Abstract
Purpose
The purpose of the study was to investigate nailfold microvascular morphology in exfoliation syndrome with or without glaucoma (XFS/XFG) compared with primary open-angle glaucoma (POAG) and control subjects using nailfold capillary videomicroscopy.
Patients and methods
We used a JH-1004 capillaroscope to perform nailfold capillary videomicroscopy on the fourth and fifth digit of the non-dominant hand. We enrolled 56 XFS/XFG patients, 87 POAG patients, and 75 control subjects. Masked observers graded the videos for hemorrhages, avascular zones ≥200 microns (μm), and degree of microvascular tortuosity on a four-point subjective scale. Multivariable odds ratios, 95% confidence intervals and P-for trends for assessing the relation between morphological changes and POAG or XFS/XFG were obtained from logistic regression analyses. We also assessed this relation with XFS/XFG compared with POAG in multivariable models.
Results
After adjusting for multiple covariates, nailfold hemorrhages, avascular zones ≥200 μm, and higher degree of vascular tortuosity were more common in XFS/XFG vs controls (P-for trend ≤0.0001) and in POAG vs controls (P-for trend ≤0.01). For each 100 capillaries, the number of hemorrhages was similar (P-for trend=0.91) between XFS/XFG and POAG patients; however, there were more avascular zones per 100 capillaries with borderline significance (P-for trend=0.04) in the XFS/XFG group. XFS/XFG patients had more tortuosity than POAG patients; specifically, having a tortuosity score ≥1.5 was associated with a 4.4-fold increased odds of XFS/XFG (95% confidence interval: 1.5–13.3) relative to a tortuosity score <1.0 (P-for trend=0.005).
Conclusion
A high degree of nailfold capillary tortuosity is a distinct non-ocular feature associated with XFS/XFG compared with either POAG or controls.
Introduction
Exfoliation syndrome (XFS) is a systemic disorder with high ocular morbidity in which extracellular fibrillar deposits (exfoliation material; XFM) accumulate in various tissues. XFM is particularly apparent on slit lamp biomicroscopic examination of the lens capsule, zonules, pupillary margin, corneal endothelium, and anterior chamber angle.1, 2, 3 Electron microscopy has revealed that XFM is present in most of the connective tissues of the anterior segment and ocular adnexa3 and in many non-ocular tissues such as the skin, heart, and lungs.3, 4, 5 XFM along with liberated uveal pigment lodge in the trabecular meshwork and may produce elevated intraocular pressure (IOP), optic nerve damage, and exfoliation glaucoma (XFG).6
Although XFM associated with blood vessels is not readily apparent clinically, many abnormalities in ocular and systemic vasculature are present in XFS/XFG. Fluorescein angiography reveals hypoperfusion, apparent microneovascularization, fluorescein leakage, and vascular anastomosis of iris vessels.7, 8, 9, 10, 11 There is apparent microneovascularization of the limbus as well as ectasia and tortuosity of ciliary vessels.12 Central retinal vein occlusions occur more commonly in XFS vs controls.13, 14 Systemically, vascular changes include a lower ankle brachial index in XFS vs controls, which suggests an accompanying peripheral vascular disease in XFS.15 Fingertip cutaneous capillary blood flow measured by laser Doppler flowmetry is lower in XFG compared with POAG and controls and the duration of cold-induced blood flow reduction as well as recovery periods are longer in XFG compared with POAG and controls.16 However, reports of systemic vascular comorbidities in XFS/XFG remain inconsistent17, 18 and further study is necessary to describe vascular changes in XFS/XFG.
Nailfold capillary videomicroscopy allows convenient in vivo visualization of the microvasculature at the junction of the fingernail and skin. Nailfold capillary videomicroscopy is used for diagnostic purposes in rheumatology19 and has served a role in defining vascular changes in primary open-angle glaucoma (POAG).20, 21, 22 Although nailfold capillary hemodynamics in XFS have been assessed,16 the nailfold capillary morphology in XFS/XFG has never been studied. The nailfold capillary bed shares the same hairpin loop architecture as the iris microvasculature23, 24 and thus may serve as a useful surrogate to gain further insight into the systemic manifestations of XFS/XFG. Therefore, we assessed nailfold capillary morphological features in XFS/XFG compared with either POAG or controls.
Materials and methods
Study design and sample
We performed a clinic-based, cross-sectional study of nailfold capillary videomicroscopy in 56 XFS/XFG, 87 POAG, and 75 control subjects. These participants were recruited to take part in the study from the Massachusetts Eye and Ear's Glaucoma and Comprehensive Ophthalmology Services in Boston, Massachusetts, USA from June 2014 to March 2016. All subjects were informed of the research proceedings and gave consent for participation in writing. This study was approved by Massachusetts Eye and Ear's Institutional Review Board and adhered to the Declaration of Helsinki.
All subjects were between the ages of 40 and 80 years at recruitment. No subjects had autoimmune connective tissue diseases such as systemic lupus erythematosus, systemic sclerosis, dermatomyositis, rheumatoid arthritis, and Sjogren's syndrome, as these conditions are associated with nailfold capillary morphological abnormalities.25, 26, 27, 28 No subjects had manifest diabetic retinopathy, which may indicate systemic microvasculopathy.29 XFS/XFG patients demonstrated XFM on slit lamp examination by an ophthalmologist at recruitment. POAG patients had no secondary cause for elevated IOP on anterior segment examination and no secondary cause for optic nerve disease on posterior segment examination. XFG and POAG patients demonstrated visual field loss consistent with nerve fiber layer pathology on reliable standard automated perimetry (Humphrey Visual Field 24-2 SITA standard) defined as fixation loss rate <33% and false-positive and negative rates <20% each. IOP was not used to define glaucoma. Control subjects had the following features: negative family history of glaucoma, IOP measurements ≤21 mm Hg, cup-disc ratio ≤0.6, cup-disc ratio asymmetry ≤0.1, normal slit lamp examinations, and full confrontational visual fields.
Nailfold capillary data collection and assessment
Nailfold capillaroscopy was performed with a JH-1004 capillaroscope at × 280 magnification (Jiahua Electronic Instrument Co., Jiangsu, China) on each subject's non-dominant fourth and fifth digits. The fourth and fifth digits of the non-dominant hand were selected to reduce the likelihood of minor traumas influencing the nailfold capillary morphology. Cedarwood oil was applied to the nailfold to facilitate clear microscopic imaging of the underlying vessels. The entire nailfold area on each of the two fingers was scanned and each video was stored for subsequent analysis.
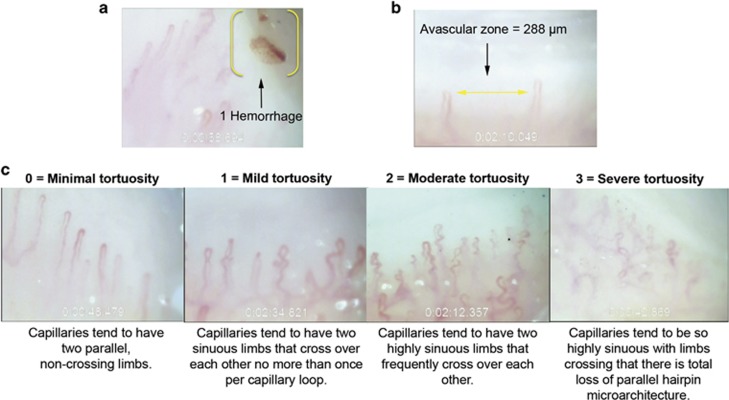
Readers masked to disease status analyzed the nailfold videos. For each subject, readers recorded the total number of capillaries sampled, counted hemorrhages and avascular zones ≥200 microns (μm), and rated the degree of microvascular tortuosity (Figure 1). The total number of capillaries sampled was used to normalize the number of hemorrhages and avascular zones to 100 capillaries per subject. Hemorrhages were defined as extracapillary blood or hemosiderin deposits. Avascular zones were defined as regions in the capillary bed displaying no capillaries for a horizontal distance with respect to the cuticle ≥200 μm. Tortuosity was assessed using a four-point subjective rating scale with the qualitative criteria shown in Figure 1. A tortuosity value (0, 1, 2, or 3) was assigned to vessels imaged at the 1- and 2-min marks of each nailfold capillary video. As two nailfold capillary videos were obtained for each subject, four separate tortuosity ratings were obtained for each subject and the mean was calculated ('mean tortuosity score'). We also imported the nailfold videos as image sequences into ImageJ software (available in the public domain at http://rsbweb.nih.gov/ij; National Institutes of Health, Bethesda, MD, USA) to obtain two alternative objective measures of tortuosity (a measure of vessel cross-over and a measure of vessel sinuosity) using methods described in the Supplementary Figure. Intraclass correlation coefficients (ICC) for all morphological assessments of nailfold capillaries showed good to excellent inter- and intra-rater reliability (inter-rater ICC≥0.74 for all features; intra-rater ICC≥0.91 for all features).
Figure 1.
Capillaroscopic images of a nailfold hemorrhage (a), an avascular zone ≥200 μm (b), and the four-point subjective tortuosity rating scale used for assessment (c).
Covariate data collection
At the time of nailfold capillaroscopy, we measured blood pressure (HEM-907XL, Omron Electronics LLC, Hoffman Estates, IL, USA), weight (HBF-400, Omron Electronics LLC), and height. Blood pressure was used to calculate mean arterial pressure (MAP), and height and weight were used to calculate body mass index (BMI) in weight in kilograms divided by height in meters squared. The following information was extracted from the medical record corresponding to the date of capillaroscopy and was adjusted for in multivariable logistic regression: age, sex, race, presence of a family history of glaucoma, history of type 1 or 2 diabetes mellitus, current use of antiplatelet or anticoagulant medication, smoking status (never, ever, or current), history of any rheumatic conditions not listed as exclusion criteria (such as osteoarthritis and gout), and evidence of any non-skin cancer malignancy.
Statistical methods
All statistical tests were two sided, and the significance level was P<0.0167 to account for three pairwise comparisons. SAS package 9.4 (SAS Institute Inc., Cary, NC, USA) was used for all statistical analyses. Normality assumptions were justified for each statistical test.
In univariate analyses, we used unpaired t-tests to compare means of continuous variables (age, BMI, and MAP) between XFS/XFG vs controls, POAG vs controls, and XFS/XFG vs POAG. χ2 tests were used to assess distribution differences of categorical variables (sex, race/ethnicity, family history of glaucoma, diabetes, using antiplatelet or anticoagulant medication, smoking, arthritis, and non-skin cancer malignancy) between the groups.
We used Mantel–Haenszel χ2 tests to assess the linear associations of nailfold capillary features across ordinal categories of increasing severity in XFS/XFG vs controls, POAG vs controls, and XFS/XFG vs POAG.
We performed multivariable-adjusted logistic regression models for each of the following pairwise comparisons: XFS/XFG vs controls, POAG vs controls, and XFS/XFG vs POAG. Multivariable odds ratios (OR) and 95% confidence intervals (95% CI) were obtained from a basic multivariable model accounting for the following covariates: age, sex, and race (family history of glaucoma was also added for the XFS/XFG vs POAG comparison), referred to as model 1. Model 2 was an expanded multivariable model accounting for the covariates in model 1 and the following clinical features: BMI, arthritis, diabetes, smoking (never, ever, or current), MAP, use of antiplatelet or anticoagulant medication, and presence of non-skin cancer malignancy. We performed multivariable tests for trends in our fully adjusted models (model 2) across three ordinal categories for each nailfold capillary feature.
Post hoc sample size calculations were based on univariate differences between XFS/XFG vs controls for each nailfold capillary feature, assuming a 1 : 1 sampling ratio and a type 1 error rate of 5%. We had at least 80% power to detect statistically significant differences between XFS/XFG and controls with 52 participants per group for any nailfold capillary feature.
Results
XFS/XFG patients were significantly older (mean age 72.2 years) than the POAG (mean age 64.8 years) and control subjects (mean age 64.8 years; P<0.0001 for both comparisons; Table 1). Both the XFS/XFG and POAG patients had lower BMI than controls (P=0.0007 and 0.04, respectively). More XFS/XFG patients were Caucasian (94.6%) compared with the POAG (80.5%) or control subjects (81.3%) and the distribution of patients across racial groups was most different between the XFS/XFG and POAG cohorts (P=0.02). XFS/XFG subjects were less likely to have a family history of glaucoma than were POAG patients (P=0.03). Controls had more diabetes than POAG patients (P=0.02). XFS/XFG were more commonly past or current cigarette smokers compared with controls (P=0.02) and were more likely to have rheumatic conditions not listed as exclusion criteria (such as osteoarthritis or gout) vs controls (P=0.03). Other demographic and clinical features between XFS/XFG, POAG, and control groups did not differ significantly. All differences were adjusted for in multivariable analyses.
Table 1. Demographic and clinical features in exfoliation syndrome/exfoliation glaucoma (XFS/XFG), primary open-angle glaucoma (POAG), and control subjects.
| Demographic and clinical features | XFS/XFG (n=56) | POAG (n=87) | Controls (n=75) | P-value for XFS/XFG vs Controls | P-value for POAG vs Controls | P-value for XFS/XFG vs POAG |
|---|---|---|---|---|---|---|
| Age in years, mean (SD) | 72.2 (5.3) | 64.8 (8.3) | 64.8 (9.4) | <0.0001a | 0.98a | <0.0001a |
| Body mass index in kg/m2, mean (SD) | 25.2 (4.6) | 26.7 (4.5) | 28.3 (5.4) | 0.0007a | 0.04a | 0.06a |
| Mean arterial pressure in mm Hg, mean (SD) | 93.9 (13.7) | 93.1 (11.9) | 94.6 (12.2) | 0.78a | 0.43a | 0.69a |
| Sex, n (%) | 0.32b | 0.43b | 0.77b | |||
| Male | 25 (44.6) | 41 (47.1) | 40 (53.3) | |||
| Female | 31 (55.4) | 46 (52.9) | 35 (46.7) | |||
| Race/ethnicity, n (%) | 0.07b | 0.30b | 0.02b | |||
| Caucasian | 53 (94.6) | 70 (80.5) | 61 (81.3) | |||
| African American/Asianc | 1 (1.8) | 14 (16.1) | 8 (10.7) | |||
| Hispanic/other | 2 (3.6) | 3 (3.5) | 6 (8.0) | |||
| Family history of glaucoma, n (%) | 21 (37.5) | 49 (56.3) | 0 (0.0) | NA | NA | 0.03b |
| Diabetes, n (%) | 12 (21.4) | 10 (11.5) | 19 (25.3) | 0.60b | 0.02b | 0.11b |
| Using antiplatelet or anticoagulant medication, n (%) | 25 (44.6) | 38 (43.7) | 33 (44.0) | 0.94b | 0.96b | 0.90b |
| Smoking, n (%) | 0.02b | 0.08b | 0.63b | |||
| Never | 32 (57.1) | 56 (64.4) | 60 (80.0) | |||
| Ever | 21 (37.5) | 26 (29.9) | 12 (16.0) | |||
| Current | 3 (5.4) | 5 (5.8) | 3 (4.0) | |||
| Arthritis,d n (%) | 15 (26.8) | 13 (14.9) | 9 (12.0) | 0.03b | 0.58b | 0.08b |
| Malignancy (non-skin cancer), n (%) | 10 (17.9) | 8 (9.2) | 7 (9.3) | 0.15b | 0.97b | 0.12b |
Abbreviation: NA, not available.
t-test.
χ2 test.
African Americans and Asians are grouped to permit statistical analysis.
Arthritic entities such as osteoarthritis or gout. No patients have rheumatoid arthritis or systemic autoimmune connective tissue diseases, which were exclusion criteria.
Univariate descriptive analyses of nailfold capillary features in relation to subject type (Table 2) showed that all microvascular abnormalities examined were more common in XFS/XFG vs controls and in POAG vs controls, whereas avascular zones and microvascular tortuosity were more common in XFS/XFG vs POAG. About 60.8% of XFS/XFG patients presented with any nailfold capillary hemorrhages compared with 71.3% of POAG patients and 32.0% of controls. About 80.3% of XFS/XFG patients, 59.7% of POAG patients, and 20.0% of controls had any avascular zones ≥200 μm. Finally, 75.0% of XFS/XFG patients, 55.1% of POAG patients, and 33.4% of controls had mean tortuosity scores ≥1.0 (mild or worse). Data from two additional tortuosity assays (vessel cross-over and vessel sinuosity) are consistent with the findings of higher degrees of tortuosity in XFS/XFG vs controls, POAG vs controls, and XFS/XFG vs POAG (Supplementary Table 1).
Table 2. Descriptive analyses of nailfold microvascular features in exfoliation syndrome/exfoliation glaucoma (XFS/XFG), primary open-angle glaucoma (POAG), and control subjects.
| Nailfold microvascular features | XFS/XFG (n=56) | POAG (n=87) | Controls (n=75) | P-valuea for XFS/XFG vs Controls | P-valuea for POAG vs Controls | P-valuea for XFS/XFG vs POAG |
|---|---|---|---|---|---|---|
| Number of hemorrhages per 100 capillaries, n (%) | <0.0001 | <0.0001 | 0.64 | |||
| 0.0 | 22 (39.3) | 25 (28.7) | 51 (68.0) | |||
| >0.0 and <1.0 | 10 (17.9) | 28 (32.2) | 15 (20.0) | |||
| ≥1.0 | 24 (42.9) | 34 (39.1) | 9 (12.0) | |||
| Number of avascular zones ≥200 μm per 100 capillaries, n (%) | <0.0001 | <0.0001 | 0.001 | |||
| 0.0 | 11 (19.6) | 35 (40.2) | 60 (80.0) | |||
| >0.0 and <0.5 | 6 (10.7) | 17 (19.5) | 7 (9.3) | |||
| ≥0.5 | 39 (69.6) | 35 (40.2) | 8 (10.7) | |||
| Mean tortuosity score,b n (%) | <0.0001 | 0.02 | 0.0004 | |||
| <1.0 | 14 (25.0) | 39 (44.8) | 50 (66.7) | |||
| ≥1.0 and <1.5 | 12 (21.4) | 29 (33.3) | 14 (18.7) | |||
| ≥1.5 | 30 (53.6) | 19 (21.8) | 11 (14.7) |
Mantel–Haenszel χ2 test.
Tortuosity scores assigned at the 1-min and 2-min time points on both fingers assessed. Mean tortuosity score is the average of four tortuosity ratings per patient.
In the fully adjusted multivariable logistic regression models (Table 3), having ≥1.0 hemorrhage relative to no hemorrhages per 100 capillaries was associated with 15.3-fold increased odds of XFS/XFG relative to controls (95% CI: 3.8–60.8) and 6.8-fold increased odds of POAG vs controls (95% CI: 2.7–17.4). Tests of linear association across ordinal categories of hemorrhages were significant for both XFS/XFG and POAG compared with controls (P-for trend <0.0001 for both). There was no statistical difference in number of hemorrhages observed per 100 capillaries between XFS/XFG and POAG groups (P-for trend=0.91). Having ≥0.5 avascular zones ≥200 μm on average per 100 capillaries relative to having no such zones was strongly associated with XFS/XFG vs controls (OR=34.4, 95% CI: 8.6–137.4) and with POAG vs controls (OR=10.4, 95% CI: 3.7–29.2) and trends for both were significant (P-for trend <0.0001). There was a borderline significant increased risk of XFS/XFG vs POAG for having ≥0.5 avascular zones ≥200 μm (OR=2.7, 95% CI: 1.0–7.4; P-for trend=0.04). Having a mean tortuosity score ≥1.5 relative to a score <1.0 was associated strongly with XFS/XFG (OR=17.9, 95% CI: 5.0–63.6) and associated moderately with POAG (OR=2.6, 95% CI: 1.0–6.8) compared with controls. The corresponding P-for trends were <0.0001 and 0.01, respectively. Having a mean tortuosity score ≥1.5 relative to a score <1.0 remained significantly associated with XFS/XFG when compared with POAG (OR=4.4, 95% CI: 1.5–13.3) with a corresponding P-for trend=0.005. Multivariable analyses using the two additional tortuosity assays (vessel cross-over and vessel sinuosity) are consistent with the findings of higher degrees of tortuosity in XFS/XFG vs controls, POAG vs controls, and XFS/XFG vs POAG (Supplementary Table 2). Nailfold capillary findings in an XFS patient, POAG patient, and a control subject are demonstrated with example capillaroscopic images in Figure 2 and corresponding sample videos presented in Supplementary Videos 1, 2, and 3.
Table 3. Multivariable logistic regression analyses of nailfold microvascular features in relation to exfoliation syndrome/exfoliation glaucoma (XFS/XFG) and primary open-angle glaucoma (POAG).
| Nailfold microvascular features |
XFS/XFG vs Controls |
POAG vs Controls |
XFS/XFG vs POAG |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Multivariable-adjusted Model 1a odds ratio (95% confidence interval) | Multivariable-adjusted Model 2b odds ratio (95% confidence interval) | P-for trend for Model 2 | Multivariable-adjusted Model 1a odds ratio (95% confidence interval) | Multivariable-adjusted Model 2b odds ratio (95% confidence interval) | P-for trend for Model 2 | Multivariable-adjusted Model 1c odds ratio (95% confidence interval) | Multivariable-adjusted Model 2d odds ratio (95% confidence interval) | P-for trend for Model 2 | |
| Number of hemorrhages per 100 capillaries | |||||||||
| 0.0 | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) | |||
| >0.0 and <1.0 | 1.1 (0.4–3.2) | 0.8 (0.2–2.9) | <0.0001 | 3.9 (1.7–9.0) | 3.7 (1.5–8.9) | <0.0001 | 0.2 (0.1–0.8) | 0.2 (0.1–0.8) | 0.91 |
| ≥1.0 | 9.5 (2.9–30.2) | 15.3 (3.8–60.8) | 7.4 (3.0–18.2) | 6.8 (2.7–17.4) | 0.7 (0.2–1.9) | 0.6 (0.2–1.7) | |||
| Number of avascular zones ≥200 μm per 100 capillaries | |||||||||
| 0.0 | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) | |||
| >0.0 and <0.5 | 4.3 (1.1–16.5) | 9.4 (1.8–46.9) | <0.0001 | 4.3 (1.6–11.6) | 5.8 (1.9–17.9) | <0.0001 | 1.1 (0.3–3.9) | 1.1 (0.2–4.7) | 0.04 |
| ≥0.5 | 21.7 (7.1–66.1) | 34.4 (8.6–137.4) | 7.3 (2.9–17.8) | 10.4 (3.7–29.2) | 3.1 (1.2–7.9) | 2.7 (1.0–7.4) | |||
| Mean tortuosity scoree | |||||||||
| <1.0 | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) | |||
| ≥1.0 and <1.5 | 3.3 (1.1–10.5) | 3.5 (1.0–13.4) | <0.0001 | 2.6 (1.2–5.8) | 3.2 (1.4–7.4) | 0.01 | 1.1 (0.3–3.0) | 0.9 (0.3–3.1) | 0.005 |
| ≥1.5 | 12.1 (4.1–35.6) | 17.9 (5.0–63.6) | 2.2 (0.9–5.2) | 2.6 (1.0–6.8) | 4.3 (1.6–11.7) | 4.4 (1.5–13.3) | |||
Abbreviation: ref, reference.
Adjusted for age, sex, and race.
Adjusted for age, sex, race, body mass index, arthritis, diabetes, smoking (never, ever, or current), mean arterial pressure, use of antiplatelet or anticoagulant medication, and malignancy (non-skin cancer).
Adjusted for age, sex, race, and family history of glaucoma.
Adjusted for age, sex, race, family history of glaucoma, body mass index, arthritis, diabetes, smoking (never, ever, or current), mean arterial pressure, use of antiplatelet or anticoagulant medication, and malignancy (non-skin cancer).
Tortuosity scores assigned at the 1-min and 2-min time points on both fingers assessed. Mean tortuosity score is the average of four tortuosity ratings per patient.
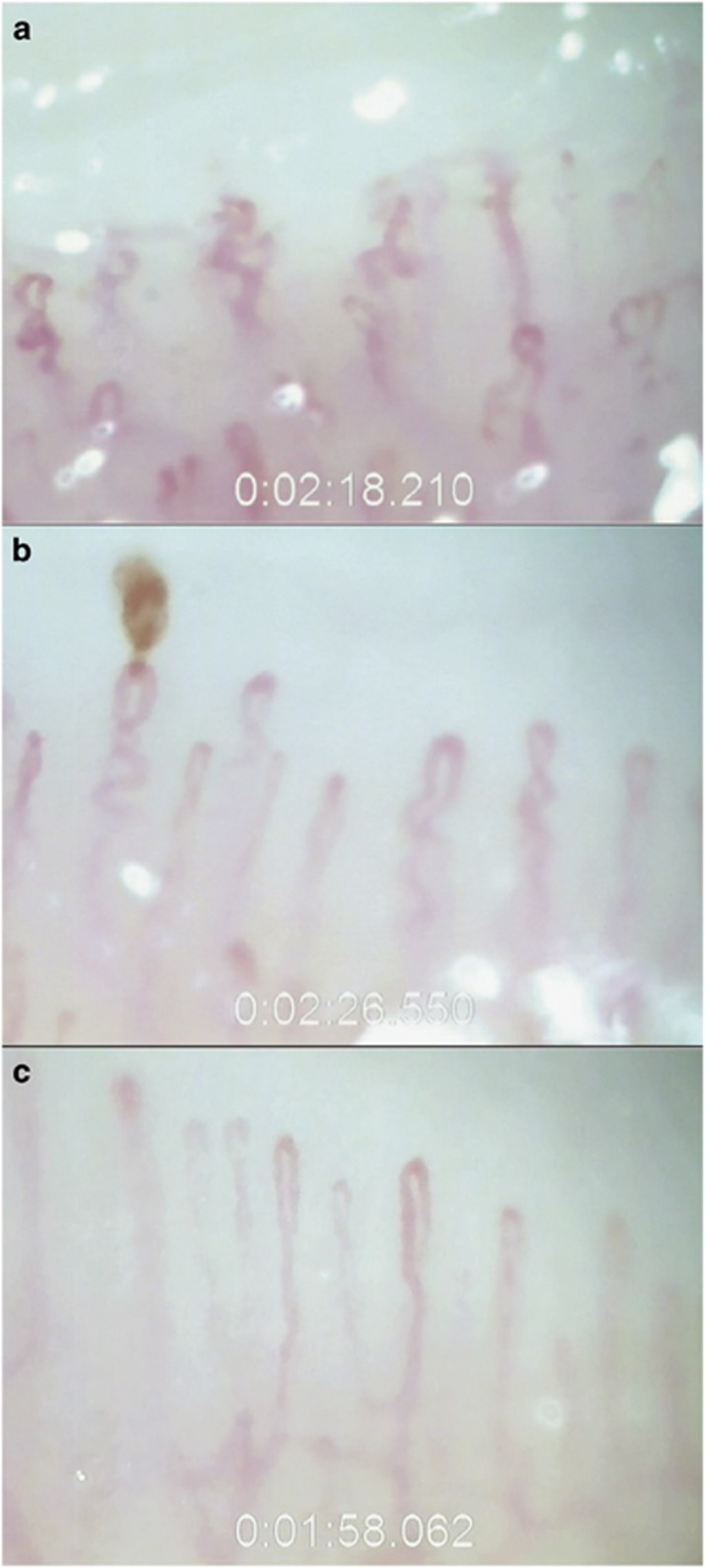
Figure 2.
Nailfold capillary presentation of a 63-year-old Caucasian male with exfoliation syndrome (a), a 68-year-old Caucasian male with primary open-angle glaucoma (b), and a 69-year-old Caucasian female control subject (c). Note the microvascular tortuosity in the XFS subject, hemorrhage in the POAG patient, and normal architecture of parallel hairpin-shaped capillaries in the control. Supplementary Material available at Eye's website shows nailfold capillary video clips from these three patients.
Discussion
We found that nailfold capillary hemorrhages, avascular zones ≥200 μm, and degree of microvascular tortuosity each had a greater association with XFS/XFG and POAG compared with controls. Tortuosity was also strongly associated with XFS/XFG when the comparison group was POAG. Avascular zones ≥200 μm were slightly more common in XFS/XFG compared with POAG. These findings represent a novel distinct non-ocular feature of XFS/XFG and demonstrate that non-ocular microvascular changes accompanying XFS/XFG may be conveniently observed in the clinic.
The processes by which nailfold capillary abnormalities develop in XFS/XFG remain unknown. Electron micrographs of iridectomy specimens have located XFM in the adventitia of iris blood vessels, often with obliterated lumens,30 and biochemical studies have determined that XFM is composed of extracellular matrix and basement membrane components.31 We speculate that accumulation of XFM in the walls of digital pre-capillary arterioles or in nailfold capillaries produces nontubular vascular lumens that alter local hemodynamics and contribute to the tortuosity, hemorrhaging, and avascularity observed in this study. Furthermore, as elastin insulates all blood vessels in the body, insufficient elastogenesis due to altered lysyl oxidase-like 1 (LOXL1) expression32 (strongly implicated in XFS/XFG33, 34) may also contribute to microvascular morphological changes. Interestingly, iris fluorescein angiography in LOXL1 knockout mice demonstrates leakage into the anterior chamber,35 and canine carotid arteries exposed to elastase exhibit marked tortuosity.36 These findings support the notion that elastin is needed to maintain vascular integrity. Moreover, the nailfold is largely unshielded from ambient solar exposure and it is known that ultraviolet radiation alters LOXL1 expression.37 Perhaps interaction between ambient ultraviolet rays and LOXL1 activity38, 39 contributes to the nailfold capillary morphological changes we observed.
Another factor that may contribute to the unique nailfold capillary tortuosity and avascularity in XFS/XFG is homocysteine (Hcy), a biomarker that is mildly, but consistently elevated in XFS/XFG aqueous humor, tears, and plasma compared with controls.17, 40 Serum Hcy may also be elevated in XFS/XFG compared with POAG,41, 42, 43 although this remains controversial.44, 45 Hcy is a non-proteinogenic amino acid that is an intermediate molecule generated during the conversion of methionine to cysteine.46 Injection of Hcy in cerebral microvessels induces dramatic vascular leakage in mice.47 Elevated Hcy is associated with endothelial cell dysfunction in atherosclerosis and small vessel disease.48, 49 Baroreflex regulation of arterial pressure is negatively correlated with plasma Hcy concentration in XFS/XFG.50 Abnormal endothelial cell function due to elevated Hcy may influence regulation of vascular tone in XFS/XFG rendering the nailfold capillaries tortuous and prone to leakage.
The presence of frequent nailfold hemorrhages in POAG and XFS/XFG deserves some attention. Previously we reported that 76.9% of 199 POAG patients had any hemorrhages using a protocol identical to the one used for this work.21 Using an entirely new patient sample we observed that 71.3% of POAG patients had similar findings. In contrast, 60.8% of XFS/XFG had any hemorrhages. We speculated that impaired nitric oxide signaling might be responsible for nailfold hemorrhages in POAG.21 Interestingly, some51, 52 but not all studies53 show that asymmetric dimethyl arginine, an inhibitor of nitric oxide synthase and contributor to endothelial cell dysfunction, is elevated in the aqueous humor and serum of XFS/XFG patients. One needs also to consider that other mechanisms may be operative in the formation of nailfold hemorrhages in XFS/XFG including a tendency for inherent blood clot formation as demonstrated by rotational thromboelastography.54
Several limitations of this nailfold capillaroscopy study merit discussion. The sample size in each group was limited and thus the OR estimates had large confidence intervals. The sample size did not permit a meaningful comparison of patients with XFS with those with XFG. Thus the study cannot clarify which, if any, nailfold capillary abnormalities are associated with XFS alone or with XFS and the related glaucoma. Nonetheless, there is compelling reason to combine data on XFS and XFG, as XFM is a biomarker for both conditions and the presence of XFS often leads to the requirement of IOP lowering treatment during follow-up.55 Our data came from a clinic-based sample where 94.6% of XFS/XFG patients were Caucasian, limiting the applicability of our conclusions to other ethnic groups. Our study was cross-sectional in nature. Therefore, we cannot determine whether nailfold capillary morphological changes precede or postdate the development of XFM in the eye. Finally, due to the nature of the capillaroscopic features that we investigated, some subjectivity is inherent in the grading of nailfold capillary videos. Although biases were minimized with the use of masked observers and inter- and intra-rater ICCs were strong, inter- and intra-reader differences invariably exist.
Our study also has several notable strengths. To investigate nailfold capillary abnormalities in XFS/XFG, we used two comparison groups: controls as well as POAG patients. Comparing XFS/XFG with POAG patients helped to identify whether the nailfold capillary abnormalities observed in XFS/XFG are driven by mechanisms that are shared with POAG or by mechanisms that are unique to XFS/XFG. Our data on severe microvascular tortuosity provide evidence that there may be a specific vascular signature for XFS/XFG. In addition, we used several multivariable models to show that abnormalities exist in XFS/XFG vs controls and in XFS/XFG vs POAG even after adjusting for demographics alone or for demographics plus systemic conditions, indicating that these abnormalities were independent predictors. Finally, we measured tortuosity using three different methods (two are presented in Supplementary Material) and found similar results in each, demonstrating the robustness of our findings.
In conclusion, we report that a high degree of nailfold microvascular tortuosity is a unique non-ocular feature of XFS/XFG compared with POAG and control subjects. Marked nailfold capillary tortuosity (≥1.5 on a scale of 0–3) is probably of limited diagnostic value as this morphological feature was present in 14.7% of controls; however, nailfold capillary microscopy could be useful to monitor treatment responses should a systemic therapy be developed for XFS. It is unclear whether or not patients with XFS/XFG exhibit tortuosity in other non-ocular microvascular beds and whether or not there are significant clinical consequences of such tortuosity. Further study is necessary to evaluate the ramifications of these non-ocular microvascular changes in XFS.
Acknowledgments
We thank Massachusetts Eye and Ear Ophthalmology Clinical Research Operations especially Ai Ren and Patricia Houlihan. This work was supported by the Harvard Glaucoma Center of Excellence (Drs Pasquale and Wiggs), NEI R01 EY020928 (Dr Wiggs), a Harvard Medical School Distinguished Scholar Award (Dr Pasquale), BrightFocus Foundation Grant G2011047 (Dr Knepper), Rosemary O' Meara and Kathleen F. Connelly Memorial Funds (Dr Knepper), the Illinois Society for the Prevention of Blindness (Dr Knepper), and the Henry Cohn Research Fund of the New York Glaucoma Research Institute (Dr Ritch). Dr Pasquale has served as a consultant to Novartis and Bausch+Lomb. He has received support to travel to the Exfoliation Glaucoma Think Tank Meetings in New York City by the Glaucoma Foundation.
Footnotes
Supplementary Information accompanies this paper on Eye website (http://www.nature.com/eye)
This data were presented in part at the 2016 American Glaucoma Society Meeting in Ft. Lauderdale, Florida.
The authors declare no conflict of interest.
Supplementary Material
References
- Prince AM, Ritch R. Clinical signs of the pseudoexfoliation syndrome. Ophthalmology 1986; 93: 803–807. [DOI] [PubMed] [Google Scholar]
- Vesti E, Kivelä T. Exfoliation syndrome and exfoliation glaucoma. Prog Retin Eye Res 2000; 19: 345–368. [DOI] [PubMed] [Google Scholar]
- Ritch R, Schlötzer-Schrehardt U. Exfoliation syndrome. Surv Ophthalmol 2001; 45: 265–315. [DOI] [PubMed] [Google Scholar]
- Schlötzer-Schrehardt UM, Koca MR, Naumann GO, Volkholz H. Pseudoexfoliation syndrome. Ocular manifestation of a systemic disorder? Arch Ophthalmol 1992; 110: 1752–1756. [DOI] [PubMed] [Google Scholar]
- Streeten BW, Li ZY, Wallace RN, Eagle RC Jr., Keshgegian AA. Pseudoexfoliative fibrillopathy in visceral organs of a patient with pseudoexfoliation syndrome. Arch Ophthalmol 1992; 110: 1757–1762. [DOI] [PubMed] [Google Scholar]
- Ritch R, Schlötzer-Schrehardt U, Konstas AG. Why is glaucoma associated with exfoliation syndrome? Prog Retin Eye Res 2003; 22: 253–275. [DOI] [PubMed] [Google Scholar]
- Parodi MB, Bondel E, Saviano S, Ravalico G. Iris indocyanine green angiography in pseudoexfoliation syndrome and capsular glaucoma. Acta Ophthalmol Scand 2000; 78: 437–442. [DOI] [PubMed] [Google Scholar]
- Battaglia Parodi M, Bondel E, Saviano S, Ravalico G. Fluorescein angiography and indocyanine green videoangiography in the iris of pseudoexfoliation syndrome. Metab Pediatr Syst Ophthalmol (1985) 1998; 21: 7–13. [PubMed] [Google Scholar]
- Brooks AM, Gillies WE. The development of microneovascular changes in the iris in pseudoexfoliation of the lens capsule. Ophthalmology 1987; 94: 1090–1097. [DOI] [PubMed] [Google Scholar]
- Parodi MB, Bondel E, Saviano S, Ravalico G. Iris fluorescein angiography and iris indocyanine green videoangiography in pseudoexfoliation syndrome. Eur J Ophthalmol 1999; 9: 284–290. [DOI] [PubMed] [Google Scholar]
- Brooks AM, Gillies WE. Fluorescein angiography and fluorophotometry of the iris in pseudoexfoliation of the lens capsule. Br J Ophthalmol 1983; 67: 249–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laatikainen L. Fluorescein angiographic studies of the peripapillary and perilimbal regions in simple, capsular and low-tension glaucoma. Acta Ophthalmol Suppl 1971; 111: 3–83. [PubMed] [Google Scholar]
- Karagiannis D, Kontadakis GA, Klados NE, Tsoumpris I, Kandarakis AS, Parikakis EA et al. Central retinal vein occlusion and pseudoexfoliation syndrome. Clin Interv Aging 2015; 10: 879–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritch R, Prata TS, de Moraes CG, Vessani RM, Costa VP, Konstas AG et al. Association of exfoliation syndrome and central retinal vein occlusion: an ultrastructural analysis. Acta Ophthalmol 2010; 88: 91–95. [DOI] [PubMed] [Google Scholar]
- Praveen MR, Shah SK, Vasavada AR, Diwan RP, Shah SM, Zumkhawala BR et al. Pseudoexfoliation as a risk factor for peripheral vascular disease: a case-control study. Eye 2011; 25: 174–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holló G, Lakatos P, Farkas K. Cold pressor test and plasma endothelin-1 concentration in primary open-angle and capsular glaucoma. J Glaucoma 1998; 7: 105–110. [PubMed] [Google Scholar]
- Pasquale LR, Borras T, Fingert JH, Wiggs JL, Ritch R. Exfoliation syndrome: assembling the puzzle pieces. Acta Ophthalmol 2016; 94: e505–e512. [DOI] [PubMed] [Google Scholar]
- Andrikopoulos GK, Alexopoulos DK, Gartaganis SP. Pseudoexfoliation syndrome and cardiovascular diseases. World J Cardiol 2014; 6: 847–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza EJ, Kayser C. Nailfold capillaroscopy: relevance to the practice of rheumatology. Rev Bras Reumatol 2015; 55: 264–271. [DOI] [PubMed] [Google Scholar]
- Park HY, Park SH, Oh YS, Park CK. Nail bed hemorrhage: a clinical marker of optic disc hemorrhage in patients with glaucoma. Arch Ophthalmol 2011; 129: 1299–1304. [DOI] [PubMed] [Google Scholar]
- Pasquale LR, Hanyuda A, Ren A, Giovingo M, Greenstein SH, Cousins C et al. Nailfold capillary abnormalities in primary open-angle glaucoma: a multisite study. Invest Ophthalmol Vis Sci 2015; 56: 7021–7028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Božic M, Sencanic PH, Spahic G, Kontic D, Markovic V, Marjanovic I et al. Is nail fold capillaroscopy useful in normotensive and primary open angle glaucoma? A pilot study. Curr Eye Res 2010; 35: 1099–1104. [DOI] [PubMed] [Google Scholar]
- Song Y, Song YJ, Ko MK. A study of the vascular network of the iris using flat preparation. Korean J Ophthalmol 2009; 23: 296–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith V. When and how to perform capillaroscopy. In: Cutolo M (ed). Atlas of Capillaroscopy in Rheumatic Diseases. Elsevier: Milan, Italy, 2011, p 34. [Google Scholar]
- Ohtsuka T. Nailfold capillary abnormalities in patients with Sjögren's syndrome and systemic lupus erythematosus. Br J Dermatol 1997; 136: 94–96. [PubMed] [Google Scholar]
- Manfredi A, Sebastiani M, Cassone G, Pipitone N, Giuggioli D, Colaci M et al. Nailfold capillaroscopic changes in dermatomyositis and polymyositis. Clin Rheumatol 2015; 34: 279–284. [DOI] [PubMed] [Google Scholar]
- Altomonte L, Zoli A, Galossi A, Mirone L, Tulli A, Martone FR et al. Microvascular capillaroscopic abnormalities in rheumatoid arthritis patients. Clin Exp Rheumatol 1995; 13: 83–86. [PubMed] [Google Scholar]
- Cortes S, Cutolo M. Capillarosecopic patterns in rheumatic diseases. Acta Reumatol Port 2007; 32: 29–36. [PubMed] [Google Scholar]
- Barchetta I, Riccieri V, Vasile M, Stefanantoni K, Comberiati P, Taverniti L et al. High prevalence of capillary abnormalities in patients with diabetes and association with retinopathy. Diabet Med 2011; 28: 1039–1044. [DOI] [PubMed] [Google Scholar]
- Ghosh M, Speakman JS. The iris in senile exfoliation of the lens. Can J Ophthalmol 1974; 9: 289–297. [PubMed] [Google Scholar]
- Zenkel M, Schlötzer-Schrehardt U. The composition of exfoliation material and the cells involved in its production. J Glaucoma 2014; 23(Suppl 1): S12–S14. [DOI] [PubMed] [Google Scholar]
- Liu X, Zhao Y, Gao J, Pawlyk B, Starcher B, Spencer JA et al. Elastic fiber homeostasis requires lysyl oxidase-like 1 protein. Nat Genet 2004; 36: 178–182. [DOI] [PubMed] [Google Scholar]
- Thorleifsson G, Magnusson KP, Sulem P, Walters GB, Gudbjartsson DF, Stefansson H et al. Common sequence variants in the LOXL1 gene confer susceptibility to exfoliation glaucoma. Science 2007; 317: 1397–1400. [DOI] [PubMed] [Google Scholar]
- Wang L, Yu Y, Fu S, Zhao W, Liu P. LOXL1 gene polymorphism with exfoliation syndrome/exfoliation glaucoma: a meta-analysis. J Glaucoma 2016; 25: 62–94. [DOI] [PubMed] [Google Scholar]
- Wiggs JL, Pawlyk B, Connolly E, Adamian M, Miller JW, Pasquale LR et al. Disruption of the blood-aqueous barrier and lens abnormalities in mice lacking lysyl oxidase-like 1 (LOXL1). Invest Ophthalmol Vis Sci 2014; 55: 856–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrin PB, Schwarcz TH, Baker WH. Mechanisms of arterial and aneurysmal tortuosity. Surgery 1988; 104: 568–571. [PubMed] [Google Scholar]
- Zenkel M, Krysta A, Pasutto F, Juenemann A, Kruse FE, Schlötzer-Schrehardt U. Regulation of lysyl oxidase-like 1 (LOXL1) and elastin-related genes by pathogenic factors associated with pseudoexfoliation syndrome. Invest Ophthalmol Vis Sci 2011; 52: 8488–8495. [DOI] [PubMed] [Google Scholar]
- Pasquale LR, Jiwani AZ, Zehavi-Dorin T, Majd A, Rhee DJ, Chen T et al. Solar exposure and residential geographic history in relation to exfoliation syndrome in the United States and Israel. JAMA Ophthalmol 2014; 132: 1439–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquale LR, Kang JH, Wiggs JL. Consideration for gene-environment interactions as novel determinants of exfoliation syndrome. Int Ophthalmol Clin 2014; 54: 29–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F, Zhang L, Li M. Plasma homocysteine, serum folic acid, serum vitamin B12, serum vitamin B6, MTHFR and risk of pseudoexfoliation glaucoma: a meta-analysis. Graefes Arch Clin Exp Ophthalmol 2012; 250: 1067–1074. [DOI] [PubMed] [Google Scholar]
- Altintas O, Maral H, Yuksel N, Karabas VL, Dillioglugil MO, Caglar Y. Homocysteine and nitric oxide levels in plasma of patients with pseudoexfoliation syndrome, pseudoexfoliation glaucoma, and primary open-angle glaucoma. Graefes Arch Clin Exp Ophthalmol 2005; 243: 677–683. [DOI] [PubMed] [Google Scholar]
- Cumurcu T, Sahin S, Aydin E. Serum homocysteine, vitamin B12 and folic acid levels in different types of glaucoma. BMC Ophthalmol 2006; 6: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Medeiros FA, Barshop BA, Weinreb RN. Total plasma homocysteine and primary open-angle glaucoma. Am J Ophthalmol 2004; 137: 401–406. [DOI] [PubMed] [Google Scholar]
- Clement CI, Goldberg I, Healey PR, Graham SL. Plasma homocysteine, MTHFR gene mutation, and open-angle glaucoma. J Glaucoma 2009; 18: 73–78. [DOI] [PubMed] [Google Scholar]
- Vessani RM, Ritch R, Liebmann JM, Jofe M. Plasma homocysteine is elevated in patients with exfoliation syndrome. Am J Ophthalmol 2003; 136: 41–46. [DOI] [PubMed] [Google Scholar]
- Chambers JC, Ueland PM, Wright M, Dore CJ, Refsum H, Kooner JS. Investigation of relationship between reduced, oxidized, and protein-bound homocysteine and vascular endothelial function in healthy human subjects. Circ Res 2001; 89: 187–192. [DOI] [PubMed] [Google Scholar]
- Lominadze D, Roberts AM, Tyagi N, Moshal KS, Tyagi SC. Homocysteine causes cerebrovascular leakage in mice. Am J Physiol Heart Circ Physiol 2006; 290: H1206–H1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon SB, Kang DW, Kim JS, Kwon SU. Homocysteine, small-vessel disease, and atherosclerosis: an MRI study of 825 stroke patients. Neurology 2014; 83: 695–701. [DOI] [PubMed] [Google Scholar]
- Lai WK, Kan MY. Homocysteine-induced endothelial dysfunction. Ann Nutr Metab 2015; 67: 1–12. [DOI] [PubMed] [Google Scholar]
- Visontai Z, Merisch B, Kollai M, Holló G. Increase of carotid artery stiffness and decrease of baroreflex sensitivity in exfoliation syndrome and glaucoma. Br J Ophthalmol 2006; 90: 563–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikropoulos DG, Mallini P, Michipouou A, Giannopoulos T, Arranz-Marquez E, Koliakos GG et al. Asymmetric dimethylo arginin (ADMA) concentration in the aqueous humor of patients with exfoliation syndrome or exfoliation glaucoma. Curr Eye Res 2013; 38: 266–270. [DOI] [PubMed] [Google Scholar]
- Tosu M, Erdurmus M, Bugdayei G, Celebi S, Alcelik A. Aqueous humour and serum concentration of asymmetric dimethyl arginine in pseudoexfoliation syndrome. Br J Ophthalmol 2012; 96: 1137–1140. [DOI] [PubMed] [Google Scholar]
- Biomter H, Puustijärvi T, Käntkanen M, Valtonen P, Teräsvirta M, Uusitalo H. Asymmetric dimethylarginine is not related to exfoliation syndrome but symmetric dimethylarginine is related to exfoliative glaucoma. Graefes Arch Clin Exp Ophthalmol 2007; 245: 204–209. [DOI] [PubMed] [Google Scholar]
- Goren Sahin D, Sahin A, Akay OM. Comparison of rotational thromboelastography findings in pseudoexfoliation syndrome patients and healthy controls. J Glaucoma 2016; 25: 879–882. [DOI] [PubMed] [Google Scholar]
- Jeng SM, Karger RA, Hodge DO, Burke JP, Johnson DH, Good MS. The risk of glaucoma in pseudoexfoliation syndrome. J Glaucoma 2007; 16: 117–121. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.