Abstract
Background
Eosinophilia has not been reported as a manifestation of a mitochondrial disorder (MID). Here, we report a patient with clinical features suggesting a MID and permanent eosinophilia, multisystem disease, and progressive hyper-creatine-kinase (CK)-emia for at least 10 years.
Materials and Methods
Methods applied included a clinical exam, blood chemical investigations, electrophysiological investigations, imaging, and invasive cardiological investigations. The patient was repeatedly followed up over several years. He required replacement cardiac surgery.
Results
In a 57-year-old male, eosinophilia was first detected at the age of 44 years and has remained almost constantly present until today. In addition to eosinophilia, he developed progressive hyper-CK-emia at the age of 47 years. His history was further positive for hepatopathy, hyperlipidemia, hypothyroidism, renal insufficiency, spontaneous Achilles tendon rupture, double vision, exercise intolerance, muscle aching, mild hypoacusis, sensory neuropathy, seizures, and mitral insufficiency/stenosis requiring valve replacement therapy, oral anticoagulation, and pacemaker implantation. Based on the multisystem nature of his abnormalities and permanent hyper-CK-emia, a MID was suspected.
Conclusion
Eosinophilia can be associated with a MID with myopathy, possibly as a reaction to myofiber necrosis. If eosinophilia is associated with progressive hyper-CK-emia and multisystem disease, a MID should be suspected.
Keywords: Mitochondrial disorder, Multiorgan disorder, Metabolic myopathy, Epilepsy, Bone marrow, Eosinophils
Introduction
Eosinophilia is a rare finding in neuromuscular disorders (NMDs) but has occasionally been reported in some of them [1, 2, 3, 4]. Excellent overviews of NMDs with eosinophilia have been provided by Schröder et al. [5] and Pellissier et al. [6]. In mitochondrial disorders (MIDs), eosinophilia has not been reported so far but, in our own experience, can be a rare hematological manifestation of nonsyndromic MIDs [7]. Here, we report a patient with clinical features of a MID and permanent eosinophilia and hyper-creatine-kinase (CK)-emia for at least 10 years.
Case Report
The patient is a 57-year-old Caucasian male (May 2015) with a height of 180 cm and a weight of 82 kg, in whom eosinophilia was first detected in August 2003 and has remained almost constantly present until today (Fig. 1). Eosinophilia from chronic infection, worms, fungi, drugs, toxins, or allergy was excluded. His individual history was positive for several items. In 1990, he had to undergo surgery for spontaneous left-sided Achilles tendon rupture. Since at least October 1999, hyperlipidemia has been noteworthy (Fig. 2), which was treated successively with fluvastatin, bezafibrate, atorvastatin, and since March 2015 with ezetimibe (10 mg/day). At the same time (October 1999), hyperuricemia was noted. In July 2001, mild renal insufficiency became apparent which has persisted until today. In June 2007, L-thyroxine was prescribed to prevent the growth of diffuse and nodular goiter but was discontinued by the patient. Later, he developed autoimmune hypothyroidism requiring L-thyroxine since March 2013. Since March 2006, elevation of the γ-glutamyl-transpeptidase has been noted. Since 2009, he has suffered from recurrent exanthema of the buttocks. In June 2013, he experienced pneumonia.
Fig. 1.
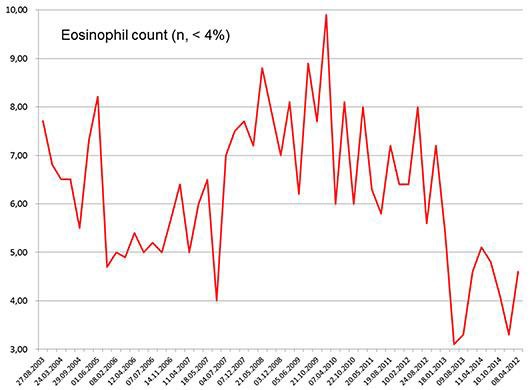
Blood eosinophil count in the presented patient over 10 years (normal values [n] <4%).
Fig. 2.
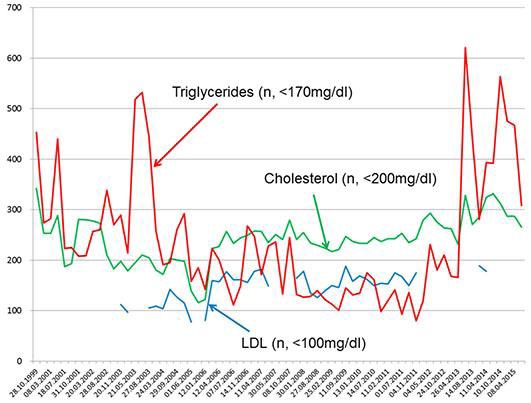
Serum triglycerides, serum cholesterol, and serum low-density lipoprotein (LDL) levels in the presented patient over 15 years. n, normal values.
Neurologic symptoms were noticed in 2002 when he recognized double vision for 3–4 min upon strenuous fixation of objects, an abnormality that has been inducible until today. In July 2005, he experienced a left-sided transient peripheral facial palsy. Since then, he has complained about mild memory impairment. At that time, also hyper-CK-emia was first recognized and has remained present until today with a tendency to increase. The maximal value was 2,088 U/l (Fig. 3). In November 2005, his wife noted a seizure-like event with a vacant stare lasting for a few seconds. In January 2006, he experienced a first tonic-clonic seizure, and carbamazepine was given. Because carbamazepine caused generalized fatigue, it was replaced by lamotrigine in April 2006. Despite changing the antiepileptic regimen, he experienced a second tonic-clonic seizure in September 2006, and lamotrigine was increased. Since then, he has only experienced recurrent focal seizures, manifesting as ascending sensory disturbance, deja vus, or smacking, with a frequency of 6 per year, and a third tonic-clonic seizure in July 2009. Since April 2006, he has recognized muscle aching after mild exercise. Neurologic exam at that time only revealed sore neck muscles and mild postural tremor. Neurologic exam in March 2009 additionally revealed reduced Achilles tendon reflexes. Electroencephalography in December 2010 was normal. In September 2013, after cardiac surgery, he experienced transient weakness for right foot extension and transient numbness of the left digit 5. Neurologic exam in November 2013 revealed mild hypoacusis, bilateral ptosis, lid edema, mild weakness for right foot extension, reduced Achilles tendon reflexes bilaterally, an exaggerated left patella tendon reflex, and postural tremor. Neurologic exam in March 2015 revealed mild hypoacusis, exaggerated tendon reflexes on the right arm, bradydiadochokinesis bilaterally with right-sided predominance, wasting of the thighs, and reduced Achilles tendon reflexes bilaterally.
Fig. 3.
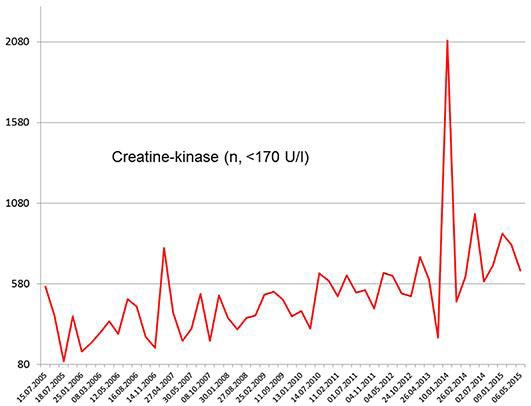
Serum creatine kinase levels in the described patient over 10 years (normal values [n] <170 U/L). Of note is the mild increase in the creatine kinase values with progression of the suspected mitochondrial disorder.
Cardiologically, he has required treatment for arterial hypertension since April 2006. Echocardiography in October 2007 revealed mitral insufficiency grade 3. Since May 2013, he has developed resting and exertional dyspnea. Echocardiography in May 2013 revealed mitral insufficiency grade 4 and mitral stenosis. Heart failure and atrial fibrillation were diagnosed, and heart failure therapy and phenprocoumon were initiated. Echocardiography revealed pulmonary hypertension (60 mm Hg) and combined mitral valve stenosis and mitral valve insufficiency grade 4; therefore, he underwent mitral valve replacement therapy (St. Jude Medical 29-mm mechanical valves) in September 2013. Coronary angiography and carotid ultrasound before surgery showed only minimal atherosclerotic alterations. Four months after cardiac surgery, he required implantation of a pacemaker because of atrioventricular block III (January 2010). Stress testing during rehabilitation after cardiac surgery had to be interrupted 2 times because of early muscle fatigue.
He had a 40-pack-year history of smoking cigarettes. The family history was positive for pulmonary embolism (father), hyperlipidemia (mother, sister), hyperuricemia (mother, sister), hypothyroidism (mother, sister, sister of mother), cataract (sister), stroke (mother), thyroid cancer (mother), osteoporosis (mother), arthrosis (mother, sister), liver cirrhosis (sister of mother), colon cancer (grandmother from the mother's side), ovarian cancer (grandmother from the mother's side), and breast cancer (grandmother from the mother's side, mother, sister of mother). His medication in April 2015 included phenprocoumon, L-thyroxine (87.5 μg/day), nebivolol (10 mg/day), lamotrigine (700 mg/day), and ezetimibe (10 mg/day). The patient did not consent with genetic diagnostics for suspected MID.
Discussion
The presented patient is interesting because of a multisystem disease affecting the central nervous system (epilepsy, mild cognitive impairment), the peripheral nerves (sensory neuropathy), the skeletal muscles (hyper-CK-emia, double vision, spontaneous tendon rupture), the endocrine system (hypothyroidism, hyperlipidemia), the ears (hypoacusis), the liver, the kidneys (renal insufficiency), and the bone marrow (eosinophilia). If all manifestations are assumed to result from a single cause, the only disorder in which such a plethora of different manifestations occurs is a MID [8]. Arguments for a MID in the presented patient are the clinical findings (episodes of double vision, epilepsy, myopathy, sensory neuropathy, spontaneous tendon rupture, renal insufficiency, hypothyroidism, hypoacusis, hepatopathy, hyperlipidemia) and the family history. Most likely, he inherited the mitochondrial multiorgan disorder syndrome from his mother who had developed hyperlipidemia, hyperuricemia, hypothyroidism, ischemic stroke, thyroid cancer, and breast cancer. Malignancy has been previously reported to be increased in MIDs [9]. Very likely, also his sister was affected, so that it is highly probable that a mutation in an mtDNA-located gene was responsible for the phenotype. Muscle biopsy has not been carried out yet because it is often negative in mildly manifesting patients and because even biochemical investigations may be negative.
Whether cardiac manifestations can be attributed to the underlying MID or not remains speculative. Most likely, he experienced a combined mitral valve cardiac defect following initially unrecognized endocarditis. Histological and bacteriological workup of the explanted valve, however, did not reveal a histological abnormality indicative of infection, and the culture was negative. A further argument against MID as the underlying cause of the valve pathology is the negative family history for cardiac disease. A rheumatologic cause of the cardiac defect was excluded since the history was negative for rheumatologic disease. He never complained about arthralgia, and there has never been inflammation with swelling, hyperthermia, or immobility of joints. A further argument against a causal relationship between suspected MID and the valve abnormality is that such a relation has not been reported before.
Eosinophilia has not been reported as a manifestation of a MID, but clinical experience suggests that eosinophilia can very well be a MID manifestation [7]. Eosinophilia in MIDs with myopathy could represent a nonspecific inflammatory reaction in response to myofiber necrosis [5]. Generally, eosinophilia in NMDs may be exclusively present within the muscle (eosinophilic myositis) or within the muscle and the peripheral blood. NMDs which have been reported in association with eosinophilic myositis include limb-girdle muscular dystrophy 2A [1, 10], limb-girdle muscular dystrophy 2C [11], Becker muscular dystrophy [12], myotonic dystrophy type 2 [13], amyotrophic lateral sclerosis [5], and inflammatory myopathy manifesting as focal eosinophilic myositis, eosinophilic polymyositis, or eosinophilic perimyositis [14]. Eosinophilia in these conditions may be idiopathic or due to parasitic infection, connective tissue disease, hematologic or nonhematological malignancy, drugs, or toxins [14]. Eosinophils may not only induce fibrosis [15], but vice versa, fibroblasts may induce eosinophilia as they are a natural source of eotaxin, a chemokine able to attract eosinophils [5].
Conclusions
This case shows that eosinophilia can be associated with myopathic MID most likely in reaction to myofiber necrosis. If eosinophilia is associated with progressive hyper-CK-emia and multisystem disease, a MID should be suspected.
Statement of Ethics
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal. The study was approved by the local ethics committee.
Disclosure Statement
The authors have no conflicts of interest to declare.
Funding Sources
No funding was received.
Author Contributions
J.F. carried out the clinical investigations and designed and drafted the report. J.H. performed clinical investigations, participated in the design and coordination, and helped to draft the manuscript. All authors have read and approved the final manuscript.
References
- 1.Oflazer PS, Gundesli H, Zorludemir S, Sabuncu T, Dincer P. Eosinophilic myositis in calpainopathy: could immunosuppression of the eosinophilic myositis alter the early natural course of the dystrophic disease? Neuromuscul Disord. 2009;19:261–263. doi: 10.1016/j.nmd.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 2.Serratrice J, Pellissier JF, Champsaur P, Weiller PJ. Fasciitis with eosinophilia: a possible causal role of angiotensin converting enzyme inhibitor. Rev Neurol (Paris) 2007;163:241–243. doi: 10.1016/s0035-3787(07)90397-5. [DOI] [PubMed] [Google Scholar]
- 3.Owens WE, 4th, Bertorini TE, Holt HT, Jr, Shadle MK. Diffuse fasciitis with eosinophilia (Shulman syndrome) J Clin Neuromuscul Dis. 2004;6:99–101. doi: 10.1097/00131402-200412000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Scola RH, Trentin AP, Fabiani G, Mücke D, Werneck LC. Peripheral neuropathy and myositis in idiopathic hypereosinophilic syndrome: case report. Arq Neuropsiquiatr. 2004;62:150–153. doi: 10.1590/s0004-282x2004000100027. [DOI] [PubMed] [Google Scholar]
- 5.Schröder T, Fuchss J, Schneider I, Stoltenburg-Didinger G, Hanisch F. Eosinophils in hereditary and inflammatory myopathies. Acta Myol. 2013;32:148–153. [PMC free article] [PubMed] [Google Scholar]
- 6.Pellissier JF, Figarella-Branger D, Serratrice G. Neuromuscular diseases with eosinophilia. Med Trop (Mars) 1998;58((4 suppl)):471–476. [PubMed] [Google Scholar]
- 7.Finsterer J. Hematological manifestations of primary mitochondrial disorders. Acta Haematol. 2007;118:88–98. doi: 10.1159/000105676. [DOI] [PubMed] [Google Scholar]
- 8.Moggio M, Colombo I, Peverelli L, Villa L, Xhani R, Testolin S, Di Mauro S, Sciacco M. Mitochondrial disease heterogeneity: a prognostic challenge. Acta Myol. 2014;33:86–93. [PMC free article] [PubMed] [Google Scholar]
- 9.Finsterer J, Krexner E. Increased prevalence of malignancy in adult mitochondrial disorders. J Med Life. 2013;6:477–481. [PMC free article] [PubMed] [Google Scholar]
- 10.Krahn M, Lopez de Munain A, Streichenberger N, Bernard R, Pécheux C, Testard H, Pena-Segura JL, Yoldi E, Cabello A, Romero NB, Poza JJ, Bouillot-Eimer S, Ferrer X, Goicoechea M, Garcia-Bragado F, Leturcq F, Urtizberea JA, Lévy N. CAPN3 mutations in patients with idiopathic eosinophilic myositis. Ann Neurol. 2006;59:905–911. doi: 10.1002/ana.20833. [DOI] [PubMed] [Google Scholar]
- 11.Baumeister SK, Todorovic S, Milić-Rasić V, Dekomien G, Lochmüller H, Walter MC. Eosinophilic myositis as presenting symptom in γ-sarcoglycanopathy. Neuromuscul Disord. 2009;19:167–171. doi: 10.1016/j.nmd.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 12.Weinstock A, Green C, Cohen BH, Prayson RA. Becker muscular dystrophy presenting as eosinophilic inflammatory myopathy in an infant. J Child Neurol. 1997;12:146–147. doi: 10.1177/088307389701200214. [DOI] [PubMed] [Google Scholar]
- 13.Meyer A, Lannes B, Carapito R, Bahram S, Echaniz-Laguna A, Geny B, Sibilia J, Gottenberg JE. Eosinophilic myositis as first manifestation in a patient with type 2 myotonic dystrophy CCTG expansion mutation and rheumatoid arthritis. Neuromuscul Disord. 2015;25:149–152. doi: 10.1016/j.nmd.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 14.Selva-O'Callaghan A, Trallero-Araguás E, Grau JM. Eosinophilic myositis: an updated review. Autoimmun Rev. 2014;13:375–378. doi: 10.1016/j.autrev.2014.01.018. [DOI] [PubMed] [Google Scholar]
- 15.Letuve S, Pretolani M. Potential role of eosinophilgranule proteins in tissue remodeling and fibrosis. In: Lee JJ, Rosenberg HF, editors. Eosinophils in Health and Disease. Amsterdam: Elsevier; 2013. pp. 393–398. [Google Scholar]


