Abstract
Aims
The type 2 diabetic heart oxidizes more fat and less glucose, which can impair metabolic flexibility and function. Increased sarcolemmal fatty acid translocase (FAT/CD36) imports more fatty acid into the diabetic myocardium, feeding increased fatty acid oxidation and elevated lipid deposition. Unlike other metabolic modulators that target mitochondrial fatty acid oxidation, we proposed that pharmacologically inhibiting fatty acid uptake, as the primary step in the pathway, would provide an alternative mechanism to rebalance metabolism and prevent lipid accumulation following hypoxic stress.
Methods and results
Hearts from type 2 diabetic and control male Wistar rats were perfused in normoxia, hypoxia and reoxygenation, with the FAT/CD36 inhibitor sulfo-N-succinimidyl oleate (SSO) infused 4 min before hypoxia. SSO infusion into diabetic hearts decreased the fatty acid oxidation rate by 29% and myocardial triglyceride concentration by 48% compared with untreated diabetic hearts, restoring fatty acid metabolism to control levels following hypoxia-reoxygenation. SSO infusion increased the glycolytic rate by 46% in diabetic hearts during hypoxia, increased pyruvate dehydrogenase activity by 53% and decreased lactate efflux rate by 56% compared with untreated diabetic hearts during reoxygenation. In addition, SSO treatment of diabetic hearts increased intermediates within the second span of the Krebs cycle, namely fumarate, oxaloacetate, and the FAD total pool. The cardiac dysfunction in diabetic hearts following decreased oxygen availability was prevented by SSO-infusion prior to the hypoxic stress. Infusing SSO into diabetic hearts increased rate pressure product by 60% during hypoxia and by 32% following reoxygenation, restoring function to control levels.
Conclusions
Diabetic hearts have limited metabolic flexibility and cardiac dysfunction when stressed, which can be rapidly rectified by reducing fatty acid uptake with the FAT/CD36 inhibitor, SSO. This novel therapeutic approach not only reduces fat oxidation but also lipotoxicity, by targeting the primary step in the fatty acid metabolism pathway.
Keywords: Metabolism, Energy, Fatty acid, Hypoxia, Glucose
1. Introduction
To power contraction the heart requires a constant supply of ATP, which can be obtained from a range of metabolic fuels. Metabolism of fatty acids produces more ATP per carbon atom but requires more oxygen per ATP generated, when compared with glucose. In type 2 diabetes, the heart becomes metabolically abnormal, oxidizing more fatty acids, and less glucose to meet its energy requirements, and this is associated with higher myocardial oxygen consumption.1–7 The primary step in myocardial fatty acid metabolism is fatty acid import into the cardiomyocyte, which is regulated by a family of sarcolemmal fatty acid transporters. Fatty acid translocase (FAT/CD36), the predominant fatty acid transporter, is increased in diabetes,8 consequently more lipid is imported into the diabetic heart fuelling increased fatty acid oxidation, lipid storage and, via the Randle effect, suppressing glucose metabolism.9,10
Strategies that modify fatty acid metabolism are of great interest for the diabetic heart. Compounds such as ranolazine, etomoxir, perhexiline, and trimetazidine, which target mitochondrial fatty acid oxidation, have been investigated predominantly in heart failure and angina.11–15 However, one concern with these mitochondrial fatty acid oxidation inhibitors is that entry of fat into the cardiomyocyte can still continue, and additional fat may accumulate as lipid within the cytosol, as reported in diabetic rats following chronic etomoxir treatment.16 Thus, a better approach would be to block fatty acid metabolism at its primary point, at the level of the sarcolemmal fatty acid uptake, by inhibiting FAT/CD36. Genetic studies with FAT/CD36-deficient mice showed particular benefit when crossed with PPARα-overexpressing mice prone to lipotoxic cardiomyopathy, as loss of FAT/CD36 reduced lipid accumulation and reversed contractile dysfunction.17 However, to exploit this genetic knowledge, therapeutic strategies that pharmacologically inhibit FAT/CD36 are needed.
Metabolic dysfunction in diabetes is regulated by complex mechanisms relating to increased fatty acid availability and insulin resistance. Metabolic changes in the diabetic heart become of greater consequence under conditions of cardiac stress, e.g. when oxygen is restricted. Published data suggest that the diabetic heart has limited metabolic flexibility to change metabolism in response to stimuli,18,19 and decreased mitochondrial energetics in response to anoxia.20,21 To acutely adapt to low oxygen availability the heart must downregulate fatty acid metabolism and increase anaerobic glycolysis, mediated in part by AMP-activated protein kinase (AMPK) activation.22 Interestingly, patients with type 2 diabetes have a number of hypoxia-associated complications, resulting in the heart being exposed, locally or systemically, to reduced oxygen supply.23–25 Therefore, understanding whether metabolic flexibility in diabetes in response to hypoxic stress is abnormal, and if it can be reinstated by pharmacological agents that suppress fatty acid uptake, is of interest.
Given the accessible location of FAT/CD36 on the extracellular surface of the cardiomyocyte, and its elevated sarcolemmal localization in the diabetic heart, FAT/CD36 may be an attractive target for metabolic intervention, as it may simultaneously reduce both fatty acid oxidation and lipid deposition. In this study, we investigate the FAT/CD36 inhibitor, sulfo-N-succinimidyl oleate (SSO) as a metabolic modulator, to study its effects in the intact heart of the type 2 diabetic rat. We questioned whether inhibition of FAT/CD36 using SSO may provide a novel pharmacological way to improve metabolism and function of the diabetic heart challenged by hypoxic stress.
2. Methods
2.1 In vivo rat model of type 2 diabetes
Male Wistar rats (n = 71, starting body weight ∼250g) were obtained from a commercial breeder (Harlan, UK). This investigation conformed to the United Kingdom Home Office guidelines under The Animal (Scientific Procedures) Act, 1986, and was approved by the University of Oxford local ethics committee.
Control rats were fed for 42 days on a standard chow diet (Harlan Laboratories). Type 2 diabetes was induced in rats by feeding a high-fat diet (60% calories from fat, Special Diet Services) for 42 days and, on day 14, a single low dose intraperitoneal injection of streptozotocin (STZ, 25 mg/kg bodyweight, in citrate buffer, pH 4)7,26–28 was given. We have previously demonstrated that it is the combination of high-fat diet and low dose STZ that induces type 2 diabetes,27 as low dose STZ alone does not impair insulin secretion, which is in contrast to type 1 diabetes models where a high dose of STZ is used to destroy the β-cells. This model of type 2 diabetes was chosen over others available as it induces hyperinsulinaemia, mild hyperglycaemia, adiposity and diastolic dysfunction,7,26,27 thereby mirroring the human disease, yet avoiding extreme hyperglycaemia and genetic manipulation present in other models.29 On day 40, control and diabetic rats had fasting blood collected from the saphenous vein to confirm hyperinsulinaemia and hyperglycaemia (blood glucose measured by Accu-check Aviva blood glucose testing system and plasma insulin concentrations measured by ELISA, R&D systems). On day 42, rats were terminally anaesthetized using an intraperitoneal injection of sodium pentobarbital (150 mg/kg bodyweight, Euthatal). Hearts were excised for perfusion and blood was collected for analysis of plasma non-esterified fatty acids (NEFA).
2.2 Isolated heart perfusion
Hearts were perfused at a constant pressure of 100 mmHg and an end-diastolic pressure of 4–8 mmHg. Hearts were perfused with Krebs-Henseleit buffer containing 11 mM glucose and 1.5% (w/v) fatty acid-free bovine serum albumin bound to 0.4 mM palmitate at 37 °C. Hearts were initially perfused in normoxia for 20 min by gassing the buffer with 95% O2 and 5% CO2 to give an oxygen partial pressure (PO2) of 413 ± 15 mmHg. Following normoxia, hearts were made acutely hypoxic by replacing the gas with 95% N2 and 5% CO2 to decrease the PO2 to 90 ± 10 mmHg for 32 min, followed by reoxygenating the buffer with 95% O2 and 5% CO2 for a further 20 min. Cardiac function was measured using a fluid-filled PVC balloon inserted into the left ventricle, attached to a bridge amplifier and PowerLab data acquisition system (ADInstruments). Left ventricular developed pressure was determined as peak systolic minus end-diastolic pressure, and rate pressure product (RPP) was the multiple of developed pressure and heart rate. Functional data were averaged over the last 5 min at the end of normoxia, hypoxia, and reoxygenation.
2.3 Sulfo-N-succinimidyl oleate
N-hydroxysulfosuccinimide sodium salt was synthesized according to the protocol of Staros.30 N-hydroxysulfosuccinimide sodium salt (2 mM), dicyclohexylcarbodiimide (2.2 mM), and oleic acid (4 mM) were dissolved in dimethylformamide overnight at room temperature, followed by stirring for 3 h at 4 °C. The product was filtered, washed three times with dimethylformamide, and sulfo-N-succinimidyl oleate (SSO) was precipitated from the solution using ∼20 volumes of ethyl acetate, filtered, and stored under vacuum in a desiccator. Following optimization of dose and time to effect, a final concentration of 0.5 mM SSO (dissolved in 200 µL DMSO) was added to the perfusion buffer. SSO was added 4 min prior to the induction of hypoxia and recirculated throughout the hypoxia-reoxygenation period. Hearts perfused with SSO were freeze-clamped at the end of reoxygenation.
2.4 Metabolic rates and tissue analysis
For measurement of glycolytic rates, buffer was supplemented with 0.2 μCi.mL−1 [5-3H]-glucose.31 For measurement of palmitate oxidation rates, buffer was supplemented with 0.2 µCi.mL−1 [9,10-3H] palmitate.31 Lactate efflux rates were measured in timed aliquots using lactate dehydrogenase. Metabolic rates were calculated during normoxia, hypoxia and reoxygenation, from perfusate samples collected at 4-min intervals for a minimum of five linear data points. In three separate groups, hearts were freeze-clamped on the cannula at the end of normoxia, following normoxia-hypoxia, or following normoxia-hypoxia-reoxygenation. Glycogen content was determined by the conversion of glycogen to glycosyl units, which were assayed using amyloglucosidase. Triglyceride content was measured following Folch extraction.32 Pyruvate dehydrogenase (PDH) activity was measured in the active form according to the protocol of Seymour and Chatham.33 PDH activity was measured as a surrogate marker for glucose oxidation, as this pathway could not be measured with the 3H-glucose label.
2.5 Metabolomics
In brief, metabolites were extracted using methanol/chloroform from ∼50 mg wet weight of powdered, frozen heart tissue.34 The organic and aqueous fractions were separated by the addition of water and chloroform. The aqueous layer was analysed subsequently for metabolomics. Mass spectrometry was performed on an AB Sciex 5500 (Warrington, UK) coupled to an Acquity ultra performance liquid chromatography (UPLC) system from Waters Ltd (Atlas Park, Manchester, UK). Part of the aqueous extract was dissolved in 70:30 acetonitrile: water containing 20 µM deoxy-glucose 6 phosphate and 20 µM [U-13C,15N] glutamate as internal standards. For chromatography of nucleotides the strong mobile phase was 100mM ammonium acetate, the weak mobile phase was acetonitrile and the LC column used was the ZIC-HILIC column from Sequant (100 × 2.1 mm, 5 µm). Metabolites were quantified using pre-defined mass transitions on the mass spectrometer in positive ion mode. For the analysis of Krebs cycle intermediates the sample from the above analysis was recovered and analysed in a second UPLC chromatography assay with the mass spectrometer in negative ion mode. The strong mobile phase was 10 mM ammonium acetate with 0.05% ammonium hydroxide and the weak mobile phase was acetonitrile, and the LC column used was a BEH amide HILIC column (100 × 2.1 mm, 1.7 µm; Waters Ltd). Compound specific parameters such as cone voltage, collision energy, and mass transitions were used for quantification of the metabolites. For the analysis of amino acids, the remaining aqueous sample was dried under nitrogen and derivatized with 3M HCl in BuOH for 15 min at 65ºC. After further drying, the sample was reconstituted in 9:1 0.1% formic acid in water/acetonitrile. For chromatography, the strong mobile phase used for analysis was acetonitrile and the weak mobile phase was 0.1% formic acid in water, and a HSS T3 column (100 × 2.1 mm, 1.7 µm) from Waters Ltd was used. Again, cone voltage, collision energy and mass transitions were optimized for each metabolite for quantification. Data are expressed in arbitrary units as the area ratio of metabolite peak relative to the internal standard, normalized to the protein content. In total, 76 metabolites were identified and quantified per heart.
2.6 Western blotting
For western blotting, protein lysates were prepared from frozen tissue, protein was loaded onto SDS-PAGE gels and separated by electrophoresis.35 Even protein loading and transfer were confirmed by normalizing to loading controls, either cyclophilin B, or ponceau staining. For measurement of sarcolemmal transporters, separation of the sarcolemmal membrane fraction from intracellular endosomes was carried out according to the established method of Luiken et al.36 Differential centrifugation was used to separate the membrane fractions, and the different fractions were analysed by western blotting for FAT/CD36 and GLUT4. As described previously, sarcolemmal fractions had three-fold enrichment of Na+K+-ATPase protein levels, whereas microsomal fractions had nine-fold enrichment of Rab4 protein levels.37 Bands were quantified using UN-SCAN-IT gel software (Silk Scientific, USA), and all samples were run in duplicate on separate gels to confirm results.
2.7 Statistics
Results, presented as means ± SEM, were considered significant at P < 0.05 (SPSS Statistics 18 and GraphPad Prism). Data are presented as scatter graphs, with n numbers displayed on graphs. Baseline function and metabolism were compared between control and diabetic hearts using an unpaired t-test, and characterization of the SSO effect in control hearts were compared by paired t-test. Metabolomic data were compared using a two-way ANOVA (two factors being diabetic status and oxygen level). Cardiac function and metabolism with SSO treatment were compared within the same oxygenation status, using a two-way ANOVA (two factors being diabetes and drug). Post-hoc comparisons were made using Tukey test with corrections made for multiple comparisons.
3. Results
3.1 Characterization of type 2 diabetic rats
There were no significant differences in body weights (377 ± 7 g control vs. 393 ± 9 g diabetic rats) or in heart weights (1.40 ± 0.03 g control vs. 1.46 ± 0.03 g diabetic rats) between groups. Epididymal fat pad weight, a marker of adiposity, was increased by 64% in diabetic rats compared with controls (5.3 ± 0.3 g vs. 8.7 ± 0.5 g in control and diabetic rats, respectively, P < 0.05). Fasting blood glucose concentrations were increased by 26% from 5.1 ± 0.3 mmol/L in control rats to 6.4 ± 0.6 mmol/L in diabetic rats (P < 0.05), and fasting plasma insulin concentrations were increased 2.7-fold from 59 ± 5 pmol/L in control rats to 163 ± 29 pmol/L in diabetic rats (P < 0.05). Plasma NEFA concentrations in the fed state were increased by 83% from 0.06 ± 0.01 mmol/L in control rats to 0.11 ± 0.01 mmol/L in diabetic rats (P < 0.05).
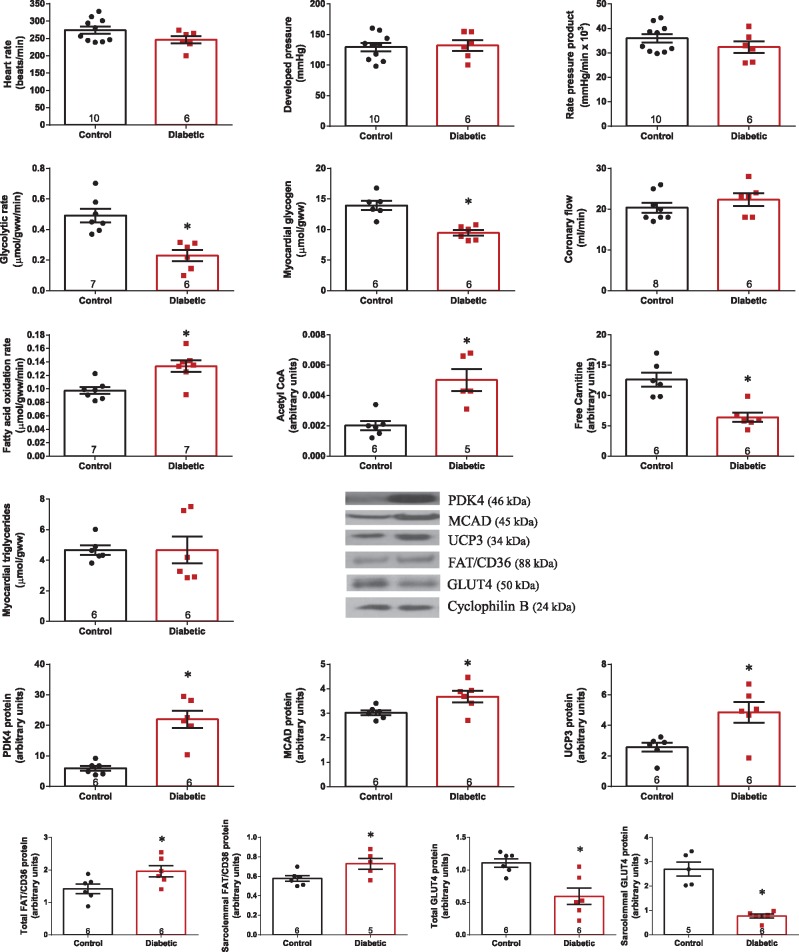
There were no differences in contractile function in type 2 diabetic hearts compared with control hearts during baseline normoxic perfusion (Figures 1 and 6). Glycolytic rate was 53% lower and myocardial glycogen concentration was decreased by 32% in diabetic hearts compared with controls (Figure 1). In contrast, fatty acid oxidation rate was increased by 37%, acetyl CoA level was 2.5-fold higher, and free carnitine was 50% lower in diabetic hearts compared with controls, but with no significant difference in myocardial triglyceride concentration. Metabolic changes in the diabetic heart are associated with increased PPARα transcriptional activity,38,39 as PPARα targets PDH kinase 4 (PDK4), medium chain acyl co-enzyme A dehydrogenase (MCAD), and uncoupling protein 3 (UCP3) were all significantly increased in the diabetic heart. In line with flux measurements and PPARα activation, whole tissue level of the fatty acid transporter FAT/CD36 was increased by 38%, and sarcolemmal content of FAT/CD36 was increased by 26% in diabetic hearts compared with control hearts. In contrast, whole tissue level of the glucose transporter GLUT4 was decreased by 46%, and sarcolemmal content of GLUT4 was decreased by 72% in diabetic hearts compared with controls.
Figure 1.
Cardiac function and substrate metabolism in control and diabetic hearts under normoxic baseline perfusion. *P < 0.05 vs. control. Acetyl CoA and free carnitine expressed as peak area ratio, relative to internal standard.
3.2 Infusion of the FAT/CD36 inhibitor, SSO, into control hearts rapidly modified metabolism without compromising function
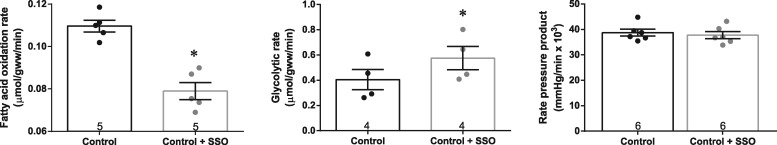
We questioned whether SSO, an inhibitor of FAT/CD36, would rapidly decrease fatty acid metabolism and promote glycolysis in the perfused heart, without having deleterious effects on function. We optimized the protocol in aerobically perfused control hearts, and found that 0.5 mM SSO decreased fatty acid oxidation rate by 28% and increased glycolytic rate by 43%, occurring within minutes of SSO addition (Figure 2). This metabolic effect of SSO occurred without compromising cardiac function, with no change in RPP (Figure 2), heart rate (279 ± 11 vs. 287 ± 9 bpm before and after SSO infusion, respectively) and developed pressure (132 ± 7 vs. 125 ± 6 mmHg before and after SSO infusion, respectively). No additional decrease in fatty acid oxidation was seen with a higher dose of SSO, and we confirmed that the DMSO-vehicle was not affecting fatty acid metabolism (see Supplementary material online, Tables S1 and S2).
Figure 2.
Substrate metabolism and cardiac function in control normoxic perfused hearts, before and after infusion of 0.5 mM SSO. *P < 0.05 vs. control without SSO.
3.3 Inhibition of FAT/CD36 in diabetic hearts normalized substrate metabolism and prevented lipid overload following hypoxic stress
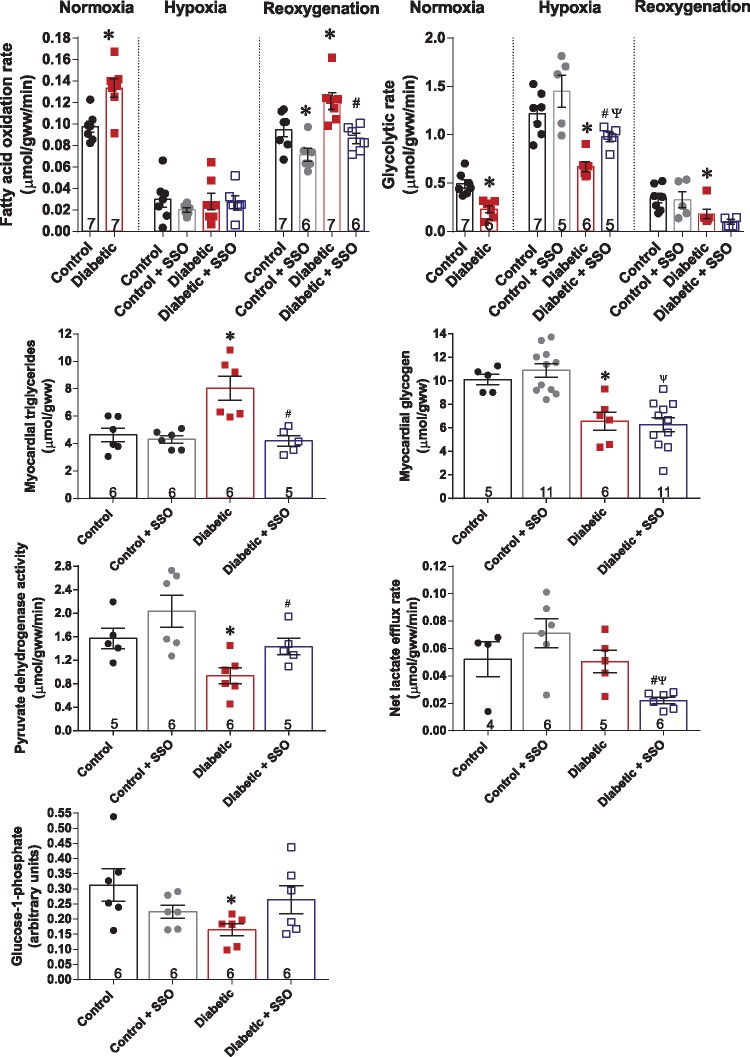
It has long been recognized that diabetic hearts do worse functionally when challenged with stress, and that abnormal metabolism plays a key role in this dysfunction. Given that diabetic hearts have elevated sarcolemmal FAT/CD36 and increased fatty acid metabolism, we questioned whether moderate inhibition of FAT/CD36 with SSO would show benefit for the diabetic heart during exposure to hypoxic stress. Therefore, we infused SSO into diabetic and control hearts 4 min prior to acute hypoxia, and recirculated it throughout the subsequent reoxygenation period. Hypoxia significantly decreased the fatty acid oxidation rate, with no difference between control and diabetic hearts, with or without SSO present (Figure 3), demonstrating this metabolic response was likely driven by low oxygen availability. In contrast, following reoxygenation SSO treatment of diabetic hearts normalized the fatty acid oxidation rate back to that found in control hearts, decreasing fatty acid oxidation by 29% compared with untreated diabetic hearts. This improvement in fatty acid oxidation at reoxygenation occurred concomitantly with an improvement of myocardial triglycerides. Whereas in untreated diabetic hearts myocardial triglyceride concentration was 73% greater than in control hearts at reoxygenation, this was restored to control levels by SSO infusion. Hypoxia significantly increased glycolytic rates in all hearts, however, in diabetic hearts glycolytic rate remained 55% lower than in control hearts, demonstrating impaired upregulating of glycolysis in response to hypoxia. This was not due to impaired p-AMPK activation and AMPK signalling in diabetic hearts during hypoxia (seeSupplementary material online, Figure S1). SSO treatment of diabetic hearts was able to increase glycolytic rate by 46% compared with untreated diabetic hearts during hypoxia (Figure 3). However, SSO had no significant effect on glycolytic rate at reoxygenation or on myocardial glycogen concentration, SSO increased PDH activity in diabetic hearts by 53%, so that it was no longer significantly different to controls. This SSO-mediated increase in PDH flux in diabetic hearts was accompanied by a significant decrease in lactate efflux rate following reoxygenation, indicating that more pyruvate was being oxidized in the mitochondria following SSO treatment of diabetic hearts. Glucose-1-phosphate level was restored to control levels by SSO-treatment of diabetic hearts.
Figure 3.
Substrate metabolism in control and diabetic hearts, with and without FAT/CD36 inhibitor SSO, during hypoxia and reoxygenation. *P < 0.05 vs. control under same oxygen condition, #P < 0.05 vs. diabetic, ΨP < 0.05 vs. control + SSO. Glucose-1-phosphate expressed as peak area ratio, relative to internal standard. Fatty acid oxidation and glycolytic rate n numbers are the same in hypoxia and reoxygenation.
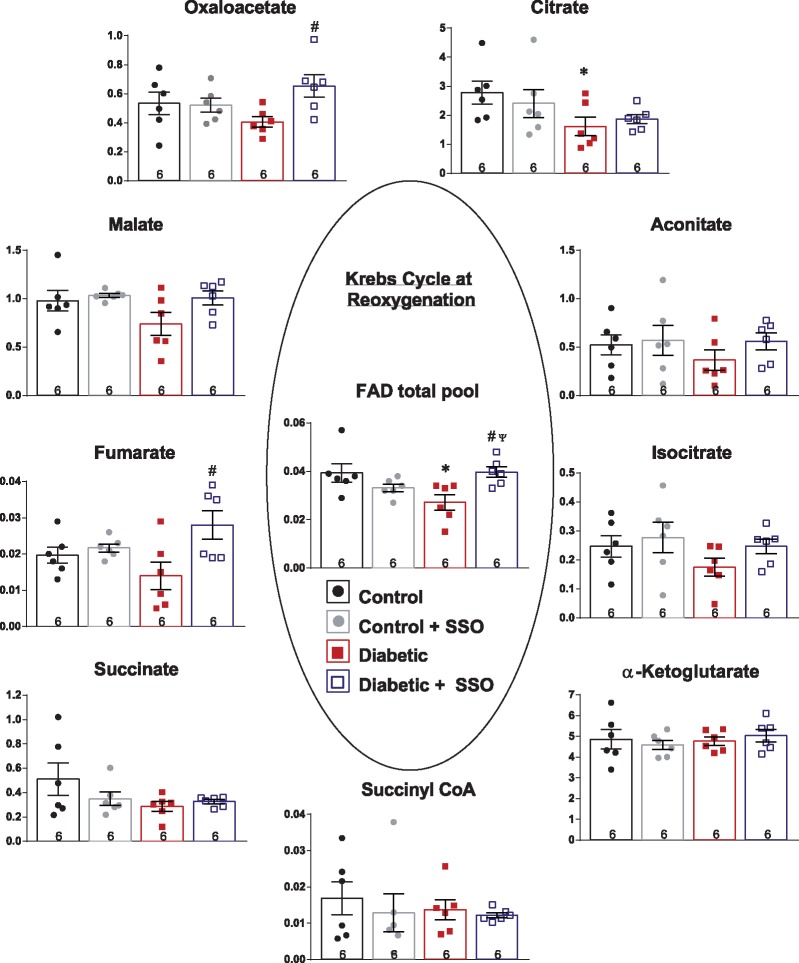
3.4 FAT/CD36 inhibition modulated Krebs cycle intermediates in diabetic hearts, predominantly within the second span of the cycle
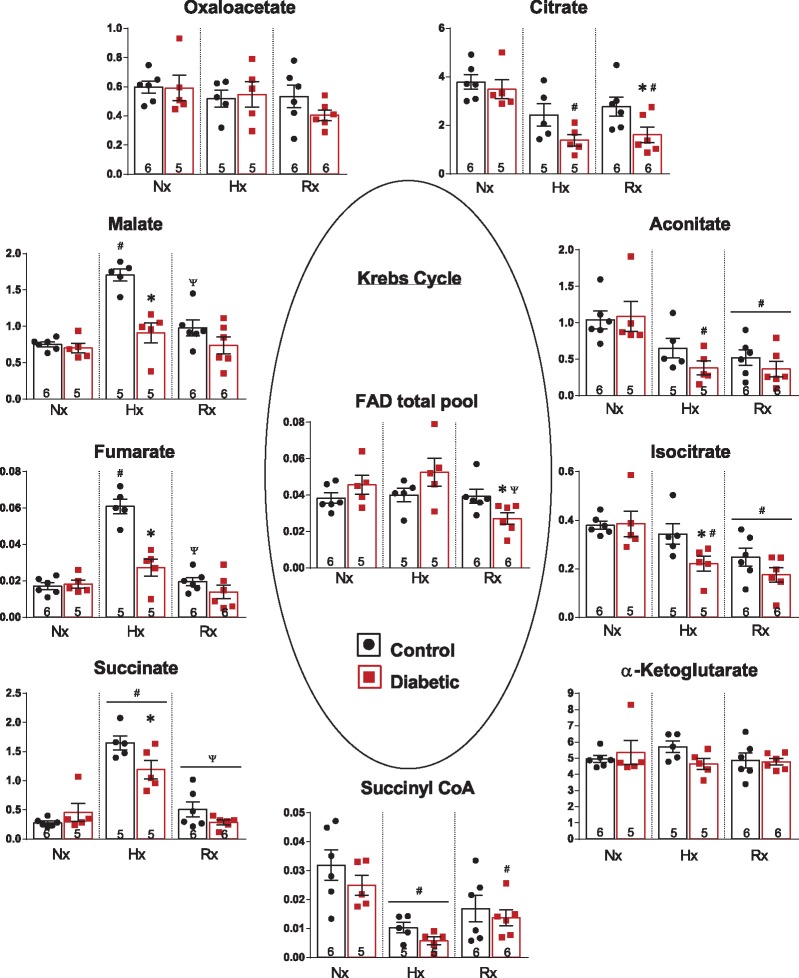
Next, we questioned whether metabolic intermediates were being changed by hypoxic stress, and whether these changes were abrogated following reoxygenation by SSO. In normoxia, there were no significant differences in any Krebs cycle intermediate between control and diabetic hearts (Figure 4). In response to hypoxia-reoxygenation, intermediates within the first span of the Krebs cycle, namely citrate, aconitate, and isocitrate, were decreased in control and diabetic hearts, with citrate significantly lower in diabetic hearts at reoxygenation, and isocitrate significantly lower in diabetic hearts after hypoxia. Sequential intermediates in the second span of the Krebs cycle, namely succinate, fumarate, and malate, were increased in control hearts, by between two- and five-fold, following hypoxia. There were significant interactions for succinate, fumarate, and malate between diabetes and oxygenation, demonstrating that in diabetic hearts these intermediates were differently modified by hypoxia-reoxygenation. In diabetic hearts, succinate, fumarate, and malate were 28–54% lower following hypoxia compared with control hearts. The total FAD pool (FAD and FADH2), essential for the oxidation of succinate to fumarate by succinate dehydrogenase, (in addition to branched chain amino acid catabolism and fatty acid oxidation), was selectively depleted by 31% in diabetic hearts following reoxygenation compared with controls. This decrease in the FAD total pool in diabetic hearts occurred independently of changes in NAD total pool, phosphocreatine or ATP, when compared with control hearts (Table 1).
Figure 4.
Myocardial levels of Krebs cycle intermediates from control and diabetic hearts under normoxia (Nx), hypoxia (Hx), and reoxygenation (Rx). *P < 0.05 vs. control under same oxygen condition, #P < 0.05 vs. normoxia within same disease state, ΨP < 0.05 vs. hypoxia within same disease state. Data expressed as peak area ratio, relative to internal standard.
Table 1.
Metabolic intermediates from control and diabetic hearts following normoxia, hypoxia and reoxygenation
| Normoxia |
Hypoxia |
Reoxygenation |
||||
|---|---|---|---|---|---|---|
| Control (n = 6) | Diabetic (n = 5) | Control (n = 5) | Diabetic (n = 5) | Control (n = 6) | Diabetic (n = 6) | |
| NAD total pool | 0.93 ± 0.05 | 0.96 ± 0.11 | 0.95 ± 0.05 | 0.89 ± 0.07 | 0.82 ± 0.12 | 0.60 ± 0.05# |
| Phosphocreatine | 36.2 ± 2.5 | 32.4 ± 4.7 | 16.3 ± 2.9# | 13.3 ± 3.5# | 38.9 ± 5.2† | 28.5 ± 3.2† |
| ATP | 8.0 ± 0.4 | 9.2 ± 0.9 | 5.4 ± 0.9 | 4.1 ± 0.7# | 4.7 ± 1.1# | 5.5 ± 0.5# |
| Adenine | 0.013 ± 0.001 | 0.013 ± 0.002 | 0.016 ± 0.000 | 0.013 ± 0.001* | 0.016 ± 0.001 | 0.011 ± 0.001* |
| Acetyl CoA | 0.0020 ± 0.0003 | 0.0050 ± 0.0007* | 0.0013 ± 0.0003 | 0.0021 ± 0.0003 | 0.0011 ± 0.0009# | 0.0006 ± 0.0002# |
| Glutathione | 0.00070 ± 0.00032 | 0.00157 ± 0.00126 | 0.00020 ± 0.00003 | 0.00018 ± 0.00005 | 0.00016 ± 0.00003 | 0.00096 ± 0.00045 |
| Oxidized glutathione | 0.202 ± 0.006 | 0.226 ± 0.015 | 0.229 ± 0.010 | 0.229 ± 0.011 | 0.184 ± 0.014# | 0.153 ± 0.011#,† |
| Leucine | 0.072 ± 0.004 | 0.097 ± 0.012 | 0.188 ± 0.011# | 0.146 ± 0.012*,# | 0.108 ± 0.013† | 0.079 ± 0.008† |
| Isoleucine | 0.060 ± 0.005 | 0.083 ± 0.011 | 0.170 ± 0.007# | 0.135 ± 0.013*,# | 0.088 ± 0.014† | 0.060 ± 0.006† |
| Phenylalanine | 0.052 ± 0.004 | 0.055 ± 0.006 | 0.080 ± 0.010 | 0.053 ± 0.005* | 0.076 ± 0.006 | 0.054 ± 0.006* |
| Lysine | 0.16 ± 0.01 | 0.32 ± 0.04* | 0.17 ± 0.02 | 0.25 ± 0.03 | 0.22 ± 0.02 | 0.22 ± 0.02 |
| Aspartate | 0.11 ± 0.01 | 0.16 ± 0.01* | 0.09 ± 0.01 | 0.14 ± 0.03 | 0.09 ± 0.01 | 0.09 ± 0.01# |
| Asparagine | 0.015 ± 0.001 | 0.027 ± 0.002* | 0.017 ± 0.001 | 0.018 ± 0.005 | 0.014 ± 0.002 | 0.015 ± 0.002 |
| Glutamate | 17.6 ± 1.3 | 19.3 ± 2.9 | 10.2 ± 0.7# | 12.2 ± 0.9# | 9.6 ± 1.4# | 7.7 ± 0.8# |
| Glutamine | 6.2 ± 0.3 | 6.6 ± 0.9 | 4.0 ± 0.3# | 4.8 ± 0.1 | 3.8 ± 0.4# | 2.6 ± 0.2#,† |
| Histidine | 0.28 ± 0.01 | 0.25 ± 0.01 | 0.25 ± 0.03 | 0.20 ± 0.02# | 0.20 ± 0.02# | 0.13 ± 0.01*,#,† |
| Alanine | 0.19 ± 0.02 | 0.22 ± 0.06 | 0.42 ± 0.03# | 0.37 ± 0.06 | 0.16 ± 0.03† | 0.11 ± 0.02† |
Data expressed as peak area ratio, relative to internal standard.
P < 0.05 vs. control at same oxygenation (highlighted in bold);
P < 0.05 vs. normoxia within same disease state;
P < 0.05 vs. hypoxia within same disease state.
SSO treatment of diabetic hearts had an anaplerotic effect on selective steps within the Krebs cycle (Figure 5). SSO treatment of diabetic hearts increased Krebs cycle intermediates in the second span of the cycle, doubling the level of fumarate and increasing oxaloacetate by 61% compared to untreated diabetic hearts. The total FAD pool, which was depleted at reoxygenation in diabetic hearts, was significantly increased by 67% by SSO treatment of diabetic hearts. Citrate level was marginally increased, so that it was no longer significantly different to controls (Figure 5).
Figure 5.
Myocardial levels of Krebs cycle intermediates at reoxygenation from control and diabetic hearts, with and without FAT/CD36 inhibitor SSO. *P < 0.05 vs. control, #P < 0.05 vs. diabetic, ΨP < 0.05 vs. control + SSO. Data expressed as peak area ratio, relative to internal standard.
A number of amino acids were modulated in diabetic hearts exposed to hypoxia/reoxygenation (Table 1 and see Supplementary material online, Table S3). Lysine, aspartate, and asparagine were increased in diabetic hearts in normoxia, leucine, isoleucine, and phenylalanine were decreased following hypoxia, and histidine and phenylalanine were decreased following reoxygenation in diabetic hearts compared with controls. Markers of the antioxidant system, reduced and oxidized glutathione, were not different between controls and diabetic hearts. None of these metabolites were modulated by SSO treatment (see Supplementary materials online, Table S4).
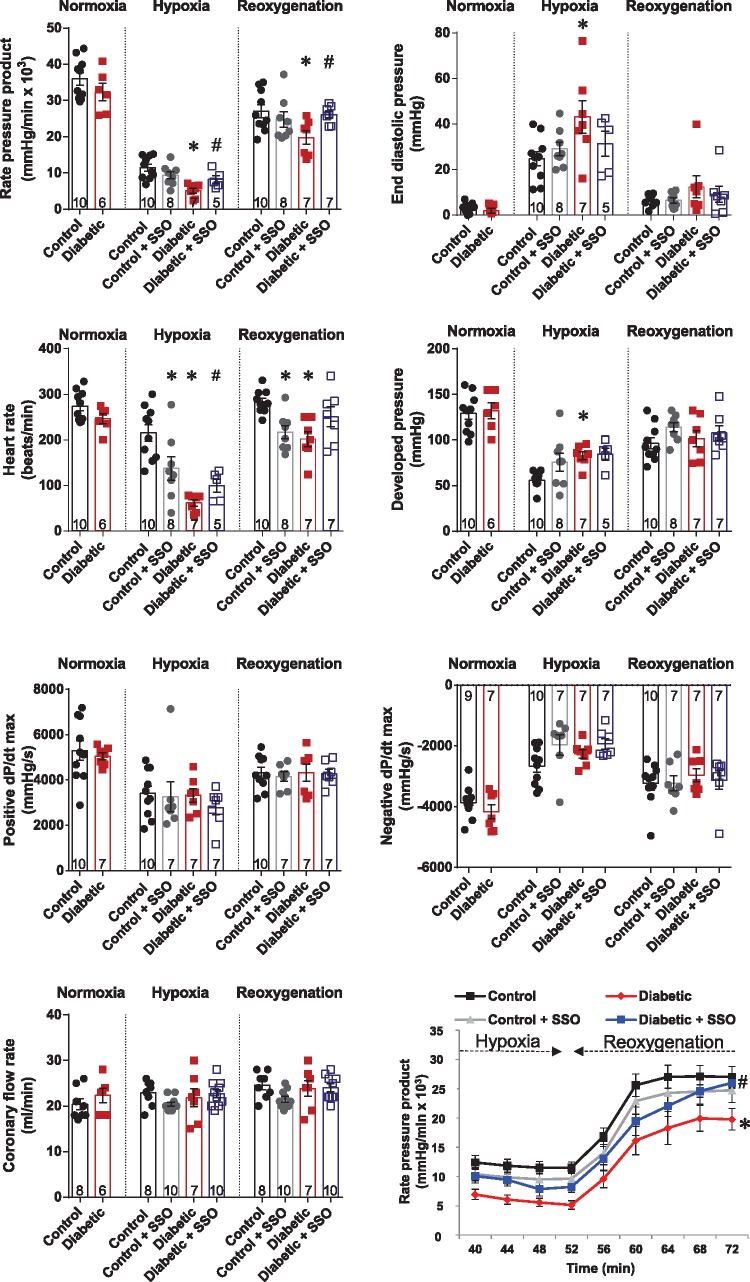
3.5 Inhibition of FAT/CD36 corrected cardiac dysfunction in diabetes
Finally, we questioned whether the beneficial metabolic effects of SSO would translate to a functional benefit during hypoxia and reoxygenation in the diabetic heart (Figure 6). At reoxygenation, RPP and developed pressure were significantly lower (P < 0.05) in both control and diabetic hearts compared with in normoxia, whereas other parameters returning to their normoxic levels. SSO had an overall beneficial effect on RPP in diabetic hearts both during hypoxia and reoxygenation. In diabetic hearts, SSO treatment increased RPP during hypoxia by 60% and during reoxygenation by 32% compared with untreated diabetic hearts. This resulted in average percentage recovery of 73% in control hearts, 67% in control hearts treated with SSO, 55% in diabetic hearts, and 69% in diabetic hearts treated with SSO. Following SSO treatment end-diastolic pressure was normalized in diabetic hearts during hypoxia, and was no longer significantly different to control hearts. SSO treatment significantly increased heart rate in diabetic hearts during hypoxia, and following reoxygenation restored heart rate in diabetic hearts to control levels. SSO had no significant effects on developed pressure, positive or negative dP/dt max, or coronary flow rates in any group.
Figure 6.
Cardiac function in control and diabetic hearts, with and without FAT/CD36 inhibitor SSO, during hypoxia and reoxygenation. *P < 0.05 vs. control, #P < 0.05 vs. diabetic. End diastolic pressure n numbers as per developed pressure.
4. Discussion
The type 2 diabetic heart had an abnormal metabolic and functional response when challenged with acute hypoxia-reoxygenation. In this study we demonstrate for the first time that moderate inhibition of FAT/CD36 can be achieved rapidly in the intact perfused heart using SSO. During hypoxic stress, SSO can promote glycolysis, decrease myocardial triglyceride deposition, and increase PDH flux in diabetic hearts. In addition, using metabolomics we were able to identify unexpected changes in metabolic intermediates, which demonstrated that SSO treatment had an anaplerotic effect to increase Krebs cycle intermediates within the second span of the cycle. These metabolic changes culminated in improved cardiac function during hypoxia and after reoxygenation, restoring function to levels found in control hearts. Thus, pharmacological inhibition of FAT/CD36 provides a mechanism to improve substrate metabolism, prevent lipid overload and restore cardiac function in diabetes.
SSO was used in the initial identification of FAT/CD36 as the plasma membrane protein responsible for fatty acid uptake,40 and has subsequently been used to define the functional significance of FAT/CD36.41–44 By administering SSO to the perfused heart we addressed two questions: whether pharmacological inhibition of FAT/CD36 was feasible and, secondly, whether rapidly suppressing fatty acid metabolism is sufficient to restore cardiac function following stress. Genetic studies have shown that FAT/CD36 deficiency can rescue cardiac lipotoxicity associated with PPARα overexpression,17 thus, translation of a genetic finding to a therapeutic angle was of great interest. As therapeutic proof of concept, incubating primary cardiomyocytes with an antibody raised to FAT/CD36 improved sarcomere shortening following exposure to a high palmitate culture media.45 In this study, we demonstrated that SSO infusion rapidly restored fatty acid oxidation rates in diabetic hearts back to control levels. In addition, SSO had the added advantage of also preventing the accumulation of intracellular triglycerides, a benefit that does not occur with compounds that target mitochondrial fatty acid oxidation, such as etomoxir, where fatty acid import and esterification may continue unhindered.16 One potential concern was that the degree of FAT/CD36 inhibition may be too great, which would result in substrate starvation and contractile dysfunction given that fatty acids are the main fuel for ATP generation. However, we have shown that SSO can be used to induce small changes in metabolism without compromising cardiac function.
In diabetic hearts, functional defects only arose when the myocardium was stressed by hypoxia, and could be reversed in minutes by changing metabolism, demonstrating that the functional deficit was not a fixed irreversible effect of the disease. In diabetic and GLUT4-deficient mice where glucose metabolism is suppressed, hypoxia induces abnormalities in electrical activity and action potential duration.46,47 This documented association between metabolism and electrical activity in the heart may contribute to the changes in heart rate in our hypoxic diabetic animals and with acute metabolic therapy. SSO treatment improved end-diastolic pressure during hypoxia in diabetic hearts, an indicator of improved relaxation and compliance following metabolic modulation.
Glucose uptake and metabolism are regulated by multiple signalling pathways, both at baseline and in response to stress.22,48 Glycolytic rates were increased to a much lesser extent in diabetic hearts than controls in response to hypoxia, which we show was not attributable to abnormal p-AMPK activation. This raised the question of whether hypoxia had already reached the maximal glycolytic response in the diabetic heart. We found this was not the case, and inhibition of FAT/CD36 provided an additional stimulus to increase glycolytic rates further in diabetic hearts. This demonstrated that increased fatty acid metabolism was responsible for suppressing anaerobic metabolism, in accordance with the Randle cycle,10 and as highlighted by the restoration of PDH activity. That SSO did not confer the same benefits to control hearts may be due to metabolic flexibility already being optimal in the healthy heart. Thus, there is inherent metabolic flexibility in the diabetic heart; but it requires simultaneous activation of multiple pathways—via the intrinsic hypoxic response, including AMPK signalling, and via extrinsic metabolic modulation. The combined effects of decreasing fatty acid metabolism and increasing glucose metabolism by SSO make it difficult to assign which of these two factors is responsible for improving functional outcomes in the diabetic heart. Given the reciprocal regulation between metabolic pathways, any process that changes one of these pathways will always modify the other, and, therefore, it is most likely that overall benefit is gained by the combination of the two.
Metabolomics is a non-hypothesis driven experimental approach, which allowed us to determine if there were any additional effects of short-term metabolic modulation, above and beyond those that would be predicted from traditional studies. Metabolomics identified that the effect of hypoxia on the Krebs cycle was not uniform, that intermediates within the first span were depleted, whereas intermediates within the second span accumulate. The enzymatic steps where these patterns change are all NAD-dependent dehydrogenase or substrate level phosphorylation steps, indicating this profile in hypoxia is likely due to mitochondrial redox-dependent regulation. The increase in succinate during hypoxia is consistent with Chouchani et al.,49 who showed an increase in succinate during ischaemia. However, in contrast to our findings, they did not identify changes in other Krebs cycle intermediates, indicating differences in the myocardial response to different stressors. Hypoxia is a less severe stressor for the heart than global ischaemia, as waste product removal and substrate delivery can still continue, which may account for these different responses. Diabetes decreased a number of intermediates within the Krebs cycle, namely citrate, isocitrate, succinate, fumarate, and malate. In agreement with our findings, Banke et al.50 demonstrated abnormal Krebs cycle flux in diabetic hearts only when stressed by ischaemia. Of interest was the increase in fumarate, oxaloacetate and the FAD pool with SSO treatment of diabetic hearts, demonstrating an additional anaplerotic benefit from metabolic therapy in diabetes.
The current findings demonstrate beneficial metabolic and functional effects of SSO for the diabetic heart, and future in vivo studies are warranted. Any changes induced by acute SSO in this study were immediate via existing proteins, whereas chronic studies may exert additional effects via transcriptional/translational changes within the heart. Potentially SSO may also reduce the deleterious effects of intracellular lipids associated with insulin resistance and cell death.51,52 However, there are a number of critical hurdles to overcome, such as systemic delivery affecting metabolism and function of other tissues, such as skeletal muscle and adipose, as well as unknown longer term effects on cardiac function.
In conclusion, type 2 diabetes impairs the metabolic and functional response to hypoxia-reoxygenation stress. Pharmacological inhibition of the sarcolemmal fatty acid transporter, FAT/CD36, provides a rapid mechanism to suppress fatty acid oxidation and promote glycolysis. Targeting the primary transport step in the pathway also prevented excessive lipid deposition and rebalanced Krebs cycle intermediates resulting in improved cardiac function in diabetic hearts following hypoxic stress.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Supplementary Material
Acknowledgements
We acknowledge Peter Ratcliffe for intellectual input on the study, and Michael Dodd and Dunja Aksentijevic for technical assistance. We thank Evangelia Shaw, whose arrival prompted this study.
Conflict of interest: none declared.
Funding
This work was supported by grants from Diabetes UK (grant number 11/0004175) and the British Heart Foundation (FS/14/65/31292 and FS/15/68/32042). This publication arises from research funded by the John Fell Oxford University Press (OUP) Research Fund. J.L.G. and J.W. acknowledges the financial support of the Medical Research Council UK (Lipid profiling and signalling MC_UP_A90_1006).
References
- 1. Rijzewijk LJ, van der Meer RW, Lamb HJ, de Jong HW, Lubberink M, Romijn JA, Bax JJ, de Roos A, Twisk JW, Heine RJ, Lammertsma AA, Smit JW, Diamant M.. Altered myocardial substrate metabolism and decreased diastolic function in nonischemic human diabetic cardiomyopathy: studies with cardiac positron emission tomography and magnetic resonance imaging. J Am Coll Cardiol 2009;54:1524–1532. [DOI] [PubMed] [Google Scholar]
- 2. Jagasia D, Whiting JM, Concato J, Pfau S, McNulty PH.. Effect of non-insulin-dependent diabetes mellitus on myocardial insulin responsiveness in patients with ischemic heart disease. Circulation 2001;103:1734–1739. [DOI] [PubMed] [Google Scholar]
- 3. Peterson LR, Herrero P, Schechtman KB, Racette SB, Waggoner AD, Kisrieva-Ware Z, Dence C, Klein S, Marsala J, Meyer T, Gropler RJ.. Effect of obesity and insulin resistance on myocardial substrate metabolism and efficiency in young women. Circulation 2004;109:2191–2196. [DOI] [PubMed] [Google Scholar]
- 4. Hafstad AD, Solevag GH, Severson DL, Larsen TS, Aasum E.. Perfused hearts from Type 2 diabetic (db/db) mice show metabolic responsiveness to insulin. Am J Physiol Heart Circ Physiol 2006;290:H1763–H1769. [DOI] [PubMed] [Google Scholar]
- 5. Mazumder PK, O’Neill BT, Roberts MW, Buchanan J, Yun UJ, Cooksey RC, Boudina S, Abel ED.. Impaired cardiac efficiency and increased fatty acid oxidation in insulin-resistant ob/ob mouse hearts. Diabetes 2004;53:2366–2374. [DOI] [PubMed] [Google Scholar]
- 6. Boardman N, Hafstad AD, Larsen TS, Severson DL, Aasum E.. Increased O2 cost of basal metabolism and excitation-contraction coupling in hearts from type 2 diabetic mice. Am J Physiol Heart Circ Physiol 2009;296:H1373–H1379. [DOI] [PubMed] [Google Scholar]
- 7. Mansor LS, Mehta K, Aksentijevic D, Carr CA, Lund T, Cole MA, Page LL, Sousa Fialho Mda L, Shattock MJ, Aasum E, Clarke K, Tyler DJ, Heather LC.. Increased oxidative metabolism following hypoxia in the type 2 diabetic heart, despite normal hypoxia signalling and metabolic adaptation. J Physiol 2016;594:307–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Luiken JJ, Arumugam Y, Bell RC, Calles-Escandon J, Tandon NN, Glatz JF, Bonen A.. Changes in fatty acid transport and transporters are related to the severity of insulin deficiency. Am J Physiol Endocrinol Metab 2002;283:E612–E621. [DOI] [PubMed] [Google Scholar]
- 9. Carley AN, Atkinson LL, Bonen A, Harper ME, Kunnathu S, Lopaschuk GD, Severson DL.. Mechanisms responsible for enhanced fatty acid utilization by perfused hearts from type 2 diabetic db/db mice. Arch Physiol Biochem 2007;113:65–75. [DOI] [PubMed] [Google Scholar]
- 10. Randle PJ, Garland PB, Hales CN, Newsholme EA.. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet 1963;1:785–789. [DOI] [PubMed] [Google Scholar]
- 11. Schmidt-Schweda S, Holubarsch C.. First clinical trial with etomoxir in patients with chronic congestive heart failure. Clin Sci 2000;99:27–35. [PubMed] [Google Scholar]
- 12. Lee L, Campbell R, Scheuermann-Freestone M, Taylor R, Gunaruwan P, Williams L, Ashrafian H, Horowitz J, Fraser AG, Clarke K, Frenneaux M.. Metabolic modulation with perhexiline in chronic heart failure: a randomized, controlled trial of short-term use of a novel treatment. Circulation 2005;112:3280–3288. [DOI] [PubMed] [Google Scholar]
- 13. Abozguia K, Clarke K, Lee L, Frenneaux M.. Modification of myocardial substrate use as a therapy for heart failure. Nat Clin Pract Cardiovasc Med 2006;3:490–498. [DOI] [PubMed] [Google Scholar]
- 14. Kosiborod M, Arnold SV, Spertus JA, McGuire DK, Li Y, Yue P, Ben-Yehuda O, Katz A, Jones PG, Olmsted A, Belardinelli L, Chaitman BR.. Evaluation of ranolazine in patients with type 2 diabetes mellitus and chronic stable angina: results from the TERISA randomized clinical trial (Type 2 Diabetes Evaluation of Ranolazine in Subjects With Chronic Stable Angina). J Am Coll Cardiol 2013;61:2038–2045. [DOI] [PubMed] [Google Scholar]
- 15. Fragasso G, Piatti Md PM, Monti L, Palloshi A, Setola E, Puccetti P, Calori G, Lopaschuk GD, Margonato A.. Short- and long-term beneficial effects of trimetazidine in patients with diabetes and ischemic cardiomyopathy. Am Heart J 2003;146:E18. [DOI] [PubMed] [Google Scholar]
- 16. Schmitz FJ, Rosen P, Reinauer H.. Improvement of myocardial function and metabolism in diabetic rats by the carnitine palmitoyl transferase inhibitor Etomoxir. Horm Metab Res 1995;27:515–522. [DOI] [PubMed] [Google Scholar]
- 17. Yang J, Sambandam N, Han X, Gross RW, Courtois M, Kovacs A, Febbraio M, Finck BN, Kelly DP.. CD36 deficiency rescues lipotoxic cardiomyopathy. Circ Res 2007;100:1208–1217. [DOI] [PubMed] [Google Scholar]
- 18. Oakes ND, Thalen P, Aasum E, Edgley A, Larsen T, Furler SM, Ljung B, Severson D.. Cardiac metabolism in mice: tracer method developments and in vivo application revealing profound metabolic inflexibility in diabetes. Am J Physiol Endocrinol Metab 2006;290:E870–E881. [DOI] [PubMed] [Google Scholar]
- 19. Mather KJ, Hutchins GD, Perry K, Territo W, Chisholm R, Acton A, Glick-Wilson B, Considine RV, Moberly S, DeGrado TR.. Assessment of myocardial metabolic flexibility and work efficiency in human type 2 diabetes using 16-[18F]fluoro-4-thiapalmitate, a novel PET fatty acid tracer. Am J Physiol Endocrinol Metab 2016;310:E452–E460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Essop MF, Anna Chan WY, Valle A, Garcia-Palmer FJ, Du Toit EF.. Impaired contractile function and mitochondrial respiratory capacity in response to oxygen deprivation in a rat model of pre-diabetes. Acta physiologica 2009;197:289–296. [DOI] [PubMed] [Google Scholar]
- 21. Mokuda O, Sakamoto Y, Ikeda T, Mashiba H.. Effects of anoxia and low free fatty acid on myocardial energy metabolism in streptozotocin-diabetic rats. Ann Nutr Metab 1990;34:259–265. [DOI] [PubMed] [Google Scholar]
- 22. Beauloye C, Bertrand L, Krause U, Marsin AS, Dresselaers T, Vanstapel F, Vanoverschelde JL, Hue L.. No-flow ischemia inhibits insulin signaling in heart by decreasing intracellular pH. Circ Res 2001;88:513–519. [DOI] [PubMed] [Google Scholar]
- 23. West SD, Nicoll DJ, Stradling JR.. Prevalence of obstructive sleep apnoea in men with type 2 diabetes. Thorax 2006;61:945–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Klein OL, Krishnan JA, Glick S, Smith LJ.. Systematic review of the association between lung function and Type 2 diabetes mellitus. Diabet Med 2010;27:977–987. [DOI] [PubMed] [Google Scholar]
- 25. Shah AD, Langenberg C, Rapsomaniki E, Denaxas S, Pujades-Rodriguez M, Gale CP, Deanfield J, Smeeth L, Timmis A, Hemingway H.. Type 2 diabetes and incidence of cardiovascular diseases: a cohort study in 1.9 million people. Lancet Diabetes Endocrinol 2015;3:105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Le Page LM, Rider OJ, Lewis AJ, Ball V, Clarke K, Johansson E, Carr CA, Heather LC, Tyler DJ.. Increasing pyruvate dehydrogenase flux as a treatment for diabetic cardiomyopathy: a combined 13C hyperpolarized magnetic resonance and echocardiography study. Diabetes 2015;64:2735–2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mansor LS, Gonzalez ER, Cole MA, Tyler DJ, Beeson JH, Clarke K, Carr CA, Heather LC.. Cardiac metabolism in a new rat model of type 2 diabetes using high-fat diet with low dose streptozotocin. Cardiovasc Diabetol 2013;12:136–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Watts LM, Manchem VP, Leedom TA, Rivard AL, McKay RA, Bao D, Neroladakis T, Monia BP, Bodenmiller DM, Cao JX, Zhang HY, Cox AL, Jacobs SJ, Michael MD, Sloop KW, Bhanot S.. Reduction of hepatic and adipose tissue glucocorticoid receptor expression with antisense oligonucleotides improves hyperglycemia and hyperlipidemia in diabetic rodents without causing systemic glucocorticoid antagonism. Diabetes 2005;54:1846–1853. [DOI] [PubMed] [Google Scholar]
- 29. Bugger H, Abel ED.. Rodent models of diabetic cardiomyopathy. Dis Model Mech 2009;2:454–66. [DOI] [PubMed] [Google Scholar]
- 30. Staros JV. N-hydroxysulfosuccinimide active esters: bis(N-hydroxysulfosuccinimide) esters of two dicarboxylic acids are hydrophilic, membrane-impermeant, protein cross-linkers. Biochemistry 1982;21:3950–3955. [DOI] [PubMed] [Google Scholar]
- 31. Lopaschuk GD, Barr RL.. Measurements of fatty acid and carbohydrate metabolism in the isolated working rat heart. Mol Cell Biochem 1997;172:137–147. [PubMed] [Google Scholar]
- 32. Folch J, Lees M, Sloane Stanley GH.. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 1957;226:497–509. [PubMed] [Google Scholar]
- 33. Seymour AM, Chatham JC.. The effects of hypertrophy and diabetes on cardiac pyruvate dehydrogenase activity. J Mol Cell Cardiol 1997;29:2771–2778. [DOI] [PubMed] [Google Scholar]
- 34. Le Belle JE, Harris NG, Williams SR, Bhakoo KK.. A comparison of cell and tissue extraction techniques using high-resolution 1H-NMR spectroscopy. NMR Biomed 2002;15:37–44. [DOI] [PubMed] [Google Scholar]
- 35. Heather LC, Cole MA, Lygate CA, Evans RD, Stuckey DJ, Murray AJ, Neubauer S, Clarke K.. Fatty acid transporter levels and palmitate oxidation rate correlate with ejection fraction in the infarcted rat heart. Cardiovasc Res 2006;72:430–437. [DOI] [PubMed] [Google Scholar]
- 36. Luiken JJ, Koonen DP, Willems J, Zorzano A, Becker C, Fischer Y, Tandon NN, Van Der Vusse GJ, Bonen A, Glatz JF.. Insulin stimulates long-chain fatty acid utilization by rat cardiac myocytes through cellular redistribution of FAT/CD36. Diabetes 2002;51:3113–3119. [DOI] [PubMed] [Google Scholar]
- 37. Heather LC, Cole MA, Atherton HJ, Coumans WA, Evans RD, Tyler DJ, Glatz JF, Luiken JJ, Clarke K.. Adenosine monophosphate-activated protein kinase activation, substrate transporter translocation, and metabolism in the contracting hyperthyroid rat heart. Endocrinology 2010;151:422–431. [DOI] [PubMed] [Google Scholar]
- 38. Finck BN, Lehman JJ, Leone TC, Welch MJ, Bennett MJ, Kovacs A, Han X, Gross RW, Kozak R, Lopaschuk GD, Kelly DP.. The cardiac phenotype induced by PPARalpha overexpression mimics that caused by diabetes mellitus. J Clin Invest 2002;109:121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Finck BN, Han X, Courtois M, Aimond F, Nerbonne JM, Kovacs A, Gross RW, Kelly DP.. A critical role for PPARalpha-mediated lipotoxicity in the pathogenesis of diabetic cardiomyopathy: modulation by dietary fat content. Proc Natl Acad Sci U S A 2003;100:1226–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Harmon CM, Luce P, Beth AH, Abumrad NA.. Labeling of adipocyte membranes by sulfo-N-succinimidyl derivatives of long-chain fatty acids: inhibition of fatty acid transport. J Membr Biol 1991;121:261–268. [DOI] [PubMed] [Google Scholar]
- 41. Harmon CM, Abumrad NA.. Binding of sulfosuccinimidyl fatty acids to adipocyte membrane proteins: isolation and amino-terminal sequence of an 88-kD protein implicated in transport of long-chain fatty acids. J Membr Biol 1993;133:43–49. [DOI] [PubMed] [Google Scholar]
- 42. Abumrad NA, el-Maghrabi MR, Amri EZ, Lopez E, Grimaldi PA.. Cloning of a rat adipocyte membrane protein implicated in binding or transport of long-chain fatty acids that is induced during preadipocyte differentiation. Homology with human CD36. J Biol Chem 1993;268:17665–17668. [PubMed] [Google Scholar]
- 43. Luiken JJ, van Nieuwenhoven FA, America G, van der Vusse GJ, Glatz JF.. Uptake and metabolism of palmitate by isolated cardiac myocytes from adult rats: involvement of sarcolemmal proteins. J Lipid Res 1997;38:745–758. [PubMed] [Google Scholar]
- 44. Coort SL, Willems J, Coumans WA, van der Vusse GJ, Bonen A, Glatz JF, Luiken JJ.. Sulfo-N-succinimidyl esters of long chain fatty acids specifically inhibit fatty acid translocase (FAT/CD36)-mediated cellular fatty acid uptake. Mol Cell Biochem 2002;239:213–219. [PubMed] [Google Scholar]
- 45. Angin Y, Steinbusch LK, Simons PJ, Greulich S, Hoebers NT, Douma K, van Zandvoort MA, Coumans WA, Wijnen W, Diamant M, Ouwens DM, Glatz JF, Luiken JJ.. CD36 inhibition prevents lipid accumulation and contractile dysfunction in rat cardiomyocytes. Biochem J 2012;448:43–53. [DOI] [PubMed] [Google Scholar]
- 46. Sohn K, Wende AR, Abel ED, Moreno AP, Sachse FB, Punske BB.. Absence of glucose transporter 4 diminishes electrical activity of mouse hearts during hypoxia. Exp Physiol 2013;98:746–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Aomine M, Nobe S, Arita M.. Increased susceptibility to hypoxia of prolonged action potential duration in ventricular papillary muscles from diabetic rats. Diabetes 1990;39:1485–1489. [DOI] [PubMed] [Google Scholar]
- 48. Montessuit C, Lerch R.. Regulation and dysregulation of glucose transport in cardiomyocytes. Biochim Biophys Acta 2013;1833:848–856. [DOI] [PubMed] [Google Scholar]
- 49. Chouchani ET, Pell VR, Gaude E, Aksentijevic D, Sundier SY, Robb EL, Logan A, Nadtochiy SM, Ord EN, Smith AC, Eyassu F, Shirley R, Hu CH, Dare AJ, James AM, Rogatti S, Hartley RC, Eaton S, Costa AS, Brookes PS, Davidson SM, Duchen MR, Saeb-Parsy K, Shattock MJ, Robinson AJ, Work LM, Frezza C, Krieg T, Murphy MP.. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature 2014;515:431–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Banke NH, Lewandowski ED.. Impaired cytosolic NADH shuttling and elevated UCP3 contribute to inefficient citric acid cycle flux support of postischemic cardiac work in diabetic hearts. J Mol Cell Cardiol 2015;79:13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yu C, Chen Y, Cline GW, Zhang D, Zong H, Wang Y, Bergeron R, Kim JK, Cushman SW, Cooney GJ, Atcheson B, White MF, Kraegen EW, Shulman GI.. Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. J Biol Chem 2002;277:50230–50236. [DOI] [PubMed] [Google Scholar]
- 52. Sparagna GC, Hickson-Bick DL, Buja LM, McMillin JB.. A metabolic role for mitochondria in palmitate-induced cardiac myocyte apoptosis. Am J Physiol Heart Circ Physiol 2000;279:H2124–H2132. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.