Abstract
AIM
To investigate the impact of adipose-derived mesenchymal stem cells (ADSCs) on cell viability and extracellular matrix (ECM) synthesis of corneal stromal cells (CSCs).
METHODS
ADSCs and CSCs were obtained from the corneas of New Zealand white rabbits and indirectly co-cultured in vitro. The proliferative capacity of CSCs in the different groups was assessed by CCK-8 assays. Annexin V-fluorescein isothiocyanate (FITC)/proliferation indices (PI) assays were used to detect the apoptosis of CSCs. The expression levels of matrix metalloproteinase (MMP), such as MMP1, MMP2, MMP9, and collagens were also evaluated by Western blot.
RESULTS
ADSCs significantly promoted proliferation and invasion of CSCs in the indirect co-culture assays. The co-cultural group displayed much higher ability of proliferation, especially under the co-culture conditions of ADSCs for 3d, compared with that CSCs cultured alone. The PI of CSCs in the co-culture system were increased approximately 3-8-fold compared with the control group. A significant change was observed in the proportions of cells at apoptosis (early and late) between the negative control group (6.34% and 2.06%) and the ADCSs-treated group (4.69% and 1.59%). The expression levels of MMPs were down regulated in the co-culture models. Compared with the control group, the decrease intensities of MMP-1, MMP-2 and MMP-9 in CSCs/ADSCs group were observed, 3.90-fold, 1.09-fold and 3.03-fold, respectively. However, the increase intensities of collagen type (I, II, III, IV, and V) in CSCs were observed in CSCs/ADSCs group, 3.47-fold, 4.30-fold, 2.35-fold, 2.55-fold and 2.43-fold, respectively, compared to that in the control group. The expressions of aldehyde dehydrogenase and fibronectin in CSCs were upregulated in the co-culture models.
CONCLUSION
ADSCs play a promotive role in CSCs' growth and invasion, which may be partially associated with MMPs decrease and collagens increase, resulting in a positive participation in the plasticity and ECM synthesis of CSCs. This provided a new insight into the extensive role of ADSCs in CSCs and a potential molecular target for corneal therapy.
Keywords: adipose-derived mesenchymal stem cell, corneal stromal cells, extracellular matrix, plasticity
INTRODUCTION
Adipose-derived mesenchymal stem cells (ADSCs) have been easily obtained from multiple cell sources in large amounts[1]–[2]. The ADSCs have the characteristics of self-renew and multi-differentiation[3]–[6]. Upon the culture media and cell generation, ADSCs have a division time of 2-4d[1]. They can interact with other cells or extracellular matrix (ECM), and those characteristics can be regulated by several factors[3]
Recently, ADSCs have many applications in tissue engineering[7]–[9]. Several studies have dedicated that stem cells could play such roles through paracrine pathways by secreting growth factors and cytokines[10]–[11]. It has been postulated that by stimulating the stem cell niche, ADSCs are able to produce stem cells and these cells have the ability to proliferate into the required cells under the certain context. They are highly reproductive and can differentiate into different cell lineages, such as adipose, bone, cartilage and neurons. Many studies have reported that ADSCs can be applicable for obesity treatments and for esthetics[12]–[13]. It also has the potential to differentiate into vascular endothelial cells, and it synthesizes and releases a variety of proangiogenic factors to promote local vascularization[14]–[16]. In addition, the ADSCs could recover the damaged cells by producing antioxidants, free radical scavengers, and heat shock proteins to the ischemic area, and delivering mitochondria to the damaged cells[17].
Most corneal diseases primarily or secondarily affect corneal stroma, which accounts for 90% of the corneal thickness, including immune or infectious diseases, ecstatic disorders, traumatic scars, corneal dystrophies, and corneal failure[18]–[19]. The current disadvantages of corneal transplantation, particularly involve in the primary immune rejection and a shortage of corneas[20]. To overcome the above problems, cell-based therapy of the cornea is a promising therapeutic approach. Recently, nonocular cells have also been used to reconstruct corneal epithelium, mainly using autologous oral mucosal epithelium[21]–[22]. Tissue engineering of functional corneal equivalents has been developed.
In the present study, our study aimed at deciphering the role of ADSCs in corneal stromal cells (CSCs) plasticity. We established the indirect co-culture system to dedicate the role of ADSCs on the proliferation of CSCs and to explore a promising strategy for corneal stromal repair.
SUBJECTS AND METHODS
Isolation and Culture Procedure of Adipose-derived Mesenchymal Stem Cells
ADSCs were isolated as previously described. ADSCs were obtained from subcutaneous adipose tissues in the groin from New Zealand white rabbits. Briefly, the freshly isolated adipose tissues were washed several times with sterile phosphate-buffered saline (PBS) with 0.1% penicillin/streptomycin for several times, and then ophthalmic scissors and ophthalmic forceps were used to excise fascia and vessels. Mince adipose tissues into small pieces and incubate them with 0.1% collagenase type I (Invitrogen, Thermo Fisher Scientific Inc., USA) in Dulbecco's modified eagle medium (DMEM)/F12 (Hyclone, GE healthcare, USA) for 30-35min at 37°C. Add an equal volume of DMEM/F12 supplemented with 10% fetal bovine serum (FBS; Wisent, Canada) to inactivate the collagenase. After centrifugation for 10min at 1500 r/min, the cellular pellet was resuspended in DMEM/F12 containing 10% FBS at 37°C with 5% CO2 in an incubator.
After 72h, cell growth was observed and recorded, collect the unadherent cells, small adipose tissues and medium into new cell culture flasks. After another 48h, according to cell growth, the unadherent cells could be inoculated directly in new culture flasks. The medium were changed for two days to eliminate unattached cells. Primary cells cultured for 14-16d and grew close to the confluence for generation. Cells were passaged by 0.25% trypsinization at the ratio of 1:2. After initial plating, the obtained ADSCs were cultured up to passage 4 and then were used for the following experiment. The humane treatments of animals were performed under the American Association for the Accreditation of Laboratory Animal Care guidelines and adhered to national and international standards.
Adipose-derived Mesenchymal Stem Cells Chondrogenic and Osteogenic Differentiation
Briefly, ADSCs (passage 3) (at a density of 1×105/well) adhering to coverslips were grown in a 6-well plate at 37°C with 5% CO2. After twenty-four hours of culture, cells were cultured in adipogenesis induction medium (AIM) consisting of DMEM with high glucose, 10% FBS, 10−6 mol/L dexamethasone, 10−2 mol/L β-glycero phosphate, and 50 µg/mL ascorbic acid, and osteogenic induction medium (OIM) containing DMEM with high glucose, 10% FBS, 10−6 mol/L dexamethasone, 10 µg/mL insulin, 60 µmol/L indomethacin, 0.5 mmol/L 3-isobutyl-1-methylxanthine (IBMX). The complete medium was changed every two weeks. Cells in the control group were treated with the medium (including 10% FBS). For fourteen days and twenty-one days of culture, the osteogenic differentiation was verified by alkaline phosphatase (ALP) detection and alizarin red staining, respectively. Two weeks after induction, the adipocyte differentiation was confirmed by oil red O staining. ADSCs were fixed in 4% cold paraformaldehyde for 20min, then fixed in isopropanol for another 5min at room temperature, stained with oil red O solution (0.5%) for 15min in the oven at 60°C. A light microscope was used to evaluate the staining results.
Isolation and Culture of Corneal Stromal Cells
CSCs were obtained from the corneas of 1-month-old New Zealand rabbits. The corneal endothelium was stripped from the cornea and incubated with 1.5% collagenase II (Invitrogen, Thermo Fisher Scientific Inc., USA) at 37°C, 45min. After the digestion is completed, add the complete medium to terminate the digestion. The medium was collected and centrifuged at 1000 rpm for 5min, 2 times. Then, CSCs obtained from the corneas of New Zealand rabbits were resuspended in basal growth medium (DMEM/F12 supplemented with 20% FBS) and plated into cell culture flasks. Cells were maintained at 37°C with 5% CO2, and the culture medium was changed every 2d. When CSCs reached confluence, they were then treated with 0.25% trypsin-EDTA, and subcultured, and seeded at a ratio of 1:2. At the fourth passage of CSCs, the medium with DMEM/F12 and 10% FBS was used to replace the previous medium. After initial expansion, the achieved CSCs were cultured up to passage 2 and then they were used for experiment.
Cellular Morphology
Individual cells (1×104 mL) (passage 2) were cultured on heat sterilized cover slips and after confluency, were observed for different periods (3, 5, 7, or 9d) using the microscope (Leica, Germany).
Indirect Co-culture
Transwell plates (Corning, Life Sciences, the Netherlands) with 0.4 µm pore polycarbonate membrane insert were used for indirect co-cultures. ADSCs were seeded in the upper compartment (1×104 cells/well) and CSCs in the lower compartment (1×104 cells/well). The above cells were cultured for 4-5d in the co-culture systerm. The group with CSCs cultured alone were used as the negative control one.
Immunofluorescence Staining and Flow Cytometry
For the immunofluorescence assay (IF), cells (1×104 cells/mL) adhering to coverslips were seeded into a 24-well plate. Twenty-four hour after induction, the cells were then fixed with cold methanol for 10min and were blocked with 5% FBS. The coverslips were incubated with primary antibodies (CD29 1:50, CD34 1:200, CD45 1:50, CD90 1:50, vimentin 1:100, and CK12 1:100) overnight at 4°C. Secondary antibodies were added and incubated at 37°C for 1h. FITC-conjugated rabbit IgG antibodies (Santa Cruz Biotechnology) were used as labels for this assay. The cells were immune labeled, washed, stained with 4′, 6-diamidino-2-phenylindole (DAPI; Zhongshan), mounted, and viewed under a fluorescence microscope (Nikon). The expression of stromal markers (CD29-PE, CD90-PE, CD34-PE, CD45-PE) on cellular membrane was also analyzed by flow cytometry.
Cell Proliferation Assay, Cell Cycle Analysis and Cell Apoptosis Assay
Cells (1×104 cells/mL) were placed in 96-well plates and cultured for different periods. Subsequently, cell counting kit (CCK)-8 (Dojindo, Japan) was used to evaluate cell proliferation according to the manufacturer's instructions. Proliferation was analyzed by optical density (OD) and the absorbance of the cell suspension was read at A450 nm. All assays were performed in triplicate. Furthermore, cells were add to 96-well culture plate (1×105 cells/mL) and treated as above mentioned. Cell cycle changes and cell apoptosis were analysed by proliferation indices (PI) staining and Annexin V-FITC/PI staining (Bestbio, Shanghai, China), respectively.
Western Blot
RIPA buffer was used to lyse cells (Beyotime, Shanghai, China) for 10min on ice, and then centrifuged at 10 000 g at 4°C to remove cell debris. After SDS-PAGE electrophoresis, equal amount (30 µg) cell extracts were transferred onto PVDF membranes (Bio-Rad, Hercules, CA, USA) followed by incubating with primary rabbit monoclonal antibody against aldehyde dehydrogenase (ALDH), matrix metalloproteinase (MMP) 1, MMP2, MMP9, collagen type (I, II, III, IV, and V). Proteins were detected using peroxidase-conjugated Affinipure secondary antibodies IgG (H+L) (1:2000; Protein Techgroupsinc, Chicago, IL, USA). Blots were detected using Image Lab™ Software, Version 5.1 (from Bio-Rad, Hercules, CA, USA).
Statistical Analysis
Data are presented as the mean±standard deviation (SD). All statistical analyses were performed using ANOVA or a two-tailed Student's t-test to compare data. All assays were performed in triplicate. P<0.05 was considered statistically significant.
RESULTS
Chondrogenic and Osteogenic Introduction of Adipose-derived Mesenchymal Stem Cells
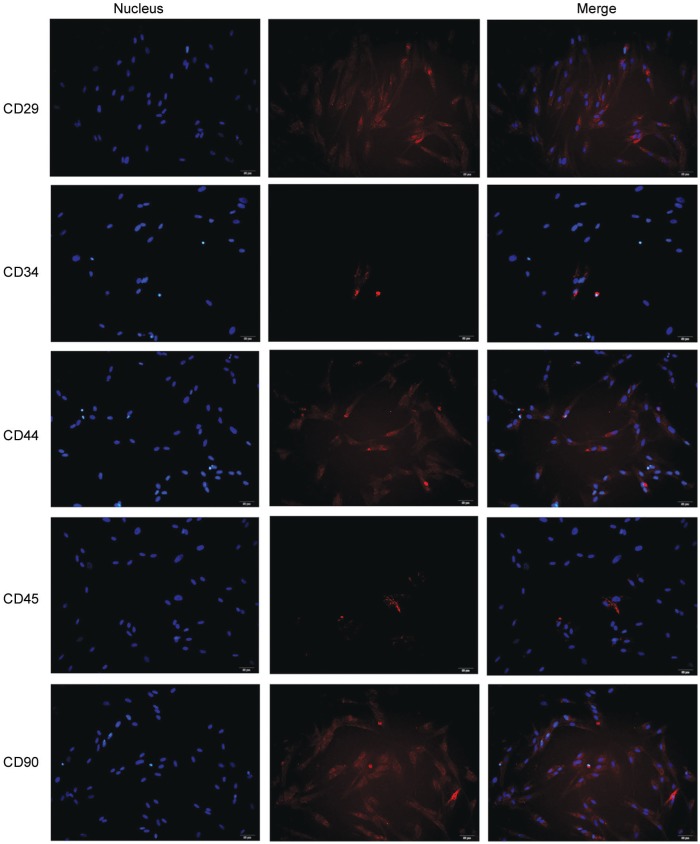
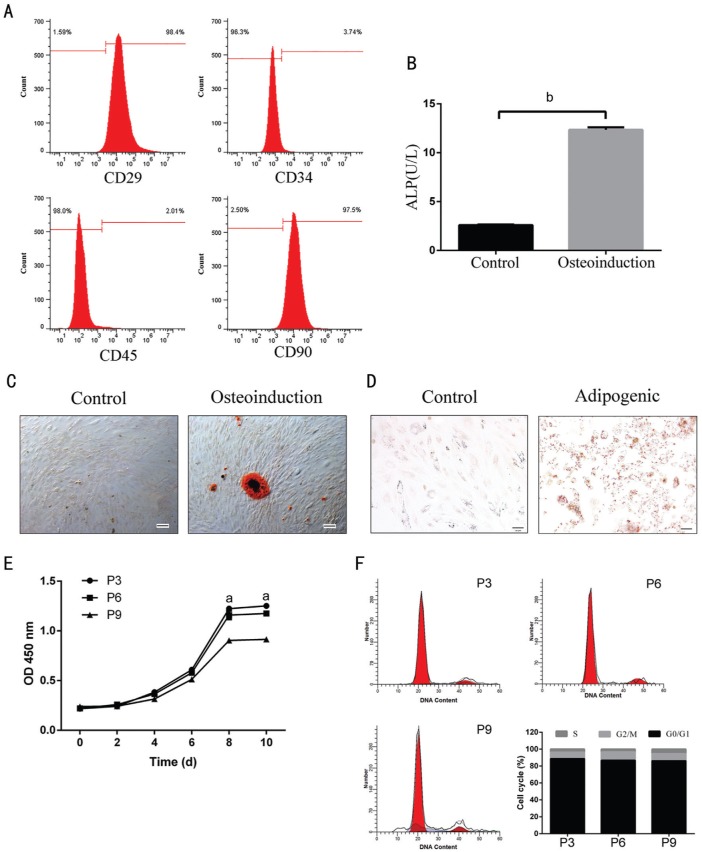
A fibroblastoid adherent and typical spindle shaped morphology was observed in the primary ADSCs. In addition, the presence of CD29, CD44 and CD90 and the absence of hematopoieticand endothelial marker CD34, CD45 were used to identify ADSCs (Figure 1). Flow cytometry showed that ADSCs were: CD29 (98.4%), CD90 (97.5%), CD34 (3.74%) and CD45 (2.01%) (Figure 2A). Moreover, a similar trend of the above markers in ADSCs was observed by IF analysis (Figure 1).
Figure 1. ADSC in vitro culture and identification.
Under the microscope, ADSCs were spindle-shaped and grew vigorously. The expression of CD29, CD90, CD34 and CD45 showed as red fluorescence within the ADSCs. DAPI was used to stain the nuclei of the cells as blue fluorescence. Original magnification: 200×. Scale bar represents 50 µm.
Figure 2. Differentiations, proliferation and alteration of cell cycle of ADSCs.
A: Flow cytometric analysis of rabbit ADSCs. After culturing for 14d, majority of isolated ADSCs expressing CD29 (98.4%) and CD90 (97.5%) characteristic of mesenchymal stem cells, showing low level of CD34 (3.74%), and CD45 (2.01%). B-D: Adipogenic and osteogenic differentiations of ADSCs. ALP detection (B) and alizarin red staining (C; 200×) and oil red O staining (D; 200×) of ADSC differentiations after day 14 or after day 21 induction of differentiation. E: The proliferation of ADSCs (passage 3, 6, and 9) was assessed by CCK-8 analysis. The ADSCs' growth curve was S-shaped. The increase of growth of ADSCs was observed as early as the third day, especially at the eight day and passage 3. F: Apoptosis phenotype was detected using flow cytometry. P3, P6, P9: Passage 3, 6, 9. aP<0.05 and bP<0.01.
We next assesed the capacity of ADSCs to differentiate into osteoblasts and adipocytes. After incubating in OIM for 2wk, ADSCs attained cuboidal morphologies and began to form nodules. These changes indicated that the osteoblastic differentiation of ADSCs had occurred. Meanwhile, they were not depositing a mineralized matrix. However, by 3wk, extensive mineralized matrix deposition was exhibited in the treated groups, as detected by ALP detection and alizarin red staining (Figure 2B, 2C), consistent with a mature osteoblast phenotype. As expected, the control groups with only ADSCs displayed no signs of cuboidal morphologies or a mineralized matrix. ADSCs exposed to AIM acquired characteristics of mature adipocytes. For 2wk of culture, cells began to accumulate small cytoplasmic lipid droplets, as denoted by positive oil red O staining. With elongation of culturing time, the lipid droplets coalesced, finally filling up most of the cytoplasm (Figure 2D). In contrast, control cultures did not assume lipid accumulation (Figure 2D). The data indicated that the ADSCs with multipotent properties have been successfully isolated.
Adipose-derived Mesenchymal Stem Cells in Vitro Culture
In vitro, ADSCs' culture was successfully obtained. ADSCs were in spindle shape with S-shaped growth curve and grew vigorously (Figure 2E). The results showed that there was no significant difference among ADSCs at different passages at a certain range. Alteration of cell cycle was detected by flow cytometry. However, the proportions of cells in cell cycle phases were similar at the end of experiment (Figure 2F).
Identification and Culture of Corneal Stromal Cells in Vitro
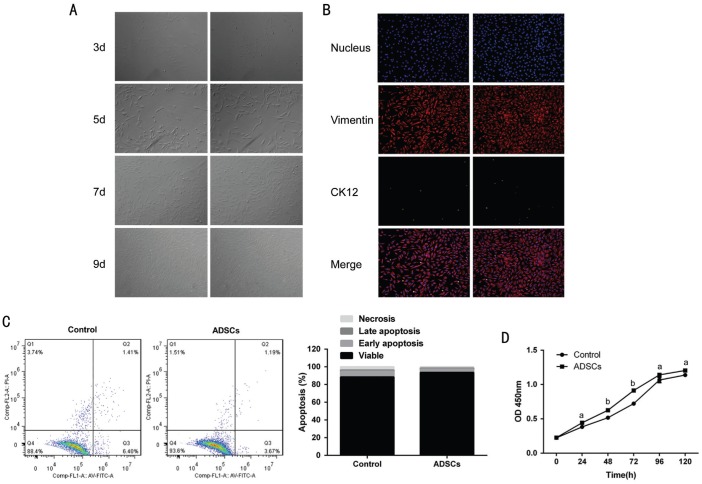
In vitro, CSCs was successfully isolated and obtained from New Zealand rabbits. After 3d of inoculation at 37°C, the cells were attached in spindle shape (round nuclear, centered); for 7d, the cells showed a fusion growth (Figure 3A). We next examined the expression of vimentin and CK12 in CSCs by IF. The CSCs showed high level of vimentin (>95%) and low level of CK12 (<5%) (Figure 3B). These data indicated that CSCs were isolated in high purity.
Figure 3. CSCs in vitro culture and identification.
A: Under the microscope, the CSCs were in the spindle-shape. Original magnification: 200×. Scale bar represents 50 µm. B: The expression of the markers vimentin+ showed red fluorescence and CK12+ showed as green fluorescence. The nuclei of the cells were stained blue with DAPI. C: Annexin V-FITC Apoptosis Detection Kit was used to detect the proportions of apoptotic cells under the co-culture conditions of ADSCs. FL1-A: Annexin V; FL3-A: PI. D: CCK-8 detection kit was used to detect the proliferation of CSCs under CSCs cultured alone or ADSCs treatment conditions. The bar graph shows mean±SD in triplicate. aP<0.05 and bP<0.01.
Promotive Effect of Adipose-derived Mesenchymal Stem Cells on the Proliferation of Corneal Stromal Cells in Vitro
To understand the ADSCs' role in proliferative kinetics of CSCs, the DNA synthesis of CSCs indirectly co-cultured with ADSCs for 3d was detected. The group with CSCs cultured alone was used as the negative control one. The co-cultural group displayed much higher ability to promote the proliferation, compared with that CSCs cultured alone (Figure 3D), especially for 3d. Additionally, the cell division rate was assessed by Flow Cytometry assays. Similarly, the PI of CSCs in the co-culture system were increased approximately 3-8-fold compared with that in the control group.
Inhibitory Effect of Adipose-derived Mesenchymal Stem Cells on the Apoptosis of Corneal Stromal Cells in Vitro
Next, the possibility of regulation of apoptosis in CSCs by ADSCs was detected. We performed apoptosis assays for CSCs and ADSCs indirect co-culture models. CSCs cultured alone were also used as the negative control. The results showed that the presence of ADSCs inhibited the apoptosis of CSCs, compared to that of the negative control groups. After indirect co-culture with ADSCs for 3d, the percentage of apoptotic cells was quantified using Annexin V-FITC apoptosis detection kit. The proportions of cells at apoptosis (early and late) and dead were calculated. A significant change was observed in the percentage of apoptotic cells under the negative control or ADCSs treatment groups. The group with ADCSs showed lower percentage of apoptotic cells than that in the control group (Figure 3C).
Inhibition of Matrix Metalloproteinase Expression in Corneal Stromal Cells and Promotion of Extracellular Matrix Synthesis by Adipose-derived Mesenchymal Stem Cells
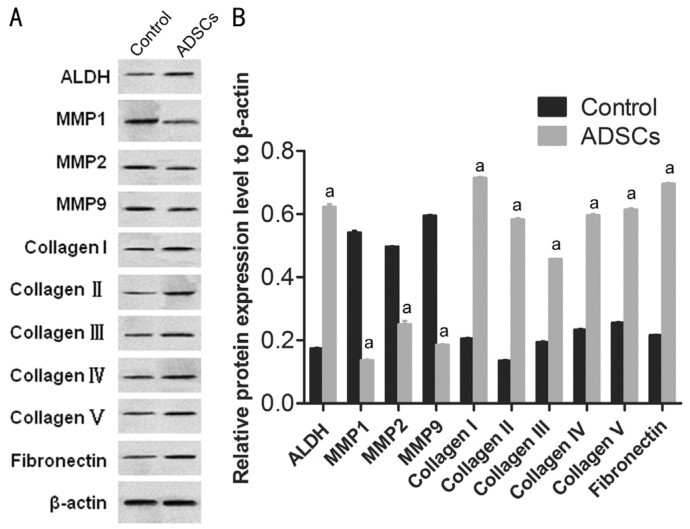
To examine the effects of ADSCs on ECM in vitro, protein detection was performed using CSCs and ADSCs. The expression of MMPs, such as MMP1, MMP2 and MMP9 were detected using Western blot. The expression levels of MMP1, MMP-2 and MMP-9 in CSCs were down regulated in the co-culture models (Figure 4A, 4B). The decrease intensities of MMP-1, MMP-2 and MMP-9 in CSCs/ADSCs group were observed, 3.90-fold, 1.09-fold and 3.03-fold, respectively. In the level of collagen type (I, II, III, IV, and V), opposite results were observed in CSCs/ADSCs group (Figure 4A, 4B). The expression of ALDH in CSCs, as a characteristic marker, was upregulated in the co-culture models and the similar results about the expression of fibronectin, as an ECM associated molecular, were observed in CSCs/ADSCs group. Based on these results, we suggested that ADSCs might be responsible for the decreased expression of MMPs and the increased expression of collagens in CSCs, which in turn promoted ECM synthesis of CSCs.
Figure 4. ADSCs suppressed the expression of MMPs and promoted ECM synthesis in CSCs.
The protein expression levels of MMPs (MMP1, MMP-2 and MMP-9), collagen type (I, II, III, IV, and V), ALDH, and fibronectin in CSCs and ADSCs-treated-CSCs determined by Western blot. β-actin were used as the internal control. aP<0.05.
DISCUSSION
Rabbits are considered as appropriate animal models to study the mechanism of ADSCs because of their blood lipid profile have the similarity with humans' metabolism[1],[5]. Our objective was to evaluate the role of rabbit ADSCs in CSCs in vitro. Subcutaneous adipose tissues in the groin were isolated from 28-day-old New Zealand rabbits by using collagenase digestion. They have the potential to differentiate into multiple cell lines. All isolated ADSCs demonstrated functional plasticity, which is proved by successfully performing osteogenic and adipocyte differentiation after addition of growth factors.
ADSCs play a major role in treating several regenerative diseases. The interest to ADSCs has been significantly generated in the field of stem cell research[23]–[24]. Recently, it has been reported that ADSCs play vital roles in revascularization[25]. Under proper inductions of ADSCs, vascular endothelial cells and vascular smooth muscle cells can be abtained by ADSCs' differentiation. Several studies have reported the ADSCs induce angiogenesis via autocrine or paracrine of angiogenic cytokines[14]–[16],[26]–[27]. By interactions between ADSCs and endothelial cells, new functional angiogenesis and revascularization were promoted.
We have focused on the effect of ADSCs on CSCs plasticity. The morphology of CSCs did not show significantly difference after cocultured with ADSCs. The data have supported that the cell lines used were ADSCs and CSCs, as both expressed the characteristic markers. After coculturing for 3d, ADSCs could apparently enhance the proliferation of CSCs; however, at the fifth day, relative disparity remained while the absolute disparity decreases. Flow cytometry results showed two distinct populations of the two cocultured cell lines. This may be indicative of an inhibitory effect of ADSCs on apoptosis in CSCs. It has been reported that coculturing leads to an increase in differentiation of stem cells. Danisovic et al[28] and Burian et al[29] have been reported that bone marrow mesenchymal stem cells differentiated into chondrocytes in a direct coculture system. The results of viability and proliferation assays in this article agreed with the recent studies. It has been reported that external damage can induce the increase of cellular viability and proliferation, the production of collagens and the release of growth factors in ADSCs and other cells. Our data were consistent with those of previous reports; the viability and proliferative ability of CSCs were increased in, the co-culture systerm, compared with those that were not exposed to ADSCs.
The ADSCs are known as an pivitol player in wound healing and tissue regeneration. In engineered tissue construct, ADSCs are often placed as seed cells to produce biologically important factors. A certain concentration of EGF treatment promoted the ADSCs' paracrine[27]. Damages resulted from topical drugs, trauma, and infection to keratocytes often result in loss of intercellular contact and keratocyte-fibroblast-myofibroblast transition, which increase the risk of corneal scar formation, corneal opacification and visual impairment[21],[30]. In our research, the treatment of a certain population of ADSCs promoted the CSCs' paracrine.
The stromal cellular layers show a vital role in the cornea. CSCs (keratocytes), as mesenchymal-derived cells, are the main cells of the corneal stroma. In adult tissues, CSCs are mitotically quiescent, with a flat, dendritic morphology, and are positive for CD34 and ALDH[31]. During corneal wound healing, CSCs are activated and transform into fibroblasts and/or myofibroblasts, losing their dendritic morphology[32], and resulting in a reduction in ALDH[33] and corneal transparency. Our data indicated that the indirect co-culture of ADSCs and CSCs increased the expression of ALDH, suggesting that ADSCs increased corneal stroma by the promotion for the proliferation of CSCs.
In human, adipocytes were found to provide energy for the promotion of cancer cells. ADSCs have no excess fat storage and there is no evidence that ADSCs provide fatty acids for tumor progression[23]. However, ADSCs have been reported to motivate the progression of a variety of solid tumors[23]. This indicated that interactions between ADSCs and other cells may be the primary mechanism involved in ADSCs' role. In addition, ADSCs show a better pro-angiogenic profile and enhance the secretion of higher levels of ECM components and MMPs. MMPs are a class of important proteases and play key roles in ECM degradation. The overexpression of MMPs had also been frequently reported in tumour tissues, and its importance in tumour angiogenesis was also well known. Through MMPs, a series of biochemical changes were triggered and it was involved in tumor metastasis, angiogenesis, and wound healing[26]. In the present study, under indirect co-culture with ADSCs, the proliferation and migration ability of CSCs, to a similar extent, was increased, but the expression of MMPs was decreased, indicating that the ECM promoting effects of ADSCs are possibly mediated by the paracrine of ADSCs and CSCs. However, this result was inconsistent with the previous reports.
Mounting evidence indicates that collagens mediate many changes in the microenvironment and regulate the non-cellular and cellular factors to facilitate tumor progression[32],[34]–[36]. In this literature, the increases of collagens were observed in co-culture supernatants; collagens (I, II, III, IV, and V) in co-cultured CSCs were up regulated; the ADSCs mediated increases in CSCs proliferation and invasion could be enhanced by the selective inhibition of MMPs and the increase of collagens. The expression of collagens and MMPs contributed to the tumor-promoting effects of ADSCs in vitro. Some signaling pathways were involved in regulating the expression of these factors in cancer, such as Hedgehog, PI3Kinase/Akt and NF-κB[37]–[39]. However, precise mechanisms involved in the elevated Collagens and reduced MMPs in CSCs mediated by ADSCs remain unclear. Besides ADSCs, CSCs might influence the secretion of ADSCs, which needs to be verified in the following studies.
In conclusion, we isolated ADSCs from subcutaneous adipose tissues in the groin from New Zealand white rabbits. Our data indicated that ADSCs enhanced growth property of CSCs, and suppressed the apoptosis of CSCs. In addition, ADSCs promoted ECM synthesis in CSCs, which may be at least partially due to the inhibition of MMPs and the promotion of Collagens level. These findings provide a novel insight into the mechanism how the ADSCs play a vital role in ECM synthesis. ADSCs significantly promoted CSCs growth and invasion, which may be associated with MMPs decrease and Collagens increase, suggesting a positive participation in the plasticity and ECM synthesis of CSCs. Our results provided further evidence of the extensive role of ADSCs in CSCs and a potential molecular target for cornea trauma therapy. Thus, a combination of a tissue-engineered human corneal endothelium coupled with co-culture with ADSCs presents a possible roadmap for the treatment of endothelial dysfunctions.
Acknowledgments
Foundation: Supported by Important Subject Fund of Science Technology Department of Zhejiang Province (No.2013C03048-1).
Conflicts of Interest: Shen T, None; Shen J, None; Zheng QQ, None; Li QS, None; Zhao HL, None; Cui L, None; Hong CY, None.
REFERENCES
- 1.Huang SJ, Fu RH, Shyu WC, Liu SP, Jong GP, Chiu YW, Wu HS, Tsou YA, Cheng CW, Lin SZ. Adipose-derived stem cells: isolation, characterization, and differentiation potential. Cell Transplant. 2013;22(4):701–709. doi: 10.3727/096368912X655127. [DOI] [PubMed] [Google Scholar]
- 2.Bunnell BA, Flaat M, Gagliardi C, Patel B, Ripoll C. Adipose-derived stem cells: isolation, expansion and differentiation. Methods. 2008;45(2):115–120. doi: 10.1016/j.ymeth.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marcus AJ, Coyne TM, Rauch J, Woodbury D, Black IB. Isolation, characterization, and differentiation of stem cells derived from the rat amniotic membrane. Differentiation. 2008;76(2):130–144. doi: 10.1111/j.1432-0436.2007.00194.x. [DOI] [PubMed] [Google Scholar]
- 4.Franco B, Vincenzo V, Alessandro DV, Tonello C, Abatangelo G. Bioengineered tendon: from tenocytes culture to ASCs (adipose derived stem cells) culture. Wound Repair Regen. 2008;16(6):A74. [Google Scholar]
- 5.Vachkova E, Bosnakovski D, Yonkova P, Grigorova N, Ivanova Z, Todorov P, Penchev G, Milanova A, Simeonova G, Stanilova S, Georgiev IP. Adipogenic potential of stem cells derived from rabbit subcutaneous and visceral adipose tissue in vitro. In Vitro Cell Dev Biol Anim. 2016;52(8):829–837. doi: 10.1007/s11626-016-0048-7. [DOI] [PubMed] [Google Scholar]
- 6.Arrigoni E, Lopa S, de Girolamo L, Stanco D, Brini AT. Isolation, characterization and osteogenic differentiation of adipose-derived stem cells: from small to large animal models. Cell Tissue Res. 2009;338(3):401–411. doi: 10.1007/s00441-009-0883-x. [DOI] [PubMed] [Google Scholar]
- 7.Mauney JR, Nguyen T, Gillen K, Kirker-Head C, Gimble JM, Kaplan DL. Engineering adipose-like tissue in vitro and in vivo utilizing human bone marrow and adipose-derived mesenchymal stem cells with silk fibroin 3D scaffolds. Biomaterials. 2007;28(35):5280–5290. doi: 10.1016/j.biomaterials.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rubin JP, Bennett JM, Doctor JS, Tebbets BM, Marra KG. Collagenous microbeads as a scaffold for tissue engineering with adipose-derived stem cells. Plast Reconstr Surg. 2007;120(2):414–424. doi: 10.1097/01.prs.0000267699.99369.a8. [DOI] [PubMed] [Google Scholar]
- 9.Locke M, Windsor J, Dunbar PR. Human adipose-derived stem cells: isolation, characterization and applications in surgery. ANZ J Surg. 2009;79(4):235–244. doi: 10.1111/j.1445-2197.2009.04852.x. [DOI] [PubMed] [Google Scholar]
- 10.Song YH, Gehmert S, Sadat S, Pinkemell K, Bai X, Matthias N, Alt E. VEGF is critical for spontaneous differentiation of stem cells into cardiomyocytes. Biochem Biophys Res Commun. 2007;354(4):999–1003. doi: 10.1016/j.bbrc.2007.01.095. [DOI] [PubMed] [Google Scholar]
- 11.Ii M, Yokoyama A, Horii M, Akimaru H, Asahara T. SDF-1 alpha mediates the therapeutic effect of human adipose-derived stem cells on acute myocardial infarction recruiting bone marrow-derived endothelial progenitor cells. Circulation. 2008;118(18):S500. [Google Scholar]
- 12.Kingham PJ, Kalbermatten DF, Mahay D, Armstrong SJ, Wiberg M, Terenghi G. Differentiation of adipose-derived stem cells to a Schwann cell phenotype. Tissue Engineering. 2007;13(7):1675. [Google Scholar]
- 13.Anghileri E, Marconi S, Pignatelli A, Cifelli P, Galie M, Sbarbati A, Krampera M, Belluzzi O, Bonetti B. Neuronal differentiation potential of human adipose-derived mesenchymal stem cells. Stem Cells Dev. 2008;17(5):909–916. doi: 10.1089/scd.2007.0197. [DOI] [PubMed] [Google Scholar]
- 14.Song YH, Shon SH, Shan MR, Stroock AD, Fischbach C. Adipose-derived stem cells increase angiogenesis through matrix metalloproteinase-dependent collagen remodeling. Integr Biol (Camb) 2016;8(2):205–215. doi: 10.1039/c5ib00277j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park HJ, Jin Y, Shin J, Yang K, Lee C, Yang HS, Cho SW. Catechol-functionalized hyaluronic acid hydrogels enhance angiogenesis and osteogenesis of human adipose-derived stem cells in critical tissue defects. Biomacromolecules. 2016;17(6):1939–1948. doi: 10.1021/acs.biomac.5b01670. [DOI] [PubMed] [Google Scholar]
- 16.Makarevich PI, Boldyreva MA, Efimenko AY, Gluhanyuk EV, Dergilev KV, Gallinger JO, Hu YC, Parfyonova YV. Therapeutic angiogenesis by subcutaneous cell sheet delivery is superior to cell injection: a study of ADSC efficacy in a model of hind limb ischemia. Molecular Therapy. 2016;24:S178. [Google Scholar]
- 17.Zhou L, Song Q, Shen J, Xu L, Xu Z, Wu R, Ge Y, Zhu J, Wu J, Dou Q, Jia R. Comparison of human adipose stromal vascular fraction and adipose-derived mesenchymal stem cells for the attenuation of acute renal ischemia/reperfusion injury. Sci Rep. 2017;7:44058. doi: 10.1038/srep44058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shimmura S, Tsubota K. Deep anterior lamellar keratoplasty. Curr Opin Ophthalmol. 2006;17(4):349–355. doi: 10.1097/01.icu.0000233953.09595.91. [DOI] [PubMed] [Google Scholar]
- 19.Terry MA, Ousley PJ. Small-incision deep lamellar endothelial keratoplasty (DLEK): six-month results in the first prospective clinical study. Cornea. 2005;24(1):59–65. doi: 10.1097/01.ico.0000133990.19027.a2. [DOI] [PubMed] [Google Scholar]
- 20.Alldredge OC, Krachmer JH. Clinical types of corneal transplant rejection. Their manifestations, frequency, preoperative correlates, and treatment. Arch Ophthalmol. 1981;99(4):599–604. doi: 10.1001/archopht.1981.03930010599002. [DOI] [PubMed] [Google Scholar]
- 21.Nishida K, Yamato M, Hayashida Y, Watanabe K, Yamamoto K, Adachi E, Nagai S, Kikuchi A, Maeda N, Watanabe H, Okano T, Tano Y. Corneal reconstruction with tissue-engineered cell sheets composed of autologous oral mucosal epithelium. N Engl J Med. 2004;351(12):1187–1196. doi: 10.1056/NEJMoa040455. [DOI] [PubMed] [Google Scholar]
- 22.Nakamura T, Inatomi T, Sotozono C, Amemiya T, Kanamura N, Kinoshita S. Transplantation of cultivated autologous oral mucosal epithelial cells in patients with severe ocular surface disorders. Br J Ophthalmol. 2004;88(10):1280–1284. doi: 10.1136/bjo.2003.038497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rigotti G, Marchi A, Sbarbati A. Adipose-derived mesenchymal stem cells: past, present, and future. Aesthetic Plastic Surgery. 2009;33(3):271–273. doi: 10.1007/s00266-009-9339-7. [DOI] [PubMed] [Google Scholar]
- 24.Wankhade UD, Shen M, Kolhe R, Fulzele S. Advances in adipose-derived stem cells isolation, characterization, and application in regenerative tissue engineering. Stem Cells Int. 2016:3206807. doi: 10.1155/2016/3206807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhong ZH, Gu HY, Peng JR, Wang WZ, Johnstone BH, March KL, Farlow MR, Du YS. GDNF secreted from adipose-derived stem cells stimulates VEGF-independent angiogenesis. Oncotarget. 2016;7(24):36829–36841. doi: 10.18632/oncotarget.9208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sahin E, Baycu C, Koparal AT, Donmez DB, Bektur E. Resveratrol reduces IL-6 and VEGF secretion from co-cultured A549 lung cancer cells and adipose-derived mesenchymal stem cells. Tumor Biol. 2016;37(6):7573–7582. doi: 10.1007/s13277-015-4643-0. [DOI] [PubMed] [Google Scholar]
- 27.Li Q, Li PH, Hou DJ, Zhang AJ, Tao CB, Li XY, Jin PS. EGF enhances ADSCs secretion via ERK and JNK pathways. Cell Biochem Biophys. 2014;69(1):189–196. doi: 10.1007/s12013-013-9769-3. [DOI] [PubMed] [Google Scholar]
- 28.Danisovic L, Varga I, Polák S, Ulicná M, Hlavacková L, Böhmer D, Vojtassák J. Comparison of in vitro chondrogenic potential of human mesenchymal stem cells derived from bone marrow and adipose tissue. Gen Physiol Biophys. 2009;28(1):56–62. [PubMed] [Google Scholar]
- 29.Burian E, Probst F, Palla B, Riedel C, Saller MM, Cornelsen M, Konig F, Schieker M, Otto S. Effect of hypoxia on the proliferation of porcine bone marrow-derived mesenchymal stem cells and adipose-derived mesenchymal stem cells in 2- and 3-dimensional culture. J Craniomaxillofac Surg. 2017;45(3):414–419. doi: 10.1016/j.jcms.2016.12.014. [DOI] [PubMed] [Google Scholar]
- 30.Arnalich-Montiel F, Pastor S, Blazquez-Martinez A, Fernandez-Delgado J, Nistal M, Alio JL, De Miguel MP. Adipose-derived stem cells are a source for cell therapy of the corneal stroma. Stem Cells. 2008;26(2):570–579. doi: 10.1634/stemcells.2007-0653. [DOI] [PubMed] [Google Scholar]
- 31.Jester JV, Moller-Pedersen T, Huang J, Sax CM, Kays WT, Cavangh HD, Petroll WM, Piatigorsky J. The cellular basis of corneal transparency: evidence for ‘corneal crystallins’. J Cell Sci. 1999;112(Pt 5):613–622. doi: 10.1242/jcs.112.5.613. [DOI] [PubMed] [Google Scholar]
- 32.Maatta M, Vaisanen T, Vaisanen MR, Pihlajaniemi T, Tervo T. Altered expression of type XIII collagen in keratoconus and scarred human cornea: increased expression in scarred cornea is associated with myofibroblast transformation. Cornea. 2006;25(4):448–453. doi: 10.1097/01.ico.0000183537.45393.1f. [DOI] [PubMed] [Google Scholar]
- 33.Funderburgh JL, Mann MM, Funderburgh ML. Keratocyte phenotype mediates proteoglycan structure: a role for fibroblasts in corneal fibrosis. J Biol Chem. 2003;278(46):45629–45637. doi: 10.1074/jbc.M303292200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou XP, Tao YQ, Wang JK, Liu DY, Liang CZ, Li H, Chen QX. Three-dimensional scaffold of type II collagen promote the differentiation of adipose-derived stem cells into a nucleus pulposus-like phenotype. J Biomed Res A. 2016;104(7):1687–1693. doi: 10.1002/jbm.a.35701. [DOI] [PubMed] [Google Scholar]
- 35.Kim I, Park H, Shin Y, Kim M. Adipogenic differentiation of adipose-derived stem cells on collagen microbeads. Tissue Engineering and Regenerative Medicine. 2009;6(4-11):924–930. [Google Scholar]
- 36.Matsumine H, Numakura K, Tsunoda S, Wang H, Matsumine R, Climov M, Giatsidis G, Sukhatme VP, Orgill DP. Adipose-derived aldehyde dehydrogenase-expressing cells promote dermal regenerative potential with collagen-glycosaminoglycan scaffold. Wound Repair Regen. 2017;25(1):109–119. doi: 10.1111/wrr.12494. [DOI] [PubMed] [Google Scholar]
- 37.Rider DA, Dombrowski C, Sawyer AA, Ng GHB, Leong D, Hutmacher DW, Nurcombe V, Cool SM. Autocrine fibroblast growth factor 2 increases the multipotentiality of human adipose-derived mesenchymal stem cells. Stem Cells. 2008;26(6):1598–1608. doi: 10.1634/stemcells.2007-0480. [DOI] [PubMed] [Google Scholar]
- 38.Kim WS, Park SH, Ahn SJ, Kim HK, Park JS, Lee GY, Kim KJ, Whang KK, Kang SH, Park BS, Sung JH. Whitening effect of adipose-derived stem cells: A critical role of TGF-beta 1. Biol Pharm Bull. 2008;31(4):606–610. doi: 10.1248/bpb.31.606. [DOI] [PubMed] [Google Scholar]
- 39.Zubkova ES, Beloglazova IB, Makarevich PI, Boldyreva MA, Sukhareva OY, Shestakova MV, Dergilev KV, Parfyonova YV, Menshikov MY. Regulation of adipose tissue stem cells angiogenic potential by tumor necrosis factor-alpha. J Cell Biochem. 2016;117(1):180–196. doi: 10.1002/jcb.25263. [DOI] [PubMed] [Google Scholar]