Abstract
Fuchs syndrome is a particular type of erythema multiforme major; the lesions are only found on the mucosae and specifically affect oral, ocular, and genital mucosae. The cause is not always immediately apparent, which is why this pathology requires a rigorous, detailed clinical examination to eliminate a differential diagnosis. The severity of the symptoms, particularly of oral and ocular symptoms, requires immediate treatment. The treatment of this pathology requires a multiple-drug regime. Through a clinical case study, the objective of this work is to help guide practitioners when diagnosing and treating this pathology as no current consensus exists on these 2 subjects. The authors present the case of a 29-year-old patient who was suffering from a recurring outbreak of Fuchs syndrome, suspected of having been triggered by Mycoplasma pneumoniae. After completing the treatment program based on colchicine and prednisolone, the patient was relieved from pain and has not suffered from any further periodic eruptions of erythema multiforme.
Keywords: Erythema multiforme, Mycoplasma pneumoniae, Oral mucosa, Glucocorticoid, Colchicine
Introduction
Erythema multiforme (EM) is a disease whose acute, bullous symptoms of autoimmune origin are generally linked to an infection with herpes simplex virus [1, 2]. Mycoplasma pneumoniae has also been suspected to trigger the disease [1, 3]. However, the role of some drugs, particularly non-steroidal anti-inflammatory drugs (NSAIDs) and penicillin, still remains a controversial issue [1]. In general, it is the male population that is affected by EM, in particular young male adults between 20 and 30 years of age. The outbreak of symptoms can be sudden and crippling. Different levels of damage to the skin and mucosa have been noted. The most commonly used method of classification differentiates between EM minor and EM major [1, 4]: (1) EM minor is characterised by a benign skin irritation and sometimes associated with lesions on one mucosa. The lesions are often on the oral mucosa. Other mucosae (genital, ocular, etc.) can be affected [5, 6]. (2) EM major corresponds to the more severe mucocutaneous forms (i.e., outbreaks on different mucosae).
A particular strain of EM major which affects the mucosa without causing damage to the skin has been described in the literature. In Germany, it is called Fuchs syndrome [7, 8]. This syndrome, which primarily affects the oral mucosa, is characterised by erythematous, erosive and ulcerative lesions which are sometimes accompanied by a yellowish coating. The prognosis is generally good. However, cases of EM major require rapid and appropriate diagnosis, followed by immediate treatment, due to the sudden onset of their painful and crippling symptoms. The difficulty in treatment is due to the fact that there is currently no consensus of opinion. Randomised controlled trials [1], which have tried to evaluate how efficient different treatments are, are rare. Tatnall et al. [9] illustrated the efficiency of long-term per-os acyclovir, and Staikuniene and Staneviciute [2] illustrated the efficiency of valacyclovir in preventing recurring post-herpetic EM outbreaks. But at the present time, no treatment has proven efficient when treating an outbreak of EM. The treatment patients receive is generally based on a multiple-drug regime [1, 7] depending on the practitioner's experience and the results of non-experimental studies [10]. In the most serious form of EM, the patient may need to be hospitalised so that the symptoms can be treated and to avoid any further complications [1]. Oral lesions can indeed lead to cases of malnutrition or fluid and electrolyte imbalances, whereas ocular lesions can cause, amongst others, symblepharon, keratoconjunctivitis sicca, ectropion and even corneal opacity.
Case Report
A 29-year-old man was transferred to the Department of Odontology by his general practitioner for an acute outbreak on his oral mucosa which prevented him from eating. The lesions had appeared 8 days earlier, and before that, the patient had suffered from a slight temperature, headaches and a sore throat. NSAIDs and cetylpyridinium lysozyme had been prescribed as a treatment. The patient also reported having suffered from successive episodes of labial herpes before the outbreak of symptoms.
General symptoms, such as asthenia and a fever, were observed. The initial cardiopulmonary examination was normal. There was no swelling of the lymph nodes, liver or spleen. During the oral examination, multiple but irregular oval-shaped erosions on an erythematous surface were noted on the palate (Fig. 1), the inside of the cheeks (Fig. 2) and the palatoglossal arches. His tongue was swollen, and an impression of his teeth around the edge could be observed (Fig. 3). A fibrous coating covered the mucosa on both sides of the tongue and across the floor of his mouth (Fig. 4). We also noted an ulceration of the urethral meatus and a bilateral conjunctivitis with no obstruction to his vision (Fig. 5). The rest of the tissue was normal. Serology for M. pneumoniae proved positive. However, herpes simplex virus by polymerase chain reaction was negative. C-reactive protein reached 54 mg/L.
Fig. 1.
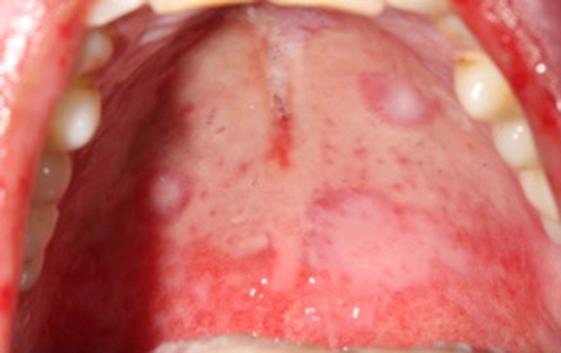
Target lesions on the palate.
Fig. 2.
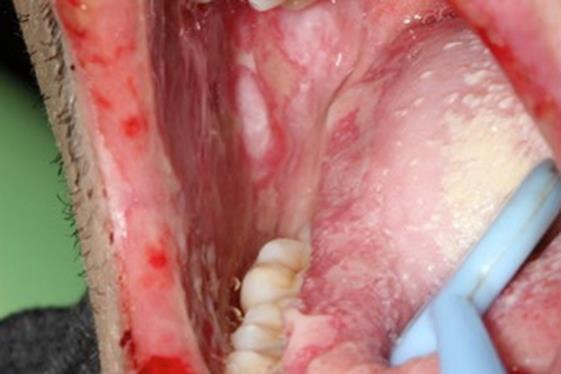
Target lesions on the cheek.
Fig. 3.
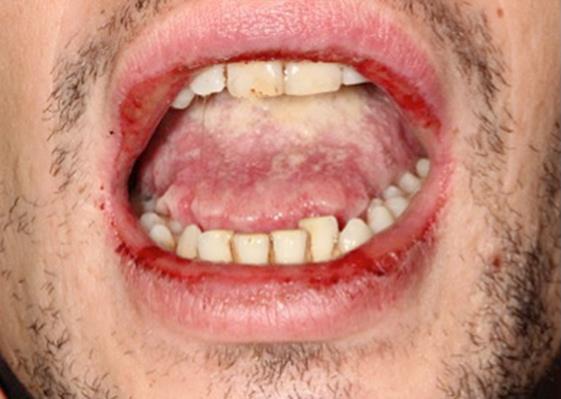
Erosions of the lips and impression of the teeth on the edges of the tongue.
Fig. 4.
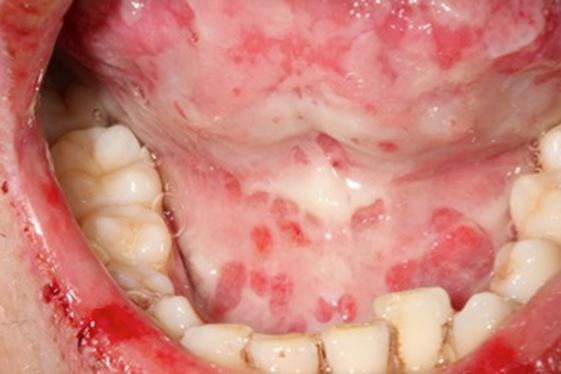
Adherent fibrin coating on the mouth floor and the ventral surface of the tongue.
Fig. 5.
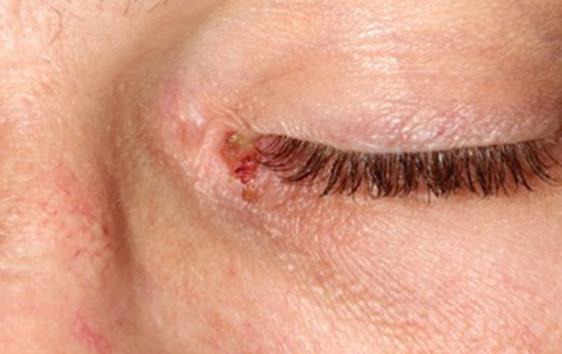
Mucosal lesion of the medial canthus.
A 7-day treatment regime was initiated made up of orodispersible prednisolone 30 mg/day and valaciclovir 500 mg morning and night. To facilitate eating and to relieve the patient from pain, lidocaine gel and tramadol were also prescribed.
On the 7-day check-up, the urethral and eye lesions had completely healed up. However, some oral erosions persisted. The decision was made to continue the prednisolone 30 mg/day for 7 days, with a recommendation to continue with the lidocaine gel and paracetamol.
A new blood test for M. pneumoniae was carried out after 15 days to eliminate a potential false positive in the absence of associated pulmonary symptoms. The IgG and IgM anti-M. pneumoniae levels corresponded to those of an acute infection.
Seven months later, the patient came back for another consultation for a similar outbreak: first, there were oral lesions, followed by conjunctivitis and fever, but this time there were no genital related symptoms. The patient was once again treated with prednisolone and valaciclovir. We noted an improvement within a few days. The prednisolone was stopped, and the valaciclovir was reduced to 500 mg/day. One week later, there was a new outbreak of EM. The valaciclovir was stopped and replaced by 1 mg/day of colchicine for 3 months. The patient has not suffered from any other outbreaks since the start of the treatment after a 7-month observation period.
Discussion
No cutaneous lesions could be found on this patient, even though the outbreaks affected different mucosae at the same time (oral, conjunctival and genital). The characteristic aspect of all the ulcerative and erosive lesions on the erythematous surface, covered by yellow coating, pointed to a diagnosis of Fuchs syndrome. In the case we are illustrating here, the ocular lesions were not as severe as in some cases in which the conjunctivitis led to blindness [1]. Lesions on the genital mucosa, as observed in this patient, are rarely documented in the literature [7, 11]. Even if the symptomatology was mainly mucosa based and the clinical aspect evoked EM lesions with no evident cause (serology for herpes simplex negative and M. pneumoniae moderately positive), the diagnosis proved difficult. Indeed, the criteria for diagnosis and classification often differ from one author to another, which makes it difficult to positively identify the pathology and consequently begin treatment [1]. The normal type of mucosal damage with EM was not really present; therefore, it was necessary to consider other types of diagnosis. Clinical elements, for example, the absence of lesions in the pharyngo-laryngeal area or on the patient's skin, allowed us to eliminate both Stevens-Johnson and Lyell syndromes [1, 7]. And in the absence of a positive viral result in serology, the numerous oval and fibrous intra-oral lesions on the cheek mucosa did not indicate viral stomatitis of the herpetic type. Despite the ulcerated aspect of the lesions, we ruled out aphthosis because of the size and quantity of the lesions. In cases of pemphigus vulgaris and cicatricial pemphigoid, the mucosal damage often precedes the cutaneous damage; but here, the oval aspect of the lesions and a negative Nikolsky result eliminated both of these pathologies. The final possibilities were neutropenic syndromes which accompany leukaemia and global medullar insufficiency or pure, toxic or drug-induced agranulocytosis, all of which provoke erosions to the mucosae; but as the patient's blood count was normal, these hypotheses were all eliminated. Finally, we retained the diagnosis of “EM major mucosa” or Fuchs syndrome [7, 8, 11, 12].
An infection with herpes simplex virus is the most common cause of EM. This infection was not formally identified upon polymerase chain reaction screening of the patient's samples [10, 13, 14]. However, we did not dismiss the diagnosis of EM. M. pneumoniae has been reported as a probable cause in cases of EM major without cutaneous damage [1, 3, 7, 8, 11, 12], and further tests proved its presence.
Concerning the case presented here, it is worth discussing steroids as a means of treatment. Prednisolone was prescribed after a full patient and serology check-up to verify that all the herpes symptoms had disappeared [1, 7]. This usage still remains controversial due to the infectious complications and delayed healing [1, 15]. In this particular case, the use of steroids seemed to relieve the pain in the oral cavity, but it is difficult to determine whether it had any effect on how long the outbreak lasted.
With no consensus on EM treatment, colchicine was prescribed for 3 months to replace the preventive treatment with valaciclovir, under which the patient had previously suffered from a recurring outbreak. Of course, we cannot draw any conclusions, but it is worth noting that the patient did not suffer from any new outbreaks with colchicine. Although the serology results were positive, the usual curative treatment for infections with M. pneumoniae (macrolides, tetracycline or quinolones [7, 8, 9]) was not initiated due to the absence of pulmonary symptoms.
This case illustrates the characteristic damage to the mucosae in a case of EM, the risk of recurring lesions and the difficulty of treatment both regarding the prevention and treatment of the outbreaks. It also outlines the difficulties of diagnosis when faced with abnormal EM symptoms which only affect the mucosae.
Statement of Ethics
The authors have no ethical conflicts to disclose.
Disclosure Statement
The authors have no conflicts of interest associated with the present work.
Acknowledgements
The authors wish to thank Ms. Victoria Giron for reading the manuscript.
References
- 1.Al-Johani KA, Fedele S, Porter SR. Erythema multiforme and related disorders. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103:642–654. doi: 10.1016/j.tripleo.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 2.Staikuniene J, Staneviciute J. Long-term valacyclovir treatment and immune modulation for herpes-associated erythema multiforme. Cent Eur J Immunol. 2015;40:387–390. doi: 10.5114/ceji.2015.54604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Canavan TN, Mathes EF, Frieden I, Shinkai K. Mycoplasma pneumoniae-induced rash and mucositis as a syndrome distinct from Stevens-Johnson syndrome and erythema multiforme: a systematic review. J Am Acad Dermatol. 2015;72:239–245. doi: 10.1016/j.jaad.2014.06.026. [DOI] [PubMed] [Google Scholar]
- 4.Huff JC, Weston WL, Tonnesen MG. Erythema multiforme: a critical review of characteristics, diagnostic criteria, and causes. J Am Acad Dermatol. 1983;8:763–775. doi: 10.1016/s0190-9622(83)80003-6. [DOI] [PubMed] [Google Scholar]
- 5.Ayangco L, Rogers RS. Oral manifestations of erythema multiforme. Dermatol Clin. 2003;21:195–205. doi: 10.1016/s0733-8635(02)00062-1. [DOI] [PubMed] [Google Scholar]
- 6.Lozada-Nur F, Gorsky M, Silverman S. Oral erythema multiforme: clinical observations and treatment of 95 patients. Oral Surg Oral Med Oral Pathol. 1989;67:36–40. doi: 10.1016/0030-4220(89)90299-5. [DOI] [PubMed] [Google Scholar]
- 7.Havliza K, Jakob A, Rompel R. Erythema multiforme majus (Fuchs syndrome) associated with Mycoplasma pneumoniae infection in two patients. J Dtsch Dermatol Ges. 2009;7:445–448. doi: 10.1111/j.1610-0387.2008.06978.x. [DOI] [PubMed] [Google Scholar]
- 8.Li K, Haber RM. Stevens-Johnson syndrome without skin lesions (Fuchs syndrome): a literature review of adult cases with Mycoplasma cause. Arch Dermatol. 2012;148:963–964. doi: 10.1001/archdermatol.2012.681. [DOI] [PubMed] [Google Scholar]
- 9.Tatnall FM, Schofield JK, Leigh IM. A double-blind, placebo-controlled trial of continuous acyclovir therapy in recurrent erythema multiforme. Br J Dermatol. 1995;132:267–270. doi: 10.1111/j.1365-2133.1995.tb05024.x. [DOI] [PubMed] [Google Scholar]
- 10.Sun Y, Chan RKW, Tan SH, Ng PPL. Detection and genotyping of human herpes simplex viruses in cutaneous lesions of erythema multiforme by nested PCR. J Med Virol. 2003;71:423–428. doi: 10.1002/jmv.10502. [DOI] [PubMed] [Google Scholar]
- 11.Meyer Sauteur PM, Gansser-Kälin U, Lautenschlager S, Goetschel P. Fuchs syndrome associated with Mycoplasma pneumoniae (Stevens-Johnson syndrome without skin lesions) Pediatr Dermatol. 2011;28:474–476. doi: 10.1111/j.1525-1470.2010.01200.x. [DOI] [PubMed] [Google Scholar]
- 12.Kennett S. Erythema multiforme affecting the oral cavity. Oral Surg Oral Med Oral Pathol. 1968;25:366–373. doi: 10.1016/0030-4220(68)90010-8. [DOI] [PubMed] [Google Scholar]
- 13.Aslanzadeh J, Helm KF, Espy MJ, Muller SA, Smith TF. Detection of HSV-specific DNA in biopsy tissue of patients with erythema multiforme by polymerase chain reaction. Br J Dermatol. 1992;126:19–23. doi: 10.1111/j.1365-2133.1992.tb08397.x. [DOI] [PubMed] [Google Scholar]
- 14.Scully C, Bagan J. Oral mucosal diseases: erythema multiforme. Br J Oral Maxillofac Surg. 2008;46:90–95. doi: 10.1016/j.bjoms.2007.07.202. [DOI] [PubMed] [Google Scholar]
- 15.Ting HC, Adam BA. Erythema multiforme-response to corticosteroid. Dermatologica. 1984;169:175–178. doi: 10.1159/000249598. [DOI] [PubMed] [Google Scholar]


