Abstract
Campylobacter jejuni infections are progressively increasing worldwide. Probiotic treatment might open novel therapeutic or even prophylactic approaches to combat campylobacteriosis. In the present study secondary abiotic mice were generated by broad-spectrum antibiotic treatment and perorally reassociated with a commensal murine Lactobacillus johnsonii strain either 14 days before (i.e. prophylactic regimen) or 7 days after (i.e. therapeutic regimen) peroral C. jejuni strain 81–176 infection. Following peroral reassociation both C. jejuni and L. johnsonii were able to stably colonize the murine intestinal tract. Neither therapeutic nor prophylactic L. johnsonii application, however, could decrease intestinal C. jejuni burdens. Notably, C. jejuni induced colonic apoptosis could be ameliorated by prophylactic L. johnsonii treatment, whereas co-administration of L. johnsonii impacted adaptive (i.e. T and B lymphocytes, regulatory T cells), but not innate (i.e. macrophages and monocytes) immune cell responses in the intestinal tract. Strikingly, C. jejuni induced intestinal, extra-intestinal and systemic secretion of pro-inflammatory mediators (such as IL-6, MCP-1, TNF and nitric oxide) could be alleviated by peroral L. johnsonii challenge. In conclusion, immunomodulatory probiotic species might offer valuable strategies for prophylaxis and/or treatment of C. jejuni induced intestinal, extra-intestinal as well as systemic pro-inflammatory immune responses in vivo.
Introduction
Human Campylobacter jejuni infections are progressively rising worldwide1, 2. Whereas C. jejuni act as commensal bacteria within the intestinal tract of wild and domestic animals, humans acquire C. jejuni usually by consumption of contaminated products derived from livestock animals or contaminated surface water via the peroral route and present with clinical symptoms of varying degree3–6. Whereas some patients suffer from rather mild malaise, others present with gastroenteritis ranging from watery diarrhea to severe ulcerative colitis with inflammatory, bloody diarrhea7. Whereas in the vast majority of cases disease resolves spontaneously, post-infectious sequelae including peripheral neuropathies such as Guillain-Barré and Miller-Fisher syndromes and reactive arthritis might develop with a latency of weeks to months8–10. Due to the scarcity of appropriate in vivo models, our understanding of the molecular mechanisms underlying Campylobacter-host interactions has been hampered for a long time6, 11. In general, mice are highly convenient for studies of bacterial pathogenicity and pathogen-host interactions. Conventionally colonized mice, however, are protected from C. jejuni infection due to their host specific microbiota composition mediating physiological colonization resistance6, 12. Our group showed previously that C. jejuni infection could be facilitated by modification of the murine intestinal microbiota6, 12, 13. Physiological colonization resistance could be overcome upon virtual eradication of the intestinal microbiota by broad-spectrum antibiotic treatment. These secondary abiotic mice could be stably infected with the pathogen and exhibited key features of human campylobacteriosis including apoptosis and pro-inflammatory immune responses in the large intestines12. Hence, secondary abiotic mice are well-suited to unravel the triangle relationship between intestinal pathogens, commensals and the host immune system in vivo 14. These findings suggest that microorganisms in the gut microbiota of our laboratory mice might contribute to colonization resistance against C. jejuni, thus providing health benefits to the host similar to probiotics15.
It is well-established that modulation of the intestinal microbiota composition by probiotic microbes is a promising strategy to prevent pathogenic colonization and subsequent infection in susceptible hosts15, 16. The reduction of the C. jejuni loads in poultry and other livestock animals is a highly preventive measure to prevent campylobacteriosis in humans. The molecular mechanisms inhibiting C. jejuni colonization are in the actual focus of research. In this context the extraordinary colonization resistance displayed by our conventional mice against C. jejuni raises intensive scientific interest. The murine intestinal microbiota is dominated by Gram-positive lactobacilli belonging to the Lacobacillaceae of the Lactobacillales within the Bacilli class of the Firmicutes 16, 17. The genus is the most diverse amongst the lactic acid bacteria, and a variety of probiotic lactobacilli including L. acidophilus/L. johnsonii, L. casei, L. rhamnosus, L. gasseri, and closely related Bifidobacterium lactis have been shown to inhibit virulence of enteropathogenic bacteria including Campylobacter, Salmonella, Shigella, enterotoxigenic E. coli or Vibrio cholerae to intestinal cells18–22. In particular for C. jeuni it has been conclusively demonstrated that L. gasseri SBT2055 inhibits invasion of epithelial cells by co-aggregation in vitro and counteracts pathogenic colonization in chicken23, 24. Furthermore, production of organic acids (mainly lactic acid) by probiotic lactobacilli exerts killing of C. jejuni in vitro and is efficiently reducing C. jejuni concentrations in vivo as shown in poultry25. Whereas Lactobacillus spp. isolated from probiotic formulations were highly effective in mediating colonization resistance against C. jejuni in mice26, bacteriocin producing L. salivarius reduced C. jejuni loads in the chicken intestinal tract27.
Given that distinct species in the dense Lactobacillus population in mice might be involved in mediating colonization resistance against C. jejuni, we isolated a commensal Lactobacillus johnsonii strain from murine fecal samples (see methods) and confirmed its antimicrobial activity against C. jejuni by using co-cultivation assays in vitro (Bachelor thesis Ulrike Escher, 2014 Beuth Hochschule, Berlin, Germany). The inhibition of C. jejuni growth by L. johnsonii was mediated via production of organic acids, as demonstrated by the loss of the antimicrobial activity against C. jejuni by neutralization of the growth media with alkalizing agents. Notably L. johnsonii (formerly termed L. acidophilus) constitutes a common commensal of the murine intestinal microbiota28. Given its scientifically confirmed probiotic potential, several L. johnsonii strains are used worldwide in probiotic products29. Most importantly, L. johnsonii inhibits the growth of C. jejuni in vitro as already known for decades30.
In the present study we investigated the potential probiotic/beneficial effects of L. johnsonii in murine campylobacteriosis by prophylactic and therapeutic treatment of C. jejuni infected secondary abiotic mice which were generated by antibiotic treatment. Results obtained by analysis of the resulting secondary abiotic animals revealed that prophylactic or therapeutic treatment of mice with L. johnsonii did not lower intestinal C. jejuni colonization, but significantly suppressed intestinal and systemic pro-inflammatory and enhanced anti-inflammatory immune responses.
Results
Intestinal colonization capacities of L. johnsonii and/or C. jejuni strain 81–176 in perorally reassociated secondary abiotic mice
In the present study we investigated the potential of a murine commensal intestinal L. johnsonii strain to reduce intestinal pathogenic burdens and to alleviate pro-inflammatory immune responses upon C. jejuni infection in vivo. In order to overcome physiological colonization resistance exerted by the conventional murine microbiota and to assure subsequent stable intestinal bacterial colonization12, we subjected conventionally raised and housed mice to broad-spectrum antibiotic treatment for eight weeks. Consequently, the murine intestinal microbiota was virtually depleted. These generated secondary abiotic mice were then perorally challenged with 108 colony forming units (CFU) of a L. johnsonii strain, that had initially been isolated from the commensal intestinal microbiota of a healthy mouse, by gavage either 14 days before (i.e. prophylactic regimen) or 7 days after (i.e. therapeutic regimen) peroral C. jejuni strain 81–176 infection (pathogenic loads of 108 CFU). At day 28 and day 21 following initial L. johnsonii and C. jejuni reassociation, respectively, mice from bacterial in vivo competition experiments were compared to mono-associated and naive mice. Following peroral challenge, either bacterial strain was able to stably colonize the murine intestinal tract with high median loads of approximately 108 to 109 CFU per gram feces (Fig. 1). Within 14 days following L. johnsonii coassociation of C. jejuni infected mice, fecal pathogenic burdens could be lowered by less than 0.5 order of magnitude (p < 0.05; Fig. 1d). Overall, both C. jejuni and L. johnsonii stably colonized the intestinal tract of secondary abiotic mice at comparable loads. Neither therapeutic nor prophylactic L. johnsonii application, however, was able to decrease intestinal pathogenic burdens in a biologically relevant fashion (Fig. 1c,d).
Figure 1.
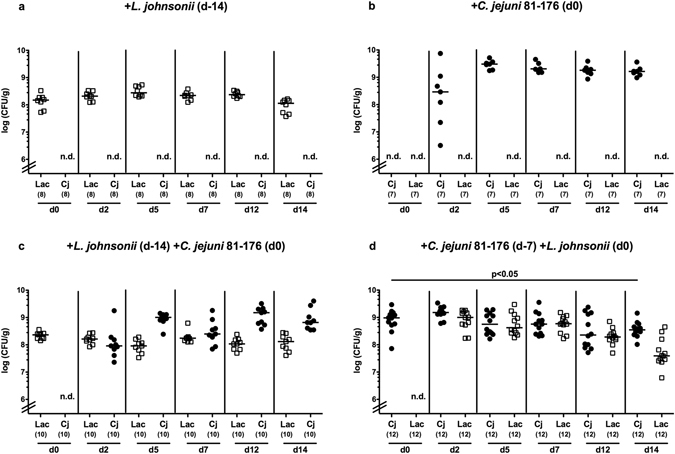
Kinetics of intestinal L. johnsonii and/or C. jejuni strain 81–176 loads in perorally reassociated secondary abiotic mice. Secondary abiotic mice were generated by broad-spectrum antibiotic treatment and perorally reassociated with (a) L. johnsonii (Lac; white squares) on day (d) −14, (b) C. jejuni strain 81–176 (Cj; black circles) on d0, (c) L. johnsonii on day −14 and C. jejuni strain 81–176 on d0, or (d) C. jejuni strain 81–176 (d-7) and L. johnsonii on d0 as described in methods. Bacterial colonization densities were assessed in fecal samples (CFU/g, colony forming units per gram) over time upon reassociation as indicated by culture. Medians (black bars) and levels of significance (p-value) determined by Mann-Whitney U test are indicated. Numbers of analyzed mice are given in parentheses. Data were pooled from three independent experiments. N.d.: not determined.
Large intestinal apoptosis following reassociation of secondary abiotic mice with C. jejuni and/or L. johnsonii
Since reassociation of secondary abiotic mice with C. jejuni and/or L. johnsonii did not affect mice macroscopically (i.e. clinically; not shown), we next investigated potential microscopic sequelae of respective bacterial challenges. Apoptosis is a well-established marker for histopathological grading of intestinal inflammation and a key feature of campylobacteriosis12. We therefore quantitatively assessed caspase3 positive cell numbers in colonic epithelia applying in situ immunohistochemistry. C. jejuni infected mice exhibited increased numbers of apoptotic cells in their large intestines as compared to naive and L. johnsonii mono-associated mice (p < 0.005–0.001; Fig. 2a; Supplemental Fig. S1). Prophylactic administration of L. johnsonii, however, was associated with approximately 50% lower apoptotic colonic epithelial cell numbers as compared to C. jejuni mono-associated mice (p < 0.05; Fig. 2a; Supplemental Fig. S1), whereas a trend towards less apoptosis could also be observed in the L. johnsonii treatment group (n.s.; Fig. 2a; Supplemental Fig. S1). Notably, L. johnsonii application alone did not induce colonic apoptosis (n.s. vs naive controls; Fig. 2a; Supplemental Fig. S1). Reassociation of gnotobiotic mice with C. jejuni and/or L. johnsonii was further accompanied by increases in colonic numbers of Ki67 positive cells indicative for cell proliferation and regeneration counteracting potential C. jejuni induced epithelial cell damage (p < 0.005–0.001; Fig. 2b; Supplemental Fig. S1). Hence, C. jejuni induced colonic apoptosis could be ameliorated by pre-treatment with L. johnsonii.
Figure 2.
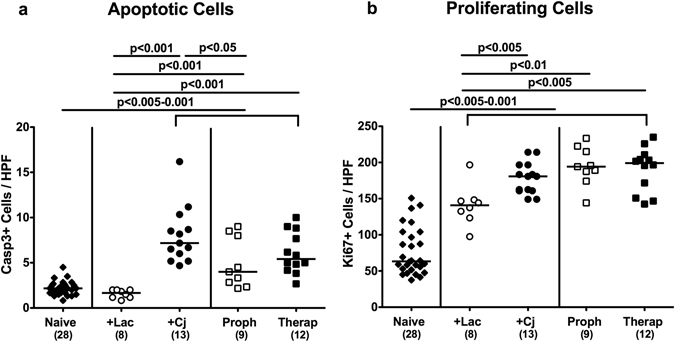
Apoptotic and proliferating cells in the colonic epithelium of C. jejuni strain 81–176 and/or L. johnsonii associated secondary abiotic mice. Secondary abiotic mice were perorally infected with C. jejuni strain 81–176 (Cj) and associated with L. johnsonii (Lac) either 14 days before (prophylactic regimen, Proph; white squares) or 7 days thereafter (therapeutic regimen, Therap; black squares) and compared to mono-associated mice (+Lac, white circles; +Cj, black circles). The average number of colonic (a) apoptotic cells (positive for caspase-3, Casp3) and (b) proliferating cells (positive for Ki67) from six high power fields (HPF, 400x magnification) per animal was determined microscopically in immunohistochemically stained colonic paraffin sections at days 21 or 28 following initial C. jejuni or L. johnsonii infection, respectively. Naive mice (black diamonds) served as uninfected controls. Medians (black bars), levels of significance (p-values) determined by one-way ANOVA test followed by Tukey post-correction test for multiple comparisons and numbers of analyzed animals (in parentheses) are indicated. Data were pooled from three independent experiments.
Large intestinal innate and adaptive immune cell responses upon reassociation of secondary abiotic mice with C. jejuni and/or L. johnsonii
Recruitment of pro-inflammatory immune cells to the site of infection is a characteristic feature of intestinal inflammation including campylobacteriosis12. We therefore quantitatively determined distinct innate as well as adaptive immune cell subsets in large intestinal ex vivo biopsies by in situ immunohistochemistry. Peroral C. jejuni infection, but not L. johnsonii application alone resulted in increased colonic numbers of T and B lymphocytes, regulatory T cells (Treg) as well as macrophages and monocytes (p < 0.001; Fig. 3; Supplemental Fig. S1). Colonic T lymphocyte numbers, however, decreased (p < 0.005), whereas conversely, Treg counts increased (p < 0.05–0.001) in bacterial competition experiments as compared to mice subjected to C. jejuni alone, irrespective whether L. johnsonii was applied prophylactically or therapeutically (Fig. 3a,b; Supplemental Fig. S1). Furthermore therapeutic, but not prophylactic L. johnsonii treatment of C. jejuni infected mice resulted in decreased colonic B lymphocyte numbers (p < 0.001; Fig. 3c; Supplemental Fig. S1). Notably, large intestinal counts of macrophages and monocytes were not affected by L. johnsonii co-association of C. jejuni infected animals (n.s.; Fig. 3d; Supplemental Fig. S1).
Figure 3.
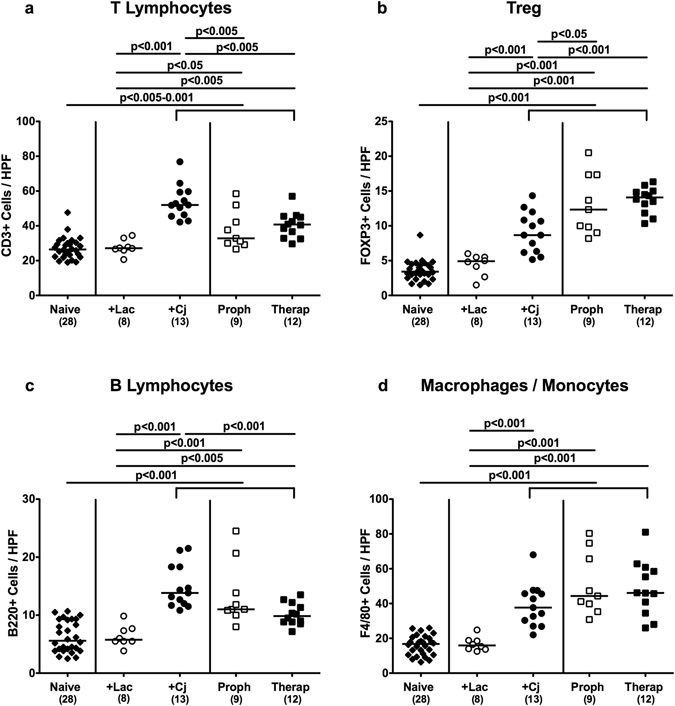
Colonic immune cell responses in C. jejuni strain 81–176 and/or L. johnsonii reassociated secondary abiotic mice. Secondary abiotic mice were perorally infected with C. jejuni strain 81–176 (Cj) and associated with L. johnsonii (Lac) either 14 days before (prophylactic regimen, Proph; white squares) or 7 days thereafter (therapeutic regimen, Therap; black squares) and compared to mono-associated mice (+Lac, white circles; +Cj, black circles). The average number of colonic (a) T lymphocytes (positive for CD3), (b) regulatory T cells (Treg; positive for FOXP3), (c) B lymphocytes (positive for B220), and (d) macrophages and monocytes (positive for F4/80) from six high power fields (HPF, 400x magnification) per animal was determined microscopically in immunohistochemically stained colonic paraffin sections at days 21 or 28 following initial C. jejuni or L. johnsonii infection, respectively. Naive mice (black diamonds) served as uninfected controls. Medians (black bars), levels of significance (p-values) determined by one-way ANOVA test followed by Tukey post-correction test for multiple comparisons and numbers of analyzed animals (in parentheses) are indicated. Data were pooled from three independent experiments.
Intestinal cytokine responses upon reassociation of secondary abiotic mice with C. jejuni and/or L. johnsonii
We further measured pro- and anti-inflammatory cytokine secretion in distinct intestinal compartments generated in bacterial in vivo competition experiments. In colonic ex vivo biopsies, IL-6 and MCP-1 concentrations increased following mono-association of secondary abiotic mice with L. johnsonii or C. jejuni, which was also true for prophylactically with L. johnsonii treated C. jejuni infected animals (p < 0.05–0.001; Fig. 4a,b). Notably, colonic secretion of respective pro-inflammatory cytokines was similar in naive and C. jejuni associated mice that were therapeutically treated with L. johnsonii (n.s.; Fig. 4a,b). Furthermore, colonic levels of the anti-inflammatory cytokine IL-10 increased upon C. jejuni infection, except for therapeutically with L. johnsonii treated animals (p < 0.05; Fig. 4c).
Figure 4.
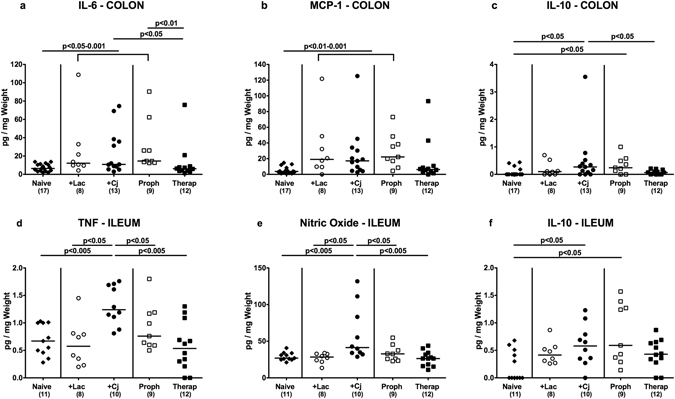
Intesinal secretion of pro- and anti-inflammatory mediators in C. jejuni strain 81–176 and/or L. johnsonii reassociated secondary abiotic mice. Secondary abiotic mice were perorally infected with C. jejuni strain 81–176 (Cj) and associated with L. johnsonii (Lac) either 14 days before (prophylactic regimen, Proph; white squares) or 7 days thereafter (therapeutic regimen, Therap; black squares) and compared to mono-associated mice (+Lac, white circles; +Cj, black circles). Colonic (a) IL-6, (b) MCP-1 and (c) IL-10 concentrations as well as ileal (d) TNF, (e) nitric oxide and (f) IL-10 secretion were determined in ex vivo biopsies derived at days 21 or 28 following initial C. jejuni or L. johnsonii infection, respectively. Naive (N) mice (black diamonds) served as uninfected controls. Medians (black bars), levels of significance (p-value) determined by one-way ANOVA test followed by Tukey post-correction test for multiple comparisons and numbers of analyzed animals (in parentheses) are indicated. Data were pooled from three independent experiments.
Even though C. jejuni has been known to primarily affect the large intestines11, we expanded our cytokine measurements to the small intestinal tract. Remarkably, C. jejuni induced increases in ileal TNF and nitric oxide (NO) secretion could be dampened to naive levels by both prophylactic and therapeutic L. johnsonii administration (p < 0.05 and p < 0.005, respectively; Fig. 4d,e). Like in the colon, IL-10 concentrations increased in the distal small intestinal tract upon C. jejuni mono-association as well as in C. jejuni infected mice that were prophylactically treated with L. johnsonii (p < 0.01 and p < 0.05, respectively; Fig. 4f).
Furthermore, NO levels were increased in mesenteric lymph nodes (MLN) of C. jejuni infected mice (p < 0.005), but could be reduced to basal (naive) levels by L. johnsonii co-administration (p < 0.05–0.001; Fig. 5a). Upon bacterial reassociation of either regimen, elevated IL-10 concentrations could be measured in MLN of secondary abiotic mice (p < 0.05–0.005; Fig. 5b).
Figure 5.
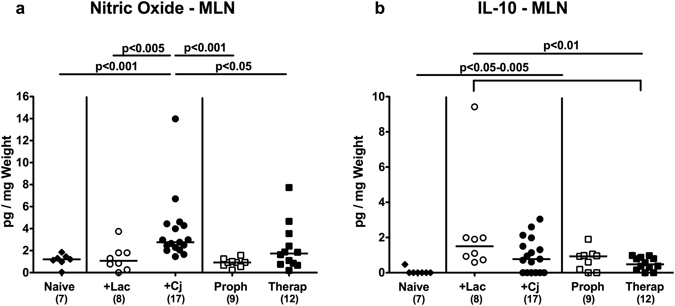
Secretion of pro- and anti-inflammatory mediators in mesenteric lymph nodes of C. jejuni strain 81–176 and/or L. johnsonii reassociated secondary abiotic mice. Secondary abiotic mice were perorally infected with C. jejuni strain 81–176 (Cj) and associated with L. johnsonii (Lac) either 14 days before (prophylactic regimen, Proph; white squares) or 7 days thereafter (therapeutic regimen, Therap; black squares) and compared to mono-associated mice (+Lac, white circles; +Cj, black circles). (a) Nitric oxide and (b) IL-10 concentrations were determined in ex vivo biopsies derived from mesenteric lymph nodes (MLN) at days 21 or 28 following initial C. jejuni or L. johnsonii infection, respectively. Naive (N) mice (black diamonds) served as uninfected controls. Medians (black bars), level of significance (p-value) determined by one-way ANOVA test followed by Tukey post-correction test for multiple comparisons and numbers of analyzed animals (in parentheses) are indicated. Data were pooled from three independent experiments.
Hence, C. jejuni induced increases in intestinal pro-inflammatory cytokine secretion could be alleviated by peroral L. johnsonii challenge. Notably, respective beneficial immunomodulatory effects were not restricted to the large intestinal tract.
Extra-intestinal cytokine responses upon reassociation of secondary abiotic mice with C. jejuni and/or L. johnsonii
We next investigated whether potential immunomodulatory properties of L. johnsonii were restricted to the intestinal tract or could additionally even be observed in an extra-intestinal compartment such as the liver. TNF concentrations increased multifold in livers upon C. jejuni infection (p < 0.01–0.001; Fig. 6a). These increases, however, could be reduced by prophylactic as well as therapeutic co-administration of L. johnsonii (p < 0.005; Fig. 6a), that was most pronounced in the latter group. Moreover, increased hepatic IL-6 secretion could be determined upon C. jejuni infection (p < 0.05; Fig. 6b), except for with L. johnsonii prophylactically treated mice. Furthermore, IL-10 concentrations were elevated in livers following C. jejuni mono-, but not co-association with L. johnsonii (Fig. 6c). Hence, alleviation of C. jejuni induced pro-inflammatory immune responses by L. johnsonii was not restricted to the intestinal tract.
Figure 6.
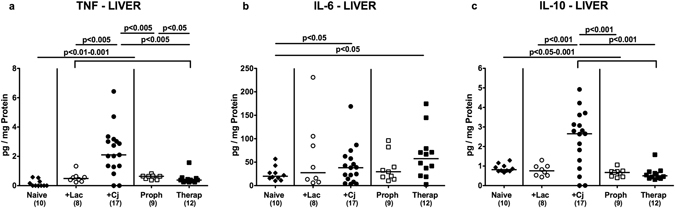
Secretion of pro- and anti-inflammatory mediators in livers of C. jejuni strain 81–176 and/or L. johnsonii reassociated secondary abiotic mice. Secondary abiotic mice were perorally infected with C. jejuni strain 81–176 (Cj) and associated with L. johnsonii (Lac) either 14 days before (prophylactic regimen, Proph; white squares) or 7 days thereafter (therapeutic regimen, Therap; black squares) and compared to mono-associated mice (+Lac, white circles; +Cj, black circles). (a) TNF, (b) IL-6 and (c) IL-10 concentrations were determined in ex vivo biopsies derived from livers at days 21 or 28 following initial C. jejuni or L. johnsonii infection, respectively. Naive (N) mice (black diamonds) served as uninfected controls. Medians (black bars), levels of significance (p-value) determined by one-way ANOVA test followed by Tukey post-correction test for multiple comparisons and numbers of analyzed animals (in parentheses) are indicated. Data were pooled from three independent experiments.
Systemic cytokine responses upon reassociation of secondary abiotic mice with C. jejuni and/or L. johnsonii
Given the beneficial effects of L. johnsonii observed in extra-intestinal tissue sites of C. jejuni infected mice we next addressed whether respective immunomodulatory “probiotic” properties could also be observed systemically. We therefore measured cytokine secretion in spleen and serum samples derived from bacterial in vivo competition experiments. Following bacterial reassociation, splenic TNF, IFN-γ, IL-6 as well as IL-10 concentrations increased multifold (p < 0.01–0.001; Fig. 7a,b,d,e). Notably, C. jejuni induced TNF levels could be lowered by prophylactic and therapeutic coassociation with L. johnsonii (p < 0.05 and 0.01, respectively; Fig. 7a). In addition, neither L. johnsonii mono-association nor prophylactic co-association of C. jejuni infected mice was associated with increased splenic MCP-1 levels (n.s. vs naive mice; Fig. 7c). Furthermore, increased TNF serum concentrations could be measured upon C. jejuni infection alone and following prophylactic, but not therapeutic L. johnsonii co-association of C. jejuni infected mice (Fig. 8a). Whereas bacterial reassociation was accompanied by increased MCP-1 concentrations in serum (p < 0.005–0.001; Fig. 8b), C. jejuni induced increases in serum MCP-1 levels could be lowered by therapeutic L. johnsonii treatment (p < 0.05; Fig. 8b). In line with this, prophylactic as well as therapeutic L. johnsonii co-association reduced C. jejuni induced increases in serum IL-6 levels to basal levels (p < 0.01 and p < 0.001, respectively; Fig. 8c). Notably, neither viable L. johnsonii nor C. jejuni could be isolated from extra-intestinal and systemic compartments including liver, spleen and blood at necropsy (not shown). Hence, L. johnsonii administration ameliorated not only intestinal and extra-intestinal, but also systemic C. jejuni induced pro-inflammatory cytokine responses.
Figure 7.
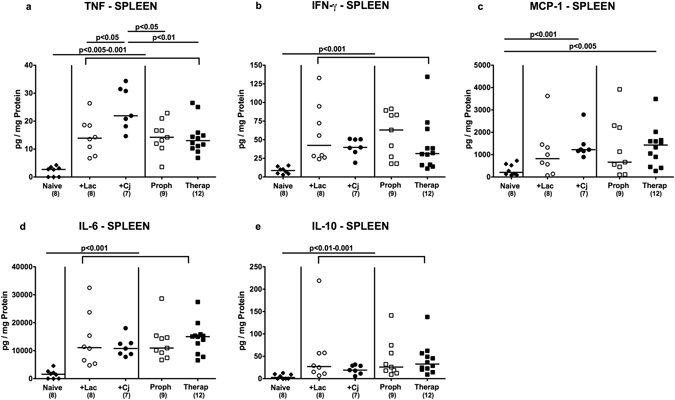
Splenic secretion of pro- and anti-inflammatory mediators of C. jejuni strain 81–176 and/or L. johnsonii reassociated secondary abiotic mice. Secondary abiotic mice were perorally infected with C. jejuni strain 81–176 (Cj) and associated with L. johnsonii (Lac) either 14 days before (prophylactic regimen, Proph; white squares) or 7 days thereafter (therapeutic regimen, Therap; black squares) and compared to mono-associated mice (+Lac, white circles; +Cj, black circles). (a) TNF, (b) INF-γ, (c) MCP-1, (d) IL-6, and (e) IL-10 concentrations were determined in ex vivo biopsies derived from spleens at days 21 or 28 following initial C. jejuni or L. johnsonii infection, respectively. Naive (N) mice (black diamonds) served as uninfected controls. Medians (black bars), levels of significance (p-value) determined by one-way ANOVA test followed by Tukey post-correction test for multiple comparisons and numbers of analyzed animals (in parentheses) are indicated. Data were pooled from three independent experiments.
Figure 8.
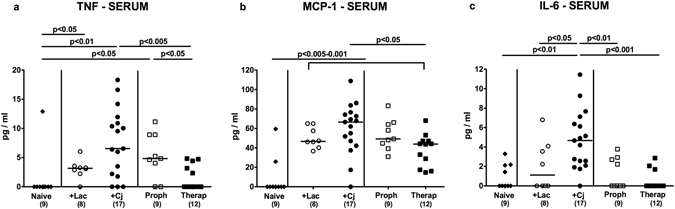
Pro-inflammatory cytokine secretion in serum of C. jejuni strain 81–176 and/or L. johnsonii reassociated secondary abiotic mice. Secondary abiotic mice were perorally infected with C. jejuni strain 81–176 (Cj) and associated with L. johnsonii (Lac) either 14 days before (prophylactic regimen, Proph; white squares) or 7 days thereafter (therapeutic regimen, Therap; black squares) and compared to mono-associated mice (+Lac, white circles; +Cj, black circles). (a) TNF, (b) MCP-1 and (c) IL-6 concentrations were determined in serum samples at days 21 or 28 following initial C. jejuni or L. johnsonii infection, respectively. Naive (N) mice (black diamonds) served as uninfected controls. Medians (black bars), levels of significance (p-value) determined by one-way ANOVA test followed by Tukey post-correction test for multiple comparisons and numbers of analyzed animals (in parentheses) are indicated. Data were pooled from three independent experiments.
Discussion
Probiotics are effective in the treatment of gastrointestinal diseases as supported by a multitude of scientific investigations including clinical studies31. Proposed mechanisms of beneficial probiotic action include modification of the intestinal microbiota32, enhancement of colonization resistance26, support of intestinal barrier functions33 as well as modulation of innate and adaptive immunity34. In case of campylobacteriosis, probiotics (in particular lactobacilli) have been shown to co-aggregate with C. jejuni thus reducing the adhesion to and invasion of the intestinal epithelial cell layers and to suppress motility which is essential for pathogenicity24, 35. The modes of actions of the corresponding probiotic formulations have been examined for decades. For instance, administration of heat killed bacteria of the closely related L. acidophilus reduced translocation of C. jejuni in infected isolator-raised germfree mice36.
Given that L. johnsonii and its relative L. acidophilus have been shown to produce several antimicrobial molecules including bacteriocins and peroxide37, we addressed in in vivo bacterial competition experiments shown here, whether pathogenic loads could be lowered upon L. johnsonii application before and/or after C. jejuni infection of secondary abiotic mice. Both probiotic and pathogenic strains could stably colonize the gastrointestinal tract at high loads upon peroral challenge. Unfortunately, neither therapeutic nor prophylactic L. johnsonii application could decrease intestinal C. jejuni burdens in a biologically relevant fashion. In a previous in vivo study applying isolator-raised germfree mice that had been reconstituted with a complex human microbiota, a complete eradication of C. jejuni from the small and large intestines could be observed upon pretreatment of human microbiota-associated mice with a probiotic mix of L. acidophilus, L. reuteri and Bifidobacterium infantis 26. One needs to take into consideration, however, that the observed contrasting pathogen-eradicative outcomes could most likely not only be explained by the quantitative and qualitative differences in the used probiotic strains, but also by substantially different immunological features of the applied mouse models. Due to the lacking contact to any bacterial ligands and subsequent absence of immunological differentiation and stimulation, isolator-raised germfree mice exhibit only poorly-developed intestinal lymphatic tissues38, 39, and need thus to be regarded as highly immunocompromized and inappropriate for dissecting commensal intestinal-pathogen-host interactions. The secondary abiotic mice used in our study, however, were born, reared and maintained under conventional conditions and exhibited a fully developed innate and adaptive immune system. Notably, recolonization of secondary abiotic mice with a single probiotic strains might not be sufficient to reconstitute the complex physiological prerequisites (i.e. colonization resistance) for effective competition with C. jejuni for nutrients and niches. Thus, rather a combination of several beneficial microbes instead of one single probiotic strain would be a more promising strategy to protect from or combat established enteropathogenic infections.
Whereas in our study re-colonized mice were not clinically/macroscopically compromized, C. jejuni infection induced colonic apoptosis and inflammation. Notably, L. johnsonii colonization alone did not induce apoptotic, but promoted proliferative/regenerative colonic epithelial responses that might counteract potential inflammation-attributed cell damage. Most importantly, the C. jejuni induced disease state could be ameliorated by treatment with L. johnsonii, which impacted apoptosis and adaptive immune responses upon C. jejuni infection. Again, L. johnsonii alone did not lead to an influx of innate or adaptive immune cells into the colonic mucosa and lamina propria. Furthermore, increases in intestinal pro-inflammatory mediators (including IL-6, NO and TNF) induced by C. jejuni infection could be alleviated by peroral L. johnsonii co-administration. Notably, respective beneficial immunomodulatory effects were not restricted to the large intestinal tract, but could also be observed in the ileum. As compared to uninfected control mice, L. johnsonii application alone further resulted in enhanced IL-10 secretion in intestinal as well as systemic compartments as shown from ex vivo biopsies taken from MLN and spleen, respectively. Our in vivo data are further supported by results from in vitro studies. Tsuda and colleagues reported, for instance, that stimulation of intestinal lymphocytes (derived from MLN of isolator-raised germfree mice) with a mix of three defined and inactivated L. johnsonii strains induced IL-10 production and regulated excessive antigen-specific cytokine responses via antigen presenting cells40. In line with this, stimulation of peritoneal macrophages with L. johnsonii also resulted in enhanced IL-10 secretion41. In weanling as well as malnourished aged mice, L. johnsonii challenge further enhanced intestinal IgA secretion, thus constituting recovery and/or modulation of accelerated immune responses within the intestinal tract42, 43.
In our study L. johnsonii administration did not only ameliorate intestinal, but also extra-intestinal such as hepatic, and most strikingly, systemic C. jejuni induced pro-inflammatory cytokine responses. In support, a study applying a murine leukemia model revealed that peroral restoration of commensal Lactobacillus spp. (that were reduced upon disease-mediated dysbiosis) resulted in decreased levels of pro-inflammatory cytokines including IL-6 and MCP-1 in muscular as well as serum samples44. Given that the efficacy of L. johnsonii as therapeutic and/or preventive measure directed against enteric diseases including infections is supported by solid data from experimental in vitro and in vivo investigations as well as clinical studies37, it has been included in probiotic products distributed worldwide (Nestlé LC1).
In conclusion, even though neither susceptibility to C. jejuni infection nor pathogenic burdens upon intestinal establishment were influenced by L. johnsonii co-administration, the anti-inflammatory properties of this single probiotic species that became overt in intestinal, extra-intestinal and, remarkably, systemic compartments of C. jejuni infected mice qualifies L. johnsonii at least as adjunct immunomodulatory application. It is rather a well-orchestrated interplay of mucosal immunity and the intestinal intraluminal milieu determined by the concert of the complex microbiota plus beneficial probiotic strains that is required to successfully combat and/or prevent from enteropathogenic infections. Future studies are needed, however, to better understand the underlying mechanisms.
Material and Methods
Ethics statement
All animal experiments were conducted according to the European Guidelines for animal welfare (2010/63/EU) with approval of the commission for animal experiments headed by the “Landesamt für Gesundheit und Soziales” (LaGeSo, Berlin, registration number G0184/12). Animal welfare was monitored twice daily by assessment of clinical conditions including weight loss.
Generation of secondary abiotic mice
Female C57BL/6j mice were reared and housed within the same specific pathogen free (SPF) unit in the Forschungseinrichtungen für Experimentelle Medizin (FEM, Charité - University Medicine Berlin). Secondary abiotic mice were generated by quintuple antibiotic treatment for eight weeks starting 10 weeks of age as described before45. Mice were kept in cages including filter tops within an experimental semi-barrier (accessible only with lab coat, overshoes, caps and sterile gloves) under standard conditions (22–24 °C room temperature, 55 ± 15% humidity, 12 h light/12 dark cycle). Mice were handled under aseptic conditions and had unlimited access to autoclaved tap water and chow (food pellets; Sniff, Soest, Germany). Murine samples were taken at comparable time points in the morning.
Bacterial in vivo competition experiments
Three days before bacterial reassociation experiments the antibiotic cocktail was replaced by sterilized tap water. Mice were then perorally challenged by gavage with either C. jejuni strain 81–176 or with a commensal L. johnsonii strain that had initially been isolated from the intestinal tract of a healthy female 3 months-old C57BL/6j wildtype mouse (respective bacterial loads of 108 CFU) as described previously12, 45. Briefly, single colonies of lactic acid bacteria were isolated from murine feces samples by cultivation of serial dilutions on MRS selective agar (Oxoid, Wesel, Germany). The capacity of the isolates to inhibit growth of C. jejuni was examined by co-cultivation assays with C. jejuni strain 81–176 on Columbia agar supplemented with 5% sheep blood (Oxoid) and in thioglycolate liquid media (Bachelor thesis Ulrike Escher, Beuth Hochschule, Berlin, Germany), according to standard microbial stabbing techniques for isolation of lactobacilli which are inhibitory for pathogens46. The isolate that mediated the most extensive growth inhibition of C. jejuni strain 81–176 in both assays was further investigated. The corresponding pleomorphic Gram-positive rods did neither produce catalase nor oxidase and were identified as L. johnsonii by molecular analysis of the complete 16S rRNA gene sequence.
For bacterial in vivo competition experiments, C. jejuni infected mice were either challenged with L. johnsonii 7 days thereafter (therapeutic regimen) or 14 days before (prophylactic regimen). Naive sex and age-matched animals served as uninfected (negative) controls. Mice were continuously kept in a sterile environment (autoclaved food and drinking water or sterile antibiotic cocktail ad libitum) and were handled under strict aseptic conditions in order to prevent from contaminations.
Clinical Score
To survey clinical signs of inflammation, a standardized cumulative clinical score (maximum 12 points), addressing the occurrence of blood in feces (0: no blood; 2: microscopic detection of blood by the Guajac method using Haemoccult, Beckman Coulter/PCD, Krefeld, Germany; 4: overt blood visible), diarrhea (0: formed feces; 2: pasty feces; 4: liquid feces), and the clinical aspect (0: normal; 2: ruffled fur, less locomotion; 4: isolation, severely compromized locomotion, pre-final aspect) was applied daily as described earlier47.
Sampling procedures
Mice were sacrificed 21 or 28 days following initial C. jejuni or L. johnsonii reassociation, respectively, by isofluran treatment (Abbott, Greifswald, Germany). Cardiac blood and tissue samples from the colon, ileum, MLN, liver and spleen were removed under sterile conditions. Intestinal samples were collected in parallel for microbiological and immunological analyses. Immunohistological changes were assessed in colonic ex vivo biopsies that had been immediately fixed in 5% formalin and embedded in paraffin. Sections (5 μm) were stained with distinct antibodies for in situ immunohistochemistry as described earlier14, 47.
Quantitative analysis of pathogenic and probiotic bacterial colonization and translocation
C. jejuni strain 81–176 and L. johnsonii were quantitated in feces over time post reassociation or upon necropsy in luminal samples taken from the colon, in homogenates of whole tissue ex vivo biopsies derived from spleen and liver (approximately 1 cm3) and in cardiac blood (approximately 1 mL). Whereas cardiac blood was analyzed by direct plating, feces, luminal colon contents and organ homogenates were dissolved in sterile phosphate buffered saline (PBS; Gibco life technologies, Paisley, UK) and serial dilutions cultured on Karmali and Columbia agar supplemented with 5% sheep blood (Oxoid) for two days at 37 °C under microaerobic conditions using CampyGen gas packs (Oxoid) for C. jejuni detection as described earlier12. L. johnsonii loads were determined on Columbia agar supplemented with 5% sheep blood, Columbia-CNA agar supplemented with colistin and nalidixic acid (both Oxoid), and MRS agar (Oxoid) in parallel and incubated under microaerobic (in jars using CampGen gas packs; Oxoid) and obligate anaerobic (in jars using Anaerogen gas packs; Oxoid) conditions for at least two days. Bacterial species were identified according to their typical morphological appearances and sequencing of the 16S rRNA genes. The detection limit of viable bacteria was ≈100 CFU per g.
Immunohistochemistry
In situ immunohistochemical analysis of colonic paraffin sections was performed as described earlier14, 47. Primary antibodies against cleaved caspase-3 (Asp175, Cell Signaling, Boston, MA, USA, 1:200), Ki67 (TEC3, Dako, Glostrup, Denmark, 1:100), CD3 (#N1580, Dako, Denmark, dilution 1:10), FOXP3 (FJK-16s, eBioscience, San Diego, CA, USA, 1:100), B220 (eBioscience, 1:200) and F4/80 (# 14–4801, clone BM8, eBioscience, 1:50) were used. For each animal the average number of positively stained cells within at least six high power fields (HPF, 400x magnification) were determined microscopically by an independent blinded investigator.
Cytokine measurements in intestinal and extra-intestinal compartments
Colonic and ileal ex vivo biopsies were cut longitudinally and washed in PBS. MLN, spleen or strips of approximately 1 cm2 of colon, ileum and liver tissues were placed in 24-flat-bottom well culture plates (Nunc, Wiesbaden, Germany) containing 500 μL serum-free RPMI 1640 medium (Gibco) supplemented with penicillin (100 U/mL) and streptomycin (100 µg/mL; PAA Laboratories, Cölbe, Germany). After 18 h at 37 °C, culture supernatants were tested for TNF, IFN-γ, MCP-1, IL-6, IL-12p70 and IL-10 by the Mouse Inflammation Cytometric Bead Assay (CBA; BD Biosciences, Heidelberg, Germany) on a BD FACSCanto II flow cytometer (BD Biosciences). NO was determined by the Griess reaction as described earlier45.
Statistical analysis
Medians, means and significance levels using appropriate tests as indicated (Mann Whitney U test and one-way ANOVA with Tukey’s post hoc test for multiple comparisons) were determined using GraphPad Prism Software v6 (La Jolla, CA, USA). Two-sided probability (p) values ≤ 0.05 were considered significant. Experiments were repeated twice.
Electronic supplementary material
Acknowledgements
We thank Michaela Wattrodt, Ursula Rüschendorf, Silvia Schulze, Alexandra Bittroff-Leben, Ines Puschendorf, Gernot Reifenberger, and the staff of the animal research facility at Charité - University Medicine Berlin for excellent technical assistance and animal breeding. This work was supported by grants from the German Research Foundation (DFG) to SB (SFB633, TP A7), UF (SFB633, TP B6), IE (SFB633, Immuco) and MMH (SFB633, TP B6), from the German Federal Ministery of Education and Research (BMBF) to SB (“Lab in a hanky”/TP1.1), and from the Federal Institute for Risk Assessment (BfR) to SB, MMH and KS (1329–526). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author Contributions
S.B.: Provided advice in design, performance of experiments and co-wrote the paper. I.E.: Performed experiments, edited the paper. U.F.: Performed experiments, edited the paper. U.E.: Suggested critical parameters in design of experiments, supplied bacterial strains, edited the paper. K.S.: Suggested critical parameters in design of experiments, supplied bacterial strains, edited the paper. M.M.H.: Designed and performed the experiments and wrote the paper. All authors reviewed the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-02436-2
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Young KT, Davis LM, Dirita VJ. Campylobacter jejuni: molecular biology and pathogenesis. Nat Rev Microbiol. 2007;5:665–679. doi: 10.1038/nrmicro1718. [DOI] [PubMed] [Google Scholar]
- 2.Dasti JI, Tareen AM, Lugert R, Zautner AE, Gross U. Campylobacter jejuni: a brief overview on pathogenicity-associated factors and disease-mediating mechanisms. Int J Med Microbiol. 2010;300:205–211. doi: 10.1016/j.ijmm.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 3.Guerry P, Szymanski CM. Campylobacter sugars sticking out. Trends Microbiol. 2008;16:428–435. doi: 10.1016/j.tim.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 4.van Asselt ED, de Jong AE, de Jonge R, Nauta MJ. Cross-contamination in the kitchen: estimation of transfer rates for cutting boards, hands and knives. J Appl Microbiol. 2008;105:1392–1401. doi: 10.1111/j.1365-2672.2008.03875.x. [DOI] [PubMed] [Google Scholar]
- 5.Lane JA, Mehra RK, Carrington SD, Hickey RM. The food glycome: a source of protection against pathogen colonization in the gastrointestinal tract. Int J Food Microbiol. 2010;142:1–13. doi: 10.1016/j.ijfoodmicro.2010.05.027. [DOI] [PubMed] [Google Scholar]
- 6.Masanta WO, et al. Modification of intestinal microbiota and its consequences for innate immune response in the pathogenesis of campylobacteriosis. Clin Dev Immunol. 2013;2013:526860–10. doi: 10.1155/2013/526860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kist M, Bereswill S. Campylobacter jejuni. Contrib Microbiol. 2001;8:150–165. doi: 10.1159/000060405. [DOI] [PubMed] [Google Scholar]
- 8.Talukder RK, Sutradhar SR, Rahman KM, Uddin MJ, Akhter H. Guillian-Barre syndrome. Mymensingh Med J. 2011;20:748–756. [PubMed] [Google Scholar]
- 9.Wakerley BR, Uncini A, Yuki N. Guillain-Barré and Miller Fisher syndromes-new diagnostic classification. Nat Rev Neurol. 2014;10:537–544. doi: 10.1038/nrneurol.2014.138. [DOI] [PubMed] [Google Scholar]
- 10.Backert, S., Tegtmeyer, N., Cróinín, T. Ó., Boehm, M. & Heimesaat, M. M. In Campylobacter 1–25 (Academic Press, 2017).
- 11.Heimesaat MM, Bereswill S. Murine infection models for the investigation of Campylobacter jejuni-host interactions and pathogenicity. Berl Munch Tierarztl Wochenschr. 2015;128:98–103. [PubMed] [Google Scholar]
- 12.Bereswill S, et al. Novel murine infection models provide deep insights into the “menage a trois” of Campylobacter jejuni, microbiota and host innate immunity. PLoS One. 2011;6:e20953. doi: 10.1371/journal.pone.0020953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haag LM, et al. Intestinal microbiota shifts towards elevated commensal Escherichia coli loads abrogate colonization resistance against Campylobacter jejuni in mice. PLoS One. 2012;7:e35988. doi: 10.1371/journal.pone.0035988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alutis ME, et al. Matrix Metalloproteinase-2 Mediates Intestinal Immunopathogenesis in Campylobacter Jejuni-Infected Infant Mice. Eur J Microbiol Immunol (Bp) 2015;5:188–198. doi: 10.1556/1886.2015.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joint, F. WHO working group report on drafting guidelines for the evaluation of probiotics in food. London, Ontario, Canada 30 (2002).
- 16.Madigan, M. T., Martinko, J. M. & Brock, T. D. Brock biology of microorganisms. (Pearson Prentice Hall, 2006).
- 17.Claesson MJ, van Sinderen D, O’Toole PW. Lactobacillus phylogenomics–towards a reclassification of the genus. Int J Syst Evol Microbiol. 2008;58:2945–2954. doi: 10.1099/ijs.0.65848-0. [DOI] [PubMed] [Google Scholar]
- 18.Bernet-Camard MF, et al. The human Lactobacillus acidophilus strain LA1 secretes a nonbacteriocin antibacterial substance(s) active in vitro and in vivo. Appl Environ Microbiol. 1997;63:2747–2753. doi: 10.1128/aem.63.7.2747-2753.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hudault S, Lievin V, Bernet-Camard MF, Servin AL. Antagonistic activity exerted in vitro and in vivo by Lactobacillus casei (strain GG) against Salmonella typhimurium C5 infection. Appl Environ Microbiol. 1997;63:513–518. doi: 10.1128/aem.63.2.513-518.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gopal PK, Prasad J, Smart J, Gill HS. In vitro adherence properties of Lactobacillus rhamnosus DR20 and Bifidobacterium lactis DR10 strains and their antagonistic activity against an enterotoxigenic Escherichia coli. Int J Food Microbiol. 2001;67:207–216. doi: 10.1016/S0168-1605(01)00440-8. [DOI] [PubMed] [Google Scholar]
- 21.Fernandez MF, Boris S, Barbes C. Probiotic properties of human lactobacilli strains to be used in the gastrointestinal tract. J Appl Microbiol. 2003;94:449–455. doi: 10.1046/j.1365-2672.2003.01850.x. [DOI] [PubMed] [Google Scholar]
- 22.Lee YK, Puong KY, Ouwehand AC, Salminen S. Displacement of bacterial pathogens from mucus and Caco-2 cell surface by lactobacilli. J Med Microbiol. 2003;52:925–930. doi: 10.1099/jmm.0.05009-0. [DOI] [PubMed] [Google Scholar]
- 23.Nishiyama K, et al. Lactobacillus gasseri SBT2055 reduces infection by and colonization of Campylobacter jejuni. PLoS One. 2014;9:e108827. doi: 10.1371/journal.pone.0108827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nishiyama K, et al. Cell surface-associated aggregation-promoting factor from Lactobacillus gasseri SBT2055 facilitates host colonization and competitive exclusion of Campylobacter jejuni. Mol Microbiol. 2015;98:712–726. doi: 10.1111/mmi.13153. [DOI] [PubMed] [Google Scholar]
- 25.Neal-McKinney JM, et al. Production of organic acids by probiotic lactobacilli can be used to reduce pathogen load in poultry. PLoS One. 2012;7:e43928. doi: 10.1371/journal.pone.0043928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wagner RD, Johnson SJ, Kurniasih Rubin D. Probiotic bacteria are antagonistic to Salmonella enterica and Campylobacter jejuni and influence host lymphocyte responses in human microbiota-associated immunodeficient and immunocompetent mice. Mol Nutr Food Res. 2009;53:377–388. doi: 10.1002/mnfr.200800101. [DOI] [PubMed] [Google Scholar]
- 27.Stern NJ, et al. Isolation of a Lactobacillus salivarius strain and purification of its bacteriocin, which is inhibitory to Campylobacter jejuni in the chicken gastrointestinal system. Antimicrob Agents Chemother. 2006;50:3111–3116. doi: 10.1128/AAC.00259-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu, X. et al. Genome Sequence of Lactobacillus johnsonii Strain W1, Isolated from Mice. Genome Announc4, doi:10.1128/genomeA.00561-16 (2016). [DOI] [PMC free article] [PubMed]
- 29.Neeser JR, et al. Lactobacillus johnsonii La1 shares carbohydrate-binding specificities with several enteropathogenic bacteria. Glycobiology. 2000;10:1193–1199. doi: 10.1093/glycob/10.11.1193. [DOI] [PubMed] [Google Scholar]
- 30.Bhatia SJ, Kochar N, Abraham P, Nair NG, Mehta AP. Lactobacillus acidophilus inhibits growth of Campylobacter pylori in vitro. J Clin Microbiol. 1989;27:2328–2330. doi: 10.1128/jcm.27.10.2328-2330.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ritchie ML, Romanuk TN. A meta-analysis of probiotic efficacy for gastrointestinal diseases. PLoS One. 2012;7:e34938. doi: 10.1371/journal.pone.0034938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Isolauri E, Sutas Y, Kankaanpaa P, Arvilommi H, Salminen S. Probiotics: effects on immunity. Am J Clin Nutr. 2001;73:444s–450s. doi: 10.1093/ajcn/73.2.444s. [DOI] [PubMed] [Google Scholar]
- 33.Ukena SN, et al. Probiotic Escherichia coli Nissle 1917 inhibits leaky gut by enhancing mucosal integrity. PLoS One. 2007;2:e1308. doi: 10.1371/journal.pone.0001308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grabig A, et al. Escherichia coli Strain Nissle 1917 Ameliorates Experimental Colitis via Toll-Like Receptor 2- and Toll-Like Receptor 4-Dependent Pathways. Infect Immun. 2006;74:4075–4082. doi: 10.1128/IAI.01449-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ding W, Wang H, Griffiths MW. Probiotics down-regulate flaA sigma28 promoter in Campylobacter jejuni. J Food Prot. 2005;68:2295–2300. doi: 10.4315/0362-028X-68.11.2295. [DOI] [PubMed] [Google Scholar]
- 36.Moyen EN, Bonneville F, Fauchere JL. [Modification of intestinal colonization and translocation of Campylobacter jejuni by erythromycin and an extract of Lactobacillus acidophilus in axenic mice] Ann Inst Pasteur Microbiol. 1986;137a:199–207. doi: 10.1016/S0769-2609(86)80024-2. [DOI] [PubMed] [Google Scholar]
- 37.Lievin-Le Moal V, Servin AL. Anti-infective activities of Lactobacillus strains in the human intestinal microbiota: from probiotics to gastrointestinal anti-infectious biotherapeutic agents. Clin Microbiol Rev. 2014;27:167–199. doi: 10.1128/CMR.00080-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Savidge TC, Smith MW, James PS, Aldred P. Salmonella-induced M-cell formation in germ-free mouse Peyer’s patch tissue. Am J Pathol. 1991;139:177–184. [PMC free article] [PubMed] [Google Scholar]
- 39.Shroff KE, Cebra JJ. Development of mucosal humoral immune responses in germ-free (GF) mice. Adv Exp Med Biol. 1995;371a:441–446. doi: 10.1007/978-1-4615-1941-6_92. [DOI] [PubMed] [Google Scholar]
- 40.Tsuda M, et al. Prior stimulation of antigen-presenting cells with Lactobacillus regulates excessive antigen-specific cytokine responses in vitro when compared with Bacteroides. Cytotechnology. 2007;55:89–101. doi: 10.1007/s10616-007-9104-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marcinkiewicz J, et al. Differential inflammatory mediator response in vitro from murine macrophages to lactobacilli and pathogenic intestinal bacteria. Int J Exp Pathol. 2007;88:155–164. doi: 10.1111/j.1365-2613.2007.00530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Inoue R, Nishio A, Fukushima Y, Ushida K. Oral treatment with probiotic Lactobacillus johnsonii NCC533 (La1) for a specific part of the weaning period prevents the development of atopic dermatitis induced after maturation in model mice, NC/Nga. Br J Dermatol. 2007;156:499–509. doi: 10.1111/j.1365-2133.2006.07695.x. [DOI] [PubMed] [Google Scholar]
- 43.Kaburagi T, et al. Effect of Lactobacillus johnsonii La1 on immune function and serum albumin in aged and malnourished aged mice. Nutrition. 2007;23:342–350. doi: 10.1016/j.nut.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 44.Bindels LB, et al. Restoring specific lactobacilli levels decreases inflammation and muscle atrophy markers in an acute leukemia mouse model. PLoS One. 2012;7:e37971. doi: 10.1371/journal.pone.0037971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Heimesaat MM, et al. Gram-negative bacteria aggravate murine small intestinal Th1-type immunopathology following oral infection with Toxoplasma gondii. J Immunol. 2006;177:8785–8795. doi: 10.4049/jimmunol.177.12.8785. [DOI] [PubMed] [Google Scholar]
- 46.Jones RJ, Hussein HM, Zagorec M, Brightwell G, Tagg JR. Isolation of lactic acid bacteria with inhibitory activity against pathogens and spoilage organisms associated with fresh meat. Food Microbiol. 2008;25:228–234. doi: 10.1016/j.fm.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 47.Heimesaat MM, et al. The role of serine protease HtrA in acute ulcerative enterocolitis and extra-intestinal immune responses during Campylobacter jejuni infection of gnotobiotic IL-10 deficient mice. Front Cell Infect Microbiol. 2014;4:77. doi: 10.3389/fcimb.2014.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


