Abstract
Aims
Ageing is associated with impairment of endothelial nitric oxide synthase (eNOS) and progressive reduction in endothelial function. A genetic study on long-living individuals—who are characterized by delays in ageing and in the onset of cardiovascular disease—previously revealed I229V (rs2070325) in bactericidal/permeability-increasing fold-containing-family-B-member-4 (BPIFB4) as a longevity-associated variant (LAV); the LAV protein enhanced endothelial NO production and vasorelaxation through a protein kinase R–like endoplasmic reticulum kinase/14-3-3/heat shock protein 90 signal. Here, we further characterize the molecular mechanisms underlying LAV-BPIFB4-dependent enhancement of vascular function.
Methods and results
LAV-BPIFB4 upregulated eNOS function via mobilization of Ca2+ and activation of protein kinase C alpha (PKCα). Indeed, the overexpression of LAV-BPIFB4 in human endothelial cells enhanced ATP-induced Ca2+ mobilization and the translocation of PKCα to the plasma membrane. Coherently, pharmacological inhibition of PKCα blunted the positive effect of LAV-BPIFB4 on eNOS and endothelial function. In addition, although LAV-BPIFB4 lost the ability to activate PKCα and eNOS in ex vivo vessels studied in an external Ca2+-free medium and in vessels from eNOS−/− mice, it still potentiated endothelial activity, recruiting an alternative mechanism dependent upon endothelium-derived hyperpolarizing factor (EDHF).
Conclusions
We have identified novel molecular determinants of the beneficial effects of LAV-BPIFB4 on endothelial function, showing the roles of Ca2+ mobilization and PKCα in eNOS activation and of EDHF when eNOS is inhibited. These results highlight the role LAV-BPIFB4 can have in restoring signals that are lost during ageing.
Keywords: Endothelium, Vascular function, Nitric oxide, BPIFB4, PKCα
1. Introduction
Endothelial nitric oxide synthase (eNOS) is a crucial enzyme for vascular physiology; its reduced activity during ageing is associated with increased susceptibility to cardio- and cerebro-vascular diseases.1–4 Moreover, in mice lacking eNOS, caloric restriction does not exert its positive effects in delaying ageing and increasing life span.5
Long-living individuals (LLIs) have a favorable genetic profile characterized by an enrichment of alleles associated with the protection from ageing and cardiovascular disease.6,7 We have recently shown for three different populations that LLIs are enriched for rs2070325 (I229V), the minor allele of bactericidal/permeability-increasing fold-containing family B member 4 (BPIFB4).8rs2070325 was one of four single-nucleotide polymorphisms on BPIFB4 that variously combined to generate BPIFB4 isoforms, such as the wild type (WT) protein and a longevity-associated variant (LAV). Of note, the LAV-BPIFB4 was associated with potentiated eNOS activity in cells, an effect correlated with increased binding of BPIFB4 to 14-3-3—through an atypical-binding site for the protein— and increased phosphorylation of BPIFB4 at serine 75—a site recognized by protein kinase R-like endoplasmic reticulum kinase (PERK). Heat shock protein 90 (HSP90) was also recruited to the LAV−14-3-3 complex as part of the eNOS activation machinery. Indeed, HSP90 co-immunoprecipitated with BPIFB4, and a specific HSP90 inhibitor blocked the potentiation of endothelial function and eNOS activation exerted by the LAV.8 Despite these findings, further characterization is needed to define how LAV-BPIFB4 transduces upstream signals to eNOS.9
On this point, we already reported that LAV-BPIFB4 enhanced acetylcholine (ACh)-evoked vasorelaxation. ACh-induced eNOS phosphorylation and activity requires capacitive Ca2+ influx.10 This function is mediated by protein kinase C alpha (PKCα), which stimulates nitric oxide (NO) production in endothelial cells and plays a role in regulating blood flow in vivo.11 In the present study, we demonstrate that PKCα is part of the signalling pathway activated by LAV-BPIFB4 to potentiate vascular function. In particular, we show that LAV-BPIFB4 activates eNOS-dependent endothelial function through Ca2+-mediated potentiation of PKCα. Moreover, when PKCα and/or eNOS is inactivated—e.g. by exposing cells to Ca2+-free media or knocking out eNOS—LAV-BPIFB4 can still enhance vasorelaxation through an endothelium-derived hyperpolarizing factor (EDHF)-mediated pathway.
2. Methods
All experiments involving animals conformed to the guidelines for the Care and Use of Laboratory Animals published with Directive 2010/63/EU of the European Parliament and were approved by the review board of IRCCS INM Neuromed (ref. number 1070/2015 PR). C57BL/6 mice were bred in our animal facility. eNOS knockout (KO) mice were obtained from the Jackson Laboratory. All effort was made to minimize the number of animals used and their suffering.
2.1 Ex vivo transfection of mouse vessels and evaluation of vascular reactivity
Mice were sacrificed by intraperitoneal injection of ketamine/xylazine (respectively, 150 and 20 mg/kg BW), and second-order branches of the mesenteric arterial tree were surgically removed and mounted on a pressure myograph for experiments.8 Endothelium-dependent relaxation was assessed by measuring the dilatory responses of mesenteric arteries to cumulative concentrations of ACh (from 10−9 to 10−5 M) in vessels pre-contracted with U46619 at a dose necessary to obtain a similar level of pre-contraction in each ring (80% of initial KCl-evoked contraction).12 Values are reported as the percentage of lumen diameter change after drug administration. Responses were tested before and after transfection.
ACh-evoked vasorelaxation was also tested in the presence of the PKCα inhibitor Gö6976 (0.5 μM) or the AKT inhibitor IL6-hydroxymethyl-chiro-inositol-2-(R)-2-O-methyl-3-O-octadecyl-sn-glycerocarbonate (10 µM) (no. 124005, Calbiochem). In some experiments, the endothelium was mechanically removed by inserting a tungsten wire into the lumen of the vessel and rotating it back and forth before mounting the vessel on the pressure myograph. Caution was taken to avoid endothelial damage.
Another experimental series was performed on vessels transfected in presence of Ca2+ and then studied in the absence of external Ca2+, using Ca2+-free Krebs, in presence of apamin (APA)—a potent inhibitor of ATP-type Ca+2-activated K+ channels and SKCa,—and charybdotoxin (CTx)—a potent and selective inhibitor of the voltage-gated Ca2+-activated K+ channel (Kv1.3) and BKCa channel (both were purchased from Sigma-Aldrich).
2.2 Fluorescence-activated cell sorting
For FACS analysis, transfected arteries were digested with type 2 collagenase (0.05%; Worthington CLS2) for 45 min at 37 °C in a shaking incubator. Freed cells were washed with PBS and passed through a 100-μm strainer (BD Falcon). Afterwards, cells were stained with anti-CD31-FITC (1:100, BD Biosciences-Pharmigen) at 4 °C for 20 min and then permeabilized with Cytofix/Cytoperm (BD Biosciences-Pharmigen) at 4 °C for 20 min. Subsequently, cells were incubated with anti-BPIFB4 (1:100; Abcam) at 4 °C for 1 h and then an allophycocyanin (APC)-conjugated anti-mouse secondary antibody (1:200; BioLegend). For non-directly conjugated antibody to BPIFB4, a staining mix without anti-BPIFB4 antibody but with inclusion of the fluorescent secondary antibody was used as negative control. Analysis of cell populations was performed using a FACS Canto II equipped with FACS Diva software (BD Biosciences) and the FlowLogic (Miltenyi Biotec) analysis program.
2.3 Production of lentiviral vectors, cell culture, and co-immunoprecipitation
BPIFB4 cDNA (WT and LAV isoforms) was cloned from pRK5 expression plasmids8 into the lentiviral vector pCDH-EF1-MSC-pA-PGK-cop-green fluorescence protein (GFP)-T2A-Puro (System Biosciences). Lentiviral particles were generated by transfection of pCDH constructs along with the packaging vectors pMD2.VSV.G, pRSV-REV, and pMDLg/pRRE (kindly provided by Prof Luigi Naldini, San Raffaele Scientific Institute, Milan, Italy) into human embryonic kidney (HEK293T) cells by calcium phosphate transfection. Lentiviral particles were concentrated by ultracentrifugation (25 000 rpm for 4 h at 4 °C) and stored at −80 °C until immediately prior to use. Lentivirus titration was performed by transducing HEK293T cells with concentrated particles in the presence of 4 μg/ml polybrene and measuring GFP expression after 3 days by flow cytometry.
For Ca2+ mobilization and confocal microscopy assay, human umbilical vein endothelial cells (HUVECs) (Lonza) were grown in complete EGM2 medium (Lonza) and infected with empty lentiviral vectors or particles encoding either WT- or LAV-BPIFB4 [at 5 multiplicity of infection (MOI)]. After 72 h, cells were selected with 2 µg/ml puromycin for 48 h.
HEK293T cells were grown in Dulbecco’s modified Eagle’s medium supplemented with 10% (v/v) fetal bovine serum and 1% non-essential amino acids at 37° C in a 5% CO2 atmosphere. For co-immunoprecipitation, 1.4 × 106 cells were plated in 10-cm dishes and transfected with pRK5 vector encoding LAV-BPIFB4 or with an empty plasmid, using Lipofectamine 2000 (Life Technologies) according to the manufacturer’s protocol. Twenty-four hours post-transfection, HEK293T cells were incubated with Gö6976 (0.3 μM) for another 24 h, harvested, and solubilized in lysis buffer (20 mM Tris-HCl pH 7.5, 650 mM NaCl, 500 mM EDTA, 250 mM EGTA, and Triton X-100). Lysates were cleared at 13 000 rpm for 20 min at 4 °C, and 700 μg protein incubated overnight with 2 μg of mouse anti-GFP (Invitrogen), mouse anti-14-3-3 (Abcam), or mouse anti-IgG (Millipore) for the control. The antibody–antigen complexes were precipitated with Glinked Sepharose protein (GE Healthcare) for 4 h at 4 °C and the beads washed three times with lysis buffer. The denatured co-immunoprecipitation products were resolved with SDS-PAGE, electro-blotted onto PVDF membranes, and hybridized with 1:1000 rabbit anti-14-3-3 (Abcam).
2.4 Western blotting
Each sample was a pool of mesenteric arteries (length, 2mm; diameter, 250 µm) excised from four mice. HUVECs infected with lentiviral particles encoding WT- BPIFB4, LAV-BPIFB4, or GFP (‘empty’ vector) were starved in serum- and growth factor-free EGM-2 for 4 h and then stimulated with 100 µM ACh for 10 min. Protein extracts were separated on 8–10% SDS-PAGE at 100 V for 1 h or on 4–12% SDS-PAGE at 100 V for 2 h and then transferred to a nitrocellulose or PVDF membrane as previously described in.8 Western blots were analysed using ImageJ software (Wayne Rasband, National Institutes of Health, USA) to determine optical density (OD) of the bands. The OD readings of phosphorylated proteins are expressed as a ratio relative to total protein or to beta-actin. All other protein expressions are normalized to account for variations in loading.
2.5 Ca2+-transient recordings
Free intracellular Ca2+ concentration ([Ca2+]i) recordings were obtained by time-resolved digital fluorescence microscopy on infected HUVECs loaded with the Ca2+ indicator X-rhod-1 AM (excitation, 550 nm; emission, 610 nm)13 to avoid overlapping of fluorescence signals due to the presence of GFP. Briefly, cells were incubated for 45 min at 37 °C with 2 μM of X-rhod-1 AM. Cells were then placed in standard mammalian Ringer solution (in mM: NaCl, 140; KCl, 2.5; CaCl2, 2; MgCl2, 2; Hepes-NaOH, 10; and glucose, 10; pH 7.3), and continuously superfused with a gravity-driven fast perfusion system (BioLogique 100). Most cells were infected and displayed clear GFP fluorescence that did not interfere with the fluorescent signal of the Ca2+ dye. Ca2+ transients were elicited by applying 100 μM ATP for 2 min.14 The time courses of Ca2+ transients were quantified by measuring at each time point the fluorescence emission in the region of interest surrounding each cell, and then transforming the obtained values as follows: ΔF/F = [F(t) − F(0)]/F(0). For each cell, the amplitude of the ATP-induced Ca2+ transient was evaluated as the difference between maximal and basal ΔF/F values.
2.6 Immunofluorescence and confocal microscopy
HUVECs infected with lentiviral particles encoding WT-BPIFB4, LAV-BPIFB4, or GFP (empty vector) were fixed in 4% paraformaldehyde in PBS for 20 min, washed twice in 50 mM NH4Cl in PBS, and permeabilized for 5 min in 0.2% Triton X-100 in PBS. Fixed cells were treated as described elsewhere.15 Immunofluorescence analysis was performed on an inverted, motorized microscope (Axio Observer Z.1) equipped with a 63X/1.4 Plan-Apochromat objective (Carl Zeiss). The attached laser-scanning unit (LSM 700 4X pigtailed laser 405-488-555-639, Carl Zeiss) enabled confocal imaging. For excitation, 405, 488, and 555 nm lasers were used. Fluorescence emission was revealed by a MBS (Main Dichroic Beam Splitter) and a VSD (Variable Secondary Dichroic Beam Splitter). Triple staining fluorescence images were acquired separately using ZEN 2012 software in the blue (Hoechst 33258), green (EGFP), and red (Alexa Fluor 594) channels at a resolution of 1024 × 1024 pixels, with the confocal pinhole set to one Airy unit, and then saved in TIFF format.
2.7 Statistical analyses
Vessel reactivity is given as mean ± S.E.M. and analysed by two-way ANOVA. Densitometry data were analysed with one-way ANOVA followed by Bonferroni posthoc analysis, as appropriate, using dedicated software (GraphPad Prism, v5.0).
3. Results
3.1 LAV-BPIFB4 activates PKCα
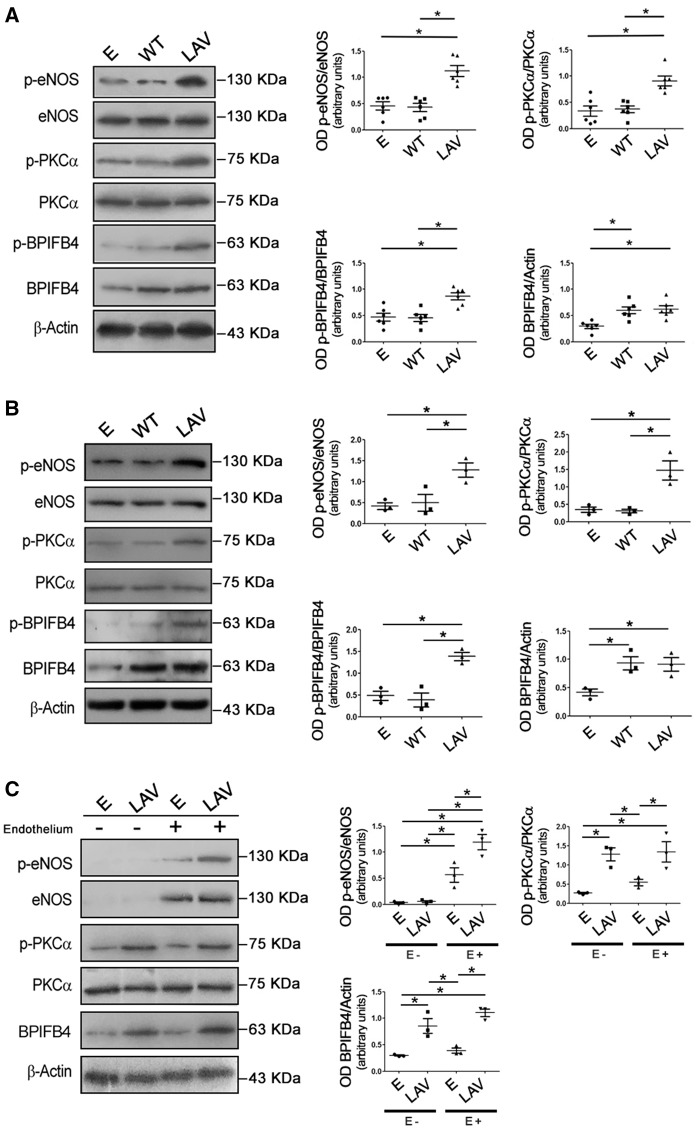
We previously reported that LAV-BPIFB4 enhances NO-mediated vasorelaxation evoked by ACh.8 ACh-evoked vasodilation of isolated mesenteric vessels has been reported to require intact PKCα activity.10 Here, found that PKCα was more phosphorylated at threonine 497—an activation site of the enzyme—in LAV-BPIFB4-overexpressing vessels than in those expressing the WT protein or only GFP (Figure 1A). Expression of LAV-BPIFB4 protein was detected through FACS analysis in 79 ± 4% CD31+ endothelial cells (data not shown). To confirm that the mechanisms recruited by LAV-BPIFB4 take place in endothelial cells, we performed Western blotting on HUVECs infected with lentiviral vectors encoding GFP (empty), WT- BPIFB4, or LAV-BPIFB4. Also in this experimental setting, overexpression of LAV-BPIFB4 was associated with activation of PKCα and eNOS (Figure 1B).
Figure 1.
Effect of LAV-BPIFB4 on PKCα/eNOS signalling. Representative Western blots of (A) ex vivo C57BL/6 mouse mesenteric arteries, (B) HUVECs, and (C) vessels with (E+) or without (E−) endothelium, transfected with an empty vector (E) or vectors for the expression of WT BPIFB4 or LAV-BPIFB4. Graphs on the right show quantification of p-eNOS (S1177), p-PKCα (T497), p-BPIFB4 (S75), and BPIFB4. Values are means ± S.E.M., n = 6 experiments for A; n = 3 experiments for B and C. Statistics was performed using one-way ANOVA, following Bonferroni’s Multiple Comparison Test. *P < 0.05.
To better characterize the role of the endothelial and smooth muscle layers, we performed experiments on endothelium-denuded vessels: the loss of endothelium was confirmed by the absence of eNOS upon Western blotting (Figure 1C) and by the absence of ACh-evoked vasorelaxation in functional studies (data not shown). Overexpression of LAV-BPIFB4 upregulated the phosphorylation of eNOS by about 2.5-fold and evoked the activation of PKCα regardless of the presence or not of endothelium (Figure 1C).
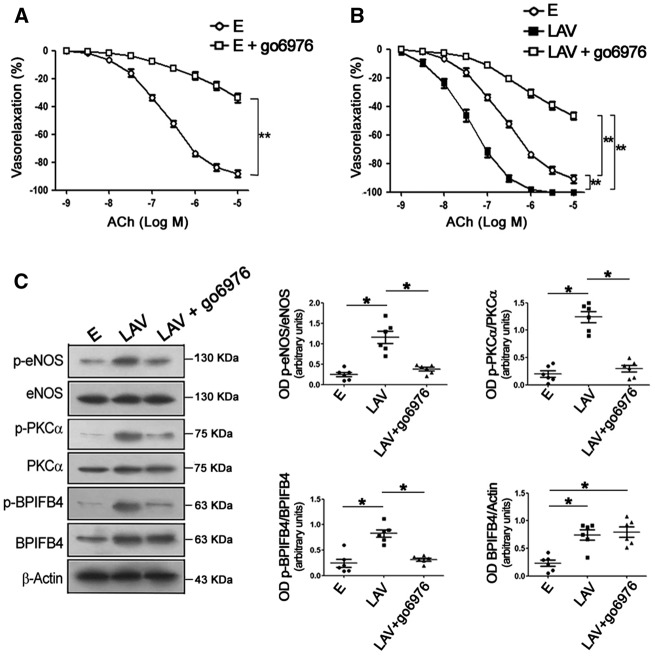
Of note, treatment with the PKCα inhibitor Gö6976 significantly blunted ACh-evoked vasorelaxation in control vessels (Figure 2A) and abolished both endothelial vasorelaxation and enhanced eNOS phosphorylation in LAV-BPIFB4-expressing vessels (Figure 2B and C). Based on these results, we can assert that PKCα is recruited by LAV-BPIFB4 to modulate eNOS and vascular tone.
Figure 2.
Inhibition of PKCα abolishes the vascular effects of LAV-BPIFB4. Dose–response curves to ACh in ex vivo C57BL/6 mouse mesenteric arteries transfected with (A) an empty vector or (B) a vector for the expression of LAV-BPIFB4, with and without the PKCα inhibitor Gö6976. Values are means ± S.E.M., n = 10 experiments per group. Statistics was performed using two-way ANOVA; **P < 0.01. (C) Western blot of transfected, ex vivo C57BL/6 mouse mesenteric arteries. Right graphs show quantification of p-eNOS (S1177), p-PKCα (T497), p-BPIFB4 (S75), and BPIFB4. Values are means ± S.E.M., n = 6 experiments. Statistics was performed using one-way ANOVA, following Bonferroni’s Multiple Comparison Test; *P < 0.05. E, empty vector; LAV, vector for the expression of LAV-BPIFB4; Gö6976, PKCα inhibitor.
3.2 BPIFB4 isoforms modulate Ca2+ influx and translocation of PKCα to membrane
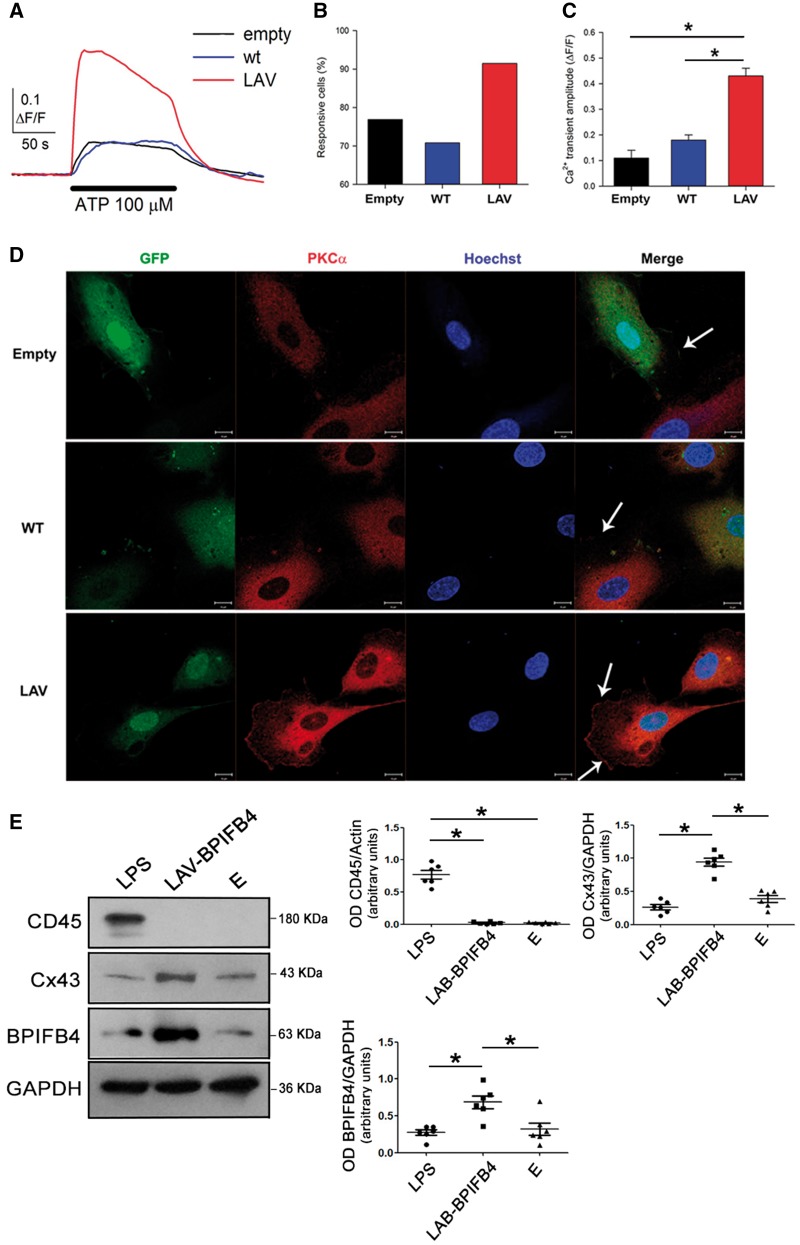
Based on the modulatory action of BPIFB4 on PKCα activity and vascular function—well-known Ca2+-dependent processes10 we investigated how agonist-induced Ca2+ mobilization was influenced by the expression of the BPIFB4 isoforms in HUVECs. ATP was used to elicit Ca2+ transients (Figure 3A), so avoiding interaction of ACh with the nicotinic receptors present on the HUVECs.16 Overexpression of the LAV-BPIFB4 isoform determined clear increases in the number of responsive cells (Figure 3B) and the mean amplitude of Ca2+ transient upon stimulation (Figure 3C). Thus, the LAV isoform clearly facilitates agonist-induced Ca2+ mobilization. Moreover, overexpression of LAV-BPIFB4 was associated with increased localization of PKCα to the plasma membrane (Figure 3D), a hallmark of its activation.17,18 The percentages of cells with membrane-localized PKCα in each setting were: empty, 6.5%; WT-BPIFB4, 10%; LAV-BPIFB4, 60%.
Figure 3.
Overexpression of LAV-BPIFB4 sensitizes endothelial cells to agonist-induced Ca2+ mobilization, and the vascular effects do not require recruitment of MNCs. (A) Typical time-courses of [Ca2+]i changes elicited by 100 μM ATP (horizontal bar, 2 min application) in HUVECs overexpressing the WT- or LAV-BPIFB4 isoforms (average from 40 cells in individual optical fields). For the empty vector, a time-course averaged from 25 individual cells in a single optical field was shown. Histograms of (B) the percentage of responding cells (n = 134, 113, and 129 cells, respectively) and (C) their mean Ca2+ transient amplitudes after ATP application (empty, n = 3 independent experiments; WT and LAV, n = 5 independent experiments). *P < 0.05; ANOVA. (D) Subcellular localization of PKCα in infected HUVECs cells. PKCα (red) was mainly cytosolic in HUVECs infected with an empty vector (Empty) and with a lentiviral vector encoding WT-BPIFB4; in contrast, PKCα was located mainly to the plasma membrane in HUVECs overexpressing LAV-BPIFB4, a clear hallmark of PKCα activation. Arrows indicate regions of plasma membrane-localized PKCα; blue, Hoechst-stained nuclei; green, GFP expression. Scale bar = 10 µm. (E) Western blot of ex vivo mouse mesenteric arteries from C57BL/6 mice treated with LPS (20 mg/kg for 16 h) and of vessels from untreated C57BL/6 mice after transfection with empty vector (E) or overexpressing LAV-BPIFB4. Right graphs show quantification of CD45, Cx43, and BPIFB4. Values are means ± S.E.M., n = 6 experiments. Statistics was performed using one way ANOVA, following Bonferroni’s Multiple Comparison Test; *P < 0.05.
Gap junctions allow exchange of Ca2+ ions between cells,19,20 and connexin-43 (Cx43) plays a prominent role in this mechanism.21 We found increased expression of Cx43 in vessels overexpressing LAV-BPIFB4 (Figure 3E). Based on this finding, we speculate that Cx43 could be involved in the effects of LAV-BPIFB4 on Ca2+ mobilization.
In previous work, we reported that mononuclear cells (MNCs) from homozygous rs2070325 carriers (which express LAV-BPIFB4) have significantly upregulated eNOS activity vs. those from heterozygous and WT carriers.8 To exclude that this mechanism was responsible for the above findings, we assessed recruitment of MNCs to vessels. Evaluation of the MNC marker CD4522 indicated that MNCs were present in vessels treated with lipopolysaccharide (LPS) (a well-known stimulus that induces MNCs recruitment)23 but not in those overexpressing LAV-BPIFB4 (Figure 3E).
3.3 LAV-BPIFB4 fails to activate PKCα and eNOS in the absence of external Ca2+
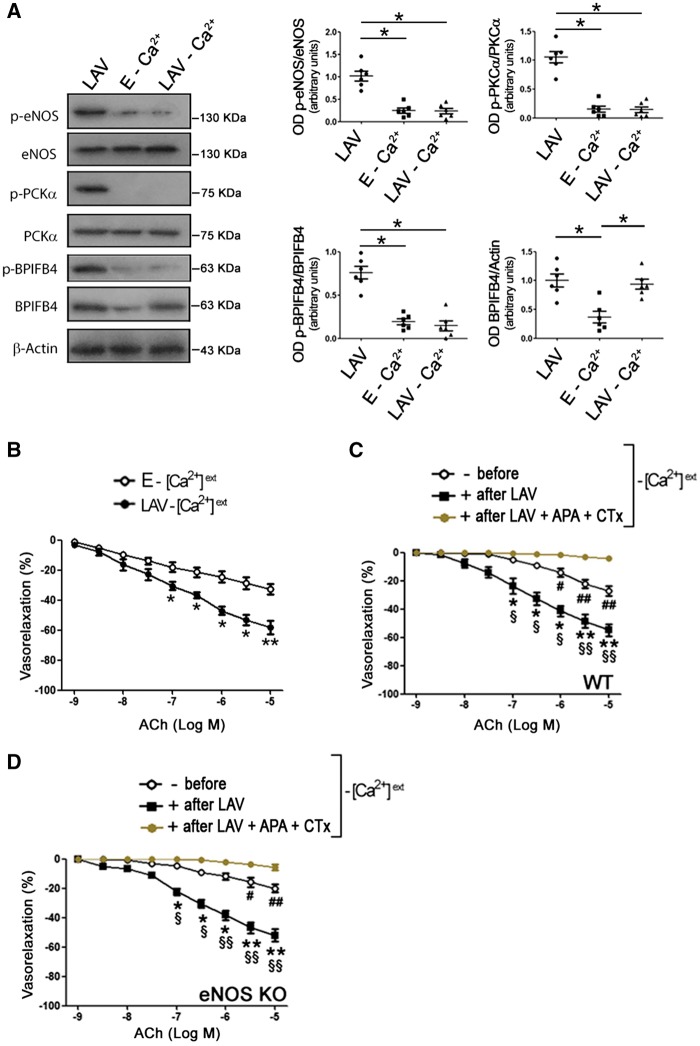
ACh-evoked eNOS phosphorylation requires influx of Ca2+.10 To clarify the role of Ca2+ in the vascular action of LAV-BPIFB4, we conducted vascular reactivity studies on mesenteric arteries in the absence of external Ca2+. In the Ca2+-free condition, LAV-BPIFB4 was hypo-phosphorylated and not able to enhance PKCα and eNOS phosphorylation (Figure 4A). However, endothelial vasorelaxation was still enhanced (Figure 4B).
Figure 4.
LAV-BPIFB4 can activate endothelial function through an EDHF-mediated mechanism. (A) Western blot of ex vivo C57BL/6 mouse mesenteric arteries transfected with LAV-BPIFB4 or empty (E) expression vectors, in the presence or absence (−Ca2+) of external Ca2+. Right graphs show quantification of p-eNOS (S1177), p-PKCα (T497), p-BPIFB4 (S75), and BPIFB4. Values are means ± S.E.M., n = 6 experiments. Statistics was performed using one way ANOVA, following Bonferroni’s Multiple Comparison Test; *P < 0.05. Dose–response curves to ACh of mouse mesenteric arteries from ex vivo WT C57BL/6 mice (B,C) or from eNOS KO mice (D) transfected with empty vector (E) or with a vector for the expression of LAV-BPIFB4 in the absence of external Ca2+ ( − [Ca2+]ext) and in the absence or presence of the EDHF inhibitors APA + CTx (100 nM each). Values are means ± S.E.M., n = 11 experiments for B; n = 8 experiments for C and D. Statistics was performed using two-way ANOVA; *P < 0.05; **P < 0.01; #P < 0.05; ##P < 0.01 vs. after LAV + APA + CTx; §P < 0.05; §§P < 0.01 vs. before.
In addition to NO, endothelium generates other mediators involved in the regulation of vascular tone, among which is EDHF.24 Thus, we inhibited EDHF release using APA—which blocks ATP-type Ca+2-activated K+ channels and SKCa—plus CTx—which blocks voltage-gated Ca2+-activated K+channels (Kv1.3) and BKCa channels. We found that when EDHF release was inhibited in the absence of external Ca2+, LAV-BPIFB4 failed to enhance endothelial vasorelaxation (Figure 4C). Taken together, these findings demonstrate that in the presence of external Ca2+, LAV-BPIFB4 enhances endothelial function via a PKCα—eNOS-mediated mechanism, whereas in the absence of Ca2+, LAV-BPIFB4 functions via an EDHF-mediated pathway. This was supported by experiments performed on eNOS−/− vessels: indeed, LAV-BPIFB4 was still able to enhance endothelial vasorelaxation in the absence of eNOS, but this effect was blunted in the presence of EDHF inhibition (Figure 4D).
3.4 PKCα is involved in a feed-forward mechanism on BPIFB4
We have previously reported that phosphorylation of serine 75 in BPIFB4 by PERK which is enhanced in the presence of the LAV isoform, induces binding to 14-3-3 and activation of eNOS.8
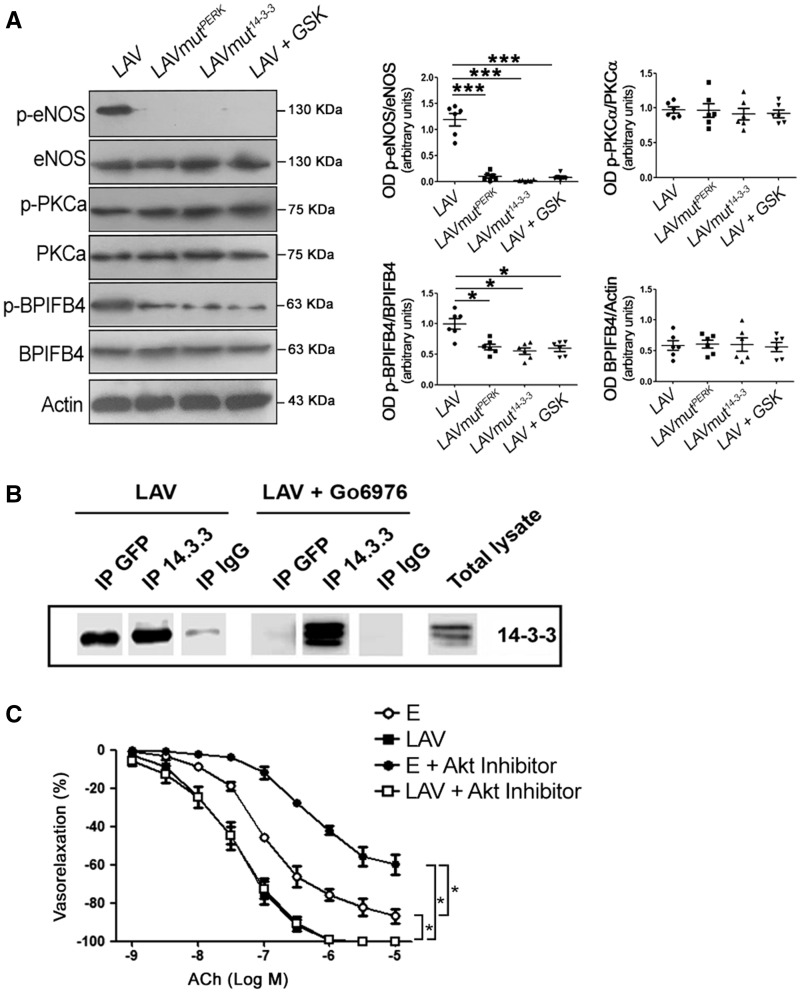
Detailed amino acid sequencing analysis revealed that serine 75 is also within a potential phosphorylation substrate motif for PKCα (amino acids 73–75: SXR/SIR). Thus, we hypothesized that PKCα contributes to eNOS activation also through its phosphorylation of BPIFB4. We first evaluated signalling in mesenteric vessels in which LAV-BPIFB4 could not be phosphorylated by PERK (namely by overexpressing a protein mutated in the PERK-phosphorylation site—LAV-BPIFB4mutPERK—or by overexpressing the LAV isoform in the presence of the PERK inhibitor GSK2606414) or bound to 14-3-3 (by overexpressing a protein mutated in the 14-3-3-binding site—LAV-BPIFB4mut14–3-3). In these setting, BPIFB4 was hypo-phosphorylated and eNOS was inactive, but PKCα remained phosphorylated (Figure 5A). When the phosphorylation of PKCα was inhibited, LAV-BPIFB4 did not co-immunoprecipitate with 14-3-3 (Figure 5B). Indeed, as shown earlier, inhibition of PKCα significantly reduced phosphorylation of LAV-BPIFB4 at serine 75 and phosphorylation of eNOS (Figure 2C), indicating that PKCα is needed for the activation of LAV-BPIFB4 and eNOS.
Figure 5.
Activation of PKCα is independent of phosphorylation of BPIFB4 by PERK at serine 75. (A) Western blot of ex vivo mouse mesenteric arteries overexpressing LAV-BPIFB4, LAV-BPIFB4mutPERK (Ser75Ala variation), LAV-BPIFB4mut14–3-3 (Ser82Asn variation), or LAV-BPIFB4 plus GSK2606414 (a PERK inhibitor). On the right, graphs of quantification of p-eNOS (S1177), p-PKC-alpha (T497), p-BPIFB4, and BPIFB4. Values are means ± S.E.M., n = 6 experiments. Statistics was performed using one-way ANOVA following Bonferroni’s Multiple Comparison Test; ***P < 0.001 vs. LAV-BPIFB4mutPERK; ***P < 0.001 vs. LAV-BPIFB4mut14–3-3; ***P < 0.001 vs. or LAV-BPIFB4 plus GSK2606414. (B) Co-immunoprecipitation of BPIFB4 and 14-3-3 in HEK293T cells overexpressing LAV-BPIFB4 tagged with GFP protein and treated with the PKCα inhibitor Gö6976. Immunoprecipitation was performed with anti-GFP (directed toward LAV-BPIFB4-GFP), anti-14-3-3, and anti-IgG (as negative control) antibodies followed by immunoblotting with anti-14-3-3 (n = 2 independent experiments). (C) Dose–response curves for ACh of ex vivo C57BL/6 mouse mesenteric arteries transfected with empty vector (E) or with LAV-BPIFB4 in the presence (+) or absence of an Akt inhibitor. Values are means ± S.E.M., n = 6 experiments. Statistics was performed using two-way ANOVA; *P < 0.05.
Because Akt signalling is one on the most important pathways modulating eNOS function,25,26 we performed experiments in the presence of Akt inhibition. In this experimental condition, vessels transfected with empty vector had significantly impaired ACh-evoked vasorelaxation, whereas those overexpressing LAV-BPIFB4 were still able to enhance ACh vasorelaxation (Figure 5C). These results clearly demonstrate that the vascular effects mediated by LAV-BPIFB4 are independent of Akt signalling.
4. Discussion
The main finding of this study is that the enhanced ability of the LAV isoform of BPIFB4 to stimulate NO production in the endothelium is due to activation of PKCα signalling. Indeed, the overexpression of LAV-BPIFB4 in HUVECs augmented Ca2+ mobilization, increasing translocation of PKCα to the plasma membrane, a step necessary for the activation of the kinase.17 In the absence of external Ca2+, the ability of LAV-BPIFB4 to enhance both PKCα and eNOS phosphorylation was abolished.
We demonstrated previously that phosphorylation of BPIFB4 at serine 75 by PERK and the binding to 14-3-3 protein are fundamental for activation of eNOS and endothelial function.8 So, to further clarify the mechanisms elicited by the LAV isoform, we investigated potential players, focusing our attention on PKCα, a major PKC family member expressed in endothelial cells and involved in regulating eNOS function.11,27 We found that inhibition of PKCα impeded the enhancing effects of LAV-BPIFB4 on eNOS and endothelial function, positioning this kinase between BPIFB4 and eNOS. Of note, mutation of LAV-BPIFB4 at its PERK-phosphorylated site or at its 14-3-3-binding site blunted eNOS activation but did not interfere with the ability to activate PKCα. These findings indicate that the activation of PKCα is independent of Ser75 phosphorylation and binding to 14-3-3.
We also found that serine 75 is within a PKC phosphorylation motif (amino acids 73–75: SXR/SIR),28 indicating that the kinase works also upstream of BPIFB4. Indeed, when we inhibited PKCα, the site serine 75 became hypo-phosphorylated and BPIFB4 did not co-immunoprecipitate with 14-3-3.
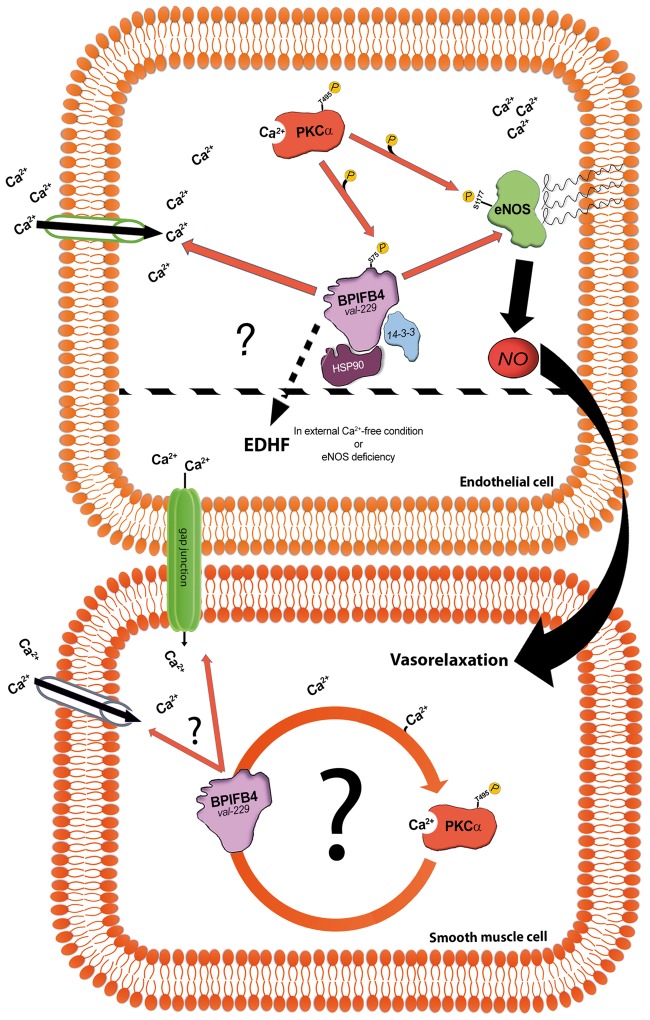
All these findings suggest a mechanism whereby the stimulation of Ca2+ influx by LAV-BPIFB4 leads to the activation PKCα, which in turn increases phosphorylation of BPIFB4 at serine 75; this hyper-phosphorylation results in enhanced binding of LAV-BPIFB4 to 14-3-3 and HSP90,8 allowing eNOS to binding with the complex and become phosphorylated by PKCα (Figure 6).
Figure 6.
Schematic of how LAV-BPIFB4 mediates its effects on the vasculature. Mechanism recruited by LAV-BPIFB4 to modulate eNOS function in endothelial cells (up). LAV-BPIFB4 enhances Ca2+ influx, leading to PKCα activation and consequential phosphorylation of LAV-BPIFB4 at serine 75. This hyper-phosphorylation results in enhanced binding of LAV-BPIFB4 to 14-3-3 and HSP90, steps necessary for interaction of the complex with eNOS and its phosphorylation by PKCα. Alternatively, in the absence of external Ca2+ or functional eNOS, LAV-BPIFB4 can still enhance vasorelaxation by stimulating EDHF signalling. Possible involvement of LAV-BPIFB4 in the activation of PKCα in smooth muscle cells (bottom). BPIFB4 val-229, LAV-BPIFB4; PKCα, protein kinase C-alpha; HSP90, heat shock protein 90; eNOS, endothelial nitric oxide synthase; NO, nitric oxide; EDHF, endothelium-derived hyperpolarizing factors; Ca2+, calcium ion.
In addition, careful analysis of our data revealed that in the absence of external Ca2+, LAV-BPIFB4 still enhanced endothelial vasorelaxation despite blunted phosphorylation of PKCα and eNOS. We found that this effect was dependent upon the recruitment of EDHF, which is known can substitute for NO in settings where eNOS is dysfunctional.29,30 To definitively clarify the concept that LAV-BPIFB4 is able to recruit alternative mechanisms protecting endothelial function in the absence of eNOS, we performed experiments on vessels from eNOS deficient mice. Also in this experimental setting, the expression of LAV-BPIFB4 was associated with enhanced endothelial vasorelaxation. More studies are needed to clarify the mechanism through which LAV-BPIFB4 mediates EDHF release.
Our findings highlight that Ca2+ mobilization is a key signal necessary for LAV-BPIFB4 to enhance endothelial NO release. The agonist-induced Ca2+ entry observed in HUVECs is an example of capacitive Ca2+ influx triggered by metabotropic receptors.10 LAV-BPIFB4 is not able to mobilize Ca2+ itself, but its expression strongly increases the amplitude of agonist-evoked Ca2+ signals, likely by interacting with one or more elements of the molecular machinery regulating [Ca2+]i. These findings also indicate a possible broader role of LAV-BPIFB4 in modulating other functions where PKCα is involved, such as control of stem cell maintenance, development, and differentiation, functions that are lost during ageing and that could be preserved in LLIs.31,32
Finally, our study defines the effects of LAV-BPIFB4 on the modulation of eNOS function in endothelial cells; we cannot exclude a contribution by smooth muscle cells in eNOS activation. Further work will be necessary to study this aspect of vasorelaxation.
5. Conclusions
The LAV of BPIFB4 has tremendous potential for exploitation as an important tool for the treatment of ageing-related diseases because of its effect on NO metabolism. Moreover, the effect of LAV-BPIFB4 on EDHF release opens up new scenarios for enhancing endothelial function via therapeutic targets other than eNOS.
Acknowledgements
We thank Prof. L. Naldini for providing the lentiviral packaging vectors.
Conflict of interest: none declared.
Funding
This work was supported by Italian Ministry of University and Research (MIUR/FIRB AUTOMED–RBAP11Z3YA to A.A.P. and PRIN- 20157ATSLF_009 to A.A.P., C.V, G.F. and L.M.). L. Milanesi is supported by Flagship “InterOmics” PB05 and A.Ferrario is a fellow of this project.
References
- 1. Forstermann U, Munzel T.. Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation 2006;113:1708–1714. [DOI] [PubMed] [Google Scholar]
- 2. Bian K, Doursout MF, Murad F.. Vascular system: role of nitric oxide in cardiovascular diseases. J Clin Hypertens (Greenwich) 2008;10:304–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Puca AA, Carrizzo A, Ferrario A, Villa F, Vecchione C.. Endothelial nitric oxide synthase, vascular integrity and human exceptional longevity. Immun Ageing 2012;9:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Forstermann U, Sessa WC.. Nitric oxide synthases: regulation and function. Eur Heart J 2012;33:829–837, 837a–837d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nisoli E, Tonello C, Cardile A, Cozzi V, Bracale R, Tedesco L, Falcone S, Valerio A, Cantoni O, Clementi E, Moncada S, Carruba MO.. Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science 2005;310:314–317. [DOI] [PubMed] [Google Scholar]
- 6. Perls TT, Wilmoth J, Levenson R, Drinkwater M, Cohen M, Bogan H, Joyce E, Brewster S, Kunkel L, Puca A.. Life-long sustained mortality advantage of siblings of centenarians. Proc Natl Acad Sci USA 2002;99:8442–8447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhang QJ, McMillin SL, Tanner JM, Palionyte M, Abel ED, Symons JD.. Endothelial nitric oxide synthase phosphorylation in treadmill-running mice: role of vascular signalling kinases. J Physiol 2009;587:3911–3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Villa F, Carrizzo A, Spinelli CC, Ferrario A, Malovini A, Maciag A, Damato A, Auricchio A, Spinetti G, Sangalli E, Dang Z, Madonna M, Ambrosio M, Sitia L, Bigini P, Cali G, Schreiber S, Perls T, Fucile S, Mulas F, Nebel A, Bellazzi R, Madeddu P, Vecchione C, Puca AA.. Genetic analysis reveals a longevity-associated protein modulating endothelial function and angiogenesis. Circ Res 2015;117:333–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kraehling JR, Sessa WC.. Enhanced eNOS activation as the fountain of youth for vascular disease: is BPIFB4 what ponce de leon was looking for? Circ Res 2015;117:309–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Adapala RK, Talasila PK, Bratz IN, Zhang DX, Suzuki M, Meszaros JG, Thodeti CK.. PKCalpha mediates acetylcholine-induced activation of TRPV4-dependent calcium influx in endothelial cells. Am J Physiol Heart Circ Physiol 2011;301:H757–H765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Partovian C, Zhuang Z, Moodie K, Lin M, Ouchi N, Sessa WC, Walsh K, Simons M.. PKCalpha activates eNOS and increases arterial blood flow in vivo. Circ Res 2005;97:482–487. [DOI] [PubMed] [Google Scholar]
- 12. Vecchione C, Aretini A, Marino G, Bettarini U, Poulet R, Maffei A, Sbroggio M, Pastore L, Gentile MT, Notte A, Iorio L, Hirsch E, Tarone G, Lembo G.. Selective Rac-1 inhibition protects from diabetes-induced vascular injury. Circ Res 2006;98:218–225. [DOI] [PubMed] [Google Scholar]
- 13. Orlandi A, Pagani F, Avitabile D, Bonanno G, Scambia G, Vigna E, et al. Functional properties of cells obtained from human cord blood CD34+ stem cells and mouse cardiac myocytes in coculture. Am J Physiol Heart Circ Physiol. 2008;294:H1541–H1549. [DOI] [PubMed] [Google Scholar]
- 14. Cortes MP, Becerra JP, Vinet R, Alvarez R, Quintana I.. Inhibition of ATP-induced calcium influx by homocysteine in human umbilical vein endothelial cells. Cell Biol Int 2013;37:600–607. [DOI] [PubMed] [Google Scholar]
- 15. Cali G, Gentile F, Mogavero S, Pallante P, Nitsch R, Ciancia G, Ferraro A, Fusco A, Nitsch L.. CDH16/Ksp-cadherin is expressed in the developing thyroid gland and is strongly down-regulated in thyroid carcinomas. Endocrinology 2012;153:522–534. [DOI] [PubMed] [Google Scholar]
- 16. Pena VB, Bonini IC, Antollini SS, Kobayashi T, Barrantes FJ.. alpha 7-type acetylcholine receptor localization and its modulation by nicotine and cholesterol in vascular endothelial cells. J Cell Biochem 2011;112:3276–3288. [DOI] [PubMed] [Google Scholar]
- 17. Newton AC. Regulation of protein kinase C. Curr Opin Cell Biol 1997;9:161–167. [DOI] [PubMed] [Google Scholar]
- 18. Denker BM, Nigam SK.. Molecular structure and assembly of the tight junction. Am J Physiol 1998;274:F1–F9. [DOI] [PubMed] [Google Scholar]
- 19. Kumar NM, Gilula NB.. The gap junction communication channel. Cell 1996;84:381–388. [DOI] [PubMed] [Google Scholar]
- 20. Dejana E, Corada M, Lampugnani MG.. Endothelial cell-to-cell junctions. FASEB J 1995;9:910–918. [PubMed] [Google Scholar]
- 21. DePaola N, Davies PF, Pritchard WF Jr, Florez L, Harbeck N, Polacek DC.. Spatial and temporal regulation of gap junction connexin43 in vascular endothelial cells exposed to controlled disturbed flows in vitro. Proc Natl Acad Sci USA 1999;96:3154–3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yamada M, Kubo H, Kobayashi S, Ishizawa K, Numasaki M, Ueda S, Suzuki T, Sasaki H.. Bone marrow-derived progenitor cells are important for lung repair after lipopolysaccharide-induced lung injury. J Immunol 2004;172:1266–1272. [DOI] [PubMed] [Google Scholar]
- 23. Ulmer AJ, Flad H, Rietschel T, Mattern T.. Induction of proliferation and cytokine production in human T lymphocytes by lipopolysaccharide (LPS). Toxicology 2000;152:37–45. [DOI] [PubMed] [Google Scholar]
- 24. Feletou M, Vanhoutte PM.. EDHF: new therapeutic targets? Pharmacol Res 2004;49:565–580. [DOI] [PubMed] [Google Scholar]
- 25. Carrizzo A, Damato A, Ambrosio M, Falco A, Rosati A, Capunzo M, Madonna M, Turco MC, Januzzi JL, De Laurenzi V, Vecchione C.. The prosurvival protein BAG3: a new participant in vascular homeostasis. Cell Death Dis 2016;7:e2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nishimatsu H, Suzuki E, Nagata D, Moriyama N, Satonaka H, Walsh K, Sata M, Kangawa K, Matsuo H, Goto A, Kitamura T, Hirata Y.. Adrenomedullin induces endothelium-dependent vasorelaxation via the phosphatidylinositol 3-kinase/Akt-dependent pathway in rat aorta. Circ Res 2001;89:63–70. [DOI] [PubMed] [Google Scholar]
- 27. Gelinas DS, Bernatchez PN, Rollin S, Bazan NG, Sirois MG.. Immediate and delayed VEGF-mediated NO synthesis in endothelial cells: role of PI3K, PKC and PLC pathways. Br J Pharmacol 2002;137:1021–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dhein S. Peptides acting at gap junctions. Peptides 2002;23:1701–1709. [DOI] [PubMed] [Google Scholar]
- 29. Huang A, Sun D, Carroll MA, Jiang H, Smith CJ, Connetta JA, Falck JR, Shesely EG, Koller A, Kaley G.. EDHF mediates flow-induced dilation in skeletal muscle arterioles of female eNOS-KO mice. Am J Physiol Heart Circ Physiol 2001;280:H2462–H2469. [DOI] [PubMed] [Google Scholar]
- 30. Parkington HC, Tare M, Coleman HA.. The EDHF story: the plot thickens. Circ Res 2008;102:1148–1150. [DOI] [PubMed] [Google Scholar]
- 31. Van Zant G, Liang Y.. The role of stem cells in aging. Exp Hematol 2003;31:659–672. [DOI] [PubMed] [Google Scholar]
- 32. Adams ER, Nolan VG, Andersen SL, Perls TT, Terry DF.. Centenarian offspring: start healthier and stay healthier. J Am Geriatr Soc 2008;56:2089–2092. [DOI] [PMC free article] [PubMed] [Google Scholar]