Abstract
AIM
To test whether homocysteine (Hcy) can influence the transcriptional profile, we hypothesized that Hcy can lead to the induction of proinflammatory molecules in the retinal cells of aging people.
METHODS
An unbiased in vitro inflammatory pathway focused study was designed employing retinal pigment epithelial (RPE) cell line, ARPE-19. Cells were cultured in the presence or absence of Hcy to capture target genes' expression profile. Three different concentrations of Hcy were added in the culture medium of confluent monolayers. cRNAs were made from the isolated total RNAs and the labeled cRNA probes were hybridized to microarrays specific for human disease pathway inflammatory cytokines, chemokines and their receptor gene micro-array panels as per manufacture's recommendations. Two Hcy up-regulated molecules: IL6 and CEBPB were further validated via Western blot analysis. Hcy's effect on ARPE-19 cellular morphology and genomic DNA integrity were also evaluated.
RESULTS
Gene microarray analyses of RPE cells in response to Hcy treatment revealed alterations in the expressions of several inflammatory gene transcripts such as CCL5, CEBPB, IL13RA2, IL15RA, IL6, IL8 and CXCL3 that were up-regulated. The transcripts for C3, CCL2, IL11RA and IL18 genes exhibited down-regulation. The IL6 and CEBPB expressions were subsequently validated at the protein levels. Treatment of the retinal cells with increasing Hcy concentration influenced their density in culture however their morphology and DNA integrity remained unaffected.
CONCLUSION
These findings suggest that Hcy can potentially mediate the expression of chemokines, cytokines and interleukins receptors in the retinal cells without having any debilitating effects on their morphology and the genomic DNA integrity.
Keywords: chemokine, choroidal neovascularization, cytokine, gene expression, hyperhomocysteinemia, inflammation, macular degeneration, retinal remodeling
INTRODUCTION
Age-related macular degeneration (AMD) is a leading cause of vision loss in the aging population worldwide. Degradation of the retinal pigment epithelium (RPE) plays a key role in the pathology of AMD. Despite recent advances, the underlying etiology of the disease is still poorly understood, which makes disease-altering or curative treatments challenging. Generally, the RPE degenerative changes start with drusen formation[1]. Drusens accumulate in part due to inflammatory immune reactions. Homocysteine (Hcy) and related sulphur-containing metabolites have been shown to exert neurotoxicity via their excitotoxic actions and oxidative stress[2] and hyperhomocysteinemia (HHcy) has been linked with various retinal neuro-vasculopathies both in animal models and humans[3]–[9]. HHcy is a risk factor for ocular diseases including AMD[10] and retinal cells induced inflammatory cytokines and chemokines can promote pathologic changes in AMD[11]. Chemotactic cytokines (chemokines) and their family members can work as dynamic organizers when induced, thereby regulating leucocyte migration and distribution during immune surveillance and inflammation. This process forms gradients that provide crucial directional cues towards the sites of injury or inflammation[12]. We therefore designed an in vitro study employing human ARPE-19 cells to test our hypothesis that Hcy can modulate transcriptional changes of inflammatory cytokines and chemokines and in turn potentially contribute in the pathogenesis of AMD process. Despite a significant progress in retinal biology, the mechanisms that lead to AMD remain unclear. Also, being a complex disease in its causation and progression, little is known about the transcriptional regulation of various inflammatory molecules in the retina of affected patients. Many theories have been put forward for mechanisms correlating with patient's age, metabolism, genetics, environmental factors, and inflammation[13]. With the advent of high-throughput methods, such as whole genome sequencing (WGS), RNA sequencing (RNA Seq) and gene expression microarrays (GEArray), there is an unprecedented opportunity to facilitate our understanding of the pathophysiological changes in the retina, RPE, and choroid. The information gained can be used to identify disease mechanisms in AMD and other related eye conditions.
Our laboratory has been elucidating the role of Hcy mediated pathologies in various human diseases for the last many years. Hcy has been reported to be elevated in plasma of patients with AMD. Earlier studies in a mouse model of HHcy, in which cystathionine-β-synthase (CBS) was deficient, revealed abnormal RPE cell morphology with features like that of AMD upon optical coherence tomography, fluorescein angiography, histological, and electron microscopic examinations. Furthermore, intravitreal injection of Hcy in normal wild type mice resulted in diffuse hyper-fluorescence, albumin leakage, and choroidal neovascularization in RPE. In vitro experiments on ARPE-19 showed that Hcy dose dependently reduced tight junction protein expression, increased fluorescein isothiocyanate dextran leakage, decreased transcellular electrical resistance, and impaired phagocytic activities. Collectively, the results demonstrated detrimental effects of excess Hcy levels (HHcy) on RPE structure and functions that could lead to the development of AMD disease like features[14]. Also, there is increasing evidence that clearly supports a role for inflammation in the AMD disease pathogenesis[15] and as a result some studies have suggested a risk association with plasma Hcy to be an independent factor for inflammation in the eye. Hcy can also alter tissue properties through generations of reactive oxygen species (ROS) that can activate matrix metalloproteinases (MMPs) which can be extruded into the matrix[16]. High levels of Hcy adversely affect the vascular endothelium and it is elevated in individuals who suffer from AMD[17]. Because Hcy levels of >15 mmol/L are considered abnormal we studied changes in retinal cells employing different concentrations of Hcy to evaluate effects on genes related with inflammatory cytokines and their receptors. In literature, transcriptome of the ciliary body, choroid including human RPE[18]–[20] in the eye have been described, but the effect of Hcy mediated transcriptional changes in RPE remains to be fully appreciated. Previously, we reported results from a pilot study to highlight the Hcy effect on RPE transcriptional changes[21]. The current study revealed interesting results suggesting the necessity to undertake further investigation in an appropriate AMD animal disease model in vivo. Based on our findings we are proposing that Hcy can serve as an important marker of inflammation in AMD as it is already being used in other medical conditions.
MATERIALS AND METHODS
ARPE-19 Cell Culture
In this study no human subjects or human medical records were used. The ARPE-19 cell line was obtained from ATCC (Manassas, VA, USA) and was handled in the biosafety level 2 laminar flow clean work station per standard cell culture practice. Briefly, the base medium was formulated with DMEM: F12. To make the complete growth medium fetal bovine serum to a final concentration of 10% was added. For sub-culturing, the spent culture medium was discarded and the cell monolayer was rinsed with 0.05% (w/v) Trypsin- 0.53 mmol/L EDTA solution to remove serum components. Cells were observed under an inverted microscope until the monolayer was dispersed. Then 6.0 to 8.0 mL of complete growth medium was added and the cells were aspirated gently by pipetting. To remove trypsin-EDTA solution, cell suspension culture was transferred to a centrifuge tube and spun at approximately 125 xg for 5min. The supernatant was discarded and cells were re-suspended in fresh growth medium and incubated at 37°C, 5% CO2 to confluency. Tissue culture medium was replaced 2 to 3 times per week. Cells were grown in sufficient quantity and then treated with different concentrations of Hcy (Sigma, St. Louis, MO, USA). Treated cell cultures were kept in the incubator for 24h at 37°C, 5% CO2. Three different concentrations were used in the Hcy exposure experiment: 0 (control), 6, 30, and 150 µmol/L.
Isolation of Total RNAs, Preparation of RNA-labeled Probes and Hybridization with Oligos Microarrays
We analyzed ARPE-19 cells to find out the molecular changes at the transcriptional level post exposure to the Hcy treatment and obtained readouts specific for the inflammatory genomic signatures employing a microarray and bioinformatics based suite using the human gene expression microarray platform (GEArray; SA Biosciences, Frederic, MD, USA) that can profile expression of 113 key cytokines and chemokines genes involved in the inflammatory immune responses. The functional gene grouping on microarrays is mentioned in Table 1. Briefly, the GEArray contains 60-mer oligonucleotides that are highly gene-specific in nature. The selected genes in this microarray have been shown to be involved in the immune cascade reactions during the process of inflammation in various human diseases. The oligos on the microarrays are specific for the genes that encode chemokines, interleukins, and TNF ligands. Total RNA extraction was done using TRIzol reagent (Invitrogen Inc., Carlsbad, CA, USA) per the manufacturer's instructions. Carryover DNA, if any, was removed by incubation with DNase I. Final products yielded a 260/280-nm ratio of 1.9 to 2.0 and a 230/260-nm ratio less than 0.5. The quality was checked via gel electrophoresis by visualization of intact 28S/18S ribosomal RNA bands and the quantity of each isolation was determined based on 260-nm absorbance. Then RNAs were converted to biotin-16-UTP labeled cRNAs as probes and used for hybridization on the Oligo GEArray nylon membranes per manufacturer's instructions. After hybridization, signals were successfully captured on the radiographic films using the chemiluminescent detection method. Finally, employing an integrated image and data analysis software (GEArray Expression Analysis Suite), the micro-arrays data were obtained and analyzed in details.
Table 1. The functional gene grouping on microarrays.
| Molecule category | Name of genes |
| Chemokines and cytokines | C5, CCL1 (I-309), CCL2 (mcp-1), CCL3 (MIP-1a), CCL4 (MIP-1b), CCL5 (RANTES), CCL7 (mcp-3), CCL8 (mcp-2), CCL11 (eotaxin), CCL13 (mcp-4), CCL15 (MIP-1d), CCL16 (HCC-4), CCL17 (TARC), CCL18 (PARC), CCL19, CCL20 (MIP-3a), CCL21 (MIP-2), CCL23 (MPIF-1), CCL24 (MPIF-2/ eotaxin-2), CCL25 (TECK), CCL26, CX3CL1 (fractalkine), CXCL1, CXCL2, CXCL3, CXCL5 (ENA-78 / LIX), CXCL6 (GCP-2), CXCL9, CXCL10 (IP-10), CXCL11 (I-TAC / IP-9), CXCL12 (SDF1), CXCL13, CXCL14, IFNA2, IFNG, IL10, IL11, IL12A, IL12B, IL13, IL15, IL16, IL17, IL17C, IL18, IL1A, IL1B, IL2, IL20, IL21, IL22, IL3, IL4, IL5, IL6, IL8, IL9, LTA, LTB, MIF, PF4, SCYE1 (endothelial monocyte-activating cytokine), SPP1, TNF, TNFSF5, XCL1 (lymphotactin) |
| Chemokine receptors and cytokine receptors | BLR1 (CXCR5), CCR1, CCR2, CCR3, CCR4, CCR5, CCR6, CCR7, CCR8, CCR9, CX3CR1, CXCR4, IL10RA, IL10RB, IL11RA, IL12RB1, IL12RB2, IL13RA1, IL13RA2, IL15RA, IL17R, IL18R1, IL1R1, IL1R2, IL1RN, IL2RA, IL2RB, IL2RG, IL5RA, IL6R, IL6ST, IL8RA, IL8RB, IL9R, XCR1 (CCXCR1) |
| Interleukins and receptors | IL10, IL10RA, IL10RB, IL11, IL11RA, IL12A, IL12B, IL12RB1, IL12RB2, IL13, IL13RA1, IL13RA2, IL15, IL15RA, IL16, IL17, IL17C, IL17R, IL18, IL18R1, IL1A, IL1B, IL1R1, IL1R2, IL1RN, IL2, IL20, IL21, IL22, IL2RA, IL2RB, IL2RG, IL3, IL4, IL5, IL5RA, IL6, IL6R, IL6ST, IL8, IL8RA, IL8RB, IL9, IL9R, TOLLIP |
| TNF ligands and receptors | LTA (LT-a), LTB (LT-b), TNF (TNF-a), TNFRSF1A (TNFR1), TNFRSF1B (TNFR2), TNFSF5 |
| Other factors involved in inflammatory response | ABCF1, BCL6, C3, C4A, CEBPB, CRP, EDG3, ICEBERG, LTB4R |
Validation of Microarray Captured Targets via Western Blotting Assay
Total cellular proteins were extracted from Hcy treated ARPE-19 cells and protein lysates were tested for CEBPB and IL-6 protein molecules following the standard Western blotting protocol. In brief, 30 µg of total protein amount of each sample was electrophoresed in 4%-12% Bis Tris Novex gels (Life Technologies, Waltham, MA, USA) and transferred to PVDF blot membranes (Bio-Rad Laboratories, Hercules, CA, USA). The membranes were blocked with 5% non-fat milk (Bio-Rad Laboratories, Hercules, CA, USA) solution and incubated with primary antibodies at 1:500 (anti-CEBPB and anti-IL-6; Santa Cruz Biotechnology, Santa Cruz, CA, USA) overnight at 4°C followed by secondary horseradish peroxidase (HRPO) conjugated anti-IgG antibodies (Cell Signaling, Beverly, MA, USA) for 1-2h. The enhanced chemiluminescence detection system (ECL plus; Pierce, Rockford, IL, USA) was used to detect bands with peroxidase activity. Beta-actin (Cell Signaling, Beverly, MA, USA) served as an internal reference for the tested proteins.
DNA Ladder Apoptosis Assay of Hcy Stimulated ARPE-19 Cultures
DNA fragmentation pattern was evaluated for the sign of apoptosis 72h after Hcy addition to the ARPE-19 cell cultures at an increasing concentration: 0.10, 0.20, 0.50, 1.0 and 5.0 mmol/L. Actinomycin-D was used to induce apoptosis in ARPE-19 cells to serve as a positive control for the Hcy treated cells. The apoptosis assay was performed with apoptotic DNA ladder isolation kit (BioVision, Inc., Milpitas, California, USA) to isolate the genomic DNAs from the Hcy treated cells. After the treatment, cells were washed with PBS and approximately 106 equivalent cells were centrifugation for 5min at 500 xg. The supernatants were carefully removed using the pipette and the cells were gently trypsinized, pelleted and 50 µL of DNA ladder extraction buffer was added with gentle pipetting. Tubes containing cells with the extraction buffer were centrifuged for 5min at 1600 xg (4500 rpm) and the supernatants were transferred to fresh tubes. The resultant pellets were extracted again with the extraction buffer. Then 5 µL enzyme “A” solution was added to the supernatants, mixed by gentle vortexing and incubated at 37°C for 10min. After that 5 µL of enzyme ‘B’ solution was added into each sample tube and incubated at 50°C for 30min. Ammonium acetate solution (5 µL) was added to each sample and mixed well. It was followed by an addition of 100 µL of isopropanol, and then sample tubes were kept at -20°C after mixing properly for 10min. The tubes were centrifuged at maximum speed (16K xg) for 10min to precipitate DNAs. The supernatants were removed and the DNA pellets were washed with 0.5 mL of 70% ethanol, centrifuged again at maximum speed (16K xg) to remove the trace amount of ethanol, and air dried for 10min at room temperature. DNA pellets were dissolved in 30 µL of DNA suspension buffer and 20 µL of it from each sample tubes were loaded onto a 1.2% agarose gel containing 0.5 µg/mL ethidium bromide in both gel and the running buffer. The gel was run at 5 V/cm for 1-2h or until the yellow dye ran to the edge of the gel. Ethidium bromide-stained DNA gel was visualized by trans-illumination with the help of UV light and photographed.
Cell Morphology Analysis of ARPE-19 Cells After Hcy Treatment
To examine the effect of Hcy on morphological changes or resultant toxicity in the ARPE-19 cells, different amounts of Hcy: 0.15, 0.50, 1.50, 5.00 and 15.0 mmol/L were added and the cultures were examined after a period of 72h and images were recorded.
RESULTS
Microarray Data Revealed Substantial Transcriptional Changes in ARPE-19 Cells by Hcy
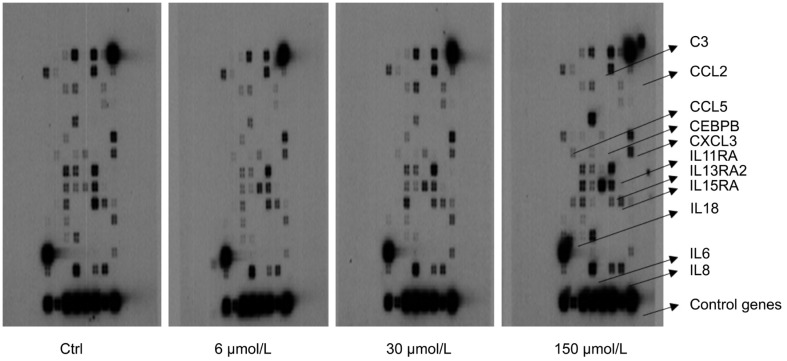
We obtained a robust inflammatory gene expression readout via microarray platform after the Hcy treatment of ARPE-19 cells (Figure 1; Table 2).
Figure 1. Inflammatory cytokines and chemokines microarray analyses of ARPE-19 cells after Hcy treatment.
In comparison to control (untreated), all Hcy (6, 30 and 150 µmol/L) treated cultures exhibited both up-regulation and down-regulation of inflammatory genes are shown by arrows.
Table 2. Classification of genes identified through gene microarray analysis after Hcy exposure.
| Gene | Gene's name | Functional gene group | Regulation (X-fold) |
| CCL5 | RANTES | Chemokine/cytokine | ↑ 2.49 |
| CEBPB | C/EBP-β | Molecules involved in inflammation | ↑ 4.42 |
| IL13RA2 | IL13 receptor-α2 | Interleukin receptor | ↑ 14.58 |
| IL15RA | IL15 receptor-α | Chemokine/cytokine receptor | ↑ 2.50 |
| IL6 | IFN-β2 | Chemokine/cytokine | ↑ 3.27 |
| IL8 | IL8 | Chemokine/cytokine | ↑ 2.60 |
| CXCL3 | GRO3 | Chemokine/cytokine | ↑ 104.60 |
| C3 | Complement component 3 | Molecules involved in inflammation | ↓ 4.34 |
| CCL2 | MCP1/SCYA2 | Chemokine/cytokine | ↓ 2.85 |
| IL11RA | IL11 receptor-α | Chemokine/cytokine receptor | ↓ 2.08 |
| IL18 | IFN gamma inducing factor | Chemokine/cytokine | ↓ 7.69 |
A selected set of differentially regulated genes along with their functional groups and fold change characteristics summarized.
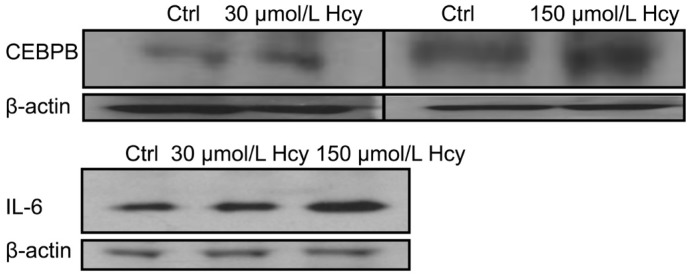
The expression profile was considered consistently changed only if the average multiple of change between the control and experimental ones was higher than two in at least three independent hybridizations from a set of three independent experiments. Under these conditions, of 113 gene-specific oligos on the chips, 7 genes were significantly upregulated during Hcy exposure; however, 4 were clearly down-regulated. Notably, CXCL3 (>than 100-fold increase) and IL13RA2 (>than 14-fold increase) tended to be preferentially altered. The resultant micro-array analyses provided a set of differentially expressed molecules of which some are already known players with respect to their roles in inflammation however we did find new ones also that might turn out to be interesting targets for exploring their potential roles in eye diseases. These might be directly or indirectly influenced by Hcy metabolism or the pathways that are amenable to Hcy plasma levels in susceptible patients. Our integrated data analyses identified top “significantly differentially expressed genes” based on the strict criteria of analysis (Figure 1, arrows). Prominent ones were: CCL5, CEBPB, IL13RA2, IL15RA, IL6, IL8, and CXCL3 which exhibited significantly higher values than other ones; C3, CCL2, IL11RA, and IL18 were downregulated after Hcy treatment. The lack of induction of the downregulated genes is currently unclear to us. From the upregulated set of transcripts that we obtained from our microarray analysis, we randomly selected two targets (CEBPB and IL6) to find out whether the same was true at the protein levels. Indeed, we did observe a dose dependent Hcy effect on both them (Figure 2).
Figure 2. Western blot assay for validation of microarray data at protein levels.
CEBPB and IL-6 molecules were tested for further validation at protein levels where ARPE-19 cultures were treated with 30 or 150 µmol/L Hcy conc. The results showed a dose dependent Hcy effect on protein expression.
Hcy did not Result in the Degradation of ARPE-19 Cells' DNA
Internucleosomal DNA fragmentation is a hallmark of apoptosis in the mammalian cells. We analyzed Hcy treated cells for detecting any sign of DNA fragmentation. Exposure of ARPE-19 cells showed no indication of apoptosis as we could not see any visible DNA laddering pattern in the samples treated even with 5 mmol/L Hcy concentration (Figure 3) implying that Hcy does not participate in the cellular death process via apoptosis at the concentrations (0.10, 0.20, 0.50, 1.00 and 5.00 mmol/L Hcy) tested in our assay.
Figure 3. Detection of apoptosis via DNA laddering pattern of ARPE-19 cells after Hcy treatment.
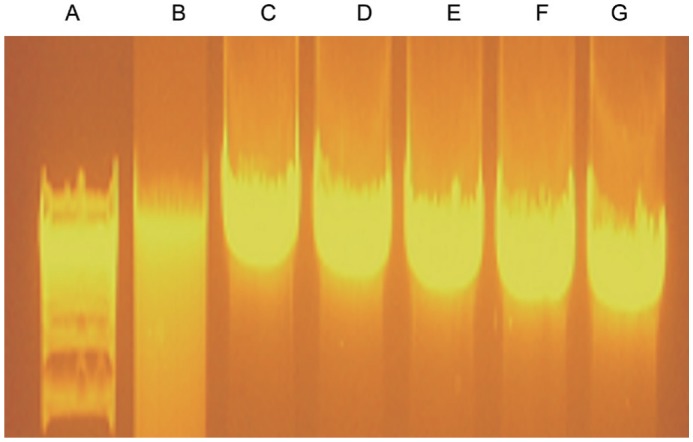
Different concentrations of Hcy: 0.10, 0.20, 0.50, 1.0 and 5.0 mmol/L were studied to gauge the effect on DNA degradation. None of the concentration induced apoptosis in this assay. Actinomycin-D served as a positive control for the induction of apoptosis. A: Kb plus marker; B: Actinomycin-D positive ctrl; C: 0.10 mmol/L Hcy; D: 0.20 mmol/L Hcy; E: 0.50 mmol/L Hcy; F: 1.00 mmol/L Hcy; G: 5.00 mmol/L Hcy.
Effect of Hcy on Cell Morphology and Viability
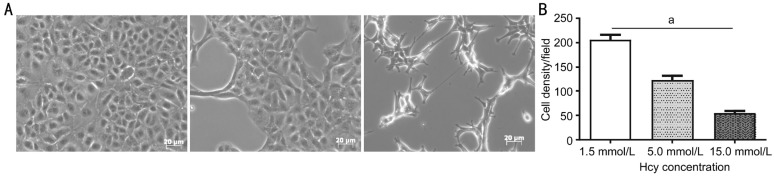
Cellular morphology and viability are direct indicators of a cell's state after treatment with an experimental agent. We decided to evaluate Hcy role on cell morphology with concentrations ranging from sub-toxic to toxic ones (e.g. 0.15, 0.50, 1.50, 5.00 and 15.0 mmol/L Hcy) to gauge the effect on the viability, overall health, and cell density of ARPE-19 culture. Compared to 1.5 mmol/L Hcy concentration (showing no deleterious effect), 5.0 and 15.0 mmol/L Hcy markedly decreased the number of cells in a dose-dependent manner (Figure 4A). When the concentration of Hcy was more than 1.5 mmol/L, there were significant differences (P<0.05) between 5.0 and 15.0 mmol/L compared to 1.5 mmol/L (Figure 4B). Apparently, Hcy did affect the morphology and cell density dynamics during the treatment period. Under the culture conditions employed, 0.50 and 1.50 mmol/L did not show any abnormal change in either cell shape or cell size. However, 5.0 mmol/L did affect the monolayer confluency and 15.0 mmol/L Hcy seemed to be notably toxic in nature 72h post Hcy treatment. Patchy areas devoid of cells were observed at both 5.0 and 15 mmol/L Hcy concentrations but cells did not appear swollen or disorganized (Figure 4A). Disorganization of monolayer continued to progress reflecting toxic effects on cell density though at 5.0 and 15 mmol/L Hcy concentrations making ARPE-19 cell layer progressively scanty. Taken together, our morphological analysis revealed toxic effects of higher Hcy concentrations on ARPE-19 cells (Figure 5).
Figure 4. Effect of Hcy on ARPE-19 cell morphology and density.
A: Various concentrations of Hcy: 1.5, 5.0 and 15.0 mmol/L were used for studying the effects on ARPE-19 cells; B: Of 1.5, 5.0 and 15.0 mmol/L doses appear to be toxic for the ARPE-19 cells in culture. aP<0.05.
Figure 5. Schematic highlighting effects of Hcy on retinal pigment epithelium.
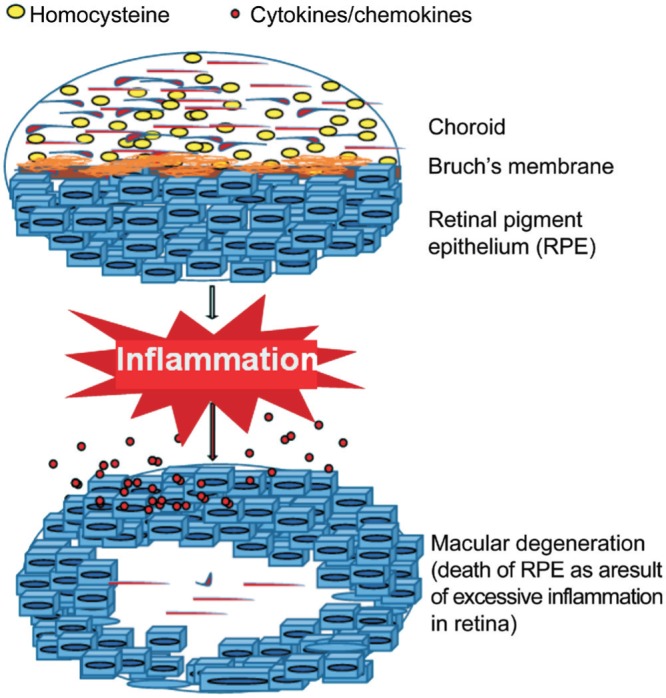
A simplified scheme depicting the potentially damaging effects in retinal cells that can initiate degenerative changes in the retina during AMD disease process.
DISCUSSION
RPE remains the prime target of diseases including AMD. Gene microarray analyses revealed alterations of several genes in retinal cells including increased expression of CCL5, IL15RA, IL8, IL6, CEBPB, IL13RA2, and CXCL3. Some were down-regulated as well, such as: IL11RA, CCL2, C3, and IL18. Elevated retinal Hcy has been shown to alter gene expression involved in endoplasmic reticular stress, N-Methyl-D-aspartate (NMDA) receptor activation, cell cycle, and apoptosis[22]. Not much is known about inflammatory chemokines and their receptor interactions in Hcy mediated retinal pathology. To our knowledge, this study is the first to report a comprehensive analysis of gene expression in ARPE-19 cells exposed to Hcy revealing 2 to 100-fold increase in various cytokines/chemokines. A few genes showed down-regulation from 2 to 7-fold in their expression. The transcriptomics findings corroborated well with the protein analysis data for two selected targets confirming both the gene and protein expressions of pro-inflammatory chemokine IL6 and the molecule involved in inflammation; CEBPB. At present, reason for the downregulation of genes by Hcy remains unclear to us. One possibility is that there is probably a temporary physiologic compensatory mechanism during Hcy exposure. Although the implications of each upregulated or downregulated target may be difficult to comprehend, the findings do open an avenue to identify new targets in ocular inflammation especially in cases where Hcy can potentially trigger inflammation in the AMD susceptible individuals. Chemokines serve important roles in regulating inflammatory processes after binding glycosoaminoglycans on endothelial cells, forming a solid-phase chemotactic gradient to attract the passing leukocytes[23]. Previous work on the expression of chemokines and their receptors in inflammatory diseases included atherosclerosis, multiple sclerosis, rheumatoid arthritis, lupus, obesity and AMD[24]. In fact, retinal degeneration upregulates chemokines whose expression is coordinated by various cell types in the retina including RPE. These chemokines in turn govern trafficking of leukocytes responsible for retinal degeneration. Recruitment and aggregation of monocytes is detrimental and is directly implicated in retinal models of AMD and diabetic retinopathy[25]. CCL2 plays role in retinal neovascularization and detachment. An earlier report described immunolocalization of CCL2 and CCL5 in retina of human diabetic eyes. A significant increase in the expression of CCL5 in diabetic retina was also described. A gene expression comparison of secretory neuroepithelia of brain choroid plexus and ocular ciliary body identified 100× fold change in IL13RA2 transcript[26]. CXCL3 regulates migration and adhesion of monocytes and mediates effects on cells by interacting with CXCR2 receptor. IL6 plays critical roles in immune response by activating Ras/MAP pathway, which regulates NFIL6 transcription. In our study Hcy increased IL6 expression more than 3-fold and this was also accompanied by a simultaneous increase in IL6 protein. Being a key factor in the modulation of immune responses, inflammatory processes, and in autoimmune diseases IL6 is also increased in AMD establishing it as a linkage for the oxidative stress via radical oxygen species in photoreceptor cells and RPE cells and also by inflammatory/autoimmune reactions in AMD[27]. It is regulated through NFκB signaling in ARPE-19 cells, other cell types and stimulates inflammatory and auto-immune reactions, atherosclerosis, Alzheimer's Disease, and arthritis. Taken together, these findings suggest that Hcy promotes RPE inflammation and that it can be mediated through the IL6 pathway, and its inhibition can render protective effects since IL6 and CCL5 are well known participants in the setting of ocular inflammation[28]. When RPE cells die they release increased amounts of IL6 followed by inflammatory changes[29]. Expressions of proinflammatory IL6 and IL8 were significantly upregulated in RPE/choroid of aged SAMP8 mice; a model that exhibited similar chronic inflammatory features of human AMD[30]. Hcy, in human monocytes, also led to expression and secretion of proinflammatory chemokines such as IL8[31].
CEBPB encodes several isoforms and is also known as IL6 dependent DNA-binding protein contributing to the regulation of acute phase response. Induction of CEBPB by inflammatory stimuli occurs during stress such as inflammation. Its binding motifs are present in the regulatory regions of various genes expressed by cells of the myelomonocytic lineages, including those encoding inflammatory cytokines like IL6, IL1β, and TNF-α, cytokines such as IL8 and IL12, the gene encoding the granulocyte colony-stimulating factor, and macrophage, granulocyte, and granulocyte-macrophage receptor genes. CEBPB knockdown prevented inflammation and p65-NFκB DNA binding, while overexpression induced NFκB, JNK activation, and pro-inflammatory cytokines expression suggesting that it is an attractive target for ameliorating inflammation[32]. CEBPB, thus plays crucial roles during inflammation by regulating the functions of both cytokine producing macrophages, granulocytes and target cells. For example, expression of IGFBP5, an important member of the IGF axis involved in cell growth and differentiation, is regulated by CEBPB during differentiation of RPE[33]. Overexpression of CEBPB leads to enhanced endothelin B receptor's promoter activity suggesting that CEBPB is important for expression of the receptor in eye as intraocular pressure (IOP) elevation led increased expression of CEBPB in retinal ganglion cell from IOP-elevated eyes[34]–[35]. IL13RA2 binds with high affinity to IL13; a pro-fibrotic cytokine leading to its internalization initiating signal transduction. Idiopathic pulmonary fibrosis with abnormal vascularity uses IL13 through JAK/STAT after binding IL13RA1/IL4RA. IL13 also binds IL13RA2, which functions as a decoy receptor.
CXCR3 expression was necessary for the IL13 mediated gene and protein upregulation of IL13RA2 demonstrating expression of CXCR3 in fibroblasts and its association with IL13RA2 indicating a requirement for CXCR3 for IL13 mediated IL13RA2 expression. The elucidation of complex relationship between these anti-fibrotic receptors and manipulation of the CXCR3-mediated regulation of IL13RA2 may represent a novel therapeutic modality in injury or inflammation[36]. IL15RA in conjunction with IL15 regulates innate and adaptive immune responses. Being a potent pro-inflammatory cytokine IL15 induces TNF-α, IL1β and other chemokines. After activation with IL15 or lipopolysaccharide, macrophages express high-affinity IL15RA. Analysis of IL15RA on human monocytes and T cells revealed that both cell types showed a significantly higher IL15RA expression in patients with rheumatoid arthritis. IL15 participates in inflammatory bowel diseases (IBD) and thus IBD patients have an increased expression of IL15RA mRNA in their inflamed mucosa. The specific receptor chain IL15RA can be expressed as a transmembranous signaling receptor, or can be cleared by a disintegrin and metalloprotease domain 17 into a neutralizing, soluble receptor; IL15RA that circulates in the sera of patients[37]. The mechanism of Hcy associated vascular injuries and toxicity in the retina such as thrombosis are not fully understood and the possible mechanisms may include platelet activation by oxidative injury, increased adhesiveness, enhanced coagulability, and vascular matrix damage[38].
Apoptosis is characterized by a series of cellular morphological changes such as cell shrinkage, retraction of cellular processes, caspase activation, chromatin condensation and DNA fragmentation[39]. Interestingly, we did not see any sign of apoptosis by DNA laddering or oligo-nucleosomal cleavage in DNA fragmentation assay after Hcy treatment of ARPE-19 cells. We do not think the reduced cell density of ARPE-19 cells at higher Hcy concentration in our study was due to necroptosis either because in contrast to apoptosis, necroptosis is characterized by ATP depletion, rupture of plasma membrane, and release of necroptosis-specific cytokine HMGB1[40]–[41]. Our ARPE-19 cell cultures were constantly maintained in complete growth medium ensuring no ATP depletion during Hcy exposure. Cell morphology to some degree reflects cell functions and thus the morphology of Hcy induced changes was monitored. We found that Hcy induced morphological alterations in retinal cells during 72h of treatment with 5.0 mmol/L concentration or above. Untreated or 0.15, 0.50, 1.50 and 5.00 mmol/L Hcy treated ARPE-19 cells looked similar in shape and size although 5.0 mmol/L had reduced cell density compared to the untreated ones. The 15 mmol/L Hcy treatment had less density and they looked more like a fibroblast type in shape and size than the untreated ones. Apparently, Hcy could exert toxic effects at the supra-physiological concentration since cells at higher Hcy concentrations (5.0 and 15 mmol/L) started showing discontinuity of monolayers without any sign of cell death. These morphological changes could in part be due to differential reorganization of cytoskeletal proteins such as actin and tubulin. Evidence suggests that cytoskeleton is critical in morphological changes[42]. Some studies have established that during cell death there is rearrangement and accumulation of cytoskeletal proteins including actin and tubulin[43]–[44]. This could be accompanied by the ability of microtubules to have spontaneous changes in polymerization and depolymerization activities during apoptosis. It is known that the organization of the actin cytoskeleton is a key element in the morphology of astrocytes and that member of the Rho family regulate their arrangement. It is noteworthy to mention here that no changes in cell morphology were observed at early time points during Hcy treatment, suggesting that the observed morphological alterations may be due to rearrangement of the cytoskeleton in ARPE-19 cells. Taken together, our data provide compelling evidence that RPE cells do not undergo apoptosis despite morphological changes in response to Hcy and the most likely reason could be the presence of various protective substances or factors in the growth medium. Elevated level of Hcy was shown to induce chronic inflammation in vascular bed, including glomerulus, and promoted glomerulosclerosis. Several laboratories, including our own, have shown that Hcy induces glomerular injury, in part mediated through the induction of inflammatory molecules[45]–[46]. Cytokine and chemokine mediated inflammation is a major factor in the development and progression of chronic kidney disease (CKD) corroborating clinical evidence that elevated Hcy levels are linked to the inflammatory state associated with CKD. Specifically, Hcy induces the expression of cytokines, such as monocyte chemoattractant protein-1 (MCP-1) and chemokines; macrophage inflammatory protein-2 (MIP-2), in cultured mesangial cells[47]–[49].
In summary, the transcriptomic messages that were discovered in our gene microarray study, several of them are known to have novel functions, are biologically relevant in many eye diseases in general and AMD in particular (Figure 5). However, it is possible that these genetic signatures may have similar expression related functions in other diseases also. These shared functional roles may help understand a variety of inflammatory ocular conditions that are both acute and chronic in nature, including AMD.
Acknowledgments
Part of this work was presented at the FASEB 2006 annual meeting in San Francisco, California, USA.
We thank Olson PK and Babbarwal A for their excellent editing.
Foundations: Supported by NIH Heart, Lung, and Blood Institute (No. HLO74815); Institute of Neurological Disorders and Stroke (No. NS-084823).
Conflicts of Interest: Singh M, None; Tyagi SC, None.
REFERENCES
- 1.Rudolf M, Clark ME, Chimento MF, Li CM, Medeiros NE, Curcio CA. Prevalence and morphology of druse types in the macula and periphery of eyes with age-related maculopathy. Invest Ophthalmol Vis Sci. 2008;49(3):1200–1209. doi: 10.1167/iovs.07-1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Viktorov IV, Aleksandrova OP, Alekseeva NY. Homocysteine toxicity in organotypic cultures of rat retina. Bull Exp Biol Med. 2006;141(4):471–474. doi: 10.1007/s10517-006-0202-4. [DOI] [PubMed] [Google Scholar]
- 3.Flott-Rahmel B, Schurmann M, Schluff P, Fingerhut R, Musshoff U, Fowler B, Ullrich K. Homocysteic and homocysteine sulphinic acid exhibit excitotoxicity in organotypic cultures from rat brain. Eur J Pediatr. 1998;157:112–117. doi: 10.1007/pl00014291. Suppl 2. [DOI] [PubMed] [Google Scholar]
- 4.Bharathselvi M, Biswas J, Selvi R, Coral K, Narayanasamy A, Ramakrishnan S, Sulochana KN. Increased homocysteine, homocysteine-thiolactone, protein homocysteinylation and oxidative stress in the circulation of patients with Eales' disease. Ann Clin Biochem. 2013;50(4):330–338. doi: 10.1177/0004563213492146. [DOI] [PubMed] [Google Scholar]
- 5.Chang HH, Lin DP, Chen YS, Liu HJ, Lin W, Tsao ZJ, Teng MC, Chen BY. Intravitreal homocysteine-thiolactone injection leads to the degeneration of multiple retinal cells, including photoreceptors. Mol Vis. 2011;17:1946–1956. [PMC free article] [PubMed] [Google Scholar]
- 6.Coral K, Raman R, Rathi S, Rajesh M, Sulochana KN, Angayarkanni N, Paul PG, Ramakrishnan S. Plasma homocysteine and total thiol content in patients with exudative age-related macular degeneration. Eye (Lond) 2006;20(2):203–207. doi: 10.1038/sj.eye.6701853. [DOI] [PubMed] [Google Scholar]
- 7.Gore AD, Rao GS, Gore MA, Desai AR. Multiple extra macular branch retinal vein occlusions in hyperhomocysteinemia. Indian J Ophthalmol. 2014;62(4):489–491. doi: 10.4103/0301-4738.132110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li J, Zhang H, Shi M, Yan L, Xie M. Homocysteine is linked to macular edema in type 2 diabetes. Curr Eye Res. 2014;39(7):730–735. doi: 10.3109/02713683.2013.877933. [DOI] [PubMed] [Google Scholar]
- 9.Markand S, Saul A, Roon P, Prasad P, Martin P, Rozen R, Ganapathy V, Smith SB. Retinal Ganglion Cell Loss and Mild Vasculopathy in Methylene Tetrahydrofolate Reductase (Mthfr)-Deficient Mice: A Model of Mild Hyperhomocysteinemia. Invest Ophthalmol Vis Sci. 2015;56(4):2684–2695. doi: 10.1167/iovs.14-16190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vine AK, Stader J, Branham K, Musch DC, Swaroop A. Biomarkers of cardiovascular disease as risk factors for age-related macular degeneration. Ophthalmology. 2005;112(12):2076–2080. doi: 10.1016/j.ophtha.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 11.Nagineni CN, Kommineni VK, Ganjbaksh N, Nagineni KK, Hooks JJ, Detrick B. Inflammatory cytokines induce expression of chemokines by human retinal cells: role in chemokine receptor mediated age-related macular degeneration. Aging Dis. 2015;6(6):444–455. doi: 10.14336/AD.2015.0323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ransohoff RM. Chemokines and chemokine receptors: standing at the crossroads of immunobiology and neurobiology. Immunity. 2009;31(5):711–721. doi: 10.1016/j.immuni.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mulero J, Manresa N, Zafrilla P, Losada M. Markers of cardiovascular risk in elderly patients with age-related macular degeneration. Clin Hemorheol Microcirc. 2014;58(3):447–453. doi: 10.3233/CH-141807. [DOI] [PubMed] [Google Scholar]
- 14.Ibrahim AS, Mander S, Hussein KA, Elsherbiny NM, Smith SB, Al-Shabrawey M, Tawfik A. Hyperhomocysteinemia disrupts retinal pigment epithelial structure and function with features of age-related macular degeneration. Oncotarget. 2016;7(8):8532–8545. doi: 10.18632/oncotarget.7384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rutar M, Natoli R, Chia RX, Valter K, Provis JM. Chemokine-mediated inflammation in the degenerating retina is coordinated by Muller cells, activated microglia, and retinal pigment epithelium. J Neuroinflammation. 2015;12:8. doi: 10.1186/s12974-014-0224-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vacek TP, Kalani A, Voor MJ, Tyagi SC, Tyagi N. The role of homocysteine in bone remodeling. Clin Chem Lab Med. 2013;51(3):579–590. doi: 10.1515/cclm-2012-0605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Axer-Siegel R, Bourla D, Ehrlich R, Dotan G, Benjamini Y, Gavendo S, Weinberger D, Sela BA. Association of neovascular age-related macular degeneration and hyperhomocysteinemia. Am J Ophthalmol. 2004;137(1):84–89. doi: 10.1016/s0002-9394(03)00864-x. [DOI] [PubMed] [Google Scholar]
- 18.Wagner AH, Anand VN, Wang WH, Chatterton JE, Sun D, Shepard AR, Jacobson N, Pang IH, Deluca AP, Casavant TL, Scheetz TE, Mullins RF, Braun TA, Clark AF. Exon-level expression profiling of ocular tissues. Exp Eye Res. 2013;111:105–111. doi: 10.1016/j.exer.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Whitmore SS, Braun TA, Skeie JM, Haas CM, Sohn EH, Stone EM, Scheetz TE, Mullins RF. Altered gene expression in dry age-related macular degeneration suggests early loss of choroidal endothelial cells. Mol Vis. 2013;19:2274–2297. [PMC free article] [PubMed] [Google Scholar]
- 20.Tian L, Kazmierkiewicz KL, Bowman AS, Li M, Curcio CA, Stambolian DE. Transcriptome of the human retina, retinal pigmented epithelium and choroid. Genomics. 2015;105(5-6):253–264. doi: 10.1016/j.ygeno.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singh M, Olson P, Grossi F, Zhang Z, Tyagi N, Moshal KS, Tyagi SC. Differential expression of inflammatory cytokines and chemokines genes by homocysteine in the human retinal pigmented epithelial cells. The FASEB Journal. 2006;(20):719–A719. Available at: http://www.fasebj.org/content/20/4/A719.3.short. [Google Scholar]
- 22.Ganapathy PS, Moister B, Roon P, Mysona BA, Duplantier J, Dun Y, Moister TK, Farley MJ, Prasad PD, Liu K, Smith SB. Endogenous elevation of homocysteine induces retinal neuron death in the cystathionine-beta-synthase mutant mouse. Invest Ophthalmol Vis Sci. 2009;50(9):4460–4470. doi: 10.1167/iovs.09-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rangasamy S, McGuire PG, Franco Nitta C, Monickaraj F, Oruganti SR, Das A. Chemokine mediated monocyte trafficking into the retina: role of inflammation in alteration of the blood-retinal barrier in diabetic retinopathy. PLoS One. 2014;9(10):108508. doi: 10.1371/journal.pone.0108508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takeda A, Baffi JZ, Kleinman ME, Cho WG, Nozaki M, Yamada K, Kaneko H, Albuquerque RJ, Dridi S, Saito K, Raisler BJ, Budd SJ, Geisen P, Munitz A, Ambati BK, Green MG, Ishibashi T, Wright JD, Humbles AA, Gerard CJ, Ogura Y, Pan Y, Smith JR, Grisanti S, Hartnett ME, Rothenberg ME, Ambati J. CCR3 is a target for age-related macular degeneration diagnosis and therapy. Nature. 2009;460(7252):225–230. doi: 10.1038/nature08151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kataoka K, Nishiguchi KM, Kaneko H, van Rooijen N, Kachi S, Terasaki H. The roles of vitreal macrophages and circulating leukocytes in retinal neovascularization. Invest Ophthalmol Vis Sci. 2011;52(3):1431–1438. doi: 10.1167/iovs.10-5798. [DOI] [PubMed] [Google Scholar]
- 26.Janssen SF, Gorgels TG, Ten Brink JB, Jansonius NM, Bergen AA. Gene expression-based comparison of the human secretory neuroepithelia of the brain choroid plexus and the ocular ciliary body: potential implications for glaucoma. Fluids Barriers CNS. 2014;11(1):2. doi: 10.1186/2045-8118-11-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zafrilla P, Losada M, Perez A, Caravaca G, Mulero J. Biomarkers of oxidative stress in patients with wet age related macular degeneration. J Nutr Health Aging. 2013;17(3):219–222. doi: 10.1007/s12603-012-0095-z. [DOI] [PubMed] [Google Scholar]
- 28.Hashida N, Ohguro N, Nakai K, Kobashi-Hashida M, Hashimoto S, Matsushima K, Tano Y. Microarray analysis of cytokine and chemokine gene expression after prednisolone treatment in murine experimental autoimmune uveoretinitis. Invest Ophthalmol Vis Sci. 2005;46(11):4224–4234. doi: 10.1167/iovs.05-0346. [DOI] [PubMed] [Google Scholar]
- 29.Szatmari-Toth M, Kristof E, Vereb Z, Akhtar S, Facsko A, Fesus L, Kauppinen A, Kaarniranta K, Petrovski G. Clearance of autophagy-associated dying retinal pigment epithelial cells - a possible source for inflammation in age-related macular degeneration. Cell Death Dis. 2016;7(9):e2367. doi: 10.1038/cddis.2016.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feng L, Cao L, Zhang Y, Wang F. Detecting Abeta deposition and RPE cell senescence in the retinas of SAMP8 mice. Discov Med. 2016;21(115):149–158. [PubMed] [Google Scholar]
- 31.Zeng X, Dai J, Remick DG, Wang X. Homocysteine mediated expression and secretion of monocyte chemoattractant protein-1 and interleukin-8 in human monocytes. Circ Res. 2003;93(4):311–320. doi: 10.1161/01.RES.0000087642.01082.E4. [DOI] [PubMed] [Google Scholar]
- 32.Neumann FJ, Ott I, Marx N, Luther T, Kenngott S, Gawaz M, Kotzsch M, Schomig A. Effect of human recombinant interleukin-6 and interleukin-8 on monocyte procoagulant activity. Arterioscler Thromb Vasc Biol. 1997;17(12):3399–3405. doi: 10.1161/01.atv.17.12.3399. [DOI] [PubMed] [Google Scholar]
- 33.Samuel W, Kutty RK, Vijayasarathy C, Pascual I, Duncan T, Redmond TM. Decreased expression of insulin-like growth factor binding protein-5 during N-(4-hydroxyphenyl)retinamide-induced neuronal differentiation of ARPE-19 human retinal pigment epithelial cells: regulation by CCAAT/enhancer-binding protein. J Cell Physiol. 2010;224(3):827–836. doi: 10.1002/jcp.22191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.He S, Minton AZ, Ma HY, Stankowska DL, Sun X, Krishnamoorthy RR. Involvement of AP-1 and C/EBPbeta in upregulation of endothelin B (ETB) receptor expression in a rodent model of glaucoma. PLoS One. 2013;8(11):79183. doi: 10.1371/journal.pone.0079183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ivanov D, Dvoriantchikova G, Barakat DJ, Nathanson L, Shestopalov VI. Differential gene expression profiling of large and small retinal ganglion cells. J Neurosci Methods. 2008;174(1):10–17. doi: 10.1016/j.jneumeth.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barnes JC, Lumsden RV, Worrell J, Counihan IP, O'Beirne SL, Belperio JA, Fabre A, Donnelly SC, Boylan D, Kane R, Keane MP. CXCR3 requirement for the interleukin-13-mediated up-regulation of interleukin-13ralpha2 in pulmonary fibroblasts. Am J Respir Cell Mol Biol. 2015;53(20):217–225. doi: 10.1165/rcmb.2013-0433OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perrier C, Arijs I, Staelens D, Breynaert C, Cleynen I, Covens K, Ferrante M, Van Assche G, Vermeire S, de Hertogh G, Schuit F, Rutgeerts P, Ceuppens JL. Interleukin-15 receptor alpha expression in inflammatory bowel disease patients before and after normalization of inflammation with infliximab. Immunology. 2013;138(1):47–56. doi: 10.1111/imm.12014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cahill M, Karabatzaki M, Meleady R, Refsum H, Ueland P, Shields D, Mooney D, Graham I. Raised plasma homocysteine as a risk factor for retinal vascular occlusive disease. Br J Ophthalmol. 2000;84(2):154–157. doi: 10.1136/bjo.84.2.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vine AK. Hyperhomocysteinemia: a risk factor for central retinal vein occlusion. Am J Ophthalmol. 2000;129(5):640–644. doi: 10.1016/s0002-9394(99)00476-6. [DOI] [PubMed] [Google Scholar]
- 40.Hanus J, Anderson C, Wang S. RPE necroptosis in response to oxidative stress and in AMD. Ageing Res Rev. 2015;24:286–298. doi: 10.1016/j.arr.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Christofferson DE, Yuan J. Necroptosis as an alternative form of programmed cell death. Curr Opin Cell Biol. 2010;22(2):263–268. doi: 10.1016/j.ceb.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brewton LS, Haddad L, Azmitia EC. Colchicine-induced cytoskeletal collapse and apoptosis in N-18 neuroblastoma cultures is rapidly reversed by applied S-100beta. Brain Res. 2001;912(1):9–16. doi: 10.1016/s0006-8993(01)02519-7. [DOI] [PubMed] [Google Scholar]
- 43.Endresen PC, Fandrem J, Eide TJ, Aarbakke J. Morphological modifications of apoptosis in HL-60 cells: effects of homocysteine and cytochalasins on apoptosis initiated by 3-deazaadenosine. Virchows Arch. 1995;426(3):257–266. doi: 10.1007/BF00191363. [DOI] [PubMed] [Google Scholar]
- 44.Ryter SW, Kim HP, Hoetzel A, Park JW, Nakahira K, Wang X, Choi AM. Mechanisms of cell death in oxidative stress. Antioxid Redox Signal. 2007;9(1):49–89. doi: 10.1089/ars.2007.9.49. [DOI] [PubMed] [Google Scholar]
- 45.Sen U, Basu P, Abe OA, Givvimani S, Tyagi N, Metreveli N, Shah KS, Passmore JC, Tyagi SC. Hydrogen sulfide ameliorates hyperhomocysteinemia-associated chronic renal failure. Am J Physiol Renal Physiol. 2009;297(2):410–419. doi: 10.1152/ajprenal.00145.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sen U, Munjal C, Qipshidze N, Abe O, Gargoum R, Tyagi SC. Hydrogen sulfide regulates homocysteine-mediated glomerulosclerosis. Am J Nephrol. 2010;31(5):442–455. doi: 10.1159/000296717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cheung GT, Siow YL, O K. Homocysteine stimulates monocyte chemoattractant protein-1 expression in mesangial cells via NF-kappaB activation. Can J Physiol Pharmacol. 2008;86(3):88–96. doi: 10.1139/y08-002. [DOI] [PubMed] [Google Scholar]
- 48.Shastry S, James LR. Homocysteine-induced macrophage inflammatory protein-2 production by glomerular mesangial cells is mediated by PI3 Kinase and p38 MAPK. J Inflamm (Lond) 2009;6:27. doi: 10.1186/1476-9255-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sen U, Givvimani S, Abe OA, Lederer ED, Tyagi SC. Cystathionine beta-synthase and cystathionine gamma-lyase double gene transfer ameliorate homocysteine-mediated mesangial inflammation through hydrogen sulfide generation. Am J Physiol Cell Physiol. 2011;300(1):155–163. doi: 10.1152/ajpcell.00143.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]