis (LC), which is widely distributed throughout the Eurasian Steppe, including the Songnen Plain and the eastern Inner Mongolian Plateau in China, is a perennial species of Gramineae1. Although LC is considered a kind of middle-quality roughage, livestock farms must purchase LC, involving thousands of kilometres of shipping transportation, thereby contributing to a large amount of additional expense. Potential alternative roughage sources must be found due to the high long-distance transportation expenses of LC and the small profit margin of the ruminant industry.
Wheat is one of the most important crops and wheat straw is the second largest biomass feedstock in the world2. However, there are more than 110 million tons of wheat straw every year in China, and most of them cannot be fully used3. Burning wheat straw results in severe resource waste and air pollution4. Many studies have been conducted to attempt to make full use of wheat straw. Both Owens et al.5 and Keady et al.6 found that feeding fermented whole-crop wheat (WCW) to beef cattle increased dry matter intake (DMI) and improved rumen fermentation. Weinbery et al.7 suggested that prolonged storage might decrease the dry matter (DM) and neutral detergent fibre (NDF) digestibility values. In addition, Geough et al.8 found that increasing the grain to straw + chaff ratios of WCW silage in the diets of finishing beef steers can serve as an effective method for enteric methane abatement. However, little information can be found about the effects of whole-crop wheat hay (WCWH) on apparent digestibility, plasma parameters and rumen fermentation of Holstein bulls. Therefore, using WCWH in place of LC is of high interest and an immediate necessity. Furthermore, replacing LC with WCWH can save energy, reduce pollution, and promote the integration of agriculture and animal husbandry.
The rumen is inhabited by diverse microbial symbionts, which play a major role in digesting and transforming a fraction of the feed into microbial proteins and volatile fatty acids9. The ruminal bacteria are closely associated with the feed, and different forages strongly influence the rumen metabolism, thus affecting the rumen microbiota10. For example, using 16S rRNA gene-cloning library technology, Kong et al.11 found different species in the bacterial communities of cows fed alfalfa or triticale diets. Yuhong et al.12 revealed that partial replacement of corn grain and cotton seed meal with ensiled mulberry leaves and sun-dried mulberry fruit pomace did not significantly affect the composition of the ruminal microflora. Thus, it is necessary to explore the changes in the rumen microbial community composition when the bulls are fed WCWH as a replacement for LC. To determine the most appropriate replacement level, multiple levels of LC replacement with WCWH were evaluated in this study. We hypothesize that WCWH can replace LC in the diet of finishing dairy bulls without negative effects. The purpose of this research was to determine the effects of WCWH as a substitute for LC on apparent digestibility, plasma parameters, rumen fermentation, and bacterial communities in Holstein bulls.
Results
Nutrient intake and apparent digestibility
The results of feed intake and apparent digestibility are listed in Table 1. The DM intake of bulls fed WCWH0 was significantly higher than that of bulls fed WCWH33 (P < 0.05), but was not different from that in the other groups. The OM intake of bulls fed WCWH0 was higher than that of bulls fed WCWH33 or WCWH100 (P < 0.05). However, bulls fed WCWH67 or WCWH100 had a higher CP intake (P < 0.001), and a lower NDF and acid detergent fibre (ADF) intake (P < 0.01) than bulls fed WCWH0. The EE intake was not significantly affected by the treatments (P > 0.05). DM, NDF and ADF apparent digestibility did not differ significantly among the dietary groups (P > 0.05). However, bulls fed WCWH100 had a significantly higher apparent digestibility of OM, CP and EE (P < 0.01) than bulls fed WCWH0.
Table 1.
The effects of treatments on feed intake and apparent digestibility of Holstein dairy bulls during the fattening period1,2.
| Item | Dietary treatment1 | |||||
|---|---|---|---|---|---|---|
| WCWH0 | WCWH33 | WCWH67 | WCWH100 | SEM3 | P-value | |
| Nutrient intake, kg/d | ||||||
| DM | 13.5a | 12.6b | 13.2ab | 12.9ab | 0.48 | 0.047 |
| OM | 12.7a | 11.7b | 12.2ab | 11.8b | 0.48 | 0.020 |
| CP | 1.51b | 1.47b | 1.65a | 1.71a | 0.06 | <0.001 |
| EE | 0.25 | 0.24 | 0.25 | 0.25 | 0.01 | 0.723 |
| NDF | 5.23a | 4.81b | 4.64bc | 4.38c | 0.21 | <0.001 |
| ADF | 2.74a | 2.61ab | 2.53b | 2.33c | 0.12 | <0.001 |
| Apparent digestibility of nutrients, % | ||||||
| DM | 76.9 | 74.2 | 77.6 | 78.5 | 2.90 | 0.191 |
| OM | 79.6bc | 77.4c | 81.4ab | 82.2a | 2.42 | <0.001 |
| CP | 74.2bc | 71.6c | 77.3ab | 81.0a | 2.89 | <0.001 |
| EE | 66.7b | 66.2b | 68.8b | 76.3a | 4.30 | 0.007 |
| NDF | 66.7 | 63.1 | 67.5 | 69.7 | 4.11 | 0.170 |
| ADF | 60.4 | 56.4 | 62.6 | 61.6 | 4.86 | 0.297 |
1WCWH0 = 0% of Leymus chinensis was replaced by whole-crop wheat hay; WCWH33 = 33% of Leymus chinensis was replaced by whole-crop wheat hay; WCWH67 = 67% of Leymus chinensis was replaced by whole-crop wheat hay; WCWH100 = 100% of Leymus chinensis was replaced by whole-crop wheat hay.
2a–c: Means within the same row without the same letter superscripts are significantly different (Tukey’s test; P < 0.05).
3SEM: Standard error of the mean.
Plasma metabolites
The effects of dietary groups on plasma metabolites are shown in Table 2. The WCWH100 treatment group had a higher level of urea N (P < 0.01) than the other dietary groups. However, no marked differences among the treatments were detected for the plasma glucose, triglyceride, cholesterol, total protein, albumin, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), and β-hydroxybutyrate (D3HB) concentrations.
Table 2.
The effects of dietary treatments on plasma biochemical parameters of Holstein dairy bulls during the fattening period1,2.
| Item | Dietary treatment1 | |||||
|---|---|---|---|---|---|---|
| WCWH0 | WCWH33 | WCWH67 | WCWH100 | SEM3 | P-value | |
| Glucose (mmol/L) | 4.22 | 4.38 | 4.40 | 4.47 | 0.22 | 0.454 |
| Triglyceride (mmol/L) | 0.17 | 0.18 | 0.16 | 0.17 | 0.03 | 0.509 |
| Cholesterol (mmol/L) | 2.56 | 2.73 | 2.69 | 2.71 | 0.28 | 0.806 |
| Total protein (g/L) | 62.6 | 64.4 | 63.1 | 64.5 | 4.30 | 0.898 |
| Albumin (g/L) | 29.8 | 31.4 | 31.0 | 31.7 | 1.67 | 0.417 |
| Urea N (mmol/L) | 2.58b | 2.77b | 2.75b | 3.15a | 0.22 | 0.007 |
| HDL-C4 (mmol/L) | 0.99 | 1.07 | 0.99 | 1.06 | 0.09 | 0.498 |
| LDL-C5 (mmol/L) | 0.82 | 0.84 | 0.85 | 0.56 | 0.09 | 0.939 |
| D3HB6 (mmol/L) | 0.22 | 0.24 | 0.20 | 0.23 | 0.03 | 0.367 |
1WCWH0 = 0% of Leymus chinensis was replaced by whole-crop wheat hay; WCWH33 = 33% of Leymus chinensis was replaced by whole-crop wheat hay; WCWH67 = 67% of Leymus chinensis was replaced by whole-crop wheat hay; WCWH100 = 100% of Leymus chinensis was replaced by whole-crop wheat hay.
2a–b: Means within the same row without the same letter superscripts are significantly different (Tukey’s test; P < 0.05).
3SEM: Standard error of the mean.
4High density lipoprotein cholesterol.
5Low density lipoprotein cholesterol.
6β-Hydroxybutyrate.
Rumen fermentation characteristics
The parameters of rumen fermentation characteristics are presented in Table 3. The pH values of rumen liquid were similar (P > 0.05) among the groups. The concentrations of NH3-N, isobutyrate and isovalerate in the WCWH100 group were higher (P < 0.01) than those in the WCWH0 group. There were no differences in the concentrations of total volatile fatty acids (TVFA), acetate, propionate, butyrate or in the acetate / propionate ratio among the dietary treatments (P > 0.05).
Table 3.
The effects of dietary treatments on rumen fermentation of Holstein dairy bulls during the fattening period1,2.
| Item | Dietary treatment1 | |||||
|---|---|---|---|---|---|---|
| WCWH0 | WCWH33 | WCWH67 | WCWH100 | SEM3 | P-value | |
| pH | 6.79 | 6.71 | 6.73 | 6.74 | 0.09 | 0.622 |
| NH3-N | 3.86c | 4.59bc | 6.34a | 5.49ab | 0.78 | <0.001 |
| TVFA4 (mmol/L) | 82.3 | 86.3 | 91.9 | 90.0 | 10.7 | 0.596 |
| VFA5 (mmol/L) | ||||||
| Acetate | 47.5 | 48.1 | 52.7 | 54.5 | 6.29 | 0.328 |
| Propionate | 20.0 | 20.4 | 21.7 | 20.8 | 3.70 | 0.925 |
| Isobutyrate | 0.32b | 0.46a | 0.48a | 0.54a | 0.08 | 0.002 |
| Butyrate | 12.9 | 15.3 | 15.0 | 12.1 | 2.51 | 0.226 |
| Isovalerate | 0.79c | 0.88bc | 1.16ab | 1.26a | 0.21 | 0.007 |
| Valerate | 0.78 | 1.20 | 0.91 | 0.82 | 0.26 | 0.108 |
| Acetate/Propionate | 2.57 | 2.46 | 2.51 | 2.74 | 0.24 | 0.390 |
1WCWH0 = 0% of Leymus chinensis was replaced by whole-crop wheat hay; WCWH33 = 33% of Leymus chinensis was replaced by whole-crop wheat hay; WCWH67 = 67% of Leymus chinensis was replaced by whole-crop wheat hay; WCWH100 = 100% of Leymus chinensis was replaced by whole-crop wheat hay.
2a–c: Means within the same row without the same letter superscripts are significantly different (Tukey’s test; P < 0.05).
3SEM: Standard error of the mean.
4Totalvolatile fatty acids.
5Volatile fatty acids.
Rumen bacteria composition in different diet groups
A total of 1,563,811 high-quality sequences, with an average of 32,579 sequences per sample, were retained after quality control and chimaera removal. These sequences, with an average length of 420 bp, were assigned to 2091 operational taxonomic units (OTUs) of rumen bacteria based on a 97% similarity cut-off. After normalization to 13,125 reads, richness estimates and diversity indices (Fig. 1, Supplementary Table 1) were evaluated. Our results indicated that richness estimates and diversity indices in the rumen microbiota did not differ significantly (P > 0.05). We further analysed the beta diversity, which is shown by principal coordinate analysis (PCoA) in Fig. 2, with colours representing different diet groups. PCoA plots of bacterial 16S rRNA showed no obvious clusters among the four diet groups using PC1 and PC2 (39.81% and 10.61%, respectively, of the explained variance). Meanwhile, the PERMANOVA results, which were analysed using the original distance matrix, indicated no marked differences among the four groups (P = 0.451). The PERMANOVA results for pair-wise tests between the groups were presented in Supplementary Table 2.
Figure 1.
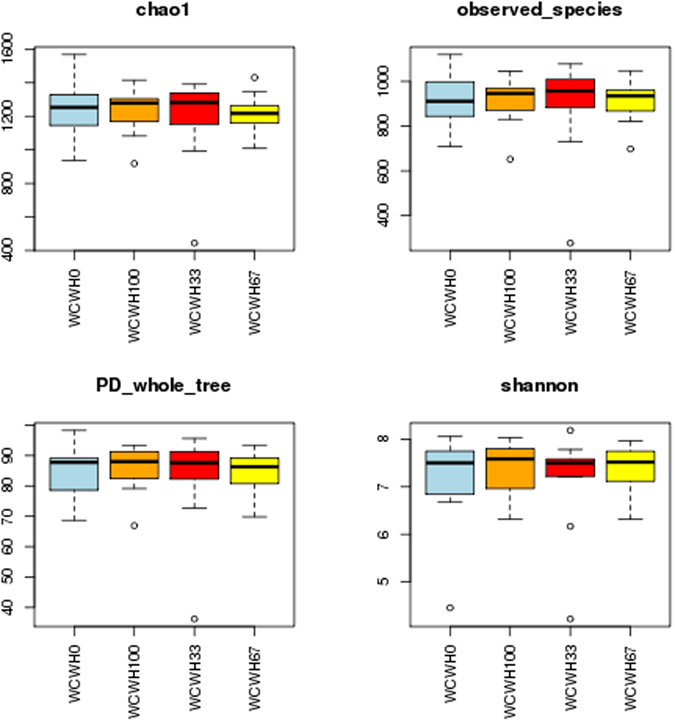
Richness estimates and diversity indices for bacteria within each diet group. The top and bottom boundaries of each box represent the 75th and 25th quartile values, respectively. The horizontal lines inside each box represent the median values.
Figure 2.
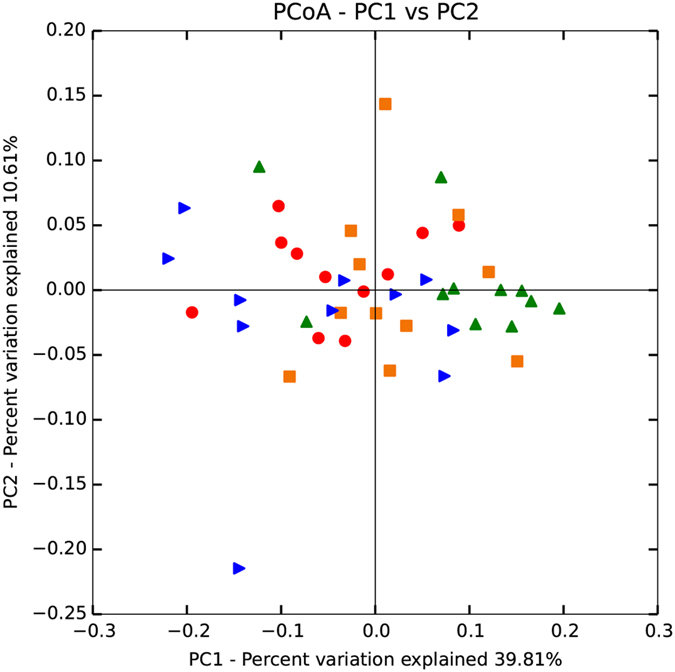
Principal Coordinate Analysis (PCoA) of rumen bacterial community structures of bulls in the four groups. The PCoA plots were constructed using the weighted UniFrac method.
Based on the taxonomic guidelines of the Silva project13, microbial compositions at the phylum and genus levels are presented in Figs 3 and 4, respectively. There were 21 identified phyla of rumen bacteria (Supplementary Table 3). In all samples, the most dominant phyla were Bacteroidetes (57.98%) and Firmicutes (35.20%), followed by Proteobacteria (1.96%) and Actinobacteria (1.35%). Minor phyla included Fibrobacteres (0.83%), Spirochaetae (0.77%), and Lentisphaerae (0.65%). The other known phyla occupied 1.15% of the rumen bacteria. The most abundant genera were Prevotella (33.86%), RC9 gut group (7.36%), Ruminococcus (6.23%), Flavonifractor (3.35%), and Succiniclasticum (2.15%). Minor genera, such as Incertae_sedis, Butyrivibrio, unidentified rumen bacterium RFN43, Saccharofermentans, Fibrobacter, Treponema, Pseudobutyrivibrio, Selenomonas, and Acetitomaculum, accounted for 1.61%, 1.47%, 1.33%, 0.91%, 0.83%, 0.70%, 0.67%, 0.62% and 0.55%, respectively, of the bacterial community. Other known genera accounted for 6.86% of the bacterial composition, and 31.51% of the sequences were unclassified at the genus level. Supplementary Table 4 shows the relative abundance of genera in all ruminal samples.
Figure 3.
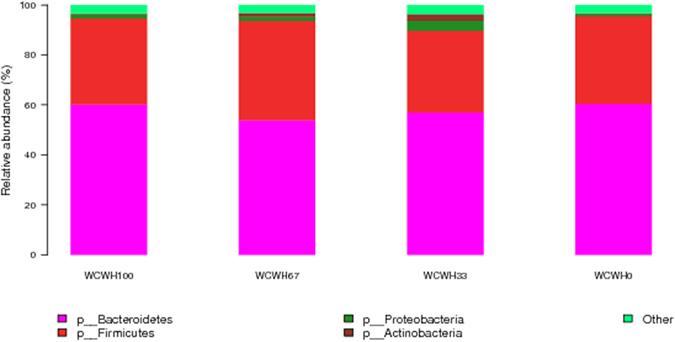
The effects of dietary treatments on the relative abundance of phyla in the ruminal bacterial community.
Figure 4.
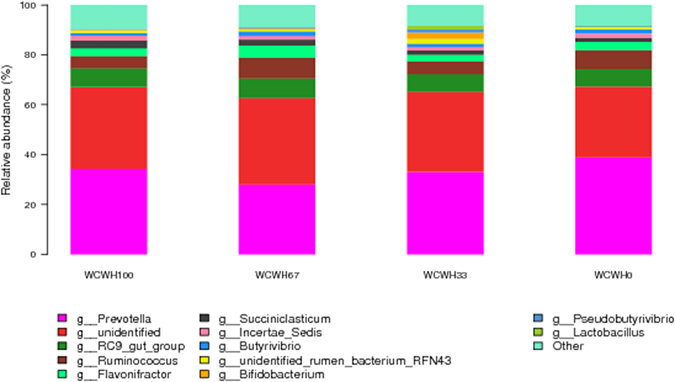
The effects of dietary treatments on the relative abundance of genera in the ruminal bacterial community.
Effects of dietary treatments on rumen microbial diversities and relative abundance
Replacing LC with WCWH did not affect the richness estimates and diversity indices (P > 0.05) (Fig. 1). The Shannon index represents species richness and evenness of the samples; Chao1 and observed species are primarily used to evaluate the richness of the microbial community.
Among all of the phyla detected, no significant differences were observed in ruminal bacteria community composition and relative abundance among the four groups (Fig. 3 and Supplementary Table 3). At the genus level, there were no significant differences among the four diet groups except for some minor genera, such as Staphylococcus, Saccharopolyspora and Laceyella (Fig. 4 and Supplementary Table 4).
Correlation analysis
The relationships between physiological / production parameters and genus abundance (representing at least 1% of the bacterial community in at least one sample) were evaluated in this study (Fig. 5). The results showed that the CP intake correlated positively with the abundance of RC9_gut_group (r = 0.998; P = 0.002), Succiniclasticum (r = 0.961; P = 0.039) and Ruminobacter (r = 0.987; P = 0.013). The apparent digestibility of ADF correlated positively with the abundance of Saccharofermentans (r = 0.956; P = 0.044), Moryella (r = 0.975; P = 0.025), Mogibacterium (r = 0.951; P = 0.049) and Desulfovibrio (r = 0.952; P = 0.048), and negatively with the abundance of Selenomonas (r = −0.980; P = 0.020). The apparent digestibility of NDF correlated positively with the abundance of Saccharofermentans (r = 0.968; P = 0.032), and negatively with the abundance of Anaeroplasma (r = −0.979; P = 0.021). The apparent digestibility of CP correlated positively with the abundance of RC9_gut_group (r = 0.961; P = 0.039) and Succiniclasticum (r = 0.975; P = 0.025). The concentration of urea N correlated negatively with the abundance of Acetitomaculum (r = −0.953; P = 0.047). The ruminal pH correlated positively with the abundance of Papillibacter (r = 0.959; P = 0.041) and Blautia (r = 0.982; P = 0.018). The concentration of NH3-N correlated positively with the abundance of the unidentified genus belonged to the phylum Firmicutes (r = 0.978; P = 0.022), Bacteroides (r = 0.968; P = 0.032), The concentration of acetate correlated positively with the abundance of RC9_gut_group (r = 0.983; P = 0.017), Succiniclasticum (r = 0.956; P = 0.044), Ruminobacter (r = 0.987; P = 0.013). The concentration of butyrate correlated positively with the abundance of Fibrobacter (r = 0.995; P = 0.005), and negatively with the abundance of Incertae_Sedis (r = −0.967; P = 0.033), Candidatus_Saccharimonas (r = −0.961; P = 0.039), and Clostridium_sensu_stricto_1 (r = −0.982; P = 0.018). The concentration of isovalerate correlated positively with the abundance of RC9_gut_group (r = 0.964; P = 0.036), Ruminobacter (r = 0.976; P = 0.024), and Bacteroides (r = 0.954; P = 0.046).
Figure 5.
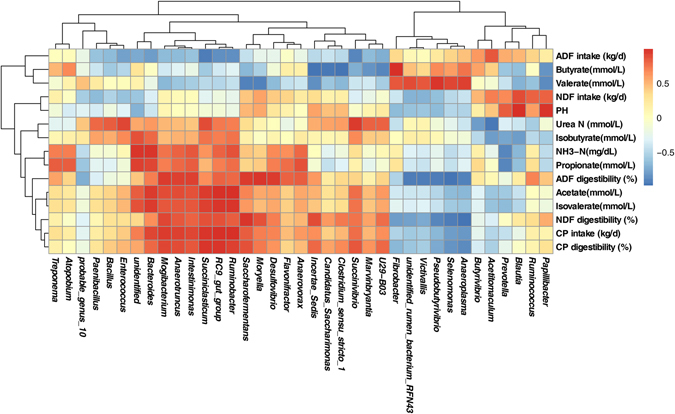
Correlation between physiological / production parameter and genus abundance. The top and left hierarchical cluster based on the corresponding correlation matrix between physiological / production parameters and genus abundance. The similar clusters were found with complete linkage method. Only the predominant bacterial genera (representing at least 1% of the bacterial community in at least one sample) are presented; Cells are coloured based on the Pearson correlation coefficient between physiological / production parameter and genus abundance. The red colour represents a positive correlation; the blue colour represents a negative correlation.
Discussion
Feed intake can be affected by both physiological factors and external factors, including dietary chemical composition, environmental conditions, and animal learning abilities14, 15. Regarding chemical components, DMI generally decreases with increasing NDF concentration16. In the present study, the WCWH0 diet had a higher NDF content but had a significantly higher DMI. This maybe because the WCWH0 diet had a numerically higher DM digestion rate and faster flow from the reticulo-rumen17. Additionally, the greater intake of OM, NDF, and ADF in the WCWH0 group may have been related to greater OM, NDF, and ADF content in the WCWH0 diet (Table 4)18, reflecting the chemical composition of the diet consumed in the case of the similar DMI. Steen et al.19 reported that digestibility was a crucial factor in regulating forage intake. However, there were lower intake of OM, NDF and ADF but higher OM digestibility and similar digestibility of NDF and ADF in the WCWH100 diet group. These findings were consistent with previous reports20, indicating that digestibility was not the dominant factor controlling forage intake. The significantly higher CP intake of bulls fed the WCWH100 diet compared with the WCWH0 diet might be caused by bulls favouring the WCWH100 diet and ingesting more.
Table 4.
Ingredients and nutrient compositions of the dietary treatments.
| Item | Dietary Treatments1 | |||
|---|---|---|---|---|
| WCWH0 | WCWH33 | WCWH67 | WCWH100 | |
| Ingredient, % (DM basis) | ||||
| corn grain | 22.3 | 24.1 | 25.9 | 27.6 |
| wheat grain | 26.5 | 23.8 | 21.1 | 18.5 |
| soybean meal | 3.45 | 2.31 | 1.14 | 0.00 |
| rapeseed meal | 0.70 | 1.54 | 2.39 | 3.23 |
| wheat bran | 0.00 | 1.14 | 2.32 | 3.47 |
| NaHCO3 | 0.55 | 0.55 | 0.55 | 0.55 |
| NaCl | 0.55 | 0.55 | 0.55 | 0.55 |
| limestone | 0.44 | 0.48 | 0.51 | 0.55 |
| mineral-vitamin premix2 | 0.55 | 0.55 | 0.55 | 0.55 |
| WCWH | 0.00 | 14.9 | 30.2 | 45.0 |
| CWR | 45.0 | 30.2 | 14.9 | 0.00 |
| Chemical composition3, % (DM basis) | ||||
| OM | 94.0 | 92.8 | 91.8 | 91.1 |
| CP | 12.1 | 12.2 | 12.2 | 12.1 |
| EE | 1.02 | 1.02 | 1.02 | 1.02 |
| NDF | 31.9 | 29.9 | 27.9 | 25.9 |
| ADF | 17.5 | 16.6 | 15.7 | 14.9 |
1WCWH0 = 0% of Leymus chinensis was replaced by whole-crop wheat hay; WCWH33 = 33% of Leymus chinensis was replaced by whole-crop wheat hay; WCWH67 = 67% of Leymus chinensis was replaced by whole-crop wheat hay; WCWH100 = 100% of Leymus chinensis was replaced by whole-crop wheat hay.
2Every kilogram of mineral–vitamin premix contained the following: 3500 IU Vitamin A, 3000 IU Vitamin D3, 45 IU Vitamin E, 63 mg Zn, 60 mg Fe, 98.6 mg Mn, 20 mg Cu, 1.1 mg I, 0.50 mg Se, and 0.45 mg Co.
3OM = organic matter; CP = crude protein; NDF = neutral detergent fibre; ADF = acid detergent fibre.
In this study, DM apparent digestibility had no significant differences among the treatments because the diets could have remained in the rumen for the similar length of time and had similar enzymatic activity, resulting in similar DM digestibility21. The higher apparent digestibility of OM and EE in the WCWH100 diet group than in the WCWH0 diet group was expected. These differences could be attributed to the high starch content of the WCWH100 diet, which was easily digested and absorbed by animals20. The higher CP digestibility in the WCWH100 group was expected and can be explained by the faster and more extensive CP degradation of wheat grain in the rumen22. Walsh, K. et al.23 observed that lower rumen pH values selectively restricted the activity of cellulolytic bacteria thereby reducing the fibre digestibility. Therefore, the undifferentiated digestibility of NDF and ADF among dietary treatments could be attributed to the normal and similar rumen pH values generating similar levels of activity of cellulolytic bacteria.
In the present study, the concentrations of plasma glucose and triglyceride were similar among the dietary treatments potentially due to the similar DM intake and ruminal propionate concentration. Jolazadeh et al.24 reported that similar DM intake had no effect on Holstein bulls plasma glucose and triglyceride concentrations. Moreover, propionate from rumen fermentation is the major substrate for hepatic gluconeogenesis in ruminants23, 25. Plasma cholesterol, HDL, and LDL were not affected by the diets and showed no hepatic or cardiac impairment in bulls26. The absence of effects of dietary treatments on the plasma albumin and total protein concentrations possibly resulted from the similar flow of microbial protein to the intestine27 and the amount of amino acids available for absorption28.
The plasma urea N was produced by protein hydrolysis and amino acid metabolism, and its concentration depends on the level of crude protein26. In the current study, the WCWH100 group had a greater plasma urea concentration than the other groups, which could be attributed to the relatively higher protein intake of WCWH29, 30. Butyrate, which comes from rumen fermentation, is mostly converted to D3HB through the rumen epithelium31. Therefore, the undifferentiated plasma D3HB concentrations among treatment diets resulted from similar butyrate concentrations in the rumen during fermentation, which is consistent with the findings of Thorp et al.32.
In this study, rumen pH values were in the normal 6.4–6.8 range33, indicating that the rumen internal environment was static to some extent when WCWH was substituted for LC. Rumen NH3-N is the degradation product of feed protein34. The greater NH3-N concentrations in the WCWH100 group resulted from the relatively higher protein intake and digestibility in the WCWH100 treatment. Fibre-degrading ruminal bacteria utilize ammonia as their primary source of N for growth35. Therefore, increasing the ammonia level improves fibre digestion in the rumen. The total VFA concentrations were unaffected among the groups, which might be the result of the same ratio of forage to concentration of treatment diets36, or might be caused by the fact that the output of total VFA was equivalent to the amount of absorption in the rumen among all treatments37. In the present research, the observed concentrations of acetate and propionate, and the acetate to propionate ratio were not significantly different, probably because of the similar ruminal pH values caused by the dietary-associated shifts38.
Isobutyrate and isovalerate levels significantly increased with increasing proportions of WCWH, which were due to the increased metabolism caused by the WCWH diets39. Consistent with our report, branched-chain VFAs (BCVFAs) are particularly required by most fibre-degrading bacteria and can increase fibre degradation40. In response to WCWH100, interesting links exist among BCVFA, NH3-N, OM and CP digestibility. The increased ruminal NH3-N concentrations suggested that the increased OM digestibility promoted the capacity of the rumen microbes to utilize the NPN (Non-Protein Nitrogen) supplied in the diets41. In addition, the increased ammonia concentrations in bulls fed the WCWH100 diet may indicate a declined utilization efficiency of NH3-N by the rumen microorganisms42, resulting from an increase in the proportion of BCVFAs observed in the WCWH100 group43. BCVFAs are mainly end products of protein fermentation and are, along with ammonia, often used as quick indicators of protein fermentation in the rumen44. Thus, the higher concentration of BCVFAs was also associated with increases in protein digestibility and ammonia concentration.
In the present study, Good’s coverage estimate was 0.98, indicating that the research recovered 98% of all OTUs computed at a 0.97 similarity. The similar richness estimates and diversity indices indicated that the rumen microbial diversity was similar among the four diet groups45. In general, diet is considered the most important determining factor of microbial composition46. In our study, the four groups had the same concentrate to forage ratio, as well as the same levels of protein and energy, and the bulls were raised under the same ambient conditions, which might account for the similarities in rumen microbial diversity. In addition, the PCoA examining the phylogenetic divergence among the OTUs did not significantly cluster, further indicating the similarities of microbial communities in different treatment groups. The rumen microbial community is a complex network and ferments the roughage ingested by the ruminant. There is a dependence among host physiology, diet composition, and microbial richness47–49. The dominant microbes must take the responsibility for a large amount of the transformation of ingested forage, especially of protein, cellulose, starch, and so on, because these are the principal energy-yielding substrates that are used for the growth of microorganisms50. In this study, the most abundant phyla were Bacteroidetes, Firmicutes, and Proteobacteria, which are the main bacterial phyla and play important roles in rumen fermentation51–53. Based on the data set presented in the current study, Bacteroidetes was more predominant than Firmicutes, consistent with the result of Jami and Mizrahi54, who reported that the rumen pH was approximately 6.51 and the Bacteroidetes proportion (50%) was higher than that of Firmicutes (43%). However, the lower rumen pH may result in a substantially decreased proportion of Bacteroidetes and an increased proportion of Firmicutes in the rumen microbial community55. Therefore, these studies indicated that the phylum Bacteroidetes maybe predominant instead of Firmicutes in the case of the normal pH range caused by our experimental diets. In our study, the Proteobacteria represented 1.96% of total bacteria in rumen. In general, the phylum Proteobacteria is dominant in the neonatal stage, followed by a sudden and sharp decline in its proportion, with Proteobacteria reaching the lowest proportion while that of Bacteroidetes becomes the highest56.
Additionally, Bacteroidales are capable of degrading cellulose, and their genomes encode degradable ability of plant polysaccharide57, 58. Therefore, the lack of differences in Bacteroidales abundance might explain the similar ADF digestibility in the current study. Within Bacteroidetes, the most dominant genus was Prevotella in the rumen, accounting for 47% of the total bacterial sequences. For ruminants, Prevotella owns the dipeptidyl peptidase type IV activity of rate-limiting, which is responsible for cleaving oligopeptides. Therefore, it plays an important role in protein metabolism, especially in breaking down oligopeptides in the rumen59. The different treatments did not affect the relative abundance of Prevotella, similar to the findings of a previous study60. Prevotella is a predominant organism in bulls fed both forage and grain48 and contributes to the majority of hereditary and metabolic variety of the microflora61.
Within the phylum Firmicutes, Ruminococcus is a predominant genus that tended to decrease numerically with increasing WCWH substitution levels. Ruminococcus contains two kinds of powerful fibre-degrading bacteria, Ruminobacter albums and Ruminococcus flavefaciens, which can produce large amounts of cellulase and hemicellulase to decompose plant fibre62. The fibre content decreased with the increasing WCWH substitution levels, and the proportion of Ruminococcus in the bacterial community decreased because of the lesser amount of substrate fibre available for them. The genus Succiniclasticum, which represents up to 2.15% of the total bacterial sequences, is involved in fermenting succinate and transforming it into propionate63. Additionally, the proportion of Succiniclasticum among the total sequences tended to increase numerically with increasing WCWH substitution level, which may indicate resource competition among the rumen bacteria64. This finding can be attributed to the ability of Succiniclasticum to decompose starch in the rumen of cattle49. In the present research, Succiniclasticum can lead to a trend of decrease in NDF digestibility with an increase in NDF content when diets have the same levels of protein and energy. In addition, this study found Butyrivibrio (belonging to Firmicutes) at 1.47% among the total bacteria in the rumen of Holstein bulls. The species of Butyrivibrio, which can produce mucosal butyrate and release butyrate close to the epithelium, may improve the bioavailability of butyrate for the host65.
Fibrobacteres species are main cellulolytic bacteria in the rumen45. In our results, the sequences of Fibrobacteres accounted for an average of only 0.83% of the total bacterial community, which was similar to a previous study by Zened et al.45. The minor and indifferent abundance of Fibrobacteres might account for the lack of change in NDF and ADF digestibility. Within the phylum Spirochaetae, the genus Treponema, which commonly exists in the rumen, is involved in degrading soluble fibre. Compared with the WCWH0 group, the other groups had not different abundance of Treponema, potentially because of similar pectin concentrations among the four treatments12.
In the present study, correlation analysis revealed that there was a relationship between nutrition intake /digestibility and rumen microbial proportion. In particular, the increased intake and apparent digestibility of CP were associated with the genera RC9_gut_group and Succiniclasticum, which belong to the phyla Bacteroidetes and Firmicutes, respectively. As reported previously, the nitrogen source in protein is essential to the proliferation of RC9_gut_group 66. Additionally, we concluded that the Succiniclasticum may play a role in the degradation of protein. In addition, both plasma metabolites and rumen metabolites were relevant to microorganisms. For instance, the correlation analysis showed that the concentrations of NH3-N and isovalerate were linked to enrichments in Bacteroides, which has been postulated to be able to degrade cellulose58. Isovalerate can be utilized by fibre-degrading ruminal bacteria and may be related to the increased ammonia concentration40, 44. In general, further studies are needed to evaluate the relationships between physiological parameters and other rumen microbes, such as protozoa, archaea, and fungi in the complex system of rumen microorganisms.
There has been no previous research examining the effects of WCWH as a substitute for LC on apparent digestibility, plasma parameters, rumen fermentation and especially the rumen microbiota of Holstein dairy bulls as outlined in our study. In conclusion, based on 16S rRNA high-throughput sequencing of ruminal microbiota, this study showed a detailed account of bacteria in the rumen and their relative abundances under the influence of the tested diets. The study revealed that using WCWH as a substitute for LC did not negatively affect apparent digestibility, plasma parameters, and the ruminal bacteria composition. However, as with many previous studies12, 45, 56, limiting the study to collect rumen fluid may result in underrepresentation of fibre-degrading microflora. Thus, we will collect both rumen fluid and solid to study microbiota in future research. The rumen microflora is a complex ecosystem that degrades forage for ruminants. It is necessary to further study whether other rumen microbes, such as protozoa, archaea, and fungi, also affect rumen characteristics.
Materials and Methods
Ethics Statement
Our experimental procedures were approved by the Animal Welfare and Ethics Committee of China Agricultural University (Permit No. DK1008). All experiments were performed according to the approved Regulations for the Administration of Affairs Concerning Experimental Animals (The State Science and Technology Commission of P. R. China, 1988).
Experimental design, animal management and diet
The feeding trial was carried out at the Xinzhicheng Dairy Farm (Zhuozhou City, Hebei Province, China). In this study, 12 Holstein dairy bulls during the fattening period (body weight = 485.0 ± 40.8 kg) were allocated to one of four dietary groups in a 4 × 4 Latin square design. Each period consisted of a 17-d adaptation period and a 5-d sampling period. All of the bulls were inoculated with vaccines, de-wormed, weighed, and marked with numbered identification tags before the start of the experiment.
The WCW was harvested at the milky ripe stage and at a stubble height of 10 to 15 cm in Zhuozhou City, Hebei Province, China. Subsequently, wheat was dried in the sun and stored for animal feed. The LC was obtained via long-distance transport from Northeast China. Experimental treatments were composed of four dietary levels of WCWH, 0, 33, 67, and 100%, as a substitute proportion of LC. The diets consisted of 55% concentrate and 44% roughage, were formulated to be isonitrogenous and isocaloric, and met the nutritional requirements of beef cattle67. Chemical constituents and ingredients of the trial diets are shown in Table 4. Bulls were fed twice daily at 7:00 and 17:00, allowing 5 to 10% orts, and fresh drinking water was provided ad libitum.
Sample Collection
During d18-d22 of each period, the roughage, concentrate, and orts were collected and weighed. Faecal samples were collected from the rectum daily at 6:00, 12:00, 18:00, and 24:00 from d19-d21. The feed, orts, and faecal samples were dried at 65 °C and smashed using a mill (Wiley, A. H. Thomas Co., Philadelphia, PA, USA) with a 1-mm screen, and then stored at −20 °C for further analysis of CP, DM, OM, NDF, ADF, and acid-insoluble ash (AIA).
On d18 of each period, blood (approximately 10 mL) was collected via the jugular vein into tubes containing Na-heparin before the morning feeding. Samples were centrifuged at 3,000 × g for 20 min at 4 °C to collect plasma, separated into three aliquots, and frozen at −20 °C for subsequent biochemical index analyses.
Approximately 100 mL of ruminal sample consisting of a mixture of liquids and solids was obtained from the oesophageal tube 2 h after morning feeding on day 22. The pH was immediately determined using a portable pH metre (HJ-90B, Aerospace Computer Company, Beijing, China). Next, 0.25 mL of metaphosphoric acid (25 g / 100 mL) was added to four aliquots of 1 ml rumen fluid, which were centrifuged at 15,000 × g at 4 °C for 15 min to determine VFA and NH3–N concentrations. Three aliquots of 1-mL samples were taken and kept in liquid N for rumen bacterial 16S rRNA analysis.
Chemical analysis
The EE, DM, and ash of feeds, refusal, and faecal samples were measured by methods No. 920.39, 934.01 and 924.05 of the Association of Official Analytical Chemists (AOAC 1990)68, respectively. The OM content was calculated using the following formula: 100- the percentage of ash. ADF and NDF were examined using the method described by Vansoest et al.69 using the Ankom Fibre Analyser (Ankom Technology, Fairport, NY). CP was measured according to the Kjeldahl method (AOAC, 1990; method 990.03).
Apparent digestibility in the digestive tract was calculated using the endogenous tracer AIA. The AIA values of the diets, orts, and faeces were analysed following the method described by Van Keulen and Young70, with the formula depicted by Zhong et al.71. The formula is as follows: D = [1 − (Ad × Nf) / (Af × Nd)] × 100, where Ad (g / kg) and Af (g / kg) represent the AIA of the diet and faeces, respectively, and Nd (g / kg) and Nf (g / kg) represent the nutrient contents of the diet and faeces, respectively.
The plasma biochemical indicators were determined using an automated biochemistry analyser (Hitachi 7020; Hitachi Co., Tokyo, Japan). β-Hydroxybutyric acid, glucose, triglyceride, total protein, and plasma urea nitrogen concentrations were determined using commercial test kits (Beijing Jiuqiang Bio-Technique Co., Beijing, China) with hydroxybutyric acid dehydrogenase, glucose oxidase, glycerophosphate oxidase, biuret, and urease, respectively. The levels of total cholesterol, albumin, HDL-C, and LDL-C in plasma were determined using an enzyme method, a bromocresol green method, and a direct measurement method, respectively.
The rumen liquid NH3–N was measured according to Bremner and Keeney72 using a spectrophotometer (UV-1700, Shimadzu Corporation, Kyoto, Japan). The VFA was quantified using a high-performance gas chromatograph (HPGC; GC-2014; Shimadzu Corporation) that was equipped with a hydrogen flame detector and a capillary column (Agilent, Technologies, Wilmington, DE, USA; 30 m long, 0.32 mm diameter, 0.50 µm film).
DNA extraction and 16S rRNA pyrosequencing
First, a 1.5 mL sample of rumen fluid was centrifuged at 1,000 × g for 10 min to discard sediment, and then the clear supernatant extract was removed by centrifugation at 12,000 × g for 10 min. Subsequently, the DNA of homogenized rumen fluid was extracted using a bacterial DNA Kit (Omega Bio-Tek, Norcross, GA, USA) according to the manufacturer’s protocol. DNA concentration and purity were evaluated by using a spectrophotometer (UV-1700, Shimadzu Corporation). Bacterial 16S rRNA genes of the V3-V4 region were amplified from extracted DNA using the barcoded primers 338 F (5′-ACTCCTACGGGAGGCAGCAG-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′). Each 50 μL PCR mixture consisted of a DNA sample (30 ng), a forward primer (2 μL; 10.0 μmol/L), a reverse primer (2 μL; 10.0 μmol / L), DNA template (4 μL; 2.5 μmol / L), sterile distilled water (36.7 μL), 10 × Pyrobest Buffer (5 μL), and Pyrobest DNA Polymerase (0.3 μL; 2.5 U / μL, TaKaRa Code: DR005A). The amplicon mixture was applied to the MiSeq Genome Sequencer (Illumina, San Diego, CA, USA) under the following conditions: 95 °C for 5 min, followed by 25 cycles of 95 °C for 30 s, 56 °C for 30 s, and 72 °C for 40 s, along with an extension at 72 °C for 10 min and storage at 4 °C. Three PCR replicates were performed for each sample. These PCR products were pooled, purified, and then quantified using a blue fluorescence quantitative system (Quanti FluorTM-ST, Promega Corporation, Madison, WI, USA). Finally, high-throughput sequencing was performed using the Illumina MiSeq platform (San Diego, CA, USA) according to the manufacturer’s instructions.
Sequence analyses
The sequences were analysed using the Quantitative Insights Into Microbial Ecology (QIIME) V1.8 pipeline73. Low-quality reads, which were selected based on sequence length, quality, primers, and tags, were removed in QIIME V1.8. The Uchime algorithm74 carried out in Usearch soft ware was applied to remove chimeric sequences. Short tags were removed using Mothur V.1.33.375. The cluster command in Mothur was used to classify the clean and high-quality sequences as bacteria. Downstream analysis (richness estimates, diversity indices and beta diversity) for OTU classification was conducted for bacteria community comparisons. The OTUs were defined by Uclust76 with a 97% similarity cut-off. To obtain corresponding species classification information of each OTU, the Ribosomal Database Project (RDP) classifier algorithm77 was used for comparative analysis of the representative OTU sequences, and all species information at various levels (phylum, genus) were annotated. Richness estimates and diversity indices including Chao 1, observed species, Good’s coverage, phylogenetic diversity whole tree (PD whole tree), and Shannon’s index were calculated using the QIIME V1.8 pipeline73. A PCoA78 based on the weighted UniFrac distances was conducted to compare all samples, and a distance-based matrices analysis (PERMANOVA) was performed to evaluate differences among samples. The vegan package in the QIIME software was used for performing PERMANOVA.
Statistical analysis
All data were statistically analysed using PROC MIXED in SAS 9.0 (SAS Institute Inc., Cary, NC, USA) according to the following model: Yijk = μ + Pi + Tj + Ck + eijk, where Yijk is the dependent variable, μ is the general mean, Pi is the fixed effect of period (i = 1, 2, 3, or 4), Tj is the fixed effect of treatment (i = 1, 2, 3, or 4), Ck is the fixed effect of cattle and eijk is the residual effect. Differences of means in different treatments were tested using Duncan’s multiple range test and considered statistically significant at P < 0.05.
Correlation analysis was performed between plasma metabolites, nutrient intake / digestibility, rumen metabolites, and microbial proportion by Pearson’s correlation test using R software79. The top and left hierarchical cluster based on the corresponding correlation matrix between physiological / production parameters and genus abundance. The similar clusters were found with complete linkage method. Significance was declared at P < 0.05.
Electronic supplementary material
Acknowledgements
This study was supported by the National Modern Agricultural Technology System (CARS-38) in China, and the Herbivore Livestock Fattening and Technique of High Quality Meat Produce Research of South China (201303144).
Author Contributions
W.N. designed the experiments and wrote the main manuscript text. Y.H. prepared Tables 1 and 2. C.X. prepared Table 3, and M.A. and H.W. revised the manuscript and analysed the results. Y.L., L.J., Q.Q., and T.S. conducted the experiments. B.C. is the corresponding author and designed the experiments. All authors reviewed the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-02258-2
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chen, S. et al. Transcriptome analysis in sheepgrass (Leymus chinensis): a dominant perennial grass of the Eurasian Steppe. Plos one8, e67974, doi:10.1371/journal.pone.0067974 (2013). [DOI] [PMC free article] [PubMed]
- 2.Talebnia F, Karakashev D, Angelidaki I. Production of bioethanol from wheat straw: An overview on pretreatment, hydrolysis and fermentation. Bioresource Technol. 2010;101:4744–4753. doi: 10.1016/j.biortech.2009.11.080. [DOI] [PubMed] [Google Scholar]
- 3.Zeng, J., Gong, D. H., Tian, Y., Wang, D. & Li, D. Study on the Process of Pretreatment and Hydrolysis of Lignocellulosic Wheat Straw Through Alkali-enzymatic Method. Academic Periodical of Farm Products Processing (2007).
- 4.Li L, et al. Wheat straw burning and its associated impacts on Beijing air quality. Science in China Series D: Earth Sciences. 2008;51:403–414. doi: 10.1007/s11430-008-0021-8. [DOI] [Google Scholar]
- 5.Owens D, McGee M, Boland T, O’Kiely P. Rumen fermentation, microbial protein synthesis, and nutrient flow to the omasum in cattle offered corn silage, grass silage, or whole-crop wheat. J Anim Sci. 2009;87:658–668. doi: 10.2527/jas.2007-0178. [DOI] [PubMed] [Google Scholar]
- 6.Keady TWJ, Lively FO, Kilpatrick DJ, Moss BW. Effects of replacing grass silage with either maize or whole-crop wheat silages on the performance and meat quality of beef cattle offered two levels of concentrates. Animal. 2007;1:613. doi: 10.1017/S1751731107685024. [DOI] [PubMed] [Google Scholar]
- 7.Weinberg ZG, Chen Y. Effects of storage period on the composition of whole crop wheat and corn silages. Anim Feed Sci Tech. 2013;185:196–200. doi: 10.1016/j.anifeedsci.2013.08.009. [DOI] [Google Scholar]
- 8.Mc Geough EJ, et al. Methane emissions, feed intake, performance, digestibility, and rumen fermentation of finishing beef cattle offered whole-crop wheat silages differing in grain content. J Anim Sci. 2010;88:2703–2716. doi: 10.2527/jas.2009-2750. [DOI] [PubMed] [Google Scholar]
- 9.Kim M, Morrison M, Yu Z. Status of the phylogenetic diversity census of ruminal microbiomes. Fems Microbiol Ecol. 2011;76:49–63. doi: 10.1111/j.1574-6941.2010.01029.x. [DOI] [PubMed] [Google Scholar]
- 10.Zhu W, et al. Effects of dietary forage sources on rumen microbial protein synthesis and milk performance in early lactating dairy cows. J Dairy Sci. 2013;96:1727–1734. doi: 10.3168/jds.2012-5756. [DOI] [PubMed] [Google Scholar]
- 11.Kong Y, Teather R, Forster R. Composition, spatial distribution, and diversityofthebacterial communities in therumenof cows feddi¡erent forages. Fems Microbiol Ecol. 2010;74:612–622. doi: 10.1111/j.1574-6941.2010.00977.x. [DOI] [PubMed] [Google Scholar]
- 12.Niu Y, et al. Effects of Diets Supplemented with Ensiled Mulberry Leaves and Sun-Dried Mulberry Fruit Pomace on the Ruminal Bacterial and Archaeal Community Composition of Finishing Steers. Plos One. 2016;11:e156836. doi: 10.1371/journal.pone.0156836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Quast C, et al. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 2012;41:D590–D596. doi: 10.1093/nar/gks1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.NRC Nutrient Requirements of beef Cattle. 7th ed. Natl. Acad. Sci. Washington, DC (2000).
- 15.Forbes, P. Integration of learning and metabolic signals into a theory of dietary choice and food intake. Pages 3–19 in Ruminant Physiology: Digestion, Metabolism, Growth and Reproduction. Cronje, P. ed. CAB Int., Wallingford, UK (2000).
- 16.Arelovich, H. M. C. S. Effects of dietary neutral detergent fiber on intakes of dry matter and net energy by dairy and beef cattle: Analysis of published data. Prof. Anim. Sci. 375–383 (2008).
- 17.Allen MS. Effects of Diet on Short-Term Regulation of Feed Intake by Lactating Dairy Cattle. J Dairy Sci. 2000;83:1598–1624. doi: 10.3168/jds.S0022-0302(00)75030-2. [DOI] [PubMed] [Google Scholar]
- 18.Rustas BO, Nadeau E, Johnsson S. Effect of stage of maturity of whole-crop barley on intake and liveweight gain by dairy steers differing in initial live weight. Grass Forage Sci. 2009;64:227–235. doi: 10.1111/j.1365-2494.2009.00688.x. [DOI] [Google Scholar]
- 19.Steen RWJ, et al. Factors affecting the intake of grass silage by cattle and prediction of silage intake. Anim. Sci. 1998;66:115–127. doi: 10.1017/S1357729800008894. [DOI] [Google Scholar]
- 20.Browne EM, Juniper DT, Bryant MJ, Beever DE. Apparent digestibility and nitrogen utilisation of diets based on maize and grass silage fed to beef steers. Anim Feed Sci Tech. 2005;119:55–68. doi: 10.1016/j.anifeedsci.2004.12.001. [DOI] [Google Scholar]
- 21.Del Bianco Benedeti P, et al. Partial Replacement of Ground Corn with Glycerol in Beef Cattle Diets: Intake, Digestibility, Performance, and Carcass Characteristics. Plos One. 2016;11:e148224. doi: 10.1371/journal.pone.0148224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herrera-saldana RE, huber JT, poore MH. Dry Matter, Crude Protein, and Starch Degradability of Five Cereal Grains1. I Dairy Sci. 1990;73:2386–2393. doi: 10.3168/jds.S0022-0302(90)78922-9. [DOI] [Google Scholar]
- 23.Walsh K, et al. Intake, digestibility and rumen characteristics in cattle offered whole-crop wheat or barley silages of contrasting grain to straw ratios. Anim Feed Sci Tech. 2009;148:192–213. doi: 10.1016/j.anifeedsci.2008.03.013. [DOI] [Google Scholar]
- 24.Jolazadeh AR, Dehghan-banadaky M, Rezayazdi K. Effects of soybean meal treated with tannins extracted from pistachio hulls on performance, ruminal fermentation, blood metabolites and nutrient digestion of Holstein bulls. Anim Feed Sci Tech. 2015;203:33–40. doi: 10.1016/j.anifeedsci.2015.02.005. [DOI] [Google Scholar]
- 25.France, J. & Dijkstra, J. Volatile fatty acid production. In Dijkstra, J., Forbes, J.M., France, J. (Eds), Quantitative Aspects of Ruminant Digestion and Metabolism 2nd ed. CABI Publishing, Wallingford, Oxfordshire, UK, 157–175 (2005).
- 26.Zhou Z, Zhou B, Ren L, Meng Q. Effect of Ensiled Mulberry Leaves and Sun-Dried Mulberry Fruit Pomace on Finishing Steer Growth Performance, Blood Biochemical Parameters, and Carcass Characteristics. plos one. 2014;9:e85406. doi: 10.1371/journal.pone.0085406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abarghuei MJ, Rouzbehan Y, Salem AZM, Zamiri MJ. Nitrogen balance, blood metabolites and milk fatty acid composition of dairy cows fed pomegranate-peel extract. Livest Sci. 2014;164:72–80. doi: 10.1016/j.livsci.2014.03.021. [DOI] [Google Scholar]
- 28.Min BR, Barry TN, Attwood GT, McNabb WC. The effect of condensed tannins on the nutrition and health of ruminants fed fresh temperate forages: a review. Anim Feed Sci Tech. 2003;106:3–19. doi: 10.1016/S0377-8401(03)00041-5. [DOI] [Google Scholar]
- 29.Owens D, McGee M, Boland T, O’Kiely P. Rumen fermentation, microbial protein synthesis, and nutrient flow to the omasum in cattle offered corn silage, grass silage, or whole-crop wheat. J Anim Sci. 2009;87:658–668. doi: 10.2527/jas.2007-0178. [DOI] [PubMed] [Google Scholar]
- 30.Yang WZ, Li YL, McAllister TA, McKinnon JJ, Beauchemin KA. Wheat distillers grains in feedlot cattle diets: Feeding behavior, growth performance, carcass characteristics, and blood metabolites. J Anim Sci. 2012;90:1301–1310. doi: 10.2527/jas.2011-4372. [DOI] [PubMed] [Google Scholar]
- 31.Bergman EN. Energy contributions of volatile fatty acids from the gastrointestinal tract in various species. Physiol Rev. 1990;70:567–590. doi: 10.1152/physrev.1990.70.2.567. [DOI] [PubMed] [Google Scholar]
- 32.Thorp CL, Wylie ARG, Steen RWJ, Shaw C, McEvoy JD. Effects of incremental changes in forage:concentrate ratio on plasma hormone and metabolite concentrations and products of rumen fermentation in fattening beef steers. J Anim Sci. 2000;71:93–109. doi: 10.1017/S1357729800054928. [DOI] [Google Scholar]
- 33.Jouany JP. Optimizing rumen functions in the close-up transition period and early lactation to drive dry matter intake and energy balance in cows. Anim Reprod Sci. 2006;96:250–264. doi: 10.1016/j.anireprosci.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 34.Wang S, Wang W, Wang J, Tan Z, Gong Y. Effects of dietary concentrate-to-forage ratio on runnen fermentation and performance of dairy COWS. Journal of Northwest A 8L F University (Nat Sci. Ed.) 2007;35:44–50. [Google Scholar]
- 35.Bryant MP. Nutritional requirements of the predominant rumen cellulolytic bacteria. Federation Proceedings. 1973;32:1809–1813. [PubMed] [Google Scholar]
- 36.Friedt AD, McAllister TA, He ML, Penner GB, McKinnon JJ. Effects of replacing barley grain with graded levels of wheat bran on rumen fermentation, voluntary intake and nutrient digestion in beef cattle. Can J Anim Sci. 2014;94:129–137. doi: 10.4141/cjas2013-139. [DOI] [Google Scholar]
- 37.Feng, Y. Ruminant nutrition Beijing: Science Press, 74–77 (2004).
- 38.Nemati M, et al. Effect of different alfalfa hay levels on growth performance, rumen fermentation, and structural growth of Holstein dairy calves. J Anim Sci. 2016;94:1141–1148. doi: 10.2527/jas.2015-0111. [DOI] [PubMed] [Google Scholar]
- 39.Cui JH, et al. Effect of urea fertilization on biomass yield, chemical composition, in vitro rumen digestibility and fermentation characteristics of forage oat straw in Tibet of China. The Journal of Agricultural Science. 2016;154:914–927. doi: 10.1017/S0021859616000198. [DOI] [Google Scholar]
- 40.Van Gylswyk NO. The effect of supplementing a low-protein hay on the cellulolytic bacteria in the rumen of sheep and on the digestibility of cellulose and hemicellulose. J. Agric. Sci. 1970;74:169–174. doi: 10.1017/S0021859600021122. [DOI] [Google Scholar]
- 41.Abdalla AL, Sutton JD, Phipps RH, Humphries DJ. Digestion in the rumen of lactating dairy cows given mixtures of urea-treated whole-crop wheat and grass silage. Anim.Sci. 1999;69:203–212. doi: 10.1017/S1357729800051249. [DOI] [Google Scholar]
- 42.Dijkstra J, Mills JAN, France J. The role of dynamic modelling in understanding the microbial contribution to rumen function. Nutr. Res. Rev. 2002;15:67–90. doi: 10.1079/NRR200237. [DOI] [PubMed] [Google Scholar]
- 43.Suárez BJ, Van Reenen CG, Stockhofe N, Dijkstra J, Gerrits WJJ. Effect of Roughage Source and Roughage to Concentrate Ratio on Animal Performance and Rumen Development in Veal Calves. J Dairy Sci. 2007;90:2390–2403. doi: 10.3168/jds.2006-524. [DOI] [PubMed] [Google Scholar]
- 44.Yang CMJ. Response of forage fiber degradation by ruminal microorganisms to branched-chain volatile fatty acids, amino acids, and dipeptides. J. Dairy Sci. 2002;85:1183–1190. doi: 10.3168/jds.S0022-0302(02)74181-7. [DOI] [PubMed] [Google Scholar]
- 45.Zened, A., Combes, S., Cauquil, L., Mariette, J. & Klopp, C. Microbial ecology of the rumen evaluated by 454 GS FLX pyrosequencing is affected by starch and oil supplementation of diets. Fems Microbiol Ecol (2013). [DOI] [PubMed]
- 46.Li RW, et al. The effect of helminth infection on the microbial composition and structure of the caprine abomasal microbiome. Sci Rep-UK. 2016;6:20606. doi: 10.1038/srep20606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jami, E., White, B. A. & Mizrahi, I. Potential Role of the Bovine Rumen Microbiome in Modulating Milk Composition and Feed Efficiency. Plos One9 (2014). [DOI] [PMC free article] [PubMed]
- 48.Pitta DW, et al. Longitudinal shifts in bacterial diversity and fermentation pattern in the rumen of steers grazing wheat pasture. ANAEROBE. 2014;30:11–17. doi: 10.1016/j.anaerobe.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 49.Henderson G, et al. Rumen microbial community composition varies with diet and host, but a core microbiome is found across a wide geographical range. Sci Rep-UK. 2015;5:14567. doi: 10.1038/srep14567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Van Soest, P. J. Nutritional Ecology of the Ruminant 2 edn (Cornell University Press, 1994).
- 51.Zhang R, Zhu W, Zhu W, Liu J, Mao S. Effect of dietary forage sources on rumen microbiota, rumen fermentation and biogenic amines in dairy cows. J Sci Food Agr. 2014;94:1886–1895. doi: 10.1002/jsfa.6508. [DOI] [PubMed] [Google Scholar]
- 52.Singh KM, et al. Microbial profiles of liquid and solid fraction associated biomaterial in buffalo rumen fed green and dry roughage diets by tagged 16S rRNA gene pyrosequencing. Mol Biol Rep. 2015;42:95–103. doi: 10.1007/s11033-014-3746-9. [DOI] [PubMed] [Google Scholar]
- 53.Wetzels SU, et al. Pyrosequencing reveals shifts in the bacterial epimural community relative to dietary concentrate amount in goats. J Dairy Sci. 2015;98:5572–5587. doi: 10.3168/jds.2014-9166. [DOI] [PubMed] [Google Scholar]
- 54.Jami EMI. Composition and Similarity of Bovine Rumen Microbiota across Individual Animals. Plos One. 2012;7:e33306. doi: 10.1371/journal.pone.0033306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hook SE, et al. Impactof subacuteruminal acidosis (SARA) adaptationand recoveryon the densityand diversityof bacteria in therumenof dairy cows. Fems Microbiol Ecol. 2011;78:275–284. doi: 10.1111/j.1574-6941.2011.01154.x. [DOI] [PubMed] [Google Scholar]
- 56.Wang L, et al. Exploring the Goat Rumen Microbiome from Seven Days to Two Years. Plos One. 2016;11:e154354. doi: 10.1371/journal.pone.0154354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pope PB, et al. Metagenomics of the Svalbard Reindeer Rumen Microbiome Reveals Abundance of Polysaccharide Utilization Loci. Plos One. 2012;7:e38571. doi: 10.1371/journal.pone.0038571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Naas AE, et al. Do Rumen Bacteroidetes Utilize an Alternative Mechanism for Cellulose Degradation? Mbio. 2014;5:e1401–e1414. doi: 10.1128/mBio.01401-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.WALLACE RJ. Conference: Altering Ruminai Nitrogen Metabolism to Improve Protein utilization. Journal of Nutrition. 1996;126:1326S–1334S. doi: 10.1093/jn/126.suppl_4.1324S. [DOI] [PubMed] [Google Scholar]
- 60.Stevenson DM, Weimer PJ. Dominance of Prevotella and low abundance of classical ruminal bacterial species in the bovine rumen revealed by relative quantification real-time PCR. Appl Microbiol Biot. 2007;75:165–174. doi: 10.1007/s00253-006-0802-y. [DOI] [PubMed] [Google Scholar]
- 61.Purushe J, et al. Comparative Genome Analysis of Prevotella ruminicola and Prevotella bryantii: Insights into Their Environmental Niche. Microb Ecol. 2010;60:721–729. doi: 10.1007/s00248-010-9692-8. [DOI] [PubMed] [Google Scholar]
- 62.Wood TM, Wilson CA, Stewart CS. Preparation of the cellulase from the cellulolytic anaerobic rumen bacterium Ruminococcus albus and its release from the bacterial cell wall. Biochem J. 1982;205:129–137. doi: 10.1042/bj2050129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vangylswyk N. Succiniclasticum ruminis gen. nov., sp. nov., a Ruminal Bacterium Converting Succinate to Propionate as the Sole Energy-Yielding Mechanism. Int. J Syst Bacteriol. 1995;45:297–300. doi: 10.1099/00207713-45-2-297. [DOI] [PubMed] [Google Scholar]
- 64.Myer PR, Smith TPL, Wells JE, Kuehn LA, Freetly HC. Rumen Microbiome from Steers Differing in Feed Efficiency. Plos One. 2015;10:e129174. doi: 10.1371/journal.pone.0129174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Baldwin RT, et al. Quantification of Transcriptome Responses of the Rumen Epithelium to Butyrate Infusion using RNA-seq Technology. Gene Regul Syst Bio. 2012;6:67–80. doi: 10.4137/GRSB.S9687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fan P, Liu P, Song P, Chen X, Ma X. Moderate dietary protein restriction alters the composition of gut microbiota and improves ileal barrier function in adult pig model. Sci Rep-UK. 2017;7:43412. doi: 10.1038/srep43412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Subcommittee On Beef Cattle Nutrition, C.O. & Animal Nutrition, N.R.C. Nutrient Requirements of Beef Cattle. National Academy Press, 103 (2000).
- 68.AOAC: Official methods of analysis. 15th edition. Washington, DC: Association of Official Analytical Chemists (1990).
- 69.Vansoest PJ, Robertson JB, Lewis BA. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. Journal Of Dairy Science. 1991;74:3583–3597. doi: 10.3168/jds.S0022-0302(91)78551-2. [DOI] [PubMed] [Google Scholar]
- 70.Van Keulen JYBA. Evaluation of acid-insoluble ash as a natural marker in ruminant digestibility studies. J Anim Sci. 1977;44:282–287. doi: 10.2527/jas1977.442282x. [DOI] [Google Scholar]
- 71.Zhong RZ, Li JG, Gao YX, Tan ZL, Ren GP. Effects of Substitution of Different Levels of Steam-Flaked Corn for Finely Ground Corn on Lactation and Digestion in Early Lactation Dairy Cows. J Dairy Sci. 2008;91:3931–3937. doi: 10.3168/jds.2007-0957. [DOI] [PubMed] [Google Scholar]
- 72.Bremner JM, Keeney DR. Steam Distillation Methods for Determination of Ammonium Nitrate and Nitrite. Anal Chim Acta. 1965;32:485–495. doi: 10.1016/S0003-2670(00)88973-4. [DOI] [Google Scholar]
- 73.Caporaso JG, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics. 2011;27:2194–2200. doi: 10.1093/bioinformatics/btr381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schloss PD, et al. Introducing mothur: Open-Source, Platform-Independent, Community-Supported Software for Describing and Comparing Microbial Communities. Appl Environ Microb. 2009;75:7537–7541. doi: 10.1128/AEM.01541-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Edgar RC. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010;26:2460–2461. doi: 10.1093/bioinformatics/btq461. [DOI] [PubMed] [Google Scholar]
- 77.Cole JR, et al. The Ribosomal Database Project: improved alignments and new tools for rRNA analysis. Nucleic Acids Res. 2009;37:D141–D145. doi: 10.1093/nar/gkn879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lozupone C, Knight R. UniFrac: a New Phylogenetic Method for Comparing Microbial Communities. Appl Environ Microbiol. 2005;71:8228–8235. doi: 10.1128/AEM.71.12.8228-8235.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.R Core Team. R: A language and environment for statistical computing (R Foundation for Statistical Computing, Vienna, Austria, 2014).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


