Abstract
The classical quorum-sensing (QS) model is based on the assumption that diffusible signaling molecules accumulate in the culture medium until they reach a critical concentration upon which expression of target genes is triggered. Here we demonstrate that the hydrophobic signal N-hexadecanoyl-L-homoserine lactone, which is produced by Paracoccus sp., is released from cells by the aid of membrane vesicles (MVs). Packed into MVs, the signal is not only solubilized in an aqueous environment but is also delivered with varying propensities to different bacteria. We propose a novel MV-based mechanism for binary trafficking of hydrophobic signal molecules, which may be particularly relevant for bacteria that live in open aqueous environments.
Many bacteria employ small secreted molecules to communicate with each other, a phenomenon often referred to as quorum sensing (QS). Among the various bacterial signals identified to date, N-acyl-homoserine lactones (AHLs) are the most common QS signals produced by Gram-negative bacteria. The production of AHLs with N-acyl side chains containing 4–18 carbons and various additional modifications have been described in >200 Gram-negative bacterial species (Wagner-Döbler et al., 2005). The classic QS model is based on the assumption that AHLs diffuse from the cell to the medium and back into the cell, and that it is the population density (that is equivalent with a critical AHL concentration) that triggers the QS regulatory cascade. However, free diffusibility was only demonstrated for the short-chain AHL N-butyryl-L-homoserine lactone (C4-HSL) and there is increasing evidence that AHLs containing longer fatty acid chains require transporters to be released from the cell (Pearson et al., 1999; Chan et al., 2007; Buroni et al., 2009). It has also been reported that long-chain AHLs, like N-hexadecanoyl-L-homoserine lactone (C16-HSL), which are typically employed by rhizobial and roseobacterial QS systems, partition with the cell envelope (Blosser-Middleton and Gray, 2001; Marketon et al., 2002; Schaefer et al., 2002; Wagner-Döbler et al., 2005; Barth et al., 2012). Recent research has highlighted the importance of membrane vesicles (MVs) for the transfer of various cellular components between cells. MV production has been demonstrated for many bacteria and recent work has also provided evidence that they are abundant in natural environments (Biller et al., 2014). These MVs have important roles in microbial and host-microbial interactions, delivering proteins and DNA (Brown et al., 2015; Kaparakis-Liaskos and Ferrero, 2015; Schwechheimer and Kuehn, 2015). The opportunistic pathogen Pseudomonas aeruginosa has been demonstrated to package the signaling molecule 2-heptyl-3-hydroxy-4-quinolone (pseudomonas quinolone signal; PQS) into membrane vesicles that serve to traffic this molecule within a population (Mashburn and Whiteley, 2005).
In this study, we demonstrate that the very hydrophobic C16-HSL signal, which is used by Paracoccus denitrificans Pd1222 for cell-to-cell communication (Schaefer et al., 2002), is released from the cells mainly by the aid of MVs. Moreover, we demonstrate that the MVs fuse with varying propensities to different bacteria, suggesting that the MVs are capable of recognizing particular cell types.
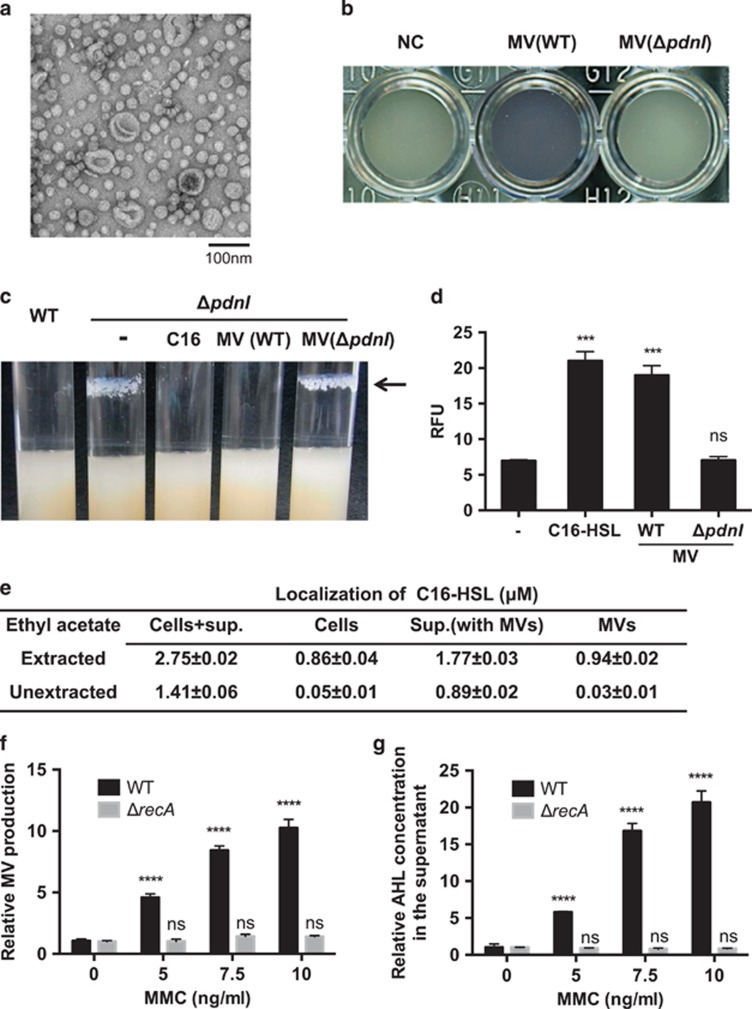
Spherical structures that are typical for MVs were observed in the supernatant of stationary phase Paracoccus denitrificans Pd1222 cultures grown in tryptic soy broth medium at 37 °C with shaking (Figure 1a). Given that the C16-HSL produced by this organism is more hydrophobic than PQS (logP of 6.05 versus 3.60, where P is the octanol-water partition coefficient), we hypothesized that this AHL signal may be associated with MVs. Culture supernatants of Pd1222 were ultracentrifuged to separate MV-associated from free C16-HSL and the MV pellet was further fractionated by density gradient ultracentrifugation. Only fractions containing MVs activated the AHL biosensor (Someya et al., 2009), indicating that C16-HSL is associated with MVs (Supplementary Figure S1). The AHL biosensor was not activated when MV samples of an AHL-null mutant, in which the luxI homolog Pden_0787 of Pd1222 had been inactivated, were tested (Figure 1b). The Pden_0787 mutant no longer produced AHLs and we named this gene pdnI (Paracoccus denitrificans luxI-homolog) (Supplementary Figure S2). These results indicate that the C16-HSL that is associated with these MVs is biologically active and can induce gene expression in a target cell.
Figure 1.
C16-HSL are associated with MVs in P. denitrificans Pd1222. (a) TEM image of MVs isolated from Pd1222. (b) C. violaceum VIR24 assay for the detection of C16-HSL. MVs isolated from Pd1222 wild-type or a pdnI mutant were analyzed. The purple pigment is indicative of the presence of C16-HSL. (c) C16-HSL inhibits Pd1222 aggregation. Arrows indicate cell aggregates of the pdnI mutant attached to the tube surface. A total of 5 μM C16-HSL or an equivalent amount of C16-HSL associated with MVs was added. The same amount of MVs derived from the wild-type or the pdnI mutant was added. (d) MV addition to a Pd1222 AHL reporter strain. A total of 5 μM C16-HSL or an equivalent amount of C16-HSL associated with MVs was added to a culture of P. denitrificans Pd1222ΔpdnI/pPLlas. Gfp expression in this strain is dependent on C16-HSL. n=3; mean±s.d. Unpaired t-test with Welch's correction. ns, not significant; ***P<0.001. (e) Quantification of C16-HSL and their localization. C16-HSL was quantified from each fractions with or without ethyl acetate extractions before measurement with UHPLC-qToF-MS. n=3; mean±s.e. (f) MV induction by MMC. MMC was added at an OD600 of 0.5, following incubation for 5 h. MV production was measured by staining with the membrane-specific dye FM4–64. n=3; mean±s.d. Significant differences with the control were determined by two-way ANOVA with Dunnett's multiple comparisons post test. ns, not significant; ****P<0.0001. (g) C16-HSL concentration in the supernatant. MVs were induced by adding MMC as mentioned before. AHL concentration in the supernatant was measured using C. violaceum VIR24/pPROBE-vioA. Relative values are shown. n=3; mean±s.d. Significant differences were determined by two-way ANOVA with Dunnett's multiple comparisons post test. ns, not significant; ****P<0.0001.
Although the functions controlled by C16-HSL in P. denitrificans have not been identified, we observed that the pdnI mutant strongly aggregates when grown at 30 °C. C16-HSL or wild-type MVs inhibited cell aggregation, while MVs of the pdnI mutant did not (Figure 1c). To examine whether MV-transported C16-HSL is delivered into Pd1222 cells, a GFP-based AHL reporter plasmid was introduced into a Pd1222 pdnI mutant. Addition of MVs to the medium induced GFP expression of the strain, suggesting that MVs that fuse with bacterial cells release their C16-HSL cargo to the cells (Figure 1d). These results indicate that C16-HSL is transported via MVs to control self-aggregation of Pd1222. Both inhibition of cell aggregation and GFP induction of the Pd1222 AHL reporter required at least 5 nM of exogenously added C16-HSL and the effects were maximal at 100–500 nM. The amount of MVs that was required to elicit expression of both phenotypes corresponded to 50 nM C16-HSL and a maximal response was observed at a concentration equivalent to 500 nM C16-HSL (Supplementary Figure S3).
Paracoccus species isolated from activated sludge also showed MV-associated AHL production (Supplementary Figure S4). Chemical analysis of the supernatant of strain AS6, identified C16-HSL as the major AHL signal (Supplementary Figure S5). MV-like structures could also be observed in activated sludge samples (Supplementary Figure S6). However, we were not able to detect AHLs from these MVs, presumably because the majority of these MVs are derived from species other than Paracoccus sp.
Quantification of C16-HSL by ultrahigh performance liquid chromatography coupled to time of flight mass spectrometry (UHPLC-qToF-MS) (Buddrus-Schiemann et al., 2014), revealed that a late-stationary phase culture of Pd1222 produced ~2.5 μM of 16-HSL, of which 64% was released to the growth medium while the remainder was associated with the cells (Figure 1e). Of the extracellular C16-HSL, 53% were found to be associated with MVs. Both MV-associated and free C16-HSL in the supernatant was able to inhibit aggregation and induce GFP expression in the Pd1222 AHL reporter strain. This indicates that in addition to free AHLs (as in the classic QS model), P. denitrificans can also use MV-associated signals to trigger the QS response and that both systems appear to operate in parallel (Supplementary Figure S7). It is worth noting that C16-HSL was hardly detectable by UHPLC-qToF-MS in MV samples unless they were extracted with ethyl acetate (Figure 1e), indicating that C16-HSL is tightly bound to MVs. The negligible amounts of C16-HSL observed in untreated MV preparations were likely extracted from MVs by acetonitrile during the UHPLC, rather than being a contamination of the MV preparation with free C16-HSL. In a late-stationary-phase culture, 5.4 × 109 MV particles per ml were detected. Assuming an equal distribution of the signal molecule and similar sizes of the MVs, each MV is associated with ~1.1 × 105 C16-HSL molecules. The threshold concentration of free C16-HSL required for biological activity was determined to be 5 nM, which corresponds to a cell density of 8.5 × 109 ml−1 (Supplementary Figure S3). Assuming a cell volume of 1 μm3, 3–350 C16-HSL molecules per cell are sufficient to trigger the QS cascade. Hence, the amount of C16-HSL associated with one MV is very likely high enough to induce the QS response in a P. denitrificans cell when it fuses with a MV.
As C16-HSL was tightly associated with MVs, we hypothesized that if C16-HSL is released via MVs, stimulation of MV production would increase the amount of C16-HSL in the supernatant. To test this hypothesis, we first examined whether MV formation by P. denitrificans can be stimulated by treatment with the DNA damaging agent mitomycin C (MMC) (Turnbull et al., 2016). The amount of MVs released into the supernatant was found to be directly proportional to the MMC concentration that is added to the culture (Figure 1f). Previous work has shown that stress-induced MV formation in P. aeruginosa is dependent on recA (Toyofuku et al., 2014; Turnbull et al., 2016). In accordance, we observed that recA is also required for stress-induced MV formation in P. denitrificans (Figure 1f). We also observed that the amount of C16-HSL in the supernatant linearly increased (R2=0.98) with the MMC concentration (Figure 1g). In contrast, MMC treatment did not affect C16-HSL concentration in the supernatant of the recA mutant (Figure 1g). Importantly, C16-HSL production was not altered by MMC treatment, indicating that C16-HSL release but not its production was stimulated (Supplementary Figure S8). These data indicate that the release of C16-HSL depends on the production of MVs.
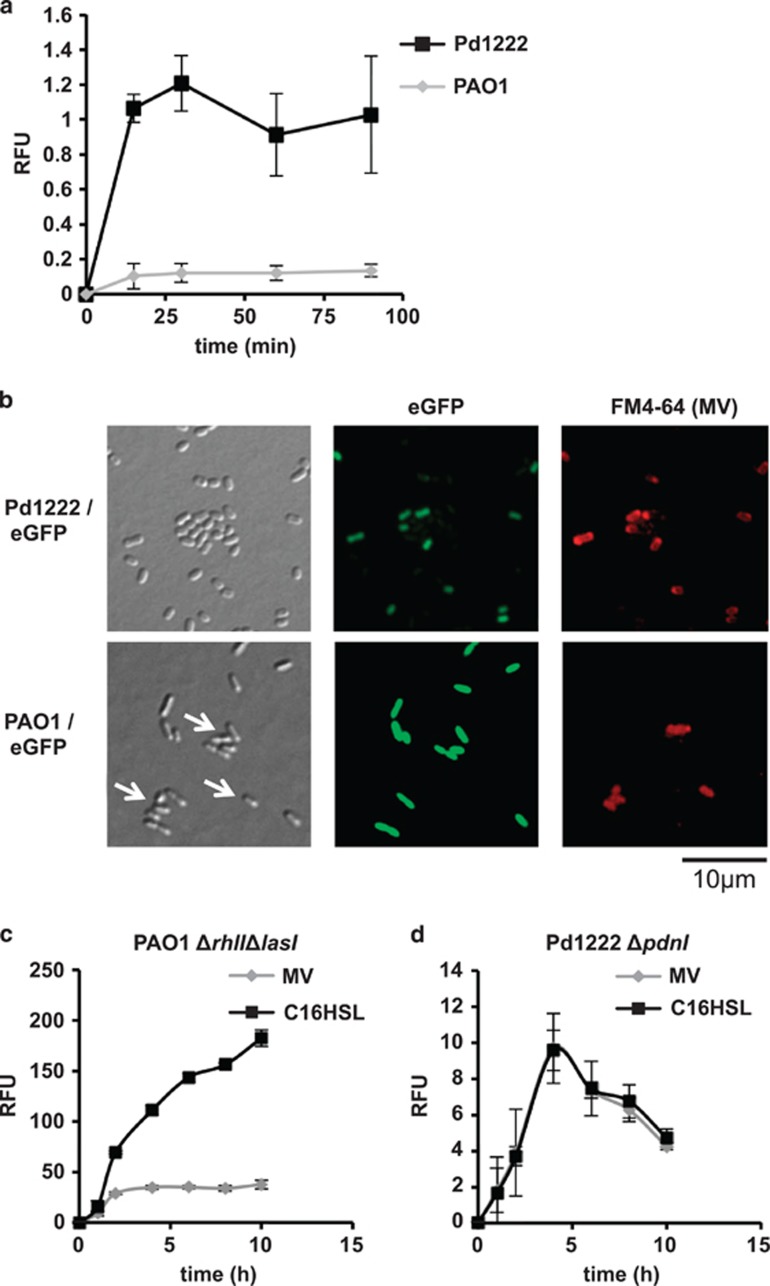
When C16-HSL was added to PBS in a polypropylene tube, it adsorbed firmly to the surface of the tube. In contrast, MV-associated C16-HSL could be easily recovered from the tubes (Supplementary Figure S9), indicating that the hydrophobic C16-HSL can be solubilized by MVs in aqueous systems. We next examined whether MVs would traffic C16-HSL to non-self cells. As an alternative target we employed the well-studied MV producer P. aeruginosa. MVs of P. denitrificans showed a low affinity for P. aeruginosa relative to P. denitrificans (Figures 2a and b). Importantly, in the P. aeruginosa background, the AHL reporter plasmid responded poorly to MV-associated C16-HSL compared to the addition of an equivalent amount of free signal molecule to the medium (Figure 2c). P. denitrificans responded well to both free and MV-associated C16-HSL (Figure 2d). We also observed that several other bacteria responded only weakly to MV-associated C16-HSL relative to free C16-HSL (Supplementary Figure S10). These results suggest that the cargo carried by P. denitrificans MVs is delivered with varying propensities to other bacteria. Further work will be required to unravel the underlying mechanism that determines the specificity of MV-associated AHL delivery.
Figure 2.
Pd1222 derived MVs traffic C16-HSL signaling. (a) Attachment of Pd1222 MVs to P. denitrificans Pd1222 or P. aeruginosa PAO1. Cells of P. denitrificans Pd1222 or P. aeruginosa PAO1 were mixed with FM4–64 labeled MVs and fusion of MVs to bacterial cells was quantified by measuring red fluorescence, that is, cells that had fused with the labelled MVs. n=3; mean±s.d. (b) MVs show higher affinity to P. denitrificans Pd1222 than P. aeruginosa PAO1 cells in a mixed culture. Pd1222 and PAO1 cells were mixed 1:1 and incubated in PBS for 3 h in the presence of FM4–64-labeled MVs. Pd1222 and PAO1 cells are marked with eGFP in the upper and lower panel, respectively. Upon fusion of labeled MVs with a bacterial cell, it becomes red fluorescent. In the upper panel the red fluorescence (that is the MV target cell) co-localizes with the green fluorescence of the marked Pd1222 cells, indicating that these cells are the preferred targets of the MVs. By contrast, red fluorescence does not co-localize with the green fluorescence of the labeled PAO1 cells (lower panel), indicating that the MVs have a very low affinity for these cells. However, in the lower panel, the red fluorescence co-localizes with the unmarked Pd1222 cells as indicated by the white arrows. (c and d) MVs traffic C16-HSL signals. 5 μM C16-HSL or an equivalent amount of C16-HSL associated with MVs were added to P. aeruginosa PAO1ΔlasIΔrhlI/pPROBE-vioA-cviR (c) or P. denitrificans Pd1222ΔpdnI/pPLlas (d). While free C16-HSL induces both biosensors, MV-associated AHLs only induce the Pd1222 biosensor, as the MVs show little affinity for PAO1 cells. n=3; mean±s.d.
Although the QS paradigm assumes free diffusibility of the signal molecule, evidence has accumulated that long-chain AHLs (Schaefer et al., 2002; Wagner-Döbler et al., 2005; Chang et al., 2012) are not diffusing out of the cell (Barth et al., 2012; Krol and Becker, 2014), in contrast to short-chain AHLs (Pearson et al., 1999). At present, very little is known how these hydrophobic signal molecules are released by the cell. A recent study of a marine Vibrio suggested that MVs can induce AHL-regulated gene expression, although the signal molecule has not been identified (Li et al., 2016). Here we show that long-chain AHLs are associated with MVs, which not only allow for their secretion but also guide their transport to specific target cells. When packed into MVs, the hydrophobic C16-HSL can be solubilized in an aqueous environment. Our data suggest that long-chain AHLs are concentrated in MVs, which will ensure that the amount of signals delivered to a target cell is sufficient to trigger its QS response. This binary signaling mechanism is fundamentally different from the classic QS model, which assumes the analog accumulation and homogenous distribution of the signal in the medium until a critical concentration is reached that induces the QS response synchronously in the majority of cells. Our data show that in a closed test tube system the amount of free AHL is sufficiently high to trigger a classical QS response and although the MV-based signaling will operate in parallel in this system, it would not be essential for QS induction. We therefore propose that the MV-based signaling is particularly valuable for trafficking hydrophobic signal molecules in natural habitats, particularly for bacteria that live in open aqueous environments where non-MV associated signals would be infinitely diluted.
Acknowledgments
MT was supported by a Grant-in-Aid for Scientific Research (25701012, 16K14795 and 16H06189) from the Ministry of Education, Culture, Sports, Science and Technology of Japan (MEXT), and from the Japanese Society for the Promotion of Science (JSPS) Postdoctoral Fellowship for Research Abroad. Financial Support by the Japan Science and Technology Agency, ERATO, CREST and ALCA, and a Grant-in-Aid for Scientific Research (60292520) from MEXT (NN) and the Swiss National Foundation (Project 3100A0-104215) (LE) is gratefully acknowledged. KM was supported by a Grant-in-Aid for Scientific Research (16J00487) from JSPS. We thank Professor David Richardson (University of East Anglia) for kindly providing us P. denitrificans Pd1222, Professor Tomohiro Morohoshi (Utsunomiya University) and Professor Tsukasa Ikeda (Utsunomiya University) for kindly providing us C. violaceum ATCC12472 and C. violaceum VIR24.
Footnotes
Supplementary Information accompanies this paper on The ISME Journal website (http://www.nature.com/ismej)
The authors declare no conflict of interest.
Supplementary Material
References
- Barth C, Jakubczyk D, Kubas A, Anastassacos F, Brenner-Weiss G, Fink K et al. (2012). Interkingdom signaling: integration, conformation, and orientation of N-acyl-L-homoserine lactones in supported lipid bilayers. Langmuir 28: 8456–8462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biller SJ, Schubotz F, Roggensack SE, Thompson AW, Summons RE, Chisholm SW. (2014). Bacterial vesicles in marine ecosystems. Science 343: 183–186. [DOI] [PubMed] [Google Scholar]
- Blosser-Middleton RS, Gray KM. (2001). Multiple N-acyl homoserine lactone signals of Rhizobium leguminosarum are synthesized in a distinct temporal pattern. J Bacteriol 183: 6771–6777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown L, Wolf JM, Prados-Rosales R, Casadevall A. (2015). Through the wall: extracellular vesicles in Gram-positive bacteria, mycobacteria and fungi. Nat Rev Microbiol 13: 620–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buddrus-Schiemann K, Rieger M, Mühlbauer M, Barbarossa MV, Kuttler C, Hense BA et al. (2014). Analysis of N-acylhomoserine lactone dynamics in continuous cultures of Pseudomonas putida IsoF by use of ELISA and UHPLC/qTOF-MS-derived measurements and mathematical models. Anal Bioanal Chem 406: 6373–6383. [DOI] [PubMed] [Google Scholar]
- Buroni S, Pasca MR, Flannagan RS, Bazzini S, Milano A, Bertani I et al. (2009). Assessment of three Resistance-Nodulation-Cell Division drug efflux transporters of Burkholderia cenocepacia in intrinsic antibiotic resistance. BMC Microbiol 9: 200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan YY, Bian HS, Tan TM, Mattmann ME, Geske GD, Igarashi J et al. (2007). Control of quorum sensing by a Burkholderia pseudomallei multidrug efflux pump. J Bacteriol 189: 4320–4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CY, Koh CL, Sam CK, Chan XY, Yin WF, Chan KG. (2012). Unusual long-chain N-acyl homoserine lactone production by and presence of quorum quenching activity in bacterial isolates from diseased tilapia fish. PLoS One 7: e44034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaparakis-Liaskos M, Ferrero RL. (2015). Immune modulation by bacterial outer membrane vesicles. Nat Rev Immunol 15: 375–387. [DOI] [PubMed] [Google Scholar]
- Krol E, Becker A. (2014). Rhizobial homologs of the fatty acid transporter FadL facilitate perception of long-chain acyl-homoserine lactone signals. Proc Natl Acad Sci USA 111: 10702–10707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Azam F, Zhang S. (2016). Outer membrane vesicles containing signaling molecules and active hydrolytic enzymes released by a coral pathogen Vibrio shilonii AK1. Environ Microbiol 18: 3850–3866. [DOI] [PubMed] [Google Scholar]
- Marketon MM, Gronquist MR, Eberhard A, González JE. (2002). Characterization of the Sinorhizobium meliloti sinR/sinI locus and the production of novel N-acyl homoserine lactones. J Bacteriol 184: 5686–5695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashburn LM, Whiteley M. (2005). Membrane vesicles traffic signals and facilitate group activities in a prokaryote. Nature 437: 422–425. [DOI] [PubMed] [Google Scholar]
- Pearson JP, Van Delden C, Iglewski BH. (1999). Active efflux and diffusion are involved in transport of Pseudomonas aeruginosa cell-to-cell signals. J Bacteriol 181: 1203–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer AL, Taylor TA, Beatty JT, Greenberg EP. (2002). Long-chain acyl-homoserine lactone quorum-sensing regulation of Rhodobacter capsulatus gene transfer agent production. J Bacteriol 184: 6515–6521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwechheimer C, Kuehn MJ. (2015). Outer-membrane vesicles from Gram-negative bacteria: biogenesis and functions. Nat Rev Microbiol 13: 605–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Someya N, Morohoshi T, Okano N, Otsu E, Usuki K, Sayama M et al. (2009). Distribution of N-acylhomoserine lactone-producing fluorescent pseudomonads in the phyllosphere and rhizosphere of potato (Solanum tuberosum L.). Microbes Environ 24: 305–314. [DOI] [PubMed] [Google Scholar]
- Toyofuku M, Zhou S, Sawada I, Takaya N, Uchiyama H, Nomura N. (2014). Membrane vesicle formation is associated with pyocin production under denitrifying conditions in Pseudomonas aeruginosa PAO1. Environ Microb 16: 2927–2938. [DOI] [PubMed] [Google Scholar]
- Turnbull L, Toyofuku M, Hynen AL, Kurosawa M, Pessi G, Petty NK et al. (2016). Explosive cell lysis as a mechanism for the biogenesis of bacterial membrane vesicles and biofilms. Nat Commun 7: 11220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner-Döbler I, Thiel V, Eberl L, Allgaier M, Bodor A, Meyer S et al. (2005). Discovery of complex mixtures of novel long-chain quorum sensing signals in free-living and host-associated marine alphaproteobacteria. Chembiochem 6: 2195–2206. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.