Abstract
AIM
To compare the corneal biomechanics of Sjögren's syndrome (SS) and non-SS dry eyes with Corneal Visualization Scheimpflug Technology (CorVis ST).
METHODS
Corneal biomechanics and tear film parameters, namely the Schirmer I test value, tear film break-up time (TBUT) and corneal staining score (CSS) were detected in 34 eyes of 34 dry eye patients with SS (SSDE group) and 34 dry eye subjects without SS (NSSDE group) using CorVis ST. The differences of the above parameters between the two groups were examined, and the relationship between corneal biomechanics and tear film parameters were observed.
RESULTS
The differences in age, sex, intraocular pressure (IOP) and central corneal thickness (CCT) were not significant between the two groups (P>0.05). The tear film parameters had significant differences between the SSDE group and NSSDE group (all P<0.05). Patients in the SSDE group had significantly lower A1-time and HC-time, but higher DA (P=0.01, 0.02, and 0.02, respectively) compared with the NSSDE group. In the SSDE group, DA was negatively correlated with TBUT (rho=-0.38, P=0.03); HC-time was negatively correlated with CSS (rho=-0.43, P=0.02). In the NSSDE group, HC-time was again negatively correlated with CSS (rho=-0.39, P=0.02).
CONCLUSION
There are differences in corneal biomechanical properties between SSDE and NSSDE. The cornea of SSDE tends to show less “stiffness”, as seen by a significantly shorter A1-time and HC-time, but larger DA, compared with the cornea of NSSDE. Biomechanical parameters can be influenced by different tear film parameters in both groups.
Keywords: corneal biomechanics, Sjögren's syndrome, dry eye
INTRODUCTION
There is a concept that dry eye can cause tear film instability and ocular surface damage by affecting the integrity of the corneal epithelium[1]. Since an intact corneal epithelium is responsible for the stability in corneal biomechanics[2], the ocular surface impairment seen in dry eye may consequently affect corneal biomechanical behavior.
Approximately 11% of dry eye patients suffer from Sjögren's syndrome (SS)[3], which is a severe systemic autoimmune disease that primarily affects exocrine glands such as salivary and lacrimal glands, leading to dryness of the main mucosal surfaces and extraglandular manifestations. Dry eye is one of the most common clinical features of SS[4]–[5]. Although the pathogenesis of SS dry eye (SSDE) and non-SS dry eye (NSSDE) are not completely understood, more severe ocular surface damage is observed in SSDE compared to NSSDE subjects[6]. Moreover, being a connective tissue disorder (CTD), SS includes lymphocytic infiltration in the exocrine glands and produces various autoantibodies, which are responsible for the tissue damage[7]–[9]. Because of the rich connective tissue in the cornea, local antigen-antibody reaction in SS subjects leads to lysis of the corneal collagen. As such, not only the epithelium, but also the stroma of the cornea can be involved[10] significantly affecting corneal biomechanics. Due to the potential differences in the mechanism of corneal damage caused by SSDE and NSSDE, it is important to further clarify the different corneal biomechanics between dry eyes with or without SS.
To date, two machines are commercially available for observing corneal biomechanics, the Ocular Response Analyzer (ORA) (Reichert, Buffalo, New York, USA) available since 2005[11] and Corneal Visualization Scheimpflug Technology (CorVis ST) (Oculus Optikgeräte GmbH, Wetzlar, Germany) since 2013[12]. With the ORA, Firat and Doganay[13] reported no association of corneal biomechanical parameters with dry eye in non-SS subjects. We have previously detected a reduced highest concavity time (HC-time) for dry eyes without SS with CorVis ST, a recently developed dynamic Scheimpflug analyzer[14]. However, there are no reports comparing corneal biomechanics between dry eyes with or without SS using CorVis ST.
Herein, we aimed to investigate the biomechanical properties of the cornea in SSDE patients and NSSDE patients with CorVis ST and determine the relationship between corneal biomechanics and tear film parameters, namely tear secretion value, tear film break-up time (TBUT) and corneal staining score (CSS), to detect the potential factors that may influence corneal biomechanical behavior. Since corneal biomechanical behavior can be altered by multiple autoimmune diseases associated with SS, such as systemic lupus erythematosus (SLE)[15], rheumatoid arthritis (RA)[16] and systemic sclerosis (SSc)[17], only primary SS patients were investigated in the current study.
SUBJECTS AND METHODS
Subjects
This study was performed in accordance with the tenets of the Declaration of Helsinki and approved by the Ethics Committee of the Chinese Academy of Medical Sciences, Peking Union Medical College Hospital. Informed consent was obtained from all patients prior to participation in the study. Patients were consecutively recruited at Peking Union Medical College Hospital.
All of the patients meet the criteria for dry eye according to the consensus for dry eye in China (2013) as described in our previous publication[14]. The dry eye patients were divided into two groups: patients with SS dry eye (SSDE group) and patients with non-SS dry eye (NSSDE group). The SSDE group comprised dry eye patients with primary SS referred to us from the Department of Rheumatology and Clinical Immunology according to criteria proposed by the American-European Consensus Group[18].
Exclusion criteria included active ocular infection, systemic diseases other than primary SS, a positive history of ocular surgery, ocular diseases (e.g. corneal dystrophy, keratoconus, glaucoma, uveitis), systemic medication and local medication use other than artificial tears, subjects with refractive error (>3 diopters spheric and >1 diopter cylindric error), recent contact lens use (within 1mo), diabetes and pregnancy.
Ocular Examinations
All subjects received a complete ophthalmic examination including measurement of best-corrected visual acuity (BCVA), slit-lamp microscopy, fundus examination, and tear film evaluations (Schirmer I test, TBUT and CSS). TBUT and CSS were observed using slit lamp biomicroscopy by masked investigator A, and a Schirmer I test was performed more than 20min after dye staining by masked investigator B.
Corneal Visualization Scheimpflug Technology Measurement
Corneal biomechanical examination was conducted using CorVis ST (Type 72100, Oculus Optikgeräte GmbH, Wetzlar, Germany) more than 20min after the above-mentioned ophthalmic examinations by masked investigator C. The working mechanism of CorVis ST has been described in our previous publication and related literature. In brief, ten phase-specific parameters were recorded during the measurement: A1-time and A2-time (the length of time from the start to the first and second applanation); A1-length and A2-length (the length of the flattened cornea at the first and second applanation); A1-Velocity (A1-V) and A2-Velocity (A2-V) (the corneal velocity during the first and second applanation); highest concavity-time (HC-time) (the length of time from the start until HC is achieved); peak distance (PD, the distance between the two peaks of the cornea at HC); HC radius (the central concave curvature at HC) and deformation amplitude (DA, the maximum amplitude from the start to when HC is achieved)[19]–[21]. Intraocular pressure (IOP), which was calculated based on the first applanation, and central corneal thickness (CCT) were also generated during the process. Only acquisitions that showed “OK” for quality of scan (QS) were analyzed. All measurements were performed between 8 and 11 a.m. to avoid diurnal variation. No eyedrops were applied 1d before measurement.
Statistical Analysis
Statistical analysis was conducted using IBM SPSS 19.0 for Windows statistical software (SPSS, Chicago, IL, USA) and GraphPad Prism 5 (GraphPad Software, Inc.). Data are expressed as mean±SD. A Shaphiro-Wilk test was used to test normal distribution. Significance was determined by two-tailed unpaired Student's t-test or Mann-Whitney U test for comparing the observed parameters of the two groups and Pearson's correlation coefficient (r) or Spearman's correlation coefficient (rho) for assessing the relationship between corneal biomechanics and tear film parameters according to data normality, and was accepted at P<0.05. Only one randomly selected eye of each patient was included in the statistical analysis in order to eliminate any potential intrasubject effects from the same patient.
RESULTS
Sixty-eight unrelated patients were recruited to the study. There were 32 female and 2 male patients, with a mean age of 52.29y (range, 29 to 72 years old) in the SSDE group (n=34). The 28 female and 6 male patients were included in the NSSDE group, with a mean age of 49.15y (range, 18 to 72 years old). The differences between the two groups with regard to age and sex were not significant (age: t=0.99, P=0.32; sex: χ2=1.28, P=0.26). Significant differences were detected between the two groups in terms of Schirmer I test value (Mann-Whitney U=306.0, P=0.002), TBUT (Mann-Whitney U=394.5, P=0.02) and CSS (Mann-Whitney U=179.5, P<0.001). The IOP and CCT values were not significantly different between the two groups (IOP: t=1.73, P=0.09; CCT: t=1.83, P=0.07). The demographic data of the study population is summarized in Table 1.
Table 1. Demographic data of the study population.
| Parameters | SSDE group (n=34) | NSSDE group (n=34) | P |
| Age (a) | 52.29±11.32 | 49.15±14.55 | 0.32a |
| Sex (F/M) | 32/2 | 28/6 | 0.26b |
| Schirmer I test (mm) | 1.79±1.39 | 3.26±1.71 | 0.002c |
| TBUT (s) | 2.38±0.95 | 3.15±1.50 | 0.02c |
| CSS (score) | 3.09±2.43 | 1.06±0.95 | <0.001c |
| IOP (mm Hg) | 13.56±2.34 | 14.56±2.46 | 0.09a |
| CCT (µm) | 525.79±30.99 | 539.06±28.63 | 0.07a |
SSDE: Sjögren's Syndrome dry eye; NSSDE: Non-Sjögren Syndrome dry eye; F: Female; M: Male; TBUT: Tear break-up time; CSS: Corneal staining score; IOP: Intraocular pressure; CCT: Central corneal thickness. at test value; bχ2-test value; cMann-Whitney U test value.
In this study, of the ten biomechanical parameters recorded by CorVis ST, three parameters, namely DA, A1-time and HC-time, showed significant differences between the SSDE group and NSSDE group. Patients in the SSDE group had significantly lower A1-time and HC-time (A1-time: t=2.52, P=0.01; HC-time: t=2.30, P=0.02) compared to the NSSDE group; while DA was significantly larger in the SSDE group compared to the NSSDE group (t=2.33, P=0.02) (Figure 1). None of the other CorVis ST parameters showed a statistically significant difference between the SSDE group and NSSDE group (P all >0.05). All parameters derived from CorVis ST in the two groups are outlined in Table 2.
Figure 1. Box and whisker plots show the distribution between the SSDE group and NSSDE group for DA (A), A1-time (B) and HC time (C).
The median for each data set is marked by the center line, with the error bars showing the minimum and maximum values of all of the data, n=34.
Table 2. CorVis ST measured parameters in the SSDE and NSSDE groups.
| Parameters | SSDE group (n=34) | NSSDE group (n=34) | t/U | P |
| A1-time (ms) | 7.40±0.30 | 7.62±0.42 | 2.52a | 0.01 |
| A2-time (ms) | 22.27±0.68 | 22.23±0.68 | 0.2a | 0.81 |
| A1-length (mm) | 1.73±0.09 | 1.75±0.05 | 506.0c | 0.38 |
| A2-length (mm) | 1.72±0.24 | 1.73±0.24 | 524.5c | 0.52 |
| A1-V (m/s) | 0.15±0.02 | 0.14±0.02 | 488.5c | 0.27 |
| A2-V (m/s) | -0.35±0.08 | -0.32±0.07 | 1.24a | 0.22 |
| HC-time (ms) | 17.27±0.83 | 17.68±0.61 | 2.30a | 0.02 |
| PD (mm) | 3.84±1.17 | 4.15±1.00 | 516.0c | 0.45 |
| HC radius (mm) | 6.89±0.88 | 7.02±0.82 | 528.5c | 0.55 |
| DA (mm) | 1.13±0.17 | 1.04±0.13 | 2.33a | 0.02 |
SSDE: Sjögren's syndrome dry eye; NSSDE: Non-Sjögren syndrome dry eye; A1-V: A1-Velocity; A2-V: A2-Velocity; HC-time: Highest concavity-time; PD: Peak distance; HC radius: Radius at HC; DA: Deformation amplitude. at-test value; cMann-Whitney U test value.
mean±SD
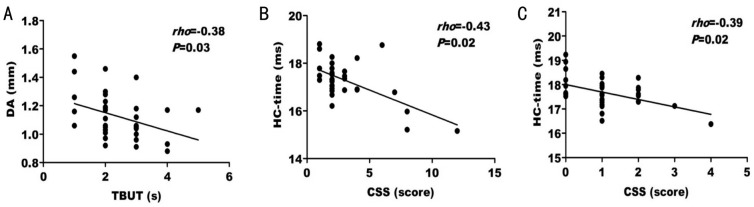
Bivariate correlation analysis was performed to detect the correlations between the above three significantly different biomechanical parameters of the cornea with tear film parameters. In the SSDE group, DA was negatively correlated with TBUT (rho=-0.38, P=0.03) (Figure 2A) where as HC-time was found to only be negatively correlated with CSS (rho=-0.43, P=0.02) (Figure 2B). In the NSSDE group, only HC-time had a significant negative correlation with CSS (rho=-0.39, P=0.02) (Figure 2C). The correlation coefficients and P values are shown in Table 3.
Figure 2. Scatter diagrams of bivariate correlation analysis.
A: Correlation between the DA and TBUT in SSDE group; B: Correlation between the HC-time and CSS in SSDE group; C: Correlation between the HC-time and CSS in non-SSDE group. rho: Spearman's correlation coefficient value.
Table 3. Factors associated with corneal parameters with bivariate correlation analysis.
| Parameters |
rho (P) of SSDE group (n=34) |
rho (P) of NSSDE group (n=34) |
||||
| DA | A1-time | HC-time | DA | A1-time | HC-time | |
| ST (mm) | -0.26 (0.14) | 0.25 (0.16) | 0.34 (0.06) | 0.19 (0.28) | 0.10 (0.58) | 0.14 (0.45) |
| TBUT (s) | -0.38 (0.03) | 0.21 (0.24) | 0.07 (0.71) | -0.01 (0.89) | 0.09 (0.61) | 0.12 (0.50) |
| CSS (score) | -0.05 (0.80) | 0.07 (0.68) | -0.43 (0.02) | -0.12 (0.52) | -0.10 (0.59) | -0.39 (0.02) |
SSDE: Sjögren's syndrome dry eye; NSSDE: Non-Sjögren's syndrome dry eye; ST: Schirmer I test; TBUT: Tear break-up time; CSS: Corneal staining score; IOP: Intraocular pressure; CCT: Central corneal thickness; DA: Deformation amplitude; A1-time: Time reaching the first applanation; HC-time: Highest concavity-time; rho: Spearman's correlation coefficient value.
DISCUSSION
Recently, the CorVis ST has allowed the assessment of corneal biomechanics in normal subjects independent of corneal morphological characteristics[22] and multiple diseased corneas, especially keratoconus (KC), glaucoma, myopic and eyes underwent corneal surgery[19],[23]–[24]. Studies have showed that, DA was significantly higher in KC and could be useful in the diagnosis and management of KC patients, particularly with crosslinking (CXL) therapy. Significantly greater A1-time, lower DA and shorter HC-time values were detected in patients with primary open angle glaucoma (POAG) than healthy controls, indicating a less deformable cornea in POAG patients[25]–[26], and changes in corneal biomechanical propertiescan be seen after long-term topical prostaglandin therapy[27]. Early areas of interest for CorVis ST included management and screening for refractive surgery. Pedersen et al[28] reported similar reduction in corneal biomechanics after the flap-based laser-assisted in situ keratomileusis (LASIK), refractive lenticule extraction (ReLEx) and the flap-free ReLEx, small incision lenticule extraction (SMILE) when evaluated by CorVis ST.
In this study, we identified significant differences with regard to three out of ten corneal biomechanical parameters between the SSDE and NSSDE group: DA, A1-time and HC-time. To the best of our knowledge, this is the first report addressing the corneal biomechanics in SSDE subjects using CorVis ST.
Patients in the SSDE group yielded a significantly shorter A1-time and HC-time, but larger DA compared to the NSSDE group. Theoretically, DA is the displacement of the corneal apex in reference to the overlaid cornea in an initial state. A1-time and HC-time are the times from the start until the first applanation and when HC is achieved, therefore, all of the above three parameters are considered to be a reflection of corneal stiffness[21],[29]. The higher DA accompanied with a lower A1-time and HC-time in the SSDE group indicated less “stiffness” of the corneal tissue in dry eyes with SS compared to dry eyes without SS.
The cornea is one of the most affected tissues in dry eye, with biomechanical properties that can be affected by the whole corneal layers and hydration[30]. Importantly, collagen in the Bowman's layer and stroma accounts for 80% of corneal dry weight and would be the major contribution to corneal biomechanical behavior[31]. Therefore, more severe corneal damage due to collagen lysis can be expected in SSDE compared to NSSDE, which consequently results in more weakened corneal biomechanics. This hypothesis is borne out by our findings that the cornea shows less “stiffness” in the SSDE group compared to the NSSDE group as represented by differences in corneal biomechanical parameters.
Although inflammation has been shown to be a major factor in the pathogenesis of dry eye, and an uncontrolled cycle of inflammation can trigger ocular surface damage in dry eyes (either SS or non-SS)[1],[32]–[33], inflammation has been shown to be more severe in SSDE patients[34]–[35], reflected by the higher level of inflammatory cytokines in tear film and also the shorter telomere length in the lacrimal gland of SSDE compared to NSSDE subjects[36]. In line with other publications[36]–[38], our study observed that dry eye associated with SS exhibited a significantly lower Schirmer I test value, shorter TBUT and higher CSS than non SS dry eye.
To investigate the potential factors affecting biomechanical properties, we observed the relationship between the above three significant biomechanical parameters and tear film parameters. The results showed that HC-time was negatively correlated with CSS in both the SSDE and NSSDE groups. As addressed by studies on keratoconus and after corneal refractive surgery, HC-time is the time from the start until the highest concavity is achieved, and a shorter HC-time may be associated with a less stiff cornea[28],[39]. CSS represents the anatomical integrity of the corneal surface and contributes to the grading of dry eye severity[1]. In the current study, we found a significantly negative effect of CSS on HC-time in the NSSDE group, which was in accordance with our previous study on dry eye without SS[14], and we also detected a similar correlation in the SSDE group. Our finding suggested that greater corneal surface damage leads to a less stiff cornea, resulting in a shorter time to achieve the highest concavity. In addition to CSS, TBUT had a significantly negative effect on DA in the SSDE group, but not in the NSSDE group, which may reflect the potential effect of TBUT on corneal compliance in SSDE subjects. To the extent of our study, subjects in SSDE group were more predisposed to be affected by tear film parameters than the NSSDE group, and further studies are needed to confirm our findings.
There were a number of limitations to this study. First, not all of the CorVis ST parameters have ideal repeatability in adults studies, although DA, A1-time and HC-time were reported to have low coefficient of variation values[12],[39]–[40] and so our results still need to be confirmed in the future studies under multiple measurements along with the improvement of the equipment design; second, due to the relatively small sample size, we didn't analyze the effect of systemic status on corneal biomechanics in the SSDE group; third, the IOP value in our study was achieved by CorVis ST instead of Goldman applanation tonometry, therefore, the effect of potential corneal collage nose due to SS on Goldman IOP was still unknown; fourth, since a number of factors such as the hydration status of the cornea over time may affect the measurement, it would be interesting to evaluate the diurnal variation of the biomechanics properties by looking at the intraclass correlation coefficient instead of only one measurement between 8 and 11 a.m. in the current study; and fifth, biomechanical properties can be affected by corneal hydration, but in dry eye patients, although no eyedrops were permitted 1d before measurement, it is still difficult to eliminate the influence caused by the use of artificial tears.
In summary, the current study showed differences in corneal biomechanical properties between SSDE and NSSDE. The cornea of SSDE tends to show less “stiffness”, represented by significantly shorter A1-time and HC-time, but larger DA, compared to the cornea of NSSDE. This finding implies that although both SSDE and NSSDE have been shown to be inflammation related diseases, different clinically relevant corneal biomechanical features could exist, which can be observed by CorVis ST. Our work may contribute to guide ophthalmological interventions and clinical therapy for SSDE and NSSDE patients in the future.
Acknowledgments
Foundation: Supported by the National Natural Science Foundation of China (No.81070755).
Conflicts of Interest: Long Q, None; Wang JY, None; Xu D, None; Li Y, None.
REFERENCES
- 1.The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007) Ocul Surf. 2007;5(2):75–92. doi: 10.1016/s1542-0124(12)70081-2. [DOI] [PubMed] [Google Scholar]
- 2.Elsheikh A, Alhasso D, Rama P. Assessment of the epithelium's contribution to corneal biomechanics. Exp Eye Res. 2008;86(2):445–451. doi: 10.1016/j.exer.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 3.Akpek EK, Klimava A, Thorne JE, Martin D, Lekhanont K, Ostrovsky A. Evaluation of patients with dry eye for presence of underlying Sjogren syndrome. Cornea. 2009;28(5):493–497. doi: 10.1097/ICO.0b013e31818d3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luciano N, Valentini V, Calabrò A, Elefante E, Vitale A, Baldini C, Bartoloni E. One year in review 2015: Sjögren syndrome. Clin Exp Rheumatol. 2015;33(2):259–271. [PubMed] [Google Scholar]
- 5.Maślińska M, Przygodzka M, Kwiatkowska B, Sikorska-Siudek K. Sjögren's syndrome: still not fully understood disease. Rheumatol Int. 2015;35(2):233–241. doi: 10.1007/s00296-014-3072-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whitcher JP, Shiboski CH, Shiboski SC, Heidenreich AM, Kitagawa K, Zhang S, Hamann S, Larkin G, McNamara NA, Greenspan JS, Daniels TE. A simplified quantitative method for assessing keratoconjunctivitis sicca from the Sjögren's Syndrome International Registry. Am J Ophthalmol. 2010;149(3):405–415. doi: 10.1016/j.ajo.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao J, Kubo S, Nakayamada S, Shimajiri S, Zhang X, Yamaoka K, Tanaka Y. Association of plasmacytoid dendritic cells with B cell infiltration in minor salivary glands in patients with Sjögren's Syndrome. Mod Rheumatol. 2016;26(5):716–724. doi: 10.3109/14397595.2015.1129694. [DOI] [PubMed] [Google Scholar]
- 8.Szabo K, Papp G, Dezso B, Zeher M. The histopathology of labial salivary glands in primary Sjögren's syndrome: focusing on follicular helper T cells in the inflammatory infiltrates. Mediators Inflamm. 2014;2014:631787. doi: 10.1155/2014/631787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Christodoulou MI, Kapsogeorgou EK, Moutsopoulos HM. Characteristics of the minor salivary gland infiltrates in Sjögren's syndrome. J Autoimmun. 2010;34(4):400–407. doi: 10.1016/j.jaut.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 10.Gregoratos ND, Bartsocas CS, Papas K. Blue sclerae with keratoglobus and brittle cornea. Br J Ophthalmol. 1971;55(6):424–426. doi: 10.1136/bjo.55.6.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luce DA. Determining in vivo biomechanical properties of the cornea with an ocular response analyzer. J Cataract Refract Surg. 2005;31(1):156–162. doi: 10.1016/j.jcrs.2004.10.044. [DOI] [PubMed] [Google Scholar]
- 12.Hon Y, Lam AK. Corneal deformation measurement using Scheimpflug noncontact tonometry. Optom Vis Sci. 2013;90(1):e1–e8. doi: 10.1097/OPX.0b013e318279eb87. [DOI] [PubMed] [Google Scholar]
- 13.Firat PG, Doganay S. Corneal hysteresis in patients with dry eye. Eye (Lond) 2011;25(12):1570–1574. doi: 10.1038/eye.2011.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Long Q, Wang J, Yang X, Jin Y, Ai F, Li Y. Assessment of corneal biomechanical properties by CorVis ST in patients with dry eye and in healthy subjects. J Ophthalmol. 2015;2015:380624. doi: 10.1155/2015/380624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yazici AT, Kara N, Yüksel K, Altinkaynak H, Baz O, Bozkurt E, Demirok A. The biomechanical properties of the cornea in patients with systemic lupus erythematosus. Eye (Lond) 2011;25(8):1005–1009. doi: 10.1038/eye.2011.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prata TS, Sousa AK, Garcia Filho CA, Doi LM, Paranhos A., Jr Assessment of corneal biomechanical properties and intraocular pressure in patients with rheumatoid arthritis. Can J Ophthalmol. 2009;44(5):602. doi: 10.3129/i09-082. [DOI] [PubMed] [Google Scholar]
- 17.Emre S, Kayikçioğlu O, Ateş H, Cinar E, Inceoğlu N, Yargucu F, Pirildar T, Oksel F. Corneal hysteresis, corneal resistance factor, and intraocular pressure measurement in patients with scleroderma using the reichert ocular response analyzer. Cornea. 2010;29(6):628–631. doi: 10.1097/ICO.0b013e3181c3306a. [DOI] [PubMed] [Google Scholar]
- 18.Shiboski SC, Shiboski CH, Criswell L, et al. American College of Rheumatology classification criteria for Sjögren's syndrome: a data-driven, expert consensus approach in the Sjögren's International Collaborative Clinical Alliance cohort. Arthritis Care Res. 2012;64(4):475–487. doi: 10.1002/acr.21591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lanza M, Cennamo M, Iaccarino S, Irregolare C, Rechichi M, Bifani M, Gironi Carnevale UA. Evaluation of corneal deformation analyzed with Scheimpflug based device in healthy eyes and diseased ones. Biomed Res Int. 2014;2014:748671. doi: 10.1155/2014/748671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Asaoka R, Nakakura S, Tabuchi H, Murata H, Nakao Y, Ihara N, Rimayanti U, Aihara M, Kiuchi Y. The Relationship between Corvis ST Tonometry Measured corneal parameters and intraocular pressure, corneal thickness and corneal curvature. PLoS One. 2015;10(10):e0140385. doi: 10.1371/journal.pone.0140385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Valbon BF, Ambrósio R, Jr, Fontes BM, Alves MR. Effects of age on corneal deformation by non-contact tonometry integrated with an ultra-high-speed (UHS) Scheimpflug camera. Arq Bras Oftalmol. 2013;76(4):229–232. doi: 10.1590/s0004-27492013000400008. [DOI] [PubMed] [Google Scholar]
- 22.Lanza M, Cennamo M, Iaccarino S, Romano V, Bifani M, Irregolare C, Lanza A. Evaluation of corneal deformation analyzed with a Scheimpflug based device. Cont Lens Anterior Eye. 2015;38(2):89–93. doi: 10.1016/j.clae.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 23.Lee R, Chang RT, Wong IY, Lai JS, Lee JW, Singh K. Assessment of corneal biomechanical parameters in myopes and emmetropes using the Corvis ST. Clin Exp Optom. 2016;99(2):157–162. doi: 10.1111/cxo.12341. [DOI] [PubMed] [Google Scholar]
- 24.Lanza M, Iaccarino S, Bifani M. In vivo human corneal deformation analysis with a Scheimpflug camera, a critical review. J Biophotonics. 2016;9(5):464–477. doi: 10.1002/jbio.201500233. [DOI] [PubMed] [Google Scholar]
- 25.Wang W, Du S, Zhang X. Corneal deformation response in patients with primary open-angle glaucoma and in healthy subjects analyzed by Corvis ST. Invest Ophthalmol Vis Sci. 2015;56(9):5557–5565. doi: 10.1167/iovs.15-16926. [DOI] [PubMed] [Google Scholar]
- 26.Lee R, Chang RT, Wong IY, Lai JS, Lee JW, Singh K. Novel parameter of corneal biomechanics that differentiate normals from glaucoma. J Glaucoma. 2016;25(6):e603–e609. doi: 10.1097/IJG.0000000000000284. [DOI] [PubMed] [Google Scholar]
- 27.Wu N, Chen Y, Yu X, Li M, Wen W, Sun X. Changes in corneal biomechanical properties after long-term topical prostaglandin therapy. PLoS One. 2016;11(5):e0155527. doi: 10.1371/journal.pone.0155527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pedersen IB, Bak-Nielsen S, Vestergaard AH, Ivarsen A, Hjortdal J. Corneal biomechanical properties after LASIK, ReLEx flex, and ReLEx smile by Scheimpflug-based dynamic tonometry. Graefes Arch Clin Exp Ophthalmol. 2014;252(8):1329–1335. doi: 10.1007/s00417-014-2667-6. [DOI] [PubMed] [Google Scholar]
- 29.Steinberg J, Katz T, Mousli A, Frings A, Casagrande MK, Druchkiv V, Richard G, Linke SJ. Corneal biomechanical changes after crosslinking for progressive keratoconus with the corneal visualization scheimpflug technology. J Ophthalmol. 2014;2014:579190. doi: 10.1155/2014/579190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Piñero DP, Alcón N. In vivo characterization of corneal biomechanics. J Cataract Refract Surg. 2014;40(6):870–887. doi: 10.1016/j.jcrs.2014.03.021. [DOI] [PubMed] [Google Scholar]
- 31.Garcia-Porta N, Fernandes P, Queiros A, Salgado-Borges J, Parafita-Mato M, González-Méijome JM. Corneal biomechanical properties in different ocular conditions and new measurement techniques. ISRN Ophthalmol. 2014;2014:724546. doi: 10.1155/2014/724546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Niu L, Zhang S, Wu J, Chen L, Wang Y. Upregulation of NLRP3 inflammasome in the tears and ocular surface of dry eye patients. PLoS One. 2015;10(5):e0126277. doi: 10.1371/journal.pone.0126277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coursey TG, de Paiva CS. Managing Sjögren's Syndrome and non-Sjögren Syndrome dry eye with anti-inflammatory therapy. Clin Ophthalmol. 2014;8:1447–1458. doi: 10.2147/OPTH.S35685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pflugfelder SC, Tseng SC, Sanabria O, Kell H, Garcia CG, Felix C, Feuer W, Reis BL. Evaluation of subjective assessments and objective diagnostic tests for diagnosing tear-film disorders known to cause ocular irritation. Cornea. 1998;17(1):38–56. doi: 10.1097/00003226-199801000-00007. [DOI] [PubMed] [Google Scholar]
- 35.Rivas L, Murube J, Shalaby O, Oroza MA, Sanz AI. Impression cytology contribution to differential diagnosis of Sjogren syndrome in the ophthalmological clinic. Arch Soc Esp Oftalmol. 2002;77(2):63–72. [PubMed] [Google Scholar]
- 36.Kawashima M, Kawakita T, Maida Y, Kamoi M, Ogawa Y, Shimmura S, Masutomi K, Tsubota K. Comparison of telomere length and association with progenitor cell markers in lacrimal gland between Sjogren syndrome and non-Sjogren syndrome dry eye patients. Mol Vis. 2011;17:1397–1404. [PMC free article] [PubMed] [Google Scholar]
- 37.Goto E, Matsumoto Y, Kamoi M, Endo K, Ishida R, Dogru M, Kaido M, Kojima T, Tsubota K. Tear evaporation rates in Sjogren syndrome and non-Sjogren dry eye patients. Am J Ophthalmol. 2007;144(1):81–85. doi: 10.1016/j.ajo.2007.03.055. [DOI] [PubMed] [Google Scholar]
- 38.Horwath-Winter J, Berghold A, Schmut O, Floegel I, Solhdju V, Bodner E, Schwantzer G, Haller-Schober EM. Evaluation of the clinical course of dry eye syndrome. Arch Ophthalmol. 2003;121(10):1364–1368. doi: 10.1001/archopht.121.10.1364. [DOI] [PubMed] [Google Scholar]
- 39.Bak-Nielsen S, Pedersen IB, Ivarsen A, Hjortdal J. Dynamic Scheimpflug-based assessment of keratoconus and the effects of corneal cross-linking. J Refract Surg. 2014;30(6):408–414. doi: 10.3928/1081597X-20140513-02. [DOI] [PubMed] [Google Scholar]
- 40.Nemeth G, Hassan Z, Csutak A, Szalai E, Berta A, Modis L., Jr Repeatability of ocular biomechanical data measurements with a Scheimpflug-based noncontact device on normal corneas. J Refract Surg. 2013;29(8):558–563. doi: 10.3928/1081597X-20130719-06. [DOI] [PubMed] [Google Scholar]