Abstract
Purpose
Adenoid cystic carcinoma (ACC) represents ~10–15% of salivary neoplasms and almost universally exhibits a lethal clinical course. ACC is also known to occur in the lacrimal gland. ACC is characterized by its heterogeneous morphology and may demonstrate tubular, cribriform, and/or solid architectural patterns. Unfortunately, these histopathological features are not specific to ACC and can be seen in other salivary gland-type neoplasms, introducing a diagnostic dilemma. The discovery of fusion transcripts has revolutionized the diagnosis, surveillance, and treatment of epithelial malignancies. In several anatomic subsites ACC is frequently characterized by a fusion transcript involving genes MYB and NFIB; more specifically, t(6;9)(q22-23;p23-24). This study explores the incidence of MYB rearrangement in cases of lacrimal gland ACC using fluorescent in situ hybridization.
Materials and methods
Retrospective clinical and histopathological review of 12 cases of lacrimal gland ACC seen at Mayo Clinic over a 25-year period (1990–2015) was performed. Demographic and clinical data were obtained from medical records. Surgical pathology archival material including H&E slides and immunostains was re-examined. Formalin-fixed paraffin-embedded material was further evaluated using immunohistochemistry when appropriate. Fluorescent in situ hybridization (FISH) using a MYB break-apart probe was applied to all histologically confirmed cases of ACC and benign salivary gland parenchyma.
Results
The median patient age was 53.6 years (range 12–64) and distributed equally by gender (six male and six female). Rearrangement of MYB was identified using FISH in seven cases (58%). Twenty-five sections of benign salivary gland parenchyma showed no evidence of MYB rearrangement. Primary surgical resection was most common treatment, and 78% of the patient received adjuvant radiation therapy. Median overall survival (OS) was 11 years. Rearrangement of MYB did not affect OS.
Conclusions
In summary, our results indicate that the MYB rearrangement defines a significant subset of lacrimal gland ACCs. Importantly, FISH for MYB rearrangement may be used as a diagnostic tool during pathological examination of lacrimal gland neoplasms. Our results showed no relationship between rearrangement status and clinical outcome. Lastly, the presence of t(6;9) in ACC may provide a platform for molecular-targeting strategies in the future.
Introduction
Primary lacrimal gland tumors are relatively rare—accounting for 5–25% of space-occupying orbital lesions.1 Adenoid cystic carcinoma (ACC) has been reported to be the most common form of malignant epithelial lacrimal gland tumor, comprising approximately 66% of malignant lesions, and has been reported to have a 15-year survival rate of only 20%.2, 3, 4 In addition, ACCs of the lacrimal gland usually carry a poor prognosis due to osseous and/or perineural invasion.1
Clinical features of lacrimal gland ACC include ocular pain in combination with globe dystopia, proptosis, and ‘S' shaped ptosis. ACC is characterized by its heterogeneous histomorphology and may demonstrate tubular, cribriform, and/or solid architectural patterns.2 Unfortunately, these histopathological features are not specific to ACC and can be observed in other salivary gland-type neoplasms, often posing a diagnostic dilemma for pathologists. Optimal patient management is controversial but includes globe-sparing surgery followed by external radiotherapy (RT), proton-beam therapy, intra-arterial chemotherapy, or radical orbital exenteration in select cases.5, 6 The differential diagnosis for ACC includes benign and malignant entities such as pleomorphic adenoma, basal cell adenoma, basal cell adenocarcinoma, and polymorphous low-grade adenocarcinoma. Needless to say, accurate pathological classification is imperative for correct clinical management and prognostic implications.
The discovery of fusion transcripts has revolutionized the diagnosis, surveillance, and treatment of epithelial malignancies. In the context of salivary gland neoplasia, fusion transcripts are currently used to define and diagnose mucoepidermoid carcinoma, mammary analog secretory carcinoma of salivary glands, hyalinizing clear cell carcinoma, and ACC. Recently, ACC has been linked to a distinguishing cytogenetic abnormality, t(6;9) (q22-23; p23-24), resulting in a fusion between the gene encoding transcription factor MYB [v-myb myeloblastosis viral oncogene homolog (avian)] and NFIB (nuclear factor IB) transcription factor in 55–92% of cases from the salivary gland and breast.7, 8, 9, 10, 11 The suspected molecular result of this genetic alteration is activation of the MYB oncogene and associated target genes involved in cell growth, apoptosis, transcription, and cell cycle regulation.7, 8 This genetic rearrangement carries potential utility for diagnostic, prognostic, and even therapeutic purposes as MYB-activated pathways are currently being explored.8
Currently, over 800 pediatric and adult cases of ACC of the lacrimal gland have been reported in literature. However, most of the articles only describe single cases or small case series due to the rarity of lacrimal gland ACC.3, 4, 12, 13, 14 In addition, the molecular pathogenesis of ACC in the lacrimal gland has yet to be widely reported. Recent literature has shown that the translocation between chromosomes 6 and 9 (q22-23; p23-24), is present in ACC of the breast and head and neck.7 However, little is known regarding MYB-NFIB genetic alterations in lacrimal gland ACC. Recent studies also suggest that MYB may be activated by other mechanisms other than gene fusion, thus further emphasizing the role of MYB in ACC of the lacrimal gland.7, 8 We sought to identify the frequency of t(6;9)(q22-23;p23-24), involving MYB and NFIB, using fluorescent in situ hybridization (FISH) in cases of ACC specifically involving the lacrimal gland and to correlate the findings with clinical parameters and outcome.
Materials and methods
The study was approved by the Mayo Clinic Institutional Review Board. Cases of ACC between 1990 and 2015 were identified in the Mayo Clinic Anatomic Pathology database. Only cases of primary lacrimal gland ACC were included. Patients whose formalin-fixed paraffin-embedded tissue was not available for analysis were excluded from study.
Clinical data
Patients' medical records were reviewed and demographic and clinical data was abstracted including age at diagnosis, gender, primary tumor site, and treatment-related information (surgery, radiotherapy, and chemotherapy). To calculate survival end points, the date of diagnosis (biopsy or excision), date of recurrence (date of biopsy, or the date of imaging or clinic visit documenting relapse), and date of death or last follow-up were recorded.
Histopathology
Archival surgical pathology material was obtained for patients previously diagnosed with ACC of the lacrimal gland at Mayo Clinic Rochester between 1990 and 2015. Histopathologic analysis was performed by two surgical pathologists (MGK and JJG). Lacrimal gland ACC cases were classified according to the predominant architectural pattern (cribriform, solid, and tubular).
Cytogenetics
MYB rearrangement was analyzed with break-apart fluorescence in situ hybridization (FISH), as previously described by Roden et al.15 Human bacterial artificial chromosomes (BACs) flanking the MYB gene region were identified using the University of California Santa Cruz (UCSC) February 2009 Assembly hg19. The 5'MYB clones (CTD-2533B20, RP11-366H19 and RP11-63K22) were labeled by nick translation with Spectrum Orange dUTP (Abbott Molecular/Vysis Products, Abbott Park, IL, USA) and the 3'MYB clones (RP11-170P19, RP11-751F23 and RP11-259A7) were labeled with SpectrumGreen dUTP (Abbott Molecular/Vysis Products, Abbott Park, IL, USA). Labeled clones were then combined to create a dual-color fusion break-apart (BAP) probe set. The BAP probe set was applied to individual slides, hybridized, and washed according to the partially automated tissue (PAT) -reduced pepsin FISH protocol.
The slides were placed in a 90 °C oven for 15 min and then deparaffinized with xylene (two times, 15 min each) at room temperature (RT), dehydrated in 100% ethanol for 5 min at RT, and placed in 10 mM Citric Acid (pH 6.0) and microwaved for 10 min. Following this, the slides were immersed in 2 × standard saline citrate (SSC) for 5 min at 37 °C followed by digestion in 0.2% pepsin working solution (1.2 grams pepsin/600 ml 0.9% NaCl pH 2.5) at 37 °C for 12 min. Immediately after digestion, the slides were dehydrated using an ethanol series (70, 85, 100%) 2 min each at RT. Working solution of BAP MYB (Mayo Clinic Laboratory developed probe, Rochester, MN, USA) was made by mixing 1μl of concentrated 5'MYB probe and 1μl of concentrated 3'MYB probe with 8μl of LSI/WCP hybridization buffer (Abbott Laboratories, Abbott Park, IL, USA). The working solution was then applied to the target areas, cover slip added, co-denatured with a ThermoBrite (Abbott Park, IL, USA) at 83 °C for 5 min, and hybridized overnight in a 37 °C humidified oven.
Following hybridization, slides were soaked in RT 2 × SSC/0.1% NP-40 to remove coverslips, placed in 2 × SSC/0.1% NP-40 at 74 °C for 2 min and then placed into RT 2 × SSC/0.1% NP-40 for 2 min. The slides were stained with 4'-6,-diamidino-2-phenylindole (DAPI) (Vector Laboratories, Burlingame, CA, USA) and coverslips were added again for microscopic review. Visualization of the FISH signals was accomplished by using fluorescence microscope and pictures were captured by using a FISH imaging system (CytoVision, Leica Biosystems, Buffalo Grove, IL, USA). Two experienced FISH technologists (DLK and SMK) independently scored 50 tumor nuclei for each case. A cutoff of >5% breaks was selected for a positive score.
Statistical analysis
Statistical analysis was performed using software JMP version 10.0 (SAS Institute, Cary, NC, USA). Survival statistics were calculated using the Kaplan–Meier method. In order to calculate OS, the date of diagnosis and date of death were used. If patient was alive or lost to follow-up then date of last follow-up was used for censoring.
Results
Histopathology
Median size of the tumor was 2.7 cm (1–6.5). In regards to the histological pattern of the 12 ACC cases, four were predominantly cribriform, three were predominantly solid, two were a mix of solid and cribriform, two were a mix of solid and tubular, and one was a mix of tubular and cribriform (Figure 1). Results are summarized in Table 1.
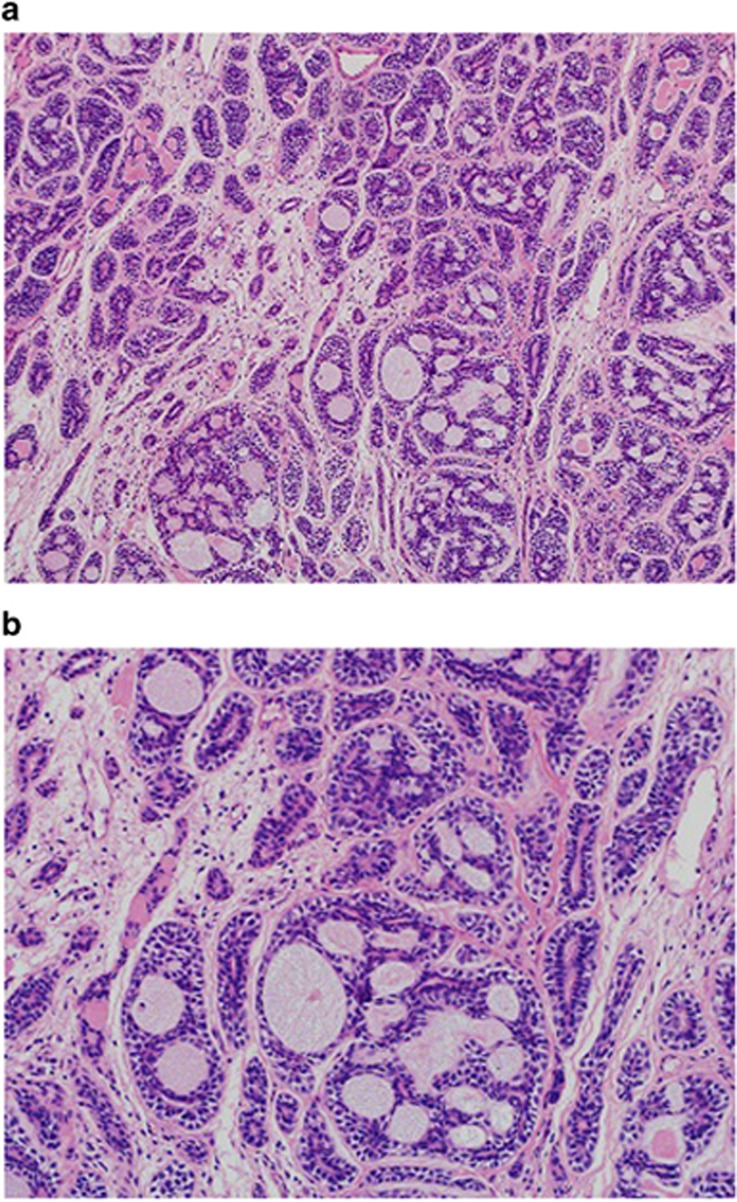
Figure 1.
Histopathology of lacrimal gland adenoid cystic carcinoma. Case showing cribriform and tubular architectural patterns (a; H&E × 200). Tumor is characterized by a dual population of myoepithelial and ductal cells (b; H&E, × 400).
Table 1. Clinical, histologic, and cytogenetic features of lacrimal gland adenoid cystic carcinoma.
| Patient | Age at diagnosis/gender | Architectural pattern | Local recurrence | Distant metastasis | MYB rearrangement | Follow-up time |
|---|---|---|---|---|---|---|
| 1 | 65 M | Solid | Yes | No | Present | DOD |
| 2 | 64 M | Solid/cribriform | Yes | Yes | Present | DOD |
| 3 | 55 M | Solid/cribriform | No | No | Present | NED 40 months |
| 4 | 41 F | Cribriform | No | No | Absent | DOD |
| 5 | 13 M | Cribriform | No | No | Absent | Lost to follow-up after 54 months |
| 6 | 55 F | Cribriform | Yes | No | Absent | AWD 137 months |
| 7 | 61 F | Cribriform | No | No | Present | NED 10 months |
| 8 | 53 F | Solid/tubular | No | No | Present | NED 11 months |
| 9 | 23 F | Solid | Yes | Yes | Absent | AWD 39 months |
| 10 | 22 M | Solid | No | No | Present | DOD |
| 11 | 60 F | Solid/tubular | No | No | Absent | NED 11 months |
| 12 | 56 M | Tubular/cribriform | No | No | Present | NED 11 months |
Abbreviations: AWD, alive with disease; DOD, died of disease; NED, no evidence of death.
Cytogenetics
Rearrangement of MYB was identified using FISH in seven cases (58% Figure 2). Rearrangement was noted in all architectural patterns (tubular, cribriform, and solid).
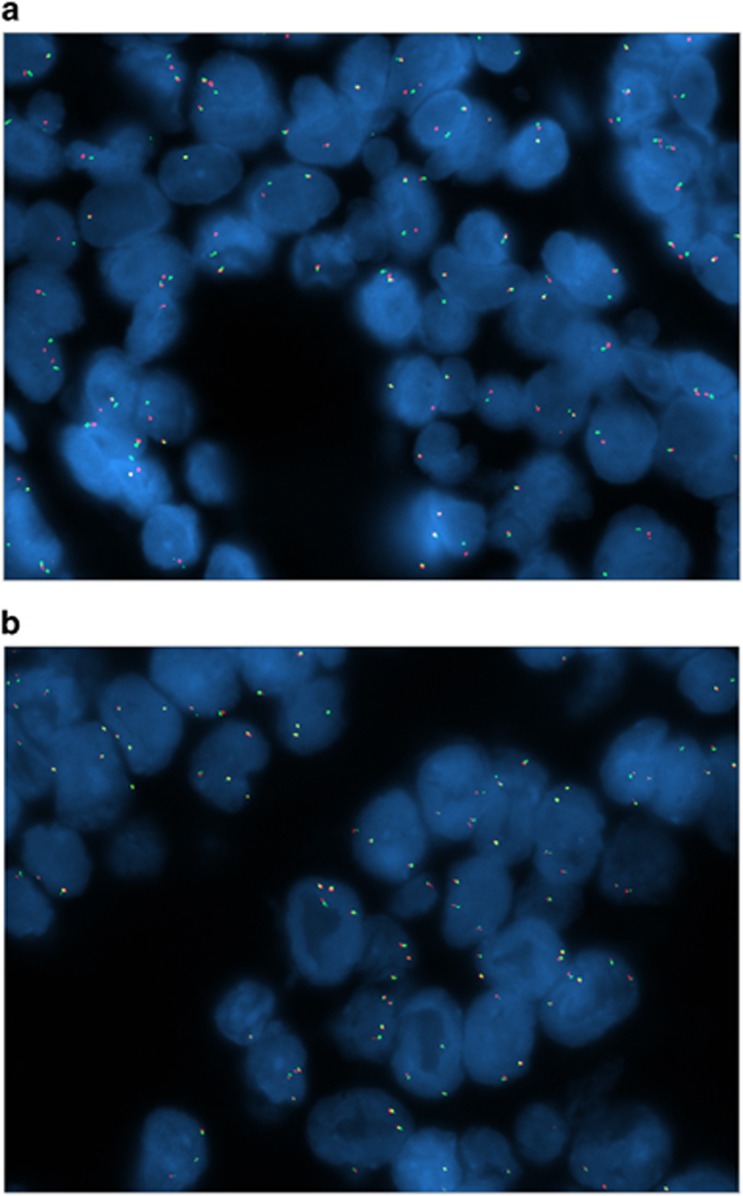
Figure 2.
FISH of lacrimal gland adenoid cystic carcinoma. Case showing rearrangement of MYB (a). Case showing no rearrangement of MYB (b).
To confirm the specificity of MYB rearrangement for ACC, FISH was also performed on 25 sections of benign salivary gland parenchyma. No rearrangement of the MYB gene region was observed in these sections.
Clinical outcome
Out of the 12 cases of lacrimal gland ACC, six were female. The median age was 54.9 years (12–65) years. Ten (83%) patients had surgical resection of the tumor as primary treatment. One patient declined treatment and one patient had no follow-up after initial diagnostic biopsy. Out of the 10 surgical patients, three patients had tumors removed with negative margins (R0), six had microscopic margins positive (R1) and one had gross residual disease at the completion of surgery (R2). Post operatively, seven (78%) patients received adjuvant radiation therapy, two patients did not, and one patient has no follow-up data after surgery. There were four locoregional recurrences and two patients developed distant disease after their surgical resection. Out of 12 patients, four have died of disease with median OS of 11 years for the whole cohort. Rearrangement of MYB did not affect OS.
Discussion
We identified MYB rearrangement in 58% of 12 cases of lacrimal gland ACC. Previous studies regarding lacrimal gland ACC are summarized in Table 2. Only one other study has shown a high frequency of MYB rearrangement in lacrimal gland ACC.3 von Holstein et al3 analyzed 14 patients with primary lacrimal gland ACC and 19 non-ACC lacrimal gland tumors using a dual-color break-apart FISH probe strategy. Their findings showed that seven out of 14 ACC cases showed MYB fusion, while all 19 non-ACC tumors displayed no fusion. Our results were similar to the findings by von Holstein et al,3 as well as to the findings by Brill et al,16 which identified a 44% frequency of MYB-NFIB fusion in ACC of multiple sites. The reason for variation in the frequency of detected MYB-NFIB fusion products is not entirely clear but likely multifactorial. Similar to von Holstein et al, our study used break-apart FISH probe strategy; Brill et al analyzed MYB-NFIB fusion products by RT-PCR. In addition, Brill et al16 found the fusion oncogene in 86% of ACC when using fresh frozen tissue in contrast to 44% when using FFPE tissue.
Table 2. Literature review of lacrimal gland ACC.
| Study (Year) | # of patients studied with ACC of lacrimal gland | Gender | Mean age at diagnosis, years (range) | # of patients alive without disease after treatment | Median follow-up, years (range) | Mean survival time |
|---|---|---|---|---|---|---|
| Font et al12 | 13 | 9M, 4F | 44 (14–80) | 2 | 8.5 Years (1–16 years) | 4.5 Years from time of initial surgery |
| Perez et al14 | 12 | 6M, 6F | 40.4 (19–52) | 4 | N/A | 77.8 Months from time of completion of treatment |
| Esmaeli et al4 | 20 | 6M, 14F | 39.5 (10–68) | 6 | 34 Months (6–264 months) | 18 Months from time of completion of treatment |
| Williams et al13 | 18 | 8M, 10F | 43 (9–69) | 10 | 26.5 Months (6–102 months) | N/A |
| von Holstein et al3 | 14 | 7M, 7F | 43 (23–67) | 5 | 7 Years (1–27 years) | Median tumor-specific survival was 8.6 years |
MYB rearrangement is considered highly specific for ACC and therefore helpful in the distinction of lacrimal gland ACC from other salivary gland carcinomas. This distinction is important as differences in prognosis and treatment are dramatic. Lacrimal gland ACC cases are reported to have a 15-year survival of 20%.2, 3, 4, 17 A study by Font et al (1998) showed that all 12 out of the lacrimal ACC cases had local recurrences ~3.5 years after surgical treatment.12
In support of the high specificity of t(6;9) for ACC, Mitani et al studied 34 non-ACCs of the salivary gland including myoepithelial carcinomas, acinic cell carcinomas, mucoepidermoid carcinomas and salivary duct carcinomas, polymorphous low-grade adenocarcinomas, and epithelial-myoepithelial carcinomas. In this study, none of these tumors harbored the t(6;9).8 West et al also evaluated a wide variety of non-ACC salivary gland tumors that included myoepithelioma, epithelial-myoepithelial carcinoma, pleomorphic adenoma/cellular pleomorphic adenoma/carcinoma ex pleomorphic adenoma, oncocytic hyperplasia/oncocytoma/oncocytic carcinoma, basal cell adenoma/adenocarcinoma, salivary duct carcinoma, and adenosquamous carcinoma as well as tumors similar to those studied by Mitani et al.9 Each of these tumors was negative for MYB rearrangement or fusion as well. Of note, only a single case of a polymorphous low-grade adenocarcinoma has been reported to harbor the t(6;9).18 We sought to confirm the specificity of MYB rearrangement for ACC in the lacrimal gland and to confirm its utility in the distinction from potential morphological mimickers in the lacrimal gland.
To test the specificity of t(6;9) for ACC, we also evaluated 25 cases with benign salivary gland parenchyma. We did not identify MYB rearrangement in any of these cases, providing additional evidence for the specificity of this genetic signature and its clinical utility in making the diagnosis of lacrimal gland ACC. However, although highly specific for ACC, the lack of this finding does not exclude a diagnosis of lacrimal gland ACC, given that MYB rearrangement is only present in a subset of these tumors.
The association of MYB-NFIB fusion with clinical features and prognosis of ACC, if any, is not entirely clear. Our results did not show any association between the presence of MYB rearrangement in lacrimal gland ACC and OS. Although our study sample size is relatively low in comparison with studies of other primary lacrimal gland carcinomas, it is quite large for lacrimal gland ACC given its overall rarity. These cases were collected over a 25-year period at a large institution. Therefore, a multicenter study is likely required to study an even larger population of ACC of the lacrimal gland to conduct reliable multivariable analysis.
The classic t(6;9) translocation may not only serve diagnostic purposes, but may potentially be of therapeutic value given its relatively high prevalence in lacrimal gland ACC. Although targeting MYB's transcriptional function itself is challenging, targeting molecules that are affected downstream of t(6;9) appears more promising. Studies by Moskaluk et al19 showed that low-passage primary ACC xenografts express spontaneously activated FGFR-1 receptors. This growth factor has been found to be upregulated in ACC cells with MYB overexpression and therefore is a candidate downstream effector of this oncoprotein.7, 20 In fact, clinical trials targeting FGF or FGFR in ACC are currently underway.21 Other potentially targetable downstream effectors of MYB include cell proliferation proteins (MYC, CD53, FGF2, VEGFA, KIT), cell cycle proteins (CCNB1, CDC2, MAD1L1), apoptosis-related markers (AP15, BCL2, BIRC3, HSPA8, SET) and cellular adhesion molecules (CD34), some of which are already targeted in clinical trials.21, 22
In conclusion, FISH for MYB rearrangement may be used as a diagnostic tool during the pathological examination of lacrimal gland neoplasms. However, a negative result does not rule out the possibility of lacrimal gland ACC. MYB rearrangement does not appear to be associated with prognosis of lacrimal gland ACC. However, multicenter studies might be helpful to confirm that finding in a larger patient population for robust statistical analysis. Lastly, the presence of t(6;9) in ACC may provide a platform for molecular-targeting strategies in the future.
Footnotes
The authors declare no conflict of interest.
References
- Weis E, Rootman J, Joly TJ, Berean KW, Al-Katan HM, Pasternak S et al. Epithelial lacrimal gland tumors: pathologic classification and current understanding. Arch Ophthalmol 2009; 127(8): 1016–1028. [DOI] [PubMed] [Google Scholar]
- Bernardini FP, Devoto MH, Croxatto JO. Epithelial tumors of the lacrimal gland: an update. Curr Opin Ophthalmol 2008; 19(5): 409–413. [DOI] [PubMed] [Google Scholar]
- von Holstein SL, Fehr A, Persson M, Therkildsen MH, Prause JU, Heegaard S et al. Adenoid cystic carcinoma of the lacrimal gland: MYB gene activation, genomic imbalances, and clinical characteristics. Ophthalmology 2013; 120(10): 2130–2138. [DOI] [PubMed] [Google Scholar]
- Esmaeli B, Ahmadi MA, Youssef A, Diba R, Amato M, Myers JN et al. Outcomes in patients with adenoid cystic carcinoma of the lacrimal gland. Ophthal Plast Reconstr Surg 2004; 20(1): 22–26. [DOI] [PubMed] [Google Scholar]
- Meldrum ML, Tse DT, Benedetto P. Neoadjuvant intracarotid chemotherapy for treatment of advanced adenocystic carcinoma of the lacrimal gland. Arch Ophthalmol 1998; 116(3): 315–321. [DOI] [PubMed] [Google Scholar]
- Tse DT, Benedetto P, Dubovy S, Schiffman JC, Feuer WJ. Clinical analysis of the effect of intraarterial cytoreductive chemotherapy in the treatment of lacrimal gland adenoid cystic carcinoma. Am J Ophthalmol 2006; 141(1): 44–53. [DOI] [PubMed] [Google Scholar]
- Persson M, Andrén Y, Mark J, Horlings HM, Persson F, Stenman G. Recurrent fusion of MYB and NFIB transcription factor genes in carcinomas of the breast and head and neck. Proc Natl Acad Sci USA 2009; 106(44): 18740–18744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitani Y, Rao PH, Futreal PA, Roberts DB, Stephens PJ, Zhao YJ et al. Novel chromosomal rearrangements and break points at the t(6;9) in salivary adenoid cystic carcinoma: association with MYB-NFIB chimeric fusion, MYB expression, and clinical outcome. Clin Cancer Res 2011; 17: 7003–7014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West RB, Kong C, Clarke N, Gilks T, Lipsick JS, Cao H et al. MYB expression and translocation in adenoid cystic carcinomas and other salivary gland tumors with clinicopathologic correlation. Am J Surg Pathol 2011; 35: 92–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetterskog D, Lopez-Garcia MA, Lambros MB, A'Hern R, Geyer FC, Milanezi F et al. Adenoid cystic carcinomas constitute a genomically distinct subgroup of triple-negative and basal-like breast cancers. J Pathol 2012; 226: 84–96. [DOI] [PubMed] [Google Scholar]
- Nordkvist A, Mark J, Gustafsson H, Bang G, Stenman G. Non-random chromosome rearrangements in adenoid cystic carcinoma of the salivary glands. Genes Chromosomes Cancer 1994; 10(2): 115–121. [DOI] [PubMed] [Google Scholar]
- Font RL, Smith SL, Bryan RG. Malignant epithelial tumors of the lacrimal gland: a clinicopathologic study of 21 cases. Arch Ophthalmol 1998; 116(5): 613–616. [DOI] [PubMed] [Google Scholar]
- Williams MD, Al-Zubidi N, Debnam JM, Shinder R, DeMonte F, Esmaeli B. Bone invasion by adenoid cystic carcinoma of the lacrimal gland: preoperative imaging assessment and surgical considerations. Ophthal Plast Reconstr Surg 2010; 26(6): 403–408. [DOI] [PubMed] [Google Scholar]
- Perez DE, Pires FR, Almeida OP, Kowalski LP. Epithelial lacrimal gland tumors: a clinicopathological study of 18 cases. Otolaryngol Head Neck Surg 2006; 134(2): 321–325. [DOI] [PubMed] [Google Scholar]
- Roden AC, Greipp PT, Knutson DL, Kloft-Nelson SM, Jenkins SM, Marks RS et al. Histopathologic and cytogenetic features of pulmonary adenoid cystic carcinoma. J Thorac Oncol 2015; 10(11): 1570–1575. [DOI] [PubMed] [Google Scholar]
- Brill LB, Kanner WA, Fehr A, Andren Y, Moskaluk CA, Loning T et al. Analysis of MYB expression and MYB-NFIB gene fusions in adenoid cystic carcinoma and other salivary neoplasms. Mod Pathol 2011; 24: 1160–1176. [DOI] [PubMed] [Google Scholar]
- Stefanyszyn MA, Hidayat AA, Pe'er JJ, Flanagan JC. Lacrimal sac tumors. Ophthal Plast Reconstr Surg 1994; 3: 169–184. [DOI] [PubMed] [Google Scholar]
- Persson F, Fehr A, Sundelin K, Löning T, Stenman G. Studies of genomic imbalances and the MYB-NFIB gene fusion in polymorphous low-grade adenocarcinoma of the head and neck. Ing J Oncol 2012; 40: 80–84. [DOI] [PubMed] [Google Scholar]
- Moskaluk CA, Baras AS, Mancuso SA, Fan H, Davidson RJ, Dirks DC et al. Development and characterization of xenograft model systems for adenoid cystic carcinoma. Lab Invest 2011; 91: 1480–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenman G, Anderson MK, Andren Y. New tricks from an old oncogene: gene fusion and copy number alterations of MYB in human cancer. Cell Cycle 2010; 9: 2986–2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon PM, Chakraborty S, Moskaluk CA, Joshi PJ, Thomas CY. Adenoid cystic carcinoma: a review of recent advances, molecular targets and clinical trials. Head Neck 2014; 38: 620–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson M, Andren Y, Moskaluk CA, Frierson Jr HF, Cooke SL, Futreal PA et al. Clinically significant copy number alterations and complex rearrangements of MYB and NFIB in head and neck adenoid cystic carcinoma. Genes Chromosomes Cancer 2012; 51: 805–817. [DOI] [PubMed] [Google Scholar]