Abstract
Background: Reducing inappropriate antibiotic prescribing in primary care is a public health priority.
Objectives: We hypothesized that a subset of patients account for the majority of antibiotic prescriptions in primary care. We investigated the relationship between the total amount of antibiotics prescribed, individual-level antibiotic use and comorbidity.
Methods: This was a cohort study using electronic health records from 1 948 390 adults registered with 385 primary care practices in the UK in 2011–13. We estimated the average number of antibiotic prescriptions per patient and the association between prescribing and comorbidity. We modelled the impact on total prescribing of reducing antibiotic use in those prescribed antibiotics most frequently.
Results: On average 30.1% (586 194/1 948 390) of patients were prescribed at least one antibiotic per year. Nine percent (174 602/1 948 390) of patients were prescribed 53% (2 091 496/3 922 732) of the total amount of antibiotics, each of whom received at least five antibiotic prescriptions over 3 years. The presence of any comorbidity increased the prescribing rate by 44% [adjusted incidence rate ratio (IRR) 1.44, 95% CI 1.43–1.45]; rates of prescribing to women exceeded those in men by 62% (adjusted IRR 1.62, 95% CI 1.62–1.63).
Conclusions: Half of antibiotics prescribed to adults in primary care were for <10% of patients. Efforts to tackle antimicrobial resistance should consider the impact of this on total prescribing.
Introduction
Reducing inappropriate antimicrobial prescribing is fundamental to national and international efforts to halt the emergence of drug resistance.1–3 In Europe >80% of antimicrobials are prescribed in the community,4 highlighting the need to reduce antimicrobial use in primary care.
Rates of antibiotic prescribing have been shown to vary widely between practices in primary care,5,6 with prescribing rates in the UK slightly less than the EU average.4 This has been interpreted as an opportunity to improve prescribing quality.7 In England, financial targets have been set to reduce total antibiotic prescribing and the use of broad-spectrum antibiotics in primary care by 2015/16, placing the onus firmly on general practitioners.7,8 However, the extent to which prescribing variation is driven by the behavioural, clinical and demographic characteristics of each individual patient remains uncertain.9
Using data from The Health Improvement Network (THIN) UK primary care database, we set out to measure variation in the rates of antibiotic prescribing between individuals and to investigate the relationship between individual-level antibiotic prescribing and age, gender and comorbidity, while accounting for variation in prescribing rates between general practices. We modelled the theoretical impact on total antibiotic prescribing of reducing antibiotic use in patients who are prescribed antibiotics frequently by calculating population-attributable fractions.
Methods
Ethics
The programme of anonymized data provision for researchers using THIN was approved in 2002. This study was approved by the scientific review committee, reference 15THIN074.
Description of the dataset
THIN is a large primary care database comprising electronic medical records from more than 12 million patients across the UK.10 In the UK, 98% of the population is registered with a general practitioner, who acts as the gatekeeper to specialist services and provides advice, treatment and prescriptions.11 Practices contributing to the THIN scheme of data collection enter demographic and clinical data every time a consultation takes place, generating a longitudinal record. Symptoms, diagnoses, treatments and referrals are recorded using a hierarchical system of >100 000 Read codes.12 Prescriptions are recorded by using Multilex (www.fdbhealth.co.uk/solutions/multilex) drug codes, which link each drug formulation to the British National Formulary (www.bnf.org), a compendium of drugs arranged by system into 15 chapters. Estimates of social deprivation are based on the Townsend score, a composite measure derived from levels of unemployment, car ownership, household overcrowding and housing tenure linked to the individual’s postcode. The THIN dataset is broadly representative of the UK population,13 with prescription and consultation rates that are comparable to national statistics and external data sources.14 The adequacy of death recording is assessed by comparing mortality rates recorded by the practice with national age and sex-standardized mortality rates.15 Thus we included data from when practices met the acceptable mortality recording criteria, and as we used data from 2011 to 2013 all practices were already fully computerized.16
Clinical definitions and statistical analysis
Patients aged between 18 and 100 years were eligible for inclusion provided they had a valid record for date of birth, gender and deprivation quintile. Patients entered the cohort on 1 January 2011 and exited the cohort on 31 December 2013. Only patients who were registered for the whole 3 year period were included in the study.
An antibiotic prescription was defined as prescription of any drug in chapter 5.1 (Antibacterial drugs) of the British National Formulary, excluding anti-TB and anti-leprotic drugs, metronidazole and tinidazole because we were primarily interested in drugs prescribed for common infections, such as respiratory, urinary tract or skin infections. We developed a list of comorbid conditions that might influence the general practitioner’s decision to prescribe an antibiotic, based on national guidance on the management of common infections in primary care.17 To identify patients with coronary artery disease, COPD, chronic kidney disease, stroke, heart failure or peripheral arterial disease recorded before 31 December 2011, we developed Read code lists based on the definitions used in the Quality and Outcomes Framework (QOF).18 We defined coronary artery disease as a QOF-defined record for secondary prevention of coronary artery disease to exclude patients who only had risk factors for coronary artery disease. We also identified individuals with asthma, high BMI and smoking. We simplified the QOF-based definition for asthma (see the code lists available as Supplementary data at JAC Online) because we were interested in a sensitive rather than specific definition of comorbidity. We defined patients as obese if they had at least one record of BMI >30 kg/m2 in the 5 years before 31 December 2011, on the assumption that the majority of obese patients fail to achieve sustained reductions in weight. Smoking use was categorized into a binary variable by defining anyone with a record of having smoked during the follow-up period, or having smoked prior to the follow-up period with no record of having stopped as a smoker, and anyone else as a non-smoker. Age was grouped as a categorical variable reflecting the patterns of antibiotic use in different age groups. We excluded children and did not collect information on prescriptions of immunosuppressive drugs because our analysis focused on the relationship between prescribing and comorbidity.
To obtain a more precise assessment of the average rate of antibiotic prescribing per patient, we used prescribing data for a 3 year period. We calculated the average rate of antibiotic prescribing per 3 years, and the equivalent annual rate, counting the total number of antibiotics prescribed per patient between 1 January 2011 and 31 December 2013. The denominator was the total number of person-years contributed by patients in the cohort over the same time period. Variation in per patient antibiotic prescribing was displayed graphically. We fitted negative binomial regression models to estimate the average number of antibiotics prescribed per patient, by age group, sex and Townsend score. To estimate the incidence rate ratios (IRRs) we generated a fully adjusted model with age, sex, Townsend score and comorbidity status (as a binary variable) and included a random effect to account for variation between practices. We used the likelihood ratio test to determine whether there was an interaction between age and sex in the fully adjusted model.
In addition, for each comorbidity and smoking, a negative binomial regression model was fitted taking antibiotic prescription rates over the 3 year period as the primary outcome, with age, sex, Townsend score and any significant interaction term as model covariates, including practice as a random effect. Multi-morbidity was defined as the presence of at least two of the comorbidities listed above.
To illustrate the potential population impact on total antibiotic prescribing of reducing antibiotic use in groups of patients who are frequently prescribed antibiotics, we calculated population-attributable fractions for patients with at least one comorbidity, for patients with multi-morbidity and for smokers. For smokers and patients with comorbidity or multi-morbidity we compared the adjusted rate of antibiotic prescribing relative to the rate of prescribing in the general population. We calculated population attributable fractions using the method described by Kleinbaum et al.19 (Table S1, available as Supplementary data at JAC Online).
Results
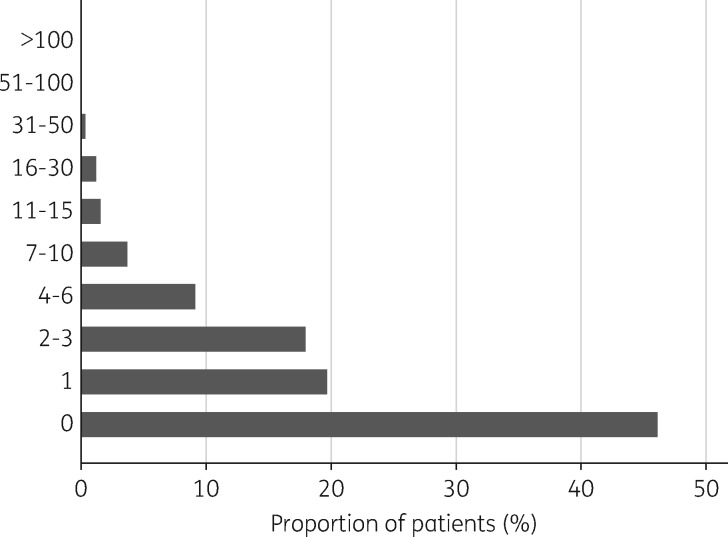
We included data from 1 948 390 adult patients who were registered with 385 general practices, representing 3 922 732 antibiotic prescriptions. Forty-nine percent were male, 35% had comorbidity and 20% were smokers. On average, 30.1% of patients were prescribed at least one antibiotic per year (580 038 patients in 2011, 598 202 patients in 2012 and 580 843 patients in 2013). Over the 3 year study period, 46.1% (899 160/1 948 390) of patients were not prescribed any antibiotics (Figure 1). Of the antibiotics, 53.3% were prescribed to 9% of registered patients (Table 1), each of whom was prescribed at least five antibiotics during the study period. Almost 10% (390 008/3 922 732) of antibiotics were prescribed to <0.5% (8549/1 948 390) of patients, each of whom received at least 30 prescriptions during the study period.
Figure 1.
Frequency of antibiotic use per patient over a 3 year period (2011–13). Forty-six percent of patients were not prescribed an antibiotic, but 7% received at least seven antibiotic prescriptions over the same time period.
Table 1.
Frequency of antibiotic prescribing in primary care and relationship to total antibiotic use
| Antibiotics prescribed per 3 years | Number of patients (cumulative %) | Number of antibiotic prescriptions (cumulative %) |
|---|---|---|
| Total | 1 948 390 | 3 922 732 |
| >30 | 8549 (0.4) | 390 008 (9.9) |
| 16–30 | 23 418 (1.6) | 486 162 (22.3) |
| 11–15 | 30 461 (3.2) | 383 589 (32.1) |
| 7–10 | 73 842 (7.0) | 601 745 (47.5) |
| 6 | 38 332 (9.0) | 229 992 (53.3) |
| 5 | 55 898 (11.8) | 279 490 (60.4) |
| 4 | 83 624 (16.1) | 334 496 (69.0) |
| 3 | 131 781 (22.9) | 395 343 (70.1) |
| 2 | 218 582 (34.1) | 437 164 (90.2) |
| 1 | 384 743 (53.9) | 384 743 (100.0) |
| 0 | 899 160 (100.0) | 0 |
The prescribing rate was 2.01 (95% CI 2.01–2.02) antibiotics per 3 years, equivalent to an average rate of 0.67 (95% CI 0.67–0.67) prescriptions per person-year. There was an interaction between age and gender so all subsequent analyses were stratified by gender (P < 0.0001). The rate of prescribing adjusted for age, social deprivation and comorbidity was 62% higher in women compared with men (adjusted IRR 1.62, 95% CI 1.62–1.63). Overall, the rates of antibiotic prescribing increased with increasing age, but this association was more marked in men compared with women (Table 2). Rates of prescribing to the most socially deprived individuals exceeded rates in the least deprived by 10% in men (adjusted IRR 1.10, 95% CI 1.09–1.12) and 19% in women (adjusted IRR 1.19, 95% CI 1.18–1.20) (Table 2).
Table 2.
Relationship between social and demographic characteristics and antibiotic prescribing
| Clinical feature | Number of patients (%) | Prescribing rate per 3 years (95% CI) | Unadjusted IRR (95% CI)a |
Adjusted IRRb (95% CI) |
||
|---|---|---|---|---|---|---|
| males | females | males | females | |||
| Age (years) | ||||||
| 18–44 | 825 266 (42.4) | 1.48 (1.47–1.49) | 1 | 1 | 1 | 1 |
| 45–64 | 715 494 (36.7) | 1.90 (1.89–1.91) | 1.50 (1.48–1.51) | 1.17 (1.16–1.18) | 1.17 (1.17–1.18) | 1.01 (1.01–1.02) |
| 65–84 | 371 860 (19.1) | 3.18 (3.16–3.21) | 2.82 (2.79–2.85) | 1.79 (1.77–1.80) | 1.72 (1.71–1.74) | 1.32 (1.32–1.33) |
| >84 | 35 770 (1.8) | 4.51 (4.42–4.60) | 4.00 (3.88–4.13) | 2.39 (2.35–2.44) | 2.26 (2.22–2.31) | 1.67 (1.64–1.69) |
| Social deprivationc | ||||||
| 1 (least deprived) | 518 734 (26.6) | 1.83 (1.82–1.84) | 1 | 1 | 1 | 1 |
| 2 | 438 753 (22.5) | 1.93 (1.92–1.95) | 1.04 (1.03–1.05) | 1.06 (1.05–1.07) | 1.01 (1.01–1.02) | 1.01 (1.02–1.03) |
| 3 | 410 325 (21.1) | 2.03 (2.02–2.05) | 1.07 (1.06–1.08) | 1.13 (1.12–1.14) | 1.04 (1.04–1.05) | 1.07 (1.06–1.08) |
| 4 | 345 464 (17.7) | 2.15 (2.14–2.17) | 1.10 (1.09–1.12) | 1.22 (1.20–1.23) | 1.08 (1.07–1.09) | 1.12 (1.11–1.13) |
| 5 (most deprived) | 235 114 (12.1) | 2.32 (2.30–2.34) | 1.16 (1.15–1.18) | 1.34 (1.32–1.35) | 1.10 (1.09-1.12) | 1.19 (1.18–1.20) |
Interaction between age and gender so all analyses stratified by gender; P < 0.0001 based on the likelihood ratio test.
IRR adjusted for all other variables in the model and comorbidity.
Quintile of deprivation.
The presence of any of the specified comorbidities increased the rate of antibiotic prescribing by more than one-third (Tables 3 and 4). The rate of antibiotic prescribing to patients with COPD was 3-fold greater than the rates of antibiotic prescribing for the general population (adjusted IRR 3.01, 95% CI 2.98–3.04). Individuals with heart failure, asthma or peripheral arterial disease were prescribed 53%–69% more antibiotics than individuals without these conditions. Compared with the general population, diabetics and individuals with coronary artery disease were prescribed 47% more antibiotics. Obesity, stroke and chronic kidney disease were all associated with more than a one-third increase in rates of antibiotic prescribing compared with individuals without these conditions.
Table 3.
Relationship between comorbidities and antibiotic prescribing
| Comorbidity | Number of patients (%) | Prescribing rate per 3 years (95% CI) | Crude IRR (95% CI) | Adjusted IRR (95% CI) | |
|---|---|---|---|---|---|
| Asthma | no | 1.85 (1.86–1.86) | 1 | 1 | |
| yes | 216 375 (11.1) | 3.28 (3.26–3.30) | 1.77 (1.76–1.78) | 1.64 (1.63–1.64) | |
| Coronary artery disease | no | 1.91 (1.91–1.92) | 1 | 1 | |
| yes | 88 391 (4.5) | 4.11 (4.07–4.15) | 2.15 (2.12–2.17) | 1.47 (1.46–1.48) | |
| Chronic kidney disease | no | 1.92 (1.91–1.92) | 1 | 1 | |
| yes | 79 030 (4.1) | 4.33 (4.28–4.37) | 2.26 (2.23–2.28) | 1.34 (1.32–1.35) | |
| COPD | no | 1.89 (1.88–1.89) | 1 | 1 | |
| yes | 36 116 (1.9) | 8.69 (8.58–8.79) | 4.60 (4.53–4.68) | 3.01 (2.98–3.04) | |
| Diabetes | no | 1.91 (1.91–1.92) | 1 | 1 | |
| yes | 107 378 (5.5) | 3.77 (3.74–3.80) | 1.97 (1.95–1.99) | 1.47 (1.46–1.48) | |
| Heart failure | no | 1.99 (1.98–1.99) | 1 | 1 | |
| yes | 14 424 (0.7) | 5.47 (5.35–5.59) | 2.75 (2.68–2.82) | 1.69 (1.66–1.72) | |
| Obesity | no | 1.82 (1.81–1.82) | 1 | 1 | |
| yes | 358 929 (18.4) | 2.87 (2.86–2.89) | 1.58 (1.57–1.59) | 1.37 (1.35–1.39) | |
| Peripheral arterial disease | no | 1.99 (1.99–1.99) | 1 | 1 | |
| yes | 16 993 (0.9) | 4.69 (4.59–4.79) | 2.36 (2.30–2.41) | 1.53 (1.50–1.55) | |
| Stroke | no | 1.99 (1.98–1.99) | 1 | 1 | |
| yes | 22 741 (1.2) | 4.41 (4.32–4.50) | 2.22 (2.17–2.27) | 1.37 (1.35–1.39) | |
| Smoker | no | 2.01 (2.01–2.02) | 1 | 1 | |
| yes | 395 824 (20.3) | 2.01 (2.00–2.02) | 1.00 (0.99–1.01) | 1.13 (1.12–1.13) | |
| Comorbidities | 0 | 1 271 513 (65.3) | 1.44 (1.44–1.45) | 1 | 1 |
| 1 | 485 168 (24.9) | 2.50 (2.49–2.51) | 1.73 (1.72–1.74) | 1.44 (1.43–1.45) | |
| 2 | 138 725 (7.1) | 3.98 (3.95–4.01) | 2.76 (2.73–2.78) | 1.93 (1.92–1.94) | |
| 3 | 52 984 (2.7) | 6.05 (5.98–6.12) | 4.19 (4.13–4.24) | 2.54 (2.51–2.56) | |
Table 4.
Relationship between antibiotic prescribing and comorbidity
| Number of antibiotics prescribed | Comorbidity |
|||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| diabetes |
chronic kidney disease |
asthma |
coronary artery disease |
heart failure |
peripheral arterial disease |
stroke/transient ischaemic attack |
COPD |
smoking |
obesity |
|||||||||||
| n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | |
| 0 | 33 188 | 30.9 | 21 907 | 27.7 | 71 388 | 33.0 | 25 473 | 28.8 | 3144 | 21.8 | 4431 | 26.1 | 6749 | 29.7 | 3917 | 10.8 | 17 8022 | 45.0 | 125 146 | 34.9 |
| 1 | 19 551 | 18.2 | 13 771 | 17.4 | 40 679 | 18.8 | 15 803 | 17.9 | 2231 | 15.5 | 2850 | 16.8 | 3845 | 16.9 | 3699 | 10.2 | 78 381 | 19.8 | 71 013 | 19.8 |
| 2–3 | 22 705 | 21.1 | 17 075 | 21.6 | 45 146 | 20.9 | 18 852 | 21.3 | 3108 | 21.5 | 3507 | 20.6 | 4676 | 20.6 | 6376 | 17.7 | 73 035 | 18.5 | 76 588 | 21.3 |
| 4–6 | 15 202 | 14.2 | 12 019 | 15.2 | 29 301 | 13.5 | 12 936 | 14.6 | 2414 | 16.7 | 2695 | 15.9 | 3272 | 14.4 | 6839 | 18.9 | 38 082 | 9.6 | 45 868 | 12.8 |
| 7–10 | 8006 | 7.5 | 6631 | 8.4 | 15 277 | 7.1 | 7134 | 8.1 | 1579 | 10.9 | 1577 | 9.3 | 1871 | 8.2 | 5610 | 15.5 | 15 946 | 4.0 | 21 370 | 6.0 |
| 11–15 | 3833 | 3.6 | 3194 | 4.0 | 6987 | 3.2 | 3504 | 4.0 | 833 | 5.8 | 878 | 5.2 | 935 | 4.1 | 3865 | 10.7 | 6460 | 1.6 | 9209 | 2.6 |
| 16–30 | 3349 | 3.1 | 2957 | 3.7 | 5615 | 2.6 | 3231 | 3.7 | 754 | 5.2 | 725 | 4.3 | 936 | 4.1 | 4096 | 11.3 | 4579 | 1.2 | 7131 | 2.0 |
| >30 | 1544 | 1.4 | 1476 | 1.9 | 1982 | 0.9 | 1458 | 1.6 | 361 | 2.5 | 330 | 1.9 | 457 | 2.0 | 1714 | 4.7 | 1319 | 0.3 | 2604 | 0.7 |
| Total | 107 378 | 100.0 | 79 030 | 100.0 | 216 375 | 100.0 | 88 391 | 100.0 | 14 424 | 100.0 | 16 993 | 100.0 | 22 741 | 100.0 | 36 116 | 100.0 | 395 824 | 100.0 | 358 929 | 100.0 |
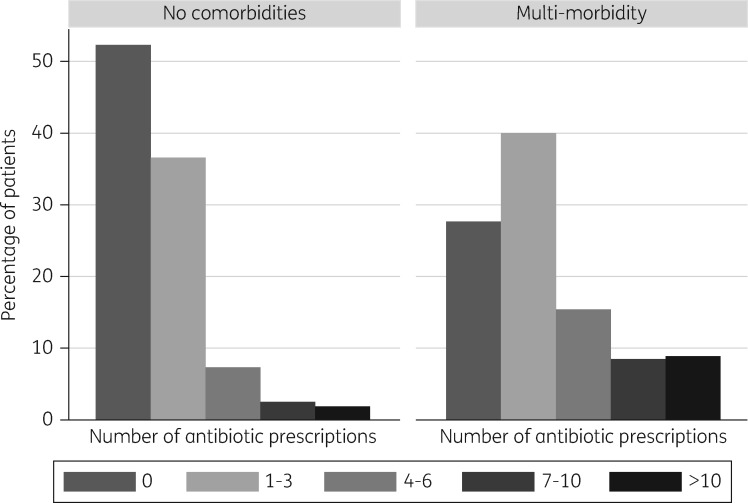
Amongst the 65% of patients without comorbidity, 52.3% (665 136/1 271 513) were not prescribed any antibiotics during the study. The percentage of patients receiving a prescription more frequently than twice per year was 4.2% (53 028/1 271 513) on average (Figure 2). By contrast, 25.3% (48 457/191 709) of patients with multi-morbidity were not prescribed any antibiotics during the 3 year study and 20.0% (38 417/191 709) were prescribed antibiotics more frequently than twice per year (Figure S1).
Figure 2.
Relationship between comorbidity and frequency of antibiotic prescribing per patient, 2011–13.
The rate of antibiotic prescribing to smokers exceeded that to non-smokers by 13% (adjusted IRR 1.13, 95% CI 1.12–1.13). During the study period 45.0% (178 022/395 824) of smokers were not prescribed any antibiotics at all and 6.0% (23 816/395 824) were prescribed antibiotics more frequently than twice per year.
At a population level, if the rate of antibiotic prescribing could be reduced in patients with multi-morbidity to the rate seen in the general population, the maximum reduction in total prescribing would be an estimated 7%. Similarly, if prescribing rates were reduced in patients with comorbidity to the rate seen in the general population, the maximum decrease in total antibiotic prescribing would be 16% over 3 years. Finally, if the rate of prescribing to smokers could be reduced to the rate seen in the general population, total antibiotic prescribing would fall by 2% over 3 years.
Discussion
In this large, individual-level study of antibiotic use and comorbidity amongst patients in primary care, more than half of the total amount of antibiotics were prescribed to 9% of patients, each of whom had at least five antibiotics in 3 years. Our results highlight the importance of high-frequency antibiotic use in a subset of patients with high rates of comorbidity.
A major strength of this study is the quality and scale of the patient information recorded in THIN, and the fact that our results can be generalized to the UK population.13 This was confirmed by our estimates of chronic disease prevalence, which were comparable to the national QOF dataset for all conditions except asthma (where we modified the QOF-based definition; see the Methods section). The limitations of our analysis reflect that the THIN dataset was devised for clinical management of patients rather than for the purpose of research, so we lacked sufficient information to estimate the appropriateness of antibiotic prescribing for patients with comorbidity. We focused on antibiotic prescribing in adults because our aim was to estimate the relationship between antibiotic use and comorbidity. We used a robust method to identify patients with chronic illnesses that might influence the general practitioner’s decision to prescribe,17 but a limitation of our study is that we did not identify patients with chronic liver or neurological disease because QOF codes for these conditions have not been developed. To provide clinically interpretable estimates of antibiotic use we only included patients who were registered with eligible general practices for the full 3 year period, which may have introduced selection bias by excluding patients who died or left the practice, potentially underestimating the association between prescribing and comorbidity. We restricted the diagnosis of comorbidity to patients with a relevant Read code recorded before 31 December 2011 to ensure that patients had sufficient follow-up time (2 years) to examine the relationship between prescribing and comorbidity. However, this may have inadvertently led to an underestimate of the association between prescribing and comorbidity as some patients will have developed comorbid conditions during follow-up. In order to model the maximal theoretical impact of reducing the antibiotic use in patients with comorbidity, we calculated population-attributable fractions, making the important assumption that comorbidity is the main driver of prescribing in these patients. In reality, the drivers of prescribing are multi-factorial and, although we took account of clustering by practice, we did not consider the role of individual-level prescriber factors in our analysis.
The fact that the rates of antibiotic prescribing vary between general practices is well established,5,6,20 but most studies that have addressed the reasons for this variation have been ecological, using practice-level data.21,22 Our estimates of the proportion of patients who are prescribed at least one antibiotic per year are comparable to annual antibiotic prevalence estimates derived from European healthcare data.23 In common with these studies, we report higher rates of prescribing in women and in older age groups.23,24 A plausible explanation for at least part of the higher rates of antibiotic prescribing in women is a higher incidence of urinary tract infection, estimated at 0.5–0.7 infections per year in women,25 and 5–8 infections per 10 000 per year in men.26 Women and the elderly are also more likely to consult their general practitioner,27 and are therefore more likely to be prescribed antibiotics.
Clearly, for some patients frequent antibiotic use is appropriate and justified, e.g. to ensure antibiotics are initiated promptly for patients with bacterial infective exacerbations of COPD through the use of antibiotic rescue packs, or through the use of prophylactic antibiotics for recurrent urinary tract infection. Nonetheless, there is some evidence that even for higher-risk patients, such as smokers or those with comorbidities with acute lower respiratory tract infection (where pneumonia is not suspected), antibiotic treatment may confer no clinically meaningful benefit.28 In one of the few studies investigating the individual-level relationship between comorbidity and antibiotic prescribing, co-existing diabetes and heart failure were independently associated with prescription of an antibiotic in patients with COPD.29 In a population-based Swedish study, patients with comorbidity (Charlson’s index of 3) were 3-fold more likely to be prescribed antibiotics compared with individuals without comorbidity. However, this study is likely to have over-estimated the relationship between antibiotic prescribing and comorbidity because comorbidity was defined by hospitalization, based on a list of ICD-10 codes.20 As many patients with comorbidities are managed in primary care without recourse to hospital, this approach will tend to underestimate the prevalence of comorbidity in primary care. By contrast, a strength of our approach is that we used definitions that were specifically designed for primary care to identify patients with relevant comorbidities. With regard to smoking, our findings agree with results from a large multicentre study, identifying the patient’s smoking status as an independent predictor of the general practitioner’s decision to prescribe an antibiotic.30 Interestingly, this study found no evidence that increased rates of antibiotic prescribing conferred any benefit to smokers.30 Whilst few clinicians would question the fact that patients with diabetes are at increased risk of infection,31 there are a range of other comorbidities, such as cardiovascular disease or obesity, that may also be considered when making the clinical decision to prescribe an antibiotic.17 There is a real need for more granular research studies to advance our understanding of whether higher rates of antibiotic use in patients with comorbidity are primarily driven by diagnostic uncertainty, exemplified by the dilemma of whether to prescribe antibiotics for an infective exacerbation of COPD, or by concerns about an overall increased susceptibility to infection in patients with comorbidities such as diabetes. Given the anticipated increases in rates of comorbidity with an ageing population, disentangling the role of comorbidity in susceptibility to and outcomes from bacterial infection may be important in the context of public health initiatives to rationalize antibiotic use in primary care.
In the UK national targets have been set to reduce total antibiotic prescribing,7 with a focus on primary prevention rather than a more targeted approach focused on reducing antibiotic prescribing to those individuals with the highest levels of antibiotic consumption. This has led to reductions in antibiotic consumption but it remains to be seen whether such efforts translate into a reduction in the incidence of antimicrobial resistance. For example, in a recent population-based study from Slovenia, a 30% reduction in antimicrobial prescribing had a variable impact on antimicrobial resistance, leading the authors to conclude that targeted interventions may be required to truly impact on antimicrobial resistance.32 Patients with frequent antibiotic exposure are likely to be at greatest risk of antimicrobial resistance, not only through their increased exposure to antibiotics,33 but also because they are more likely to be admitted to hospital, where they may be exposed to drug-resistant pathogens.34 In the context of international initiatives to reduce inappropriate antibiotic use, it may be important to assess the feasibility of reducing the rate of antibiotic prescribing to patients with the highest frequency of antibiotic use. Such efforts will require further research to characterize the temporal relationship between antibiotic prescribing and the clinical indication for the prescription.
Data sharing
The data are under licence, but the code lists are provided as Supplementary data. The data algorithms used in the analysis are available from the corresponding author.
Supplementary Material
Acknowledgements
Pilot data from this study were presented at the Farr Conference, St Andrews, 2015 (C5-1369) and at the Young Statistician’s Meeting, Exeter University, 2015.
We would like to thank Dr Susan Hopkins for providing helpful comments on the draft manuscript.
Funding
This work was funded by a Clinical Lecturer Starter Grant from the Academy of Medical Sciences (SGCL11-Shallcross).
The funder had no role in the: design and conduct of the analysis; interpretation of the data; preparation, review or approval of the manuscript; and decision to submit the manuscript for publication.
Transparency declarations
None to declare.
Author contributions
N. B. performed the statistical analyses and revised the manuscript. I. P. advised on the study design and statistical methods and revised the manuscript. G. R. provided clinical input into the study design and revised the manuscript. A. H. and L. S. developed the idea for the study. A. H. revised the manuscript. L. S. wrote the first draft of the study protocol and drafted and revised the manuscript. L. S. is the guarantor and had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Supplementary data
Code lists, Table S1 and Figure S1 are available as Supplementary data at JAC Online.
References
- 1. Department of Health. UK 5 Year Antimicrobial Resistance Strategy 2013 to 2018 https://www.gov.uk/government/publications/uk-5-year-antimicrobial-resistance-strategy-2013-to-2018.
- 2. Laxminarayan R, Matsoso P, Pant S. et al. Access to effective antimicrobials: a worldwide challenge. Lancet 2016; 387: 168–75. [DOI] [PubMed] [Google Scholar]
- 3. WHO. Antimicrobial Resistance: Global Report on Surveillance 2014 http://www.who.int/drugresistance/documents/surveillancereport/en/.
- 4. ECDC. Surveillance of Antimicrobial Consumption in Europe 2011 http://ecdc.europa.eu/en/publications/Publications/antimicrobial-consumption-europe-surveillance-2011.pdf.
- 5. Hawker JI, Smith S, Smith GE. et al. Trends in antibiotic prescribing in primary care for clinical syndromes subject to national recommendations to reduce antibiotic resistance, UK 1995-2011: analysis of a large database of primary care consultations. J Antimicrob Chemother 2014; 69: 3423–30. [DOI] [PubMed] [Google Scholar]
- 6. Gulliford MC, Dregan A, Moore MV. et al. Continued high rates of antibiotic prescribing to adults with respiratory tract infection: survey of 568 UK general practices. BMJ Open 2014; 4: e006245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. NHS England. Promote Appropriate Antimicrobial Prescribing in Primary Care 2015.https://www.england.nhs.uk/wp-content/uploads/2015/04/04-cdi-promoting-antibiotic-prescribing-pc.pdf.
- 8. National Institute for Health and Care Excellence. Antimicrobial Stewardship: Systems and Processes for Effective Antimicrobial Medicine Use 2015 https://www.nice.org.uk/guidance/ng15.
- 9. PHE. Behaviour Change and Antibiotic Prescribing in Healthcare Settings 2015 https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/405031/Behaviour_Change_for_Antibiotic_Prescribing_-_FINAL.pdf.
- 10. IMS Health. http://csdmruk.cegedim.com/our-data/our-data.shtml.
- 11. Lis Y, Mann RD.. The VAMP research multi-purpose database in the U.K. J Clin Epidemiol 1995; 48: 431–43. [DOI] [PubMed] [Google Scholar]
- 12. Chisholm J. The Read clinical classification. BMJ 1990; 300: 1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Blak BT, Thompson M, Dattani H. et al. Generalisability of The Health Improvement Network (THIN) database: demographics, chronic disease prevalence and mortality rates. Inform Prim Care 2011; 19: 251–5. [DOI] [PubMed] [Google Scholar]
- 14. Lewis JD, Schinnar R, Bilker WB. et al. Validation studies of the health improvement network (THIN) database for pharmacoepidemiology research. Pharmacoepidemiol Drug Saf 2007; 16: 393–401. [DOI] [PubMed] [Google Scholar]
- 15. Maguire A, Blak BT, Thompson M.. The importance of defining periods of complete mortality reporting for research using automated data from primary care. Pharmacoepidemiol Drug Saf 2009; 18: 76–83. [DOI] [PubMed] [Google Scholar]
- 16. Horsfall L, Walters K, Petersen I.. Identifying periods of acceptable computer usage in primary care research databases. Pharmacoepidemiol Drug Saf 2012; 22: 64–9. [DOI] [PubMed] [Google Scholar]
- 17. PHE. Managing Common Infections: Guidance for Primary Care 2016 https://www.gov.uk/government/publications/managing-common-infections-guidance-for-primary-care.
- 18. Health and Social Care Information Centre. QOF Business Rules v28.0 http://www.hscic.gov.uk/qofbrv28.
- 19. Kleinbaum D, Kupper L, Morgenstern H.. Epidemiologic Research. New York: John Wiley & Sons, 1982. [Google Scholar]
- 20. Ternhag A, Grunewald M, Naucler P. et al. Antibiotic consumption in relation to socio-demographic factors, co-morbidity, and accessibility of primary health care. Scand J Infect Dis 2014; 46: 888–96. [DOI] [PubMed] [Google Scholar]
- 21. Wang KY, Seed P, Schofield P. et al. Which practices are high antibiotic prescribers? A cross-sectional analysis. Br J Gen Pract 2009; 59: e315–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Covvey JR, Johnson BF, Elliott V. et al. An association between socioeconomic deprivation and primary care antibiotic prescribing in Scotland. J Antimicrob Chemother 2014; 69: 835–41. [DOI] [PubMed] [Google Scholar]
- 23. Brauer R, Ruigomez A, Downey G. et al. Prevalence of antibiotic use: a comparison across various European health care data sources. Pharmacoepidemiol Drug Saf 2016; 25 Suppl 1: 11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mor A, Froslev T, Thomsen RW. et al. Antibiotic use varies substantially among adults: a cross-national study from five European Countries in the ARITMO project. Infection 2015; 43: 453–72. [DOI] [PubMed] [Google Scholar]
- 25. Hooton T, Scholes D, Hughes J. et al. A prospective study of risk factors for symptomatic urinary tract infection in young women. N Engl J Med 1996; 335: 468–74 [DOI] [PubMed] [Google Scholar]
- 26. Krieger J, Ross S, Simonsen J.. Urinary tract infection in healthy university men. J Urol 1993; 149: 1046–8 [DOI] [PubMed] [Google Scholar]
- 27. Hippisley-Cox J, Vinogradova Y.. Trends in Consultation Rates in General Practice 1995/1996 to 2008/2009: Analysis of the QResearch® Database http://www.hscic.gov.uk/catalogue/PUB01077/tren-cons-rate-gene-prac-95-09-95-09-rep.pdf.
- 28. Moore M, Stuart B, Coenen S. et al. Amoxicillin for acute lower respiratory tract infection in primary care: subgroup analysis of potential high-risk groups. Br J Gen Pract 2014; 64: e75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bont J, Hak E, Birkhoff CE. et al. Is co-morbidity taken into account in the antibiotic management of elderly patients with acute bronchitis and COPD exacerbations? Fam Pract 2007; 24: 317–22. [DOI] [PubMed] [Google Scholar]
- 30. Stanton N, Hood K, Kelly MJ. et al. Are smokers with acute cough in primary care prescribed antibiotics more often, and to what benefit? An observational study in 13 European countries. Eur Respir J 2010; 35: 761–7 [DOI] [PubMed] [Google Scholar]
- 31. Pearson-Stuttard J, Blundell S, Harris T. et al. Diabetes and infection: assessing the association with glycaemic control in population-based studies. Lancet Diabetes Endocrinol 2016; 4: 148–58. [DOI] [PubMed] [Google Scholar]
- 32. Furst J, Cizman M, Mrak J. et al. The influence of a sustained multifaceted approach to improve antibiotic prescribing in Slovenia during the past decade: findings and implications. Expert Rev Anti Infect Ther 2015; 13: 279–89. [DOI] [PubMed] [Google Scholar]
- 33. Costelloe C, Metcalfe C, Lovering A. et al. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: systematic review and meta-analysis. BMJ 2010; 340: c2096. [DOI] [PubMed] [Google Scholar]
- 34. Bottle A, Aylin P, Majeed A.. Identifying patients at high risk of emergency hospital admissions: a logistic regression analysis. J R Soc Med 2006; 99: 406–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.