Abstract
De novo and inherited mutations of X-chromosome cell adhesion molecule protocadherin 19 (PCDH19) cause frequent, highly variable epilepsy, autism, cognitive decline and behavioural problems syndrome. Intriguingly, hemizygous null males are not affected while heterozygous females are, contradicting established X-chromosome inheritance. The disease mechanism is not known. Cellular mosaicism is the likely driver. We have identified p54nrb/NONO, a multifunctional nuclear paraspeckle protein with known roles in nuclear hormone receptor gene regulation, as a PCDH19 protein interacting partner. Using breast cancer cells we show that PCDH19-NONO complex is a positive co-regulator of ERα-mediated gene expression. Expression of mutant PCDH19 affects at least a subset of known ERα-regulated genes. These data are consistent with our findings that genes regulated by nuclear hormone receptors and those involved in the metabolism of neurosteroids in particular are dysregulated in PCDH19-epilepsy girls and affected mosaic males. Overall we define and characterize a novel mechanism of gene regulation driven by PCDH19, which is mediated by paraspeckle constituent NONO and is ERα-dependent. This PCDH19-NONO-ERα axis is of relevance not only to PCDH19-epilepsy and its comorbidities but likely also to ERα and generally nuclear hormone receptor-associated cancers.
Introduction
PCDH19 Female Epilepsy (PCDH19-FE) (OMIM # 300088) is a frequent seizure disorder, which until recently has been under recognized (1,2). Unlike the classical X-linked male lethal disorder, PCDH19-FE affects heterozygous girls while hemizygous males are spared. The causative gene was originally mapped to X-chromosome in 1997 (3) and later identified to be PCDH19 in 2008 (4). PCDH19 belongs to the δ2 protocadherin subfamily of the cadherin adhesion molecule super family (5). This subclass of protocadherins also includes PCDH8, PCDH10, PCDH17 and PCDH18. The respective proteins are known (or speculated) to be involved in weak homotypic cell-cell adhesion and cell migration in a calcium dependent manner and are predominantly expressed in the brain with expression restricted to specific regions (6–8).
PCDH19 is the second most frequently mutated gene in epilepsy after SCN1A that causes Dravet Syndrome (9). PCDH19-FE is focal epilepsy characterized by early onset seizures (6–36 months), which cluster, are drug resistant and are often triggered by fever (10). PCDH19-FE is clinically variable, ranging from benign focal epilepsy with normal intelligence to severe generalized or multifocal epilepsy, resembling Dravet syndrome (1,9). The frequency of seizures often decreases at/after puberty and can stop altogether during adulthood. Several other comorbidities including intellectual disability, autism, schizophrenia and attention deficit hyperactivity disorder (ADHD) are common among PCDH19-FE affected individuals (9,10). So far in excess of 100 unique PCDH19 mutations have been reported that include missense changes, premature stop codons and complete gene deletions (Supplementary Material, Fig. S1). This also includes a single case of an affected male with an early somatic mutation leading to loss of PCDH19 in approximately half of his cells (11). The majority of PCDH19 mutations are within the extracellular domain with all missense mutations clustering exclusively within this region (Supplementary Material, Fig. S1). There is no obvious difference between the phenotype of patients with partial and complete loss of PCDH19 protein (11).
Determining the pathogenic mechanism of PCDH19 variants has been a major challenge. We have recently shown that neurosteroids may play a significant role in the pathology of PCDH19-FE (12). We found that allopregnanolone levels were low in the blood of PCDH19-FE girls and that genes regulated by estrogen, progesterone and androgen receptors were among the most significantly dysregulated in patient primary skin cells (12).
Here, we show that the non-POU-domain-containing octamer binding protein (NONO)/P54nrb, a well-known multifunctional RNA- and DNA-binding nuclear protein, interacts with PCDH19. We also show that a fraction of the PCDH19 protein is localized in the cell nucleus where it interacts with NONO to co-regulate gene expression via estrogen receptor alpha (ERα). Our data show that PCDH19 variants behave as loss of function mutations in this ERα-dependent gene regulation pathway. Taken together our results suggest that PCDH19 has a gene expression regulatory function besides its postulated role in cell-cell adhesion.
Results
NONO interacts with PCDH19
PCDH19 has six extracellular cadherin (EC) repeats in the N-terminus that resemble classical cadherins, a transmembrane domain (TM) and two conserved motifs known as CM1 and CM2 in the C-terminal region of the protein (Supplementary Material, Fig. S1) that are typical of δ2 protocadherins (5). To identify PCDH19 interacting proteins, we performed a yeast two-hybrid (Y2H) screen using the C-terminal region (amino acids 706 to 1145) of mouse Pcdh19 (the least conserved region of Pcdh19 among its orthologs and paralogs) as a bait and E11 whole mouse embryo cDNA library as a prey (see Materials and Methods). Pcdh19 has been shown to be expressed in the mouse embryo during E9 to E14, a period important for early brain development (13). The Pcdh19 C-terminal region is highly similar (94% amino acid sequence identity) to human PCDH19, with the CM1 and CM2 motifs being 100% conserved (Supplementary Material, Fig. S2A).
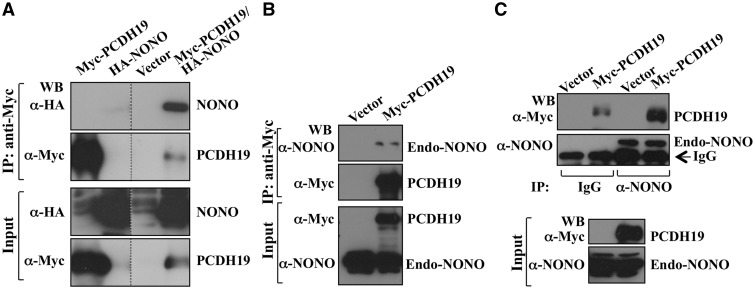
One of the Pcdh19-interacting proteins identified by our screen was Nono, a 471 amino acid 54 kDa protein. Nono is a member of the Drosophila behavior human splicing (DBHS)-family of proteins with many roles in gene regulation including binding to a long noncoding RNA to form subnuclear bodies called paraspeckles (14,15). Nono would be the first nuclear protein to directly interact with a δ2 protocadherin, a member of the cadherin superfamily which have been reported to involve in cell membrane functions (13,14,16–18). We used epitope-tagged human PCDH19 (96% amino acid sequence identical to mouse) protein for validating its interaction with NONO. Using co-immunoprecipitation experiments on HEK293T cell lysates expressing Myc-PCDH19 and HA-NONO we demonstrate that NONO and PCDH19 interact (Fig. 1A). The interaction between human NONO and PCDH19 was also validated by investigating Myc-PCDH19 interaction with the endogenous NONO. We detected the endogenous NONO in Myc-PCDH19 immunoprecipitates from HEK293T cells expressing this protein (Fig. 1B). This interaction was also confirmed by reciprocal detection of Myc-PCDH19 in NONO immunoprecipitated from the HeLa cells (Fig. 1C). Taken together these results showed that PCDH19 and NONO proteins interact in human cells.
Figure 1.
NONO interacts with PCDH19. (A) PCDH19 interaction with NONO was determined by co-immunoprecipitation assay using lysates from HEK293T cells expressing Myc-PCDH19, HA-NONO or both. Myc-PCDH19 was immunoprecitated and HA-NONO detected by western blotting with anti-HA antibody. (B) Lysates from HEK293T cells expressing Myc-tagged PCDH19 were immunoprecipitated and endogenous NONO detected by western blotting with anti-NONO antibody. (C) Endogenous NONO was immunoprecipitated from HeLa cells expressing Myc-PCDH19 and western blotted with anti-Myc antibody. Low level of Myc-PCDH19 immunoprecipitated with IgG was due to non-specific binding. Figure 1A–C was generated by cropping full-length western blots shown in Supplementary Material, Figure S10. Dotted lines separate the cropped areas of the western blots.
PCDH19 localizes in close proximity to NONO in the nucleus
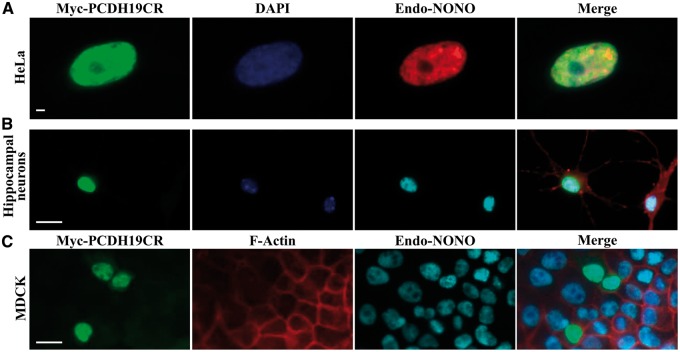
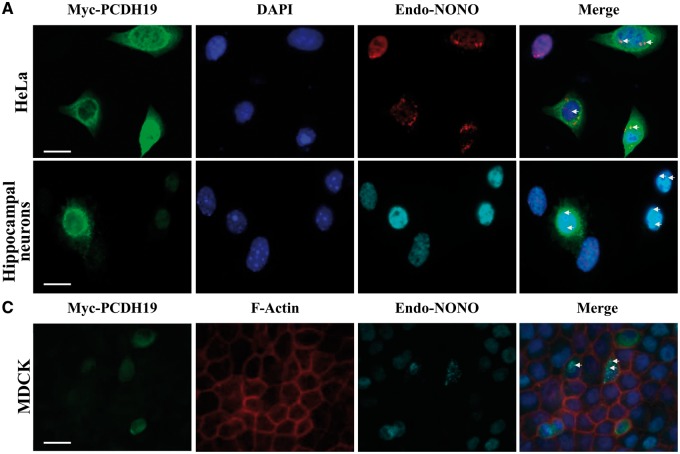
We next examined cellular localization of PCDH19 and NONO using immunofluorescence staining in cells expressing either full-length or a C-terminal PCDH19 region. In HeLa cells, the C-terminal region of PCDH19 (PCDH19CR) predominantly localized to the nucleus and in close proximity to NONO (Fig. 2A). This is consistent with the presence of predicted nuclear localization sequences (NLSs) within the PCDH19 protein (Supplementary Material, Fig. S2B). The full-length PCDH19 was predominantly perinuclear with some nucleoplasmic localization (Fig. 3A and Supplementary Material, Fig. S3). In a few NONO-speckles there appeared to be some diffused PCDH19, which overlapped with the NONO signal (Fig. 3A). NONO is a paraspeckle protein, but has also been shown to be present in the nucleoplasm (Figs. 2A and 3A) (19). A similar localization pattern was observed in mouse primary hippocampal neurons expressing the two proteins (Figs. 2B and 3B). Next, we examined localization of the two proteins in the common tight-junction cell model, Madin-Darby Canine Kidney (MDCK) cells (20,21). Unlike the classical cadherins that are known to be embedded within the cell membranes (22,23), PCDH19 was largely perinuclear and occasionally nucleoplasmic (Figs. 2C and 3C). We further examined PCDH19, NONO and ERα localization using a subcellular fractionation approach. Western blot analysis of control and Myc-PCDH19-expressing MCF-7 subcellular fractions showed that the majority of the endogenous and Myc-PCDH19 was present in the nuclear fraction along with the bulk of the cellular NONO and ERα (Supplementary Material, Fig. S4). These data suggest that PCDH19, NONO and ERα likely associate in the nucleus of the MCF-7 cells.
Figure 2.
Cellular localization of the C-terminal PCDH19 and NONO. (A) HeLa cells expressing the C-terminal Myc-PCDH19 region (Myc-PCDH19CR) showing exclusive nuclear localization of PCDH19 (green) and partial co-localization with endogenous NONO (red). (B) Mouse primary hippocampal neurons expressing Myc-PCDH19CR showing PCDH19 (green) and endogenous NONO (cyan) nuclear localization. (C) MDCK cells expressing Myc-PCDH19CR showing predominantly nuclear and a small amount of cytoplasmic localization (green). Myc-PCDH19CR-endogenous NONO (cyan) nuclear co-localization was also observed. Cell nuclei and F-actin were visualized by staining with DAPI (blue) and phalloidin (red), respectively. Scale bars = 20 µm. Images are representative of >200 cells examined.
Figure 3.
Cellular localization of full-length PCDH19 and NONO. (A) HeLa cells expressing full-length Myc-PCDH19 showing cytoplasmic and nuclear localization of PCDH19 (green) and co-localization of PCDH19 and endogenous NONO (red) in the nucleus. (B) Mouse primary hippocampal neurons expressing Myc-PCDH19 (full-length) (green) showing punctuate expression and enriched in the cell soma in a perinuclear pattern with some basal expression in the nucleus with endogenous NONO (cyan). (C) MDCK cells expressing full-length Myc-PCDH19 showing predominantly cytoplasmic with some nuclear localization and a small amount of membrane localization (green). In cells expressing PCDH19, some PCDH19-endogenous NONO (cyan) co-localization was observed (indicated by arrows). F-actin and cell nuclei were visualized by staining with phalloidin (red) and DAPI (blue), respectively. Scale bars = 20 μm. Images are representative of >200 cells examined.
PCDH19 affects ERα transcriptional function
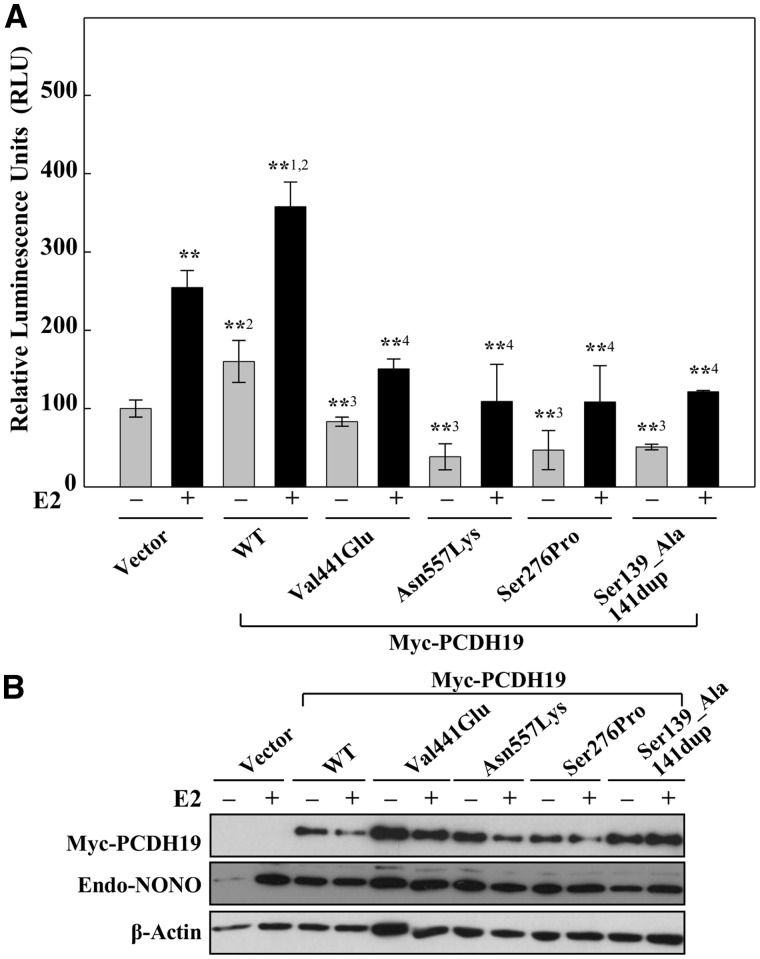
In our previous study, we gained indirect evidence of a likely role of nuclear hormone receptors in the pathology of PCDH19-FE (12). To examine this further we took advantage of various breast cancer cell lines including MCF-7. MCF-7 cells express endogenous PCDH19 as well as the estrogen receptor alpha (ERα or ESR1), progesterone receptor (PGR) and androgen receptor (AR) (Supplementary Material, Fig. S5). ERα has been of particular interest because it is a well-known steroid receptor implicated in epilepsy, psychiatric and behavioral disorders (24–26). Previously, we revealed that approximately one third of the significantly dysregulated genes in primary skin fibroblasts of PCDH19-FE girls have experimentally validated or predicted Estrogen Response Element (ERE) motifs in or near their promoters (12). To test whether PCDH19 influences ERα function, we used an ERE reporter assay. This assay employs an ER-responsive luciferase (ERE-LUC) construct, in which the firefly luciferase coding sequence is cloned downstream of three tandem ERE repeats. We observed significantly increased luciferase reporter activity in MCF-7 cells ectopically expressing the wild type PCDH19 protein (Fig. 4A). This was further enhanced by the addition of the ERα ligand estradiol (E2) (Fig. 4A). Significant reduction in the ERE-LUC reporter activity in MCF-7 cells with PCDH19 knockdown indicated that regulation of ERα transcriptional activity was PCDH19-dependent (Supplementary Material, Fig. S6). Taken together the data showed that the PCDH19 protein enhances transcription from the ERE, and given that this effect is further enhanced in the presence of E2 supported a direct role of PCDH19 in ERα function.
Figure 4.
The effect of PCDH19 variants on ERα-mediated gene expression. (A) Luciferase activity was assayed in MCF-7 cells transfected with reporter containing three copies of vitellogenin Estrogen Response Element (3× ERE TATA luc) and either control, wild-type (WT) or mutant (MT) Myc-PCDH19 expression vectors. Cells were initially cultured in charcoal-stripped FCS medium for 16 h and then for 6 h in the presence or absence of 10 nM estradiol (E2). Data are expressed as relative luciferase activity ± SD from four or more independent experiments. **P < 0.01 comparing +E2 versus–E2 vector control, **1P < 0.01 comparing +E2 versus –E2 WT, **2P < 0.01 comparing +E2 and –E2 WT versus vector or **3P < 0.01 comparing –E2 WT versus –E2 MT. **4P < 0.01 comparing +E2 WT versus +E2 MT using Bonferroni adjusted planned comparisons. (B) Levels of PCDH19 and endogenous-NONO protein were determined by western blotting with anti-Myc and anti-NONO antibodies. β-Actin was used as a loading control.
Next, we aimed to determine whether selected PCDH19-FE disease-associated missense mutations [(Val441Glu, Asn557Lys, Ser276Pro) (4,27) and a small in-frame duplication (Ser139_Ala141dup) (28)] have an effect. Whilst the wild-type PCDH19 enhanced transcription from the ERE-LUC reporter, none of the mutants did (Fig. 4). Addition of E2 increased the luciferase reporter expression for all PCDH19 variants tested, when compared to the empty vector transfected cells cultured without E2. To assess the physiological relevance of PCDH19 on ERα-mediated gene transcription, the effect of the wild-type or Asn557Lys PCDH19 was examined using four endogenous bona fide ERα target genes AKR1C3, APOD, ENC1 and OXTR. Overexpression of the wild-type PCDH19 led to an increase in the expression of these genes, an effect which was lost in the cells expressing the Asn557Lys mutant (Supplementary Material, Fig. S7). Overall, mutations of the extracellular domain of PCDH19 lead to altered transcriptional regulation of ERE-LUC reporter and select endogenous cellular gene targets of ERα.
PCDH19 effect on ERE-LUC is ERα-dependent
To determine if the effect of PCDH19 on the luciferase reporter activity from the ERE-LUC plasmid is ERα-dependent, we compared the effect of PCDH19 between ERα deficient MDA-MB-231 and ERα positive MCF-7 cells (Supplementary Material, Fig. S8). We assayed ERE-LUC transcriptional activity in cells ectopically-expressing Ser139_Ala141dup (28) or wild-type PCDH19 and cultured in the presence and absence of the ERα ligand E2. While in the ERα positive MCF-7 cells the wild-type PCDH19 led to a significant increase of luciferase reporter expression in the presence or absence of E2 ligand, this effect was not detected in the ERα negative MDA-MB-231 cells irrespective of E2 addition (Supplementary Material, Fig. S8A). To further elucidate that the effect of PCDH19 on ERE-LUC reporter activity is ERα-dependent, we performed ERE-LUC reporter assay in MCF-7 cells with and without ERα knockdown and with or without exogenous Myc-PCDH19 expression. While PCDH19 positively regulated the ERE-LUC reporter activity in the control siRNA transfected cells, this effect was significantly reduced in cells treated with ERα siRNA (Supplementary Material, Fig. S9). Together, these data show that the PCDH19-mediated increase in ERE-LUC transcriptional activity is ERα-dependent.
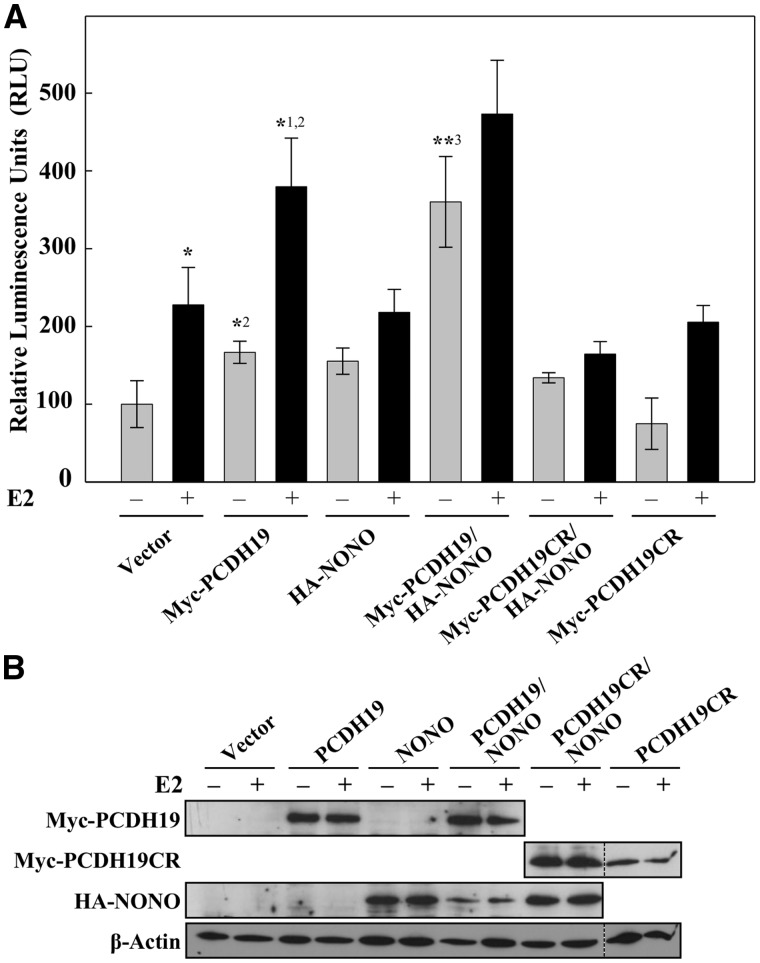
PCDH19 regulates ERα through NONO
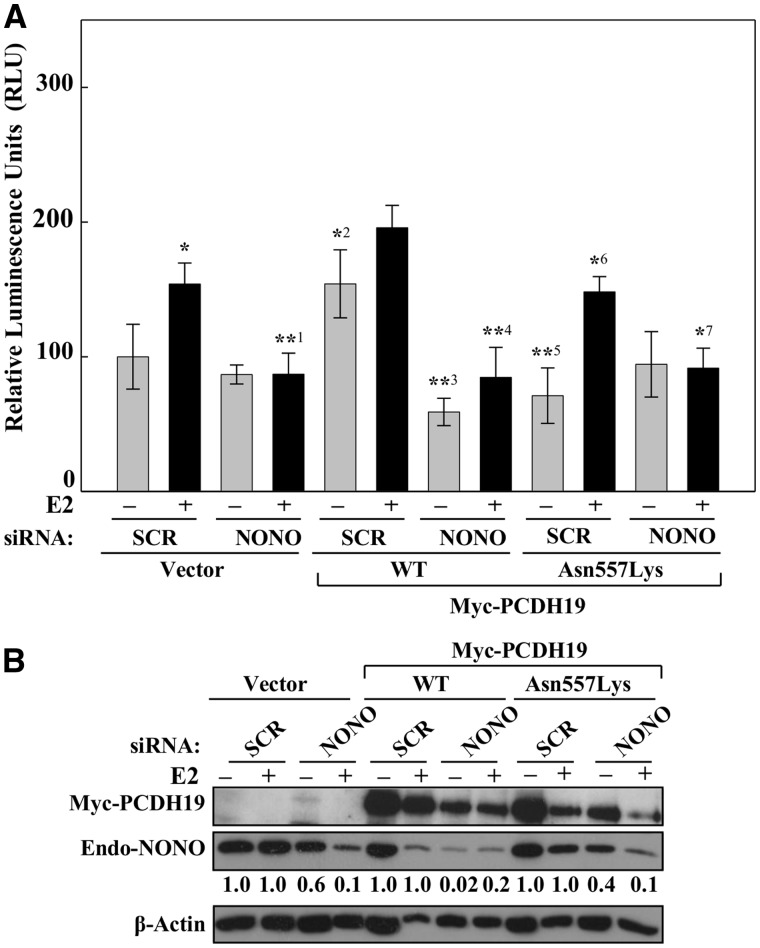
NONO is reported to be involved in the transcriptional regulation of PGR (29) and AR (30,31) but so far there is no direct evidence functionally linking NONO to ERα. We asked whether NONO was required for the PCDH19-mediated ERα-dependent regulation of the ERE-LUC reporter activity. LUC reporter activity was tested in the MCF-7 cells expressing wild type PCDH19, NONO individually or together in the presence or absence of E2. Overexpression of PCDH19 led to increased ERE-LUC reporter activity that was further enhanced in the presence of E2 (Fig. 5A). Interestingly, we observed that the presence of both PCDH19 and NONO enhanced the ERE-LUC reporter activity even further (Fig. 5A). This combined effect suggests the importance of both PCDH19 and NONO in the ERα-mediated gene expression regulation. Interestingly, the C-terminal PCDH19 fragment, the region which we showed to interact with NONO, did not show a noticeable effect on the ERE-LUC reporter activity. This suggested that while the C-terminal domain of PCDH19 is crucial for PCDH19-NONO interaction, the N-terminal cadherin domain is required for ERα-dependent gene regulation. To confirm the role of NONO in PCDH19-ERα-mediated regulation of ERE-LUC reporter activity we performed reporter assay in NONO depleted cells expressing vector, wild-type or Asn557Lys PCDH19. Whereas wild-type PCDH19 increased the ERE-LUC reporter activity (also see Fig. 4A) in SCR siRNA treated cells, this activity was significantly reduced in the NONO siRNA treated cells (Fig. 6A and B). The reporter activity of Asn557Lys PCDH19 was comparable to SCR or NONO siRNA treated cells transfected with vector alone (Fig. 6A and B). Taken together, these findings demonstrate that NONO is required for PCDH19-ERα-mediated gene regulation.
Figure 5.
PCDH19 and NONO affect ERα-mediated gene expression. (A) MCF-7 cells were transfected with 3 × ERE TATA luc and control plasmids, wild-type Myc-PCDH19, Myc-PCDH19CR, wild-type HA-NONO, Myc-PCDH19/HA-NONO or Myc-PCDH19CR/HA-NONO expression vectors. Cells were cultured in charcoal-stripped FCS for 16 h and then for 6 h in the presence or absence of 10 nM E2. Data are expressed as relative luciferase activity ± SD from three or more independent experiments. *P < 0.05 comparing +E2 versus –E2 vector control, *1P < 0.05 comparing +E2 versus –E2 WT, *2P < 0.01 comparing +E2 WT versus +E2 vector and –E2 WT versus –E2 vector, **3P < 0.01 comparing –E2 WT/NONO versus –E2 WT using Bonferroni adjusted planned comparisons. (B) Western blot of MCF-7 cell extracts expressing wild-type Myc-PCDH19 or Myc-PCDH19CR were probed with anti-Myc antibody. Transfected HA-NONO was detected using anti-HA antibody while endogenous NONO using anti-NONO antibody. β-Actin was used as a loading control. Figure 5B was generated by cropping full-length western blots shown in Supplementary Material Figure S10. Dotted lines separate the cropped images from different western blots. As HA-NONO and Myc-PCDH19CR were detected at approximately the same size, different immunoblots were used to visualize the two proteins.
Figure 6.
NONO is required for PCDH19 effect on ERα-mediated gene expression. (A) MCF-7 cells were transfected with NONO or control siRNA for 24 h and subsequently co-transfected with expression plasmids (control, wild-type or mutant Myc-PCDH19) in conjunction with 3× ERE TATA luc reporter plasmid. Cells were cultured in charcoal-stripped medium for 16 h and then for 6 h in the presence or absence of 10 nM E2. Data are expressed as relative luciferase activity ± SD from three or more independent experiments. *P < 0.05 comparing +E2 versus –E2 SCR vector control, *1P < 0.05 comparing +E2 SCR vector control versus +E2 NONO siRNA vector control, *2P < 0.01 comparing -E2 SCR vector control versus -E2 SCR WT, **3P < 0.01 comparing –E2 SCR WT versus –E2 NONO siRNA WT, **4P < 0.01 comparing +E2 SCR WT versus +E2 NONO siRNA WT, **5P < 0.01 comparing –E2 SCR MT versus –E2 SCR WT, *6P < 0.05 comparing +E2 SCR MT versus +E2 SCR WT, *7P < 0.05 comparing +E2 NONO siRNA MT versus +E2 SCR siRNA MT. (B) Western blot of MCF-7 cell extracts expressing wild-type or mutant Myc-PCDH19 were probed with anti-Myc antibody. Endogenous NONO was detected using anti-NONO antibody and β-Actin was used as a loading control. The level of NONO was measured relative to β-actin signal by the ImageJ program and the level of NONO knockdown for each treatment relative to SCR control (fixed at 1) is shown.
Discussion
Since the identification of PCDH19 as the gene responsible for PCDH19-FE (4), there has been little progress in the understanding of the molecular mechanisms driving the disease. PCDH19 is a member of the cadherin super family of cell adhesion proteins that play important roles in the brain morphogenesis and wiring including maintenance and plasticity of neuronal circuits that are important in learning and memory (32). In the present study, we identified a nuclear protein NONO as a novel interactor of PCDH19. This was surprising given our current understanding that Pcdh19/PCDH19 primarily functions as a cell-cell adhesion molecule (5). NONO is a member of the DBHS family of proteins which form essential structural component of ribonucleoprotein bodies found in the interchromatin space of mammalian cell nuclei called paraspeckles (14,15). Paraspeckle proteins including p54nrb/NONO, splicing factor proline-and glutamine-rich (SFPQ/PSF), paraspeckle protein 1 (PSPC1) and fused in sarcoma protein (FUS/TLS) have been shown to interact and form complexes with one another and are involved in both transcriptional and posttranscriptional regulation and DNA repair (30). Therefore interaction of two proteins previously shown to be localized in two different cellular compartments seemed unlikely. While we are yet to determine the specific region of interaction between PCDH19 and NONO, we demonstrate that the PCDH19 is present in the perinuclear and nucleoplasmic region of the cell and in close proximity with NONO. NONO is a known co-regulator of steroid hormone nuclear receptors such as PGR and AR (30,31). Additionally, PGR knockdown has been shown to downregulate PCDH19 (33). These data are consistent with the role of PCDH19-NONO interaction in the regulation of steroid hormone receptors and also agree with our recently published findings showing nuclear hormone receptor regulated gene involvement in the pathogenesis of PCDH19-FE (12).
It is well known that the seizures in PCDH19-FE are associated with fever. As such it might be intriguing to speculate that this association could be, at least partly, modulated by NONO. It has been recently shown that silencing NONO expression in H295R human adrenocortical cells decreases the ability of the cells to increase intracellular cAMP production and subsequent cortisol biosynthesis in response to adrenocorticotropin hormone (ACTH) stimulation (34). It has also been shown that stressors like high ambient temperature result in the greatest rise in plasma ACTH as well as corticosterone synthesis and secretion in rats (35). As such it is plausible to speculate that this axis of PCDH19-NONO-ACTH is not working properly in PCDH19-FE, which may explain increased vulnerability of the PCDH19-FE girls to fever (or stress in general) and its associated effects, i.e. seizures.
A number of other PCDH19-interacting proteins have been identified recently; for example N-cadherin (36,37), Nck-associated protein 1 (NAP1) (38,39) and cytoplasmic FMR1 interacting protein 2 (CYFIP2) (38,39). Interestingly, NAP1 and CYFIP2, like NONO, were shown to interact with the C-terminal region of PCDH19 (38,39). Notably, we used mouse and human PCDH19 while other studies used chicken and zebrafish Pcdh19. NAP1 and CYFIP2 proteins have been shown to form part of the WAVE regulatory complex (WRC) that controls actin cytoskeletal dynamics within the cell (39,40). In addition, WRC can also interact with the C-terminal region of a number of protocadherins including PCDH8, PCDH10, PCDH12, PCDH17, PCDH18, PCDHα6 and PCDH19 (39). While this may facilitate a potential role of PCDH19 in actin cytoskeletal dynamics by associating with the WRC, the molecular mechanism mediating this initial association between PCDH19-WRC-induced rearrangements in the actin-cytoskeletal networks has not yet been established. These data along with our results suggested involvement of PCDH19 in more than one cellular pathway. We therefore postulated that while one role of PCDH19 is in cell-cell adhesion and migration that is most likely mediated by PCDH19 interaction with WRC involving actin cytoskeletal networks (39), PCDH19 may also act as a signalling molecule linking cell adhesion and cell migration to gene expression regulation. The evidence presented here suggests that PCDH19 and NONO interact and regulate gene expression, at least partly through ERα nuclear steroid hormone receptor.
ER signalling has been associated with the activation of multiple neuroprotective pathways (41). Although ERs are predominantly intracellular, ∼5% of cellular ERα and ERβ are associated with the membrane (42,43). Here they interact with neurotransmitters and growth factor receptors that are involved in non-genomic ER signalling and as such indirect transcriptional regulation (41,42). PCDH19, as a member of the cadherin superfamily of cell adhesion molecules, is assumed to be a cell membrane protein. However, this has not yet been demonstrated. Using subcellular fractionation studies of the MCF-7 cells, we show that PCDH19, NONO and ERα are present predominantly in the nuclear fraction (Supplementary Material, Fig. S4). It is plausible that these proteins associate with each other in at least MCF-7 cells in the nucleus, to regulate ERα transcriptional activity.
We have previously shown that the levels of the neurosteroid allopregnanolone are reduced in PCDH19-FE compared to normal control girls (12). The enzymes involved in reducing progesterone to allopregnanolone (through an intermediate metabolite 5α-DHP) are the aldo-keto reducatases (AKR1C1-4). We have shown that at least AKR1C3 mRNA (and likely also AKR1C2) and protein were reduced in PCDH19-FE patients. The 17β-hydroxysteroid dehydrogenases (HSD) enzymatic properties of AKR1C3 are responsible for reducing androstenedione to testosterone that could then be converted to estradiol by cytochrome P450 aromatase enzyme in the brain (44). It appeared that the reduced expression of AKR1C3 not only affected the allopregnanolone level, but also testosterone, and in turn, estradiol (E2). In most tissues, endogenous ER expression is controlled by the abundance of estrogen; ER expression is low in the presence of lower estrogen levels and high when estrogen is high (44,45). For example, treatment of temporal lobe epilepsy patients with P450-inducing anti-epileptic drugs (phenytoin and carbamazepine) increased the level of E2 in patients causing a significant upregulation of ERα mRNA and proteins in their hippocampal pyramidal neurons (46,47). Here, we show that wild-type PCDH19 associates with NONO and, directly or indirectly, affects ERα-mediated gene expression, which is further enhanced in the presence of E2. In contrast, PCDH19-FE disease-associated variants including three missense mutations and one in-frame duplication abolished this function of PCDH19 or even led to lower than basal levels of the reporter expression. From clinical, patient phenotype perspective, there are no obvious yet identified differences between the group of patients with missense mutations (as tested here) and those with protein truncating mutations or entire gene deletions (10,11). This suggests that all of the currently reported PCDH19 mutations are loss of function mutations, which most likely lead, among other consequences, to altered ERα-regulated developmental and functional gene expression.
Estrogen signaling has been established to be crucial for normal brain development (48) and often with gender specific effects (49). ERα and its ligand estradiol (E2) have been shown to play important roles in various diseases including epilepsy, neurodegenerative disorders, psychiatric disorders, behavioral disorders and cancers (24,26,50,51). The role of estrogens in seizures susceptibility has been widely studied and is highly controversial. Findings among different studies are inconsistent due to biological complexity and variations in the experimental settings (26,52). In general, estrogens have proconvulsant and epileptogenic properties in both human and animal models (53–56). This is particularly well-demonstrated in women with catamenial epilepsy, where seizure activity has been linked to fluctuating estrogen and progesterone levels during the menstrual cycle (56). In contrast, but consistent with our findings, evidence also supports a role for estrogen in anticonvulsant effect under specific conditions (25). An example of this is in a recent study demonstrating that neonatal E2 treatment prevented epilepsy in the ARX model of X-linked infantile spasms syndrome. E2 treatment also restored certain classes of interneurons in the somatosensory cortex, e.g. those expressing a known ERα target gene, neuropeptide Y (NPY) as well as other, e.g. ARX-regulated genes like Shox2 (57). Since the seizure onset and offset age of PCDH19-FE patients fall between the periods of low and high estrogen activity, respectively, PCDH19 mutations that resulted in lower ERα transcriptional activity will potentially affect these patients primarily during low estrogen period (when ERα activity is at the minimal level).
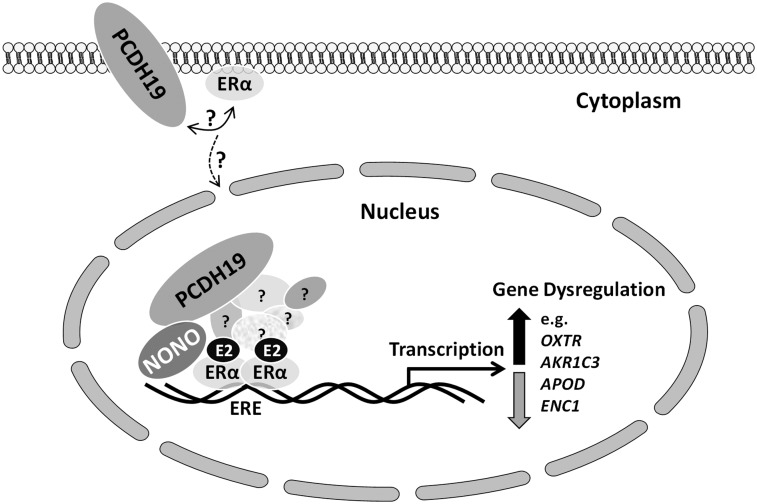
In summary, we have identified NONO as a novel PCDH19 interacting protein, which appears to be important in PCDH19-mediated ERα-dependent gene regulation (Fig. 7). This together with our recent finding of neurosteroid allopregnanolone deficiency in PCDH19-FE girls further underscores the importance of steroid nuclear hormone receptor, and ERα specifically, signaling in epilepsy and likely also in autism, intellectual disability and other behaviours. Our study of a novel regulatory pathway of ERα regulation by a cell adhesion molecule has also potential relevance to other, apparently unrelated disorders like breast cancer and opens new possibilities of intervention.
Figure 7.
Proposed model of PCDH19-mediated co-regulation of gene expression with NONO and ERα. PCDH19 is postulated to be a membrane bound protein of which a proportion (this work) is present in the nucleus. Our model suggests that the nuclear PCDH19 interacts with NONO and cooperates with ERα and its associated (in this case largely unknown) proteins to regulate target gene expression (e.g. OXTR, AKR1C3, APOD or ENC1, see Supplementary Material, Fig. S7). Whether PCDH19 interacts with ERα, directly or indirectly at the cell membrane or in the nucleus, remains to be determined. Estradiol = E2; ERE = Estrogen Response Element; ? = unknown proteins, protein-protein interactions or signalling pathways.
Materials and Methods
Cell culture and transfection
Human embryonic kidney cells (HEK293T, ATCC CRL-1573) were cultured in Dulbecco's modified Eagle's medium (Sigma) containing 10% fetal bovine serum (Life Technologies), 2 mM glutamine, 0.2% (w/v) sodium bicarbonate, penicillin (1.2 mg/ml) and streptomycin (1.6 mg/ml). The cells were transfected using Lipofectamine 2000 (Life Technologies) according to the manufacturer's protocol and harvested 24 h post-transfection. Human cervical carcinoma (HeLa) cells were cultured, transfected with Lipofectamine 2000 (Life Technologies) and harvested in the same manner. MCF-7 (human mammary adenocarcinoma, ECACC# 86012803) and MDA-MB-231 (human mammary adenocarcinoma, ATCC# HTB-26) cells were cultured in Dulbecco's modified Eagle's medium as described above.
Yeast two-hybrid screen
The Yeast two-hybrid screening was performed using the Matchmaker Gold Yeast Two-Hybrid system (Clontech) according to the manufacturer's instructions. The C-terminal fragment containing the alternative spliced exon 2 and the conserved domains CM1 and CM2 of mouse Pcdh19 cDNA cytoplasmic region (amino acid 708 to 1146) (NM_001105246.1) was amplified with primers 5′- cacacacacacaggatcc ccATG GAG TC GCTCCTGCTGCCGGTGCT-3′ and 5′- cacacacacacacacagcggccgcT TAGAGAACGATATCCTTCAGACGCTTCACAC-3′ and cloned in-frame with the GAL4 DNA binding sequence within the pGBKT7 vector at EcoRI and BamHI sites to generate pGBKT7 + Pcdh19CR yeast bait expression construct. Sequencing verified the orientation and integrity of the mouse Pcdh19 cDNA sequences. This bait construct was then transformed into two strains of yeast (bait in Y2H Gold and prey in Y187) together with an E11 embryo Clontech’s Mate & Plate library as prey and Pcdh19CR as bait.
Generation of expression constructs
The full-length mouse Pcdh19 (NM_001105246.1) and human PCDH19 (NM_001184880) cDNA was amplified from mouse and human brain cDNA, respectively. The mouse Pcdh19 was amplified with primers 5′- cacacacacaca ggatccccATGGAGTCTCTCC TGCTGCCGGTGC-3′ and 5′- cacacacacacacacagcggccgcTTAG AG AA CGATATCCTTCAGACG-3′ and the human PCDH19 using the primers 5′- cacacacacacagg atccccATGGAGTCGCTCCTG CTGCCG GTGCT-3′ and 5′- cacacaca cacac acagcggccgcTTAGAGAACGA TA TCCT TCAGACGCTTCACAC-3′. The PCR products were cloned at BamHI and NotI in the pCMV-Myc plasmid (Clontech) for mammalian expression. Orientation and integrity of both human and mouse Pcdh19 was confirmed by sequencing. The double-tagged PCDH19 (Myc-tagged at the N-terminus and FLAG-tagged at the C-terminus) was generated by PCR amplification using: 5′- ccgtggagctgatagcgagaaagtc -3′ and 5′ cacacacacaGCGGCC GCttacttgtcgtcatcgtctttgtagtcgagaacgatatccttcagacgcttc -3′ oligonucleotide primers. The PCR product was subcloned into pCMV-Myc-PCDH19 expression construct at NsiI and NotI sites. Similar double-tagged constructs for expression of four different PCDH19 missense mutations (Val441Glu, Asn557Lys, Ser276Pro) were generated by subcloning the fragments containing the mutations into the pCMV-Myc-PCDH19-FLAG at HindIII and KpnI sites. PCDH19 duplication construct was generated by subcloning a PCDH19 fragment containing S139_A141dup into the pCMV-Myc-PCDH19-FLAG at the BglII and KpnI sites. Sequencing was performed to verify the presence of the mutations.
Primers for PCR amplification of the mouse and human NONO coding region were designed using the published murine Nono cDNA (CT010241) and human NONO (CR456761) sequence, respectively. The murine Nono was amplified from mouse brain cDNA with primers 5′-cacacacacacgaatt cccATGCAGAG CAA TA AAGCCTTTAACT-3′ and 5′-cacacacacacaggtaccATATCGGCG GC GTTTATTTGGAGC-3′. The PCR product was then cloned into pCMV-HA plasmid at the EcoRI and KpnI sites. The human NONO was amplified from human brain cDNA with primers 5′- cacacacacaga att cgg ATGCAGAGTAATAAAACTTTTAACTT-3′ and 5′- caca caca ca cac gcgccgcTTATTAGTATCGGCG ACGTTTG TTT GG GG-3′ and cloned into pCMV-HA plasmid at the EcoRI and NotI sites. Sequencing verified the orientation and integrity of both human and mouse NONO expression plasmids.
Immunoprecipitation and western blot analysis
For western blotting, protein lysates were prepared by sonicating the cells in 50 mM Tris-HCl pH 7.4, 150 mM NaCl, 2 mM Na3VO4, 10mM NaF, 0.8% Triton X-100 and protease inhibitors (Complete, Roche Applied Science). Protein concentration was determined by Pierce BCA Protein Assay Kit (Thermo Scientific). Lysates from HEK293T cells expressing the Myc-PCDH19, HA-NONO or both were prepared in 50 mM Tris-HCl pH 8.0, 150 mM NaCl, 50 mM NaF, 0.1 mM Na3VO4, 1% Triton-X-100 and 1× protease inhibitors. The lysates were incubated with Myc-conjugated agarose beads (Sigma), washed once with the lysis buffer, three times with wash buffer (50 mM Tris-HCl pH 8.0, 150 mM NaCl, 1% NP40, 0.5% Sodium deoxycholate and 0.1% SDS), eluted with 1× SDS-loading buffer, subjected to SDS-PAGE and western blotting. The target proteins were detected with appropriate primary antibodies and horseradish peroxidase-conjugated anti-mouse (Pierce) or anti-rabbit (Pierce) secondary antibodies using an enhanced chemiluminescence kit (ECL, Amersham Biosciences). β-Tubulin was employed as a loading control using rabbit anti-β-tubulin antibody (Abcam, Cambridge, UK). Mouse anti-Myc (9E10; Santa Cruz Biotech), mouse anti-HA (clone HA-7; Sigma), mouse anti-HSP90 (AC88; Enzo Life Sciences), mouse anti-β-Actin (clone AC-74; Sigma), mouse anti-FLAG (clone M2; Sigma), mouse KCNT1 (clone S3-26; Sigma), rabbit anti-PCDH19 (Bethyl Laboratories), rabbit anti-NONO (Bethyl Laboratories), rabbit anti-β-Tubulin (Abcam), rabbit anti-ERα (Santa Cruz Biotech) and rabbit anti-Histone H3 (Abcam) primary antibodies were used in the present study.
Subcellular fractionation
MCF-7 cells transfected with control or wild-type Myc-PCDH19 expression plasmids for 24 h were used for subcellular fractionation. Briefly, the cells were swollen in 0.01 M Tris-HCl, pH 7.4, 0.01 M NaCl, 1.5 mM MgCl2 and 1 × protease inhibitors on ice for 10 min and then resuspended in the same buffer with 0.2% Triton-X-100. Cell membranes were broken using a Dounce homogenizer and centrifuged at 1500 rpm for 10 min to pellet nuclei. Nuclei were lysed in 1× lysis buffer (50 mM Tris-HCl pH 7.4, 150 mM NaCl, 1mM EDTA, 1× protease inhibitors). The supernatants were centrifuged at 70 000 rpm for 30 min to separate the cell membranes from the cytoplasmic fraction. Supernatant was collected as a cytoplasmic fraction and the cell membrane pellets were resuspended in lysis buffer with 0.8% Triton-X-100 or 1% DDM.
Immunofluorescence
5 × 104 HeLa cells, 1.5 × 105 MDCK cells or 0.5 × 105 mouse primary hippocampal neurons (E18.5) were plated per well of a 12-well culture dish onto poly-L-lysine coated coverslips and transfected with either full-length or C-terminal Myc-PCDH19 expression constructs. Next day, the cells were fixed in 4% paraformaldehyde/1× PBS for 10 min, permeabilized with 0.2% Triton-X-100/1× PBS for 5 min, blocked in 10% horse serum/1× PBS for 1h and then incubated with either anti-Myc, anti-NONO or both antibodies. Primary antibodies were visualized with alexa-488 conjugated anti-rabbit (for anti-Myc) or alexa-555 conjugated anti-mouse IgG (for anti-NONO) (Thermo Scientific). All images were acquired at either 20× or 60× magnification using AxioVision Rel. 4.8 (Carl Zeiss).
Quantitative RT-PCR (qPCR)
qPCR analyses were performed using the following profiles: initial denaturation at 95°C for 10 min followed by 40 cycles of denaturation at 95°C for 15 s and annealing at 60°C for 30 s). Melt curve was included each run as quality control checkpoint. All primers were designed to generate a specific 70–250 bp amplicon (primers available on request). Data were acquired using StepOne Real-Time PCR System and software V 2.0 (Applied Biosystems) and expression of mRNA was normalized against either hypoxanthine-guanine phosphoribosyltransferase (HPRT) or actin, beta (ACTB).
Dual luciferase reporter assay
MCF-7 cells were transfected with luciferase reporter containing three copies of vitellogenin Estrogen Response Element (3× ERE TATA luc) and control, wild-type PCDH19, mutant PCDH19, C-terminal PCDH19 region, NONO, wild-type PCDH19 and NONO or C-terminal PCDH19 region and NONO expression vectors. MDA-MB-231 cells were transfected with luciferase reporter containing 3× ERE TATA luc and control, wild-type PCDH19 or mutant PCDH19 expression vectors. pRL-TK (Renilla) (Promega) was included in each transfection as transfection control. Cells were cultured in media containing 10% charcoal-stripped FCS for 16 h and then in the presence or absence of 10 nM estradiol (E2) for 6h. Cells were lysed with the 1× lysing buffer and subjected to dual-luciferase reporter assay (Promega) according to the manufactures protocol. NONO and PCDH19 protein expression was determined by western blotting.
siRNA knockdown of NONO
MCF-7 cells were transfected with either NONO (annealed 5′- GGAAGCCAGCUGCUCGGAAAGCUCUUU -3′ and 5′- AGAGCU UUCCG AGCAGCUGGCUUCCUU -3′) or scrambled (annealed 5′-UUCUCCGAACGUGUCACGUTT-3′ and 5′-ACGUGACAC GUU CGG AGAATT-3′) (GenePharma) using Lipofectamine RNAiMAX reagent according to the manufacturer's specifications (Life Technologies). Following 24 h of siRNA transfection, MCF-7 cells were transfected with luciferase reporter containing 3× ERE TATA luc and control, wild-type PCDH19 or mutant PCDH19 expression vectors. Cells were cultured in charcoal-stripped FCS in the presence or absence of 10 nM E2, lysed and subjected to dual-luciferase reporter assay (Promega). NONO and PCDH19 protein expression was determined by western blotting.
Supplementary Material
Supplementary Material is available at HMG online.
Supplementary Material
Acknowledgements
The Women’s and Children’s Hospital Foundation Grant to D. H. P. is gratefully acknowledged. The 3× ERE TATA luc reporter plasmid from Prof. Donald McDonnell, Duke University School of Medicine, Durham, NC 27710, USA (Addgene 11354) is also acknowledged.
Conflict of Interest statement. None declared.
Funding
This study was supported by Australian National Health and Medical Research Council (NHMRC) Program Grant (628952) and Research Fellowship (1041920) to J.G. and Insieme per la Ricerca PCDH19 – ONLUS. Funding to pay the Open Access publication charges for this article was provided by National Health and Medical Research Council of Australia.
References
- 1. Scheffer I.E., Turner S.J., Dibbens L.M., Bayly M.A., Friend K., Hodgson B., Burrows L., Shaw M., Wei C., Ullmann R.. et al. (2008) Epilepsy and mental retardation limited to females: an under-recognized disorder. Brain, 131, 918–927. [DOI] [PubMed] [Google Scholar]
- 2. Juberg R.C., Hellman C.D. (1971) A new familial form of convulsive disorder and mental retardation limited to females. J. Pediatr., 79, 726–732. [DOI] [PubMed] [Google Scholar]
- 3. Ryan S.G., Chance P.F., Zou C.H., Spinner N.B., Golden J.A., Smietana S. (1997) Epilepsy and mental retardation limited to females: an X-linked dominant disorder with male sparing. Nat. Genet., 17, 92–95. [DOI] [PubMed] [Google Scholar]
- 4. Dibbens L.M., Tarpey P.S., Hynes K., Bayly M.A., Scheffer I.E., Smith R., Bomar J., Sutton E., Vandeleur L., Shoubridge C.. et al. (2008) X-linked protocadherin 19 mutations cause female-limited epilepsy and cognitive impairment. Nat. Genet., 40, 776–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kahr I., Vandepoele K., van Roy F. (2013) Delta-protocadherins in health and disease. Prog. Mol. Biol. Transl. Sci., 116, 169–192. [DOI] [PubMed] [Google Scholar]
- 6. Krishna K.K., Hertel N., Redies C. (2011) Cadherin expression in the somatosensory cortex: evidence for a combinatorial molecular code at the single-cell level. Neuroscience, 175, 37–48. [DOI] [PubMed] [Google Scholar]
- 7. Hertel N., Redies C. (2011) Absence of layer-specific cadherin expression profiles in the neocortex of the reeler mutant mouse. Cereb. Cortex, 21, 1105–1117. [DOI] [PubMed] [Google Scholar]
- 8. Kim S.Y., Chung H.S., Sun W., Kim H. (2007) Spatiotemporal expression pattern of non-clustered protocadherin family members in the developing rat brain. Neuroscience, 147, 996–1021. [DOI] [PubMed] [Google Scholar]
- 9. Depienne C., LeGuern E. (2012) PCDH19-related infantile epileptic encephalopathy: an unusual X-linked inheritance disorder. Hum. Mutat., 33, 627–634. [DOI] [PubMed] [Google Scholar]
- 10. Higurashi N., Nakamura M., Sugai M., Ohfu M., Sakauchi M., Sugawara Y., Nakamura K., Kato M., Usui D., Mogami Y.. et al. (2013) PCDH19-related female-limited epilepsy: further details regarding early clinical features and therapeutic efficacy. Epilepsy Res., 106, 191–199. [DOI] [PubMed] [Google Scholar]
- 11. Depienne C., Bouteiller D., Keren B., Cheuret E., Poirier K., Trouillard O., Benyahia B., Quelin C., Carpentier W., Julia S.. et al. (2009) Sporadic infantile epileptic encephalopathy caused by mutations in PCDH19 resembles Dravet syndrome but mainly affects females. PLoS Genet., 5, e1000381.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tan C., Shard C., Ranieri E., Hynes K., Pham D.H., Leach D., Buchanan G., Corbett M., Shoubridge C., Kumar R.. et al. (2015) Mutations of protocadherin 19 in female epilepsy (PCDH19-FE) lead to allopregnanolone deficiency. Hum. Mol. Genet., 24, 5250–5259. [DOI] [PubMed] [Google Scholar]
- 13. Gaitan Y., Bouchard M. (2006) Expression of the delta-protocadherin gene Pcdh19 in the developing mouse embryo. Gene Expr. Patterns, 6, 893–899. [DOI] [PubMed] [Google Scholar]
- 14. Bond C.S., Fox A.H. (2009) Paraspeckles: nuclear bodies built on long noncoding RNA. J. Cell Biol., 186, 637–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Passon D.M., Lee M., Rackham O., Stanley W.A., Sadowska A., Filipovska A., Fox A.H., Bond C.S. (2012) Structure of the heterodimer of human NONO and paraspeckle protein component 1 and analysis of its role in subnuclear body formation. Proc. Natl. Acad. Sci. USA, 109, 4846–4850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liu Q., Chen Y., Kubota F., Pan J.J., Murakami T. (2010) Expression of protocadherin-19 in the nervous system of the embryonic zebrafish. Int. J. Dev. Biol., 54, 905–911. [DOI] [PubMed] [Google Scholar]
- 17. Hertel N., Redies C., Medina L. (2012) Cadherin expression delineates the divisions of the postnatal and adult mouse amygdala. J. Comp. Neurol., 520, 3982–4012. [DOI] [PubMed] [Google Scholar]
- 18. Park Y., Lee J.M., Hwang M.Y., Son G.H., Geum D. (2013) NonO binds to the CpG island of oct4 promoter and functions as a transcriptional activator of oct4 gene expression. Mol. Cells, 35, 61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mircsof D., Langouet M., Rio M., Moutton S., Siquier-Pernet K., Bole-Feysot C., Cagnard N., Nitschke P., Gaspar L., Znidaric M.. et al. (2015) Mutations in NONO lead to syndromic intellectual disability and inhibitory synaptic defects. Nat. Neurosci., 18, 1731–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gonzalez-Mariscal L., Chavez de Ramirez B., Cereijido M. (1985) Tight junction formation in cultured epithelial cells (MDCK). J. Membr. Biol., 86, 113–125. [DOI] [PubMed] [Google Scholar]
- 21. Prozialeck W.C., Lamar P.C. (1997) Cadmium (Cd2+) disrupts E-cadherin-dependent cell-cell junctions in MDCK cells. In Vitro Cell. Dev. Biol. Anim., 33, 516–526. [DOI] [PubMed] [Google Scholar]
- 22. Guillaume E., Comunale F., Do Khoa N., Planchon D., Bodin S., Gauthier-Rouviere C. (2013) Flotillin microdomains stabilize cadherins at cell-cell junctions. J. Cell Sci., 126, 5293–5304. [DOI] [PubMed] [Google Scholar]
- 23. Kurrle N., Vollner F., Eming R., Hertl M., Banning A., Tikkanen R. (2013) Flotillins directly interact with gamma-catenin and regulate epithelial cell-cell adhesion. PLoS One, 8, e84393.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zorumski C.F., Paul S.M., Izumi Y., Covey D.F., Mennerick S. (2013) Neurosteroids, stress and depression: potential therapeutic opportunities. Neurosci. Biobehav. Rev., 37, 109–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Velisek L., Nebieridze N., Chachua T., Veliskova J. (2013) Anti-seizure medications and estradiol for neuroprotection in epilepsy: the 2013 update. Recent Pat. CNS Drug Discov., 8, 24–41. [DOI] [PubMed] [Google Scholar]
- 26. Scharfman H.E., MacLusky N.J. (2014) Sex differences in the neurobiology of epilepsy: a preclinical perspective. Neurobiol. Dis., 72 Pt B, 180–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hynes K., Tarpey P., Dibbens L.M., Bayly M.A., Berkovic S.F., Smith R., Raisi Z.A., Turner S.J., Brown N.J., Desai T.D.. et al. (2010) Epilepsy and mental retardation limited to females with PCDH19 mutations can present de novo or in single generation families. J. Med. Genet., 47, 211–216. [DOI] [PubMed] [Google Scholar]
- 28. Depienne C., Trouillard O., Bouteiller D., Gourfinkel-An I., Poirier K., Rivier F., Berquin P., Nabbout R., Chaigne D., Steschenko D.. et al. (2011) Mutations and deletions in PCDH19 account for various familial or isolated epilepsies in females. Hum. Mutat., 32, E1959–E1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dong X., Yu C., Shynlova O., Challis J.R., Rennie P.S., Lye S.J. (2009) p54nrb is a transcriptional corepressor of the progesterone receptor that modulates transcription of the labor-associated gene, connexin 43 (Gja1). Mol. Endocrinol., 23, 1147–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kuwahara S., Ikei A., Taguchi Y., Tabuchi Y., Fujimoto N., Obinata M., Uesugi S., Kurihara Y. (2006) PSPC1, NONO, and SFPQ are expressed in mouse Sertoli cells and may function as coregulators of androgen receptor-mediated transcription. Biol. Reprod., 75, 352–359. [DOI] [PubMed] [Google Scholar]
- 31. Dong X., Sweet J., Challis J.R., Brown T., Lye S.J. (2007) Transcriptional activity of androgen receptor is modulated by two RNA splicing factors, PSF and p54nrb. Mol. Cell. Biol., 27, 4863–4875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Redies C., Hertel N., Hubner C.A. (2012) Cadherins and neuropsychiatric disorders. Brain Res., 1470, 130–144. [DOI] [PubMed] [Google Scholar]
- 33. Cloke B., Huhtinen K., Fusi L., Kajihara T., Yliheikkila M., Ho K.K., Teklenburg G., Lavery S., Jones M.C., Trew G.. et al. (2008) The androgen and progesterone receptors regulate distinct gene networks and cellular functions in decidualizing endometrium. Endocrinology, 149, 4462–4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lu J.Y., Sewer M.B. (2015) p54nrb/NONO regulates cyclic AMP-dependent glucocorticoid production by modulating phosphodiesterase mRNA splicing and degradation. Mol. Cell. Biol., 35, 1223–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Djordjevic J., Cvijic G., Davidovic V. (2003) Different activation of ACTH and corticosterone release in response to various stressors in rats. Physiol. Res., 52, 67–72. [PubMed] [Google Scholar]
- 36. Emond M.R., Biswas S., Blevins C.J., Jontes J.D.. A complex of Protocadherin-19 and N-cadherin mediates a novel mechanism of cell adhesion. J. Cell Biol., 195, 1115–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Biswas S., Emond M.R., Jontes J.D. (2010) Protocadherin-19 and N-cadherin interact to control cell movements during anterior neurulation. J. Cell Biol., 191, 1029–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tai K., Kubota M., Shiono K., Tokutsu H., Suzuki S.T. (2010) Adhesion properties and retinofugal expression of chicken protocadherin-19. Brain Res., 1344, 13–24. [DOI] [PubMed] [Google Scholar]
- 39. Chen B., Brinkmann K., Chen Z., Pak C.W., Liao Y., Shi S., Henry L., Grishin N.V., Bogdan S., Rosen M.K. (2014) The WAVE regulatory complex links diverse receptors to the actin cytoskeleton. Cell, 156, 195–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nakao S., Platek A., Hirano S., Takeichi M. (2008) Contact-dependent promotion of cell migration by the OL-protocadherin-Nap1 interaction. J. Cell Biol., 182, 395–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Arevalo M.A., Azcoitia I., Garcia-Segura L.M. (2015) The neuroprotective actions of oestradiol and oestrogen receptors. Nat. Rev. Neurosci., 16, 17–29. [DOI] [PubMed] [Google Scholar]
- 42. Levin E.R., Hammes S.R. (2016) Nuclear receptors outside the nucleus: extranuclear signalling by steroid receptors. Nat. Rev. Mol. Cell Biol., 17, 783–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Micevych P., Dominguez R. (2009) Membrane estradiol signaling in the brain. Front. Neuroendocrinol., 30, 315–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Brann D.W., Dhandapani K., Wakade C., Mahesh V.B., Khan M.M. (2007) Neurotrophic and neuroprotective actions of estrogen: basic mechanisms and clinical implications. Steroids, 72, 381–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Verdier-Sevrain S., Yaar M., Cantatore J., Traish A., Gilchrest B.A. (2004) Estradiol induces proliferation of keratinocytes via a receptor mediated mechanism. FASEB J., 18, 1252–1254. [DOI] [PubMed] [Google Scholar]
- 46. Meyer R.P., Hagemeyer C.E., Knoth R., Kaufmann M.R., Volk B. (2006) Anti-epileptic drug phenytoin enhances androgen metabolism and androgen receptor expression in murine hippocampus. J. Neurochem., 96, 460–472. [DOI] [PubMed] [Google Scholar]
- 47. Killer N., Hock M., Gehlhaus M., Capetian P., Knoth R., Pantazis G., Volk B., Meyer R.P. (2009) Modulation of androgen and estrogen receptor expression by antiepileptic drugs and steroids in hippocampus of patients with temporal lobe epilepsy. Epilepsia, 50, 1875–1890. [DOI] [PubMed] [Google Scholar]
- 48. Varea O., Escoll M., Diez H., Garrido J.J., Wandosell F. (2013) Oestradiol signalling through the Akt-mTORC1-S6K1. Biochim. Biophys. Acta, 1833, 1052–1064. [DOI] [PubMed] [Google Scholar]
- 49. Carrer H.F., Cambiasso M.J. (2002) Sexual differentiation of the brain: genes, estrogen, and neurotrophic factors. Cell. Mol. Neurobiol., 22, 479–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Schumacher M., Weill-Engerer S., Liere P., Robert F., Franklin R.J., Garcia-Segura L.M., Lambert J.J., Mayo W., Melcangi R.C., Parducz A.. et al. (2003) Steroid hormones and neurosteroids in normal and pathological aging of the nervous system. Prog. Neurobiol., 71, 3–29. [DOI] [PubMed] [Google Scholar]
- 51. Key T.J. (1995) Hormones and cancer in humans. Mutat. Res., 333, 59–67. [DOI] [PubMed] [Google Scholar]
- 52. Veliskova J. (2006) The role of estrogens in seizures and epilepsy: the bad guys or the good guys? Neuroscience, 138, 837–844. [DOI] [PubMed] [Google Scholar]
- 53. Logothetis J., Harner R., Morrell F., Torres F. (1959) The role of estrogens in catamenial exacerbation of epilepsy. Neurology, 9, 352–360. [DOI] [PubMed] [Google Scholar]
- 54. Woolley C.S. (2000) Estradiol facilitates kainic acid-induced, but not flurothyl-induced, behavioral seizure activity in adult female rats. Epilepsia, 41, 510–515. [DOI] [PubMed] [Google Scholar]
- 55. Veliskova J. (2007) Estrogens and epilepsy: why are we so excited? Neuroscientist, 13, 77–88. [DOI] [PubMed] [Google Scholar]
- 56. Verrotti A., Laus M., Coppola G., Parisi P., Mohn A., Chiarelli F. (2010) Catamenial epilepsy: hormonal aspects. Gynecol. Endocrinol., 26, 783–790. [DOI] [PubMed] [Google Scholar]
- 57. Olivetti P.R., Maheshwari A., Noebels J.L. (2014) Neonatal estradiol stimulation prevents epilepsy in Arx model of X-linked infantile spasms syndrome. Sci. Transl. Med., 6, 220ra212.. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.