Abstract
Non-typeable Haemophilus influenzae (NTHi) is the most common bacterial cause of infection of the lower airways in adults with chronic obstructive pulmonary disease (COPD). Infection of the COPD airways causes acute exacerbations, resulting in substantial morbidity and mortality. NTHi has evolved multiple mechanisms to establish infection in the hostile environment of the COPD airways, allowing the pathogen to persist in the airways for months to years. Persistent infection of the COPD airways contributes to chronic airway inflammation that increases symptoms and accelerates the progressive loss of pulmonary function, which is a hallmark of the disease. Persistence mechanisms of NTHi include the expression of multiple redundant adhesins that mediate binding to host cellular and extracellular matrix components. NTHi evades host immune recognition and clearance by invading host epithelial cells, forming biofilms, altering gene expression and displaying surface antigenic variation. NTHi also binds host serum factors that confer serum resistance. Here we discuss the burden of COPD and the role of NTHi infections in the course of the disease. We provide an overview of NTHi mechanisms of persistence that allow the pathogen to establish a niche in the hostile COPD airways.
Keywords: non-typeable Haemophilus influenzae, chronic obstructive pulmonary disease, persistent infection, mechanisms of persistence, immune evasion
A review of mechanisms of persistence used by non-typeable Haemophilus influenzae to infect the airways of individuals with chronic obstructive pulmonary disease
INTRODUCTION
Infections by non-typeable Haemophilus influenzae (NTHi) are a major cause of exacerbations in adults with chronic obstructive pulmonary disease (COPD) (Murphy 2015). Exacerbations of COPD and otitis media are the most prevalent infections caused by NTHi. These infections result in missed time from work, emergency room visits, hospitalizations and respiratory failure (Guarascio et al. 2013; Murphy 2015; Anderson 2016). Nasopharyngeal colonization provides a reservoir of strains that infect the lower airways of individuals with COPD and cause acute exacerbations (King 2012). NTHi persists in the airways of individuals with COPD for months to years, contributing to chronic airway inflammation that results in worsening of symptoms (Murphy et al. 2004; Desai et al. 2014). NTHi infections and persistent infection of the lower airways expedites disease progression (Anzueto 2010; Finney et al. 2014). No vaccine is yet available to prevent NTHi infection in COPD (Murphy et al. 2009; Murphy 2015). Here we discuss NTHi infections in the context of COPD with emphases on defined mechanisms of persistence, including adhesins, biofilms, intracellular survival, IgA protease, genetic variability, nutrient acquisition, toxin-antitoxins and outer membrane vesicles. We describe how these factors contribute to persistent infection and provide avenues of research that will advance our understanding of NTHi infection in COPD. Understanding molecular mechanisms of pathogenesis will guide future research that will lead to the development of therapeutic and preventive interventions. Better ways to treat and prevent these infections in the setting of COPD will have an important impact on the burden of disease caused by NTHi infections.
CHRONIC OBSTRUCTIVE PULMONARY DISEASE AND NON-TYPEABLE HAEMOPHILUS INFLUENZAE
Chronic obstructive pulmonary disease
COPD is a debilitating respiratory disease and a major cause of morbidity and mortality (Finney et al. 2014; Anderson 2016). COPD affects 24 million Americans and is the third leading cause of death in the USA and the world (Jemal et al. 2005; Decramer, Janssens and Miravitlles 2012). The direct annual healthcare costs from COPD in the USA are estimated to be $30 billion, and an additional $20 billion from indirect costs (Guarascio et al. 2013).
The primary etiological factor in COPD is exposure to tobacco smoke (Puljic and Pahl 2004; Sethi and Murphy 2008; Guarascio et al. 2013; Finney et al. 2014; Anderson 2016; Lopez-Campos, Tan and Soriano 2016). Other important causes include tobacco exposure from secondary and tertiary smoking, occupational exposure to dust, noxious fumes and vapors, and outdoor and indoor pollution, especially burning biomass fuels indoors for cooking and heating (Finney et al. 2014; Anderson 2016; Lopez-Campos, Tan and Soriano 2016). Interestingly, individuals who have never smoked comprise 10%–30% of all COPD patients (Puljic and Pahl 2004; Lopez-Campos, Tan and Soriano 2016). Approximately 30%–40% of disease is related to causes other than tobacco smoke, including genetic predisposition, damaged airways due to prenatal maternal smoke exposure and childhood infection (Anderson 2016; Lopez-Campos, Tan and Soriano 2016).
COPD is a common, preventable disease characterized by persistent respiratory symptoms and airflow limitation (Adamson et al. 2011; Guarascio et al. 2013). COPD is an umbrella term that includes emphysema, bronchiolitis/small airways disease and chronic bronchitis (Puljic and Pahl 2004; Adamson et al. 2011; Anderson 2016). Emphysema results in destruction of lung parenchyma, specifically in the alveoli. The damage to the alveoli results in decreased surface area for gas exchange, restricting oxygen uptake and carbon dioxide release. Elastin fibers normally function as springs to keep alveoli open during expiration, but these too are destroyed in emphysema, causing alveoli to collapse and trap air within the lungs, particularly during exercise (Anderson 2016). Bronchiolitis, or small airway disease, manifests as inflammation and fibrosis of the smaller airways, further limiting the airflow. Chronic bronchitis is excessive mucus in the airway lumen due to goblet cell metaplasia and hypersecretion of mucus (Adamson et al. 2011; Anderson 2016).
Impact of exacerbations
The course of COPD is characterized by a progressive decline in lung function, with intermittent acute exacerbations of worsening symptoms (Fernaays et al. 2006b; Anzueto 2010; Finney et al. 2014; Murphy 2015). These periods of acute exacerbation are clinically defined by increased sputum production, sputum purulence and dyspnea compared to baseline symptoms (Sethi and Murphy 2008; Murphy and Parameswaran 2009; Finney et al. 2014; Murphy 2015). Exacerbations become more frequent as COPD progresses, increasing from an average of 2.68 events per year in patients with ‘moderate’ COPD to 3.43 events per year in patients with ‘severe’ COPD, as defined by the GOLD (Global Initiative for Chronic Obstructive Lung Disease) standards (Anzueto 2010; GOLD-Report 2017).
An acute exacerbation of COPD lasts between 7 and 10 days, but some symptoms may last longer. At 8 weeks, 20% of patients have not recovered to their pre-exacerbation state. Patients who experience frequent exacerbations, defined as having two or more exacerbations per year, have worse health status and higher morbidity compared to patients with less frequent exacerbations (Seemungal et al. 2000).
Recurrent exacerbations are associated with an accelerated progressive loss of lung function (Eldika and Sethi 2006; Anzueto 2010). COPD exacerbations result in approximately 110 000 deaths and more than 500 000 hospitalizations per year in the USA (Anzueto 2010). Exacerbations are the largest cost for the treatment of COPD, with over $18 billion spent in direct costs annually (Anzueto 2010; GOLD-Report 2017). Hospitalizations account for 58% of the total cost, followed by medication at 32.3% (Anzueto 2010).
The gradual loss of lung function associated with COPD may be attributed in part to an increased concentration of inflammatory markers found in sputum, even during clinically stable periods, suggestive of persistent inflammation and continued lung damage (Anzueto 2010). Neutrophils are attracted to the airway lumen during exacerbations, and increased concentration of neutrophils in sputum correlated with rapid decline in FEV1 over a 15-year follow-up study (Sethi et al. 2008; Anzueto 2010).
Infection in the course of COPD
Infection is the predominant cause of exacerbations; bacteria, viruses and atypical bacteria (Mycoplasma and Chlamydia) are implicated in up to 80% of acute exacerbations, with air pollution and other environmental factors accounting for the other 20% (Eldika and Sethi 2006; Sethi and Murphy 2008). Bacteria play a direct causative role in acute exacerbations, and are responsible for 50% of all acute exacerbations. Bacteria are detected in clinically significant concentrations in the lower airways of 4% of healthy adults, 29% of adults with COPD and 54% of adults during exacerbations of COPD (Sethi and Murphy 2008). In a longitudinal study that involved collecting monthly sputum samples from patients with COPD, an exacerbation was diagnosed in 33% of visits at which a new bacterial strain was isolated compared with 15.4% of visits at which no new strain was present (Sethi et al. 2002). Hence, the acquisition of new strains of bacteria plays an essential role in the pathogenesis of exacerbations (Sethi and Murphy 2008).
In addition to causing acute exacerbations, bacteria are often present in the lower airways of COPD patients during clinically stable periods (Murphy et al. 2004). Pathogens are detected in 25%–50% of sputum, bronchial brushings, bronchoalveolar lavage and bronchial biopsy specimens from adults with stable COPD. Bacteria in the airways promote inflammation, damage to the lower airways and elicit host responses (Sethi and Murphy 2008).
NTHi in exacerbations of COPD
The most common bacterial pathogens that cause exacerbations include NTHi, Moraxella catarrhalis, Streptococcus pneumoniae and Pseudomonas aeruginosa (Sethi and Murphy 2008). NTHi is the most common bacterial pathogen isolated from the lower airways in COPD patients, both during exacerbations and clinically stable periods (Bandi et al. 2001; Sethi et al. 2002). NTHi infections are responsible for approximately half of bacterial exacerbations, translating to between 20% and 30% of all acute exacerbations of COPD (Sethi and Murphy 2008; Finney et al. 2014; King and Sharma 2015).
Characteristics and identification of Haemophilus influenzae
Haemophilus influenzae is non-motile Gram negative of the family Pasteurellaceae that is pleomorphic in shape, with morphologies ranging from coccobacillus to filamentous bacillus (Herbert, Crook and Moxon 1998). Haemophilus influenzae is a fastidious facultative anaerobe that requires both Factor X (hemin) and Factor V (nicotinamide adenine dinucleotide—NAD) for growth. Humans are the only known reservoir of H. influenzae (Agrawal and Murphy 2011).
Strains of H. influenzae are generally classified as either encapsulated or non-encapsulated based on the presence of a polysaccharide capsule comprised of polyribosylribitol phosphate (Agrawal and Murphy 2011; De Chiara et al. 2014; Langereis and de Jonge 2015). Encapsulated strains produce one of six capsular serotypes (a–f) determined by their specific reactivity to sera. Typeable strains are mainly responsible for invasive diseases (Agrawal and Murphy 2011; Langereis and de Jonge 2015). The capsule provides resistance to complement killing and phagocytosis during invasive infection (Noel et al. 1992). Haemophilus influenzae type b (Hib) strains were the most common cause of invasive disease, as they were once responsible for severe morbidity and mortality in children prior to the introduction of the Hib conjugate vaccines (Agrawal and Murphy 2011; Langereis and de Jonge 2015). Encapsulated non-type b strains, including types a, c, e and f, are less common causes of invasive infection in selected populations (Tsang et al. 2014).
Non-encapsulated strains lack capsule and therefore do not react to sera against any capsular type rendering them non-typeable. Non-typeable strains primarily cause mucosal infections, though there are a growing number of reports of invasive NTHi infections (Langereis and de Jonge 2015). Encapsulated strains that cause disease are clonal, whereas the population of non-typeable strains that cause both localized and invasive infections is genetically diverse (Meats et al. 2003; Erwin et al. 2005; De Chiara et al. 2014).
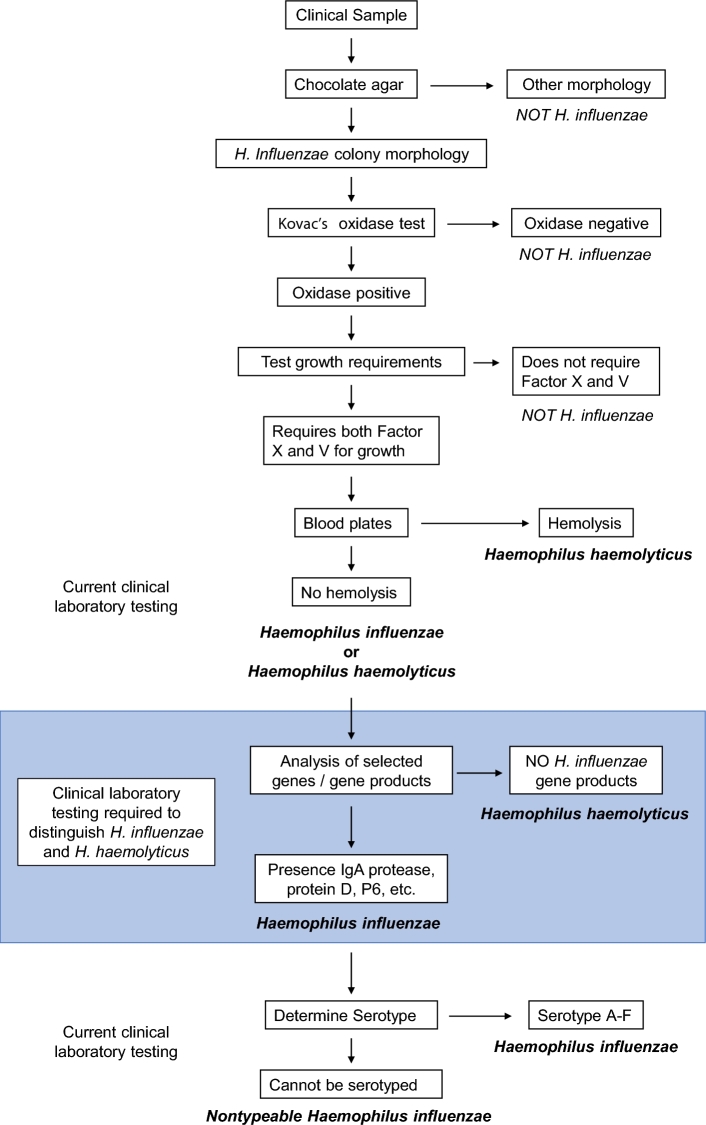
The fastidious growth requirements of hemin and NAD are used to identify H. influenzae in the clinical microbiology laboratory. Chocolate agar, incubated between 33°C and 37°C and 5%–10% CO2, is commonly used to isolate Haemophilus species in clinical samples. Colonies of H. influenzae appear translucent, tannish, moist, smooth and convex, with a distinct odor described as ‘mousy’ or ‘bleachlike’. Encapsulated strains of H. influenzae grow larger and more mucoid than non-typeable strains. Haemophilus influenzae is catalase and oxidase positive. Testing for hemin and NAD requirements using impregnated disks or strips or Haemophilus ID Quad plates are used widely in clinical microbiology laboratories. Haemophilus influenzae is unable to convert delta-aminolevulinic acid into porphyrins or porphobilinogen, hence is negative for the porphyrin test as well (Mahon, Lehman and Manuselis 2011).
Haemophilus haemolyticus and H. influenzae both require hemin and NAD. Based on clinical microbiology laboratory standards, the two species are distinguished from one another by the presence of hemolysis by strains of H. haemolyticus (Murray et al.2007; Garcia 2010). However, recent work has established that not all strains of H. haemolyticus are hemolytic (Murphy et al. 2007; Murray et al.2007). Thus, H. haemolyticus strains are frequently misidentified as H. influenzae by the standard methods that are used widely in clinical microbiology laboratories (Murphy et al. 2007; Price et al. 2015; Hu et al. 2016). This confusion has broad implications on the accurate assessment of clinical diagnosis, epidemiological studies regarding carriage, prevalence of antibiotic resistance and assessment of the role of Haemophilus species in disease (Price et al. 2015; Hu et al. 2016). Up to 40% of isolates from sputum samples identified as NTHi using widely accepted clinical microbiology laboratory standards are actually H. haemolyticus (Murphy et al. 2007; Hu et al. 2016).
Additional testing beyond an assessment of hemolysis is required for strains of Haemophilus species that require hemin and NAD for growth (Fig. 1). Differences in several features between the species can be used, including the P6 protein, the presence of the IgA protease gene, protein D and others. Genetic methods discriminate H. influenzae from H. haemolyticus, but recombination between the species may confound such methods (Price et al. 2015). A dual-target approach increases sensitivity and specificity in the accurate discrimination of H. haemolyticus and H. influenzae (Hu et al. 2016). Distinguishing between NTHi and H. haemolyticus has important implications in understanding the role of NTHi infection in COPD because NTHi causes exacerbations whereas H. haemolyticus does not (Murphy et al. 2007).
Figure 1.
Identification of NTHi in the clinical microbiology laboratory. Shaded area represents additional laboratory testing required to reliably differentiate NTHi from H. haemolyticus, currently indistinguishable by standard laboratory testing. Factor V = nicotinamide-adenine-dinucleotide (NAD), Factor X = hemin.
Clinical manifestations at primary and secondary sites
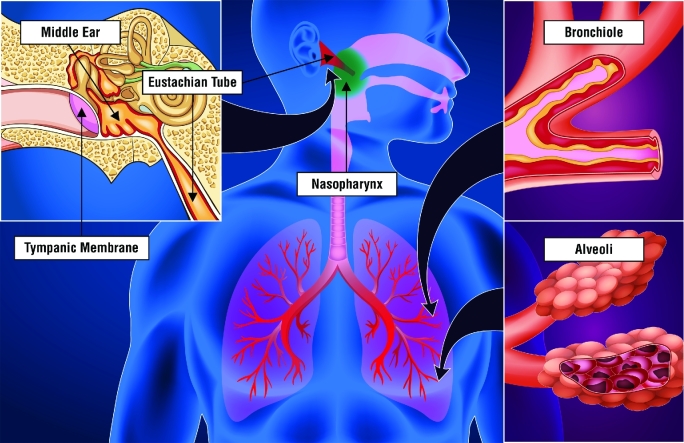
NTHi has evolved as a highly adaptive bacterium that colonizes and infects mucosal surfaces of the human respiratory airways. NTHi colonizes the nasopharynx of the human host as a ‘primary site of colonization’. The primary site acts as a reservoir for strains to ascend to the middle ear or descend to the lower airways to cause infections at these ‘secondary sites of infection’ (Fig. 2) (Finney et al. 2014; Murphy 2015; Duell, Su and Riesbeck 2016).
Figure 2.
Representation of NTHi primary site colonization of the nasopharynx (green) and secondary site infections of the middle ear and lower airways (red). NTHi colonizes the nasopharynx as a commensal organism. In children, NTHi ascends the Eustachian tube to infect the middle ear causing otitis media. During otitis media, the middle ear space, which is normally air filled, contains an inflammatory exudate due to the invading NTHi, resulting in a classic inflamed and bulging tympanic membrane. In adults with COPD, NTHi descends to the lower airways to infect the small airways of the lung. Airflow is restricted in the bronchioles and alveoli of the COPD airways due to chronic inflammation and swelling and collapsed alveolar sacks due to tissue destruction, respectively. NTHi infection of the COPD lower airways contributes to airway inflammation and increased mucous production, further contributing to restricted airflow.
Nasopharyngeal colonization in the first 2 years of life is predominated by one strain of NTHi with an average turnover of 2 months (Faden et al. 1996). This primary site is a reservoir for transmission of NTHi strains from host to host via aerosolized droplets (King 2012). NTHi infections of upper airway secondary sites, often precipitated by a preceding viral upper respiratory tract infection, cause otitis media and sinusitis by contiguous spread (Murphy 2003; Agrawal and Murphy 2011). Lower airway secondary site infections by NTHi cause acute exacerbations in individuals with COPD and bronchiectasis, and community acquired pneumonia (Sethi et al. 2002; Murphy 2003; King et al. 2006; Agrawal and Murphy 2011; King 2012; King and Sharma 2015). Causes of the shift of primary site colonization to secondary site infection are not clearly defined, but preceding viral infection is a frequent contributing factor (Bakaletz 2016; Duell, Su and Riesbeck 2016). Preceding viral infection or viral co-infection leads to the development of NTHi otitis media and pneumonia in animal models (Langereis et al. 2012; Unger et al. 2012; Wong et al. 2013; Bakaletz 2016). Secondary sites are often seen as dead-end infections since dissemination of the bacterium from host to host occurs predominantly via the nasopharynx (Langereis and Hermans 2013). Thus, survival at secondary sites is critical for the bacterial population. Underlying health conditions such as cystic fibrosis and COPD permit such secondary site infection and persistence in the lower airways (King 2012; Murphy 2015).
Host airway immunity in healthy individuals versus those with COPD
The large surface area of the human airways provides ample opportunities for invading pathogens to infect and cause disease. Infections caused by invading pathogens must overcome innate and humoral immune effectors present in the airways of healthy individuals (Finney et al. 2014; King and Sharma 2015). COPD is characterized by diminished airway immune system function and altered responses to pathogens allowing NTHi to establish infection and persist in individuals with COPD compared to healthy hosts (Sethi and Murphy 2008; King et al. 2013; Finney et al. 2014; King and Sharma 2015).
Innate immune effectors: The lower airways contain complement proteins as well as antimicrobial peptides and enzymes, which include lysozyme, lactoferrin, cathelicidin and defensins. Innate immune effectors are among the first lines of defense against pathogens invading the airways. These antimicrobial molecules disrupt membrane integrity of pathogens by forming pores that lyse the bacterial cell (Laube et al. 2006; Underwood and Bakaletz 2011; Pandya and Wilkes 2014). Detection of increased levels of circulating and sputum C3a and C5a complement proteins, potent inflammatory inducers, in individuals with COPD contribute to disease pathogenesis such as prolonged exacerbation recovery times (Pandya and Wilkes 2014; Westwood et al. 2016). Dysregulated expression of antimicrobial peptides in the individuals with COPD contributes to both increased bacterial airway colonization susceptibility and increased epithelial lung cell damage during exacerbations (Merkel et al. 2005; Paone et al. 2011; Hiemstra et al. 2016). Combined with the dysregulation of innate immune effectors in the COPD airways, NTHi has evolved virulence mechanisms to resist killing by these innate immune effectors. NTHi binds human serum factor H and vitronectin to inhibit complement cascades that confers serum resistance (Barthel et al. 2012; Langereis, de Jonge and Weiser 2014; Rosadini et al. 2014). Additionally, NTHi possess the susceptibility to antimicrobial peptides transporter that confers resistance to antimicrobial peptides (Mason et al. 2006; Shelton et al. 2011).
Innate physical barriers: The lower airway epithelium of healthy individuals is covered in a thin layer of mucous produced by goblet cells. The airway lumen contains mucin proteins and antimicrobial peptides that trap and kill bacterial cells (Laube et al. 2006; Linden et al. 2008). Rapid turnover of the mucous layer clears bacteria bound to this protective layer to prevent infection. Turnover of the mucous layer is mediated by coordinated beating of ciliated airway epithelium that act to ascend the mucous out of the airways to clear invading pathogens (Finney et al. 2014). Airway tissue destruction observed in COPD airways diminishes the number of ciliated cells and exposes underlying extracellular matrix (ECM) to pathogens (Anderson 2016; Duell, Su and Riesbeck 2016). Cigarette smoke further inhibits cilia function, making clearance of bacteria bound to mucous less efficient. Mucous production is increased in COPD airways, resulting in further reduction of mucus clearance by the remaining ciliated cells (Alikhan and Lee 2014; Finney et al. 2014). Both lipooligosaccharide (LOS) and protein D of NTHi inhibit cilia activity, further reducing the efficacy of the mucociliary immune function (Denny 1974; Janson et al. 1999; King and Sharma 2015). All of these alterations in innate immune physical barriers provide opportunities for NTHi to establish airway infection.
Innate cellular effectors: Resident macrophages in human airways patrol the airway epithelium and phagocytose bacteria and dead epithelial cells (Finney et al. 2014; King and Sharma 2015). During infection, neutrophils are recruited to the airway lumen to provide a first line of defense against invading bacteria. Neutrophil recruitment is regulated by chemokines secreted by epithelial cells during inflammatory responses to bacterial LOS, peptidoglycan fragments and proteins (Leake et al. 1994; Berenson et al. 2005; Choi et al. 2014; King and Sharma 2015). In normal human airways resident macrophages and infiltrating neutrophils prevent or limit infection by invading bacteria such as NTHi. Alveolar macrophages from adults with COPD are impaired in their ability to phagocytose NTHi (Berenson et al. 2006, 2013). The impairment of phagocytic function is attributed to reduced Toll-like receptor (TLR)-2 and TLR-4 signaling, important for recognition of pathogen-associated molecular patterns of proteins and LOS (Berenson et al. 2014). In addition, medications taken by patients with COPD, such as anticholinergics, contribute to reduced phagocytic activity of alveolar macrophages (Berenson, Kruzel and Sethi 2016). Though corticosteroids are used to reduce inflammation, NTHi induces a glucocorticoid-resistant inflammatory response in alveolar macrophages irrespective of COPD (Cosio et al. 2015). Thus, medications taken by those with COPD should be taken into consideration when studying immune responses.
Adaptive immune response effectors: Production of NTHi-specific antibodies contributes to pathogen clearance (Kennedy et al. 2000; King et al. 2008). Individuals with hypogammaglobinemia are more susceptible to NTHi infections, illuminating the role of antibodies in protection (Kainulainen et al. 1999). Antibodies provide direct bactericidal activity through activation of complement cascades and indirectly through increased opsonophagocytosis (Choi et al. 2015; Winter and Barenkamp 2015). While both healthy individuals and those with COPD generate NTHi-specific antibodies upon exposure to NTHi, those affected by COPD do not always clear the pathogen from the lower airways (Groeneveld et al. 1990; Murphy et al. 2004; Sethi et al. 2004; Novotny et al. 2009). Despite antibody production, NTHi persists in the airways of adults with COPD (Murphy et al. 2004). This lack of clearance despite abundant NTHi-specific antibodies may be due to (i) the important role of altered innate immunity, which antibodies cannot overcome; (ii) residency in a biofilm, which provides protection from antibodies; (iii) intracellular survival, which makes bacterial cells unavailable to binding by antibodies; and/or (iv) immunoglobulin isotypes that are not effective in clearing pathogens (Clementi, Hakansson and Murphy 2014; Gunn, Bakaletz and Wozniak 2016; Staples et al. 2016). Development of adaptive immune responses that generate functional antibodies that will induce bactericidal and opsonophagocytic killing are therefore paramount in providing protection against NTHi infection (Langereis and Weiser 2014; Choi et al. 2015; Winter and Barenkamp 2015).
The context in which antigens are presented by antigen presenting cells to adaptive immune cells is important for polarizing protective T-cell responses. A T-helper type 1 polarized response provides clearing immunity to NTHi compared to a T-helper type 2 polarized response (King et al. 2003). Individuals with COPD have impaired T-helper type 1 polarizing responses and increased levels of regulatory T cells that act to limit immune responses (Knobloch et al. 2011; Kalathil et al. 2014). The host may limit immune responses to reduce immune dependent host tissue damage, but adversely affect NTHi-specific T-effector cell proliferation (Abe et al. 2002; Kalathil et al. 2014). Differently polarized responses in the COPD airways may contribute to variable production of antibodies that are less effective in clearing NTHi than those isotypes produced in otherwise healthy people (Choi et al. 2015; Winter and Barenkamp 2015; Staples et al. 2016). Overall, a more comprehensive understanding of the host immune response to NTHi in COPD will be critical in order to develop successful approaches to vaccine development and other interventions to prevent and treat NTHi infection in COPD.
NTHi MECHANISMS OF PERSISTENCE IN THE RESPIRATORY TRACT
Adhesins
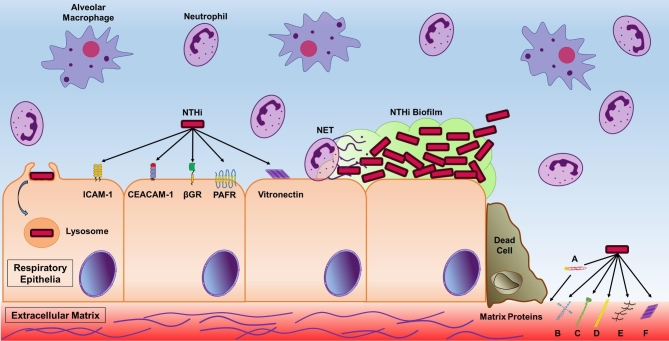
Bacterial adherence to host factors is an important step in colonization and infection. Attachment to host mucosal mucins, cells and ECM permits NTHi to colonize primary sites and establish infection in secondary sites (Duell, Su and Riesbeck 2016). This crucial step permits bacteria to invade host cells, form microcolonies that mature into biofilms or bind ECM proteins that become accessible when tissue damage arises from pathologies such as COPD (Fig. 3). Attachment, invasion of host cells and biofilm formation are major virulence mechanisms that contribute to persistent infection (Arce et al. 2009; Clementi, Hakansson and Murphy 2014). NTHi harnesses multiple surface-exposed adhesin proteins and LOS moieties that contribute to attachment to host factors (Swords et al. 2000; Duell, Su and Riesbeck 2016). The abundance of adhesins permits NTHi to adhere to the variety of host cellular populations and mucosae in primary and secondary sites (Table 1).
Figure 3.
Model of persistent NTHi infection in the COPD small airways. In the small airways of individuals with COPD NTHi attaches to respiratory epithelial cells via multiple adhesins. Known host cell-NTHi adhesin receptors include ICAM-1, CEACAM-1, β-glucan receptor (βGR), platelet activating factor receptor (PAFR) and a vitronectin receptor that binds host vitronectin bound by NTHi. NTHi adhesins also bind to ECM proteins exposed as a result of epithelial cell damage. NTHi binds to the host ECM indirectly by binding host plasminogen (A) and through direct interactions of NTHi adhesins and host laminin (B), collagen IV (C), fibronectin (D), proteoglycans (E) and vitronectin (F). Attachment to respiratory epithelia permits intracellular invasion and evasion from host immune effectors. Attachment to respiratory epithelial and the ECM permits NTHi biofilm formation. Biofilm residency contributes to evasion from host immune effectors including resident alveolar macrophages and recruited neutrophils. Biofilms offer resistance to NET, of which components can be incorporated into the NTHi biofilms. NTHi utilizes intracellular invasion, biofilm formation and additional virulence persistence mechanisms to evade host immune recognition and clearance.
Table 1.
Specificity of binding of NTHi adhesins to host cells and host receptors.
| NTHi adhesin | Host cell | Receptor | Reference(s) |
|---|---|---|---|
| Protein | |||
| Protein D (PD) | Human monocytes Normal human bronchial epithelia Type II pneumocytes | Ahren et al. (2001a); Johnson et al. (2011) | |
| Outer membrane protein 5 fimbriae (P5) | Bronchiolar epithelia Type II pneumocytes Alveolar macrophages | ICAM-1 CEACAM-1 | Avadhanula et al. (2006); Bookwalter et al. (2008); Euba et al. (2015) |
| High molecular weight proteins 1 and 2 (HMW1/2) | Pharyngeal epithelia Chang cells Macrophages | St Geme et al. (1993); Noel et al. (1994); Vuong et al. (2013) | |
| Haemophilus influenzae adhesion (Hia) | Primary human bronchial epithelia Type II pneumocytes Chang cells | Barenkamp and St Geme (1996); St Geme and Cutter (2000); Baddal et al. (2015) | |
| Haemophilus adhesion and penetration (Hap) | Bronchiolar epithelia Type II pneumocytes Alveolar macrophages Chang cells | Fink et al. (2003); Euba et al. (2015) | |
| Type IV pilus subunit A (PilA) | Primary human bronchial epithelia | ICAM-1 | Jurcisek et al. (2007); Novotny and Bakaletz (2016) |
| Protein E (PE) | Bronchial epithelia Type II pneumocytes Pharyngeal epithelia Chang cells | Indirect via vitronectin | Ronander et al. (2009); Ikeda et al. (2015) |
| Protein 4 (P4) | Primary human bronchial epithelia Pharyngeal epithelia Bronchiolar Epithelia Type II pneumocytes | Su et al. (2016) | |
| Protein F (PF) | Primary human bronchial epithelia Type II pneumocytes | Jalalvand et al. (2013) | |
| Opacity-associated protein A (OapA) | Chang cells | Prasadarao et al. (1999) | |
| Sensitivity to antimicrobial peptides protein (SapA) | Chinchilla middle ear epithelia Primary human bronchial epithelia | Raffel et al. (2013) | |
| LOS moiety | |||
| Phosphocholine (ChoP) | Bronchiolar epithelia | Platelet activating factor receptor | Swords et al. (2000) |
| Unknown | Human monocytes Human eosinophils Type II pneumocytes | β-Glucan receptor (Dectin-1) | Ahren et al. (2001b, 2003); Heyl et al. (2014) |
Mucin: Mucins are high molecular weight glycoproteins that are constitutively expressed and secreted into the mucosa (Lamblin et al. 2001). NTHi outer membrane proteins P2 and P5 fimbriae bind to mucin proteins, allowing bacteria to attach to the mucosa (Reddy et al. 1996, 1997). Attachment to mucin proteins facilitates subsequent penetration of the mucosal layer and provides an opportunity to bind to host epithelial cells or exposed ECM in areas of tissue damage (Clementi and Murphy 2011; Baddal et al. 2015; Duell, Su and Riesbeck 2016). This initial adherence to mucosa is crucial for establishment of colonization and infection.
Host macrophages and epithelial cells: In the course of colonization or infection, NTHi binds to host macrophage or monocytic cells as well as epithelial cells from different respiratory tissue types (St Geme and Falkow 1990; Ahren et al. 2001b). Adherence to monocytes is in part mediated by protein D (PD), which leads to invasion and intracellular persistence rather than phagocytic killing (Craig et al. 2001; Ahren et al. 2001a,b). The PD antigen also mediates binding to respiratory epithelial cells. Binding to these cell types may not be a result of a direct interaction with PD and the host cell, but rather through PD-dependent decoration of LOS by a phosphocholine (ChoP) moiety that mediates binding to host cell platelet activating factor receptor (PAFR) (Swords et al. 2000; Johnson et al. 2011).
Earlier work reported that NTHi primarily bound non-ciliated primary human bronchial cells (Ketterer et al. 1999). Recent work showed that NTHi bound ciliated cells in addition to non-ciliated cells of well-differentiated normal human bronchial epithelia (Baddal et al. 2015). The fate of NTHi attached to epithelial includes biofilm formation or host cellular invasion and persistence as shown in Fig. 3 (Clementi and Murphy 2011). Multiple proteinaceous and polysaccharide NTHi surface constituents mediate attachment to a variety of host cells. A summary of the host cell binding capacities of NTHi adhesins is provided in Table 1. In particular, the P5 antigen binds to host epithelial cells via interactions with the host cell ICAM-1 and CEACAM-1 (Avadhanula et al. 2006; Bookwalter et al. 2008). The type IV pilus cap protein (PilA) also binds host ICAM-1 (Jurcisek et al. 2007; Novotny and Bakaletz 2016). The conserved NTHi protein E (PE) contributes to attachment to a variety of epithelial cells from the lower and upper airways, which induces expression of ICAM-1, resulting in an abundance of ICAM-1 receptors to which other NTHi adhesins may adhere (Ronander et al. 2008, 2009). NTHi binding to cells is partly dependent on the host cell β-glucan receptors, though the NTHi ligand has yet to be identified (Ahren et al. 2001b, 2003; Clementi and Murphy 2011). The multiplicity of binding interactions between several protein and saccharide NTHi components contributing to host cell adherence, summarized in Table 1, emphasizes the importance of attachment for colonization or infection in the host (Duell, Su and Riesbeck 2016).
ECM proteins: Several NTHi adhesins that bind host cell components also bind extracellular host components (Table 2). ECM proteins become accessible to bacterial pathogens when there is tissue damage at the site of infection (Fig. 3) (Fink, Green and St Geme 2002). This is seen in COPD as airway tissue is damaged due to chronic inflammation and preceding viral infections (Sethi and Murphy 2008; Bakaletz 2016). Attachment to ECM proteins anchors bacteria at the site to establish infection. Proteins of the ECM that NTHi cellular components bind include proteoglycans, fibronectin, collagen, laminin and vitronectin (Noel, Love and Mosser 1994; Fink, Green and St Geme 2002; Hallstrom et al. 2011; Jalalvand et al. 2013; Su et al. 2016). Table 2 outlines the NTHi adhesin interactions with host ECM components as well as serum factors.
Table 2.
Specificity of binding of NTHi surface antigens to extracellular matrix (ECM) and serum factors.
| ECM component | Serum factor | |||||||
|---|---|---|---|---|---|---|---|---|
| Surface protein | Proteoglycan | Laminin | Collagen IV | Fibronectin | Factor H | Vitronectin | Plasminogen | References |
| P5 | • | Langereis, de Jonge, and Weiser. (2014); Rosadini et al. (2014) | ||||||
| HMW1/2 | • | Noel, Love and Mosser (1994) | ||||||
| Hap | • | • | • | Fink et al. (2002) | ||||
| PE | • | • | • | Hallstrom et al. (2011); Barthel et al. (2012) | ||||
| P4 | • | • | • | Su et al. (2016) | ||||
| PF | • | • | Jalalvand et al. (2013); Su et al. (2013) | |||||
Serum factors: Serum factors such as factor H, plasminogen and vitronectin are present at primary and secondary sites and are bound by different NTHi adhesins (Table 2). Binding of factor H and vitronectin mediates serum resistance of bacterial pathogens. These serum factors inhibit complement cascades that would otherwise deposit pore-forming proteins that lyse cells. Bacterial binding of these bactericidal inhibitors shields and protects the bacteria from killing (Hallstrom and Riesbeck 2010; Barthel et al. 2012; Langereis, de Jonge and Weiser 2014). Protein E binds plasminogen and upon subsequent cleavage into plasmin permits interaction with host ECM, further contributing to adherence (Barthel et al. 2012). Vitronectin binding by NTHi adhesins not only provides serum resistance, but contributes to indirect attachment to host ECM and respiratory epithelial cells, aiding in attachment and persistent infection (Ikeda et al. 2015).
Several NTHi surface proteins are multifunctional adhesins that participate in bacterial cell attachment to host cells, ECM proteins and serum factors to resist killing by host innate immune effectors (Tables 1 and 2). Collectively, the redundancy of these adhesins and their multifaceted roles in infection allow NTHi to establish persistent infection (Fig. 3).
Biofilms
Biofilms are microbial communities housed in an organized matrix of extracellular polymeric substance consisting of extracellular DNA, proteins and polysaccharides (Swords 2012; Gunn, Bakaletz and Wozniak 2016). Biofilm formation is a virulence mechanism implemented by a number of human airway-associated pathogens to establish persistent infections in both healthy hosts and those with underlying conditions (Hoiby, Ciofu and Bjarnsholt 2010; Bakaletz 2012; Langereis and Hermans 2013; Gunn, Bakaletz and Wozniak 2016; Lewis and Torres 2016). NTHi forms biofilms in experimental otitis media, in patients with otitis media and in the lower airways of patients with cystic fibrosis (Hall-Stoodley et al. 2006; Starner et al. 2006; Jurcisek and Bakaletz 2007; Post 2015; Idicula et al. 2016). Biofilms may act as reservoirs of bacteria that can re-infect secondary sites (Langereis and Hermans 2013; Tikhomirova and Kidd 2013; Duell, Su and Riesbeck 2016). Targeting selected NTHi-specific biofilm-expressed factors prevents and resolves experimental otitis media (Bakaletz 2012; Novotny et al. 2015, 2016). Resolution and prevention of NTHi infection by targeting biofilm components illustrates the important role that NTHi biofilms play in infection of secondary sites.
Much of the current understanding of NTHi biofilm formation during infection is in the context of otitis media. Currently, there is a lack of direct evidence for NTHi biofilm formation in the lower airways of patients with COPD. Furthermore, no sufficient animal model allows NTHi to establish persistent lower airway infection similar to the months of carriage observed in adults with COPD, limiting analyses evaluating the role of biofilm formation on persistent lower airway infection in COPD (Murphy et al. 2004; Langereis and Hermans 2013; Murphy 2015). However, clinical isolates of NTHi from adults with COPD form biofilms in vitro and sera from these adults recognizes the peroxiredoxin-glutaredoxin NTHi antigen that is expressed during biofilm formation (Murphy et al. 2005; Juneau et al. 2015). Additionally, Pang et al. (2008) used a mouse model pre-treated with a bolus of elastase in the lungs to simulate COPD airway pathologies to show impaired pulmonary clearance of NTHi in the lower airways up to 72 h post infection. After 48 h, multicellular NTHi communities observed in lung tissue cryosections resembled the pattern of biofilm-associated colonies. These studies provide additional, but indirect, support that biofilms are formed in vivo during infection of the lower airways of hosts with COPD (Pang et al. 2008).
Secondary sites of infections are hostile environments for invading bacteria due to the presence of multiple immune effectors. Residency within a biofilm affixed to host cellular surfaces offers protection from the plethora of host immune effectors and antibiotic treatment (Fig. 3) (Slinger et al. 2006; Bakaletz 2012; Swords 2012; Gunn, Bakaletz and Wozniak 2016).
Innate immune effector cells and biofilms: Neutrophils and macrophages at secondary sites act to clear invading pathogens. The structure of the extracellular polymeric substance of NTHi biofilms provides steric hindrance against phagocytosis (Langereis and Hermans 2013). Additionally, biofilm-associated NTHi are resistant to clearance by neutrophil extracellular traps (NETs). NTHi cellular components promote NET formation. For example, expression of NTHi LOS moieties that are known to play a role in biofilm formation is associated with induction of NETs by NTHi. Biofilm-associated and planktonic NTHi survive within NETs and are resistant to killing by infiltrating neutrophils (Hong et al. 2009; Juneau et al. 2011). Resistance to oxidative stress effectors from neutrophils in NETs is conferred by the transcriptional upregulation of oxidative stress mediators, peroxiredoxin-glutaredoxin and catalase (Juneau et al. 2015). The presence of NET components in NTHi biofilms contributes to biofilm mass, further limiting diffusion of antimicrobial immune effectors and promoting persistent infection (Langereis and Hermans 2013).
Innate and humoral immune antimicrobial effectors and biofilms: Secondary sites of infection contain complement mediators, immunoglobulins, and host antimicrobial enzymes and peptides (Laube et al. 2006; Underwood and Bakaletz 2011). Extracellular DNA of NTHi biofilms sequesters human β-defensin-3 and reduces its antimicrobial activity (Jones, McGillivary and Bakaletz 2013). Complement proteins produced by secondary site epithelial cells or macrophages contribute to clearance of pathogens (Figueira et al. 2007; Pandya and Wilkes 2014). Pathogen-specific immunoglobulins mediate bacterial killing by direct bactericidal activity or via opsonophagocytosis (Neary and Murphy 2006; Khan, Kaur and Pichichero 2012; Winter and Barenkamp 2014). The biofilm matrix protects NTHi from these innate and humoral immune effectors. The biofilm biomass limits diffusion of small antimicrobial peptides and accessibility of other effectors to reduce their effectiveness (Izano, Shah and Kaplan 2009; Gunn, Bakaletz and Wozniak 2016). Additionally, IgA1 protease that cleaves host IgA is present at the apical surface of the biofilm acting to shield bacteria from secretory immunoglobulin A (Webster et al. 2006). Biofilms play a key role in pathogenesis by protecting NTHi from this cohort of first line of defense innate and acquired humoral immune effectors at secondary infection sites.
Biofilms and antibiotic treatment: Antibiotics are used to treat otitis media and acute exacerbations of COPD. Though antibiotics are effective, they do not always clear persistent bacterial infections at secondary sites (Bakaletz 2012; Pettigrew et al. 2016). Biofilm-associated NTHi show increased resistance to a spectrum of antibiotics (Slinger et al. 2006). Interestingly, β-lactam antibiotics stimulate NTHi biofilm formation in vitro (Wu et al. 2014). Limited diffusion of the antibiotics into the biofilm biomass is hypothesized to increase antibiotic resistance of biofilm bacteria. This hypothesis has recently been challenged by the observation that resistance to antibiotics is independent of biofilm size (Reimche et al. 2016). Biofilm-resident bacteria have a unique transcriptome profile and are characterized as metabolically inactive. The limited metabolic activity of biofilm bacteria is hypothesized to diminish the efficacy of antibiotics that target metabolic processes (Bakaletz 2012; Post et al. 2014). Given recent findings, the metabolic profile of biofilm bacteria may play a more significant role in antibiotic resistance than limited diffusion of antibiotics. Subinhibitory concentrations of azithromycin inhibit NTHi biofilm formation and disrupt established biofilms in vitro (Starner et al. 2008). Despite this finding, our group found that 19% of NTHi strains from adults with COPD given a course of azithromycin treatment developed a fourfold increase in MIC during persistent infection (Pettigrew et al. 2016). The increase in MIC of these strains resulted in these strains becoming resistant to azithromycin. This observation suggests that, in addition to biofilm formation, other mechanisms, such as intracellular persistence, may play a significant role in persistence at secondary sites during antibiotic treatment.
The significance of NTHi biofilms during infection and persistence is highlighted by their importance in experimental otitis media (Bakaletz 2012). In addition to this model, in vitro static and continuous flow biofilm models provide insights into factors associated with biofilm formation, maintenance and potential therapies. Expanding the available in vitro models to include host cell components such as a substratum of living or fixed epithelial cells or ECM proteins would incorporate key host attachment factors and better simulate in vivo conditions (Marks, Parameswaran and Hakansson 2012; Baddal et al. 2015).
Intracellular survival
NTHi is classically considered an extracellular pathogen, but multiple lines of evidence demonstrate its role as a facultative intracellular bacterium as well (St Geme and Falkow 1990; Bandi et al. 2001; Clementi, Hakansson and Murphy 2014; Mell et al. 2016). NTHi also penetrates between epithelial cells and resides in the intercellular space of epithelial tissues (van Schilfgaarde et al. 1999; Mell et al. 2016). NTHi can persist for months to years in the airways in adults with COPD (Murphy et al. 2004). The observation that expectorated sputum cultures remain negative in spite of the presence of NTHi supports that the intracellular location provides temporary niches or long-term reservoirs for chronic NTHi infection by providing protection from immune pressures (Clementi, Hakansson and Murphy 2014).
NTHi invades human epithelial cells of adenoid and respiratory tissues, subepithelial cells and macrophages both in vitro and in vivo (St Geme and Falkow 1990; Forsgren et al. 1994, 1996; Ahren et al. 2001a). In one study, intracellular NTHi was identified in bronchial biopsies in 0% of healthy adults, 33% of clinically stable COPD patients and 87% of patients who were experiencing exacerbations of COPD (Bandi et al. 2001). These observations suggest a role for intracellular infection by NTHi both in the pathogenesis of exacerbations of COPD and as a mechanism for persistent airway infection (Bandi et al. 2001; Finney et al. 2014).
The genome of NTHi does not contain specialized secretion systems for the active invasion of host cells, suggesting that the organism relies on host pathways to invade host cells and tissues (Clementi and Murphy 2011; Clementi, Hakansson and Murphy 2014). Host cell internalization of NTHi involves actin, tubulin and the formation of lamellipodia and microvilli, which engulf the bacteria into vesicles (Holmes and Bakaletz 1997; Ketterer et al. 1999; Clementi, Hakansson and Murphy 2014). Internalization is also facilitated by macropinocytosis and PAFR-mediated endocytosis in epithelial cells, utilizing the bacterial ligand ChoP (Ketterer et al. 1999; Swords et al. 2000; Clementi, Hakansson and Murphy 2014). Multiple host pathways terminate in lysosomes, including phagocytosis, macropinocytosis, clathrin- or receptor-mediated endocytosis, lipid raft-mediated endocytosis, autophagy, secretion, transcytosis and paracytosis (Clementi and Murphy 2011). NTHi is found within lysosomes, indicating that the pathogen has evolved mechanisms to avoid or neutralize this deadly fate for intracellular survival (Clementi and Murphy 2011; Clementi, Hakansson and Murphy 2014; Mell et al. 2016). A diagrammatic model of NTHi small airway persistence is shown in Fig. 3.
IgA protease
IgA binds bacteria to prevent mucosal attachment to epithelial cells, inactivate toxins and inhibit agglutination (King 2012; Murphy et al. 2015). A limited number of exclusively human mucosal pathogens including Haemophilus influenzae, Streptococcus pneumoniae, Neisseria meningitidis and N. gonorrhoeae express IgA proteases, which cleaves the hinge region of human IgA1, the predominant IgA subclass of the respiratory mucosa (Plaut et al. 1975; Kilian, Mestecky and Schrohenloher 1979; Kilian, Mestecky and Russell 1988). Cleavage of the hinge region releases Fc and Fab fragments, inhibiting the protective functions of the immunoglobulin (Kilian et al. 1996; Murphy et al. 2015).
All strains of NTHi contain the igaA gene that encodes IgA protease, and ∼40% contain a second distinct gene, igaB, that encodes a second IgA protease, IgA B protease (Fernaays et al. 2006a; Murphy et al. 2015). In a study of 297 strains of NTHi from blood, cerebrospinal fluid, middle ear fluid, sputum of adults with COPD and the nasopharynx of children, the proportion of NTHi isolates that contained the igaB gene varied by site of isolation. Strains isolated from COPD sputum samples had a higher proportion with the igaB gene (45%) compared to middle ear isolates from children (31%). Only 9% of strains from children with asymptomatic nasopharyngeal colonization had the igaB gene (Fernaays et al. 2006a). These observations suggest that igaB plays a role in virulence in NTHi infection in COPD.
IgA proteases play a role in bacterial infection of NTHi not only by cleavage of human IgA1 to evade mucosal immunity, but also by mediating intracellular persistence (Clementi, Hakansson and Murphy 2014). The second IgA protease in NTHi, IgA B protease, facilitates intracellular survival by cleavage of the IgA1-like hinge region of lysosome-associated membrane protein 1, a glycoprotein in lysosomes that mediates microbicidal activity (Lin et al. 1997; Clementi, Hakansson and Murphy 2014). This alternative function of IgA protease promotes survival of intracellular pathogens by disrupting lysosomal acidification, hence increasing the organism's ability to survive. The observation that an igaB knockout mutant died more rapidly in intracellular survival assays of respiratory epithelial cells compared to the wild-type strain further supports this conclusion. Thus, IgA B protease is required for optimal intracellular persistence of NTHi (Clementi, Hakansson and Murphy 2014).
A recent discovery revealed that the NTHi genome has two variants of each igaA and igaB gene, each encoding a protease with its own specific cleavage site of the IgA1 hinge region (Murphy et al. 2015). While the IgA-B protease appears to play a more important role than IgA-A protease in intracellular survival, the more subtle implications of the four types of IgA protease on NTHi pathogenesis and persistence are yet to be fully elucidated. Future work will be important to understand the role of IgA proteases as a mechanism of persistence by NTHi in the airways of adults with COPD.
Genetic variation
In adapting to different sites of infection and persisting in hostile environments, NTHi differentially regulates the expression of virulence factors (Qu et al. 2010; Baddal et al. 2015). NTHi only has four two-component signaling pathways compared to the over 40 in Escherichia coli (Fleischmann et al. 1995). This observation suggests that NTHi also uses mechanisms other than two-component signaling to alter gene expression during colonization of primary sites and infection of secondary sites. NTHi utilizes antigenic variation, phase variation and epigenetic variation as mechanisms of genetic variation to infect and persist in the human respiratory tract.
Antigenic variation: Surface-exposed epitopes of antigens are the targets of protective host antibodies that are bactericidal or opsonic (Sethi et al. 2004; Choi et al. 2015; Winter and Barenkamp 2015). NTHi demonstrates enormous genetic heterogeneity among different strains, including the surface epitopes of antigens, resulting in induction of strain-specific antibody responses (Gilsdorf, Marrs and Foxman 2004; Sethi et al. 2004; De Chiara et al. 2014). This genetic heterogeneity among strains accounts, in part, for the frequently observed reinfection of the lower airways of adults with COPD by different strains of NTHi (Sethi et al. 2002, 2004).
Surface-exposed epitopes of antigens of NTHi demonstrate substantial variation during persistent infection (Duim et al. 1997; Hiltke, Sethi and Murphy 2002; Hiltke et al. 2003). Antigenic variation is observed in surface-exposed epitopes of NTHi proteins, including: outer membrane proteins P1, P2 and P5 as a result of point mutations, insertions and deletions, and/or recombination of exogenous DNA (Sikkema and Murphy 1992; Duim et al. 1997; Bolduc et al. 2000; Hiltke et al. 2003; Gilsdorf, Marrs and Foxman 2004). Variation in surface-exposed epitopes identifies these antigens as being under immune selective pressure from the host, which promotes diversifying selection in the persisting population (Sikkema and Murphy 1992; Duim et al. 1997; Bolduc et al. 2000; Hiltke et al. 2003). Antigenic variation of surface epitopes facilitates persistence by evading protective antibody recognition (Duim et al. 1997; Sethi et al. 2002, 2004; Murphy et al. 2004).
Mutations that lead to variation in epitope sequences may be generated by natural mistakes in DNA replication or by damaged DNA that is incorrectly corrected by DNA repair enzymes. DNA-damaging agents, such as reactive oxygen species from inflammatory responses, are present at secondary sites. NTHi must tolerate or repair damaged DNA to survive (Sweetman, Moxon and Bayliss 2005). NTHi possess the conical DNA repair mechanism genes found in E. coli, except for translesion synthesis DNA repair polymerases (Fleischmann et al. 1995; Sweetman, Moxon and Bayliss 2005). The absence of such repair mechanisms indicates that NTHi relies on other DNA repair systems to correct DNA damaged bases. Hypermutator phenotype NTHi isolates are observed in patients with cystic fibrosis due to mutations of the mutS mismatch repair gene (Watson, Burns and Smith 2004). Further investigations of the role of NTHi DNA damage repair during persistent infection will be important to understand the role of DNA repair in antigenic variation.
Phase variation: Phase variation is a stochastic and reversible mechanism of gene expression determined by the number of simple sequence repeats (SSR) in transcriptional promoter regions or within genes. Tetranucleotide repeats are the predominant SSR in the NTHi genome (Power et al. 2009). The number of SSR repeats regulates gene expression by slipped-strand mispairing during DNA replication. For example, phase variation of the IgA-B2 protease gene expression is observed during experimental human nasopharyngeal colonization and lower airway persistence in adults with COPD as a result of slipped strand mispairing of a 7 nucleotide repeat upstream of the gene (Poole et al. 2013; Murphy et al. 2015). LOS synthesis genes undergo phase variation that alters LOS expression profiles that are important for colonization and evasion of opsonophagocytosis (Weiser, Love and Moxon 1989; Poole et al. 2013; Langereis and Weiser 2014). The HMW1/2 and Hia adhesins, which are important for host colonization, are also under regulation by phase variation (Giufre et al. 2008; Atack et al. 2015a,b).
Epigenetic gene regulation: Another regulator of expression is DNA methyltransferases, which epigenetically regulate expression of virulence genes (Atack et al. 2015b; Brockman et al. 2016; Tan et al. 2016; VanWagoner et al. 2016). NTHi possess phase variable type III restriction modification-DNA methyltransferases (modA genes), of which there are several allelic variations among strains (Fox et al. 2007; Tan et al. 2016). The expression of these methyltransferases alters expression of genes, independent of genetic sequence of virulence gene promoters. Thus, genome sequence analyses will not elucidate the complete picture of virulence factor expression (Tan et al. 2016). Continuing research in this area of epigenetic regulation will be important for understanding virulence gene expression during infection of secondary sites by NTHi compared to primary site nasopharyngeal colonization.
Nutrient acquisition
The hostile environment of COPD airways is deficient in nutrients and contains high levels of reactive oxygen species, and antimicrobial peptides (Qu et al. 2010). To survive in this nutrient-deficient environment, NTHi undergoes adaptations in metabolic pathways, including those that mediate uptake of iron and other trace metals, to alter cellular function to acquire critical nutrients (Finney et al. 2014; Duell, Su and Riesbeck 2016). Iron is limited in the host, and hence microbes need to sequester iron for survival (Duell, Su and Riesbeck 2016). Iron-dependent fur-regulated genes are virulence factors that contribute to longer, more severe, infection by regulating iron within the cell (Harrison et al. 2013; Szelestey et al. 2013; Duell, Su and Riesbeck 2016). Deletion of four hemoglobin-binding genes from NTHi results in a delayed onset of otitis media with reduced duration of infection compared to wild type in a chinchilla model (Morton et al. 2004). Heme acquisition genes are more prevalent in middle ear strains of NTHi than colonizing throat strains (Hariadi et al. 2015). Collectively, these data indicate that heme acquisition genes mediate important virulence mechanisms in NTHi infection.
NTHi has uptake systems for other trace metals that are less well studied than those that mediate iron uptake. Additional adaptations of NTHi include the production of urease and antioxidants to survive and cause persistent infection in lower airways (Murphy and Brauer 2011). These pathways represent ‘nutritional virulence factors’ that facilitate airway infection caused by NTHi. As more of such mechanisms are elucidated, these pathways represent potentially high value targets for the development of novel therapies to eradicate NTHi from the airways in COPD.
Toxin–antitoxin systems
Toxin–antitoxin (TA) systems in NTHi have been the subject of recent study and appear to play a role in persistent infection. TA systems consist of a stable toxin and labile antitoxin (Ren et al. 2012; Wen, Behiels and Devreese 2014). The TA module is encoded in an operon, which results in co-transcription and co-translation of the toxin and the antitoxin (Wen, Behiels and Devreese 2014). TA systems are classified into types based on the nature of the antitoxin and their mode of action (Ren et al. 2012). Toxins are proteins, and antitoxins are either small RNA molecules or proteins that inhibit the toxin by directly preventing its action or by controlling toxin production (Wen, Behiels and Devreese 2014). Type II TA systems, which include a protein antitoxin, are two-gene operons with the antitoxin gene upstream of the toxin gene.
TA modules are activated upon stressful environmental conditions such as nutrient limitation, antibiotic therapy or oxidative stress. Certain proteases, for example, Lon or Clp proteases, degrade the antitoxin, and the stable toxin is free to facilitate growth arrest (Ren et al. 2012). Toxin functions to facilitate growth arrest by mechanisms including ribonuclease activity, for example. Upon removal of the stressful condition, antitoxin synthesis is resumed, and counteracts toxin activity (Ren et al. 2012, 2014).
In NTHi, type II TA modules play a role in persistent infection (Cline, Saleem and Daines 2012; Ren et al. 2012, 2014; Hamilton et al. 2014; Jin, Pavelka and Butler 2015; Walling and Butler 2016). Virulence-associated protein (vap) genes have been identified in NTHi as TA modules. NTHi mutants lacking the vapBC-1 locus, the vapXD locus and both TA loci demonstrated marked attenuation of survival in primary human respiratory epithelial tissues and the chinchilla model, supporting that these loci contribute to persistence in vitro and in vivo models of otitis media (Ren et al. 2012). Another study examining the toxAvapA locus, a homolog of the host inhibition of growth (higBA) gene pair, demonstrates a similar result, supporting that this type II TA system is also beneficial for persistence of the organism in otitis media (Ren et al. 2014). Analysis of transcriptional changes of NTHi and ciliated human bronchial epithelium in an in vitro model of infection demonstrates an increase in NTHi virulence factor expression by 6 h, especially in the conserved TA family components including higBA, vapBC-1, vapBC-2 and stbDE (Baddal et al. 2015).
The hypothesis regarding the role of TA systems in persistent infection includes the creation of ‘persister’ phenotype bacteria and biofilm formation. Persister cells are those from a homogeneous population that survive various stressors; they are not mutants but phenotypic variants. The role of TA systems in biofilms has been debated. Biofilms themselves serve as reservoirs or niches for the persister phenotypes. Alternatively, bacterial TA systems could facilitate altruistic programmed cell death of individual microbes to allow a small population of persisters to continue in a biofilm (Wen, Behiels and Devreese 2014).
While TA loci appear to modulate persistence of NTHi in models of otitis media, the effect of TA loci in NTHi persistence in a COPD context has not been studied. Given the observations in models of otitis media, TA systems should be explored as a persistence mechanism in the setting of COPD.
Outer membrane vesicles
Gram-negative bacteria shed vesicles derived from their outer membranes. These vesicles contain outer membrane proteins, LOS and periplasmic proteins in the lumen of the vesicle. In a number of species, outer membrane vesicles (OMVs) contribute to pathogenesis (Roier et al. 2016). NTHi-produced OMVs, were first described as transformasomes because they bind and contain DNA and their production was increased during competence development (Deich and Hoyer 1982; Kahn, Barany and Smith 1983). Transfer of DNA functions as a virulence mechanism by delivering additional virulence factors to recipient strains, resulting in altered antigenic epitopes of surface-exposed antigens that further contribute to immune evasion (Hiltke et al. 2003; Gilsdorf, Marrs and Foxman 2004). Binding of DNA by OMVs may serve as a nucleation site for cell aggregation and biofilm formation, since extracellular DNA is critical for biofilm structure and maintenance (Jurcisek and Bakaletz 2007; Sharpe et al. 2011; Duell, Su and Riesbeck 2016; Novotny et al. 2016).
OMVs act as intercellular delivery systems between NTHi and the host, inducing host responses. NTHi OMV bind to epithelial cells and elicit inflammatory cytokine and defensin secretion. Stimulation of inflammatory responses independent of direct NTHi–host cell interaction compounds the pathologies of inflammatory damage during infection, especially in individuals with COPD (Sharpe et al. 2011). Induction of inflammatory responses, which normally act to clear invading pathogens, may also act as a decoy response that localizes inflammation to tissue sites independent of the presence of viable NTHi (Duell, Su and Riesbeck 2016).
NTHi OMVs induce robust proliferation and antibody production from peripheral blood B cells by IgD B-cell receptor activation. Though antibodies are produced upon OMVs and B-cell interaction, the antibodies do not recognize NTHi (Deknuydt, Nordstrom and Riesbeck 2014). Induction of such non-specific antibody responses is another mechanism whereby OMVs act as a decoy of the immune system, permitting invading NTHi to establish infection and persist.
CONCLUSIONS AND FUTURE DIRECTIONS
As a major pathogen in COPD, NTHi takes advantage of altered host innate immunity to colonize and infect the respiratory tract. NTHi infection has a profound impact on the course of the disease by causing acute exacerbations, which result in substantial global morbidity, mortality and healthcare costs. A more subtle, but important effect of NTHi infection, is the induction of airway inflammation that contributes to the progressive loss of lung function that is a hallmark of COPD. NTHi employs a myriad of mechanisms to evade host immunity, including biofilm formation, intracellular survival, genetic variation and many others.
Future investigations should be directed toward understanding molecular mechanisms of pathogenesis of NTHi, an exclusively human pathogen that causes infection in a disease that is unique to humans (Table 3). Such work will identify potential therapeutic targets to use as novel approaches to prevent and treat infections in COPD. Approaches may include systemic or topical drugs to eradicate the organism from the airways, immunomodulators and surface-exposed virulence factors as vaccine antigens. Preventing NTHi infection in the setting of COPD would have an enormous impact in prolonging life and relieving suffering for people with COPD.
Table 3.
Future directions for research to advance understanding of persistent NTHi infection in COPD.
| Area of study | Goals of future work |
|---|---|
| Adhesins | • Identify host cell receptors of NTHi adhesins |
| Biofilm formation | • Investigate COPD airways for NTHi biofilms |
| • Simulate in vivo conditions in current in vitro models | |
| • Establish animal model of persistent NTHi infection | |
| Intracellular survival | • Assess the impact of host cell invasion and intracellular survival on duration of persistence |
| IgA proteases | • Characterize role of IgA protease variants in NTHi persistence |
| Genetic variation | • Comprehensively identify targets of epigenetic regulation |
| • Assess role of DNA repair mechanisms on genetic variation | |
| • Assess vaccine candidates based on genetic variation | |
| Nutrient acquisition | • Characterize NTHi nutritional virulence factors |
| Toxin-antitoxin (TA) systems | • Elucidate the role of TA systems in NTHi persistence in the context of COPD |
| Outer membrane vesicles (OMVs) | • Characterize role of OMV during persistence |
| Immune response | • Define protective immune responses to NTHi in COPD |
| • Identify vaccine formulations that elicit protective immune responses |
Acknowledgments
We would like to thank Mr. Sean Murphy for creation of Fig. 2 and Dr. Antonia Perez for careful review of the manuscript.
Author Contributions
CPA and MCG equally contributed to writing of this manuscript. CPA, MCG, and TFM contributed to the design, writing, and editing of the manuscript.
FUNDING
This work was supported by NIH grant R01 AI19641 (TFM) and by the National Center for Advancing Translational Sciences award UL1TR001412 to the University at Buffalo.
REFERENCES
- Abe Y, Murphy TF, Sethi S et al. Lymphocyte proliferative response to P6 of Haemophilus influenzae is associated with relative protection from exacerbations of chronic obstructive pulmonary disease. Am J Resp Crit Care 2002;165:967–71. [DOI] [PubMed] [Google Scholar]
- Adamson J, Haswell LE, Phillips G et al. In Vitro Models of Chronic Obstructive Pulmonary Disease (COPD). In Martin-Loeches Ignacio, (ed.), Bronchitis (Rijeka: InTech; ), Ch. 03. 2011. [Google Scholar]
- Agrawal A, Murphy TF. Haemophilus influenzae infections in the H. influenzae type b conjugate vaccine era. J Clin Microbiol 2011;49:3728–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahren IL, Eriksson E, Egesten A et al. Nontypeable Haemophilus influenzae activates human eosinophils through beta-glucan receptors. Am J Resp Cell Mol Biol 2003;29:598–605. [DOI] [PubMed] [Google Scholar]
- Ahren IL, Janson H, Forsgren A et al. Protein D expression promotes the adherence and internalization of non-typeable Haemophilus influenzae into human monocytic cells. Microb Pathog 2001a;31:151–8. [DOI] [PubMed] [Google Scholar]
- Ahren IL, Williams DL, Rice PJ et al. The importance of a beta-glucan receptor in the nonopsonic entry of nontypeable Haemophilus influenzae into human monocytic and epithelial cells. J Infect Dis 2001b;184:150–8. [DOI] [PubMed] [Google Scholar]
- Alikhan MM, Lee FE. Understanding nontypeable Haemophilus influenzae and chronic obstructive pulmonary disease. Curr Opin Pulm Med 2014;20:159–64. [DOI] [PubMed] [Google Scholar]
- Anderson GP. Advances in understanding COPD. F1000Res 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anzueto A. Impact of exacerbations on COPD. Eur Respir Rev 2010;19:113–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arce FT, Carlson R, Monds J et al. Nanoscale structural and mechanical properties of nontypeable Haemophilus influenzae biofilms. J Bacteriol 2009;191:2512–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atack JM, Srikhanta YN, Fox KL et al. A biphasic epigenetic switch controls immunoevasion, virulence and niche adaptation in non-typeable Haemophilus influenzae. Nat Commun 2015b;6:7828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atack JM, Winter LE, Jurcisek JA et al. Selection and counterselection of Hia expression reveals a key role for phase-variable expression of Hia in infection caused by nontypeable Haemophilus influenzae J Infect Dis 2015a;212:645–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avadhanula V, Rodriguez CA, Ulett GC et al. Nontypeable Haemophilus influenzae adheres to intercellular adhesion molecule 1 (ICAM-1) on respiratory epithelial cells and upregulates ICAM-1 expression. Infect Immun 2006;74:830–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddal B, Muzzi A, Censini S et al. Dual RNA-seq of nontypeable Haemophilus influenzae and host cell transcriptomes reveals novel insights into host-pathogen cross talk. MBio 2015;6:e01765–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakaletz LO. Bacterial biofilms in the upper airway—evidence for role in pathology and implications for treatment of otitis media. Paediatr Respir Rev 2012;13:154–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakaletz LO. Viral-bacterial co-infections in the respiratory tract. Curr Opin Microbiol 2016;35:30–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandi V, Apicella MA, Mason E et al. Nontypeable Haemophilus influenzae in the lower respiratory tract of patients with chronic bronchitis. Am J Resp Crit Care 2001;164:2114–9. [DOI] [PubMed] [Google Scholar]
- Barenkamp SJ, St Geme JW 3rd. Identification of a second family of high-molecular-weight adhesion proteins expressed by non-typable Haemophilus influenzae. Mol Microbiol 1996;19:1215–23. [DOI] [PubMed] [Google Scholar]
- Barthel D, Singh B, Riesbeck K et al. Haemophilus influenzae uses the surface protein E to acquire human plasminogen and to evade innate immunity. J Immunol 2012;188:379–85. [DOI] [PubMed] [Google Scholar]
- Berenson CS, Garlipp MA, Grove LJ et al. Impaired phagocytosis of nontypeable Haemophilus influenzae by human alveolar macrophages in chronic obstructive pulmonary disease. J Infect Dis 2006;194:1375–84. [DOI] [PubMed] [Google Scholar]
- Berenson CS, Kruzel RL, Eberhardt E et al. Phagocytic dysfunction of human alveolar macrophages and severity of chronic obstructive pulmonary disease. J Infect Dis 2013;208:2036–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berenson CS, Kruzel RL, Eberhardt E et al. Impaired innate immune alveolar macrophage response and the predilection for COPD exacerbations. Thorax 2014;69:811–8. [DOI] [PubMed] [Google Scholar]
- Berenson CS, Kruzel RL, Sethi S. The impact of exogenous factors on respiratory pathogen-induced innate alveolar macrophage responses in COPD. Immunol Invest 2016;45:130–47. [DOI] [PubMed] [Google Scholar]
- Berenson CS, Murphy TF, Wrona CT et al. Outer membrane protein P6 of nontypeable Haemophilus influenzae is a potent and selective inducer of human macrophage proinflammatory cytokines. Infect Immun 2005;73:2728–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolduc GR, Bouchet V, Jiang RZ et al. Variability of outer membrane protein P1 and its evaluation as a vaccine candidate against experimental otitis media due to nontypeable Haemophilus influenzae: an unambiguous, multifaceted approach. Infect Immun 2000;68:4505–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookwalter JE, Jurcisek JA, Gray-Owen SD et al. A carcinoembryonic antigen-related cell adhesion molecule 1 homologue plays a pivotal role in nontypeable Haemophilus influenzae colonization of the chinchilla nasopharynx via the outer membrane protein P5-homologous adhesin. Infect Immun 2008;76:48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockman KL, Jurcisek JA, Atack JM et al. ModA2 phasevarion switching in nontypeable Haemophilus influenzae increases the severity of experimental otitis media. J Infect Dis 2016;214:817–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Cox AD, Li J et al. Activation of innate immune responses by Haemophilus influenzae lipooligosaccharide. Clin Vaccine Immunol 2014;21:769–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Nix EB, Gaultier GN et al. Naturally occurring bactericidal antibodies specific for Haemophilus influenzae lipooligosaccharide are present in healthy adult individuals. Vaccine 2015;33:1941–7. [DOI] [PubMed] [Google Scholar]
- Clementi CF, Hakansson AP, Murphy TF. Internalization and trafficking of nontypeable Haemophilus influenzae in human respiratory epithelial cells and roles of IgA1 proteases for optimal invasion and persistence. Infect Immun 2014;82:433–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clementi CF, Murphy TF. Non-typeable Haemophilus influenzae invasion and persistence in the human respiratory tract. Front Cell Infect Microbiol 2011;1:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline SD, Saleem S, Daines DA. Regulation of the vapBC-1 toxin-antitoxin locus in nontypeable Haemophilus influenzae. PLoS One 2012;7:e32199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosio BG, Jahn A, Iglesias A et al. Haemophilus influenzae induces steroid-resistant inflammatory responses in COPD. BMC Pulm Med 2015;15:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig JE, Cliffe A, Garnett K et al. Survival of nontypeable Haemophilus influenzae in macrophages. FEMS Microbiol Lett 2001;203:55–61. [DOI] [PubMed] [Google Scholar]
- De Chiara M, Hood D, Muzzi A et al. Genome sequencing of disease and carriage isolates of nontypeable Haemophilus influenzae identifies discrete population structure. P Natl Acad Sci USA 2014;111:5439–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decramer M, Janssens W, Miravitlles M. Chronic obstructive pulmonary disease. Lancet 2012;379:1341–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deich RA, Hoyer LC. Generation and release of DNA-binding vesicles by Haemophilus influenzae during induction and loss of competence. J Bacteriol 1982;152:855–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deknuydt F, Nordstrom T, Riesbeck K. Diversion of the host humoral response: a novel virulence mechanism of Haemophilus influenzae mediated via outer membrane vesicles. J Leukocyte Biol 2014;95:983–91. [DOI] [PubMed] [Google Scholar]
- Denny FW. Effect of a toxin produced by Haemophilus influenzae on ciliated respiratory epithelium. J Infect Dis 1974;129:93–100. [DOI] [PubMed] [Google Scholar]
- Desai H, Eschberger K, Wrona C et al. Bacterial colonization increases daily symptoms in patients with chronic obstructive pulmonary disease. Ann Am Thorac Soc 2014;11:303–9. [DOI] [PubMed] [Google Scholar]
- Duell BL, Su YC, Riesbeck K. Host-pathogen interactions of nontypeable Haemophilus influenzae: from commensal to pathogen. FEBS Lett 2016;590:3840–53. [DOI] [PubMed] [Google Scholar]
- Duim B, Bowler LD, Eijk PP et al. Molecular variation in the major outer membrane protein P5 gene of nonencapsulated Haemophilus influenzae during chronic infections. Infect Immun 1997;65:1351–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldika N, Sethi S. Role of nontypeable Haemophilus influenzae in exacerbations and progression of chronic obstructive pulmonary disease. Curr Opin Pulm Med 2006;12:118–24. [DOI] [PubMed] [Google Scholar]
- Erwin AL, Nelson KL, Mhlanga-Mutangadura T et al. Characterization of genetic and phenotypic diversity of invasive nontypeable Haemophilus influenzae. Infect Immun 2005;73:5853–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euba B, Moleres J, Viadas C et al. Relative contribution of P5 and Hap surface proteins to nontypable Haemophilus influenzae interplay with the host upper and lower airways. PLoS One 2015;10:e0123154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faden H, Duffy L, Williams A et al. Epidemiology of nasopharyngeal colonization with nontypeable Haemophilus influenzae in the first two years of life. Acta Otolaryngol Suppl 1996;523:128–9. [PubMed] [Google Scholar]
- Fernaays MM, Lesse AJ, Cai X et al. Characterization of igaB, a second immunoglobulin A1 protease gene in nontypeable Haemophilus influenzae. Infect Immun 2006a;74:5860–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernaays MM, Lesse AJ, Sethi S et al. Differential genome contents of nontypeable Haemophilus influenzae strains from adults with chronic obstructive pulmonary disease. Infect Immun 2006b;74:3366–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueira MA, Ram S, Goldstein R et al. Role of complement in defense of the middle ear revealed by restoring the virulence of nontypeable Haemophilus influenzae siaB mutants. Infect Immun 2007;75:325–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink DL, Buscher AZ, Green B et al. The Haemophilus influenzae Hap autotransporter mediates microcolony formation and adherence to epithelial cells and extracellular matrix via binding regions in the C-terminal end of the passenger domain. Cell Microbiol 2003;5:175–86. [DOI] [PubMed] [Google Scholar]
- Fink DL, Green BA, St Geme JW 3rd. The Haemophilus influenzae Hap autotransporter binds to fibronectin, laminin, and collagen IV. Infect Immun 2002;70:4902–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finney LJ, Ritchie A, Pollard E et al. Lower airway colonization and inflammatory response in COPD: a focus on Haemophilus influenzae. Int J Chron Obstruct Pulmon Dis 2014;9:1119–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischmann RD, Adams MD, White O et al. Whole-genome random sequencing and assembly of Haemophilus influenzae Rd. Science 1995;269:496–512. [DOI] [PubMed] [Google Scholar]
- Forsgren J, Samuelson A, Ahlin A et al. Haemophilus influenzae resides and multiplies intracellularly in human adenoid tissue as demonstrated by in situ hybridization and bacterial viability assay. Infect Immun 1994;62:673–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsgren J, Samuelson A, Borrelli S et al. Persistence of nontypeable Haemophilus influenzae in adenoid macrophages: a putative colonization mechanism. Acta Otolaryngol 1996;116:766–73. [DOI] [PubMed] [Google Scholar]
- Fox KL, Dowideit SJ, Erwin AL et al. Haemophilus influenzae phasevarions have evolved from type III DNA restriction systems into epigenetic regulators of gene expression. Nucleic Acids Res 2007;35:5242–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia LS. Clinical Microbiology Procedures Handbook, 3rd edn Washington, DC: ASM, 2010, 2540. [Google Scholar]
- Gilsdorf JR, Marrs CF, Foxman B. Haemophilus influenzae: genetic variability and natural selection to identify virulence factors. Infect Immun 2004;72:2457–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giufre M, Carattoli A, Cardines R et al. Variation in expression of HMW1 and HMW2 adhesins in invasive nontypeable Haemophilus influenzae isolates. BMC Microbiol 2008;8:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOLD-Report. Global Strategy for Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease (2017 Report), in Agusti AG, Vogelmeier C (ed.), Global Initiative for Chronic Obstructive Lung Disease. 2017, available at http://goldcopd.org.
- Groeneveld K, Eijk PP, van Alphen L et al. Haemophilus influenzae infections in patients with chronic obstructive pulmonary disease despite specific antibodies in serum and sputum. Am Rev Respir Dis 1990;141 (5 Pt 1):1316–21. [DOI] [PubMed] [Google Scholar]
- Guarascio AJ, Ray SM, Finch CK et al. The clinical and economic burden of chronic obstructive pulmonary disease in the USA. Clinicoecon Outcomes Res 2013;5:235–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn JS, Bakaletz LO, Wozniak DJ. What's on the outside matters: the role of the extracellular polymeric substance of Gram-negative biofilms in evading host immunity and as a target for therapeutic intervention. J Biol Chem 2016;291:12538–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall-Stoodley L, Hu FZ, Gieseke A et al. Direct detection of bacterial biofilms on the middle-ear mucosa of children with chronic otitis media. JAMA 2006;296:202–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallstrom T, Riesbeck K. Haemophilus influenzae and the complement system. Trends Microbiol 2010;18:258–65. [DOI] [PubMed] [Google Scholar]
- Hallstrom T, Singh B, Resman F et al. Haemophilus influenzae protein E binds to the extracellular matrix by concurrently interacting with laminin and vitronectin. J Infect Dis 2011;204:1065–74. [DOI] [PubMed] [Google Scholar]
- Hamilton B, Manzella A, Schmidt K et al. Analysis of non-typeable Haemophilous influenzae VapC1 mutations reveals structural features required for toxicity and flexibility in the active site. PLoS One 2014;9:e112921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariadi NI, Zhang L, Patel M et al. Comparative profile of heme acquisition genes in disease-causing and colonizing nontypeable Haemophilus influenzae and Haemophilus haemolyticus. J Clin Microbiol 2015;53:2132–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison A, Santana EA, Szelestey BR et al. Ferric uptake regulator and its role in the pathogenesis of nontypeable Haemophilus influenzae. Infect Immun 2013;81:1221–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert MA, Crook D, Moxon ER. Molecular methods for Haemophilus influenzae. Methods Mol Med 1998;15:243–63. [DOI] [PubMed] [Google Scholar]
- Heyl KA, Klassert TE, Heinrich A et al. Dectin-1 is expressed in human lung and mediates the proinflammatory immune response to nontypeable Haemophilus influenzae. MBio 2014;5:e01492–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiemstra PS, Amatngalim GD, van der Does AM et al. Antimicrobial peptides and innate lung defenses: role in infectious and noninfectious lung diseases and therapeutic applications. Chest 2016;149:545–51. [DOI] [PubMed] [Google Scholar]
- Hiltke TJ, Sethi S, Murphy TF. Sequence stability of the gene encoding outer membrane protein P2 of nontypeable Haemophilus influenzae in the human respiratory tract. J Infect Dis 2002;185:627–31. [DOI] [PubMed] [Google Scholar]
- Hiltke TJ, Schiffmacher AT, Dagonese AJ et al. Horizontal transfer of the gene encoding outer membrane protein P2 of nontypeable Haemophilus influenzae, in a patient with chronic obstructive pulmonary disease. J Infect Dis 2003;188:114–7. [DOI] [PubMed] [Google Scholar]
- Hoiby N, Ciofu O, Bjarnsholt T. Pseudomonas aeruginosa biofilms in cystic fibrosis. Future Microbiol 2010;5:1663–74. [DOI] [PubMed] [Google Scholar]
- Holmes KA, Bakaletz LO. Adherence of non-typeable Haemophilus influenzae promotes reorganization of the actin cytoskeleton in human or chinchilla epithelial cells in vitro. Microb Pathog 1997;23:157–66. [DOI] [PubMed] [Google Scholar]
- Hong W, Juneau RA, Pang B et al. Survival of bacterial biofilms within neutrophil extracellular traps promotes nontypeable Haemophilus influenzae persistence in the chinchilla model for otitis media. J Innate Immun 2009;1:215–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu F, Rishishwar L, Sivadas A et al. Comparative genomic analysis of Haemophilus haemolyticus and nontypeable Haemophilus influenzae and a new testing scheme for their discrimination. J Clin Microbiol 2016;54:3010–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idicula WK, Jurcisek JA, Cass ND et al. Identification of biofilms in post-tympanostomy tube otorrhea. Laryngoscope 2016;126:1946–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda M, Enomoto N, Hashimoto D et al. Nontypeable Haemophilus influenzae exploits the interaction between protein-E and vitronectin for the adherence and invasion to bronchial epithelial cells. BMC Microbiol 2015;15:263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izano EA, Shah SM, Kaplan JB. Intercellular adhesion and biocide resistance in nontypeable Haemophilus influenzae biofilms. Microb Pathog 2009;46:207–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalalvand F, Su YC, Morgelin M et al. Haemophilus influenzae protein F mediates binding to laminin and human pulmonary epithelial cells. J Infect Dis 2013;207:803–13. [DOI] [PubMed] [Google Scholar]
- Janson H, Carlén, B, Cervin A et al. Effects on the ciliated epithelium of protein D-producing and -nonproducing nontypeable Haemophilus influenzae in nasopharyngeal tissue cultures. J Infect Dis 1999;180:737–46. [DOI] [PubMed] [Google Scholar]
- Jemal A, Ward E, Hao Y et al. Trends in the leading causes of death in the United States, 1970-2002. JAMA 2005;294:1255–9. [DOI] [PubMed] [Google Scholar]
- Jin G, Pavelka MS Jr, Butler JS. Structure-function analysis of VapB4 antitoxin identifies critical features of a minimal VapC4 toxin-binding module. J Bacteriol 2015;197:1197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RW, McGillivary G, Denoel P et al. Abrogation of nontypeable Haemophilus influenzae protein D function reduces phosphorylcholine decoration, adherence to airway epithelial cells, and fitness in a chinchilla model of otitis media. Vaccine 2011;29:1211–21. [DOI] [PubMed] [Google Scholar]
- Jones EA, McGillivary G, Bakaletz LO. Extracellular DNA within a nontypeable Haemophilus influenzae-induced biofilm binds human beta defensin-3 and reduces its antimicrobial activity. J Innate Immun 2013;5:24–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juneau RA, Pang B, Weimer KE et al. Nontypeable Haemophilus influenzae initiates formation of neutrophil extracellular traps. Infect Immun 2011;79:431–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juneau RA, Pang B, Armbruster CE et al. Peroxiredoxin-glutaredoxin and catalase promote resistance of nontypeable Haemophilus influenzae 86-028NP to oxidants and survival within neutrophil extracellular traps. Infect Immun 2015;83:239–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurcisek JA, Bakaletz LO. Biofilms formed by nontypeable Haemophilus influenzae in vivo contain both double-stranded DNA and type IV pilin protein. J Bacteriol 2007;189:3868–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurcisek JA, Bookwalter JE, Baker BD et al. The PilA protein of non-typeable Haemophilus influenzae plays a role in biofilm formation, adherence to epithelial cells and colonization of the mammalian upper respiratory tract. Mol Microbiol 2007;65:1288–99. [DOI] [PubMed] [Google Scholar]
- Kahn ME, Barany F, Smith HO. Transformasomes: specialized membranous structures that protect DNA during Haemophilus transformation. P Natl Acad Sci USA 1983;80:6927–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kainulainen L, Nikoskelainen J, Vuorinen T et al. Viruses and bacteria in bronchial samples from patients with primary hypogammaglobulinemia. Am J Resp Crit Care 1999;159(4 Pt 1):1199–204. [DOI] [PubMed] [Google Scholar]
- Kalathil SG, Lugade AA, Pradhan V et al. T-regulatory cells and programmed death 1+ T cells contribute to effector T-cell dysfunction in patients with chronic obstructive pulmonary disease. Am J Resp Crit Care 2014;190:40–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy BJ, Novotny LA, Jurcisek JA et al. Passive transfer of antiserum specific for immunogens derived from a nontypeable Haemophilus influenzae adhesin and lipoprotein D prevents otitis media after heterologous challenge. Infect Immun 2000;68:2756–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketterer MR, Shao JQ, Hornick DB et al. Infection of primary human bronchial epithelial cells by Haemophilus influenzae: macropinocytosis as a mechanism of airway epithelial cell entry. Infect Immun 1999;67:4161–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan MN, Kaur R, Pichichero ME. Bactericidal antibody response against P6, protein D, and OMP26 of nontypeable Haemophilus influenzae after acute otitis media in otitis-prone children. FEMS Immunol Med Mic 2012;65:439–47. [DOI] [PubMed] [Google Scholar]
- Kilian M, Mestecky J, Russell MW. Defense mechanisms involving Fc-dependent functions of immunoglobulin A and their subversion by bacterial immunoglobulin A proteases. Microbiol Rev 1988;52:296–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilian M, Mestecky J, Schrohenloher RE. Pathogenic species of the genus Haemophilus and Streptococcus pneumoniae produce immunoglobulin A1 protease. Infect Immun 1979;26:143–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilian M, Reinholdt J, Lomholt H et al. Biological significance of IgA1 proteases in bacterial colonization and pathogenesis: critical evaluation of experimental evidence. Apmis 1996;104:321–38. [DOI] [PubMed] [Google Scholar]
- King P. Haemophilus influenzae and the lung (Haemophilus and the lung). Clin Transl Med 2012;1:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King P, Holdsworth S, Freezer N et al. Bronchiectasis. Intern Med J 2006;36:729–37. [DOI] [PubMed] [Google Scholar]
- King PT, Hutchinson PE, Johnson PD et al. Adaptive immunity to nontypeable Haemophilus influenzae. Am J Resp Crit Care 2003;167:587–92. [DOI] [PubMed] [Google Scholar]
- King PT, Lim S, Pick A et al. Lung T-cell responses to nontypeable Haemophilus influenzae in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol 2013;131:1314–21.e14. [DOI] [PubMed] [Google Scholar]
- King PT, Ngui J, Gunawardena D et al. 'Systemic humoral immunity to non-typeable Haemophilus influenzae. Clin Exp Immunol 2008;153:376–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King PT, Sharma R. The lung immune response to nontypeable Haemophilus influenzae (lung immunity to NTHi). J Immunol Res 2015;2015:706376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knobloch J, Schild K, Jungck D et al. The T-helper cell type 1 immune response to gram-negative bacterial infections is impaired in COPD. Am J Resp Crit Care 2011;183:204–14. [DOI] [PubMed] [Google Scholar]
- Lamblin G, Degroote S, Perini JM et al. Human airway mucin glycosylation: a combinatory of carbohydrate determinants which vary in cystic fibrosis. Glycoconj J 2001;18:661–84. [DOI] [PubMed] [Google Scholar]
- Langereis JD, de Jonge MI. Invasive disease caused by nontypeable Haemophilus influenzae. Emerg Infect Dis 2015;21:1711–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langereis JD, de Jonge MI, Weiser JN. Binding of human factor H to outer membrane protein P5 of non-typeable Haemophilus influenzae contributes to complement resistance. Mol Microbiol 2014;94:89–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langereis JD, Hermans PW. Novel concepts in nontypeable Haemophilus influenzae biofilm formation. FEMS Microbiol Lett 2013;346:81–9. [DOI] [PubMed] [Google Scholar]
- Langereis JD, Stol K, Schweda EK et al. Modified lipooligosaccharide structure protects nontypeable Haemophilus influenzae from IgM-mediated complement killing in experimental otitis media. MBio 2012;3:e00079–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langereis JD, Weiser JN. Shielding of a lipooligosaccharide IgM epitope allows evasion of neutrophil-mediated killing of an invasive strain of nontypeable Haemophilus influenzae. MBio 2014;5:e01478–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laube DM, Yim S, Ryan LK et al. Antimicrobial peptides in the airway. Curr Top Microbiol Immunol 2006;306:153–82. [DOI] [PubMed] [Google Scholar]
- Leake ER, Holmes K, Lim DJ et al. Peptidoglycan isolated from nontypeable Haemophilus influenzae induces experimental otitis media in the chinchilla. J Infect Dis 1994;170:1532–8. [DOI] [PubMed] [Google Scholar]
- Lewis ER, Torres AG. The art of persistence-the secrets to Burkholderia chronic infections. Pathog Dis 2016;74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L, Ayala P, Larson J et al. The Neisseria type 2 IgA1 protease cleaves LAMP1 and promotes survival of bacteria within epithelial cells. Mol Microbiol 1997;24:1083–94. [DOI] [PubMed] [Google Scholar]
- Linden SK, Sutton P, Karlsson NG et al. Mucins in the mucosal barrier to infection. Mucosal Immunol 2008;1:183–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Campos JL, Tan W, Soriano JB. Global burden of COPD. Respirology 2016;21:14–23. [DOI] [PubMed] [Google Scholar]
- Mahon CR, Lehman DC, Manuselis G. Haemophilus and other fastidious Gram-Negative bacilli. In: Wurm-Cutter E. (ed.) Textbook of Diagnostic Microbiology, 4th edn Maryland Heights: Missouri Elsevier Health Sciences, 2011;395–414. [Google Scholar]
- Marks LR, Parameswaran GI, Hakansson AP. Pneumococcal interactions with epithelial cells are crucial for optimal biofilm formation and colonization in vitro and in vivo. Infect Immun 2012;80:2744–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason KM, Bruggeman ME, Munson RS et al. The non-typeable Haemophilus influenzae Sap transporter provides a mechanism of antimicrobial peptide resistance and SapD-dependent potassium acquisition. Mol Microbiol 2006;62:1357–72. [DOI] [PubMed] [Google Scholar]
- Meats E, Feil EJ, Stringer S et al. Characterization of encapsulated and noncapsulated Haemophilus influenzae and determination of phylogenetic relationships by multilocus sequence typing. J Clin Microbiol 2003;41:1623–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mell JC, Viadas C, Moleres J et al. Transformed recombinant enrichment profiling rapidly identifies HMW1 as an intracellular invasion locus in Haemophilus influenza. PLoS Pathog 2016;12:e1005576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkel D, Rist W, Seither P et al. Proteomic study of human bronchoalveolar lavage fluids from smokers with chronic obstructive pulmonary disease by combining surface-enhanced laser desorption/ionization-mass spectrometry profiling with mass spectrometric protein identification. Proteomics 2005;5:2972–80. [DOI] [PubMed] [Google Scholar]
- Morton DJ, Bakaletz LO, Jurcisek JA et al. Reduced severity of middle ear infection caused by nontypeable Haemophilus influenzae lacking the hemoglobin/hemoglobin-haptoglobin binding proteins (Hgp) in a chinchilla model of otitis media. Microb Pathog 2004;36:25–33. [DOI] [PubMed] [Google Scholar]
- Murphy TF. Respiratory infections caused by non-typeable Haemophilus influenzae. Curr Opin Infect Dis 2003;16:129–34. [DOI] [PubMed] [Google Scholar]
- Murphy TF. Vaccines for nontypeable Haemophilus influenzae: the future is now. Clin Vaccine Immunol 2015;22:459–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy TF, Brauer AL. Expression of urease by Haemophilus influenzae during human respiratory tract infection and role in survival in an acid environment. BMC Microbiol 2011;11:183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy TF, Brauer AL, Schiffmacher AT et al. Persistent colonization by Haemophilus influenzae in chronic obstructive pulmonary disease. Am J Resp Crit Care 2004;170:266–72. [DOI] [PubMed] [Google Scholar]
- Murphy TF, Brauer AL, Sethi S et al. Haemophilus haemolyticus: a human respiratory tract commensal to be distinguished from Haemophilus influenzae. J Infect Dis 2007;195:81–9. [DOI] [PubMed] [Google Scholar]
- Murphy TF, Faden H, Bakaletz LO et al. Nontypeable Haemophilus influenzae as a pathogen in children. Pediatr Infect Dis J 2009;28:43–8. [DOI] [PubMed] [Google Scholar]
- Murphy TF, Kirkham C, Jones MM et al. 'Expression of IgA proteases by Haemophilus influenzae in the respiratory tract of adults with chronic obstructive pulmonary disease. J Infect Dis 2015;212:1798–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy TF, Kirkham C, Sethi S et al. Expression of a peroxiredoxin-glutaredoxin by Haemophilus influenzae in biofilms and during human respiratory tract infection. FEMS Immunol Med Mic 2005;44:81–9. [DOI] [PubMed] [Google Scholar]
- Murphy TF, Parameswaran GI. Moraxella catarrhalis, a human respiratory tract pathogen. Clin Infect Dis 2009;49:124–31. [DOI] [PubMed] [Google Scholar]
- Murray PR, Baron EJ, Jorgensen JH et al. Manual of Clinical Microbiology, 9th edn Washington, DC: ASM, 2007, 2488. [Google Scholar]
- Neary JM, Murphy TF. Antibodies directed at a conserved motif in loop 6 of outer membrane protein P2 of nontypeable Haemophilus influenzae recognize multiple strains in immunoassays. FEMS Immunol Med Mic 2006;46:251–61. [DOI] [PubMed] [Google Scholar]
- Noel GJ, Barenkamp SJ, St Geme JW 3rd et al. High-molecular-weight surface-exposed proteins of Haemophilus influenzae mediate binding to macrophages. J Infect Dis 1994b;169:425–9. [DOI] [PubMed] [Google Scholar]
- Noel GJ, Hoiseth SK, Edelson PJ. Type b capsule inhibits ingestion of Haemophilus influenzae by murine macrophages: studies with isogenic encapsulated and unencapsulated strains. J Infect Dis 1992;166:178–82. [DOI] [PubMed] [Google Scholar]
- Noel GJ, Love DC, Mosser DM. High-molecular-weight proteins of nontypeable Haemophilus influenzae mediate bacterial adhesion to cellular proteoglycans. Infect Immun 1994;62:4028–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novotny LA, Adams LD, Kang DR et al. Epitope mapping immunodominant regions of the PilA protein of nontypeable Haemophilus influenzae (NTHI) to facilitate the design of two novel chimeric vaccine candidates. Vaccine 2009;28:279–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novotny LA, Bakaletz LO. Intercellular adhesion molecule 1 serves as a primary cognate receptor for the Type IV pilus of nontypeable Haemophilus influenzae. Cell Microbiol 2016;18:1043–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novotny LA, Jurcisek JA, Goodman SD et al. Monoclonal antibodies against DNA-binding tips of DNABII proteins disrupt biofilms in vitro and induce bacterial clearance in vivo. EBioMedicine 2016;10:33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novotny LA, Jurcisek JA, Ward MO Jr et al. Antibodies against the majority subunit of type IV Pili disperse nontypeable Haemophilus influenzae biofilms in a LuxS-dependent manner and confer therapeutic resolution of experimental otitis media. Mol Microbiol 2015;96:276–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandya PH, Wilkes DS. Complement system in lung disease. Am J Resp Cell Mol 2014;51:467–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang B, Hong W, West-Barnette SL et al. Diminished ICAM-1 expression and impaired pulmonary clearance of nontypeable Haemophilus influenzae in a mouse model of chronic obstructive pulmonary disease/emphysema. Infect Immun 2008;76:4959–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paone G, Conti V, Vestri A et al. Analysis of sputum markers in the evaluation of lung inflammation and functional impairment in symptomatic smokers and COPD patients. Dis Markers 2011;31:91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettigrew MM, Tsuji BT, Gent JF et al. Effect of fluoroquinolones and macrolides on eradication and resistance of Haemophilus influenzae in chronic obstructive pulmonary disease. Antimicrob Agents Ch 2016;60:4151–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaut AG, Gilbert JV, Artenstein MS et al. Neisseria gonorrhoeae and neisseria meningitidis: extracellular enzyme cleaves human immunoglobulin A. Science 1975;190:1103–5. [DOI] [PubMed] [Google Scholar]
- Poole J, Foster E, Chaloner K et al. Analysis of nontypeable haemophilus influenzae phase-variable genes during experimental human nasopharyngeal colonization. J Infect Dis 2013;208:720–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post DM, Held JM, Ketterer MR et al. Comparative analyses of proteins from Haemophilus influenzae biofilm and planktonic populations using metabolic labeling and mass spectrometry. BMC Microbiol 2014;14:329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post JC. Direct evidence of bacterial biofilms in otitis media. Laryngoscope 2001;111:2083–94. [DOI] [PubMed] [Google Scholar]
- Power PM, Sweetman WA, Gallacher NJ et al. Simple sequence repeats in Haemophilus influenzae. Infect Genet Evol 2009;9:216–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasadarao NV, Lysenko E, Wass CA et al. Opacity-associated protein A contributes to the binding of Haemophilus influenzae to chang epithelial cells. Infect Immun 1999;67:4153–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price EP, Sarovich DS, Nosworthy E et al. Haemophilus influenzae: using comparative genomics to accurately identify a highly recombinogenic human pathogen. BMC Genomics 2015;16:641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puljic R, Pahl A. Smoke induced changes in epithelial cell gene expression: development of an in vitro model for COPD. Altex 2004;21:3–7. [PubMed] [Google Scholar]
- Qu J, Lesse AJ, Brauer AL et al. Proteomic expression profiling of Haemophilus influenzae grown in pooled human sputum from adults with chronic obstructive pulmonary disease reveal antioxidant and stress responses. BMC Microbiol 2010;10:162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffel FK, Szelestey BR, Beatty WL et al. The Haemophilus influenzae Sap transporter mediates bacterium-epithelial cell homeostasis. Infect Immun 2013;81:43–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy MS, Bernstein JM, Murphy TF et al. Binding between outer membrane proteins of nontypeable Haemophilus influenzae and human nasopharyngeal mucin. Infect Immun 1996;64:1477–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy MS, Murphy TF, Faden HS et al. Middle ear mucin glycoprotein: purification and interaction with nontypable Haemophilus influenzae and Moraxella catarrhalis. Otolaryngol Head Neck Surg 1997;116:175–80. [DOI] [PubMed] [Google Scholar]
- Reimche JL, Kirse DJ, Whigham AS et al. Resistance of nontypeable Haemophilus influenzae biofilms is independent of biofilm size. Pathog Dis 2016;75:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren D, Kordis AA, Sonenshine DE et al. The ToxAvapA toxin-antitoxin locus contributes to the survival of nontypeable Haemophilus influenzae during infection. PLoS One 2014;9:e91523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren D, Walker AN, Daines DA. Toxin-antitoxin loci vapBC-1 and vapXD contribute to survival and virulence in nontypeable Haemophilus influenzae. BMC Microbiol 2012;12:263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roier S, Zingl FG, Cakar F et al. A novel mechanism for the biogenesis of outer membrane vesicles in Gram-negative bacteria. Nat Commun 2016;7:10515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronander E, Brant M, Eriksson E et al. Nontypeable Haemophilus influenzae adhesin protein E: characterization and biological activity. J Infect Dis 2009;199:522–31. [DOI] [PubMed] [Google Scholar]
- Ronander E, Brant M, Janson H et al. Identification of a novel Haemophilus influenzae protein important for adhesion to epithelial cells. Microbes Infect 2008;10:87–96. [DOI] [PubMed] [Google Scholar]
- Rosadini CV, Ram S, Akerley BJ. Outer membrane protein P5 is required for resistance of nontypeable Haemophilus influenzae to both the classical and alternative complement pathways. Infect Immun 2014;82:640–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seemungal TA, Donaldson GC, Bhowmik A et al. 'Time course and recovery of exacerbations in patients with chronic obstructive pulmonary disease. Am J Resp Crit Care 2000;161:1608–13. [DOI] [PubMed] [Google Scholar]
- Sethi S, Evans N, Grant BJ et al. New strains of bacteria and exacerbations of chronic obstructive pulmonary disease. N Engl J Med 2002;347:465–71. [DOI] [PubMed] [Google Scholar]
- Sethi S, Murphy TF. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med 2008;359:2355–65. [DOI] [PubMed] [Google Scholar]
- Sethi S, Wrona C, Eschberger K et al. Inflammatory profile of new bacterial strain exacerbations of chronic obstructive pulmonary disease. Am J Resp Crit Care 2008;177:491–7. [DOI] [PubMed] [Google Scholar]
- Sethi S, Wrona C, Grant BJ et al. Strain-specific immune response to Haemophilus influenzae in chronic obstructive pulmonary disease. Am J Resp Crit Care 2004;169:448–53. [DOI] [PubMed] [Google Scholar]
- Sharpe SW, Kuehn MJ, Mason KM. Elicitation of epithelial cell-derived immune effectors by outer membrane vesicles of nontypeable Haemophilus influenzae. Infect Immun 2011;79:4361–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton CL, Raffel FK, Beatty WL et al. Sap transporter mediated import and subsequent degradation of antimicrobial peptides in Haemophilus. PLoS Pathog 2011;7:e1002360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikkema DJ, Murphy TF. Molecular analysis of the P2 porin protein of nontypeable Haemophilus influenzae. Infect Immun 1992;60:5204–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slinger R, Chan F, Ferris W et al. Multiple combination antibiotic susceptibility testing of nontypeable Haemophilus influenzae biofilms. Diagn Microbiol Infect Dis 2006;56:247–53. [DOI] [PubMed] [Google Scholar]
- St Geme JW 3rd, Cutter D. The Haemophilus influenzae Hia adhesin is an autotransporter protein that remains uncleaved at the C terminus and fully cell associated. J Bacteriol 2000;182:6005–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Geme JW 3rd, Falkow S. Haemophilus influenzae adheres to and enters cultured human epithelial cells. Infect Immun 1990;58:4036–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Geme JW 3rd, Falkow S, Barenkamp SJ. High-molecular-weight proteins of nontypable Haemophilus influenzae mediate attachment to human epithelial cells. P Natl Acad Sci USA 1993;90:2875–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staples KJ, Taylor S, Thomas S et al. Relationships between mucosal antibodies, non-typeable Haemophilus influenzae (NTHi) infection and airway inflammation in COPD. PLoS One 2016;11:e0167250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starner TD, Shrout JD, Parsek MR et al. Subinhibitory concentrations of azithromycin decrease nontypeable Haemophilus influenzae biofilm formation and Diminish established biofilms. Antimicrob Agents Ch 2008;52:137–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starner TD, Zhang N, Kim G et al. Haemophilus influenzae forms biofilms on airway epithelia: implications in cystic fibrosis. Am J Resp Crit Care 2006;174:213–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su YC, Jalalvand F, Morgelin M et al. Haemophilus influenzae acquires vitronectin via the ubiquitous Protein F to subvert host innate immunity. Mol Microbiol 2013;87:1245–66. [DOI] [PubMed] [Google Scholar]
- Su YC, Mukherjee O, Singh B et al. Haemophilus influenzae P4 interacts with extracellular matrix proteins promoting adhesion and serum resistance. J Infect Dis 2016;213:314–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweetman WA, Moxon ER, Bayliss CD. Induction of the SOS regulon of Haemophilus influenzae does not affect phase variation rates at tetranucleotide or dinucleotide repeats. Microbiology 2005;151(Pt 8):2751–63. [DOI] [PubMed] [Google Scholar]
- Swords WE. Nontypeable Haemophilus influenzae biofilms: role in chronic airway infections. Front Cell Infect Microbiol 2012;2:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swords WE, Buscher BA, Ver Steeg Ii K et al. Non-typeable Haemophilus influenzae adhere to and invade human bronchial epithelial cells via an interaction of lipooligosaccharide with the PAF receptor. Mol Microbiol 2000;37:13–27. [DOI] [PubMed] [Google Scholar]
- Szelestey BR, Heimlich DR, Raffel FK et al. Haemophilus responses to nutritional immunity: epigenetic and morphological contribution to biofilm architecture, invasion, persistence and disease severity. PLoS Pathog 2013;9:e1003709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan A, Atack JM, Jennings MP et al. The capricious nature of bacterial pathogens: phasevarions and vaccine development. Front Immunol 2016;7:586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tikhomirova A, Kidd SP. Haemophilus influenzae and Streptococcus pneumoniae: living together in a biofilm. Pathog Dis 2013;69:114–26. [DOI] [PubMed] [Google Scholar]
- Tsang RS, Bruce MG, Lem M et al. A review of invasive Haemophilus influenzae disease in the Indigenous populations of North America. Epidemiol Infect 2014;142:1344–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underwood M, Bakaletz L. Innate immunity and the role of defensins in otitis media. Curr Allergy Asthma Rep 2011;11:499–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger BL, Faris AN, Ganesan S et al. Rhinovirus attenuates non-typeable Hemophilus influenzae-stimulated IL-8 responses via TLR2-dependent degradation of IRAK-1. PLoS Pathog 2012;8:e1002969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Schilfgaarde M, Eijk P, Regelink A et al. Haemophilus influenzae localized in epithelial cell layers is shielded from antibiotics and antibody-mediated bactericidal activity. Microb Pathog 1999;26:249–62. [DOI] [PubMed] [Google Scholar]
- VanWagoner TM, Atack JM, Nelson KL et al. The modA10 phasevarion of nontypeable Haemophilus influenzae R2866 regulates multiple virulence-associated traits. Microb Pathog 2016;92:60–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuong J, Wang X, Theodore JM et al. Absence of high molecular weight proteins 1 and/or 2 is associated with decreased adherence among non-typeable Haemophilus influenzae clinical isolates. J Med Microbiol 2013;62(Pt 11):1649–56. [DOI] [PubMed] [Google Scholar]
- Walling LR, Butler JS. Structural determinants for antitoxin identity and insulation of cross talk between homologous toxin-antitoxin systems. J Bacteriol 2016;198:3287–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson ME Jr, Burns JL, Smith AL. Hypermutable Haemophilus influenzae with mutations in mutS are found in cystic fibrosis sputum. Microbiology 2004;150(Pt 9):2947–58. [DOI] [PubMed] [Google Scholar]
- Webster P, Wu S, Gomez G et al. Distribution of bacterial proteins in biofilms formed by non-typeable Haemophilus influenzae. J Histochem Cytochem 2006;54:829–42. [DOI] [PubMed] [Google Scholar]
- Weiser JN, Love JM, Moxon ER. The molecular mechanism of phase variation of H. influenzae lipopolysaccharide. Cell 1989;59:657–65. [DOI] [PubMed] [Google Scholar]
- Wen Y, Behiels E, Devreese B. Toxin-Antitoxin systems: their role in persistence, biofilm formation, and pathogenicity. Pathog Dis 2014;70:240–9. [DOI] [PubMed] [Google Scholar]
- Westwood JP, Mackay AJ, Donaldson G et al. The role of complement activation in COPD exacerbation recovery. ERJ Open Res 2016;2:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter LE, Barenkamp SJ. Antibodies to the HMW1/HMW2 and Hia adhesins of nontypeable haemophilus influenzae mediate broad-based opsonophagocytic killing of homologous and heterologous strains. Clin Vaccine Immunol 2014;21:613–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter LE, Barenkamp SJ. Naturally acquired HMW1- and HMW2-specific serum antibodies in adults and children mediate opsonophagocytic killing of nontypeable Haemophilus influenzae. Clin Vaccine Immunol 2015;23:37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong SM, Bernui M, Shen H et al. Genome-wide fitness profiling reveals adaptations required by Haemophilus in coinfection with influenza A virus in the murine lung. P Natl Acad Sci USA 2013;110:15413–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Li X, Gunawardana M et al. Beta- lactam antibiotics stimulate biofilm formation in non-typeable haemophilus influenzae by up-regulating carbohydrate metabolism. PLoS One 2014;9:e99204. [DOI] [PMC free article] [PubMed] [Google Scholar]