Abstract
The galactoside-binding protein galectin-3 is increasingly recognized as an important player in cancer development, progression, and metastasis via its interactions with various galactoside-terminated glycans. We have shown previously that circulating galectin-3, which is increased up to 30-fold in cancer patients, promotes blood-borne metastasis in an animal cancer model. This effect is partly attributable to the interaction of galectin-3 with unknown receptor(s) on vascular endothelial cells and causes endothelial secretion of several metastasis-promoting cytokines. Here we sought to identify the galectin-3-binding molecule(s) on the endothelial cell surface responsible for the galectin-3-mediated cytokine secretion. Using two different galectin-3 affinity purification processes, we extracted four cell membrane glycoproteins, CD146/melanoma cell adhesion molecule (MCAM)/MUC18, CD31/platelet endothelial cell adhesion molecule-1 (PECAM-1), CD144/VE-cadherin, and CD106/Endoglin, from vascular endothelial cells. CD146 was the major galectin-3-binding ligand and strongly co-localized with galectin-3 on endothelial cell surfaces treated with exogenous galectin-3. Moreover, galectin-3 bound to N-linked glycans on CD146 and induced CD146 dimerization and subsequent activation of AKT signaling. siRNA-mediated suppression of CD146 expression completely abolished the galectin-3-induced secretion of IL-6 and G-CSF cytokines from the endothelial cells. Thus, CD146/MCAM is the functional galectin-3-binding ligand on endothelial cell surfaces responsible for galectin-3-induced secretion of metastasis-promoting cytokines. We conclude that CD146/MCAM interactions with circulating galectin-3 may have an important influence on cancer progression and metastasis.
Keywords: carbohydrate-binding protein, cytokine, endothelium, galectin, IL-6, CD146, MCAM, galectin-3
Introduction
Galectin-3 is a galactoside-binding protein that is expressed by many types of human cells. Overexpression of galectin-3 commonly occurs in most types of cancer (1, 2) and is increasingly recognized to influence cancer cell-cell and cancer-microenvironment communications and contributes to cancer development, progression, and metastasis as a result of galectin-3 interaction with various galactose-terminated glycans carried on the cell surface and in the extracellular matrix (2).
Earlier studies by us as well as by other groups have revealed that the concentration of circulating galectin-3 is markedly increased, up to 30-fold, in the bloodstream of cancer patients, including those with colorectal, breast, lung, bladder, pancreatic, and head and neck cancers and melanoma (2–8). Patients with metastatic disease tend to have higher concentrations of circulating galectin-3 than those with only localized tumors (3). Our recent study has revealed that increased circulating galectin-3 is an important promoter of blood-borne metastasis in a mouse model (9). This effect of galectin-3 is shown to be partly attributed to galectin-3 interaction with the oncofetal Thomsen-Friedenreich (galactoseβ1,3-N-acetyl-galactosamine α-, TF) disaccharide expressed on the cancer-associated transmembrane mucin protein MUC1 (10, 11), which is expressed by the majority of epithelial cancer cells (12). The galectin-3-MUC1 interaction induces MUC1 cell-surface polarization, leading to exposure of the underlying adhesion molecules. This results in increased tumor cell heterotypic adhesion to the vascular endothelium as well as increased tumor cell homotypic aggregation to form tumor emboli (13). Circulating galectin-3 is also shown to promote metastasis by interaction with still unknown molecules on the endothelial cell surface and induces endothelial secretion of the cytokines IL-6, G-CSF,2 GM-CSF, and intercellular adhesion molecule 1 (sICAM-1) (14).
Proinflammatory cytokines such as IL-6 and G-CSF are important metastasis promoters and are known to enhance various steps in the metastatic cascade, including cancer-endothelial adhesion, cell proliferation, migration, and angiogenesis (15, 16). We showed that the increased secretion of these cytokines from the blood vascular endothelium generated by exogenous galectin-3, at concentrations similar to those seen in cancer patients, increases the expression of various adhesion molecules on the endothelial cell surface, including integrins and E-selectin, either in an endocrine and/or paracrine way, resulting in increased cancer cell-endothelial adhesion, endothelial cell migration, and angiogenesis (14). The galectin-3-binding ligand(s) on the endothelial cell surface responsible for galectin-3-mediated endothelial secretion of these metastasis-promoting cytokines, however, has remained unknown. We report in this study that CD146/melanoma cell adhesion molecule (MCAM, also known as MUC18) is the galectin-3-binding ligand on the endothelial cell surface responsible for circulating galectin-3-mediated endothelial secretion of cytokines.
Results
Galectin-3 induces cytokine secretion by micro- and macrovascular endothelial cells
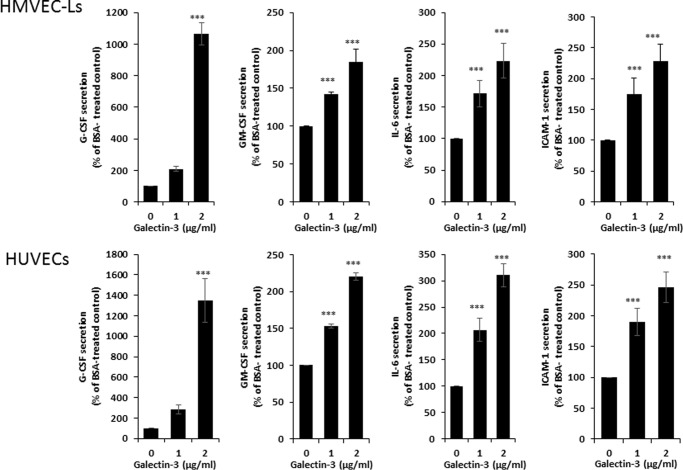
Our earlier study has reported that circulating galectin-3 induces the secretion of IL-6, G-CSF, GM-CSF, and ICAM-1 from human microvascular endothelial cells (14). Before proceeding to identify the endothelial cell-surface galectin-3-binding molecules for galectin-3-induced cytokine secretion, we first compared the effect of galectin-3 on the secretion of these cytokines from human micro- and macrovascular endothelial cells. It was found that the presence of galectin-3 at concentrations similar to those seen in cancer patients (3) increased the secretion of IL-6, G-CSF, GM-CSF, and ICAM-1 from human microvascular lung endothelial cells (HMVEC-Ls) and also from macrovascular human umbilical vein endothelial cells (HUVECs) (Fig. 1). This suggests that the effect of galectin-3 on endothelial secretion of these cytokines can occur in both micro- and macrovasculature.
Figure 1.
Galectin-3 induces cytokine secretion from both human micro- and macrovascular endothelial cells. A and B, HMVEC-Ls (A) and HUVECs (B) were treated with BSA or galectin-3 for 24 h, and the concentrations of G-CSF, GM-CSF, IL-6, and ICAM-1 in the culture medium were determined. Data are presented as mean ± S.E. of at least three independent experiments, each in triplicate. ***, p < 0.001.
Four endothelial cell membrane glycoproteins were extracted by galectin-3 affinity purification
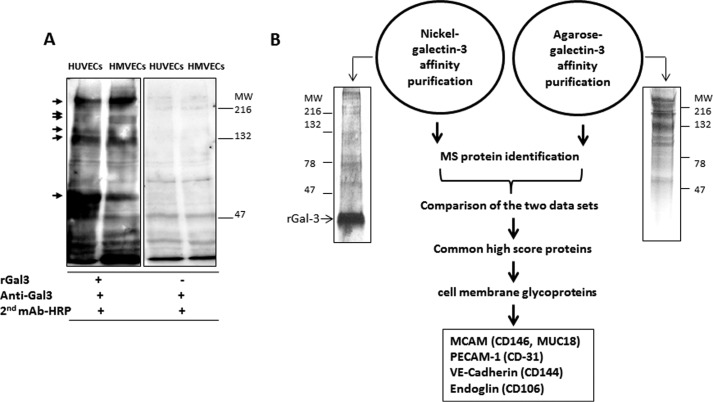
Galectin-3 blotting/overlay demonstrated similar binding of galectin-3 to several proteins in HUVECs and HMVEC-Ls (Fig. 2A). Two galectin-3 affinity purification approaches, His-tagged recombinant galectin-3 conjugated to nickel and to agarose, were used to extract galectin-3-binding proteins from endothelial cells. After removal of unbound proteins, bound proteins were eluted from the columns with lactose. Various proteins ranging from ∼50 to ∼250 kDa were seen to be extracted by both affinity purification processes, as shown by silver staining following SDS-PAGE (Fig. 2B). Mass spectrometry analysis revealed a number of cell membrane and cytosolic proteins. The top scored cell membrane proteins in both the galectin-3-nickel and galectin-3-agarose affinity extracts were CD146/MCAM (MUC18), CD31/platelet endothelial cell adhesion molecule (PECAM-1), CD144/VE-cadherin, and CD106/Endoglin (11∼28 peptides of each protein).
Figure 2.
Identification of endothelial cell surface galectin-3-binding proteins. A, galectin-3 blotting/overlay shows similar binding of galectin-3 to a number of proteins in HUVECs and HUVECs. MW, molecular weight. B, galectin-3 affinity purification and protein identification. HUVEC cell lysates were separated by SDS-PAGE and stained by silver staining (left panel) or applied to nickel- or agarose-conjugated galectin-3 columns. Bound proteins were released by lactose and identified by mass spectrometry or separated by SDS-PAGE and stained by silver staining (right panel).
CD146/MCAM is the major galectin-3-binding protein on the endothelial cell surface
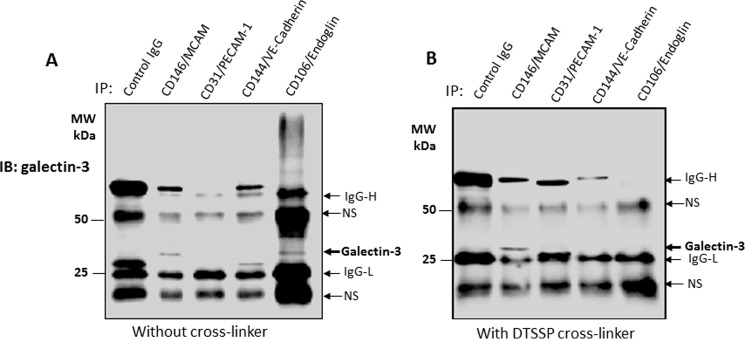
To confirm the interaction of galectin-3 with these cell membrane proteins, we treated the cells with galectin-3, followed by immunoprecipitation with antibodies against each of the four identified proteins. Galectin-3 was predominately found in the immunoprecipitates of CD146/MCAM and CD106/Endoglin (Fig. 3A), suggesting possible binding ligands among these proteins. In addition to galectin-3 and the antibody/immunoglobulin heavy and light chains, two other protein bands were also seen in the blots of the immunoprecipitates (Fig. 3). As these two protein bands occurred in all immunoprecipitates, including the control IgG immunoprecipitates, they reflect nonspecific antibody/immunoglobulin binding.
Figure 3.
Identification of CD146/MCAM as the major galectin-3-binding protein. A and B, HUVECs were treated with galectin-3 (4 μg) for 60 min. The cells were then treated without (A) or with (B) DTSSP cross-linker before immunoprecipitation (IP) with antibodies against MCAM, PECAM-1, PE-Cadherin, or endoglin, followed by immunoblotting (IB) with anti-galectin-3 antibody and peroxidase-conjugated secondary antibody. Co-immunoprecipitated galectin-3 and the heavy (IgG-H) and light (IgG-L) chains of the antibody/immunoglobulin used in the immunoprecipitation are indicated by arrows. Additional arrows show nonspecific (NS) antibody/immunoglobulin binding. MW, molecular weight.
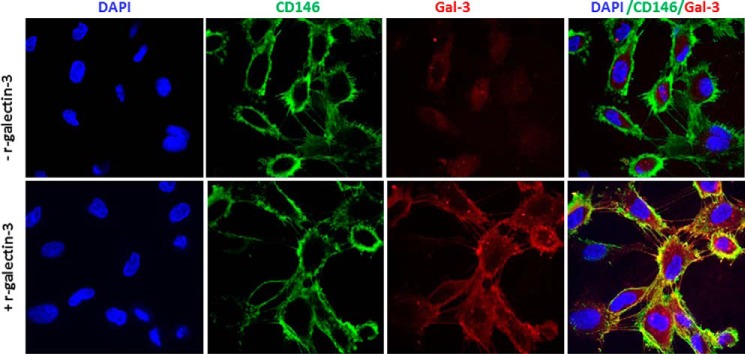
To further determinate the interaction of galectin-3 with these cell membrane proteins, we cross-linked the cell-surface proteins with a cleavable cross-linker, DTSSP (3,3′-dithiobis(sulfosuccinimidyl propionate), after treatment of the cells with recombinant galectin-3. Subsequent immunoprecipitation with antibodies against each of these proteins revealed the presence of galectin-3 only in the CD146/MCAM immunoprecipitates (Fig. 3B). This indicates that CD146/MCAM represents the major galectin-3-binding ligand on the endothelial cell surface. Analysis by confocal microscopy showed weak expression of endogenous galectin-3 by the cells (Fig. 4). Strong cell-surface co-localization of CD146/MCAM with galectin-3 occurred when exogenous galectin-3 was introduced (Fig. 4).
Figure 4.
Co-localization of exogenous galectin-3 with CD146/MCAM in HUVECs. After treatment of HUVECs without (top panel) or with (bottom panel) galectin-3 (4 μg/ml) for 60 min, the cells were fixed, and CD146/MCAM (green) and galectin-3 (red) immunohistochemistry was conducted and followed by confocal microscopy. Cell nuclei were stained by DAPI (blue).
Galectin-3 binds to N-linked glycans expressed on CD146/MCAM
To gain insight into the interaction of galectin-3 with CD146/MCAM, endothelial cells were first treated with N-glycanase (PNGaseF) or O-glycanase (from Streptococcus pneumoniae) before treatment with recombinant galectin-3. The cells were lysed and immunoprecipitated with anti-CD146/MCAM antibody. Treatment of the cells with N-glycanase reduced the size of the CD146/MCAM molecular weight in SDS-PAGE as a result of reduction of N-linked sugar chains (Fig. 5, top panel). Reduction of N-glycans on CD146/MCAM substantially reduced (35%) the presence of galectin-3 in the immunoprecipitates (Fig. 5, bottom panel), whereas treatment of the cells with O-glycanase showed no effect on the galectin-3 presence in CD146/MCAM immunoprecipitates. This suggests that galectin-3 binds predominantly to N-linked glycans on CD146/MCAM.
Figure 5.
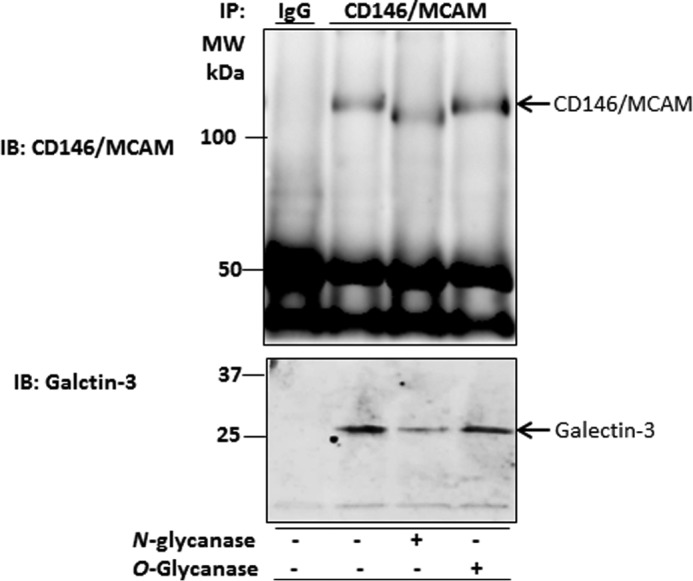
Galectin-3 binds to N-linked glycans on CD146/MCAM. After immunoprecipitation (IP) of HUVECs with anti-CD146 antibody, the immunoprecipitates were treated with PNGaseF or O-glycanase before electrophoresis and immunoblotting (IB) with CD146/MCAM and galectin-3 antibody. MW, molecular weight.
Suppression of CD146/MCAM expression abolishes galectin-3-induced cytokine secretion
To determine whether CD146/MCAM is the functional ligand responsible for galectin-3-induced cytokine secretion of the endothelium, we suppressed CD146/MCAM expression by siRNA. siRNA treatment caused an 80% reduction of cellular CD146/MCAM expression in comparison with cells treated with control siRNA (Fig. 6A). Suppression of CD146/MCAM expression completely abolished the effect of galectin-3 on endothelial secretion of IL-6 and G-CSF (Fig. 6, B and C). This indicates that CD146/MCAM is the galectin-3-binding receptor responsible for galectin-3-induced endothelial secretion of the cytokines.
Figure 6.
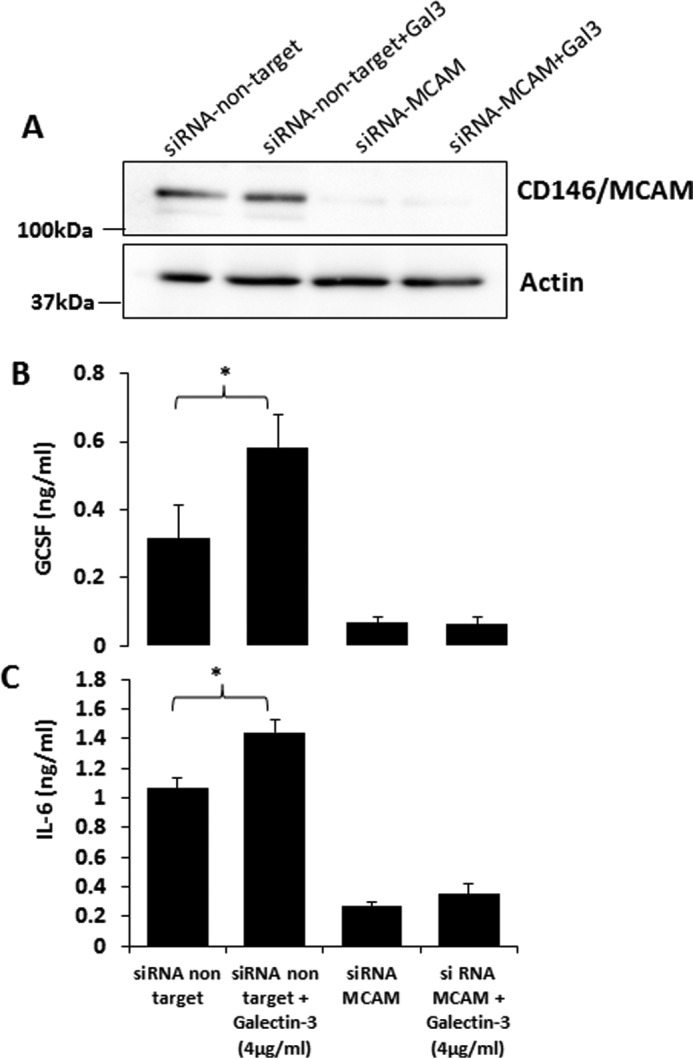
Suppression of CD146/MCAM expression abolishes galectin-3-induced cytokine secretion. A–C, HUVECs were treated with control or CD146/MCAM siRNA for 36 h. The cells were then either lysed and analyzed for CD146/MCAM expression by CD146/MCAM immunoblotting (A) or further treated with galectin-3 (4 μg/ml) for 24 h before the levels of G-CSF (B) or IL-6 (C) in the culture medium were determined. *, p < 0.5 (unpaired t test).
Galectin-3 induces CD146/MCAM dimerization and activation of downstream AKT signaling
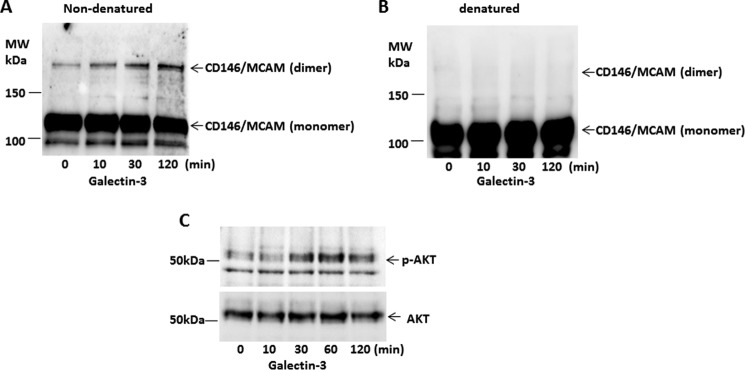
CD146/MCAM activity is known to be associated with CD146/MCAM dimerization (17, 18) and downstream activation of AKT signaling (19–21). To determine whether the galectin-3-CD146/MCAM interaction affects CD146/MCAM activity, we assessed CD146/MCAM dimerization and AKT activation in the cell response to galectin-3. Introduction of galectin-3 caused a time-dependent increase in CD146/MCAM dimerization that was detected under non-denatured (Fig. 7A) but not denatured SDS-PAGE conditions (Fig. 7B). Galectin-3 treatment also caused a similarly time-dependent increase in AKT phosphorylation (Fig. 7C) as CD146/MCAM dimerization.
Figure 7.
Galectin-3 induces CD146/MCAM dimerization and downstream AKT signaling. A–C, HUVECs were treated with galectin-3 (4 μg/ml) for various times. The cells were lysed and applied to SDS-PAGE under non-denatured (A) or denatured (B) conditions and probed by anti-CD146/MCAM antibody. Cellular proteins were also probed with antibodies against p-AKT and AKT (C). MW, molecular weight.
Galectin-3 activates several downstream signaling pathways
To gain further insight into the global effect of galectin-3 on endothelial cytokine secretion, we compared the activity of 46 tyrosine kinases in cell response to galectin-3 using a human phospho-kinase array. Phosphorylation of several tyrosine kinases was substantially increased in response to 24-h treatment of endothelial cells with galectin-3 (Fig. 8). These include HSP60 (39-fold), WINK1 (13-fold), p27 (6.9-fold), β-catenin (4.4-fold), c-jun (4-fold), p70 S6 kinase (3.6-fold), Creb (2.2-fold), p53 (Ser-392 and Ser-15, 2.8-fold and 1.8-fold, respectively), and PRAS40 (1.5-fold). This suggests that endothelial secretion of cytokines in response to galectin-3, which was shown previously to take several hours (14) and is shown in this study to be associated with binding of galectin-3 to cell-surface CD146/MCAM (Fig. 6, 7), may involve multiple signaling pathways. The increased phosphorylation of PRAS40, a substrate of AKT, after 24-h treatment of the cells with galectin-3 is in keeping with the increase in CD146/MCAM dimerization and AKT activation.
Figure 8.
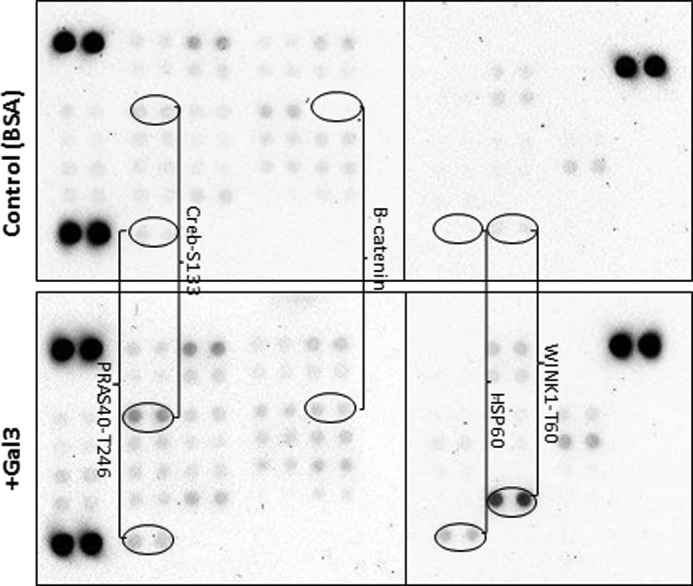
Activation of multiple downstream signaling pathways. HUVECs were treated with BSA (top panel) or galectin-3 (4 μg/ml) (bottom panel) for 24 h before the cells were lysed and analyzed by the Profiler human phospho-kinase array.
Discussion
This study shows that galectin-3, at concentrations similar to those found in the blood of cancer patients, induces secretion of the cytokines IL-6, G-CSF, GM-CSF, and ICAM-1 from both micro- and macrovascular endothelial cells. CD146/MCAM is identified as the galectin-3-binding ligand on the endothelial surface responsible for the galectin-3-mediated secretion of cytokines. Galectin-3 binds to N-glycans on CD146/MCAM and induces CD146/MCAM dimerization and downstream AKT activation.
CD146/MCAM, also known as MUC18 (22), is a type I transmembrane glycoprotein that contains eight putative N-glycosylation sites on its extracellular domain (17). Its glycosylation accounts for about 35% of the molecular weight (23). CD146/MCAM was previously considered as an endothelial cell marker and has been used for the identification of circulating endothelial cells (24). It is now known to be also expressed by melanoma cells (17) and is associated with melanoma metastasis (25–27). There are two CD146/MCAM isoforms, CD146-long and CD146-short. The CD146-long form contains a 63-amino acid intracellular domain including two putative endocytic domains and five potential protein kinase recognition sites, and the CD146-short form contains a truncated cytoplasmic tail lacking both of the endocytosis motifs and one of the protein kinase sites. Endothelial cells express both the long and short forms, whereas melanoma cells primarily express the long isoform.
CD146 is a member of the immunoglobulin superfamily of cell adhesion molecules and a key cell adhesion protein on the vascular endothelial cell surface. It is involved in various cell-cell and cell-matrix interactions in vascular endothelial activities, such as cell migration and angiogenesis (17) and also engages in outside-in signaling. Although the natural CD146 ligand still remains elusive, its engagement is known to activate several signaling pathways, including PI3K/AKT, p38, and PKC (17). CD146/MCAM interaction with VEGFR-2 on the endothelial cell surface has been shown to activate AKT and p38 signaling and increase cell migration (21). Interaction of CD146 with Laminin-411 facilitates Th17 cell entry into the central nervous system (28). Binding of Netrin-1 to CD146/MCAM was reported to activate an array of downstream signaling and increase endothelial cell proliferation, migration, and angiogenesis (29). Recently, CD146 was reported to interact with exogenously introduced galectin-1 in endothelial cells and protects against galectin-1-induced cell apoptosis (30). Endogenous galectin-3 expression in HUVECs was found to be low (Fig. 4). Strong interaction and co-localization of CD146/MCAM with galectin-3 occurred when exogenous galectin-3 was introduced (Figs. 3 and 4).
It remains to be determined exactly how CD146/MCAM activation in response to galectin-3 binding induces endothelial secretion of cytokines. The rapid activation of AKT indicates its involvement at the initial stage of signaling transduction. The activation of cellular WINK1, β-catenin, and HSP60 after 24-h galectin-3 treatment suggests the possible involvement of multiple downstream signaling pathways at later stages of the process. Toll-like receptor 2-associated IL-6 secretion from megakaryocytes has been shown to be associated with Wnt β-catenin signaling (31), and the presence of humanized anti-HSP60 antibody suppresses IL-6 secretion from peripheral blood mononuclear cells (PBMCs) (32). These studies suggest a role of WNT and HSP signaling in galectin-3-mediated cytokine secretion.
In addition to CD146/MCAM, CD31/PECAM-1 and CD144/VE-cadherin were also seen in this study to be extracted by galectin-3 affinity purification from endothelial cells (Figs. 2 and 3). CD146 has been reported to be able to interact with VEGF receptor 2 (VEGFR-2) and induce activation of AKT (21). Interestingly, a complex formed by CD31/VE-cadherin and CD144/PECAM is reported to be associated with VEGFR-2 in endothelial cells (33), and their interaction affects VEGFR-2-associated AKT signaling. It is therefore possible that CD146/MCAM, CD31/VE-cadherin and CD144/PECAM may form a supercomplex on the endothelial cell surface through VEGFR-2. This may explain the extraction of these four proteins by galectin-3 affinity purification (Fig. 2) even though galectin-3 may only bind to CD146/MCAM (Fig. 3). The discovery that exogenous galectin-3 increases AKT activation (Fig. 7), a downstream CD146/MCAM signaling that is also associated with VEGFR-2 activation, is in keeping with this.
CD146/MCAM is thus the functional galectin-3 binding ligand on the endothelial cell surface responsible for galectin-3-induced endothelial secretion of metastasis-promoting cytokines. Many previous studies have shown that higher serum levels of proinflammatory cytokines (e.g. IL-6 and TNFα) correlate with advanced metastatic stages and poor survival in various types of cancer (34). These cytokines enhance various cell activities, including proliferation, invasion, angiogenesis, and metastasis (15, 35). The increased secretion of IL-6, G-CSF, and other cytokines from the vascular endothelium induced by interaction of circulating galectin-3 with endothelial CD146/MCAM in cancer may therefore have an important influence on cancer progression and metastasis.
Experimental procedures
Materials
Human IL-6 and G-CSF ELISA kits were purchased from Peprotech (London, UK). Antibodies against CD146/MCAM (MAB932), CD144/PECAM-1 (BBA7), CD31/VE-Cadherin (MAB9381), Galectin-3 (MAB1154), biotinylated-anti-Galectin-3 (BAF1154), and Proteome Profiler human phospho-kinase array kits (Ary003b) were from R&D Systems (Abingdon, UK). Antibodies against Endoglin (SC-18838) and pan-actin 5 were from Santa Cruz Biotechnology (Heidelberg, Germany) and Neomarkers (Fremont, CA), respectively. Antibodies against AKT (9272S) and phospho-AKT (Thr(P)-308, 13038S) were purchased from Cell Signaling Technology (Hitchin, UK). DTSSP was purchased from Thermo Fisher Scientific (Runcorn, UK).
Cells
HMVEC-Ls and HUVECs were obtained from Lonza (Basel, Switzerland) and cultured in EGMTM and EGM-2TM-MV medium, respectively. Cells with less than six passages were used in all experiments.
Cytokine quantification
HUVECs or HMVEC-Ls were seeded in 12-well plates at 5 × 104 cells/well and cultured for 24 h at 37 °C before introduction of recombinant galectin-3 for 24 h. The culture medium was collected and centrifuged at 1000 rpm to remove any cell debris. The supernatant was used for determination of IL-6 and G-CSF concentration using the IL-6 and G-CSF ELISA kits according to the instructions of the manufacturer.
Production of recombinant galectin-3
Full-length recombinant human galectin-3 and His-tagged recombinant human galectin-3 were produced in Escherichia coli as described previously (36).
Galectin-3 affinity purification
Confluent HUVECs were washed once with 100 mm lactose/PBS and twice with PBS before being lysed in lysis buffer (PBS, 0.5% Triton X-100, 0.5% Nonidet P-40 (v/v), and protease inhibitors). The lysate was collected and sonicated three times for 20 s on ice. The lysate was cleared by centrifugation at 16,000 × g for 10 min at 4 °C and before application to galectin-3 affinity columns. The galectin-3-nickel column was prepared by injection of 12 mg of His-tagged recombinant galectin-3 to a His-Trap HP column (GE Healthcare). Galectin-3-agarose affinity beads were prepared by conjugating 30 mg of recombinant galectin-3 to 12.5 ml of NHS-agarose slurry beads (Pierce) according to the instructions of the manufacturer instructions.
After removal of the unbound galectin-3 by three washes with PBS, the cell lysate was applied to the column three times. After three washes with PBS, the bound proteins were eluted with 0.2 m lactose/PBS. The eluate was dialysed at 4 °C for 24 h against distilled water. The samples were freeze-dried and analyzed by SDS-PAGE followed by silver staining or by mass spectrometry.
Mass spectrometry and protein identification
Sample preparation
A proportion of the freeze-dried eluate from both the galectin-3-agarose and galectin-3-nickel columns was reconstituted in 500 μl of 25 mm ammonium bicarbonate (NH4HC03). 10 μl of Strataclean resin (Agilent) was added to the sample, followed by gentle vortexing for 1 min. The samples were centrifuged at 2000 × g for 2 min, and the protein-depleted sample was aspirated and the resin washed three times with 1 ml of 25 mm NH4HC03. The resin was resuspended in 80 μl of the same buffer, 5 μl of 1% (w/v) Rapigest (Waters) was added, and the samples were placed in a heating block set at 80 °C for 10 min. Samples were reduced by the addition of 5 μl of 60 mm dithiothreitol in 25 mm NH4HCO3 and incubated at 60 °C for 10 min. The samples were alkylated by addition of 180 mm iodoacetaminde in 25 mm NH4HCO3, followed by incubation at room temperature in the dark for 30 min. Trypsin (Promega Gold, 5 μl) was added from a 0.2 mg/ml stock solution in 50 mm acetic acid (∼1:50 enzyme to protein ratio), and the samples were incubated at 37 °C overnight with continuous mixing.
Mass spectrometry analysis
Data-dependent LC/MS-MS analyses were conducted on a QExactive quadrupole-Orbitrap mass spectrometer coupled to a Dionex Ultimate 3000 RSLC nano-liquid chromatograph. The sample digest from the galectin-3 affinity chromatography was analyzed using a 50-min LC/MS method. The digest (1 μl) was loaded onto a trapping column (Acclaim PepMap 100 C18, 75 μm × 2 cm, 3-μm packing material, 100 Å) using a loading buffer of 0.1% (v/v) TFA, 2% (v/v) acetonitrile in water for 7 min at a flow rate of 9 μl/min. The trapping column was then set in line with an analytical column (EASY-Spray PepMap RSLC C18, 75 μm × 50 cm, 2-μm packing material, 100 Å), and the peptides were eluted using a linear gradient of 96.2% A (0.1% (v/v) formic acid):3.8% B (0.1% (v/v) formic acid in water:acetonitrile (80:20, v/v)) to 50% A:50% B over 30 min at a flow rate of 300 nl/min, followed by washing at 1% A:99% B for 5 min and re-equilibration of the column to starting conditions. A shorter, 30-min LC/MS method was used for the digest from His-galectin-3 affinity chromatography because of the lower sample complexity. The column was maintained at 40 °C, and the effluent was introduced directly into the integrated nano-electrospray ionization source operating in positive ion mode. The mass spectrometer was operated in data-dependent acquisition (DDA) mode with survey scans between m/z 300–2000 acquired at a mass resolution of 70,000 (full width at half-maximum) at m/z 200. The maximum injection time was 250 ms, and the automatic gain control was set to 1e6. The 10 most intense precursor ions with charge states between 2+ and 5+ were selected for MS-MS with an isolation window of 2 m/z units. The maximum injection time was 100 ms, and the automatic gain control was set to 1e5. Fragmentation of the peptides was by higher-energy collisional dissociation using a normalized collision energy of 28%. Dynamic exclusion of m/z values to prevent repeated fragmentation of the same peptide was used with an exclusion time of 20 s.
Raw data files were searched in Proteome Discoverer (v1.4) against the UniProt human reviewed database (20,187 sequences) using the Mascot search engine. Trypsin was specified as the protease, with one missed cleavage allowed and with fixed carbamidomethyl modification for cysteine and variable oxidation modification for methionine. A precursor mass tolerance of 10 ppm and a fragment ion mass tolerance of 0.01 Da were applied. A peptide false discovery rate of 1–5% was applied.
Immunofluorescence and confocal microscopy
Subconfluent HUVECs or HMVEC-Ls were seeded into 12-well plates inserted with glass coverslips. After 24-h culture, the cells were treated with either galectin-3 or BSA (4 μg/ml) for 1 h. After washing with PBS and fixing with 4% paraformaldehyde, the cells were incubated with 1% BSA for 1 h before application of biotinylated anti-galectin-3 (1:1000) or anti-MCAM (1:1000) in 2% BSA/PBS (w/v) for 1 h at room temperature. After three washes with PBS, the cells were incubated with FITC-conjugated (1:2000) secondary antibody or Texas Red-Avidin (1:2000) for 1 h at room temperature. The cells were washed five times in PBS before being mounted using DAPI-containing fluorescent mounting medium (Vector Laboratories, Burlingame, CA). The slides were analyzed with a 3i confocal microscope (Marianas SDC, 3i Imaging) and Slidebook 6 Reader version 6.0.4 (Intelligent-imaging).
Co-immunoprecipitation
90% confluent HUVECs were first treated with galectin-3 (4 μg/ml) for 1 h at 37 °C before being washed with ice-cold PBS and lysed with lysis buffer (1% Triton X-100 in PBS) on ice for 10 min. In some of the experiments, after treatment with galectin-3 (4 μg/ml), the cells were washed and cross-linked with 2 mm DTSSP for 1 h on ice. After quenching the reaction with 20 mm Tris buffer for 15 min, the cells were lysed and centrifuged at 10,000 × g for 10 min at 4 °C. The supernatants were collected and precleared with 30 μl of protein-A/G Plus-agarose beads (sc-2003, Santa Cruz Biotechnology) for 1 h at 4 °C. The lysates were incubated with anti-MCAM antibody (2 μg/ml) or control isotype control IgG at 4 °C overnight. After addition of 30 μl of protein-A/G Plus-agarose beads for 4 h, the beads were washed four times with ice-cold PBS with 1% Triton X-100 (v/v). Proteins were eluted from the beads by boiling in SDS sample buffer and analyzed by SDS-PAGE and Western blotting.
Western blotting
Cell lysates or immunoprecipitates were separated by SDS-PAGE and transferred to nitrocellulose membrane. The membranes were blocked in 5% BSA (w/v)/0.05% Tween 20/TBS before incubation with primary antibodies against MCAM, PECAM-1, VE-Cadherin, Endoglin, galectin-3, or actin at a concentration of 1:1000 overnight at 4 °C. After three washes with 0.05% Tween 20/TBS, the blots were incubated with peroxidase-conjugated anti-mouse or anti rabbit antibodies (1:5000) for 1 h. After six washes with 0.05% Tween 20/TBS, the blots were developed using a chemiluminescence Super Signal kit and visualized with the Molecular Imager® Gel DocTM XR system (Bio-Rad).
Protein deglycosylation
Following immunoprecipitation as described above, protein-A/G Plus-agarose beads were washed twice with ice-cold 1% Triton X-100/PBS before incubation with 5 units of PNGaseF (F8435, Sigma) or 10 milliunits O-glycanase (GK80090, Prozyme) overnight at 37 °C. The beads were washed twice with PBS, and proteins were eluted from the beads by boiling in SDS sample buffer and analyzed by SDS-PAGE and Western blotting.
siRNA protein knockdown
Fifty percent confluent HMVEC-Ls were cultured in 12-well plates for 24 h. MCAM siRNA (150 pmol) (siGenome SMARTpool human MCAM, Thermo Fisher) or control non-target siRNA (siGenome control siRNA #1, Thermo Fisher) were incubated with 100 μl of serum-free medium for 5 min. DharmaFect Transfection Reagent 4 (Thermo Fisher) (2.5 μl in 100 μl of serum-free medium) was then introduced for 20 min at room temperature before 800 μl of EGM2 medium was added. After 16 h incubation, the transfection medium was replaced with fresh medium for 24 h. The cells were then either lysed by SDS sample buffer for subsequent analysis of MCAM expression by immunoblotting or introduced with galectin-3 for 24 h at 37 °C before the IL-6 and G-CSF concentrations in the culture media were determined by ELISA.
CD146/MCAM dimerization
HMVEC-Ls were cultured in 12-well plates at 5 × 104 cells/well for 1 day before treatment with galectin-3 (4 μg/ml) for 0, 10, 30, and 60 min at 37 °C. Cells were washed in ice-cold PBS and lysed in SDS sample buffer with or without β-mercaptoethanol before analysis by SDS-PAGE and Western blotting.
Protein phospho-kinase array
90% confluent HUVECs were treated with 4 μg/ml galectin-3 or BSA for 24 h. Cells were washed, lysed, and analyzed using the Proteome Profiler human phospho-kinase array kit (Ary003b) according to the instructions of the manufacturer. Membranes were visualized with the Molecular Imager® Gel DocTM XR system and analyzed using Image Lab software version 5.2.1 (Bio-Rad).
Statistical analysis
Unpaired t test for single comparison or one-way analysis of variance followed by Bonferroni test for multiple comparisons (SPSS 24t) were used where appropriate. Differences were considered significant when p < 0.05.
Author contributions
F. C. performed and analyzed the experiments shown in Figs. 2–8 and contributed to drafting the manuscript. W. W. performed and analyzed the experiments shown in Fig. 1 and part of Fig. 2. M. Z. produced the recombinant galectin-3. D. S. performed the mass spectrometry analysis for protein identification. R. B. and J. M. R. contributed to the design and interpretation of data. L. G. Y. designed the study, contributed to the interpretation of data, and drafted the manuscript. All authors reviewed the results and approved the final version of the manuscript.
This work was supported by North West Cancer Research Fund Grant CR975. The authors declare that they have no conflicts of interest with the contents of this article.
- G-CSF
- granulocyte colony-stimulating factor
- DTSSP
- 3,3′-dithiobis(sulfosuccinimidyl propionate)
- MCAM
- melanoma cell adhesion molecule
- HMVEC-L
- human microvascular lung endothelial cell
- HUVEC
- human umbilical vein endothelial cell
- PECAM
- platelet endothelial cell adhesion molecule
- ICAM
- intercellular adhesion molecule.
References
- 1. Liu F. T., and Rabinovich G. A. (2005) Galectins as modulators of tumour progression. Nat. Rev. Cancer 5, 29–41 [DOI] [PubMed] [Google Scholar]
- 2. Newlaczyl A. U., and Yu L. G. (2011) Galectin-3: a jack-of-all-trades in cancer. Cancer Lett. 313, 123–128 [DOI] [PubMed] [Google Scholar]
- 3. Barrow H., Guo X., Wandall H. H., Pedersen J. W., Fu B., Zhao Q., Chen C., Rhodes J. M., and Yu L. G. (2011) Serum galectin-2, -4, and -8 are greatly increased in colon and breast cancer patients and promote cancer cell adhesion to blood vascular endothelium. Clin. Cancer Res. 17, 7035–7046 [DOI] [PubMed] [Google Scholar]
- 4. Iurisci I., Tinari N., Natoli C., Angelucci D., Cianchetti E., and Iacobelli S. (2000) Concentrations of galectin-3 in the sera of normal controls and cancer patients. Clin. Cancer Res. 6, 1389–1393 [PubMed] [Google Scholar]
- 5. Sakaki M., Oka N., Nakanishi R., Yamaguchi K., Fukumori T., and Kanayama H. O. (2008) Serum level of galectin-3 in human bladder cancer. J. Med. Invest. 55, 127–132 [DOI] [PubMed] [Google Scholar]
- 6. Saussez S., Glinoer D., Chantrain G., Pattou F., Carnaille B., André S., Gabius H. J., and Laurent G. (2008) Serum galectin-1 and galectin-3 levels in benign and malignant nodular thyroid disease. Thyroid 18, 705–712 [DOI] [PubMed] [Google Scholar]
- 7. Vereecken P., Zouaoui Boudjeltia K., Debray C., Awada A., Legssyer I., Sales F., Petein M., Vanhaeverbeek M., Ghanem G., and Heenen M. (2006) High serum galectin-3 in advanced melanoma: preliminary results. Clin. Exp. Dermatol. 31, 105–109 [DOI] [PubMed] [Google Scholar]
- 8. Senapati S., Chaturvedi P., Chaney W. G., Chakraborty S., Gnanapragassam V. S., Sasson A. R., and Batra S. K. (2011) Novel interaction of MUC4 and galectin: potential pathobiological implications for metastasis in lethal pancreatic cancer. Clin. Cancer Res. 17, 267–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhao Q., Guo X., Nash G. B., Stone P. C., Hilkens J., Rhodes J. M., and Yu L. G. (2009) Circulating galectin-3 promotes metastasis by modifying MUC1 localization on cancer cell surface. Cancer Res. 69, 6799–6806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yu L. G., Andrews N., Zhao Q., McKean D., Williams J. F., Connor L. J., Gerasimenko O. V., Hilkens J., Hirabayashi J., Kasai K., and Rhodes J. M. (2007) Galectin-3 interaction with Thomsen-Friedenreich disaccharide on cancer-associated MUC1 causes increased cancer cell endothelial adhesion. J. Biol. Chem. 282, 773–781 [DOI] [PubMed] [Google Scholar]
- 11. Sindrewicz P., Lian L.-Y., and Yu L.-G. (2016) Interaction of the oncofetal Thomsen-Friedenreich antigen with galectins in cancer progression and metastasis. Front. Oncol. 6, 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Taylor-Papadimitriou J., Burchell J., Miles D. W., and Dalziel M. (1999) MUC1 and cancer. Biochim. Biophys. Acta 1455, 301–313 [DOI] [PubMed] [Google Scholar]
- 13. Zhao Q., Barclay M., Hilkens J., Guo X., Barrow H., Rhodes J. M., and Yu L. G. (2010) Interaction between circulating galectin-3 and cancer-associated MUC1 enhances tumour cell homotypic aggregation and prevents anoikis. Mol. Cancer 9, 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen C., Duckworth C. A., Zhao Q., Pritchard D. M., Rhodes J. M., and Yu L. G. (2013) Increased circulation of galectin-3 in cancer induces secretion of metastasis-promoting cytokines from blood vascular endothelium. Clin. Cancer Res. 19, 1693–1704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kowanetz M., Wu X., Lee J., Tan M., Hagenbeek T., Qu X., Yu L., Ross J., Korsisaari N., Cao T., Bou-Reslan H., Kallop D., Weimer R., Ludlam M. J., Kaminker J. S., et al. (2010) Granulocyte-colony stimulating factor promotes lung metastasis through mobilization of Ly6G+Ly6C+ granulocytes. Proc. Natl. Acad. Sci. U.S.A. 107, 21248–21255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wei L. H., Kuo M. L., Chen C. A., Chou C. H., Lai K. B., Lee C. N., and Hsieh C. Y. (2003) Interleukin-6 promotes cervical tumor growth by VEGF-dependent angiogenesis via a STAT3 pathway. Oncogene 22, 1517–1527 [DOI] [PubMed] [Google Scholar]
- 17. Wang Z., and Yan X. (2013) CD146, a multi-functional molecule beyond adhesion. Cancer Lett. 330, 150–162 [DOI] [PubMed] [Google Scholar]
- 18. Bu P., Zhuang J., Feng J., Yang D., Shen X., and Yan X. (2007) Visualization of CD146 dimerization and its regulation in living cells. Biochim. Biophys. Acta 1773, 513–520 [DOI] [PubMed] [Google Scholar]
- 19. Nodomi S., Umeda K., Saida S., Kinehara T., Hamabata T., Daifu T., Kato I., Hiramatsu H., Watanabe K. I., Kuwahara Y., Iehara T., Adachi S., Konishi E., Nakahata T., Hosoi H., and Heike T. (2016) CD146 is a novel marker for highly tumorigenic cells and a potential therapeutic target in malignant rhabdoid tumor. Oncogene 35, 5317–5327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang J., Tang X., Weng W., Qiao Y., Lin J., Liu W., Liu R., Ma L., Yu W., Yu Y., Pan Q., and Sun F. (2015) The membrane protein melanoma cell adhesion molecule (MCAM) is a novel tumor marker that stimulates tumorigenesis in hepatocellular carcinoma. Oncogene 34, 5781–5795 [DOI] [PubMed] [Google Scholar]
- 21. Jiang T., Zhuang J., Duan H., Luo Y., Zeng Q., Fan K., Yan H., Lu D., Ye Z., Hao J., Feng J., Yang D., and Yan X. (2012) CD146 is a coreceptor for VEGFR-2 in tumor angiogenesis. Blood 120, 2330–2339 [DOI] [PubMed] [Google Scholar]
- 22. Sers C., Riethmüller G., and Johnson J. P. (1994) MUC18, a melanoma-progression associated molecule, and its potential role in tumor vascularization and hematogenous spread. Cancer Res. 54, 5689–5694 [PubMed] [Google Scholar]
- 23. Shih I. M., Elder D. E., Speicher D., Johnson J. P., and Herlyn M. (1994) Isolation and functional characterization of the A32 melanoma-associated antigen. Cancer Res. 54, 2514–2520 [PubMed] [Google Scholar]
- 24. Erdbruegger U., Dhaygude A., Haubitz M., and Woywodt A. (2010) Circulating endothelial cells: markers and mediators of vascular damage. Curr. Stem Cell Res. Ther. 5, 294–302 [DOI] [PubMed] [Google Scholar]
- 25. Ruma I. M., Putranto E. W., Kondo E., Murata H., Watanabe M., Huang P., Kinoshita R., Futami J., Inoue Y., Yamauchi A., Sumardika I. W., Youyi C., Yamamoto K., Nasu Y., Nishibori M., et al. (2016) MCAM, as a novel receptor for S100A8/A9, mediates progression of malignant melanoma through prominent activation of NF-κB and ROS formation upon ligand binding. Clin. Exp. Metastasis 33, 609–627 [DOI] [PubMed] [Google Scholar]
- 26. Jiang G., Zhang L., Zhu Q., Bai D., Zhang C., and Wang X. (2016) CD146 promotes metastasis and predicts poor prognosis of hepatocellular carcinoma. J. Exp. Clin. Cancer Res. 35, 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jouve N., Bachelier R., Despoix N., Blin M. G., Matinzadeh M. K., Poitevin S., Aurrand-Lions M., Fallague K., Bardin N., Blot-Chabaud M., Vely F., Dignat-George F., and Leroyer A. S. (2015) CD146 mediates VEGF-induced melanoma cell extravasation through FAK activation. Int. J. Cancer 137, 50–60 [DOI] [PubMed] [Google Scholar]
- 28. Flanagan K., Fitzgerald K., Baker J., Regnstrom K., Gardai S., Bard F., Mocci S., Seto P., You M., Larochelle C., Prat A., Chow S., Li L., Vandevert C., Zago W., et al. (2012) Laminin-411 is a vascular ligand for MCAM and facilitates TH17 cell entry into the CNS. PLoS ONE 7, e40443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tu T., Zhang C., Yan H., Luo Y., Kong R., Wen P., Ye Z., Chen J., Feng J., Liu F., Wu J. Y., and Yan X. (2015) CD146 acts as a novel receptor for netrin-1 in promoting angiogenesis and vascular development. Cell Res. 25, 275–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jouve N., Despoix N., Espeli M., Gauthier L., Cypowyj S., Fallague K., Schiff C., Dignat-George F., Vély F., and Leroyer A. S. (2013) The involvement of CD146 and its novel ligand Galectin-1 in apoptotic regulation of endothelial cells. J. Biol. Chem. 288, 2571–2579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Undi R. B., Sarvothaman S., Narasaiah K., Gutti U., and Gutti R. K. (2016) Toll-like receptor 2 signalling: significance in megakaryocyte development through Wnt signalling cross-talk and cytokine induction. Cytokine 83, 245–249 [DOI] [PubMed] [Google Scholar]
- 32. Ulmansky R., Landstein D., Moallem E., Loeb V., Levin A., Meyuhas R., Katzavian G., Yair S., and Naparstek Y. (2015) A humanized monoclonal antibody against heat shock protein 60 suppresses murine arthritis and colitis and skews the cytokine balance toward an anti-inflammatory response. J. Immunol. 194, 5103–5109 [DOI] [PubMed] [Google Scholar]
- 33. Kitazume S., Imamaki R., Ogawa K., and Taniguchi N. (2014) Sweet role of platelet endothelial cell adhesion molecule in understanding angiogenesis. Glycobiology 24, 1260–1264 [DOI] [PubMed] [Google Scholar]
- 34. Pop V. V., Seicean A., Lupan I., Samasca G., and Burz C. C. (2017) IL-6 roles: molecular pathway and clinical implication in pancreatic cancer: a systematic review. Immunol. Lett. 181, 45–50 [DOI] [PubMed] [Google Scholar]
- 35. Ara T., and Declerck Y. A. (2010) Interleukin-6 in bone metastasis and cancer progression. Eur. J. Cancer 46, 1223–1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Duckworth C. A., Guimond S. E., Sindrewicz P., Hughes A. J., French N. S., Lian L. Y., Yates E. A., Pritchard D. M., Rhodes J. M., Turnbull J. E., and Yu L. G. (2015) Chemically modified, non-anticoagulant heparin derivatives are potent galectin-3 binding inhibitors and inhibit circulating galectin-3-promoted metastasis. Oncotarget 6, 23671–23687 [DOI] [PMC free article] [PubMed] [Google Scholar]