Abstract
AIM
To investigate the effect of activating transcription factor-3 (ATF3)-deletion on apoptosis of cultured retinal ganglion cells (RGCs).
METHODS
Three ATF3 siRNA (ATF3-rat-651, ATF3-rat-319, ATF3-rat-520) were constructed, and were transiently transfected into RGC-5 cells. Quantitative real-time polymerase chain reaction (PCR) was used to examine ATF3 expression and the most effective ATF3 siRNA was selected for further studies. Flow cytometry was applied to investigate the effects of ATF3 deletion on RGC-5 apoptosis under elevated hydrostatic pressure. Quantitative real-time PCR and Western blot were performed to validate differentially expressed genes and proteins in ATF3-knockdown RGC-5 cells.
RESULTS
ATF3 specific siRNA effectively down-regulated ATF3 expression and significantly inhibited cell apoptosis in RGC-5 cells. Quantitative real-time PCR and Western blot confirmed that ATF3 knockdown remarkably decreased Jun-B and increased c-Jun at both mRNA and protein levels in RGC-5 cells.
CONCLUSION
ATF/cAMP-response element-binding family of transcription factors may be involved in the development of glaucoma and could be novel treatment targets for glaucoma.
Keywords: ATF3, elevated hydrostatic pressure, apoptosis, glaucoma
INTRODUCTION
It's known to all that the first leading cause of irreversible blindness and the second leading cause of blindness and is glaucoma, which affects approximately 70 million people in the world[1]–[2]. It is commonly agreed that the most significant risk factor for enhanced retinal ganglion cells (RGCs) death in glaucoma is the high intraocular pressure (HIOP)[2]–[3]. It has been reported that both in rats and in glaucoma patients the elevated intraocular pressure (IOP) increased apoptosis rate of RGCs[4]–[6]. The appearance of structural damage at the optic nerve head is typically following the elevated IOP, including cupping of the optic disc, neuroretinal rim thinning and sectoral retinal nerve fiber layer (RNFL) thinning, indicating the pathological progression of the disease within the retina as a whole. However, the role of increased IOP in cellular damage in RGCs is still unknown.
Pressure is vital for cell integrity and cellular functions. Aberrant pressures beyond physiological limits can cause disease. For example, glaucoma, a common ocular disease, is frequently and closely associated with raised IOP[7]. One of the characteristics of glaucoma and the main factor leading to visual functional damage is HIOP[6]. In addition, it is reported that the elevated IOP in acute glaucoma is positively related with apoptosis of the neurons. However, little is known about the relationship between apoptosis and IOP in RGCs[8].
Mounting evidence implied that the gene of activating transcription factor-3 (ATF3) is an immediate early gene induced by extensive stress signals in various cell types, encoding a member of the ATF/cAMP-response element-binding protein family of transcription factors. In quiescent cells, ATF3 has a low expression level, but is increased in the presence of chemical toxin, physiological stresses as well as cellular signals, including cytokines, chemokines, growth factors/hormones[9]–[11]. Therefore, ATF3 has been described as an adaptive response gene that is implicated in a variety of cellular processes. Moreover, growing data implicated ATF3 as an important regulator of apoptosis and cell proliferation. Consistently, including c-Jun and Jun-B, which are known as immediate early genes, are often induced in the microarray analysis as the same cluster as ATF3[12]–[14].
Interestingly, ATF3 has been recently found to be upregulated in a rat glaucoma model of RGC layer[15]. Considering the above data, we wonder if ATF3 is involved in apoptosis of RGC with HIOP. Our purpose of this study was to find the alteration of ATF3 expression in RGCs and to explore the underlying cellular and molecular mechanisms. We hope that the results will lead to a better understanding of the cellular mechanism of early apoptosis of RGC and add to our understanding of glaucomatous injury and provide novel therapeutic strategies for glucoma.
SUBJECTS AND METHODS
Cell Culture and Reagents
RGC-5, the rat RGC line, which was cultured in Dulbecco's modified Eagle's medium containing 10% fetal calf serum, supplemented with 100 µg/mL streptomycin and 100 U/mL penicillin (Sigma-Aldrich, St. Louis, MO, USA) in humidified incubator with 5% CO2 at 374°C, The cell density was around 80% when exposed to elevated hydrostatic pressure.
Pressure System
A pressurized incubator was designed to treat the cells to elevated hydrostatic pressure as previously described[16]. The pressure chamber was connected via a regulator to a source of 5% CO2/95% oxygen air. The arrangement provided constant hydrostatic pressure within ±1 mm Hg ranging from 0 to 100 mm Hg. Gas to the chamber was warmed and humidified by bubbling through water. Both the water flask and the pressure chamber were maintained at 374°C by placing them inside an electronically controlled conventional incubator. The advantages of our device include easily and accurately control of the gas flow and the pressure using the flow meter and the regulator. To examine the effect elevated hydrostatic pressure on cellular processes, every pressure was maintained for 24h. Control RGC-5 cells were incubated simultaneously in 5% CO2 at 374°C.
Transient Transfection
For transient transfection experiments, cells were seeded in 6-well culture dishes, 24h later, cells were transfected with 100 pmol/L small interfering RNA (siRNA) specific for rat ATF3 or non-target siRNA using lipofectamine 2000 (lipo2000) according to the manufacturer's protocol. After 48h, cells were subjected to 20, 40 and 70 mm Hg hydrostatic pressure for 24h, respectively. After that, cells were prepared for real-time polymerase chain reaction (PCR) and cell apoptosis analysis.
Flow Cytometry
Cell apoptosis was assessed with flow cytometry using an Annexin V FITC/PI double staining kit. Cells were treated with elevated hydrostatic pressure for 24h, washed twice in phosphate buffer solution, and then resuspended in binding buffer (10 mmol/L HEPES/ NaOH pH 7.4, 140 mmol/L NaCl, 2.5 mmol/L CaCl2. Fluorescein isothiocyanate-conjugated annexin V (Annexin V-FITC) solution and propidium iodide solution were added in cell suspension. The cells were incubated for 15min at room temperature in the dark, and then washed with binding buffer and analyzed with flow cytometry using Cell Quest software. Experiments were repeated at least 3 times (n≥3).
Real-time Polymerase Chain Reaction
After application of elevated hydrostatic pressure, the cells were harvested and quantitative real-time PCR was performed. Total RNA was extracted with TriZol reagent and cDNA was generated using a PrimeScript RT reagent kit according to manufacturer's instructions from 1 µg of total RNA. Quantitative real-time PCR was performed with SYBR Premix ExTaq II in a real-time 7500 detection system. GAPDH was used as a positive control. The primer sequences (forward/reverse) were as follows: GAPDH, 5′-TGCCACTCAGAAGACTGTGGATG-3′/5′-GCCTGCTTCACCACCTTCTTGAT-3′; ATF-3, 5′-GCGAAGACTGGAGCAAAATG3′/5′- AGGTGTCAGGTT AGCAAAATCC-3′. The PCR program was set as follows: 954°C for 30s, followed by 40 cycles of a 954°C denaturation for 5s, 604°C for 34s, and 954°C extension for 15s, 604°C for 30s, 954°C for 15s. A standard curve for each gene was generated from serial dilutions of PCR products to monitor amplification efficiency and to relatively quantify mRNA abundance. The ΔCt values were calculated by Ctgene-CtGAPDH. The relative expression level of each gene was expressed as a fold change of 2−ΔΔCt. Each experiment was carried out three times.
Protein Isolation and Western Blot Analysis
About 120 µL cell lysis buffer [60 mmol/L of Tris, 2% sodium dodecyl sulfate (SDS), 100 mmol/L of 2-mercaptoethanol, and 0.01% bromophenol blue] was added to each cell samples. An equal amount of protein (30 µg) was separated on a 12% SDS-PAGE gels running at a constant 115 V for 120min, and transferred onto PVDF (0.45 mm pore) membranes (Invitrogen) running at a constant 100 V for 70min. After blocking, the membranes were incubated with primary antibodies overnight at 4°C. The antibodies were diluted as follows: β-actin (1:500, Abcam, Cambridge, MA, USA), ATF3 (1:1000, Abcam, Cambridge, MA, USA), Jun-B (1:1000, Abcam, Cambridge, MA, USA) ATF3 (1:1000, Abcam, Cambridge, MA, USA), and c-Jun ATF3 (1:1000, Abcam, Cambridge, MA, USA). Horseradish peroxidase-conjugated anti-rabbit IgG secondary antibody was incubated at room temperature for 2h at a 1:5000 dilution. An enhanced chemiluminescence kit (Amersham Bioscience, Piscataway, NJ, USA) was used to detect blotting signals following the manufacturer's instructions. β-actin in each sample was measured as an internal control. Gel-Pro Analyzer software (Media Cybernetics) was used to analyze the data. Experiments were repeated 3 times per protein per treatment group (n=3).
Statistic Analysis
SPSS 17.0 was used for statistical analyses. All experiments were performed at least in triplicate. The data are presented as the mean±SD. Comparison of two experimental conditions was evaluated using the unpaired student's t-test. Comparison of three experimental conditions was evaluated using one-way ANOVA and the Bonferroni t-test. P<0.05 was considered to be statistically significant.
RESULTS
Optimum Dosage of siRNA and Lipo2000 for the Most Effective Activating Transcription Factor-3 Knockdown
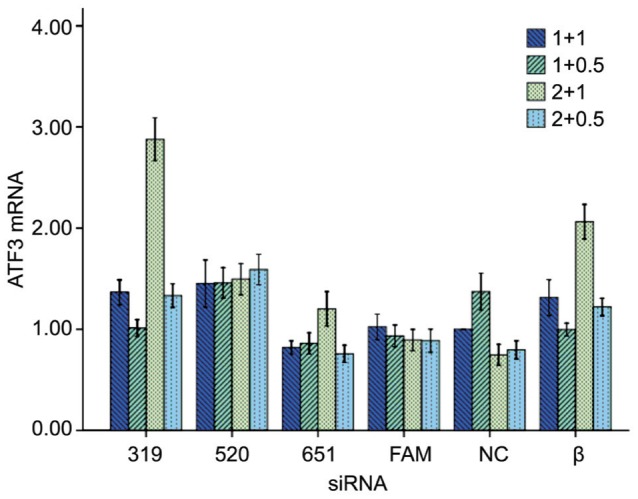
In order to inhibit ATF3 expression, we designed three sequences of ATF3-specific siRNA including ATF3-rat-319 (siRNA 319), ATF3-rat-520 (siRNA 520), ATF3-rat-651 (siRNA 651). At the same time, we also designed positive control group rat-Actb-324 (β), negative control group (NC) and negative control fluorescent tags group (FAM). Then we transfected these siRNAs with transfection agent lipo2000 with different doses. We perfromed real-time PCR to choose the optimum dosage of siRNA and lipo2000 and found that different amount of siRNA and lipo2000 resulted in different ATF3 mRNA expression except siRNA 520. The optimum doses of siRNA and lipo2000 are respectively 1 µL siRNA + 0.5 µL lipo2000 for siRNA 319, 2 µL siRNA + 0.5 µL lipo2000 for siRNA 651, 2 µL siRNA + 1 µL lipo2000 for NC, 1 µL siRNA + 0.5 µL lipo2000 for siRNA β (Figure 1).
Figure 1. The optimum dosage of the siRNA and lipo2000 were used in the following experiments.
Selection of the Most Effective siRNA
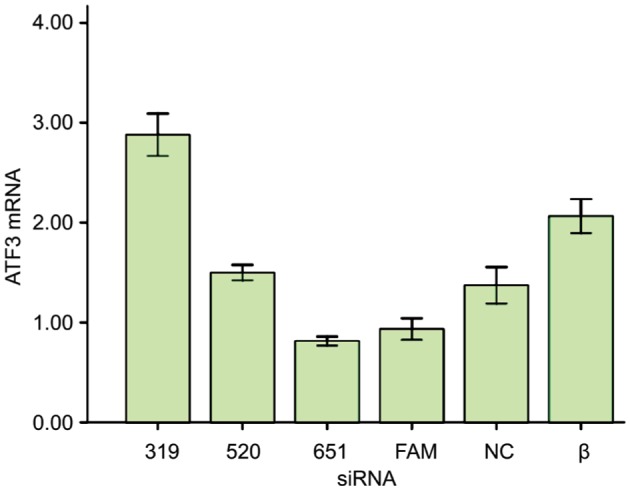
According to the data obtained above, siRNA 319, siRNA 520, siRNA 651, FAM, NC and siRNA β were transiently transfected into RGC-5 cells. The interfering effectiveness of ATF3 siRNA in RGC-5 cells was assayed by quantitative real-time PCR. As shown in Figure 2, ATF3 gene expression in siRNA 651-transfected RGC-5 cells showed a significant decrease compared with other groups (P<0.01, n=3). Therefore, we used siRNA 651 for ATF3 knockdown in the following experiments.
Figure 2. The ATF3 RNA expression of different siRNAs.
Suppression of Activating Transcription Factor-3 depletion on Apoptosis of Retinal Ganglion Cells
Flow cytometry was used to identify cell apoptosis in this study. In RGC-5 cells transfected with negative control siRNA, the percentages of apototic cells are respectively 11.17%±2.77% (0 mm Hg), 15.44%±4.62% (20 mm Hg), 19.13%±4.75% (40 mm Hg) and 46.53%±10.84% (70 mm Hg) under different pressure exposure. In contrast, the apototic percentages of cells transfected with ATF3 specific siRNA are 3.33%±0.81% (20 mm Hg), 7.10%±3.25% (40 mm Hg) and 30.30%±4.80% (70 mm Hg) under different pressure exposure, respectively. There was a significant decrease of apoptotic rates in ATF3 depleted RGC-5 cells compared with the control cells (P<0.01, n=3) (Figure 3). These results suggested that downregulation of ATF3 suppressed apoptosis of RGC-5 cells.
Figure 3. The ATF3 RNA express of siRNA 651 in different pressures.

Efficiency of Activating Transcription Factor-3 Specific siRNA in Retinal Ganglion Cells on Varied Hydrostatic Pressure
In RGC-5 cells without siRNA transfection, ATF3 mRNA expression was increased with elevated pressure exposure. After siRNA 651 transfection, ATF3 mRNA expression was reduced in the 20 and 40 mm Hg pressure groups, but was increased in the 70 mm Hg pressure group (Figure 3). These data indicated that siRNA 651 could inhibit ATF3 expression under 20 and 40 mm Hg pressure but not 70 mm Hg.
Effect of Activating Transcription Factor-3 Depletion on mRNA Expression of Jun-B and c-Jun by Elevated Hydrostatic Pressure
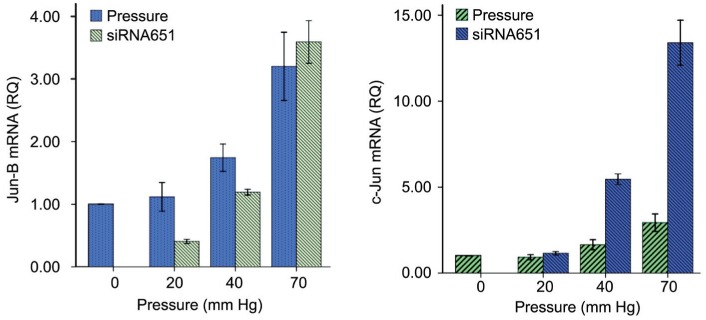
In RGC-5 cells transfected with negative control siRNA, the mRNA expression of Jun-B increased remarkbly in cells exposed to elevated pressure compared to cells in non-pressurized control group (0 vs 40 vs 70 mm Hg). However, the increased mRNA expression of Jun-B induced by elevated pressure was attenuated by ATF3 depletion. As shown in Figure 5, in RGC-5 cells transfected with ATF3 specific siRNA, Jun-B mRNA expression remarkably decreased in cells exposed to elevated pressure of 20 and 40 mm Hg, but not in cells treated with pressure of 70 mm Hg. As expected, ATF3 depletion significantly increased c-Jun mRNA expression in RGC-5 cells exposed to elevated pressure compared to cells treated with non-specific siRNA, especially under 40 and 70 mm Hg (Figure 4).
Figure 5. The protein expression of Jun-B and c-Jun in RGC-5 with siRNA in different pressures.
Figure 4. The mRNA expression of Jun-B and c-Jun with siRNA in different pressures.
Effect of Activating Transcription Factor-3 depletion on protein expression of Jun-B and c-Jun in Retinal Ganglion Cells to Elevated Pressure
We further examined the effects of ATF3 depletion on RGC-5 cells treated with elevated pressure of 40 mm Hg. As shown in Figure 5, the expression of Jun-B protein significantly decreased in 40 mm Hg compared with that in 0 mm Hg. However, Jun-B protein level increased after ATF3 siRNA interference (P<0.01). There was no evident difference in the expression of c-Jun protein between 40 and 0 mm Hg (P=0.990, but after siRNA651 transfection, its expression level increased significantly (P<0.01).
DISCUSSION
The RGC-5 cell line is a transformed RGC line exhibiting some characteristics of RGCs, expressing Brn-3C, Thy-1, NMDA receptor, neuritin, synaptophysin, as well as GABA-B receptor, without expression of glial fibrillary acidic protein, HPC-1, nor 8A-1[17]–[22]. RGC-5 cell line is widely used in the research of glaucoma.
HIOP is defined as one of the characteristics of glaucoma and mounting studies have confirmed that continuous rise of IOP could cause the death of RGCs and retinal pigment epithelium cells, and finally result in blindness[23]–[24]. Additionally, elevated IOP in acute glaucoma is positively related with apoptosis of RGCs.
ATF3 is an immediate early gene induced by extensive stress signals including elevated pressure, and is implicated as a critical regulator of cell proliferation and apoptosis at least partly through regulation of the AP-1 family members. Interestingly, ATF3 has been recently found to be upregulated in RGC layer of a rat glaucoma model[21].
Thus, in the present study, we treated RGC-5 cells with elevated pressure for 24h to model glaucoma in vitro, and explored the effects of ATF3 depletion on apoptosis induced by elevated pressure. Furthermore, we screened mRNA and protein expressions of two important AP-1 members, Jun-B and c-Jun, which are potential ATF3 regulated genes. As expected, RGCs did not express appreciable levels of ATF3, c-Jun or Jun-B at ambient pressure, but c-Jun protein is induced by elevated pressure in RGCs and Jun-B protein is induced in the same temporal pattern. As indicated in the previous studies that demonstrate a role for disease-related activation of c-Jun in vivo models of glaucoma[10],[25].
According to our results, we found that different dosage of siRNA and transfection agent would lead to different transfection effeciency even in the same cell line. The transfecion effeciency depends on the ratio of siRNA and transfection agent (2:1) to a great extent instead of the greater dosage. Among three ATF3-specific siRNAs, siRNA 651 showed the highest interference effeciency in RGC-5 cells. Flow cytometry assay found that the proportion of apoptotic cells decreased obviously as ATF3 expression reduced under the same pressure, demonstrating ATF3 played an important role in pressure-induced apoptosis in RGC-5 cells. In our study, we put our focus on the role of ATF3 in the early apoptosis. In addition, previous research confirmed that ATF3 was the most obvious factor for early damage in early glaucoma animal models using microarray analysis. Therefore, based on our results and previous data, we conclude that ATF3 may play an important role in the early damage in glaucoma.
Flow cytometry assay showed ATF3 siRNA can protect RGC-5 cells from the early apoptosis under pressure intervention, but the role of ATF3 depletion depended on the pressure level. Under small pressure, for example 20 and 40 mm Hg, ATF3 depletion can effectively reduce the apoptosis rate and protect cells from damage of pressure. Meanwhile, under higher pressure, for example 70 mm Hg, ATF3 depletion only slightly ameliorated pressure induced apoptosis. The results indicated that ATF3 efficiently attenuated small pressure induced apoptosis, but to a less extent in cells exposed to high pressure. Furthermore, we performed PCR analysis to assess the efficiency of siRNA mediated ATF3 depletion under various levels of pressure and to screen for apoptosis related mRNAs regulated by ATF3. We found ATF3 was greatly down-regulated in cells exposed to pressure of 20 and 40 mm Hg, but not under pressure of 70 mm Hg. In consistent with previous studies, we found two members of AP-1 family, Jun-B and c-Jun, were regulated by ATF3 depletion in RGC-5 cells exposed to 20 and 40 mm Hg pressure. The mRNA expression of Jun-B greatly decreased while the levels of c-Jun increased in ATF3 depleted cells. Considering the AP-1 family members as vital regulators of apoptosis, the results implicated that ATF3 depletion protected RGC-5 cells from elevated pressured induced apoptosis through Jun-B and c-Jun. At the same time, in further experiments, real-time PCR showed ATF3 and Jun-B mRNA expression were reduced by ATF3 depletion in the 20 and 40 mm Hg pressure groups, but not in the 70 mm Hg pressure group. In contrast, the mRNA expression of c-Jun increased. Compared with the detection of early apoptosis, we suggest ATF3 and Jun-B mediated RGC-5 apoptosis which was caused by pressure. The c-Jun played a protective role in the early apoptosis caused by stress in RGC-5 cells. In this study, the change trend of ATF3 was different from c-Jun, the reason might be that ATF3 siRNA depleted ATF3 expression, and c-Jun form different source dimers ATF3 decrease, so the c-Jun mRNA and protein were increased.
Acknowledgments
We thank Dr Ling-Ling Wu for technical advice; Dong-Xia Fan, Xiao-Jie Chen and Xin-Zhu Jia for correcting English usage on this manuscript.
Conflicts of Interest: Sun MM, None; Wang YC, None; Li Y, None; Guo XD, None; Chen YM, None; Zhang ZZ, None.
REFERENCES
- 1.Zhuo YH, Wei YT, Bai YJ, Duan S, Lin MK, Saragovi HU, Ge J. Pro370Leu MYOC gene mutation in a large Chinese family with juvenile-onset open angle glaucoma: correlation between genotype and phenotype. Mol Vis. 2008;14:1533–1539. [PMC free article] [PubMed] [Google Scholar]
- 2.Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90(3):262–267. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guo L, Moss SE, Alexander RA, Ali RR, Fitzke FW, Cordeiro MF. Retinal ganglion cell apoptosis in glaucoma is related to intraocular pressure and IOP-induced effects on extracellular matrix. Invest Ophthalmol Vis Sci. 2005;46(1):175–182. doi: 10.1167/iovs.04-0832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agar A, Li S, Agarwal N, Coroneo MT, Hill MA. Retinal ganglion cell line apoptosis induced by hydrostatic pressure. Brain Res. 2006;1086(1):191–200. doi: 10.1016/j.brainres.2006.02.061. [DOI] [PubMed] [Google Scholar]
- 5.Nakazawa T, Nakazawa C, Matsubara A, Noda K, Hisatomi T, She H, Michaud N, Hafezi-Moghadam A, Miller JW, Benowitz LI. Tumor necrosis factor-alpha mediates oligodendrocyte death and delayed retinal ganglion cell loss in a mouse model of glaucoma. J Neurosci. 2006;26(49):12633–12641. doi: 10.1523/JNEUROSCI.2801-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miglior S, Bertuzzi F. Relationship between intraocular pressure and glaucoma onset and progression. Curr Opin Pharmacol. 2013;13(1):32–35. doi: 10.1016/j.coph.2012.09.014. [DOI] [PubMed] [Google Scholar]
- 7.Huang C, Cen LP, Liu L, Leaver SG, Harvey AR, Cui Q, Pang CP, Zhang M. Adeno-associated virus-mediated expression of growth-associated protein-43 aggravates retinal ganglion cell death in experimental chronic glaucomatous injury. Mol Vis. 2013;19:1422–1432. [PMC free article] [PubMed] [Google Scholar]
- 8.Shang L, Huang JF, Ding W, Chen S, Xue LX, Ma RF, Xiong K. Calpain: a molecule to induce AIF-mediated necroptosis in RGC-5 following elevated hydrostatic pressure. BMC Neurosci. 2014;15:63. doi: 10.1186/1471-2202-15-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thompson MR, Xu D, Williams BR. ATF3 transcription factor and its emerging roles in immunity and cancer. J Mol Med. 2009;87(11):1053–1060. doi: 10.1007/s00109-009-0520-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou ZJ, McCall MA. Retinal ganglion cells in model organisms: development, function and disease. J Physiol. 2008;586(18):4343–4345. doi: 10.1113/jphysiol.2008.160838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hai T, Wolford CC, Chang YS. ATF3, a hub of the cellular adaptive-response network, in the pathogenesis of diseases: is modulation of inflammation a unifying component? Gene Expr. 2010;15(1):1–11. doi: 10.3727/105221610x12819686555015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu D, Chen J, Hai T. The regulation of ATF3 gene expression by mitogen-activated protein kinases. Biochem J. 2007;401(2):559–567. doi: 10.1042/BJ20061081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tan SZ, Sampat K, Rasool S, Nolan D. Unilateral acute angle closure glaucoma. BMJ Case Rep. 2013;2013(pii):bcr2012007836. doi: 10.1136/bcr-2012-007836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miyazaki K, Inoue S, Yamada K, Watanabe M, Liu Q, Watanabe T, Adachi MT, Tanaka Y, Kitajima S. Differential usage of alternate promoters of the human stress response gene ATF3 in stress response and cancer cells. Nucleic Acids Res. 2009;37(5):1438–1451. doi: 10.1093/nar/gkn1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo Y, Johnson EC, Cepurna WO, Dyck JA, Doser T, Morrison JC. Early gene expression changes in the retinal ganglion cell layer of a rat glaucoma model. Invest Ophthalmol Vis Sci. 2011;52(3):1460–1473. doi: 10.1167/iovs.10-5930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ju WK, Kim KY, Lindsey JD, Angert M, Patel A, Scott RT, Liu Q, Crowston JG, Ellisman MH, Perkins GA, Weinreb RN. Elevated hydrostatic pressure triggers release of OPA1 and cytochrome C, and induces apoptotic cell death in differentiated RGC-5 cells. Mol Vis. 2009;15:120–134. [PMC free article] [PubMed] [Google Scholar]
- 17.Surgucheva I, Weisman AD, Goldberg JL, Shnyra A, Surguchov A. Gamma-synuclein as a marker of retinal ganglion cells. Mol Vis. 2008;14:1540–1548. [PMC free article] [PubMed] [Google Scholar]
- 18.Xiang M, Zhou L, Macke JP, Yoshioka T, Hendry SH, Eddy RL, Shows TB, Nathans J. The Brn-3 family of POU-domain factors: primary structure, binding specificity, and expression in subsets of retinal ganglion cells and somatosensory neurons. J Neurosci. 1995;15(7 Pt 1):4762–4785. doi: 10.1523/JNEUROSCI.15-07-04762.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang W, Fileta J, Guo Y, Grosskreutz CL. Downregulation of Thy1 in retinal ganglion cells in experimental glaucoma. Curr Eye Res. 2006;31(3):265–271. doi: 10.1080/02713680500545671. [DOI] [PubMed] [Google Scholar]
- 20.Liu CJ, Chaturvedi N, Barnstable CJ, Dreyer EB. Retinal Thy-1 expression during development. Invest Ophthalmol Vis Sci. 1996;37(7):1469–1473. [PubMed] [Google Scholar]
- 21.Gan L, Wang SW, Huang Z, Klein WH. POU domain factor Brn-3b is essential for retinal ganglion cell differentiation and survival but not for initial cell fate specification. Dev Biol. 1999;210(2):469–480. doi: 10.1006/dbio.1999.9280. [DOI] [PubMed] [Google Scholar]
- 22.Wang SW, Gan L, Martin SE, Klein WH. Abnormal polarization and axon outgrowth in retinal ganglion cells lacking the POU-domain transcription factor Brn-3b. Mol Cell Neurosci. 2000;16(2):141–156. doi: 10.1006/mcne.2000.0860. [DOI] [PubMed] [Google Scholar]
- 23.Le PV, Tan O, Chopra V, Francis BA, Ragab O, Varma R. Regional correlation among ganglion cell complex, nerve fiber layer, and visual field loss in glaucoma. Invest Ophthalmol Vis Sci. 2013;54(6):4287–4295. doi: 10.1167/iovs.12-11388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Banitt MR, Ventura LM, Feuer WJ, Savatovsky E, Luna G, Shif O, Bosse B, Porciatti V. Progressive loss of retinal ganglion cell function precedes structural loss by several years in glaucoma suspects. Invest Ophthalmol Vis Sci. 2013;54(3):2346–2352. doi: 10.1167/iovs.12-11026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levkovitch-Verbin H, Quigley HA, Martin KR, Harizman N, Valenta DF, Pease ME, Melamed S. The transcription factor c-jun is activated in retinal ganglion cells in experimental rat glaucoma. Exp Eye Res. 2005;80(5):663–670. doi: 10.1016/j.exer.2004.11.016. [DOI] [PubMed] [Google Scholar]