Abstract
AIM
To evaluate changes of proinflammatory cytokines in aqueous humor of patients with acute primary angle-closure (APAC) and age-related cataracts.
METHODS
Twenty eyes of 20 APAC patients and 15 eyes of 15 age-related cataract patients were included in this cross-sectional study. Aqueous humor samples were collected prospectively. The levels of 20 proinflammatory cytokines were evaluated in the aqueous humor of the APAC and cataract patients using the multiplex bead immunoassay technique. Clinical data were collected for correlation analysis.
RESULTS
Seven of the 20 proinflammatory cytokines included in the magnetic bead panel were detectable in both APAC eyes and cataract eyes: interleukin (IL)-10, IL-12, IL-15, IL-21, IL-6, chemokine (C-C motif) ligand 20, and tumor necrosis factor alpha (TNF-α). IL-27 was only detectable in APAC eyes. Compared with the cataract eyes, the APAC eyes had significantly elevated concentrations of IL-12 (P=0.036), IL-15 (P=0.001), IL-6 (P=0.012), and IL-27 (only detectable in APAC eyes). Age was positively correlated with IL-12 (P=0.022) and IL-6 (P=0.037), and time elapsed between APAC onset and aqueous humor samples collection was positively correlated with IL-15 (P=0.037), IL-27 (P=0.040), and TNF-α (P=0.042).
CONCLUSION
Several proinflammatory cytokines including IL-12, IL-15, IL-6 and IL-27, were elevated in the APAC eyes and may be implicated in its pathologic mechanism.
Keywords: acute primary angle-closure, aqueous humor, inflammation, cytokines
INTRODUCTION
Acute primary angle closure (APAC), one of the most serious ophthalmic emergencies, is characterized by a sudden rise in intraocular pressure (IOP) and structural damage, accompanied by severe inflammation in the anterior segment[1]. Studies have revealed that infectious inflammation and autoimmune inflammation have a role in the physiopathology of acute secondary angle closure, possibly by forming posterior synechiae leading to pupillary block and peripheral anterior synechiae[2]–[3]. However, it has not been fully elucidated how proinflammatory conditions in the anterior segment contribute to the onset and development of APAC.
Our previous studies showed that when compared with the cataract group, some general inflammatory cytokines in the aqueous, including interleukin (IL)-6, IL-8, granulocyte-colony stimulating factor, monocyte chemoattractant protein (MCP)-1, MCP-3, and vascular endothelial growth factor (VEGF), were significantly higher in primary angle-closure glaucoma patients[4]. However, studying only these inflammatory cytokines that mostly emerge at the peak of inflammation is insufficient to understand the exact role of inflammation in the onset and progression of APAC. Therefore, further exploration of proinflammatory cytokines which are more likely implicated in the early stage of inflammation may serve to provide a better understanding of the pathogenesis and development of APAC.
In this study, we focused on 20 proinflammatory cytokines in the aqueous humor of APAC eyes and investigated the possible roles of proinflammatory cytokines in the pathologic mechanism of APAC.
SUBJECTS AND METHODS
Subjects and Enrollment Criteria
The Ethical Review Committee of Zhongshan Ophthalmic Center approved this study. Informed consent forms were signed by all participants in this study in accordance with the tenets of the Declaration of Helsinki.
All subjects were from the Chinese Han population and fulfilled the following inclusion criteria[5]: 1) presence of at least two of the following symptoms: ocular or periocular pain, nausea, and/or vomiting, and an antecedent history of intermittent blurring of vision with halos; 2) IOP of at least 22 mm Hg (as measured by Goldmann applanation tonometry); 3) presence of at least three of the following signs of conjunctival injection, corneal epithelial edema, mid-dilated unreactive pupil, and shallow anterior chamber; 4) presence of an occluded angle in the affected eye, verified by gonioscopy. An ultrasound biomicroscopy (UBM) examination was performed on all of the eyes to confirm the presence of a narrow-angle pupillary block component. One eye of each subject was included. All eyes underwent thorough ophthalmic examinations, including IOP measurement (Goldmann applanation tonometry), slit-lamp biomicroscopy, gonioscopy, fundus examination, B-scanning and UBM.
All of the APAC eyes underwent peripheral iridectomy or trabeculectomy. We standardized the antiglaucomatous medication treatment for APAC as described in our previous study[6]: topical pilocarpine 1% four times daily; topical β-blocker (timolol 0.5%) twice daily and/or brinzolamide (Azopt; Alcon Laboratories, Elkridge, MD, USA) and/or topical α-2 agonists (Alphagan; Allergan, Inc., Irvine, CA, USA); topical steroids; oral acetazolamide 250 mg three times daily; and intravenous mannitol 20% at 1-2 g/kg four hours after the initiation of the treatment when IOP was not reduced by 20% from the initial IOP, unless contraindicated by systemic disease (e.g. congestive heart failure).
Patients with age-related cataracts who underwent routine cataract surgery were included as controls. The enrolled cataract patients had normal IOP readings.
Patients were excluded in the presence of any of the following criteria: a secondary acute attack due to lens subluxation, trauma, tumor, uveitis, iris neovascularization, or any obvious cataract leading to an intumescent lens; a known systemic inflammatory, autoimmune, or immunosuppressive disease; a preexisting ocular disease (e.g. retinal artery occlusion, retinal vein occlusion, age-related macular degeneration, diabetic retinopathy); or a history of previous ocular surgery. Patients with histories of chronic or intermittent angle closure were also excluded.
Aqueous Humor Collection
In accordance with the procedure described in our previous study[7], aqueous humor samples (50-100 µL) were collected as follows: 1) samples were collected from the APAC eyes at the start of trabeculectomy or peripheral iridectomy; 2) samples were collected from the cataract eyes at the start of cataract surgery. To avoid the influence of surgical traumatic damage to the blood-aqueous barrier (BAB), all samples were obtained prior to any conjunctival or intraocular manipulation. All samples were immediately stored at -80°C for later analysis.
Cytokine Analysis
Cytokine concentrations were analyzed using a multiplex bead-based immunoassay system (MILLIPLEX® MAP Human Th17 Magnetic Bead Panel Kit; Luminex Corp., Austin, TX, USA). The assays were performed according to the instruction manual, using a Bio-Plex® suspension array system (Bio-Plex 200; Bio-Rad, Hercules, CA, USA). The kit was used for the simultaneous quantification of the following cytokines: IL-17A, IL-17F, IL-10, chemokine (C-C motif) ligand 20 (CCL20)/macrophage inflammatory protein 3a, IL-12P70, IL-13, IL-15, IL-22, IL-9, IL-33, IL-21, IL-4, IL-23, IL-5, IL-6, IL-17E/IL-25, IL-27, IL-31, tumor necrosis factor alpha (TNF-α), and IL-28A. A 25 µL volume of aqueous sample was used in each reaction. The minimum detectable concentration of the assay kit for these analytes varies from 0.3 pg/mL to 0.099 ng/mL, in accordance with the instruction manual.
Statistical Analysis
All the data in this study were processed and analyzed by SPSS (Version 17.0; SPSS, Chicago, IL, USA). The differences in cytokine levels between the two groups were analyzed with the Mann-Whitney U test. Correlations between the cytokines and the clinical data, including age, IOP, and elapsed time, were calculated using Spearman's correlation test. P<0.05 was considered statistically significant.
RESULTS
Basic Clinical Characteristics of the Subjects
Twenty eyes with APAC and 15 eyes with age-related cataracts were included in this study. The basic clinical characteristics of the APAC and cataract patients are summarized in Table 1. The mean age of the APAC patients and the cataract patients were 62.1±6.5y and 70.2±7.7y, respectively (P=0.002). We enrolled cataract patients with larger age because they were less likely to develop APAC in later life. As expected, the APAC group had a higher IOP1 value (the highest IOP ever measured; 41.0±13.2 mm Hg) than the cataract group (13.5±3.4 mm Hg) (P<0.001). However, the IOP2 value (IOP measured just before surgery; 17.7±10.7 mm Hg) was not significantly higher than that of the cataract group (13.5±3.4 mm Hg) (P=0.152). Time elapsed between APAC onset and aqueous humor samples collection was 9.0±7.6d (range, 1-30d).
Table 1. The basic clinical characteristics of the subjects.
| Characteristic | APAC | Cataract | P |
| No. of eyes | 20 | 15 | - |
| Age, a (SD) | 62.1 (6.5) | 70.2 (7.7) | 0.002 |
| Sex, M/F | 3/17 | 8/7 | 0.027a |
| IOP1, mm Hg (SD) | 41.0 (13.2) | 13.5 (3.4) | <0.001 |
| IOP2, mm Hg (SD) | 17.7 (10.7) | 13.5 (3.4)1 | 0.152 |
| Elapsed time, d (SD) | 9.0 (7.6) | N/A | - |
SD: Standard deviation; IOP1: The highest intraocular pressure that ever measured; IOP2: The intraocular pressure measured at the time before surgery; Elapsed time: Time elapsed between APAC onset and aqueous humor samples collection; N/A: No data collect. 1The intraocular pressure in the cataract patients was only measured once before surgery, so the IOP2 is the same with IOP1; aThe difference of the sex distribution between the two groups was measured by χ2 test.
Aqueous Cytokines
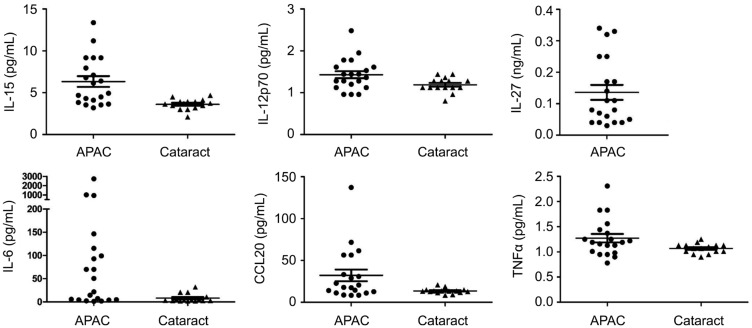
The aqueous concentrations of 20 proinflammatory cytokines were measured in both the cataract and APAC groups (Table 2). In this study, 20 proinflammatory cytokines were included in the magnetic bead panel. Only IL-10, CCL20, IL-12, IL-15, IL-21, IL-6, and TNFα were detectable in both APAC eyes and cataract eyes; IL-27 was only detectable in APAC eyes; the levels of the other 12 cytokines were under the minimum detectable concentration of this panel. Compared to the cataract group, the APAC group had significantly elevated concentrations of IL-12 (1.4±0.4 pg/mL, P=0.036), IL-15 (6.3±2.9 pg/mL, P=0.001), IL-6 (269.8±648.2 pg/mL, P=0.012), and IL-27 (0.14±0.11 ng/mL, only detectable in APAC eyes). The CCL20 and TNFα levels were also higher, with the P values (CCL20: P=0.057; TNF: P=0.056) almost reaching the bound of 0.05 (Figure 1; Table 2).
Table 2. The levels of different cytokines in aqueous humor.
| Cytokine | APAC | Cataract | aP |
| IL-10 (ng/mL) | 0.52 (0.21) | 0.51 (0.20) | 0.65 |
| CCL20 (pg/mL) | 32.2 (31.5) | 13.5 (3.1) | 0.057 |
| IL-12 (pg/mL) | 1.4 (0.4) | 1.2 (0.2) | 0.036 |
| IL-15 (pg/mL) | 6.3 (2.9) | 3.6 (0.6) | 0.001 |
| IL-21 (pg/mL) | 3.5 (2.8) | 2.1 (0.6) | 0.203 |
| IL-6 (pg/mL) | 269.8 (648.2) | 8.2 (9.1) | 0.012 |
| IL-27 (ng/mL) | 0.14 (0.11) | - | - |
| TNFα (ng/mL) | 1.3 (0.4) | 1.1 (0.1) | 0.056 |
SD: Standard deviation. aDifferences between groups were tested by Mann-Whitney U test.
mean (SD)
Figure 1. Scatterplots showing the levels of CCL20, IL-12, IL-15, IL-6, IL-27, and TNFα in aqueous humor of APAC and cataract patients.
Compared to the cataract group, the APAC group had significantly elevated concentrations of IL-12 (P=0.036), IL-15 (P=0.001), IL-6 (P=0.012) and IL-27 (P=0.002). In addition, the levels of CCL20 and TNFα were also higher, whose P values (CCL20: P=0.057; TNFα: P=0.056) almost reached the bound of 0.05.
Correlation Analysis
The correlations between cytokine levels and age, elapsed time, and IOP2 are shown in Table 3. No correlation was found between aqueous cytokine levels and IOP2. However, age was positively correlated with IL-12 [correlation coefficient (CC)=0.507, P=0.022] and IL-6 (CC=0.469, P=0.037) levels, and time elapsed between APAC onset and aqueous humor samples collection was positively correlated with IL-15 (CC=0.469, P=0.037), IL-27 (CC=0.463, P=0.04), and TNFα (CC=0.458, P=0.042) levels.
Table 3. Factors influence the levels of Th-17 related cytokines in aqueous humor.
| Cytokine | Age |
IOP2 |
Elapsed time |
|||
| CC | P | CC | P | CC | P | |
| CCL20 | 0.0398 | 0.083 | 0.105 | 0.66 | 0.253 | 0.281 |
| IL-12 | 0.507 | 0.022 | -0.051 | 0.829 | 0.286 | 0.222 |
| IL-15 | 0.271 | 0.248 | 0.273 | 0.244 | 0.469 | 0.037 |
| IL-6 | 0.469 | 0.037 | 0.16 | 0.5 | 0.054 | 0.82 |
| IL-27 | 0.344 | 0.137 | 0.175 | 0.461 | 0.463 | 0.04 |
| TNFα | 0.41 | 0.072 | 0.074 | 0.758 | 0.458 | 0.042 |
The relation among the levels of the cytokines with age, IOP2 and elapsed time was calculated by Spearman's correlation. CC: Correlation coefficient.
DISCUSSION
APAC is an ocular emergency that can cause permanent damage to the optic nerve and anterior segment structures if it is not controlled. However, the pathogenesis mechanism of APAC has not yet been full elucidated. We put forward that the proinflammatory microenvironment in the anterior segment might play an important role in the onset and development of APAC. We used multiplex bead immunoassays, which can simultaneously detect multiple cytokines in small-volume clinical samples, and found that APAC eyes had significantly elevated concentrations of IL-12, IL-15, IL-6, IL-27, CCL20, and TNFα. The levels of some cytokines were related to duration of the disease or age of the APAC patient.
Both IL-12 and IL-27 belong to the IL-12 family and were upregulated in the aqueous humor of the APAC eyes in our study. IL-12 is a proinflammatory cytokine that has key roles in the development of Th1 cells and the subsequent immune cascades[8]–[9]. While IL-27 has an anti-inflammatory effect by suppressing monocyte migration and neutrophil trafficking[10], another key function is to regulate early inflammatory events during acute inflammation[11]. Therefore, IL-27 might be a protecting effector molecule of APAC. IL-12 and IL-27 performed inverse functions on inflammatory events in APAC eyes. How did interactions between IL-12 and IL-27 modulate early inflammation and its later development in APAC patients? These questions need to be investigated further.
Monocyte/macrophage lineage and many other antigen-presenting cells can produce IL-15, which could stimulate the production and secretion of IL-8 by neutrophils, leading to further recruitment of neutrophils to the inflammatory site and augmenting the inflammation[12]. IL-15 is one of the earliest cytokines to be secreted promptly during an acute inflammatory state[13]. In the present study, IL-15 was upregulated in the aqueous humor of APAC eyes; it might trigger early inflammation in the anterior segment and contribute to the onset of APAC. Targeting the IL-15 pathway might serve to control the early proinflammatory state in the anterior segment of eyes and prevent APAC.
IL-6, which is promptly and transiently produced in response to tissue injuries and immune reactions, issues a warning signal in the event of tissue damage[14]. In the current study, the aqueous level of IL-6 was higher in the APAC eyes than in the control cataract eyes, which may contribute to inflammatory damage in anterior segment of APAC eyes.
IL-12, IL-15, IL6, and IL-27 are all closely associated with immune privilege within the eye. BAB dysfunction can contribute to the pathophysiology of inflammatory ocular diseases by allowing the leakage of molecules and inflammatory cells from the blood into the anterior segment of the eye[15]. Studies have shown that IL-12 can abrogate immune privilege within the anterior chamber by activating cytolytic T cells and inducing Th1 cells, which mediate delayed-type hypersensitivity[16]. IL-15, together with TNF, can potentially increase the permeability of the blood-brain barrier (BBB) by decreasing expression of the tight junction protein claudin-2[17]. The structure of the BAB is very similar to that of the BBB; therefore, IL-15 and TNF might increase BAB permeability in APAC eyes. In addition, IL-6 can induce excess production of VEGF, leading to enhanced angiogenesis and increased vascular permeability[18]. To the contrary, it has been suggested that IL-27 contributes to ocular immune privilege presenting as BAB and blood-retina barrier[19]. It is possible that IL-12, IL-15, and IL-6 permeate the aqueous humor immediately through the BAB when damage occurs to the BAB structure. In turn, these cytokines might cooperate to promote disruption of the BAB, thus raising BAB permeability and leading to greater vascular leakage of molecules and inflammatory cells into the anterior segment of the eye. The inflammatory cells and these molecules in the aqueous humor may promote the onset of APAC by forming posterior synechiae, leading to pupillary block and peripheral anterior synechiae, and then may also exacerbate damage in APAC eyes directly. Conversely, IL-27 may serve as a protective factor in the remission state of APAC by maintaining ocular immune privilege. The different or even inverse roles of these cytokines in the pathologic mechanism of APAC through modulating the pathophysiological conditions of the BAB are complicated and need to be investigated further.
In the present study, unexpectedly, the IOP measured just before surgery was not significantly correlated with the levels of any of the cytokines we detected. This is probably because the IOP before surgery was reduced by the antiglaucoma drugs. However, interestingly, the time elapsed between APAC onset and aqueous humor samples collection was positively correlated with levels of IL-27, IL-15, and TNFα. As mentioned above, IL-15, together with TNF, can increase BAB permeability, while IL-27 may serve to maintain ocular immune privilege. The balance of these cytokines may play an important role in the remission stage of inflammation in the anterior segment. Another finding was that age was positively correlated with levels of IL-12 and IL-6, both of which can induce or promote inflammation in eyes. This finding might indicate that it becomes easier for IL-12 and IL-6 to permeate aqueous humor through the BAB with increasing age. On one hand, these upregulated proinflammatory cytokines in aqueous humor might augment inflammation and exacerbate the damage associated with APAC. On the other hand, this result may provide another possible explanation for the higher incidence of APAC in older populations.
Some potential limitations of this study should be mentioned. First, the use of topical antiglaucoma medications may influence the aqueous immune homeostasis. In the present study, the duration of medication use varied in the APAC patients due to different disease durations before surgery, which may be one of the factors generating the difference in cytokine levels. Another limitation was the sample size, as the number of subjects enrolled was relatively low. Larger patient populations might help strengthen the results and conclusions of this study.
In summary, several proinflammatory cytokines, including IL-12, IL-15, IL-6 and IL-27, were upregulated in the aqueous humor of APAC eyes compared with control eyes with cataracts. These upregulated aqueous cytokines may be implicated in the pathogenesis and progression of APAC, but the exact mechanism need to be investigated further.
Acknowledgments
Authors' Contributions: All authors conceived of and designed the experimental protocol. Liu YM and Chen SD collected the data. All authors were involved in the analysis. Liu YM wrote the first draft of the manuscript. Zhang XL and Liu YM reviewed and revised the manuscript and produced the final version. All authors read and approved the final manuscript.
Foundations: Supported by the National Natural Science Foundation of China (No.81670847; No.81600728).
Conflicts of Interest: Liu YM, None; Chen SD, None; Li XY, None; Huang WB, None; Li F, None; Wang JW, None; Li YQ, None; Zhang XL, None.
REFERENCES
- 1.Pokhrel PK, Loftus SA. Ocular emergencies. Am Fam Physician. 2007;76(6):829–836. [PubMed] [Google Scholar]
- 2.Sng CC, Barton K. Mechanism and management of angle closure in uveitis. Curr Opin Ophthalmol. 2015;26(2):121–127. doi: 10.1097/ICU.0000000000000136. [DOI] [PubMed] [Google Scholar]
- 3.Pandey A, Balekudaru S, Venkatramani DV, George AE, Lingam V, Biswas J. Incidence and management of glaucoma in vogt koyanagi harada disease. J Glaucoma. 2016;25(8):674–680. doi: 10.1097/IJG.0000000000000400. [DOI] [PubMed] [Google Scholar]
- 4.Huang W, Chen S, Gao X, Yang M, Zhang J, Li X, Wang W, Zhou M, Zhang X, Zhang X. Inflammation-related cytokines of aqueous humor in acute primary angle-closure eyes. Invest Ophthalmol Vis Sci. 2014;55(2):1088–1094. doi: 10.1167/iovs.13-13591. [DOI] [PubMed] [Google Scholar]
- 5.Friedman DS, Chew PT, Gazzard G, Ang LP, Lai YF, Quigley HA, Seah SK, Aung T. Long-term outcomes in fellow eyes after acute primary angle closure in the contralateral eye. Ophthalmology. 2006;113(7):1087–1091. doi: 10.1016/j.ophtha.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 6.Chen S, Huang W, Wang J, Zhang J, Wang W, Zhou M, Gao X, Zhang X. Soluble CD44 and vascular endothelial growth factor levels in patients with acute primary angle closure. Acta Ophthalmol. 2015;93(4):e261–e265. doi: 10.1111/aos.12564. [DOI] [PubMed] [Google Scholar]
- 7.Zhang X, Li A, Ge J, Reigada D, Laties AM, Mitchell CH. Acute increase of intraocular pressure releases ATP into the anterior chamber. Exp Eye Res. 2007;85(5):637–643. doi: 10.1016/j.exer.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 8.Vignali DA, Kuchroo VK. IL-12 family cytokines: immunological playmakers. Nat Immunol. 2012;13(8):722–728. doi: 10.1038/ni.2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Teng MW, Bowman EP, McElwee JJ, Smyth MJ, Casanova JL, Cooper AM, Cua DJ. IL-12 and IL-23 cytokines: from discovery to targeted therapies for immune-mediated inflammatory diseases. Nat Med. 2015;21(7):719–729. doi: 10.1038/nm.3895. [DOI] [PubMed] [Google Scholar]
- 10.Diveu C, McGeachy MJ, Boniface K, Stumhofer JS, Sathe M, Joyce-Shaikh B, Chen Y, Tato CM, McClanahan TK, de Waal Malefyt R, Hunter CA, Cua DJ, Kastelein RA. IL-27 blocks RORc expression to inhibit lineage commitment of Th17 cells. J Immunol. 2009;182(9):5748–5756. doi: 10.4049/jimmunol.0801162. [DOI] [PubMed] [Google Scholar]
- 11.Wirtz S, Tubbe I, Galle PR, Schild HJ, Birkenbach M, Blumberg RS, Neurath MF. Protection from lethal septic peritonitis by neutralizing the biological function of interleukin 27. J Exp Med. 2006;203(8):1875–1881. doi: 10.1084/jem.20060471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cassatella MA, McDonald PP. Interleukin-15 and its impact on neutrophil function. Curr Opin Hematol. 2000;7(3):174–177. doi: 10.1097/00062752-200005000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Stacey AR, Norris PJ, Qin L, Haygreen EA, Taylor E, Heitman J, Lebedeva M, DeCamp A, Li D, Grove D, Self SG, Borrow P. Induction of a striking systemic cytokine cascade prior to peak viremia in acute human immunodeficiency virus type 1 infection, in contrast to more modest and delayed responses in acute hepatitis b and c virus infections. J Virol. 2009;83(8):3719–3733. doi: 10.1128/JVI.01844-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scheller J, Garbers C, Rose-John S. Interleukin-6: from basic biology to selective blockade of pro-inflammatory activities. Semin Immunol. 2014;26(1):2–12. doi: 10.1016/j.smim.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 15.Kong X, Liu X, Huang X, Mao Z, Zhong Y, Chi W. Damage to the blood-aqueous barrier in eyes with primary angle closure glaucoma. Mol Vis. 2010;16:2026–2032. [PMC free article] [PubMed] [Google Scholar]
- 16.Uno T, Streilein JW, Ksander BR. The effect of IL-12 on immune privilege of the eye. Ann N Y Acad Sci. 1996;795:426–428. doi: 10.1111/j.1749-6632.1996.tb52712.x. [DOI] [PubMed] [Google Scholar]
- 17.Pan W, Wu X, He Y, Hsuchou H, Huang EY, Mishra PK, Kastin AJ. Brain interleukin-15 in neuroinflammation and behavior. Neurosci Biobehav Rev. 2013;37(2):184–192. doi: 10.1016/j.neubiorev.2012.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tzeng HE, Tsai CH, Chang ZL, Su CM, Wang SW, Hwang WL, Tang CH. Interleukin-6 induces vascular endothelial growth factor expression and promotes angiogenesis through apoptosis signal-regulating kinase 1 in human osteosarcoma. Biochem Pharmacol. 2013;85(4):531–540. doi: 10.1016/j.bcp.2012.11.021. [DOI] [PubMed] [Google Scholar]
- 19.Amadi-Obi A, Yu CR, Liu X, Mahdi RM, Clarke GL, Nussenblatt RB, Gery I, Lee YS, Egwuagu CE. TH17 cells contribute to uveitis and scleritis and are expanded by IL-2 and inhibited by IL-27/STAT1. Nat Med. 2007;13(6):711–718. doi: 10.1038/nm1585. [DOI] [PubMed] [Google Scholar]