Abstract
When activated through toll-like receptors (TLRs), macrophages generate IL-33, an IL-1 family member that induces innate immune responses through ST2 signaling. LPS, a TLR4 ligand, induces macrophages to generate prostaglandin E2 (PGE2) through inducible COX-2 and microsomal PGE2 synthase 1 (mPGES-1) (1). We demonstrate that IL-33 production by bone marrow-derived murine macrophages (bmMFs) requires the generation of endogenous PGE2 and the intrinsic expression of EP2 receptors to amplify NF-κB-dependent, LPS-induced IL-33 expression via exchange protein activated by cAMP (EPAC). Compared with WT cells, bmMFs lacking either mPGES-1 or EP2 receptors displayed reduced LPS-induced IL-33 levels. A selective EP2 agonist and, to a lesser extent, EP4 receptor agonist potentiated LPS-induced IL-33 generation from both mPGES-1-null and WT bmMFs, whereas EP1 and EP3 receptor agonists were inactive. The effects of PGE2 depended on cAMP, were mimicked by an EPAC-selective agonist, and were attenuated by EPAC-selective antagonism and knockdown. LPS-induced p38 MAPK and NF-κB activations were necessary for both IL-33 production and PGE2 generation, and exogenous PGE2 partly reversed the suppression of IL-33 production caused by p38 MAPK and NF-κB inhibition. Mice lacking mPGES-1 showed lower IL-33 levels and attenuated lung inflammation in response to repetitive Alternaria inhalation challenges. Cumulatively, our data demonstrate that endogenous PGE2, EP2 receptors, and EPAC are prerequisites for maximal LPS-induced IL-33 expression and that exogenous PGE2 can amplify IL-33 production via EP2 and EP4 receptors. The ubiquitous induction of mPGES-1-dependent PGE2 may be crucial for innate immune system activation during various IL-33 driven pathologic disorders.
Keywords: cAMP, cytokine, innate immunity, macrophage, prostaglandin, bone marrow macrophages, EPAC, IL-33, PGE2, mPGES-1
Introduction
IL-33 is a recently discovered member of the IL-1 cytokine family that binds to TLR/IL1R2 superfamily receptor ST2 (2–4). IL-33 is constitutively expressed in the nuclei of epithelial cells at barrier sites such as the skin, lung, and intestine and is also expressed by fibroblastic reticular cells and endothelial cells within lymphoid tissues (5). It interacts with histones, promotes chromatin compaction, and retards NF-κB transcriptional activity (5). It can also be released in response to cellular damage or activation and, after proteolytic processing to an active cytokine, can bind to a heterotrimeric receptor composed of T1/ST2 and IL-1RAcP, influencing both innate and adaptive type 2 immune responses (2). In addition to barrier cells, macrophages and dendritic cells can inducibly express IL-33 and contribute to the amplification of inflammatory responses in sepsis, cardiovascular disease, and allergy (6–9). However, little is known about the mechanisms that control such inducible IL-33 expression.
Among the resident hematopoietic cells involved in innate immunity, macrophages are noteworthy for inducible expression of IL-33 in response to LPS in vitro (10–12) and also upon infection with H3N1 or by TLR7/8 agonist stimulation (13). These same stimuli also induce macrophages to generate prostaglandin E2 (PGE2), a lipid mediator that plays critical roles in pyrexia, pain sensation, and inflammation. LPS-stimulated macrophages generate PGE2 from endogenous arachidonic acid that is converted sequentially to prostaglandin H2 (PGH2) and then to PGE2, primarily by the inducible enzymes COX-2 and microsomal PGE2 synthase 1 (mPGES-1), respectively (1). When released, PGE2 acts through four types of E prostanoid receptors (EP1-EP4) and can function as both autocrine as well as paracrine mediators (14). In addition to generating PGE2, macrophages also express EP receptors and respond to endogenous and exogenous PGE2 ex vivo. In particular, by eliciting signaling through cAMP and PKA-dependent transcription factors, PGE2 can amplify the production of IL-6 by macrophages and of IL-23 by dendritic cells but can also suppress the generation of IL-23 and IL-12 by human monocytes. Although exogenous PGE2 can potentiate IL-33 expression by dendritic cells, no studies have addressed whether it regulates IL-33 expression by macrophages. Given the potent capacity for macrophages to generate PGE2, we hypothesized that mPGES-1-dependent endogenous PGE2 may regulate IL-33 production in response to LPS/TLR4-dependent activation and have feedback effects in the presence of stimuli.
In this study, we show that mPGES-1-derived endogenous PGE2 amplifies the generation of IL-33 by bmMFs upon stimulation by LPS. Early PGE2 signaling through EP2 receptors and EPAC, but not PKA, drives this amplification, which is synergistic with the requisite p38 MAPK and NF-κB pathways needed to induce IL-33 expression. In addition, exogenous PGE2 can markedly enhance the LPS-stimulated IL-33 production through both EP2 and EP4 receptors. We also demonstrate that endogenous PGE2 is required for maximal induction of lung IL-33 expression and the attendant immunopathology in response to intranasal Alternaria alternata (Alternaria) inhalation. These amplification mechanisms may also play an important role in sepsis, viral infections, and other circumstances in which PGE2 and IL-33 play roles in orchestrating innate immunologic responses.
Results
Endogenous PGE2 is required for maximal IL-33 production in response to LPS
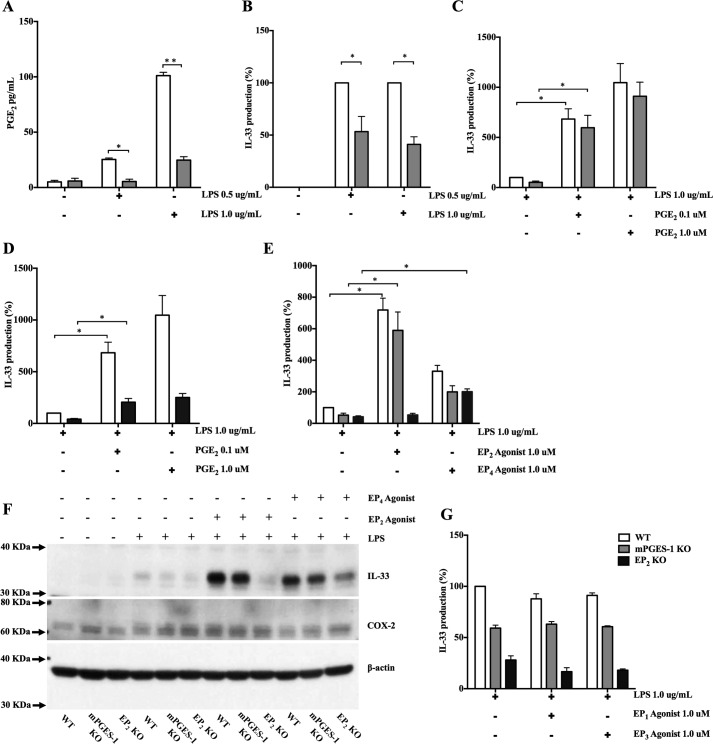
To determine the role of endogenous PGE2 in LPS-mediated IL-33 production, we stimulated bmMFs with LPS at concentrations of 0.5 and 1.0 μg/ml. Supernatants were collected for the measurement of PGE2, and cell lysates were used to measure IL-33 by ELISA. Preliminary experiments demonstrated that IL-33 protein was not detected in resting, unstimulated bmMFs and showed maximal LPS-stimulated IL-33 protein expression by WT macrophages at 8 h (data not shown). Stimulation with LPS dose-dependently induced the release of PGE2 by WT cells, which was significantly reduced in cells lacking mPGES-1 (Fig. 1A). Peak induction of IL-33 protein was significantly impaired in bmMFs from mPGES-1 knock-out mice (Fig. 1B and supplemental Fig. 1A). Exogenous PGE2 did not induce IL-33 production on its own but restored IL-33 generation by mPGES-1 knock-out cells to levels equivalent to WT cells at concentrations as low as 0.001 μm (supplemental Fig. 1B) and also further potentiated IL-33 production by both mPGES-1 KO and WT cells at doses of 0.1 μm and higher (Fig. 1C and supplemental Fig. 1B).
Figure 1.
Endogenous PGE2 is required for IL-33 production in response to LPS and it signals through EP2 and EP4. A, PGE2 levels in cell supernatants from LPS-stimulated WT and mPGES-1 KO bmMFs after 8 h. B, IL-33 protein percentage production levels in corresponding cell lysates by ELISA. C, potentiation of IL-33 production by exogenous PGE2 at concentrations in mPGES-1 KO and WT cells. D, exogenous PGE2 induction to LPS-stimulated to EP2 KO bmMFs. E, enhanced IL-33 production by the EP2 receptor-selective agonist AE1-259-01 and the selective EP4 receptor agonist AE-329. F, WB showing LPS-induced IL-33 protein and COX-2 levels in WT, mPGES-1 KO, and EP2 KO cells in response to EP2 and EP4 agonists. G, IL-33 production in the presence of the EP1 receptor-selective agonist D004 and the EP3 receptor agonist AE248. Data are presented as mean ± S.E. of at least three independent experiments. Statistical significance was determined using unpaired t test and one-sample t test (comparing fixed 100%). p < 0.05 was considered statistically significant. *, p < 0.05; **, p < 0.005.
To determine whether the requirement for endogenous PGE2 to amplify IL-33 generation was due to EP receptor signaling, we used a combination of molecular and pharmacologic approaches. LPS stimulation of bmMFs lacking EP2 receptors demonstrated significantly lower IL-33 protein expression compared with WT cells, similar to the response of the mPGES-1 KO cells (supplemental Fig. 1B and Fig. 1, A and D). However, the potentiation of IL-33 expression by EP2 KO cells in response to exogenous PGE2 was ∼80% reduced compared with WT (Fig. 1D) and mPGES-1 KO cells (supplemental Fig. 1B). Stimulation of WT and mPGES-1 KO bmMFs with the EP2 receptor-selective agonist AE1-259-01 (1.0 μm) in combination with LPS significantly enhanced IL-33 production but failed to do so in EP2 KO cells (Fig. 1E). The selective EP4 receptor agonist AE-329 (1.0 μm) modestly potentiated LPS-induced IL-33 expression in all three genotypes (Fig. 1E). Similar inductions in LPS-induced IL-33 protein by EP2 and EP4 agonists in WT and mPGES-1 KO cells were also observed by Western blotting (WB) (Fig. 1F). EP2 KO cells responded to the EP4 receptor agonist but not to the EP2 agonist (Fig. 1F). Neither the EP1 receptor-selective agonist D004 (1.0 μm) nor the EP3 receptor agonist AE248 (1.0 μm) had an effect on LPS-stimulated production of IL-33 (Fig. 1G).
LPS induces the coordinate expression of PGE2 pathway constituents needed to amplify IL-33 expression
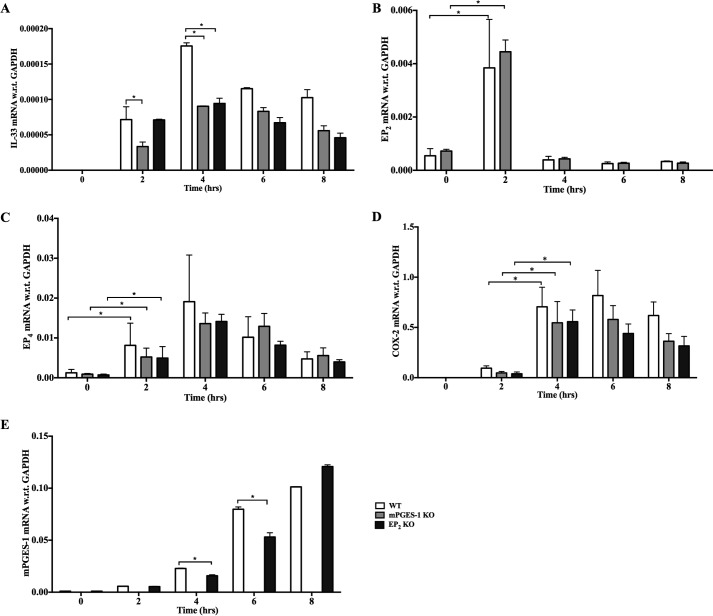
To assess the potential relationship between IL-33 induction and the inducible constituents of the PGE2 synthetic pathway, we monitored the expression of the relevant transcripts by qPCR following stimulation of bmMFs with LPS. LPS-induced expression of IL-33 mRNA in WT cells was evident at 2 h and peaked at 4 h. The induction was significantly blunted in both mPGES-1 KO and EP2 KO cells compared with WT cells (Fig. 2A). Stimulation of WT cells with LPS elicited a strong, transient up-regulation of EP2 receptor mRNA peaking at 2 h (Fig. 2B) and also induced a more sustained increase in EP4 receptor mRNA that peaked at 4 h (Fig. 2C). LPS induced increases in both COX-2 (Fig. 2D) and mPGES-1 (Fig. 2E) mRNA expression that peaked at 4 and 8 h, respectively. The absence of EP2 receptors did not significantly alter the induced expression of COX-2, mPGES-1, or EP4, and the absence of mPGES-1 did not alter the expression of COX-2 or either receptor (Fig. 2, B–E).
Figure 2.
LPS induces coordinate expression of PGE2 pathway constituents needed to amplify IL-33 expression. A–E, we stimulated bmMFs with LPS (1.0 μg/ml) and collected RNA every 2 h until 8 h and monitored (A) IL-33, (B) EP2 receptor, (C) EP4 receptor, (D) COX-2, and (E) mPGES-1 mRNA levels. Data are presented as mean ± S.E. of three independent experiments. Statistical significance was determined using unpaired t test. p < 0.05 was considered statistically significant. *, p < 0.05.
cAMP mediates potentiation of LPS-induced IL-33 production
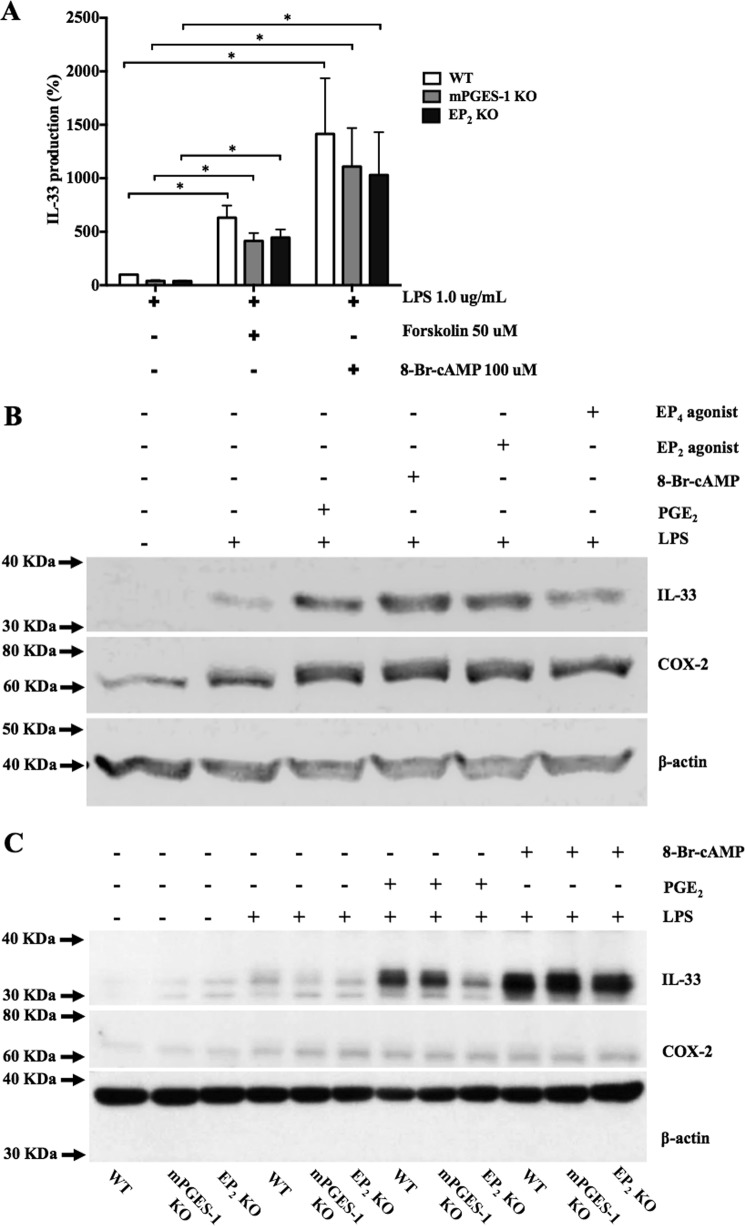
Both EP2 and EP4 receptors activate adenylyl cyclase to induce cAMP accumulation (15). To determine whether cAMP was involved in potentiation of IL-33 production, we stimulated bmMFs with LPS in the absence or presence of the pharmacologic adenyl cyclase activator forskolin (50.0 μm) or a cell-permeable cAMP analog, 8-bromo-cAMP (100.0 μm). Both agents significantly potentiated LPS-induced IL-33 expression in mPGES-1 KO and EP2 KO cells and also potentiated the response of WT cells (Fig. 3A). WBs confirmed that PGE2, 8-bromo-cAMP, EP2, and EP4 agonists each increased the induction of LPS-induced IL-33 protein levels in WT cells (Fig. 3B). EP2 KO cells responded to 8-bromo-cAMP but showed a reduced response to PGE2 (Fig. 3C). Neither forskolin nor 8-bromo-cAMP altered the LPS-induced increases in COX-2 protein (Fig. 3, B and C).
Figure 3.
cAMP mediates potentiation of LPS-induced IL-33 production. A, IL-33 production by LPS-stimulated (1.0 μg/ml) bmMFs in the presence of 50.0 μm forskolin and 100 μm 8-bromo-cAMP (8-Br-cAMP). Data are presented as mean ± S.E. of three independent experiments. Statistical significance was determined using one-sample t test (comparing fixed 100%). p < 0.05 was considered statistically significant. *, p < 0.05. B, WB showing LPS-induced IL-33 protein levels in WT cells further enhanced by addition of PGE2, 8-Br-cAMP, EP2 and EP4 agonists. C, Western blots showing the effects of PGE2 and 8-Br-cAMP on IL-33 and COX-2 expression in cells from the indicated genotypes.
PGE2-enhanced IL-33 production involves EPAC but not PKA or PI3K
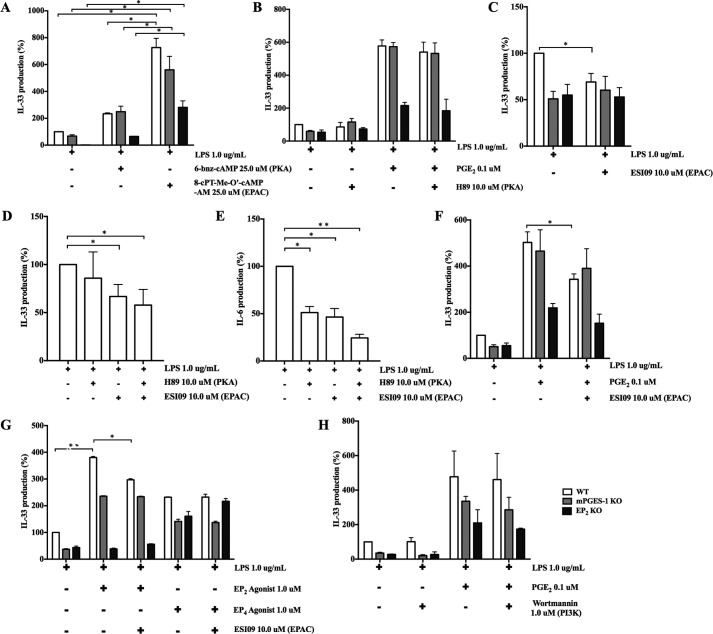
cAMP activates PKA and downstream transcription factors such as CREB. To determine whether PKA and associated transcription factors were involved in IL-33 induction, we used cAMP analogs selective for PKA and EPAC, respectively. Both the PKA-selective cAMP analogue (6-bnz-cAMP, 25.0 μm) and the EPAC-selective cAMP analogue 8-pCPT-2-O-Me-cAMP-AM (25.0 μm) potentiated the production of IL-33 by LPS-stimulated bmMFs (Fig. 4A), although the potentiation was significantly greater for 8-pCPT-2-O-Me-cAMP-AM. To investigate further, we stimulated bmMFs in the presence or absence of PKA inhibitors. H89 (10.0 μm) failed to inhibit the production of IL-33 by LPS-stimulated bmMFs of any genotype and also did not alter its potentiation by exogenous PGE2 (Fig. 4B). Similarly, a second PKA inhibitor, KT5720, failed to alter LPS-induced IL-33 production (supplemental Fig. 1C). Moreover, the modest potentiation of IL-33 production by 6-bnz-cAMP was insensitive to H89 and KT5720, suggesting an off-target effect of this agonist (supplemental Fig. 1D). Stimulation of WT bmMFs with LPS in the presence of an EPAC-selective inhibitor (ESI09, 10.0 μm) significantly decreased the production of IL-33 to a level comparable with those of mPGES-1 KO and EP2 KO bmMFs and did not alter IL-33 generation in either knockout strain (Fig. 4C). The addition of H89 to ESI09 produced no further inhibition of LPS-induced IL-33 production compared with ESI09 alone (Fig. 4D). In contrast, LPS-induced production of IL-6 was significantly inhibited by both H89 and ESI09, with an additive effect of the two inhibitors (Fig. 4E). ESI09 also modestly but significantly reduced PGE2- and AE1-259-01-enhanced IL-33 production by WT bmMFs (Fig. 4, F and G) but had no effect on potentiation by AE-329 (Fig. 4G).
Figure 4.
PGE2-enhanced IL-33 production involves EPAC but not PKA or PI3K. A, IL-33 production by LPS-stimulated bmMFs (1.0 μg/ml) in the presence of selective cAMP analogs for PKA and EPAC. B, levels of IL-33 production by LPS-stimulated bmMFs with or without PGE2 in the presence of the PKA inhibitor H89 (10.0 μm). C, effect of ESI09 (10.0 μm) on IL-33 production by LPS-stimulated bmMFs. D, effects of H89 and ESI09 alone and in combination on LPS-induced IL-33 production by WT bmMFs. E, effects of H89 and ESI09 on LPS-induced IL-6 production by the same samples as in D. F, effects of ESI09 on PGE2-enhanced IL-33 production by bmMFs. G, effects of ESI09 on potentiation of IL-33 production by WT bmMFs stimulated with AE1-259-01 but not with AE-329. H, effect of wortmannin (1.0 μm) on LPS-induced IL-33 production and its potentiation by PGE2. Data are presented as mean ± S.E. of at least three independent experiments. Statistical significance was determined using one-sample t test (comparing fixed 100%). p < 0.05 was considered statistically significant. *, p < 0.05; **, p < 0.005.
Given the ability of EP4 receptors to elicit PI3K activation (16–18), we examined the effect of wortmannin (1.0 μm) on LPS-induced IL-33 production and its potentiation by PGE2. Wortmannin had no effect on LPS-stimulated IL-33 production by bmMFs, irrespective of the absence or presence of PGE2 (Fig. 4H). Additionally, we used inhibitors of PKB/AKT signaling that is downstream of PI3K, namely FPA 124 (100.0 nm) and 10-DEBC (5.0 μm), and again found no effect on IL-33 production both in absence or presence of PGE2 (supplemental Fig. 1, E and F).
Gene silencing confirms that PGE2 enhances IL-33 production in part through EPAC
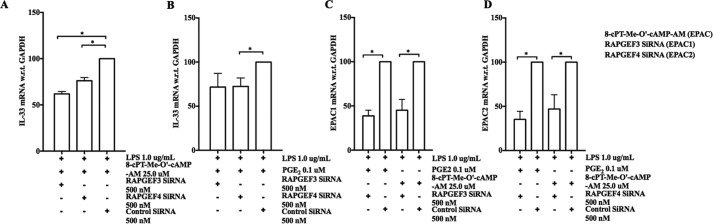
To verify the involvement of EPAC in the potentiation of LPS-induced IL-33 expression, we performed EPAC gene-silencing studies. We used Rapgef3 and Rapgef4 siRNA pools to inhibit the expression of EPAC1 and EPAC2, respectively. We studied the effect of EPAC knockdown on enhancement of LPS-induced IL-33 expression by PGE2 (0.1 μm) and 8-pCPT-2-O-Me-cAMP-AM (25.0 μm) in WT bmMFs. Compared with bmMFs transfected with control siRNA, bmMFs transfected with EPAC1 or EPAC2 siRNAs displayed significantly diminished potentiation of IL-33 mRNA expression in the presence of 8-pCPT-2-O-Me-cAMP-AM (Fig. 5A). Transfection with EPAC2 siRNA also significantly decreased the expression of IL-33 by LPS-stimulated cells in the presence of PGE2, whereas EPAC1 siRNA produced a modest statistically insignificant effect (Fig. 5B). qPCR confirmed that each siRNA pool successfully suppressed the expression of their respective targets (Fig. 5, C and D). Co-transfection of bmMFs with both siRNAs resulted in profound suppression (∼70%) of IL-33 expression. However, the efficacy of EPAC2 knockdown under these conditions was severely limited (data not shown).
Figure 5.
Gene silencing confirms that PGE2 enhances IL-33 production in part through EPAC. A and B, effect of EPAC knockdown on 8-pCPT-2-O-Me-cAMP-AM-enhanced (A) or PGE2-enhanced (B) IL-33 production. C and D, mRNA levels of EPAC1 (C) and EPAC2 (D) in the presence of the respective siRNAs in PGE2/8-pCPT-2-O-Me-cAMP-AM-stimulated bmMFs. Data are presented as mean ± S.E. of three independent experiments. Statistical significance was determined using unpaired t test. p < 0.05 was considered statistically significant. *, p < 0.05.
PGE2-mediated potentiation of LPS-stimulated IL-33 production is independent of p38 MAPK and NF-κB activation
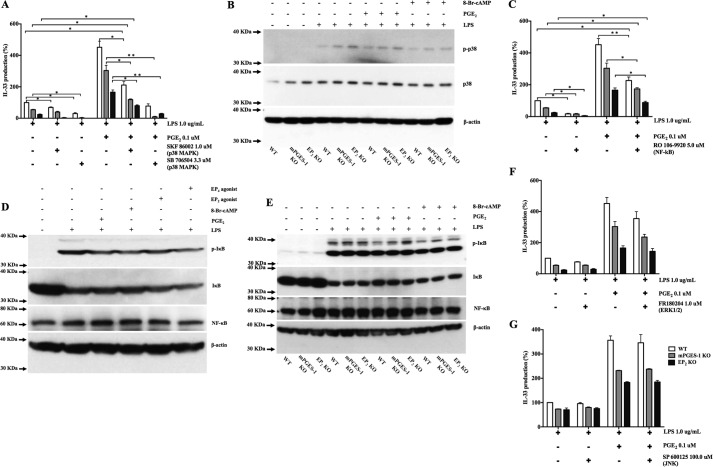
Stimulation of macrophages with LPS via TLR4 activates p38 MAPK and NF-κB, which synergize to induce the expression of proinflammatory cytokines and enzymes (19, 20). We therefore sought to determine whether endogenous or exogenous PGE2 modulated MAPK or NF-κB activation to potentiate LPS-induced IL-33 expression. Two different inhibitors of p38 MAPK, SKF 86002 (1.0 μm) and SB 706504 (3.3 μm), inhibited LPS-induced IL-33 production (Fig. 6A). Stimulation with LPS induced p38 phosphorylation in all three bmMF genotypes (Fig. 6B). The NF-κB inhibitor RO 106-9920 (5.0 μm) significantly suppressed the LPS-induced expression of IL-33 in all three genotypes (Fig. 6C). LPS stimulation strongly induced phosphorylation of IκB in WT cells (Fig. 6D) and also in both KOs (Fig. 6E). Both the p38 inhibitor SB 706504 and the NF-κB inhibitor RO 106-9920 significantly suppressed LPS-induced production of PGE2 (supplemental Fig. 1G). Exogenous PGE2 partially reversed the suppression of IL-33 induction by both p38 inhibition and NF-κB inhibition (Fig. 6, A and C). Neither PGE2 nor selective EP2 or EP4 agonists altered the phosphorylation of p38 (Fig. 6B) or IκB, whereas 8-Br-cAMP tended to decrease p38 phosphorylation (Fig. 6D). Neither the ERK1/2 inhibitor FR 180204 (1.0 μm) nor the JNK inhibitor SP 600125 (100.0 μm) altered LPS-induced IL-33 expression in any genotype either in the absence or presence of PGE2 (Fig. 6, F and G).
Figure 6.
PGE2-mediated potentiation of LPS-stimulated IL-33 production is independent of p38 MAPK and NF-κB activation. A, effect of the p38 MAPK inhibitors SKF 86002 and SB706504 on IL-33 production by LPS (1.0 μg/ml) with or without PGE2. B, protein lysates from WT, mPGES-1 KO, and EP2 KO bmMFs 30 min after LPS stimulation were analyzed by WB, showing levels of phospho-p38 MAPK in WT, mPGES-1 KO, and EP2 KO bmMFs. C, IL-33 production by bmMFs in the presence of NF-κB inhibitor. D, NF-κB activation levels upon LPS stimulation in WT bmMFs in the presence of other selective agonists. E, WB analysis showing NF-κB activation by IκB phosphorylation in WT, mPGES-1 KO, and EP2 KO cells upon LPS stimulation and PGE2 addition. F, IL-33 levels with the ERK1/2 inhibitor FR180204 (1.0 μm) in the absence or presence of PGE2. G, effect of the JNK signaling inhibitor SP 600125 (100.0 μm) on IL-33 levels. Data are presented as mean ± S.E. of at least three independent experiments. Statistical significance was determined using one-sample t test (comparing fixed 100%). p < 0.05 was considered statistically significant. *, p < 0.05; **, p < 0.005.
Endogenous PGE2 is necessary for inducible IL-33 expression and type 2 immunopathology in response to Alternaria
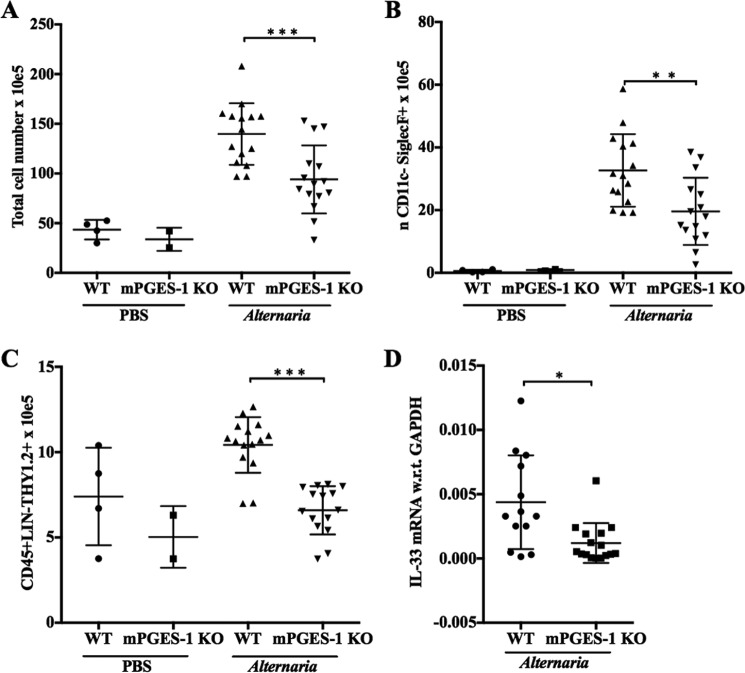
Alternaria alternata is a mold that induces severe asthma exacerbations in humans and marked IL-33-dependent immunopathology in mice. To determine whether PGE2 is involved in this immunopathology, we administered an extract of Alternaria to WT and mPGES-1 KO mice intranasally. Compared with WT mice, mPGES-1-null mice showed significantly reduced lung inflammation, as indicated by total lung cells and eosinophils (Fig. 7, A and B). The numbers of group 2 innate lymphoid cells (ILC2s), a major target of IL-33, were reduced in the lungs of mPGES-1 KO mice compared with WT mice (Fig. 7C). Single-lung cell suspensions from Alternaria-treated mPGES-1 KO mice expressed significantly lower levels of IL-33 mRNA than WT controls (Fig. 7D), whereas IL-33 transcripts from PBS-challenged WT and mPGES-1 KO mouse cells were below detection levels. Similar trends in inflammation, ILC2 expansion, and IL-33 expression were identified in EP2 receptor knock-out mice (n = 1 experiment, data not shown).
Figure 7.
Endogenous PGE2 is necessary for inducible IL-33 expression and type 2 immunopathology in response to Alternaria. WT and mPGES-1 KO mice were treated with four intranasal doses of Alternaria (12 μg) over 9 days. On day 10, mice from the indicated groups were euthanized for the studies. A–C, total lung cell numbers (A), total lung eosinophils (B), and lung ILC2 numbers (C) in WT and mPGES-1 KO mice in PBS and Alternaria-challenged mice. D, qPCR quantification of the IL-33 mRNA transcript in lung single-cell suspensions in WT and mPGES-1 KO Alternaria-challenged mice (IL-33 transcripts were below detection levels in PBS-challenged mice). Data are presented as mean ± S.D. of at least three independent experiments. Statistical significance was determined using an unpaired t test. *, p < 0.05; **, p < 0.005; ***, p < 0.0005.
Discussion
Originally identified as a DNA-binding protein, IL-33 is now well recognized as an inducer and amplifier of innate immune responses by activating ST2-expressing hematopoietic and structural cells. Although IL-33 is strongly implicated in type 2 immune responses to allergens and parasites, driving ILC2 expansion and cytokine production, it is also elevated in the serum of patients with myocardial infarction, congestive heart failure, and trauma/septic shock (21). Experimental models also demonstrate that IL-33 is critical for the induction of local inflammation at the onset of colitis and LPS-induced endotoxic shock (8), both of which also involve prominent production of PGE2 (22, 23). Macrophages are important innate hematopoietic effector cells that are major sources of PGE2 during inflammatory responses because of their capacity for inducible expression and function of COX-2 and mPGES-1. Macrophages can also generate IL-33 inducibly, and this induction can be facilitated by pharmacologic enhancement of intracellular cAMP levels. Because PGE2 can modulate the generation of certain cytokines through both autocrine and paracrine pathways (and frequently through cAMP), we sought to determine whether the endogenous PGE2 synthetic pathway and EP receptors mediated an amplification step for IL-33 expression in response to LPS, the ligand for TLR4, and a key inducer of macrophage activation in sepsis and antimicrobial responses.
We induced IL-33 protein and mRNA expression by bmMFs, which do not constitutively express IL-33, using LPS as a physiologically relevant TLR4 ligand. To identify potential contributions from endogenous PGE2, we compared the responses of WT cells to those derived from mice lacking mPGES-1. mPGES-1 KO cells showed significantly reduced LPS-mediated induction of IL-33 at both the RNA and protein levels (Figs. 1B and 2A) as well as the anticipated impairment in PGE2 generation during the same time frame compared with WT control cells (Fig. 1A). The impairment of IL-33 induction in bmMFs lacking EP2 receptors, which bind PGE2 with high affinity, was comparable (>50% of the control, Fig. 1D) with that observed in mPGES-1 KO cells (Fig. 1B). Notably, EP2 receptor mRNA expression was rapidly induced by LPS (Fig. 2B), peaking before the maximal induction of COX-2 (Fig. 2D), mPGES-1 (Fig. 2E), and IL-33 (Fig. 2A) mRNAs. Both exogenous PGE2 (Fig. 1C) and an EP2 receptor-selective agonist (Fig. 1, E and F) restored LPS-induced IL-33 expression in mPGES-1 KO cells, but not in EP2 receptor KO cells, to WT levels. Collectively, these observations are consistent with a critical role for endogenous PGE2 in augmenting IL-33 expression, with EP2 receptors being functionally dominant. The rapid up-regulation of EP2 receptor expression by LPS may ensure maximal cellular sensitivity to PGE2 prior to the peak levels of its production, permitting a substantial autocrine contribution to IL-33 expression.
Because both EP2 and EP4 receptors activate adenylyl cyclases, we suspected that the cAMP-dependent effectors PKA and/or EPAC would account for the effects of endogenous and exogenous PGE2. Indeed, pharmacologic activation of adenylyl cyclase with forskolin or stimulation with the cell-permeable cAMP mimic 8-Br-cAMP enhanced the LPS-induced expression of IL-33 in all three genotypes (Fig. 3A-3C), effectively bypassing the deficiency in endogenous EP receptor signaling. TLR4 signaling activates the PKA-dependent transcription factor CREB in peritoneal macrophages and a macrophage cell line, and this activation (and the associated IL-33 generation) is potentiated by cAMP-elevating agents. Several lines of evidence suggest that this is not the most relevant pathway involved in PGE2-mediated potentiation of IL-33 production by bmMFs. First, the absence of mPGES-1 and EP2 receptors did not alter LPS-induced CREB phosphorylation, and neither exogenous PGE2 nor 8-Br-cAMP potentiated this phosphorylation (supplemental Fig. 2A). Second, two different PKA inhibitors (H89 and KT5720, Fig. 4B and supplemental Fig. 1C, respectively) failed to suppress LPS-induced IL-33 expression in any genotype either in the absence or presence of exogenous PGE2. In contrast, H89 substantially suppressed the induced expression of IL-6, another cAMP-facilitated cytokine, under the same conditions (Fig. 4, D and E). Third, potentiation of LPS-induced IL-33 generation by a PKA-selective cAMP analogue was modest compared with that induced by the EPAC-selective agonist (Fig. 4A) and was not inhibited by either PKA antagonist, suggesting an off-target effect (supplemental Fig. 1D).
In contrast to the lack of effect of PKA inhibition, EPAC inhibition suppressed the LPS-induced expression of IL-33 in WT cells (Fig. 4C) to levels equivalent to those induced in cells lacking either mPGES-1 or EP2 receptors. Notably, EPAC inhibition also decreased the potentiation of LPS-induced IL-33 by exogenous PGE2 (Fig. 4F) and by an EP2 agonist (Fig. 4G) but not by an EP4 agonist (Fig. 4G), suggesting the involvement of an additional EP4-driven pathway activated by exogenous PGE2. The results of the siRNA knockdown studies were consistent with a significant contribution from EPAC to the PGE2- and cAMP-dependent potentiation of LPS-induced IL-33 expression (Fig. 5). We performed EPAC1 and EPAC2 double knockdown as well by electroporating both EPAC1 and EPAC2 siRNAs to WT bmMFs. As expected, EPAC1 and EPAC2 double knockdown significantly inhibited the production of both IL-33 mRNA and IL-6 secretion (∼70% for each), but there was a limitation of the assay, as EPAC1 and EPAC2 double electroporation reduced the efficiency of EPAC2 knockdown (data not shown). Thus, although endogenous PGE2 promotes LPS-induced IL-33 expression primarily through EP2 receptors and EPAC, exogenous PGE2 can activate an additional cAMP-dependent pathway that depends, at least in part, on EP4 receptors. Furthermore, this additional pathway does not involve PI3K (Fig. 4H) or its downstream effectors (Supplemental Figs. 1, D and E, and 2B).
TLR signaling elicits expression of cytokines and other proinflammatory proteins by activating NF-κB-dependent transcription and p38 MAPK-dependent mRNA stabilization. Our studies suggest that these pathways were also essential for LPS-mediated induction of IL-33 expression (Fig. 6, A–E) but were not modulated by PGE2 or EP receptor signaling. The lack of difference in LPS-induced IκB and p38 phosphorylation between WT, EP2 KO, and mPGES-1 KO cells demonstrates that endogenous PGE2 is not required for full activation of these pathways. Indeed, both exogenous PGE2 and pharmacologic activation of cAMP-dependent signaling pathways slightly attenuated p38 and NF-κB activation. The modulation of NF-κB by cAMP pathways is complex and dependent on both stimulus and cell type, and our findings are consistent with previous reports regarding LPS-stimulated macrophages (24). Importantly, as shown in supplemental Fig. 1B, exogenous PGE2 elicits significant potentiation of LPS-induced IL-33 production at low nanomolar doses. The exquisite sensitivity of these cells to potentiation of IL-33 production by PGE2 likely permits the EPAC-dependent signal to override the modest PGE2-dependent suppression of the requisite NF-κB and p38 signals. Notably, although selective inhibitors of NF-κB and p38 markedly suppressed LPS-induced IL-33 expression, exogenous PGE2 still induced substantial potentiation of IL-33 expression under these conditions. Given that synergism between signaling pathways is most evident at submaximal levels of input, the modest suppression of NF-κB by PGE2 may enhance the extent to which its subsequent activation of EPAC amplifies the response. Because NF-κB and p38 activation are also required for endogenous PGE2 production (supplemental Fig. 1G), the effect of the inhibitors is likely to reflect a loss of both direct (IL-33 transcription and mRNA stabilization) and indirect (potentiation by endogenous PGE2) inputs required for maximal IL-33 expression.
Finally, we sought an in vivo context to determine whether PGE2 is necessary to amplify IL-33-driven immunopathology. Although a single dose of Alternaria extract to naive mice induces the release of preformed IL-33 from epithelial cells, repeated intranasal dosing up-regulates lung IL-33 expression (and attendant ILC2 expansion and eosinophil recruitment) in part by inducing IL-33-expressing macrophages (25–29). Indeed, lung inflammation, ILC2 expansion, and IL-33 expression were all lower in mPGES-1 KO mice than in WT controls (Fig. 7). These findings support the physiological function of PGE2 in amplifying IL-33 expression, which, in turn, drives the downstream IL-33-dependent effectors.
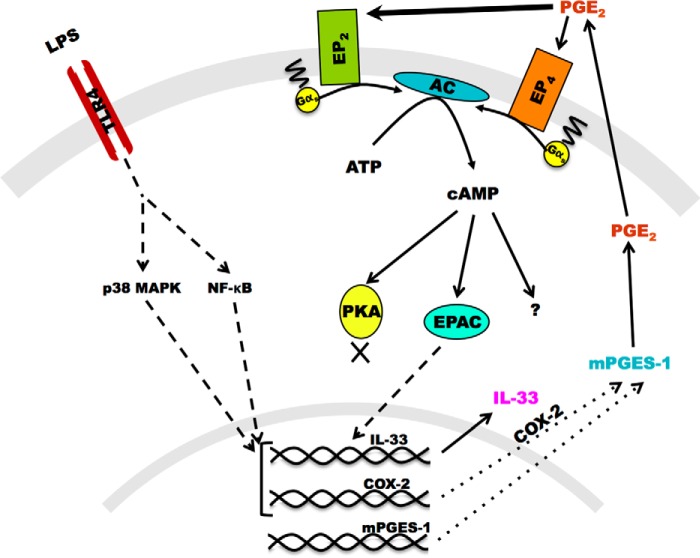
Our study demonstrates that endogenous PGE2, produced through the inducibly expressed COX-2/mPGES-1 system, uses EP2 receptors and EPAC to orchestrate a pathway that amplifies IL-33 expression downstream of p38 and NF-κB in mouse bmMFs (Fig. 8). To our knowledge, this is the first study showing the involvement of exogenous PGE2 in up-regulating LPS-induced IL-33 production in macrophages. This autocrine system may facilitate IL-33-dependent features during early innate immune responses. We postulate that PGE2 derived from paracrine sources may amplify macrophage production of IL-33 during Th2 inflammation, inflammatory processes in sepsis, asthma, inflammatory bowel disease, and endotoxin shock, where PGE2 production is an integral part of the pathology (22, 23, 30, 31). COX-2 inhibitors, which prevent the synthesis of PGE2, show efficacy in inflammatory diseases such as arthritis but can also increase the risk of adverse cardiovascular events. Given that IL-33 is proinflammatory in many contexts but also cardioprotective, it is tempting to speculate that some of the observed clinical effects of COX-2 inhibitors may reflect the loss of PGE2-dependent amplification mechanisms for IL-33 expression.
Figure 8.
Schematic of the tentative pathway involved in IL-33 augmentation by PGE2. Shown is a schematic of IL-33 augmentation by LPS-stimulated bmMFs in response to PGE2 acting through EPAC but not PKA.
Experimental procedures
Materials
RPMI 1640 and Dulbecco's PBS were purchased from Corning (Manassas, VA). FBS was purchased from Sigma-Aldrich (St. Louis, MO) and was used after heat inactivation and filtration. Pharmacological agents, including forskolin, 8-bromo-cAMP sodium salt, H89 HCl, 8-pCPT-2-O-Me-cAMP-AM, 6-Bnz-cAMP sodium salt, ESI09, wortmannin, FPA 124, 10-DEBC HCl, FR180204, SKF 86002 diHCl, SB706504, SP 600125, RO 106–9920, and KT5720, were purchased from Tocris Biosciences (Bristol, UK). LPS from Escherichia coli 0111:B4 was obtained from Sigma-Aldrich. PGE2 and PGE2 ELISA kits were purchased from Cayman Chemicals (Ann Arbor, MI). The prostanoid receptor agonists EP1-EP4 were purchased from Ono Pharmaceuticals (Osaka, Japan). Different ELISA kits were purchased: IL-33 was from R&D Systems (Minneapolis, MN), IL-6 from eBiosciences (San Diego, CA), and phospho-Akt and phospho-CREB from Cell Signaling Technology (Danvers, MA). Antibodies for Western blotting were purchased as follows: IL-33 (AF3626) from R&D Systems; COX-2 (4842), β-actin (8457), phospho-ERK1/2 (4377S), total ERK1/2 (9102S), phospho-p38 MAPK (4511), total p38 MAPK (8690), phospho-IκB (9246), total IκB (4814), and NF-κB (8242) from Cell Signaling Technology; and all HRP-conjugated secondary antibodies (170-6515 and 172-1034) from Bio-Rad (Hercules, CA). For gene-silencing experiments, EPAC (Rapgef3), EPAC2 (Rapgef4), and the control pool of non-targeting siRNAs were procured from GE Dharmacon (Lafayette, CO). The primers used in this study were synthesized from IDT Technologies (Coralville, IA) as the sequences provided in supplemental Fig. 1F, except for the qPCR primers for Rapgef3 and Rapgef4, which were ordered from Qiagen (Valencia, CA). For in vivo studies, the following reagents were purchased: A. alternata cellular extract (Greer Laboratories, Lenoir, NC), Collagenase IV (Worthington), DNase I (Roche), rat anti-mouse CD16/CD32 (BD Biosciences), CD45 PercPCy5 (clone 30-F11, BioLegend, San Diego, CA), CD19 FITC (6D5, BioLegend), CD3 FITC (145–2C11, BioLegend), CD11b FITC (M1/70, BioLegend), CD11c PE-Cy7 FITC (N418, BioLegend), Ly6G/C FITC (RB6-8C5, eBioscience, San Diego, CA), Nk1.1 FITC (PK136, BioLegend), FceR1 FITC (MAR1, BioLegend), Siglec-F APC (E50-2440, BD Biosciences), and Thy 1.2 APC (53–2.1, eBioscience).
Bone marrow macrophages
All mouse strains used were described previously (14) and were on the C57BL/6 background and housed at Charles River. Bone marrow was harvested humanely from 6- to 8-week-old female mice with procedures approved by the Animal Care and Use Committee of the Dana-Farber Cancer Institute. Bone marrow cells were cultured with 50 ng/ml of recombinant murine MCSF (rmMCSF) for 7 days at 37 °C in a humidified CO2 (5%) incubator with fresh medium changed on day 2 and day 5. On day 7, bmMFs were harvested by using StemPro Accutase (Thermo Fisher Scientific, Grand Island, NY) and plated on tissue culture plates at 1 × 106 cells/ml in RPMI 1640 complete medium. When adhered, cells were stimulated with LPS with or without pharmacological agents for 8 h unless stated otherwise. Supernatants were collected for measurements of PGE2 and IL-6, and cells were lysed with Pathscan cell lysis buffer from Cell Signaling Technology by two cycles of freeze-thaw, and lysates were analyzed either by ELISA or WB.
ELISA and Western blotting
ELISAs for IL-6, PGE2 IL-33, and phospho-CREB, and phospho-AKT were done according to the protocol of the manufacturer. WT bmMFs produced varying quantities (40–150 pg/ml) of IL-33 in response to LPS, well within the detection limits of the ELISA kit (IL-33 ELISA, R&D Systems, detection range of 31.2–2000 pg/ml). Because the relative potentiation of IL-33 production by PGE2 (its reductions in mPGES-1 and EP2 KO cells) showed minimal interexperimental variability, we normalized the data to LPS-stimulated WT cells for each experiment and displayed the mean data as a percent change. Representative amounts of IL-33 produced by bmMFs upon LPS stimulation are shown in supplemental Fig. 1A. For WB, lysates were prepared and quantified using BCA protein estimation from Thermo Fisher Scientific. 30–50 μg of total protein for each sample with 4× NuPage sample preparation buffer was run on Novex 4–20% Tris/glycine gels from Thermo Fisher Scientific and post-run transferred onto a methanol-charged PVDF membrane. Membranes were blocked for 2 h with 5% skim milk in TBST (TBS-Tween 20) and processed further according to the recommendations of the manufacturer for primary and secondary antibodies.
Real-time PCR and gene silencing
For real-time PCR studies, kits and reagents from Qiagen were used. RNAs were extracted using the RNeasy kit and post-quantification subjected to first-strand cDNA synthesis using the RT2 first strand kit. qPCR assays were done with the respective primers using RT2 SYBR Green qPCR Master Mix on Stratagene qPCR systems. Relative mRNA levels were determined in comparison with GAPDH. For Rapgef3- and Rapgef4-silencing experiments, ON-TARGETplus pools of the respective siRNAs, along with a non-targeting control, were electroporated to bmMFs at a concentration of 500 nm/1 × 106 cells using the Nucleofector mouse macrophage electroporation kit from Lonza (Atlanta, GA). 24 h after electroporation, cells were supplied with RPMI 1640 complete medium with rmMCSF and allowed to recover for another 24 h. After a total of 48 h, cells were subjected to LPS stimulation along with other agonists.
Intranasal inhalation challenge and flow cytometry
C57B/L6 WT and mPGES-1 KO or EP2 KO mice (9–11 weeks old) were given 12 μg of A. alternata cellular extract (Alternaria) in 20 μl of PBS or PBS alone by intranasal inhalation on days 0, 3, 6, and 9 as described previously (32) and euthanized 18 h after the last dose. Lungs were manually divided into pieces ∼1.0 mm3 in size, followed by digestion in RPMI containing 428 units/ml Collagenase IV and 20 mg/ml DNase I (30 min, 37 °C). After digestion, suspensions were strained and passed through 70 μm sterile filters and washed with PBS, followed by RBC lysis. After RBC lysis, the suspensions from individual mice were washed, strained again, and counted (for total cell counts). At this point, about 2 million cells from each mouse were aliquoted for RNA extraction later. From the remaining cells, 1 million cells per mouse were washed again and blocked (1 h, 4 °C) with 1% rat anti-mouse CD16/CD32 (BD Biosciences) and 10% FBS and then stained (1 h, 4 °C) with appropriate antibodies. Single-cell suspensions were stained with CD45 PercPCy5.5, lineage antibodies-FITC (CD19, CD3, CD11b, CD11c, Ly6G/C, Nk1.1, and FceR1), Siglec-F PE, and Thy 1.2 APC. Post-staining, cells were fixed with 4% paraformaldehyde, and data were acquired using a FACSCanto II flow cytometer with FACSDiva software. Analysis was done with FlowJo (Tree Star, Ashland, OR).
Statistical analysis
Data are presented as mean ± S.E. of at least three independent experiments unless otherwise stated. Statistical significance was determined using unpaired t test and one-sample t test (comparing fixed 100%). p <0.05 was considered statistically significant (*, p < 0.05; **, p < 0.005).
Author contributions
J. A. B. supervised the study. J. A. B., S. K. S., and B. B. designed the study and analyzed the results. S. K. S. carried out all experiments except for the IL-6 ELISA, which was done by H. R. The manuscript was written by S. K. S. and J. A. B. All authors approved the final version.
Supplementary Material
Acknowledgments
We thank Amanda Paskavitz for technical assistance.
This work was supported by National Institutes of Health Grants AI078908, AI095219, AT002782, AI082369, HL111113, and HL117945 and by the Vinik family. The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This article contains supplemental Figs. 1 and 2.
- TLR
- toll-like receptor
- PGE2
- prostaglandin E2
- mPGES
- microsomal prostaglandin E2 synthase
- EP
- E prostanoid
- bmMF
- bone marrow-derived murine macrophage
- EPAC
- exchange protein activated by cAMP
- WB
- Western blotting
- qPCR
- quantitative PCR
- CREB
- cAMP-response element-binding protein
- ILC2
- group 2 innate lymphoid cell
- rmMCSF
- recombinant murine macrophage colony-stimulating factor
- 6-bnz-cAMP
- N6-benzoyladenosine-3′, 5′-cyclic monophosphate
- 8-pCPT-2-O-Me-cAMP AM
- 8-(4-chlorophenylthio)-2′-O-methyladenosine-3′, 5′-cyclic monophosphate
- FPA 124
- dichloro[(2Z)-2-[(4-oxo-4H-1-benzopyran-3-yl)methylene]hydrazinecarbothioamide
- 10-DEBC
- 10-[4′-(N,N-Diethylamino)butyl]-2-chlorophenoxazine hydrochloride
- APC
- allophycocyanin.
References
- 1. Duan Y., Chen F., Zhang A., Zhu B., Sun J., Xie Q., and Chen Z. (2014) Aspirin inhibits lipopolysaccharide-induced COX-2 expression and PGE2 production in porcine alveolar macrophages by modulating protein kinase C and protein tyrosine phosphatase activity. BMB Rep. 47, 45–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hardman C., and Ogg G. (2016) Interleukin-33, friend and foe in type-2 immune responses. Curr. Opin. Immunol. 42, 16–24 [DOI] [PubMed] [Google Scholar]
- 3. Baekkevold E. S., Roussigné M., Yamanaka T., Johansen F.-E., Jahnsen F. L., Amalric F., Brandtzaeg P., Erard M., Haraldsen G., and Girard J.-P. (2003) Molecular characterization of NF-HEV, a nuclear factor preferentially expressed in human high endothelial venules. Am. J. Pathol. 163, 69–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang P., Zhu F., and Konstantopoulos K. (2010) Prostaglandin E 2 induces interleukin-6 expression in human chondrocytes via cAMP/protein kinase A- and phosphatidylinositol 3-kinase-dependent NF-κB activation. Am. J. Physiol. Cell Physiol. 298, 1445–1456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schwartz C., O'Grady K., Lavelle E. C., and Fallon P. G. (2016) Interleukin 33: an innate alarm for adaptive responses beyond Th2 immunity-emerging roles in obesity, intestinal inflammation, and cancer. Eur. J. Immunol. 10.1002/eji.201545780 [DOI] [PubMed] [Google Scholar]
- 6. Mirchandani A. S., Salmond R. J., and Liew F. Y. (2012) Interleukin-33 and the function of innate lymphoid cells. Trends Immunol. 33, 389–396 [DOI] [PubMed] [Google Scholar]
- 7. Schmitz J., Owyang A., Oldham E., Song Y., Murphy E., McClanahan T. K., Zurawski G., Moshrefi M., Qin J., Li X., Gorman D. M., Bazan J. F., and Kastelein R. A. (2005) IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity 23, 479–490 [DOI] [PubMed] [Google Scholar]
- 8. Oboki K., Ohno T., Kajiwara N., Arae K., Morita H., Ishii A., Nambu A., Abe T., Kiyonari H., Matsumoto K., Sudo K., Okumura K., Saito H., and Nakae S. (2010) IL-33 is a crucial amplifier of innate rather than acquired immunity. Proc. Natl. Acad. Sci. U.S.A. 107, 18581–18586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nabe T., Wakamori H., Yano C., Nishiguchi A., Yuasa R., Kido H., Tomiyama Y., Tomoda A., Kida H., Takiguchi A., Matsuda M., Ishihara K., Akiba S., Ohya S., Fukui H., et al. (2015) Production of interleukin (IL)-33 in the lungs during multiple antigen challenge-induced airway inflammation in mice, and its modulation by a glucocorticoid. Eur. J. Pharmacol. 757, 34–41 [DOI] [PubMed] [Google Scholar]
- 10. Polumuri S. K., Jayakar G. G., Shirey K. A., Roberts Z. J., Perkins D. J., Pitha P. M., and Vogel S. N. (2012) Transcriptional regulation of murine IL-33 by TLR and non-TLR agonists. J. Immunol. 189, 50–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ohno T., Oboki K., Kajiwara N., Morii E., Aozasa K., Flavell R. A., Okumura K., Saito H., and Nakae S. (2009) Caspase-1, caspase-8, and calpain are dispensable for IL-33 release by macrophages. J. Immunol. 183, 7890–7897 [DOI] [PubMed] [Google Scholar]
- 12. Yanagawa Y., Hiraide S., Iizuka K., and Yanagawa Y. (2016) Cyclic AMP signaling enhances lipopolysaccharide sensitivity and interleukin-33 production in RAW264. 7 macrophages. Microbiol. Immun. 10.1111/1348-0421.12381 [DOI] [PubMed] [Google Scholar]
- 13. Chang Y. J., Kim H. Y., Albacker L. A., Baumgarth N., McKenzie A. N., Smith D. E., Dekruyff R. H., and Umetsu D. T. (2011) Innate lymphoid cells mediate influenza-induced airway hyper-reactivity independently of adaptive immunity. Nat. Immunol. 12, 631–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liu T., Laidlaw T. M., Katz H. R., and Boyce J. A. (2013) Prostaglandin E2 deficiency causes a phenotype of aspirin sensitivity that depends on platelets and cysteinyl leukotrienes. Proc. Natl. Acad. Sci. U.S.A. 110, 16987–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yanagawa Y., Suzuki M., Matsumoto M., and Togashi H. (2011) Prostaglandin E2 enhances IL-33 production by dendritic cells Yoshiki. Immunol. Lett. 141, 55–60 [DOI] [PubMed] [Google Scholar]
- 16. Fujino H., West K. A., and Regan J. W. (2002) Phosphorylation of glycogen synthase kinase-3 and stimulation of T-cell factor signaling following activation of EP2 and EP4 prostanoid receptors by prostaglandin E2. J. Biol. Chem. 277, 2614–2619 [DOI] [PubMed] [Google Scholar]
- 17. Fujino H., Xu W., and Regan J. W. (2003) Prostaglandin E2 induced functional expression of early growth response factor-1 by EP4, but not EP2, prostanoid receptors via the phosphatidylinositol 3-kinase and extracellular signal-regulated kinases. J. Biol. Chem. 278, 12151–12156 [DOI] [PubMed] [Google Scholar]
- 18. Yokoyama U., Iwatsubo K., Umemura M., Fujita T., and Ishikawa Y. (2013) The prostanoid EP4 receptor and its signaling pathway. Pharmacol. Rev. 65, 1010–1052 [DOI] [PubMed] [Google Scholar]
- 19. Shi Q., Cao J., Fang L., Zhao H., Liu Z., Ran J., Zheng X., Li X., Zhou Y., Ge D., Zhang H., Wang L., Ran Y., and Fu J. (2014) Geniposide suppresses LPS-induced nitric oxide, PGE2 and inflammatory cytokine by down-regulating NF-κB, MAPK and AP-1 signaling pathways in macrophages. Int. Immunopharmacol. 20, 298–306 [DOI] [PubMed] [Google Scholar]
- 20. Búfalo M. C., Ferreira I., Costa G., Francisco V., Liberal J., Cruz M. T., Lopes M. C., Batista M. T., and Sforcin J. M. (2013) Propolis and its constituent caffeic acid suppress LPS-stimulated pro-inflammatory response by blocking NF-κB and MAPK activation in macrophages. J. Ethnopharmacol. 149, 84–92 [DOI] [PubMed] [Google Scholar]
- 21. Lander F., Hufnagel M., and Berner R. (2010) Antimikrobielle therapie der sepsis in der pediatrie. Pädiatrische praxis 75, 233–245 [Google Scholar]
- 22. Nakatsuji M., Minami M., Seno H., Yasui M., Komekado H., Higuchi S., Fujikawa R., Nakanishi Y., Fukuda A., Kawada K., Sakai Y., Kita T., Libby P., Ikeuchi H., Yokode M., and Chiba T. (2015) EP4 receptor-associated protein in macrophages ameliorates colitis and colitis-associated tumorigenesis. PLoS Genet. 11, 1–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li W., Li H., Mu Q., Zhang H., Yao H., Li J., and Niu X. (2014) Protective effect of sanguinarine on LPS-induced endotoxic shock in mice and its effect on LPS-induced COX-2 expression and COX-2 associated PGE 2 release from peritoneal macrophages. Int. Immunopharmacol. 22, 311–317 [DOI] [PubMed] [Google Scholar]
- 24. Gerlo S., Kooijman R., Beck I. M., Kolmus K., Spooren A., and Haegeman G. (2011) Cyclic AMP: a selective modulator of NF-κB action. Cell Mol. Life Sci. 68, 3823–3841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Anderson E. L., Kobayashi T., Iijima K., Bartemes K. R., Chen C.-C., and Kita H. (2016) IL-33 Mediates reactive eosinophilopoiesis in response to airborne allergen exposure. Allergy 10.1111/all.12861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Halim T. Y., Steer C. A., Mathä L., Gold M. J., Martinez-Gonzalez I., McNagny K. M., McKenzie A. N., and Takei F. (2014) Group 2 innate lymphoid cells are critical for the initiation of adaptive T helper 2 cell-mediated allergic lung inflammation. Immunity 40, 425–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lund S., Walford H. H., and Doherty T. A. (2013) Type 2 innate lymphoid cells in allergic disease. Curr. Immunol. Rev. 9, 214–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McKenzie A. N., Spits H., and Eberl G. (2014) Innate lymphoid cells in inflammation and immunity. Immunity 41, 366–374 [DOI] [PubMed] [Google Scholar]
- 29. Woo Y., Jeong D., Chung D. H., and Kim H. Y. (2014) The roles of innate lymphoid cells in the development of asthma. Immune Netw. 14, 171–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Beltrán C. J., Núñez L. E., Díaz-Jiménez D., Farfan N., Candia E., Heine C., López F., González M. J., Quera R., and Hermoso M. A. (2010) Characterization of the novel ST2/IL-33 system in patients with inflammatory bowel disease. Inflamm. Bowel Dis. 16, 1097–1107 [DOI] [PubMed] [Google Scholar]
- 31. Kim H. Y., Chang Y. J., Subramanian S., Lee H. H., Albacker L. A., Matangkasombut P., Savage P. B., McKenzie A. N. J., Smith D. E., Rottman J. B., Dekruyff R. H., and Umetsu D. T. (2012) Innate lymphoid cells responding to IL-33 mediate airway hyperreactivity independently of adaptive immunity. J. Allergy Clin. Immunol. 129, 216–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Doherty T. A., Khorram N., Chang J. E., Kim H.-K., Rosenthal P., Croft M., and Broide D. H. (2012) STAT6 regulates natural helper cell proliferation during lung inflammation initiated by Alternaria. AJP Lung Cell Mol. Physiol. 303, L577–L588 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.