Abstract
Food-borne trans-fatty acids (TFAs) are mainly produced as byproducts during food manufacture. Recent epidemiological studies have revealed that TFA consumption is a major risk factor for various disorders, including atherosclerosis. However, the underlying mechanisms in this disease etiology are largely unknown. Here we have shown that TFAs potentiate activation of apoptosis signal-regulating kinase 1 (ASK1) induced by extracellular ATP, a damage-associated molecular pattern leaked from injured cells. Major food-associated TFAs such as elaidic acid (EA), linoelaidic acid, and trans-vaccenic acid, but not their corresponding cis isomers, dramatically enhanced extracellular ATP-induced apoptosis, accompanied by elevated activation of the ASK1-p38 pathway in a macrophage-like cell line, RAW264.7. Moreover, knocking out the ASK1-encoding gene abolished EA-mediated enhancement of apoptosis. We have reported previously that extracellular ATP induces apoptosis through the ASK1-p38 pathway activated by reactive oxygen species generated downstream of the P2X purinoceptor 7 (P2X7). However, here we show that EA did not increase ATP-induced reactive oxygen species generation but, rather, augmented the effects of calcium/calmodulin-dependent kinase II-dependent ASK1 activation. These results demonstrate that TFAs promote extracellular ATP-induced apoptosis by targeting ASK1 and indicate novel TFA-associated pathways leading to inflammatory signal transduction and cell death that underlie the pathogenesis and progression of TFA-induced atherosclerosis. Our study thus provides insight into the pathogenic mechanisms of and proposes potential therapeutic targets for these TFA-related disorders.
Keywords: apoptosis, apoptosis signal-regulating kinase 1 (ASK1), macrophage, P2X7, p38 MAPK
Introduction
trans-Fatty acids (TFAs)4 are unsaturated fatty acids that contain one or more carbon-carbon trans double bonds. Most TFAs are produced during the industrial food manufacturing processes, mainly through partial hydrogenation of their cis isomers, hereafter called cis-fatty acids (CFAs), present in biological sources, including vegetable and fish oils (1). CFAs have a wide range of beneficial effects on health, such as anti-inflammatory, antidiabetic, and cardioprotective effects (2–5). On the other hand, TFAs have been reported to have adverse effects on health; evidence from epidemiological studies has shown the association of TFA intake with systemic inflammation, diabetes, and cardiovascular diseases (CVDs) (6). The underlying mechanisms of these TFA-related disorders have been explained by the biological actions of TFAs analogous to those of saturated fatty acids (SFAs) because SFAs have similar physical properties as TFAs and are well established as a risk factor for these disorders, in which the underlying mechanisms have been extensively studied (6). For instance, increased intake of TFAs as well as SFAs reduces endothelial function (7, 8), and up-regulates the level of plasma cytokines (9, 10) and low-density lipoprotein cholesterol (a major cause of cholesterol accumulation and inflammation in the artery wall) (11), thereby contributing to the pathogenesis of CVDs. Nevertheless, TFAs have been known as more potent risk factors for CVDs compared with SFAs, which decrease the level of cardioprotective high-density lipoprotein cholesterol (12). Moreover, a previous report demonstrated that TFAs, but not SFAs, decrease membrane fluidity and particularly inhibit insulin-induced antilipolytic effects and glucose transport in adipocytes (13). Thus, TFAs have specific adverse biological effects that are probably associated with a risk of TFA-related disorders. However, to date, there have been few studies of the biological effects of TFAs at the cellular or molecular level, and the underlying mechanisms of the disorders associated with TFA intake remain largely unknown.
Among TFA-related disorders, TFAs are most strongly associated with atherosclerosis, a major cause of CVDs, which is shown by a number of epidemiological studies and research using a mouse model (12, 14–16). Atherosclerosis is regarded as an inflammatory disease because macrophages play major roles in its pathogenesis and progression; monocytes circulating in the blood are recruited to athero-prone arterial sites, differentiate into macrophages, and finally become so-called foam cells in response to oxidized lipoproteins, which contribute to plaque formation and pro-inflammatory responses in atherosclerotic lesions (17). Apoptotic macrophages increase in advanced atherosclerotic lesions because of insufficient clearance of apoptotic macrophages through phagocytosis by neighboring macrophages, called efferocytosis (17, 18). The remaining apoptotic macrophages eventually undergo necrotic cell death, called secondary necrosis, resulting in the release of proinflammatory molecules, such as damage-associated molecular patterns, from the macrophages and the amplification of proinflammatory responses, leading to the progression of atherosclerosis (17, 18). Extracellular ATP, one of the damage-associated molecular patterns leaked from damaged tissues or cells, is a potent inducer of macrophage apoptosis through the P2X purinoceptor 7 (P2X7) receptor coupled with ion fluxes (19, 20). We have previously reported the mechanism by which the ATP-P2X7 receptor axis mediates apoptosis; ATP-induced P2X7 receptor activation causes reactive oxygen species (ROS) generation, which induces activation of apoptosis signal-regulating kinase 1 (ASK1)-p38 MAPK pathway, thereby leading to apoptosis (21). A recent study showed that the P2X7 receptor is highly expressed in macrophages in atherosclerotic lesions and that knockdown of the P2X7 receptor in mice reduced plaque formation and plasma cytokine levels, suggesting the involvement of ATP-P2X7 receptor signaling in atherosclerosis progression (22). It is assumed that TFAs exacerbate atherosclerosis by promoting macrophage apoptosis downstream of ATP-P2X7 receptor signaling, which contributes to the onset of CVDs; however, no study has been carried out to examine this assumption.
In this study, we show that elaidic acid (EA, C18.1 9T), the most abundant TFA in foods (1), but not oleic acid (OA, C18.1 9C) as a cis isomer of EA, drastically enhanced extracellular ATP-induced cell death by causing hyperactivation of the ASK1-p38 pathway. Palmitic acid (PA, C16.0), a typical SFA in foods, also enhanced extracellular ATP-induced p38 activation and cell death but clearly to a lesser extent than EA. Other TFAs, including linoelaidic acid (LEA, C18.2, 9T12T) and trans-vaccenic acid (TVA, C18.1 11T), but not their cis isomers, also significantly enhanced extracellular ATP-induced p38 activation and cell death. These results demonstrate a novel TFA-specific biological action for inflammatory signal transduction and cell death, which explains the pathogenesis and progression of atherosclerosis associated with TFAs.
Results
EA drastically enhances extracellular ATP-induced p38 activation and cell death
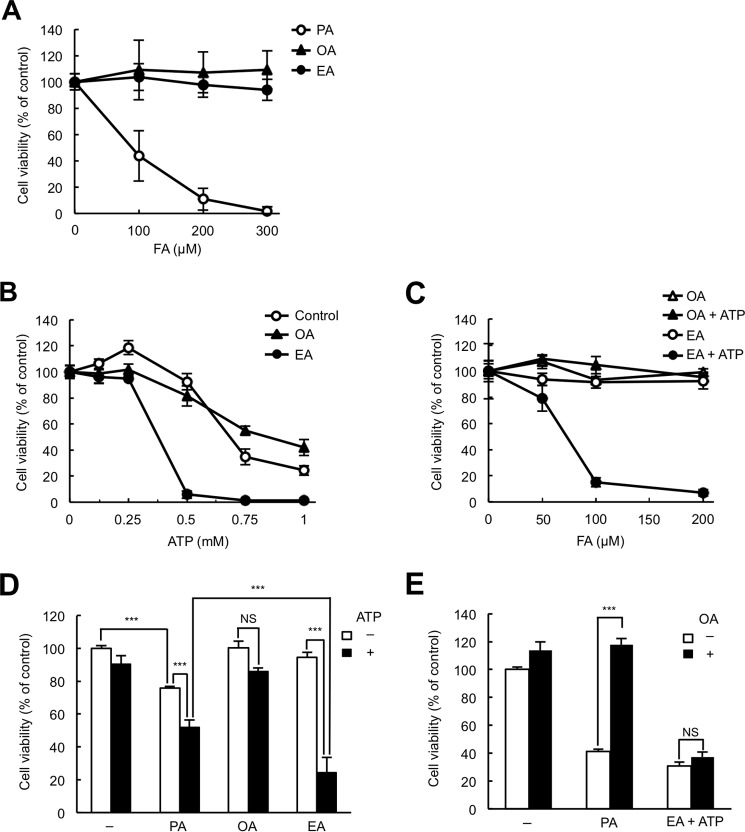
To examine whether TFAs potentiate macrophage apoptosis induced by extracellular ATP, we compared the effects of EA (the most abundant TFA in processed foods) and OA (its cis isomer) on ATP-induced cell death in a macrophage-like murine cell line, RAW264.7. First we checked the cytotoxicity of EA and OA for RAW264.7 cells. As shown in Fig. 1A, treatment of EA and OA did not change the viability of RAW264.7 cells in the range of 0–300 μm, whereas treatment of PA, an SFA that is known to have severe cytotoxic effects, caused a significant decrease in cell viability in a concentration-dependent manner, indicating that EA and OA have no cytotoxic effect on RAW264.7 cells, at least up to 300 μm. We next pretreated RAW264.7 cells with 200 μm EA or OA, stimulated them with ATP in the range of 0–1 mm, and then subjected them to a cell viability assay. Interestingly, we found that stimulation with 0.5 mm ATP severely reduced the viability of EA-pretreated cells (less than 10% cell viability) but not that of control or OA-pretreated cells (about 80–90% cell viability) (Fig. 1B). Moreover, when cells were stimulated with 0.5 mm ATP, pretreatment with EA markedly reduced cell viability in a concentration-dependent manner, whereas OA did not at concentrations of 0–200 μm (Fig. 1C), indicating that pretreatment with EA but not OA drastically enhances ATP-induced cell death.
Figure 1.
EA promotes extracellular ATP-induced cell death. A, RAW264.7 cells were treated with the indicated concentrations of PA, OA, or EA for 24 h and subjected to a cell viability assay. The data shown are the mean ± S.D. FA, fatty acid. B, RAW264.7 cells were pretreated with or without 200 μm fatty acid as indicated for 12 h, stimulated with various concentrations of ATP for 6 h, and then subjected to a cell viability assay. The data shown are the mean ± S.D. C, RAW264.7 cells were pretreated with various concentrations of OA or EA for 12 h, stimulated with 0.5 mm ATP for 6 h, and then subjected to a cell viability assay. The data shown are the mean ± S.D. D, RAW264.7 cells were pretreated with 100 μm PA, OA, or EA for 12 h, stimulated with 0.5 mm ATP for 6 h, and then subjected to a cell viability assay. The data shown are the mean ± S.D. Significant differences were determined by one-way ANOVA followed by Tukey-Kramer test. ***, p < 0.001; NS, not significant. E, RAW264.7 cells were pretreated with 200 μm PA or EA in the presence or absence of 100 μm OA for 12 h, and then EA-pretreated cells were stimulated with 0.5 mm ATP for 6 h and subjected to a cell viability assay. The data shown are the mean ± S.D. Significant differences were determined by one-way ANOVA followed by Tukey-Kramer test. ***, p < 0.001.
Because SFAs such as PA are known to have several similar biological actions as TFAs (6), we examined whether PA pretreatment also enhances ATP-induced cell death. As shown in Fig. 1A, 100 μm PA pretreatment alone decreased cell viability, and subsequent ATP stimulation also induced slight but significant cell death (Fig. 1D). Nevertheless, EA enhanced ATP-induced cell death to a much larger extent than PA (Fig. 1D), suggesting that EA is a more potent enhancer of ATP-induced cell death than PA. CFAs are known to have protective effects against cytotoxicity induced by SFAs (23). We therefore examined the possibility that CFAs may also suppress TFA-mediated enhancement of ATP-induced cell death. PA-induced cell death was fully suppressed by co-treatment with OA, whereas treatment with OA failed to suppress ATP-induced cell death enhanced by EA (Fig. 1E), suggesting that EA enhances ATP-induced cell death through a mechanism different from that of PA-induced cell death.
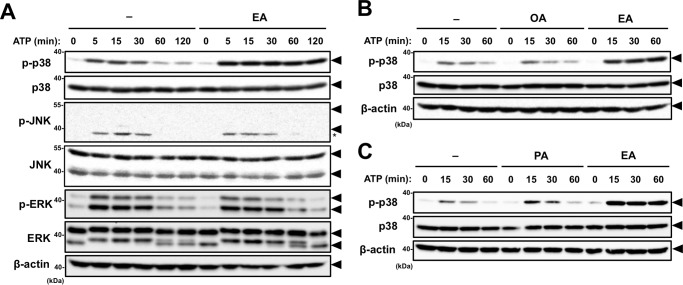
We have previously reported that extracellular ATP induces apoptosis through the p38 pathway activated by P2X7 receptor-mediated ROS generation (21). To investigate whether EA-mediated enhancement of cell death induced by extracellular ATP depends on the activation of p38, RAW264.7 cells were stimulated with a nonlethal dose of ATP (0.5 mm) in the presence or absence of EA, and then the phosphorylation status of p38 was monitored by immunoblotting of cell lysates. As shown in Fig. 2A, pretreatment with EA (200 μm) substantially enhanced ATP-induced p38 phosphorylation. In contrast, ATP-induced phosphorylation of other MAPKs, such as JNK and ERK, was not altered by EA pretreatment, suggesting that there is a highly specific mechanism by which EA potentiates ATP-induced p38 activation (Fig. 2A). Unlike EA, OA failed to enhance ATP-induced p38 activation (Fig. 2B). Pretreatment with PA faintly enhanced ATP-induced p38 activation compared with pretreatment with the same concentration of EA (Fig. 2C). These data show that, among the tested fatty acids, EA most potently enhanced ATP-induced activation of p38, which correlates well with the rate of extracellular ATP-induced cell death shown in Fig. 1D.
Figure 2.
EA enhances p38 activation induced by extracellular ATP. A and B, RAW264.7 cells were pretreated with 200 μm OA or EA for 12 h and then stimulated with 0.5 mm ATP for the indicated periods. Cell lysates were subjected to immunoblotting with the indicated antibodies. Arrowheads indicate bands corresponding to the indicated proteins, and the asterisk indicates a nonspecific band. C, RAW264.7 cells were treated with 100 μm PA or EA for 12 h, and stimulation and immunoblot analysis were performed as in A and B. Arrowheads indicate bands corresponding to the indicated proteins.
EA promotes ATP-induced apoptosis through the ASK1-p38 pathway downstream of the P2X7 receptor
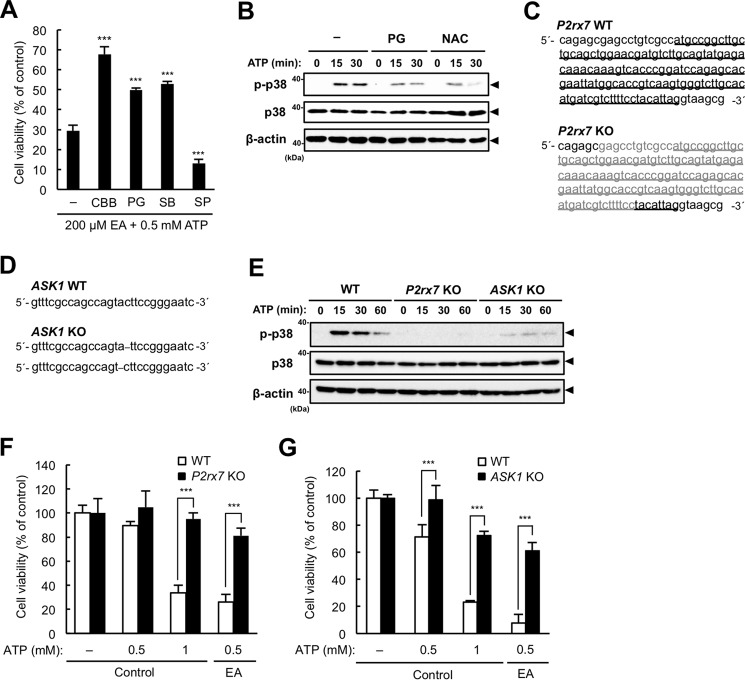
We next examined whether EA enhances ATP-induced cell death through ROS-dependent activation of p38 downstream of the P2X7 receptor. As shown in Fig. 3A, the enhanced ATP-induced cell death mediated by EA was significantly suppressed by pretreatment with either the P2X7 receptor inhibitor Coomassie Brilliant Blue G-250 (CBB), the antioxidant propyl gallate (PG), or the p38 inhibitor SB203580 but not with the JNK inhibitor SP600125, suggesting that ROS-dependent activation of p38 downstream of the P2X7 receptor is responsible for the enhancement of cell death. Moreover, immunoblot analysis showed that antioxidants such as PG and N-acetylcysteine (NAC) apparently suppressed extracellular ATP-induced p38 activation in the presence of EA (Fig. 3B), suggesting that the enhanced p38 activation by EA is dependent on ATP-induced ROS generation. To further confirm the importance of P2X7 receptor signaling on EA-mediated enhancement of ATP-induced cell death, we established P2rx7 and ASK1 KO cell lines that harbor frameshift mutations in all gene loci encoding the P2X7 receptor and ASK1, respectively (Fig. 3, C and D). In these KO cell lines, both extracellular ATP-induced phosphorylation of p38 (Fig. 3E) and cell death (Fig. 3, F and G) were almost completely suppressed. Collectively, these data suggest that the enhancement of ATP-induced cell death by EA depends on the activation of the ASK1-p38 pathway and ROS generation downstream of the P2X7 receptor.
Figure 3.
EA-mediated enhancement of ATP-induced cell death is dependent on the ASK1-p38 pathway downstream of the P2X7 receptor. A, RAW264.7 cells were pretreated with 200 μm EA for 12 h in the presence of various inhibitors, including the P2X7 receptor inhibitor CBB (1 mm), the antioxidant PG (20 μm), the p38 inhibitor SB203580 (SB, 20 μm), and the JNK inhibitor SP600125 (SP, 20 μm), stimulated with 0.5 mm ATP for 4 h, and then subjected to a cell viability assay. The data are represented as the percentage of viability of the cells treated with 200 μm EA without ATP stimulation and shown as mean ± S.D. Significant differences were determined by one-way ANOVA followed by Tukey-Kramer test. ***, p < 0.001 (versus control without inhibitors). B, RAW264.7 cells were pretreated with 200 μm EA for 12 h in the presence of 0.5 mm NAC or 20 μm PG and then stimulated with 0.5 mm ATP for the indicated periods. Cell lysates were subjected to immunoblotting with the indicated antibodies. Arrowheads indicate bands corresponding to the indicated proteins. C and D, DNA sequences around the gRNA target sites of P2rx7 (C) and ASK1 (D) genes in WT and KO RAW264.7 cells generated by using the CRISPR/Cas9 system. In P2rx7 KO cells, all P2rx7 loci are deleted at the same position (gray letters), in which the start codon is included. The underline indicates exon 1 (C). In ASK1 KO cells, frameshift deletions (dashes) are introduced in exon 5 of ASK1 loci (D). E, WT, P2rx7 KO, and ASK1 KO cells were pretreated with 200 μm EA, and stimulation and immunoblot analysis were performed as for Fig. 2, A and B. F and G, P2rx7 WT and KO cells (F) and ASK1 WT and KO cells (G) were pretreated with or without 200 μm EA for 12 h, stimulated with the indicated concentration of ATP for 6 h, and then subjected to a cell viability assay. The data shown are the mean ± S.D. Significant differences were determined by one-way ANOVA followed by Tukey-Kramer test. ***, p < 0.001.
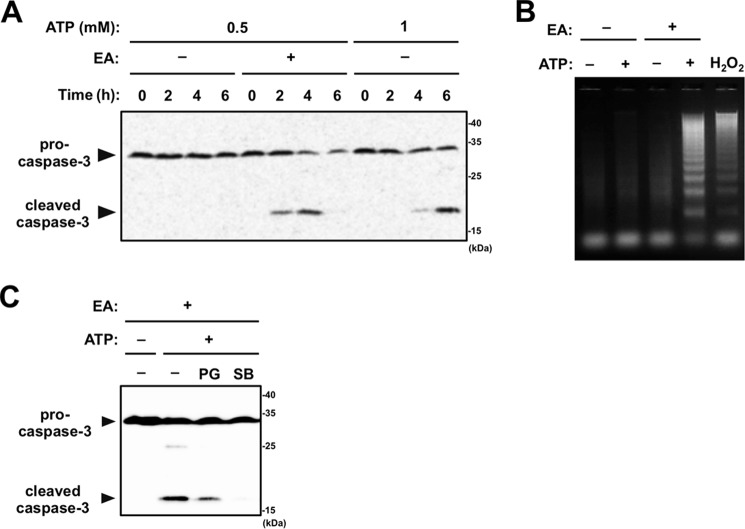
To examine whether ATP-induced cell death enhanced by EA is apoptotic cell death, RAW264.7 cells were stimulated with 0.5 mm ATP with or without EA, and caspase-3 activation was assessed by immunoblotting of the cleaved (activated) form of caspase-3 in cell lysates. Stimulation of 0.5 mm ATP induced cleavage of caspase-3 only in the presence of EA (Fig. 4A, fifth through eighth lanes), which was a similar level as observed with stimulation of 1 mm ATP in the absence of EA (Fig. 4A, ninth through twelfth lanes). Only stimulation of 1 mm ATP was reported previously to induce apoptosis (21). Moreover, a DNA ladder assay demonstrated that 0.5 mm ATP treatment clearly induced apoptosis only when EA was present, as well as H2O2 treatment, which is generally known to induce apoptosis (Fig. 4B). These data suggest that EA promotes apoptosis induced by extracellular ATP. To confirm the contribution of ROS-dependent p38 activation to accelerated apoptosis, we further examined the requirement of ROS and p38 for enhancement of ATP-induced caspase-3 activation. Indeed, extracellular ATP-induced cleavage of caspase-3 was significantly suppressed either by the antioxidant PG or the p38 inhibitor SB203580 (Fig. 4C), supporting the notion that the enhancement of ROS-dependent p38 activation contributes to the EA-mediated increase in ATP-induced apoptosis.
Figure 4.
EA promotes apoptosis induced by extracellular ATP. A, RAW264.7 cells were pretreated with or without 200 μm EA for 12 h and then stimulated with 0.5 or 1 mm ATP for the indicated periods. Cell lysates were subjected to immunoblotting with an anti-caspase-3 antibody. B, RAW264.7 cells were pretreated with or without 200 μm EA, stimulated with vehicle, 0.5 mm ATP, or 0.5 mm H2O2 for 4 h, and then subjected to a DNA fragmentation assay. C, RAW264.7 cells were pretreated with 200 μm EA for 12 h in the presence of either the antioxidant PG or the p38 inhibitor SB203580 (SB), stimulated with 0.5 mm ATP for 4 h, and then subjected to immunoblotting with an anti-caspase-3 antibody.
EA enhances ATP-induced ASK1 activation without increased ROS generation
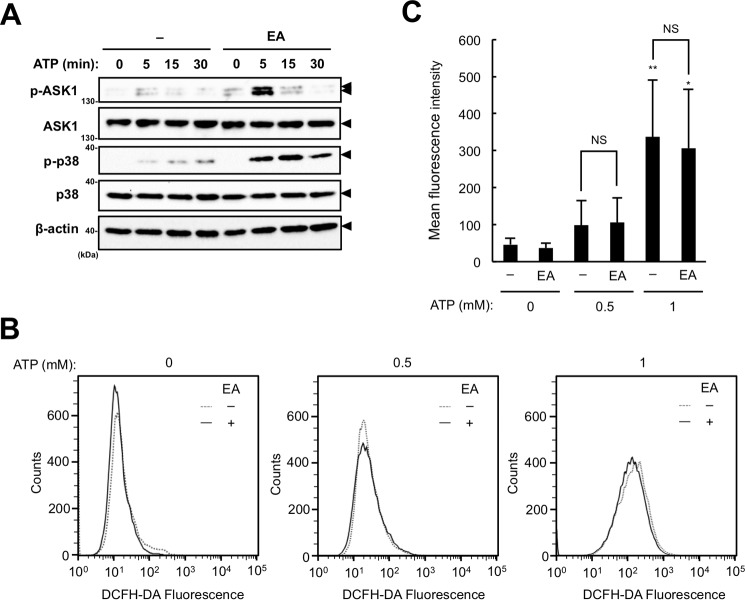
ASK1 is directly activated in response to ROS through the change in redox status of its binding partner thioredoxin (Trx). In unstimulated cells, reduced Trx binds to ASK1 and negatively regulates its activation. After ROS are produced, Trx is oxidized and dissociates from ASK1, inducing autophosphorylation (activation) of ASK1 (24). To examine whether EA enhances extracellular ATP-induced ASK1 activation, we assessed the phosphorylation status of ASK1 in RAW264.7 cells stimulated with 0.5 mm ATP with or without EA. Immunoblot analysis revealed that pretreatment with EA significantly increased extracellular ATP-induced phosphorylation of ASK1 compared with untreated cells, which correlated well with p38 activation (Fig. 5A). To determine whether EA affects extracellular ATP-induced ROS generation, we next assessed extracellular ATP-induced ROS generation in the presence or absence of EA. However, as shown in Fig. 5, B and C, FACS analysis using the ROS indicator 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA) showed that EA did not affect extracellular ATP-induced ROS generation at all (Fig. 5, B and C). These data therefore suggest that EA potentiates extracellular ATP-induced ASK1 activation by a mechanism distinct from ROS generation.
Figure 5.
EA enhances ATP-induced ASK1 activation without increased ROS generation. A, RAW264.7 cells were pretreated with or without 200 μm EA for 12 h and then stimulated with 0.5 or 1 mm ATP for the indicated periods. Cell lysates were subject to immunoblotting with the indicated antibodies. Arrowheads indicate bands corresponding to the indicated proteins. B and C, RAW264.7 cells were pretreated with or without 200 μm EA for 12 h, treated with 20 μm DCFH-DA for 30 min, and then stimulated with 0.5 mm ATP for 5 min. The amount of intracellular ROS was analyzed by FACS analysis, and a representative FACS plot (B) and mean ± S.D. (C) of four independent experiments are shown. Significant differences were determined by one-way ANOVA followed by Tukey-Kramer test. *, p < 0.05; **, p < 0.01 (versus control without ATP stimulation); NS, not significant.
CaMKII participates in the EA-mediated enhancement of ASK1 activation
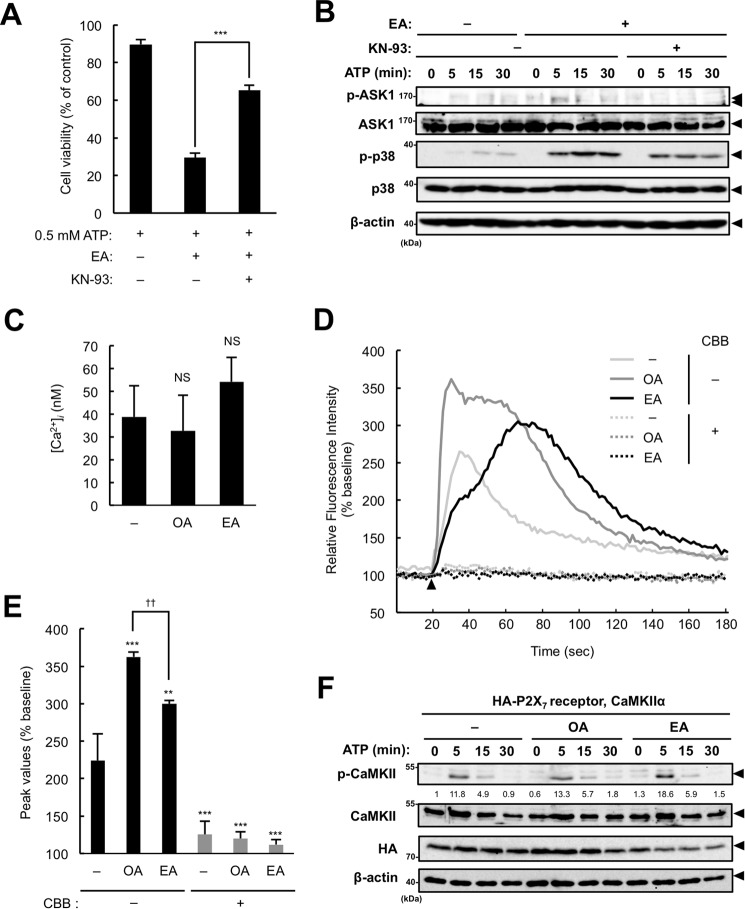
Extracellular ATP induces Ca2+ influx through the P2X7 receptor and leads to activation of Ca2+-dependent enzymes, including calcium/calmodulin-dependent kinase II (CaMKII) (25). Both ASK1 and CaMKII are closely linked to the pathogenesis and progression of CVDs (26, 27), and, more importantly, CaMKII functions as an activator of ASK1 (28). Therefore, it can be assumed that EA enhances ATP-induced ASK1 activation by positively regulating CaMKII activation. To address this possibility, we examined whether the CaMKII-specific inhibitor KN-93 prevents the increase in ATP-induced cell death mediated by EA. As shown in Fig. 6A, KN-93 significantly suppressed the EA-mediated promotion of ATP-induced cell death. Furthermore, KN-93 also markedly suppressed the enhancement of ATP-induced activation of ASK1 and p38 mediated by EA (Fig. 6B). These results suggest that CaMKII is involved in EA-mediated ASK1 activation. Given that EA enhances ATP-induced CaMKII activation, it is possible that P2X7 receptor-mediated Ca2+ influx is increased in the presence of EA. We therefore examined whether EA promotes ATP-induced Ca2+ influx using a Ca2+ indicator, Fluo-4. First we confirmed that intracellular free Ca2+ concentrations were not altered by OA or EA pretreatment (Fig. 6C). We then found that EA-pretreated cells showed a higher increase in Ca2+ influx than control cells, and observed a time delay of the Ca2+ influx in EA-pretreated cells for unknown reasons (Fig. 6, D and E). On the other hand, OA-pretreated cells showed a much higher increase than EA-pretreated cells (Fig. 6, D and E) even though OA failed to potentiate the ATP-induced p38 activation (Fig. 2B). In addition, the increased Ca2+ influx by pretreatment with EA or OA was almost completely inhibited by a P2X7 receptor inhibitor, CBB, suggesting that both fatty acids affect P2X7 receptor-mediated Ca2+ influx (Fig. 6D). However, considering that ATP-induced ERK activation, which is reported to depend on P2X7 receptor-mediated Ca2+ influx (29), was not altered in the presence of EA, as shown in Fig. 2A, the increased Ca2+ influx by EA appears not to influence downstream signaling. Collectively, these observations suggest that EA promotes CaMKII-dependent activation of the ASK1-p38 pathway independent of the increased Ca2+ influx. We next investigated the effect of EA on ATP-induced CaMKII autophosphorylation at Thr-286, which is known to positively regulate CaMKII activity by enhancing its interaction with Ca2+/calmodulin (30) and also by enabling its sustained activation independent of Ca2+/calmodulin (31–33). We transfected HEK293A cells with plasmids expressing the P2X7 receptor and CaMKIIα, pretreated with OA or EA, and then stimulated them with ATP. Immunoblot analysis showed that ATP-induced CaMKII phosphorylation at Thr-286 in EA-pretreated cells was increased compared with control or OA-pretreated cells (Fig. 6F). These results imply the presence of EA-specific mechanisms that activate ASK1 through CaMKII activation.
Figure 6.
CaMKII participates in the EA-mediated enhancement of ASK1 activation. A, RAW264.7 cells were pretreated with 200 μm EA for 12 h, treated with the CaMKII inhibitor KN-93 (10 μm) 30 min before stimulation with 0.5 mm ATP for 6 h, and then subjected to a cell viability assay. Data are presented as the percentage of viability of the cells treated with 200 μm EA without ATP stimulation and shown as mean ± S.D. Significant differences were determined by one-way ANOVA followed by Tukey-Kramer test. ***, p < 0.001. B, RAW264.7 cells were pretreated with 200 μm EA for 12 h and then treated with the CaMKII inhibitor KN-93 (5 μm) 30 min before stimulation with 0.5 mm ATP for the indicated periods. Cell lysates were subjected to immunoblotting with the indicated antibodies. Arrowheads indicate bands corresponding to the indicated proteins. C–E, RAW264.7 cells were pretreated with 200 μm EA for 12 h, loaded with Fluo-4/AM for 1 h, and then subjected to measurements of basal intracellular free Ca2+ concentrations ([Ca2+]i, mean ± S.D.) (C) and ATP-induced Ca2+ influx (D and E). The cells were stimulated with 0.5 mm ATP at 18 s after starting measurements (arrowhead), and the fluorescence was monitored intermittently for 3 min. Representative data of three independent experiments are shown as the percentage of basal fluorescent intensity (D), and the average peak values of the relative fluorescence intensity are shown as mean ± S.D. Significant differences were determined by one-way ANOVA followed by Tukey-Kramer test. NS, not significant (versus control) (C); **, p < 0.01; ***, p < 0.001 (versus control without CBB); ††, p < 0.01 (E). F, HEK293A cells were transfected with the HA-P2X7 receptor and CaMKII, pretreated with 200 μm OA or EA for 12 h, and then stimulated with 3 mm ATP for the indicated periods. Cell lysates were subjected to immunoblotting with the indicated antibodies. The values below the p-CaMKII blot indicate the relative phosphorylation levels of p-CaMKII normalized with total CaMKII levels. Arrowheads indicate bands corresponding to the indicated proteins.
Other TFAs also enhance ATP-induced cell death
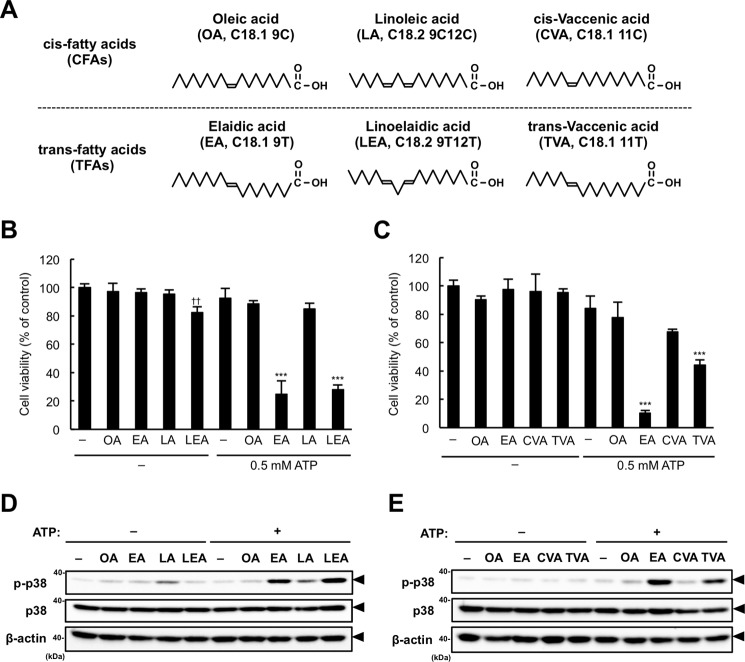
To examine whether the property of EA to enhance ATP-induced cell death is common among other TFA species, we assessed the effects of the following fatty acids on ATP-induced cell death: two other major TFAs in foods, LEA and TVA (1), and their cis isomers, linoleic acid (LA, C18.2 9C12C) and cis-vaccenic acid (CVA, C18.1 11C), respectively (Fig. 7A). LEA, but not LA, dramatically enhanced ATP-induced cell death at the same level as EA, although LEA showed a subtle cytotoxicity even without ATP (Fig. 7B). TVA pretreatment also showed a relatively small but significant enhancement of ATP-induced cell death, which was not observed in CVA-pretreated cells (Fig. 7C). Furthermore, both LEA and TVA, but not their cis isomers LA and CVA, respectively, enhanced ATP-induced p38 activation in a similar manner as EA (Fig. 7, D and E), which correlates with their ability to promote cell death (Fig. 7, B and C). These data suggest that the promoting effect of EA on ATP-induced apoptosis is a common feature among TFA species.
Figure 7.
Other TFAs also induce ATP-induced cell death. A, structures of the fatty acids tested. In parentheses, the following information about fatty acids is shown in a conventional manner: abbreviation, the number of carbon atoms, the number of carbon-carbon double bonds, and the positions of double bonds from the carboxyl-terminal. T, trans; C, cis). B and C, RAW264.7 cells were pretreated with the indicated fatty acids at 200 μm for 12 h, stimulated with 0.5 mm ATP for 6 h, and then subjected to a cell viability assay. The data shown are the mean ± S.D. Significant differences were determined by one-way ANOVA followed by Tukey-Kramer test. ††, p < 0.01 (versus control without ATP); ***, p < 0.001 (versus control with 0.5 mm ATP). D and E, RAW264.7 cells were pretreated with the indicated fatty acids at 200 μm for 12 h and then stimulated with 0.5 mm ATP for 1 h. Cell lysates were subjected to immunoblotting with the indicated antibodies. Arrowheads indicate bands corresponding to the indicated proteins.
Discussion
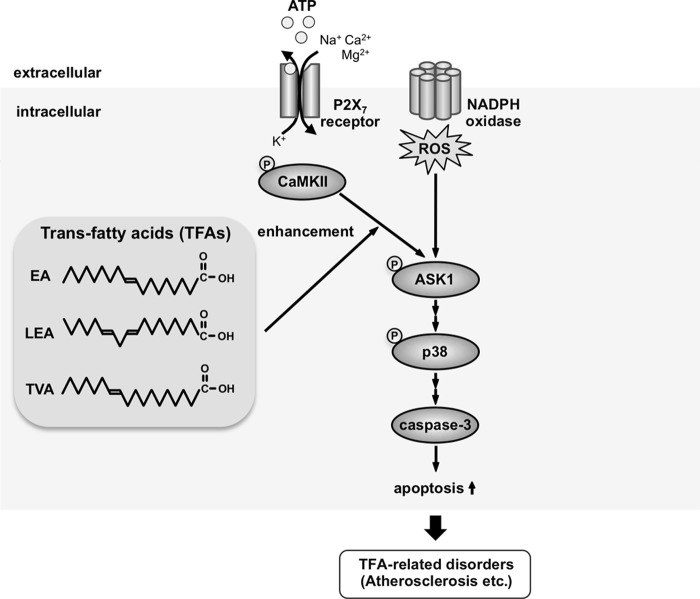
In this study, we have shown that TFAs such as EA, LEA, and TVA, but not their cis isomers, promote extracellular ATP-induced activation of the ASK1-p38 pathway and subsequent apoptosis. The proposed model is shown in Fig. 8. This is the first report showing the promoting effect of fatty acids (at least several TFAs and PA) on ATP-induced apoptosis and, notably, such a clearly trans form-specific biological effect of unsaturated fatty acids on inflammatory responses. We have further shown that EA promotes ATP-induced cell death far more efficiently than PA (Fig. 1D). Accumulating epidemiological evidence has shown that TFAs are more closely linked to CVDs than SFAs (12), and a recent mouse model study has revealed that the intake of TFAs induces more severe atherosclerosis than intake of SFAs (14). Notably, extracellularly released ATP and the P2X7 receptor are linked to the progression of atherosclerosis (22, 34), which is caused by the accumulation of apoptotic macrophages in atherosclerotic lesions. Taken together, our findings raise the intriguing possibility that TFAs promote macrophage apoptosis in atherosclerotic lesions, thereby facilitating atherosclerosis development and CVDs, which should be investigated in future studies.
Figure 8.
A proposed model for TFA-mediated enhancement of ATP-induced apoptosis. ATP binding to the P2X7 receptor leads to the generation of ROS through the activation of NADPH oxidases, which subsequently induces the activation of the ASK1-p38 pathway, resulting in caspase-3 activation and apoptosis, as we have shown previously (21). Meanwhile, TFAs promote extracellular ATP-induced p38 activation and apoptosis by potentiating activation of ASK1 mediated by CaMKII. The excessive apoptosis by TFAs may exceed the phagocytic capacity of macrophages, leading to the development of TFA-related disorders, including atherosclerosis.
With respect to the actual amount of TFAs in human bodies, several lines of evidence have shown that the plasma level of EA is ∼10 μm, and that of the whole TFAs is ∼40 μm (35, 36). Although, to the best of our knowledge, there is no report showing the amount of TFAs in human tissues, several reports have shown that of EA in the livers of rodents. Rodent livers contain 30–60 mg/g of liver total fatty acids (37–39), and the proportion of EA in total fatty acids is 1–3% (40–42). Taken together, the concentration of EA in rodent livers is estimated to be 1–6 mm. Thus, the concentrations of fatty acids in tissues such as livers are generally much higher than those in plasma, and the concentrations of TFAs used by us here (50–300 μm) would be physiologically relevant to the human condition.
EA promotes extracellular ATP-induced activation of ASK1 without affecting ROS generation (Fig. 5). Moreover, we found that EA specifically enhances p38 activation induced by ATP (Fig. 2B) but not that induced by lipopolysaccharide or H2O2 (data not shown), although p38 is activated downstream of ROS-induced ASK1 activation in all cases (21, 43). These data collectively suggest that EA has a specific role as an enhancer of extracellular ATP-induced ASK1 activation. We demonstrated that the CaMKII-specific inhibitor KN-93 strongly suppressed ATP-induced activation of ASK1 and p38 (Fig. 6B) and subsequent cell death (Fig. 6A). Moreover, we found that EA, but not OA, augments ATP-induced CaMKII autophosphorylation at Thr-286 (Fig. 6F), which positively regulates CaMKII activity. CaMKII has been shown previously to directly phosphorylate ASK1 at an unidentified residue other than Thr-845 (the critical autophosphorylation site), which promotes ASK1 activation (28). Therefore, it is assumed that EA enhances ATP-induced CaMKII autophosphorylation at Thr-286, thereby promoting CaMKII-dependent ASK1 activation. Although the mechanism by which EA potentiates ATP-induced CaMKII autophosphorylation remains to be elucidated, EA may down-regulate the expression or activities of CaMKII phosphatases such as protein phosphatase 1 (PP1) and PP2A (44). Otherwise, EA may increase O-GlcNAcylation of CaMKII, which has been implicated to be positively associated with its autophosphorylation status (45). Further studies will be needed to clarify the molecular mechanism for TFA-mediated enhancement of ASK1 activation through CaMKII.
Apoptosis induced by ATP-P2X7 receptor signaling has been observed in various types of immune cells other than macrophages, including thymocytes, dendritic cells, and mast cells (46). TFAs may be able to promote extracellular ATP-induced apoptosis in these immune cells in a similar manner as in macrophages, although the signaling pathway that mediates apoptosis in these cells remains to be elucidated. Indeed, in a mouse mast cell line, P-815, ATP-induced p38 activation, and cell death was significantly enhanced by EA (data not shown). Expression of the P2X7 receptor is not limited in immune cells but also found in neuronal cells and glial cells, like microglia, astrocytes, and oligodendrocytes (47). The P2X7 receptor mediates ATP-induced cell death in these cells, which is considered to play a causative role in several neurodegenerative disorders, including Huntington's disease and Alzheimer's disease (47). Several lines of evidence have shown that intake of TFAs can increase the risk of locomotor ataxia and cognitive disorders, including Alzheimer's disease (48–51). Taken together, TFAs may promote neuronal cell death through ATP-P2X7 receptor signaling and thereby contribute to the development of these disorders. Thus, the promotion of cell death induced by extracellular ATP, which is the newly found biological effect of TFAs, leads to the elucidation of the pathogenetic mechanisms of TFA-related disorders. Moreover, our findings propose the ROS-dependent ASK1-p38 pathway, causing ATP-induced cell death, as a potential diagnostic and therapeutic target to prevent and combat TFA-related disorders.
Experimental procedures
Reagents
ATP and KN-93 were purchased from Sigma. CBB was purchased from Fluka. SB203580 was purchased from Santa Cruz Biotechnology. SP600125, NAC, and H2O2 were purchased from Wako.
Plasmids
The cDNA encoding the mouse P2X7 receptor was obtained by PCR using cDNA from RAW264.7 cells as a template and inserted into pcDNA3.2 with an HA tag plasmid. The cDNA encoding CaMKIIα inserted into the pCAGGS plasmid was constructed as in a previous report (52).
Cell culture and transfection
HEK293A and RAW264.7 cells were cultured in Dulbecco's modified Eagle's medium and RPMI 1640, respectively, containing 10% heat-inactivated fetal bovine serum and 1% penicillin-streptomycin solution in 5% CO2 at 37 °C. Plasmid transfection was performed using polyethylenimine “Max” (PEI-MAX, Polysciences) according to the instructions of the manufacturer.
Preparation and treatment of fatty acids
Fatty acids, including PA and OA (Nacalai Tesque), EA (Sigma), LA and LEA (Cayman Chemical), and CVA and TVA (Olbracht Serdary Research Laboratories), were prepared as described previously with minor modifications (53). Briefly, fatty acids were dissolved in 0.1 n NaOH at 70 °C and then conjugated with fatty acid-free BSA (Sigma, pH 7.4) at 55 °C for 10 min to make 5 mm BSA-conjugated fatty acid stock solutions containing 10% BSA. Cells were treated with various concentrations of BSA-conjugated fatty acids by diluting stock solutions (the final BSA concentration in medium was set to 1%).
Immunoblot analysis
Cells were lysed in ice-cold lysis buffer containing 20 mm Tris-HCl (pH 7.4), 150 mm NaCl, 1% Triton X-100, 10% glycerol, and 1% protease and phosphatase inhibitor mixtures (Nacalai Tesque). After centrifugation, the cell extracts were resolved by SDS-PAGE and analyzed as described previously (21). The antibodies used were against phospho-p38, p38, phospho-JNK, JNK, phospho-ERK, ERK, caspase-3, phospho-ASK1 (Cell Signaling Technology), ASK1 (Santa Cruz Biotechnology), phospho-CaMKII, CaMKII (54), HA (Roche), and β-actin (Wako). The blots were developed with ECL (Merck Millipore).
Cell viability assay
RAW264.7 cells were seeded on 96-well plates. After any stimulation or treatment, cell viability was determined using a 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt/phenazine methosulfate assay (Cell Titer 96 cell proliferation assay, Promega) according to the protocol of the manufacturer. The absorbance was read at 492 nm using a microplate reader (Multiskan Ascent, Thermo Electron Corp.). Data were normalized to control (100%) without stimulus unless noted otherwise.
DNA fragmentation assay
The DNA fragmentation assay was performed as described previously (21). Briefly, stimulated cells were collected and suspended in lysis buffer (20 mm Tris-HCl (pH 7.5), 10 mm EDTA, and 0.5% Triton X-100), and the lysates were incubated at room temperature for 10 min, followed by centrifugation at 12,000 × g for 10 min. The supernatants were incubated with 0.2 mg/ml proteinase K and 0.1 mg/ml RNase A for 1 h at 42 °C, purified with phenol/chloroform extraction and ethanol precipitation, and separated on an agarose gel.
Generation of knockout cell lines
P2rx7- and MAP kinase kinase kinase 5 (MAP3K5, also known as ASK1) knockout cells were generated using the CRISPR/Cas9 system (55, 56). Two guide RNAs (gRNAs) were designed to target a region in exon 1 of the P2rx7 gene (5′-CGGATCCAGAGCACGAATTA-3′) and in exon 5 of the ASK1 gene (5′-GGTATGGATTCCCGGAAGTA-3′) using CRISPRdirect (57). A gRNA-encoding oligonucleotide was cloned into the lentiCRISPRv2 plasmid (Addgene) (58), and the plasmid was transfected with HEK293A cells together with a packaging plasmid, psPAX2, and an envelope plasmid, pVSV-G. The supernatants were collected and used to infect RAW264.7 cells, and then infected cells were selected with puromycin and cloned by limiting dilution. To determine the mutations of P2rx7 and ASK1 in cloned cells, the genomic sequence around the target region was analyzed by PCR-direct sequencing using extracted DNA from each clone as a template and the following primers: 5′-CGGTGTGTTTCCTTTGGCTG-3′ and 5′-TTACTGTTTCCTCCCAGCGG-3′ for P2rx7 and 5′-GTCATGCGTTTTCCTC-3′ and 5′-ATATTGTCTACCCGTTGC-3′ for ASK1.
Quantification of ROS generation
ATP-induced ROS generation was quantified by FACS analysis as described previously (21), using DCFH-DA (Sigma). FACS analysis was performed using EC800 (Sony) for flow cytometry and FlowJo (Tree Star) for analyzing data.
Measurement of intracellular Ca2+ levels
ATP-induced Ca2+ influx into cells was assessed using the Calcium Kit II-Fluo 4 (Dojindo) according to the instructions of the manufacturer. Fluorescence intensity was measured at 485-nm excitation/518-nm emission using a microplate reader (Flexstation3, Molecular Devices). For assessing intracellular free Ca2+ concentrations ([Ca2+]i), Fluo-4 fluorescence under Ca2+-saturated (Fmax) and Ca2+-free (Fmin) conditions was measured for cells treated with either 0.1% Triton X-100 plus 2 mm CaCl2 or 10 mm EGTA, respectively, in Krebs-Ringer-HEPES buffer (10 mm HEPES (pH 7.4), 11.5 mm glucose, 0.1% BSA, 130 mm NaCl, 4.7 mm KCl, 4 mm NaHCO3, 1.2 mm KH2PO4, 1.2 mm MgSO4, and 1.8 mm CaCl2), and [Ca2+]i was calculated using the following equation: [Ca2+]i = KD(F − Fmin)/(Fmax − F), KD = 345 nm.
Author contributions
Y. H., K. F., J. A., T. N., and A. M. conceived and designed the experiments. Y. H., M. T., Y. K., K. K., H. K., and K. M. performed the experiments. Y. H., M. T., Y. K., K. K., H. K., K. M., Y. S., Y. Y., K. F., J. A., T. N., and A. M. analyzed the data. Y. H., M. T., Y. K., T. N., and A. M. wrote the paper.
Acknowledgments
We thank all members of the Laboratory of Health Chemistry for helpful discussions.
This work was supported by the Nagai Memorial Research Scholarship from the Pharmaceutical Society of Japan, KAKENHI from the Japan Society for the Promotion of Science, and the Ministry of Education, Culture, Sports, Science and Technology. The authors declare that they have no conflicts of interest with the contents of this article.
- TFA
- trans-fatty acid
- CFA
- cis-fatty acid
- CVD
- cardiovascular disease
- SFA
- saturated fatty acid
- P2X7
- P2X purinoceptor 7
- ROS
- reactive oxygen species
- ASK1
- apoptosis signal-regulating kinase 1
- EA
- elaidic acid
- OA
- oleic acid
- PA
- palmitic acid
- LEA
- linoelaidic acid
- TVA
- trans-vaccenic acid
- CBB
- Coomassie Brilliant Blue G-250
- PG
- propyl gallate
- NAC
- N-acetylcysteine
- Trx
- thioredoxin
- DCFH-DA
- 2′,7′-dichlorodihydrofluorescein diacetate
- CaMKII
- calcium/calmodulin-dependent kinase II
- LA
- linoleic acid
- CVA
- cis-vaccenic acid
- gRNA
- guide RNA
- ANOVA
- analysis of variance.
References
- 1. Gebauer S. K., Psota T. L., and Kris-Etherton P. M. (2007) The diversity of health effects of individual trans fatty acid isomers. Lipids 42, 787–799 [DOI] [PubMed] [Google Scholar]
- 2. Ouellet V., Weisnagel S. J., Marois J., Bergeron J., Julien P., Gougeon R., Tchernof A., Holub B. J., and Jacques H. (2008) Dietary cod protein reduces plasma C-reactive protein in insulin-resistant men and women. J. Nutr. 138, 2386–2391 [DOI] [PubMed] [Google Scholar]
- 3. Jiménez-Gómez Y., López-Miranda J., Blanco-Colio L. M., Marín C., Pérez-Martínez P., Ruano J., Paniagua J. A., Rodríguez F., Egido J., and Pérez-Jiménez F. (2009) Olive oil and walnut breakfasts reduce the postprandial inflammatory response in mononuclear cells compared with a butter breakfast in healthy men. Atherosclerosis 204, e70–e76 [DOI] [PubMed] [Google Scholar]
- 4. Micallef M. A., and Garg M. L. (2009) Anti-inflammatory and cardioprotective effects of n-3 polyunsaturated fatty acids and plant sterols in hyperlipidemic individuals. Atherosclerosis 204, 476–482 [DOI] [PubMed] [Google Scholar]
- 5. Shapiro H., Theilla M., Attal-Singer J., and Singer P. (2011) Effects of polyunsaturated fatty acid consumption in diabetic nephropathy. Nat. Rev. Nephrol. 7, 110–121 [DOI] [PubMed] [Google Scholar]
- 6. Estadella D., da Penha Oller do Nascimento C. M., Oyama L. M., Ribeiro E. B., Dâmaso A. R., and de Piano A. (2013) Lipotoxicity: effects of dietary saturated and transfatty acids. Mediators Inflamm. 2013, 137579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lopez-Garcia E., Schulze M. B., Meigs J. B., Manson J. E., Rifai N., Stampfer M. J., Willett W. C., and Hu F. B. (2005) Consumption of trans fatty acids is related to plasma biomarkers of inflammation and endothelial dysfunction. J. Nutr. 135, 562–566 [DOI] [PubMed] [Google Scholar]
- 8. Iwata N. G., Pham M., Rizzo N. O., Cheng A. M., Maloney E., and Kim F. (2011) Trans fatty acids induce vascular inflammation and reduce vascular nitric oxide production in endothelial cells. PLoS ONE 6, e29600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Baer D. J., Judd J. T., Clevidence B. A., and Tracy R. P. (2004) Dietary fatty acids affect plasma markers of inflammation in healthy men fed controlled diets: a randomized crossover study. Am. J. Clin. Nutr. 79, 969–973 [DOI] [PubMed] [Google Scholar]
- 10. Han S. N., Leka L. S., Lichtenstein A. H., Ausman L. M., Schaefer E. J., and Meydani S. N. (2002) Effect of hydrogenated and saturated, relative to polyunsaturated, fat on immune and inflammatory responses of adults with moderate hypercholesterolemia. J. Lipid Res. 43, 445–452 [PubMed] [Google Scholar]
- 11. Michas G., Micha R., and Zampelas A. (2014) Dietary fats and cardiovascular disease: putting together the pieces of a complicated puzzle. Atherosclerosis 234, 320–328 [DOI] [PubMed] [Google Scholar]
- 12. Mensink R. P., Zock P. L., Kester A. D., and Katan M. B. (2003) Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a meta-analysis of 60 controlled trials. Am. J. Clin. Nutr. 77, 1146–1155 [DOI] [PubMed] [Google Scholar]
- 13. Ibrahim A., Natrajan S., and Ghafoorunissa R. (2005) Dietary trans-fatty acids alter adipocyte plasma membrane fatty acid composition and insulin sensitivity in rats. Metabolism 54, 240–246 [DOI] [PubMed] [Google Scholar]
- 14. Machado R. M., Nakandakare E. R., Quintao E. C., Cazita P. M., Koike M. K., Nunes V. S., Ferreira F. D., Afonso M. S., Bombo R. P., Machado-Lima A., Soriano F. G., Catanozi S., and Lottenberg A. M. (2012) Omega-6 polyunsaturated fatty acids prevent atherosclerosis development in LDLr-KO mice, in spite of displaying a pro-inflammatory profile similar to trans fatty acids. Atherosclerosis 224, 66–74 [DOI] [PubMed] [Google Scholar]
- 15. Bassett C. M., McCullough R. S., Edel A. L., Maddaford T. G., Dibrov E., Blackwood D. P., Austria J. A., and Pierce G. N. (2009) Trans-fatty acids in the diet stimulate atherosclerosis. Metabolism 58, 1802–1808 [DOI] [PubMed] [Google Scholar]
- 16. Siddiqui R. A., Harvey K. A., Ruzmetov N., Miller S. J., and Zaloga G. P. (2009) n-3 fatty acids prevent whereas trans-fatty acids induce vascular inflammation and sudden cardiac death. Br. J. Nutr. 102, 1811–1819 [DOI] [PubMed] [Google Scholar]
- 17. Moore K. J., Sheedy F. J., and Fisher E. A. (2013) Macrophages in atherosclerosis: a dynamic balance. Nat. Rev. Immunol. 13, 709–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tabas I. (2010) Macrophage death and defective inflammation resolution in atherosclerosis. Nat. Rev. Immunol. 10, 36–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Coddou C., Yan Z., Obsil T., Huidobro-Toro J. P., and Stojilkovic S. S. (2011) Activation and regulation of purinergic P2X receptor channels. Pharmacol. Rev. 63, 641–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wiley J. S., Sluyter R., Gu B. J., Stokes L., and Fuller S. J. (2011) The human P2X7 receptor and its role in innate immunity. Tissue Antigens 78, 321–332 [DOI] [PubMed] [Google Scholar]
- 21. Noguchi T., Ishii K., Fukutomi H., Naguro I., Matsuzawa A., Takeda K., and Ichijo H. (2008) Requirement of reactive oxygen species-dependent activation of ASK1-p38 MAPK pathway for extracellular ATP-induced apoptosis in macrophages. J. Biol. Chem. 283, 7657–7665 [DOI] [PubMed] [Google Scholar]
- 22. Peng K., Liu L., Wei D., Lv Y., Wang G., Xiong W., Wang X., Altaf A., Wang L., He D., Wang H., and Qu P. (2015) P2X7R is involved in the progression of atherosclerosis by promoting NLRP3 inflammasome activation. Int. J. Mol. Med. 35, 1179–1188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Leamy A. K., Egnatchik R. A., and Young J. D. (2013) Molecular mechanisms and the role of saturated fatty acids in the progression of non-alcoholic fatty liver disease. Prog. Lipid Res. 52, 165–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Saitoh M., Nishitoh H., Fujii M., Takeda K., Tobiume K., Sawada Y., Kawabata M., Miyazono K., and Ichijo H. (1998) Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J. 17, 2596–2606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. León D., Hervás C., and Miras-Portugal M. T. (2006) P2Y1 and P2X7 receptors induce calcium/calmodulin-dependent protein kinase II phosphorylation in cerebellar granule neurons. Eur. J. Neurosci. 23, 2999–3013 [DOI] [PubMed] [Google Scholar]
- 26. Hayakawa R., Hayakawa T., Takeda K., and Ichijo H. (2012) Therapeutic targets in the ASK1-dependent stress signaling pathways. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 88, 434–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mattiazzi A., Bassani R. A., Escobar A. L., Palomeque J., Valverde C. A., Vila Petroff M., and Bers D. M. (2015) Chasing cardiac physiology and pathology down the CaMKII cascade. Am. J. Physiol. Heart Circ. Physiol. 308, H1177–H1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Takeda K., Matsuzawa A., Nishitoh H., Tobiume K., Kishida S., Ninomiya-Tsuji J., Matsumoto K., and Ichijo H. (2004) Involvement of ASK1 in Ca2+-induced p38 MAP kinase activation. EMBO Rep. 5, 161–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gendron F. P., Neary J. T., Theiss P. M., Sun G. Y., Gonzalez F. A., and Weisman G. A. (2003) Mechanisms of P2X7 receptor-mediated ERK1/2 phosphorylation in human astrocytoma cells. Am. J. Physiol. Cell Physiol. 284, C571–C581 [DOI] [PubMed] [Google Scholar]
- 30. Meyer T., Hanson P. I., Stryer L., and Schulman H. (1992) Calmodulin trapping by calcium-calmodulin-dependent protein kinase. Science 256, 1199–1202 [DOI] [PubMed] [Google Scholar]
- 31. Lai Y., Nairn A. C., and Greengard P. (1986) Autophosphorylation reversibly regulates the Ca2+/calmodulin-dependence of Ca2+/calmodulin-dependent protein kinase II. Proc. Natl. Acad. Sci. U.S.A. 83, 4253–4257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Miller S. G., and Kennedy M. B. (1986) Regulation of brain type II Ca2+/calmodulin-dependent protein kinase by autophosphorylation: a Ca2+-triggered molecular switch. Cell 44, 861–870 [DOI] [PubMed] [Google Scholar]
- 33. Yang E., and Schulman H. (1999) Structural examination of autoregulation of multifunctional calcium/calmodulin-dependent protein kinase II. J. Biol. Chem. 274, 26199–26208 [DOI] [PubMed] [Google Scholar]
- 34. Ferrari D., Vitiello L., Idzko M., and la Sala A. (2015) Purinergic signaling in atherosclerosis. Trends Mol. Med. 21, 184–192 [DOI] [PubMed] [Google Scholar]
- 35. Mori K., Ishida T., Yasuda T., Hasokawa M., Monguchi T., Sasaki M., Kondo K., Nakajima H., Shinohara M., Shinke T., Irino Y., Toh R., Nishimura K., and Hirata K. (2015) Serum trans-fatty acid concentration is elevated in young patients with coronary artery disease in Japan. Circ. J. 79, 2017–2025 [DOI] [PubMed] [Google Scholar]
- 36. Vesper H. W., Kuiper H. C., Mirel L. B., Johnson C. L., and Pirkle J. L. (2012) Levels of plasma trans-fatty acids in non-Hispanic white adults in the United States in 2000 and 2009. JAMA 307, 562–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cenedella R. J. (1971) Effects of Su-13437, a new hypolipidemic drug, upon synthesis in vivo of hepatic and carcass total fatty acids and total cholesterol. Lipids 6, 475–480 [DOI] [PubMed] [Google Scholar]
- 38. Chung M. Y., Mah E., Masterjohn C., Noh S. K., Park H. J., Clark R. M., Park Y. K., Lee J. Y., and Bruno R. S. (2015) Green tea lowers hepatic COX-2 and prostaglandin E2 in rats with dietary fat-induced nonalcoholic steatohepatitis. J. Med. Food 18, 648–655 [DOI] [PubMed] [Google Scholar]
- 39. Druart C., Neyrinck A. M., Dewulf E. M., De Backer F. C., Possemiers S., Van de Wiele T., Moens F., De Vuyst L., Cani P. D., Larondelle Y., and Delzenne N. M. (2013) Implication of fermentable carbohydrates targeting the gut microbiota on conjugated linoleic acid production in high-fat-fed mice. Br. J. Nutr. 110, 998–1011 [DOI] [PubMed] [Google Scholar]
- 40. Dorfman S. E., Laurent D., Gounarides J. S., Li X., Mullarkey T. L., Rocheford E. C., Sari-Sarraf F., Hirsch E. A., Hughes T. E., and Commerford S. R. (2009) Metabolic implications of dietary trans-fatty acids. Obesity 17, 1200–1207 [DOI] [PubMed] [Google Scholar]
- 41. Hussein O., Grosovski M., Lasri E., Svalb S., Ravid U., and Assy N. (2007) Monounsaturated fat decreases hepatic lipid content in non-alcoholic fatty liver disease in rats. World J. Gastroenterol. 13, 361–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Liu W. H., Lin C. C., Wang Z. H., Mong M. C., and Yin M. C. (2010) Effects of protocatechuic acid on trans fat induced hepatic steatosis in mice. J. Agric. Food Chem. 58, 10247–10252 [DOI] [PubMed] [Google Scholar]
- 43. Matsuzawa A., Saegusa K., Noguchi T., Sadamitsu C., Nishitoh H., Nagai S., Koyasu S., Matsumoto K., Takeda K., and Ichijo H. (2005) ROS-dependent activation of the TRAF6-ASK1-p38 pathway is selectively required for TLR4-mediated innate immunity. Nat. Immunol. 6, 587–592 [DOI] [PubMed] [Google Scholar]
- 44. Strack S., Barban M. A., Wadzinski B. E., and Colbran R. J. (1997) Differential inactivation of postsynaptic density-associated and soluble Ca2+/calmodulin-dependent protein kinase II by protein phosphatases 1 and 2A. J. Neurochem. 68, 2119–2128 [DOI] [PubMed] [Google Scholar]
- 45. Erickson J. R., Pereira L., Wang L., Han G., Ferguson A., Dao K., Copeland R. J., Despa F., Hart G. W., Ripplinger C. M., and Bers D. M. (2013) Diabetic hyperglycaemia activates CaMKII and arrhythmias by O-linked glycosylation. Nature 502, 372–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Idzko M., Ferrari D., and Eltzschig H. K. (2014) Nucleotide signalling during inflammation. Nature 509, 310–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tewari M., and Seth P. (2015) Emerging role of P2X7 receptors in CNS health and disease. Ageing Res. Rev. 24, 328–342 [DOI] [PubMed] [Google Scholar]
- 48. Morris M. C., Evans D. A., Bienias J. L., Tangney C. C., Bennett D. A., Aggarwal N., Schneider J., and Wilson R. S. (2003) Dietary fats and the risk of incident Alzheimer disease. Arch. Neurol. 60, 194–200 [DOI] [PubMed] [Google Scholar]
- 49. Morris M. C., Evans D. A., Bienias J. L., Tangney C. C., and Wilson R. S. (2004) Dietary fat intake and 6-year cognitive change in an older biracial community population. Neurology 62, 1573–1579 [DOI] [PubMed] [Google Scholar]
- 50. Morris M. C., Evans D. A., Tangney C. C., Bienias J. L., Schneider J. A., Wilson R. S., and Scherr P. A. (2006) Dietary copper and high saturated and trans fat intakes associated with cognitive decline. Arch. Neurol. 63, 1085–1088 [DOI] [PubMed] [Google Scholar]
- 51. Teixeira A. M., Dias V. T., Pase C. S., Roversi K., Boufleur N., Barcelos R. C., Benvegnú D. M., Trevizol F., Dolci G. S., Carvalho N. R., Quatrin A., Soares F. A., Reckziegel P., Segat H. J., Rocha J. B., et al. (2012) Could dietary trans fatty acids induce movement disorders? Effects of exercise and its influence on Na+K+-ATPase and catalase activity in rat striatum. Behav. Brain Res. 226, 504–510 [DOI] [PubMed] [Google Scholar]
- 52. Shioda N., Sawai M., Ishizuka Y., Shirao T., and Fukunaga K. (2015) Nuclear translocation of calcium/calmodulin-dependent protein kinase IIδ3 promoted by protein phosphatase-1 enhances brain-derived neurotrophic factor expression in dopaminergic neurons. J. Biol. Chem. 290, 21663–21675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Cousin S. P., Hugl S. R., Wrede C. E., Kajio H., Myers M. G. Jr, and Rhodes C. J. (2001) Free fatty acid-induced inhibition of glucose and insulin-like growth factor I-induced deoxyribonucleic acid synthesis in the pancreatic β-cell line INS-1. Endocrinology 142, 229–240 [DOI] [PubMed] [Google Scholar]
- 54. Fukunaga K., Goto S., and Miyamoto E. (1988) Immunohistochemical localization of Ca2+/calmodulin-dependent protein kinase II in rat brain and various tissues. J. Neurochem. 51, 1070–1078 [DOI] [PubMed] [Google Scholar]
- 55. Mali P., Yang L., Esvelt K. M., Aach J., Guell M., DiCarlo J. E., Norville J. E., and Church G. M. (2013) RNA-guided human genome engineering via Cas9. Science 339, 823–826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Cong L., Ran F. A., Cox D., Lin S., Barretto R., Habib N., Hsu P. D., Wu X., Jiang W., Marraffini L. A., and Zhang F. (2013) Multiplex genome engineering using CRISPR/Cas systems. Science 339, 819–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Naito Y., Hino K., Bono H., and Ui-Tei K. (2015) CRISPRdirect: software for designing CRISPR/Cas guide RNA with reduced off-target sites. Bioinformatics 31, 1120–1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sanjana N. E., Shalem O., and Zhang F. (2014) Improved vectors and genome-wide libraries for CRISPR screening. Nat. Methods 11, 783–784 [DOI] [PMC free article] [PubMed] [Google Scholar]