Abstract
AIM
To evaluate ocular penetration of topically applied 1% tigecycline.
METHODS
Forty-two New Zealand White rabbits were divided into 3 groups. A 50 µL drop of 1% tigecycline was administered in group 1. In groups 2 and 3, the drop was administered every 15min for 60min (keratitis protocol). Aqueous humor samples in groups 1 and 2 were collected under general anesthesia at 15, 30, 45, 60, 120, and 180min after the last drop. All animals in group 3 were euthanatized. Cornea, vitreous and blood samples were collected 60 and 120min after the last drop. Tigecycline concentrations were measured using high performance liquid chromatography-mass spectrometry (LC-MS/MS).
RESULTS
The peak aqueous humor tigecycline concentration [mean 0.73±0.14 mg/L (SD) and 2.41±0.14 mg/L, respectively] occurred 45min after topical drug application in groups 1 and 2. Group 3 mean values in the cornea, and vitreous, were 3.27±0.50 µg/g, and 0.17±0.10 mg/L at 60min and 3.17±0.77 µg/g and 0.20±0.07 mg/L at 120min, respectively. Tigecycline serum concentrations were negligible.
CONCLUSION
Tigecycline levels in the aqueous humor in groups 1 and 2, and in the cornea in group 3 exceeded the minimum inhibitory concentrations of most gram-positive organisms that cause bacterial keratitis and endophthalmitis.
Keywords: antibiotic, cornea, pharmacokinetics, aqueous humor, rabbit eyes
INTRODUCTION
Bacterial keratitis and endophthalmitis are still some of the main causes of ocular morbidity and visual disability. Frequent causes of bacterial keratitis and endophthalmitis are gram positive pathogens, especially staphylococcus aureus and coagulase negative staphylococci[1]–[2]. Bacterial resistance to most popular antibiotic agents is a growing problem[3]–[5]. Cases of resistance to vancomycin in gram positive bacteria (enterococcus sp., bacillus sp. and staphylococcus sp.) related with endophthalmitis were reported[3],[6]–[7]. Bacteria that resistant to fluoroquinolone arised instantly after the presentation and extensive utilization of these antibiotics in the 1990s and has significantly rised in the last two decades[4]–[5]. For this reason new agents are required to control future ocular bacterial infection cases.
Tigecycline is a derivative of minocycline. It is a glycylcycline, a novel class of antibiotics developed to accomplish tetracycline specific resistance mechanisms. In vitro, tigecycline is active against a wide spectrum of bacteria, including multidrug resistant (MDR) bacteria, and it is FDA approved for the therapy of complicated intraabdominal infections, community-acquired pneumonia, and complicated skin infections[8]. Since this novel antibiotic has low bioavailability after oral application, it is available solely as an intravenous formulation. This lipophilic antibiotic has linear pharmacokinetics, spreads out quickly and has a extensive volume of distribution, pointing out wide tissue penetration[9].
Tigecycline inhibits protein synthesis by binding reversibly to the 30S ribosomal subunit with a fivefold higher affinity than tetracyclines[10]. This augmented ribosomal binding facilitates tigecycline to accomplish resistance produced by ribosomal protection[8]. Tigecycline has a wide spectrum of action towards anaerobic and aerobic gram positive and negative bacteria, including multiple classes of antimicrobials resistant microorganisms[8]. The minimum inhibitory concentrations (MICs) of tigecycline which demonstrates sensitivity are 2 mg/L or less for enterobacteriaceae, 0.25 mg/L or less for Streptococcus spp; including pneumoniae and vancomycin-susceptible enterococcus faecalis, 0.5 mg/L or less for Staphylococcus aureus, and 4 mg/L or less for anaerobes. The Tigecycline Evaluation and Surveillance Trial demonstrated that tigecycline was very effective towards most gram-positive bacteria, with the inclusion of 100% of the methicillin sensitive S aureus, 99% of the methicillin resistant S aureus, and 98% of the vancomycin resistant enterococcus. 95% of enterobacteriaceae were found to be sensitive to tigecycline at the FDA susceptibility breakpoint of 2 mg/L or less[11]–[12].
This antibiotic sustained in vitro activity towards MDR gram negative bacteria, including Klebsiella spp. and Escherichia coli. that produce wide spectrum b-lactamases and enterobacteriaceae strains that produce carbapenemase and showed high action towards acinetobacter baumanii with MIC 50/90 results of 0.5/2 mg/L[11],[13]–[14]. It was also found to be effective towards community-acquired infectious bacteria such as H influenza and S pneumoniae regardless of penicillin sensitivity or production of b-lactamase, with MIC 90 values of lower than 0.5 mg/L[11],[14]. It was found that pseudomonas aeruginosa are naturally resistant to tigecycline and it has restricted action towards providencia spp. and proteus spp[15].
In this experimental rabbit eye model study, it was intended to investigate the penetration of tigecycline into the cornea, aqueous humor and vitreous after topical application.
MATERIALS AND METHODS
This study was made at Konya Training and Research Hospital, Department of Ophthalmology, Konya, Turkey. Forty-two rabbits (New Zealand), aged 17-19wk (weight around 3.0 to 3.5 kg), were included. They divided into 3 groups and each group received 1 of 3 dosing regimens. In order to reduce the number of rabbit, right and left eyes of 30 rabbits constituted groups 1 and 2 respectively. Right eyes of twelve rabbits constituted group 3. An authorized breeding centre (Konya Necmettin Erbakan University, Application and Research Centre of Experimental Medicine) provided the rabbits. They were kept under standardized conditions in individual cages with steady temperature (21°C) and daylight cycle (12h light/24h). Initially, examination with a handheld slit lamp was made in all eyes. Rabbits with healthy eyes were included. Rabbits had free access to pellets (ad libitum) and water. Rabbits were treated in accordance with the Association for Research in Vision and Ophthalmology's (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research.
Preparation of the Antibiotic for Topical Administration
Pure powder of tigecycline (Tygacil, Wyeth, Turkey) was reconstituted in sterile water for injection to make a concentration 10 mg/mL (1%). The pH value of 1% aqueous solution of tigecycline was found to be 7.7-8.2[16]. Since the color of tigecycline powder is orange, the reconstituted solution has a yellow to orange color.
Groups
Group 1
Topical tigecycline solution (10 mg/mL, 1%) was applied. In order to prevent any runoff of the drug after instillation into the inferior conjunctival fornix, the eyelids were kindly held closed for a while after application. The volume of a drop was 50-µL (500 µg) and a single drop was applied with a micropipette into the right eye of each rabbit (n=30).
Group 2
A 50-µL drop of the preparation was administered every 15min for 60min to left eyes (keratitis protocol) (n=30).
Group 3
A 50-µL drop of the preparation was administered every 15min for 60min to right eyes (keratitis protocol) (n=12). Half of the rabbits in this group were euthanatized 60min after the last drop and the other half at 120min. The overdose was a mixture of ketamine and barbiturate (75 mg/kg) injected in the auricular vein.
Specimen Gathering
All eyes were examined after application of drug with a portable slit lamp for signs of ocular surface toxicity such as punctate epitheliopathy, conjunctival hyperemia and chemosis. Specimens were taken under ketamine induced general anesthesia (15 mg/kg) given through an auricular vein catheter. In group 1 (right eye) and group 2 (left eye), aqueous humor specimens were gathered at 15 (n=5), 30 (n=5), 45 (n=5), 60 (n=5), 120 (n=5), and 180min (n=5) after the last drop of tigecycline. In group 3, after the rabbits were euthanatized, specimens of cornea and vitreous from right eyes and blood were gathered at 60 (n=6), and 120min (n=6) after the last drop. Specimens were keeped at -80°C until analysis.
After 1 drop of oxybuprocaine was administered, 0.2 mL aqueous humor was withdrawn using a 30-gauge needle attached on a 1 mL syringe driven at the limbus.
The 0.2 mL vitreous was aspirated with a 25-gauge needle attached on a 5 mL syringe, driven at 3.0 mm from limbus, and guided toward the centre of the bulbus oculi[17]. Care was taken to avoid bleeding when the needle was introduced.
The corneas were excised using microsurgical instruments and washed with a sterile balanced salt solution before being dried, scaled, and frozen at -80°C.
Analytical procedures
Tigecycline levels were evaluated in accordance with a high performance liquid chromatography mass spectrometry (LC-MS/MS) method defined before[18]. To evaluate tigecycline levels in serum, vitreous, cornea, and aqueous humor, a sensitive and selective liquid chromatography mass spectrometry method used with a liquid chromatograph mass spectrometer device (Shimadzu, LCMS-8030 Triple Quadrupole)[18]. Positive ion mode was used in this device. Ions were analyzed by multiple reaction monitoring. A tigecycline hydrate standard was obtained from Sigma Aldrich (Germany, PZ0021-5 mg). Specimen preparation: after thawing at room temperature (22°C), 200 µL of the specimen was diluted with 600 µL of acetonitrile, followed by vortex mixing and centrifuged for 5min at 10 000 g. The supernatants were delivered to clean tubes. The supernatants were evaporated to dryness under a mild nitrogen current in a heating block at 45°C, and the remnants were redissolved in 200 µL of formic acid 0.1% (vol/vol) in water and analyzed by LC-MS/MS. The rate of flow was 0.2 mL/min, and the injection volume was 20 µL. The calibration curve ranged from 0.012 to 15 µg/mL, with a lower limit of quantitation of 0.012 µg/mL.
Means and medians of values were calculated by using a computer program (SPSS, Inc. Statistical Package for Social Science, version 15.0).
RESULTS
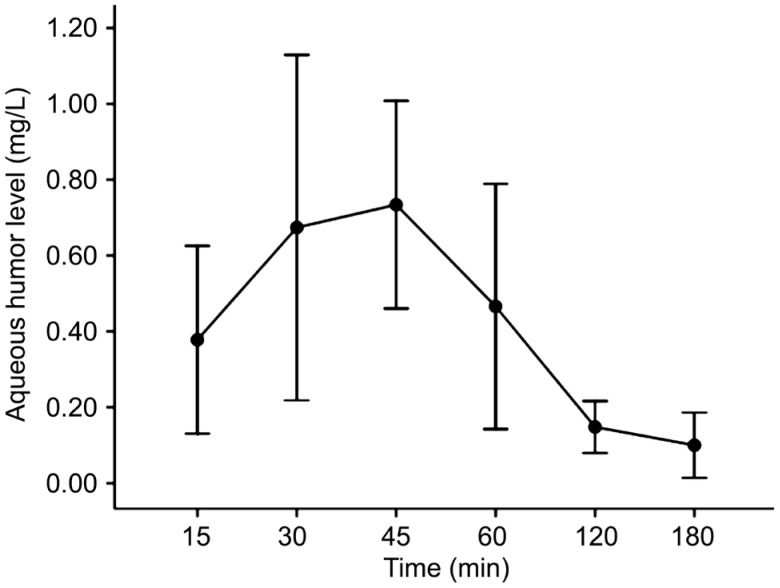
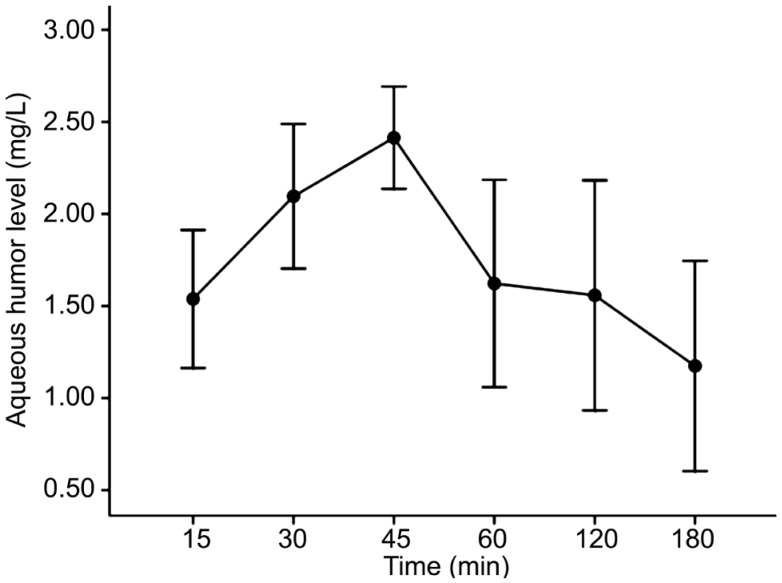
The aqueous humor concentrations reached a mean peak of 0.73±0.14 mg/L at 45min in group 1 (Figure 1, Table 1). In group 2, the mean peak value was 2.41±0.14 mg/L at 45min (Figure 2, Table 1).
Figure 1. Aqueous humor versus time concentration curve of single drop of topical 1% tigecycline (500 µg).
Table 1. Tigecycline concentrations in aqueous humor after topical 1% tigecycline.
| Time (min) | Aqueous humor (mg/L) |
|
| Single drop protocol | Multiple drop protocol | |
| 15 | 0.38±0.12 (0.34) | 1.54±0.32 (1.58) |
| 30 | 0.67±0.23 (0.74) | 2.09±0.20 (2.10) |
| 45 | 0.73±0.14 (0.75) | 2.41±0.14 (2.40) |
| 60 | 0.46± 0.16 (0.48) | 1.62±0.28 (1.56) |
| 120 | 0.15±0.03 (0.16) | 1.56±0.31 (1.58) |
| 180 | 0.10±0.04 (0.10) | 1.17±0.29 (1.20) |
Values expressed as mean±standard deviation (median).
Figure 2. Aqueous humor versus time concentration curve of tigecycline (2 mg) after topical administration according to a keratitis protocol.
The mean tigecycline concentrations in cornea were 3.27± 0.50 µg/g at 60min and 3.17±0.77 µg/g at 120min in group 3. This value was 2.02-fold higher at 60min and 2.03-fold higher at 120min than in the aqueous humor in group 2.
The mean vitreous concentrations of tigecycline were 0.17±0.10 mg/L at 60min and 0.20±0.07 mg/L at 120min in group 3.
Ocular surface adverse effects such as punctate epitheliopathy, conjunctival hyperemia and chemosis were not observed after application of tigecycline in all groups.
DISCUSSION
After topical administration of 1% tigecycline in rabbit eyes, efficient penetration in the aqueous humor in groups 1 and 2 and in the cornea in group 3 was found. These measured values were higher than the MICs for most potential bacteria suggesting that the topical application of tigecycline may be a useful practice for the management of keratitis. In group 3, tigecycline could not achieve enough penetration in the vitreous to reach a value higher than the MIC levels of most causative bacteria related with endophthalmitis.
Severe bacterial keratitis typically requires intensive antimicrobial therapy. Empirical therapy is usually done with a 15min to hourly instillation of topical fluoroquinolone or a fortified aminoglycoside-cephalosporin combination[19]–[20]. Empirical treatment of bacterial keratitis is necessary when awaiting the outcome of culture and sensitivity testing, or where culture facilities are unavailable. The antibiotic regimen chosen should be of a sufficiently broad spectrum to cover likely pathogens while considering bacterial prevalence, antibiotic sensitivities and geographically-specific epidemiological data[21]. Despite the publication of numerous clinical trials, there is no consensus as to which topical antibiotics and which regimen (i.e. monotherapy or combination therapy) provide superior clinical outcomes[22]. Besides fluoroquinolone and fortified aminoglycoside-cephalosporin combinations, vancomycin is another popular topically used fortified antibiotic for treatment of keratitis. However, bacterial ocular infections resistant to vancomycin were reported recently[23]–[26]. One of these studies presented three cases of bacterial keratitis. In one patient, corneal infection secondary to vancomycin-resistant Enterococcus faecalis was unresponsive to topical vancomycin but responded well to topical 0.2% linezolid[23]. The treatment was switched to linezolid because of pain and discomfort attributed to vancomycin in two patients[23]. Since it was found that tigecycline was highly active against vancomycin-resistant enterococcus isolates[11]–[12], it can be used topically with vancomycin.
Tigecycline belongs to the tetracycline antibiotic family. A number of non-antibiotic effects of doxycycline or minocycline were reported in many studies. These include antioxidant[27], antiangiogenetic[28]–[29], wound healing promoter[30], antiapoptotic[31]–[32], neuroprotective[33]–[34], antimetastatic[35], and antiinflammatory effects[31],[36]–[38]. Similarly, recent experimental studies suggested that tigecycline had a modulatory effect on matrix metalloproteinase-9 (MMP-9)[30], as well as anti-inflammatory[39], anti-angiogenic[40], and antiapoptotic[41], activity. In our study, it was found that up to 3.27 µg/g concentration of tigecycline penetrated into the corneal tissue after the keratitis protocol. Based on this finding topical tigecycline can be an alternative to oral doxycycline which has potential systemic side effects, in order to suppress MMP-9 activity in corneal inflammatory disorders such as chemical burns. In our study, we used 1% concentration based on previous studies[42]–[43]. After topical application, adverse reactions such as conjunctival chemosis, hyperemia or corneal epithelium toxicity were not observed in any groups. However, further experimental animal studies are needed to evaluate the optimum dose.
Our study had some limitations. Rabbit eye model were used in our study. Rabbit eyes differ from human eyes in several ways. Firstly the cornea of rabbit is thinner (0.4 vs 0.52 mm) and has large diameter (15 vs 12 mm)[44]–[45]. Additionally, the tear turnover and blink rates are lower in rabbits than humans. These disparities produce higher drug penetrations of drugs into rabbit eyes[44]–[45]. Also, our experiments were performed using healthy animals, and the results could vary significantly in eyes with inflammation.
In conclusion, aqueous humor penetration of tigecycline seems to be well in the in groups 1 and 2 and the cornea penetration in group 3. This value may cover the MICs of many bacteria. Further studies are needed to evaluate the penetration and tolerability of tigecycline in the human eye.
Acknowledgments
Foundation: Supported by Konya Training and Research Hospital (KTRH-32, Konya, Turkey).
Conflicts of Interest: Sakarya Y, None; Sakarya R, None; Ozcimen M, None; Goktas S, None; Ozcimen S, None; Alpfidan I, None; Ivacik IS, None; Erdogan E, None; Cetinkaya S, None; Bukus A, None.
REFERENCES
- 1.Green M, Apel A, Stapleton F. Risk factors and causative organisms in microbial keratitis. Cornea. 2008;27(1):22–27. doi: 10.1097/ICO.0b013e318156caf2. [DOI] [PubMed] [Google Scholar]
- 2.Ly CN, Pham JN, Badenoch PR, Bell SM, Hawkins G, Rafferty DL, McClellan KA. Bacteria commonly isolated from keratitis specimens retain antibiotic susceptibility to fluoroquinolones and gentamicin plus cephalothin. Clin Exp Ophthalmol. 2006;34(1):44–50. doi: 10.1111/j.1442-9071.2006.01143.x. [DOI] [PubMed] [Google Scholar]
- 3.Khera M, Pathengay A, Jindal A, Jalali S, Mathai A, Pappuru RR, Relhan N, Das T, Sharma S, Flynn HW. Vancomycin-resistant Gram-positive bacterial endophthalmitis: epidemiology, treatment options, and outcomes. J Ophthalmic Inflamm Infect. 2013;3(1):46. doi: 10.1186/1869-5760-3-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scoper SV. Review of third- and fourth-generation fluoroquinolones in ophthalmology: in vitro and in vivo efficacy. Adv Ther. 2008;25(10):979–994. doi: 10.1007/s12325-008-0107-x. [DOI] [PubMed] [Google Scholar]
- 5.McDonald M, Blondeau JM. Emerging antibiotic resistance in ocular infections and the role of fluoroquinolones. J Cataract Refract Surg. 2010;36(9):1588–1598. doi: 10.1016/j.jcrs.2010.06.028. [DOI] [PubMed] [Google Scholar]
- 6.Pathengay A, Moreker MR, Puthussery R, Ambatipudi S, Jalali S, Majji AB, Mathai A, Husssain N, Dave V, Sharma S, Das T. Clinical and microbiological review of culture proven endophthalmitis caused by multidrug resistant bacteria in patients seen at tertiary eye care center in southern India. Retina. 2011;31(9):1806–1811. doi: 10.1097/IAE.0b013e31820f4b9d. [DOI] [PubMed] [Google Scholar]
- 7.Das MK, Pathengay A, Shah GY, Koday NK. Vancomycin-resistant coagulase negative Staphylococcus endophthalmitis following cataract surgery. J Cataract Refract Surg. 2011;37(10):1908–1909. doi: 10.1016/j.jcrs.2011.07.023. [DOI] [PubMed] [Google Scholar]
- 8.Zuckerman JM, Qamar F, Bono BR. Macrolides, ketolides, and glycylcyclines: azithromycin, clarithromycin, telithromycin, tigecycline. Infect Dis Clin North Am. 2009;23(4):997–1026. doi: 10.1016/j.idc.2009.06.013. [DOI] [PubMed] [Google Scholar]
- 9.Meagher AK, Ambrose PG, Grasela TH, Ellis-Grosse EJ. The pharmacokinetic and pharmacodynamic profile of tigecycline. Clin Infect Dis. 2005;41:S333–340. doi: 10.1086/431674. Suppl 5. [DOI] [PubMed] [Google Scholar]
- 10.Bergeron J, Ammirati M, Danley D, James L, Norcia M, Retsema J, Strick CA, Su WG, Sutcliffe J, Wondrack L. Glycylcyclines bind to the high-affinity tetracycline ribosomal binding site and evade Tet(M)- and Tet(O)-mediated ribosomal protection. Antimicrob Agents Chemother. 1996;40(9):2226–2228. doi: 10.1128/aac.40.9.2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoban DJ, Bouchillon SK, Johnson BM, Johnson JL, Dowzicky MJ, Tigecycline Evaluation and Surveillance Trial (TEST Program) Group In vitro activity of tigecycline against 6792 Gram-negative and Gram-positive clinical isolates from the global Tigecycline Evaluation and Surveillance Trial (TEST Program, 2004) Diagn Microbiol Infect Dis. 2005;52(3):215–227. doi: 10.1016/j.diagmicrobio.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 12.Bouchillon SK, Hoban DJ, Johnson BM, Johnson JL, Hsiung A, Dowzicky MJ, Tigecycline Evaluation and Surveillance Trial (TEST) Group In vitro activity of tigecycline against 3989 Gram-negative and Gram-positive clinical isolates from the United States Tigecycline Evaluation and Surveillance Trial (TEST Program; 2004) Diagn Microbiol Infect Dis. 2005;52(3):173–179. doi: 10.1016/j.diagmicrobio.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 13.Castanheira M, Sader HS, Deshpande LM, Fritsche TR, Jones RN. Antimicrobial activities of tigecycline and other broad-spectrum antimicrobials tested against serine carbapenemase- and metallo-beta-lactamase-producing Enterobacteriaceae: report from the SENTRY Antimicrobial Surveillance Program. Antimicrob Agents Chemother. 2008;52(2):570–573. doi: 10.1128/AAC.01114-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhanel GG, DeCorby M, Nichol KA, Wierzbowski A, Baudry PJ, Karlowsky JA, Lagacé-Wiens P, Walkty A, Mulvey MR, Hoban DJ, Canadian Antimicrobial Resistance Alliance Antimicrobial susceptibility of 3931 organisms isolated from intensive care units in Canada: Canadian National Intensive Care Unit Study, 2005/2006. Diagn Microbiol Infect Dis. 2008;62(1):67–80. doi: 10.1016/j.diagmicrobio.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 15.Slover CM, Rodvold KA, Danziger LH. Tigecycline: a novel broad-spectrum antimicrobial. Ann Pharmacother. 2007;41(6):965–972. doi: 10.1345/aph.1H543. [DOI] [PubMed] [Google Scholar]
- 16.Product monograph of tigecycline. Available at http://www.pfizer.ca/en/our_products/products/monograph/225.
- 17.Saleh M, Jehl F, Dory A, Lefevre S, Prevost G, Gaucher D, Sauer A, Speeg-Schatz C, Bourcier T. Ocular penetration of topically applied linezolid in a rabbit model. J Cataract Refract Surg. 2010;36(3):488–492. doi: 10.1016/j.jcrs.2009.09.036. [DOI] [PubMed] [Google Scholar]
- 18.Ozcimen M, Sakarya Y, Ozcimen S, Goktas S, Sakarya R, Alpfidan I, Erdogan E. Pharmacokinetics of intravenously administered tigecycline in eye compartments: an experimental study. Graefes Arch Clin Exp Ophthalmol. 2014;252(12):1993–1997. doi: 10.1007/s00417-014-2784-2. [DOI] [PubMed] [Google Scholar]
- 19.American Academy of Ophthalmology Cornea/External Disease Panel . San Francisco, CA: American Academy of Ophthalmology; 2013. Preferred practice pattern guidelines: bacterial keratitis-limited revision. Available at https://www.aao.org/preferred-practice-pattern/bacterial-keratitis-ppp--2013. Accessed on July 11, 2015. [Google Scholar]
- 20.International Council of Ophthalmology . International Council of Ophthalmology; 2007. International clinical guidelines: bacterial keratitis. Accessed on March 12, 2015; Available at http://www.icoph.org/downloads/ICOBactKeratitisMa.pdf. [Google Scholar]
- 21.Shah A, Sachdev A, Coggon D, Hossain P. Geographic variations in microbial keratitis: an analysis of the peer-reviewed literature. Br J Ophthalmol. 2011;95(6):762–767. doi: 10.1136/bjo.2009.169607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McDonald EM, Ram FS, Patel DV, McGhee CN. Topical antibiotics for the management of bacterial keratitis: an evidence-based review of high quality randomised controlled trials. Br J Ophthalmol. 2014;98(11):1470–1477. doi: 10.1136/bjophthalmol-2013-304660. [DOI] [PubMed] [Google Scholar]
- 23.Tu EY, Jain S. Topical linezolid 0.2% for the treatment of vancomycin-resistant or vancomycin-intolerant gram-positive bacterial keratitis. Am J Ophthalmol. 2013;155(6):1095–1098.e1. doi: 10.1016/j.ajo.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 24.Hernandez-Camarena JC, Bautista-de Lucio VM, Navas A, Ramirez-Miranda A, Graue-Hernandez EO. Delayed-onset post-keratoplasty endophthalmitis caused by vancomycin resistant Enterococcus faecium. Case Report Ophthalmol. 2012;3(3):370–374. doi: 10.1159/000344006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bains HS, Weinberg DV, Feder RS, Noskin GA. Postoperative vancomycin-resistant Enterococcus faecium endophthalmitis. Arch Ophthalmol. 2007;125(9):1292–1293. doi: 10.1001/archopht.125.9.1292. [DOI] [PubMed] [Google Scholar]
- 26.Maguen E, Morgan MA. A case of vancomycin-resistant enterococcus conjunctivitis and its clinically successful topical treatment. Cornea. 2007;26(2):223–224. doi: 10.1097/01.ico.0000243957.29898.13. [DOI] [PubMed] [Google Scholar]
- 27.Kraus RL, Pasieczny R, Lariosa-Willingham K, Turner MS, Jiang A, Trauger JW. Antioxidant properties of minocycline: neuroprotection in an oxidative stress assay and direct radical-scavenging activity. J Neurochem. 2005;94(3):819–827. doi: 10.1111/j.1471-4159.2005.03219.x. [DOI] [PubMed] [Google Scholar]
- 28.Samtani S, Amaral J, Campos MM, Fariss RN, Becerra SP. Doxycycline-mediated inhibition of choroidal neovascularization. Invest Ophthalmol Vis Sci. 2009;50(11):5098–5106. doi: 10.1167/iovs.08-3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cox CA, Amaral J, Salloum R, Guedez L, Reid TW, Jaworski C, John-Aryankalayil M, Freedman KA, Campos MM, Martinez A, Becerra SP, Carper DA. Doxycycline's effect on ocular angiogenesis: an in vivo analysis. Ophthalmology. 2010;117(9):1782–1791. doi: 10.1016/j.ophtha.2010.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simonetti O, Cirioni O, Lucarini G, Orlando F, Ghiselli R, Silvestri C, Brescini L, Rocchi M, Provinciali M, Guerrieri M, Di Primio R, Giacometti A, Offidani A. Tigecycline accelerates staphylococcal-infected burn wound healing through matrix metalloproteinase-9 modulation. J Antimicrob Chemother. 2012;67(1):191–201. doi: 10.1093/jac/dkr440. [DOI] [PubMed] [Google Scholar]
- 31.Kelly KJ, Sutton TA, Weathered N, Ray N, Caldwell EJ, Plotkin Z, Dagher PC. Minocycline inhibits apoptosis and inflammation in a rat model of ischemic renal injury. Am J Physiol Renal Physiol. 2004;287(4):F760–766. doi: 10.1152/ajprenal.00050.2004. [DOI] [PubMed] [Google Scholar]
- 32.Krady JK, Basu A, Allen CM, Xu Y, LaNoue KF, Gardner TW, Levison SW. Minocycline reduces proinflammatory cytokine expression, microglial activation, and caspase-3 activation in a rodent model of diabetic retinopathy. Diabetes. 2005;54(5):1559–1565. doi: 10.2337/diabetes.54.5.1559. [DOI] [PubMed] [Google Scholar]
- 33.Levkovitch-Verbin H, Kalev-Landoy M, Habot-Wilner Z, Melamed S. Minocycline delays death of retinal ganglion cells in experimental glaucoma and after optic nerve transection. Arch Ophthalmol. 2006;124(4):520–526. doi: 10.1001/archopht.124.4.520. [DOI] [PubMed] [Google Scholar]
- 34.Zhang C, Lei B, Lam TT, Yang F, Sinha D, Tso MO. Neuroprotection of photoreceptors by minocycline in light-induced retinal degeneration. Invest Ophthalmol Vis Sci. 2004;45(8):2753–1759. doi: 10.1167/iovs.03-1344. [DOI] [PubMed] [Google Scholar]
- 35.Saikali Z, Singh G. Doxycycline and other tetracyclines in the treatment of bone metastasis. Anticancer Drugs. 2003;14(10):773–778. doi: 10.1097/00001813-200311000-00001. [DOI] [PubMed] [Google Scholar]
- 36.Yrjänheikki J, Tikka T, Keinänen R, Goldsteins G, Chan PH, Koistinaho J. A tetracycline derivative, minocycline, reduces inflammation and protects against focal cerebral ischemia with a wide therapeutic window. Proc Natl Acad Sci USA. 1999;96(23):13496–13500. doi: 10.1073/pnas.96.23.13496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yrjänheikki J, Keinänen R, Pellikka M, Hökfelt T, Koistinaho J. Tetracyclines inhibit microglial activation and are neuroprotective in global brain ischemia. Proc Natl Acad Sci USA. 1998;95(26):15769–15774. doi: 10.1073/pnas.95.26.15769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Amin AR, Attur MG, Thakker GD, Patel PD, Vyas PR, Patel RN, Patel IR, Abramson SB. A novel mechanism of action of tetracyclines: effects on nitric oxide synthases. Proc Natl Acad Sci USA. 1996;93(24):14014–14019. doi: 10.1073/pnas.93.24.14014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dunston CR, Griffiths HR, Lambert PA, Staddon S, Vernallis AB. Proteomic analysis of the anti-inflammatory action of minocycline. Proteomics. 2011;11(1):42–51. doi: 10.1002/pmic.201000273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goktas S, Erdogan E, Sakarya R, Sakarya Y, Yılmaz M, Ozcimen M, Unlukal N, Alpfidan I, Tas F, Erdogan E, Bukus A, Ivacık IS. Inhibition of corneal neovascularization by topical and subconjunctival tigecycline. J Ophthalmol. 2014;2014:452685. doi: 10.1155/2014/452685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yagnik RM, Benzeroual KE. Tigecycline prevents LPS-induced release of pro-inflammatory and apoptotic mediators in neuronal cells. Toxicol In Vitro. 2013;27(2):686–693. doi: 10.1016/j.tiv.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 42.Goktas S, Kurtoglu MG, Sakarya Y, Ugurluoglu C, Ozcimen M, Sakarya R, Alpfidan I, Ivacık IS, Erdogan E, Bukus A. New therapy option for treatment of methicillin-resistant staphylococcus aureus keratitis: tigecycline. J Ocul Pharmacol Ther. 2015;31(2):122–127. doi: 10.1089/jop.2014.0052. [DOI] [PubMed] [Google Scholar]
- 43.Romanowski EG, Yates KA, O'Connor KE, et al. Seattle, Washington, USA: ARVO 2013 Annual Meeting; The comparison of topical RPX-978 (an Ophthalmic Formulation of Tigecycline) to topical vancomycin in a MRSA rabbit keratitis model. http://iovs.arvojournals.org/article.aspx?articleid=2149139. Accessed on July 11, 2015. [Google Scholar]
- 44.Owen GR, Brooks AC, James O, Robertson SM. A novel in vivo rabbit model that mimics human dosing to determine the distribution of antibiotics in ocular tissues. J Ocul Pharmacol Ther. 2007;23(4):335–342. doi: 10.1089/jop.2006.0123. [DOI] [PubMed] [Google Scholar]
- 45.Proksch JW, Granvil CP, Siou-Mermet R, Comstock TL, Paterno MR, Ward KW. Ocular pharmacokinetics of besifloxacin following topical administration to rabbits, monkeys, and humans. J Ocul Pharmacol Ther. 2009;25(4):335–344. doi: 10.1089/jop.2008.0116. [DOI] [PubMed] [Google Scholar]