Abstract
Background
Breast cancer is a disease with diverse clinical symptoms, molecular profiles, and its nature to response its therapeutic treatments. Radiotherapy (RT), along with surgery and chemotherapy is a part of treatment in breast cancer. The aim of present study was to investigate pre and post treatment effects of radiotherapy in serum fatty acids and its lipids profile in patients with breast cancer.
Methods
In this comparative as well as follow up study, Serum fatty acids were performed by gas chromatography to investigate fatty acids and Microlab for analysis of lipid profile.
Results
Among serum free and total fatty acids the major saturated fatty acids (SFAs) in serum lipids of breast cancer patients (pre and post treated) were stearic acid (18:0) and palmitic acid (16:0). These fatty acids contributed about 35-50% of total fatty acids. The decreased concentrations of linoleic acid (C18:2) and arachidonic acid (C20:4) with a lower ratio of C18:2/C18:1 was found in pretreated breast cancer patients as compared to controls. The n-3/n-6 ratio of breast cancer patients was decreased before treatment but it was 35% increased after treatment. In addition, plasma activity of D6 desaturase was increased in the breast cancer patients, while the activity of D5 desaturase was decreased. Increased levels of SFAs, monounsaturated fatty acids (MUFAs) and decreased polyunsaturated fatty acids (PUFAs) levels in breast cancer patients (pre and post treated) as compared to controls. Serum total cholesterol (TC) (224.4 mg/dL) and low density lipoprotein cholesterol (LDL-C) (142.9 mg/dL) were significantly increased in pretreated breast cancer patients but after the radiotherapy treatment, the TC (150.2 mg/dL) and LDL-C (89.8 mg/dL) were decreased.
Conclusion
It seems that RT would have played a potential role in the treatment of BC. After RT the serum levels of PUFAs, TC, and LDL-C are improved. Our study reinforces the important role of RT in the management of BC.
The level of PUFAs, TC, and LDL-C can be used as the biomarkers for early diagnosis in individuals with risk of breast cancer.
Electronic supplementary material
The online version of this article (doi:10.1186/s12944-017-0481-y) contains supplementary material, which is available to authorized users.
Keywords: Radiotherapy, Breast cancer, Lipids, Fatty acids, Gas chromatography
Background
Breast cancer (BC) is a disease with diverse molecular profiles, clinical behavior and response to therapy [1]. Radiotherapy (RT) is being used to treat cancer patients, since very long time. Now a day, more than half of all the cancer patients receive RT at one point during their treatment. RT after surgery plays an important role in the treatment of early and advance stage in BC. Recurrence is the main problem in the management of BC, but RT lowers the local recurrence, improves survival rates and controls the growth of cancerous cells in BC [2]. Modern RT techniques have been developed for the improvement of temporary tolerability along with a reduction in tissue damage in BC patients [3].
Dietary lipids are found to have an association of BC recurrence and survival of cancerous cells. Lipids as the part of cell membranes signal molecules and energy substrates play a vital role in BC. Previous studies reported a correlation of fat and suggested that dietary fat plays an important role in the incidence of BC, in an animal model [4]. Dietary fat is directly associated with BC as the high fat intake is composed of fatty acids which have distinctive biophysical and chemical properties that can influence on BC disease and normal health [5, 6]. Saturated fatty acids (SFAs) and monounsaturated fatty acids (MUFAs) are reported with the possible association with increased risk of BC in rodents [7] and humans [8]. It is proved that BC synthesizes endogenously 95% of FAs for nutritional lipid supply. SFAs and MUFAs positively associated are reported with the risk of BC [9]. Polyunsaturated fatty acids (PUFAs) are an important part of the diet, sufficient amount of PUFAs in the diet is essential because they are structural components of cell membrane and play an important role in cell signaling, metabolism, regulation of gene expression and inflammation [10]. Some animal studies found that n-6 PUFAs are associated with tumor enhancing effects while n-3 PUFAs shows protective effects [7]. The n-3 PUFAs may be associated with several mechanisms that counteract carcinogenic processes [11, 12]. Another study found that saturated fat increased the risk of BC but they did not found a significant association of total PUFAs [13] or n-3 PUFAs intake [14]. An experimental study reported that n-3 PUFAs in comparison to n-6 PUFAs shows inhibitory effects on BC [15].
Lipoproteins are the distributors of both endogenous and exogenous lipids across the tissues. It is therefore possible that lipoproteins can play a fundamental role in the progression of cancer via lipids supply to malignant cells and tumors. The level of plasma lipids reflects the dietary lipid intake in individuals. There are several reports of increased plasma lipid levels such as total lipids (TL), phospholipids (PL), triglycerides (TG), total cholesterol (TC) and low density lipoproteins (LDL-C) in BC patients [16]. Therefore, the present study was aimed to determine the possible effect of RT on serum fatty acids and lipid profiling in BC patients (before and after treatment), in comparison to healthy individuals.
Methods
Blood serum samples were collected from Nuclear Institute of Medicine and Radiotherapy (NIMRA), Jamshoro, Pakistan. One hundred and thirty female patients with BC aged 25 to 65 years were enrolled before starting the radiotherapy during January, 2014 to July, 2015. The dietary habits of patients were almost same according to nutritionist because they were admitted at Nuclear Institute of Medicine Radiotherapy, Jamshoro, Pakistan for completing the therapy of 6 cycles. The female patients selected were those who carry BC, since 1 year and the tumor size of patients were also almost same. Firstly, we collected the blood sample before starting the radiotherapy, when a female patient was agreed for this procedure of treatment. After completion of all six cycles of radiotherapy, the blood samples of same female BC patients were collected as post treated patients. Those female BC patients who were treated with radiotherapy elsewhere and continued their therapy at NIMRA were excluded from the study. Those females who discontinued their radiotherapy due to any reason or having other complications due to radiotherapy and all male BC patients were excluded from the present study. We also collected fifty blood samples from untreated normal volunteer female subjects. The controls met the criteria of having negative personal and family history of any cancer disease, age and gender matched, non- smokers, having normal BMI (non- obese) were included in present study. The females with cardiovascular diseases, diabetes and hyperlipidemia were excluded from the study. All the participant females signed written informed consents before sample collection; they were also consented for publication. The study was carried out by following the regulations of Institutional Ethnical Committee, Institute of Biochemistry, University of Sindh, Jamshoro, Pakistan under their permission letter.
The blood samples from all participants were collected in fasting condition. Serum was separated and stored at −40 °C until analyzed further. Fatty acids and lipid profiling were performed by gas chromatograph 8700 (Perkin–Elmer Ltd., England) and Vital Scientific Microlab 300 (Germany), respectively. Fatty acid contents such as free fatty acids (FFAs) and total fatty acids (TFAs) were analyzed before and after RT. The FFAs and TFAs samples were prepared as per reported method [17]. Briefly, gas chromatograph, model 8700 fitted with non-bonded biscynopylsiloxane stationary-phase, polar capillary column Rt-2560(100 m × 0.25 mm) with a film thickness of 0.2 μm (Supelco, PA, USA) and flame ionization detector was used. Oxygen free nitrogen was used as a carrier gas at a flow rate of 3.5 ml/min. The initial temperature of the oven was adjusted at 120 °C/4 min, which was raised up to 220 °C for 20 min. The injector and detector temperature were set at 260 °C and 270 °C, respectively. 2 μl sample was injected from injector and peaks were measured by comparing with standards supplied by Fluka Chemika (Buchs, Switzerland). Thirteen fatty acids of three different groups are given as follows;
SFAs: C12:0 (lauric acid) C14:0 (myristic acid), C15:0 (pentadecyclic acid)C16:0 (palmitic acid), and C18:0 (Stearic acid).
MUFAs: C14:1 (myristoleic acid), C16:1 (palmitoleic acid), and C18:1 (oleic acid).
PUFAs: C18:2 (linoleic acid), C18:3 (α-linolenic acid), C20:3 (Dihomo-γ-linolenic acid), C20:4 (Arachidonic acid), and C22:6 (docosahexaenoic acid). The composition of FA’s was reported by relative percentage of the total peak area.
D5 Desaturase was calculated as 20:4n-6/20:3n-6 ratio. D6 Desaturase was calculated as 20:3n-6/18:2n-6 ratio [18]. Lipid profile contains total cholesterol (TC), triglycerides (TG), high density lipoprotein cholesterol (HDL-C), low density lipoprotein cholesterol (LDL-C) and very low density lipoprotein cholesterol (VLDL-C) which were analyzed by using kit method (Merck, Germany) in microlab 300 [19].
Statistical analysis
The values obtained were expressed as mean ± SD. To compare data among the age groups ANOVA was applied and to compare the patients data with controls and patients data for comparison between pre and post treated, student’s t-test was applied by using SPSS 15.2 software. p value less than 0.05 was considered as significant.
Results
Free fatty acid levels with n-3 and n-6 classes of serum lipids in breast cancer patients (pre and post treated) were compared with controls (Table 1)(data is presented in Additional files 1, 4 and 9). The SFAs in serum lipids of BC patients (pre and post treated) were significantly increased, stearic acid (C18:0) and palmitic acid (C16:0) were the major SFAs (Fig. 1a). In MUFAs, a significant increase in the concentration of oleic acid (C18:1) was observed in BC patients (Fig. 1b). In PUFAs, decreased concentrations of linoleic acid (C18:2) and Arachidonic acid (C20:4) were observed in BC patients as compared to controls (Fig. 1c). Lower ratio of C18:2/C18:1, whereas, a decrease in n-3/n-6 ratio was observed in BC patients but improvement in n-3/n-6 was found after RT treatment. In addition, plasma activity of D6 desaturase was increased in the breast cancer patients while the activity of D5 desaturase was decreased (Table 1).
Table 1.
Free fatty acid profile of BC patients (pre and post) in comparison with controls
| Free fatty acids | Controls | Pre-treated BC patients | Post-treated BC patients |
|---|---|---|---|
| C-14: 0 | 1.26 ± 0.96 | 1.30 ± 0.30 | 0.81 ± 0.44 |
| C-15: 0 | ND | 0.57 ± 0.24 | ND |
| C-16: 0 | 18.17 ± 5.06 | 23.0 ± 2.86* | 28.2 ± 2.47* |
| C-18: 0 | 13.89 ± 3.56 | 21.8 ± 2.00* | 15.9 ± 1.76¶ |
| C-14: 1 | 0.75 ± 0.49 | 1.86 ± 0.51 | 0.04 ± 0.01 |
| C-16: 1 | 2.619 ± 1.37 | 1.85 ± 0.48 | 2.66 ± 0.96 |
| C-18: 1 | 19.71 ± 2.276 | 27.3 ± 1.63* | 26.5 ± 2.01* |
| C-18: 2 (n-6) | 29.14 ± 3.28 | 20.2 ± 1.65* | 19.84 ± 2.37* |
| C-18: 3 (n-3) | 1.54 ± 0.79 | 1.21 ± 0.836* | 0.76 ± 0.40* |
| C-20: 3 (n-6) | 0.65 ± 0.3 | 0.39 ± 0.19 | 0.6 ± 0.47 |
| C-20: 4 (n-6) | 7.76 ± 2.40 | 1.09 ± 0.52* | 2.7 ± 1.55*¶ |
| C-22: 6 (n-3) | 1.18 ± 0.79 | 0.47 ± 0.24 | 1.88 ± 1.74 |
| C-18:0: C-18:1 | 0.719 ± 0.24 | 0.79 ± 0.06 | 0.605 ± 0.090 |
| C-18:2: C-18:1 | 1.5 ± 0.28 | 0.074 ± 0.03 | 0.75 ± 0.118 |
| C-18:3: C-18:1 | 0.071 ± 0.033 | 2.8 ± 0.97* | 0.008 ± 0.002*¶ |
| n-3: n-6 | 7.4 ± 1.41 | 2.80 ± 1.01* | 3.43 ± 1.71*¶ |
| Sat: unsat FA’s | 35.2 ± 6.05 | 25.0 ± 1.34* | 26.6 ± 3.47* |
| D5 Desaturase | 21.2 ± 19.1 | 3.83 ± 3.4* | 7.84 ± 1.2 |
| D6 Desaturase | 0.019 ± 0.009 | 0.03 ± 0.02 | 0.02 ± 0.013 |
Values represent mean ± standard deviation of triplicates. The different symbol on the same row indicates the Significant difference at p < 0.05. *p < 0.05 shows the comparison between pre and post treated BC patients with controls, ¶ p < 0.05 shows the comparison between pre-treated BC patients and post treated BC patients. Lauric acid (C12:0), Myristic acid (C14:1), myristoleic acid (C14:1), Pentadecyclic acid (C15:0), palmitic acid (C16:0), palmitoleic acid (C16:1), stearic acid (C18:0), oleic acid (C18:1), linoleic acid (C18:2), α-linolenic acid (C18:3), C20:3 (Dihomo-γ-linolenic acid), arachidonic acid (C20:4) and docosahexaenoic acid (DHA (C22:6). D5 Desaturase was calculated as 20:4n-6/20:3n-6 ratio. D6 Desaturase was calculated as 20:3n-6/18:2n-6 ratio. Sat: unsat FA’s shows saturated and unsaturated fatty acids ratio
Fig. 1.
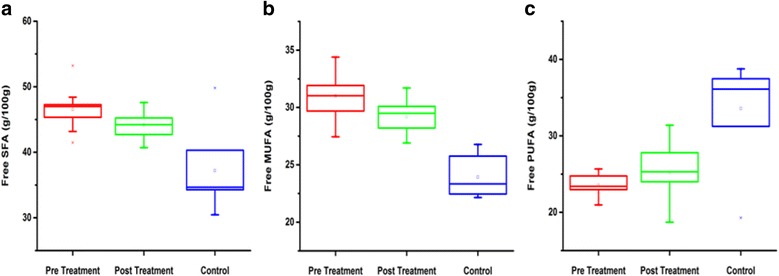
Detailed evaluation of free form of fatty acids. a Shows free SFAs level in pre-treated and post-treated as compared with control. b Shows free MUFAs level in pre and post-treatment patients as compared with control. c Shows Free PUFAs level between pre-post-treatment and control subjects
In addition, same results were observed in total fatty acids as well (Table 2)(data is presented in Additional files 2, 3 and 10). Total SFAs levels were significantly increased in BC (pre and post treated) patients as compared to controls (Fig. 2a). MUFAs were also elevated in BC patients as compared to controls (Fig. 2b). In contrast, PUFAs were reduced in BC (pre and post treated) patients as compared to controls (Fig. 2c). The Significant variation (P < 0.05) was observed in free fatty acids including SFAs and PUFAs.
Table 2.
Total fatty acid profile of BC patients (pre and post) in comparison with controls
| Total fatty acid | Controls | Pre-treated BC patients | Post-treated BC patients |
|---|---|---|---|
| C-12: 0 | ND | 0 ± 0 | 0.42 ± 0.04 |
| C-14: 0 | 1.26 ± 0.96 | 1.34 ± 0.30 | 2.03 ± 1.06 |
| C-15: 0 | 0.80 ± 0.13 | 0.47 ± 0.22 | 1.86 ± 1.18 |
| C-16: 0 | 18.2 ± 5.06 | 22.3 ± 2.57* | 20.7 ± 2.73* |
| C-18: 0 | 14.6 ± 3.56 | 23.1 ± 2.43* | 22.9 ± 2.36* |
| C-14: 1 | 0.80 ± 0.74 | 1.19 ± 0.60 | 0.86 ± 0.42 |
| C-16: 1 | 2.62 ± 1.37 | 1.72 ± 0.43* | 1.34 ± 0.76* |
| C-18: 1 | 19.7 ± 2.276 | 25.4 ± 1.6* | 24.6 ± 2.32* |
| C-18: 2 (n-6) | 29.1 ± 3.28 | 20.64 ± 1.94* | 22.1 ± 2.39* |
| C-18: 3 (n-3) | 0.98 ± 0.76 | 1.69 ± 0.60* | 2.36 ± 1.85* |
| C-20: 3 (n-6) | 1.58 ± 0.87 | 0.55 ± 0.30 | 0.72 ± 0.69 |
| C-20: 4 (n-6) | 7.72 ± 2.42 | 1.24 ± 0.46* | 1.59 ± 0.96* |
| C-22: 6 (n-3) | 0.46 ± 0.30 | 0.51 ± 0.19 | 1.15 ± 0.89 |
| C-18:0: C-18:1 | 0.72 ± 0.24 | 0.91 ± 0.10 | 0.93 ± 0.14 |
| C-18:2: C-18:1 | 1.5 ± 0.28 | 0.82 ± 0.09 | 0.91 ± 0.14 |
| C-18:3: C-18:1 | 0.05 ± 0.03 | 0.07 ± 0.02 | 0.10 ± 0.09 |
| n-3: n-6 | 9.8 ± 3.99 | 3.4 ± 0.86* | 4.62 ± 1.95* |
| Sat: unsat FA’s | 41.0 ± 4.50 | 26.2 ± 2.02* | 29.1 ± 3.22* |
| D5 Desaturase | 7.75 ± 6.02 | 2.66 ± 1.25* | 3.53 ± 3.45* |
| D6 Desaturase | 0.026 ± 0.014 | 0.05 ± 0.03 | 0.03 ± 0.02 |
* p < 0.05 shows the comparison pre and post treated BC patients with controls, ¶ p < 0.05 shows the comparison of pretreated BC patients with post treated BC patients. Values represent mean ± standard deviation of triplicates. The different symbol on the same row indicates the Significant difference at p < 0.05. Lauric acid (C12:0), Myristic acid (C14:1), myristoleic acid (C14:1), Pentadecyclic acid (C15:0), palmitic acid (C16:0), palmitoleic acid (C16:1), stearic acid (C18:0), oleic acid (C18:1), linoleic acid (C18:2), α-linolenic acid (C18:3), C20:3 (Dihomo-γ-linolenic acid), arachidonic acid (C20:4), and docosahexaenoic acid (DHA (C22:6).D5 Desaturase was calculated as 20:4n-6/20:3n-6 ratio.D6 Desaturase was calculated as 20:3n-6/18:2n-6 ratio. Sat: unsat FA’s shows saturated and unsaturated fatty acids ratio
Fig. 2.
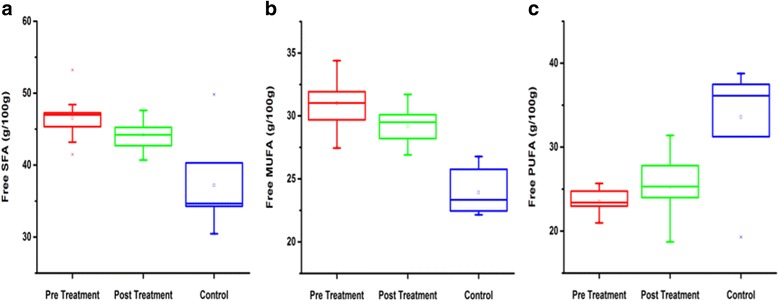
Detailed evaluation of total form of fatty acids. The SFAs (a), MUFAs (b) were elevated and PUFAs (c) was lower in Breast cancer (pre and post treated) patients in contrast with controls. The Significant variation (P < 0.05) was found in total fatty acids including SFAs and PUFAs
Levels of TC, LDL-C, HDL-C, VLDL and TG were also examined (Table 3). Where, serum TC and LDL-C in pretreated BC patients were significantly increased as compared to controls (Fig. 3), while, after RT treatment TC and LDL-C levels were decreased significantly (Fig. 4)(data is presented in Additional files 5, 6, 7 and 8). Whereas TG and VLDL-C increased in post treated BC patients as compared with controls but within normal ranges. The non-significant level of HDL-C and high level of TC were also found affected on TC: HDL-C ratio (Table 3).
Table 3.
Comparison of lipid profile in BC (pre and post treated) patients with controls
| Lipid parameter (mg/dL) | Controls n = 50 | Pre-treated BC patients n = 130 | Post-treated BC patients n = 130 |
|---|---|---|---|
| TC (<200) | 137.88 ± 24.45 | 224.4 ± 25.21* | 150.2 ± 22.19* ¶ |
| TG (<150) | 114.42 ± 24.74 | 144.7 ± 21.28 | 146.2 ± 27.42 |
| HDL (<40) | 56.56 ± 14.77 | 55.96 ± 16.97 | 43.36 ± 5.62 ¶ |
| LDL(<100) | 54.16 ± 12.71 | 142.9 ± 25.04* | 89.83 ± 26.68* ¶ |
| TL (450-1000) | 509.72 ± 37.97 | 718.1 ± 53.74* | 579.7 ± 55.59* ¶ |
| VLDL(<30) | 22.67 ± 4.75 | 28.95 ± 4.25* | 31.49 ± 5.71* |
| TC:HDL(4.0) | 2.712 ± 0.84 | 4.48 ± 1.60 | 3.54 ± 0.72 |
| TC:LDL (1-6) | 2.70 ± 0.91 | 1.59 ± 0.17* | 1.78 ± 0.53 *¶ |
| HDL:LDL (0.5) | 1.06 ± 0.45 | 0.39 ± 0.145* | 0.51 ± 0.14 * |
*p < 0.01 shows the comparison between pre-treated and post treated BC patients with controls, ¶ p < 0.01 shows the comparison between pre-treated BC patients with post treated BC patients
Fig. 3.
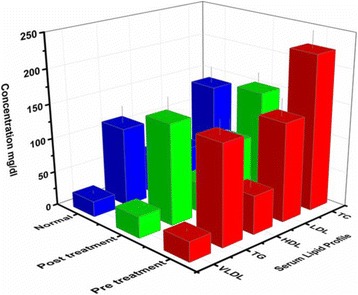
Serum lipid profile of pre and post treatment BC patients in comparison with control: The figure pretreated that the patients have significant positive association of high level of TC(224.4 ± 25.21), low level of HDL-C(55.96 ± 16.97) and high level of LDL-C (142.9 ± 25.04) with Breast cancer disease (Red bar). After treatment the level of TC and LDL-C decreases significantly. (Green Bar)
Fig. 4.
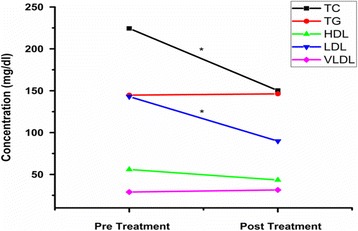
Showed the comparison between pre-treated and post-treated BC patients. Represented that TC and LDL levels are decreased in post-treatment as compared to post-treatment BC patients. While as, TG, HDL and VLDL levels showed no significant change
Discussion
In present study, the effective role of RT was examined. Significant differences were observed in FAs and lipid profile in serum samples from patients with BC before and after RT treatment. The levels of PUFAs were increased, whereas, TC and LDL-C levels were significantly decreased after the RT treatment. SFAs levels were also found to be associated with the risk of BC (Fig. 1a–c and 2a–c). In SFAs, stearic and palmitic acid while, in MUFAs oleic acid were found significantly increased in BC (pre and post treated). D6 desaturase activity was higher in BC patients, while the n-3/n-6 ratio and D5 desaturase activity were lower in BC patients compared to controls (Tables 1 and 2).
Metabolic pathways as well as the dietary intake influence the FAs composition. Arachidonic acid originates from both diet and elongation desaturation process of its precursor, linoleic acid. The D5 and D6 desaturases are the key enzymes not only for this pathway, but also play a role in the n-3 fatty acid pathway. So, the indirect information was collected from the lipid composition analysis which provides a suitable and simple model for studying FAs metabolism [20]. Lipid autacoids which produced endogenously, locally acting eicosanoids play a key role in the tissue homeostasis and have recently implicated in cancer. These eicosanoids were generated by distinct enzymatic systems initiated by cyclooxygenase, cytochrome p450 and lipooxygenase [21, 22]. In another study, excess of SFAs were found cytotoxic to BC cells [23]. Hilvo et al. (2011) measured the lipid composition in tissue membrane of BC and observed the high concentration of phosphatidylcholine, which is a palmitate containing species in BC in comparison to normal breast tissues [24]. Results of the previous study showed increased concentrations of lumina A and B subtypes of all phospholipid (PL) classes like ethanolamine, phosphatidyl inositol and choline in BC. In these PL, acyl group analysis showed a high level of C16:0 and C18:1 FAs due to increasing in de novo synthesis as their source. High concentration of SFAs content in cultured cell membranes decreased the permeability of cell membrane, sensitivity and number of insulin receptors, due to which insulin resistance increased. Insulin resistance is related to the increase in free IGF-I and decrease in IGF binding protein I. According to epidemiological studies, increased free IGF-I and decreased IGF protein I increase the risk of BC. This phenomenon has combined effect with a high level of estradiol stored in fatty acid esters, which may enhance BC [25]. SFAs can also increase the risk of BC by increasing insulin resistance [26].
In another study, the high consumption of animal fat, meat and SFAs was found to be associated with increased risk of BC [27, 28]. A meta-analysis of 10 case control studies [13] and some animal studies, [29] have shown that MUFAs play a positive role in the pathogenesis of BC. MUFAs also have different effect on BC [30]. In addition, our data advocates that there is no correlation between PUFAs levels and BC. Whereas, Serini et al. [31] proposed that n-3 PUFA plays a significant role in the incidence of BC by the growth of tumor cells and increase cell replication process, by interfering cell cycle. Some studies show that n-3 PUFAs prevent BC by influencing the enzyme activity [32, 33]. Some of the case-control studies have reported positive [33, 34] relation of n-6 fatty acids intake with breast cancer risk. n-6 fatty acids by competition with n-3 fatty acids to produce eicosanoids increase the risk of breast cancer and also because of having many double bonds are easily oxidized and enhance cellular damage [35]. Moreover, a meta-analysis of cohort studies [36] and also a systematic review [37] showed an inverse association of n-3 fatty acids with breast cancer risk. In this mentioned meta-analysis very long chain n-3 PUFAs intake, which was estimated by using the composition of fatty acids in biological samples such as adipose tissue, erythrocyte membranes, serum and plasma showed a protective effect on breast cancer [36]. The low ratio of n-3/n-6 PUFAs promotes the pathogenesis of BC [38].
In the present study, high level of TC and LDL-C were observed before RT, but, after treatment TC and LDL-C levels were decreased, significantly (Fig. 4). According to a study, significant benefits of radiotherapy were reported previously. Overall, 16% absolute decrease in the recurrence of BC and 4% decrease in the death rate by BC were observed [2]. Present study showed a strong association of TC and LDL-C to BC (Table 3). Lipids are known to play an important role in tumor development and progression, as Cancer cells need lipids for membrane biogenesis and protein modifications [39]. The cancer cells that are not rapidly proliferating require increased amounts of lipids for enhanced signaling and resistance to apoptosis [6]. Similar results were found by other researchers and proposed that high serum TC level plays a significant role in Breast carcinogenesis [40–43]. Elkhadrawy et al. [43] found a contrary association of TC in BC patients, but LDL-C level was also found increased in BC patients in this study. High dietary fat intakes have been found to be positively related to breast cancer in many epidemiological studies. High concentration of plasma LDL-C is more vulnerable to oxidation, cause of higher lipid peroxidation in BC patients [44].
In our study, no association between HDL-C and BC was observed (Fig. 3). Similarly, Bhat et al. [45] also reported no significant changes for HDL-C in BC patients as compared to controls. Inverse association for HDL-C was found by Borelli R, et al. [46] and argued that HDL-C is a biochemical index which can be associated with increased risk of BC. BC patients whom were reported with a high level of blood LDL-C were also found with higher levels of oxidized LDL-C. The oxidized LDL-C is related with increased risk of BC, while HDL-C is less susceptible to peroxidation due to its lipids and apoprotein content. Hence, HDL do not produce reactive oxygen species and acts as an anti-carcinogen [47].TG was not significantly increased in BC patients in our findings, while another study found a significant increase in TG levels in BC patients [48].
Conclusion
In conclusion, RT is an effective treatment and plays an important role in the lipid profile and fatty acid management in most patients with BC. The improvement in n-3/n-6 was found after treatment, as evident from the results of post treated patients. After treatment, the n-3/n-6 ratio was increased by 35%. RT benefits depend on the advances in both surgery and systemic treatment. It also contributes benefit for the treatment of BC to reduce breast cancer mortality rates. So, it is expected that RT will play significant role in the health care system.
Additional files
Serum free fatty acids in pre treated BC patients. (PDF 165 kb)
Serum total fatty acid concentrations in Pre treated BC patients. (PDF 166 kb)
Serum total fatty acid concentrations in controls. (PDF 158 kb)
Serum free fatty acid concentrations in controls. (PDF 158 kb)
Serum lipid profile of pre treated BC patients. (PDF 162 kb)
Serum lipid profile of post treated BC patients. (PDF 154 kb)
Serum lipid profile of controls. (PDF 232 kb)
GC-FID Chromatogram showing serum total fatty acid profile of pre-treated BC patients. (PDF 233 kb)
Serum free fatty acids in post-treated BC patients. (PDF 159 kb)
Serum total fatty acids in post treated BC patients. (PDF 402 kb)
Acknowledgments
We are highly thankful to the staff of Nuclear Institute of Medicine and Radiotherapy (NIMRA), Jamshoro, Pakistan for their cooperation in collecting samples and Sadia Qamar Arain for herhelp in GC analysis.
Funding
The present research work was carried out under the support of departmental facilities at Institute of Biochemistry University of Sindh and National Centre of Excellence in Analytical Chemistry, Jamshoro, Pakistan.
Availability of data and materials
Data sheet of serum lipid profile and fatty acid composition of control and Breast cancer is attached as Additional file 1 and representative GC chromatograms are attached as Additional file 2.
Authors’ contributions
NAC acquired the permission from Ethical Committee. Sampling was done by SS and NT with the help of the staff at Nuclear Institute of Medicine and Radiotherapy (NIMRA), Jamshoro, Pakistan. SS and FNT carried out the serum lipid analysis by Gas chromatography and compiled the results. While NAC helped in Microlab analysis. The manuscript was drafted by SS, MY, FNT and NAC. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interest.
Consent for publication
Participants were informed for data sharing while their name and identity will be hidden as per consent.
Ethics approval and consent to participate
The study was approved by ethical committee of Institute of the Biochemistry University of Sindh, Jamshoro. All patients were well informed in their local languages and with their full knowledge and understanding a written consent was signed in their familiar languages, in the case of illiterate patients, verbal information was provided to the patient and consent was taken with thumb impressions in the presence of their trustworthy witnesses and enrolled in the study. The direct benefits received as participants were free analysis of lipid profile and fatty acid composition, counted as social service in public benefit.
Disclaimers
Authors state that views expressed in the submitted manuscript are their own work.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- FAMEs
Fatty acid methyl esters
- FAs
Fatty acids
- FID
Flame ionization detector
- GC
Gas chromatography
- HDL-C
High density lipoprotein cholesterol
- LDL-C
Low density lipoprotein cholesterol
- MUFAs
Monounsaturated fatty acids
- PUFAs
Poly unsaturated fatty acids
- SFAs
Saturated fatty acids
- TC
Total cholesterol
- TG
Triglycerides
- VLDL-C
Very low density lipoprotein cholesterol
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1186/s12944-017-0481-y) contains supplementary material, which is available to authorized users.
Contributor Information
Sana Shaikh, Email: sanashaikhmehmood@yahoo.com.
Naseem Aslam Channa, Phone: +92-3332618354, Email: nachanna2000@gmail.com.
Farha Naz Talpur, Email: farahtalpur@hotmail.com.
Muhammad Younis, Email: m_younisbioc@yahoo.com.
Naila Tabassum, Email: nailageo@yahoo.com.
References
- 1.Bosch A, Eroles P, Zaragoza R, Viña JR, Lluch A. Triple-negative breast cancer: molecular features, pathogenesis, treatment and current lines of research. Cancer Treat Rev. 2010;36(3):206–215. doi: 10.1016/j.ctrv.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 2.Kunkler I. Re: Locoregional radiation therapy in patients with high-risk breast cancer receiving adjuvant chemotherapy: 20-year results of the British Columbia randomized trial. J Natl Cancer Inst. 2005;97(15):1162–1163. doi: 10.1093/jnci/dji216. [DOI] [PubMed] [Google Scholar]
- 3.Reimer T, Gerber B. Quality-of-life considerations in the treatment of early-stage breast cancer in the elderly. Drugs Aging. 2010;27(10):791–800. doi: 10.2165/11584700-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 4.Wynder EL, et al. Breast cancer: weighing the evidence for a promoting role of dietary fat. J Natl Cancer Inst. 1997;89(11):766–775. doi: 10.1093/jnci/89.11.766. [DOI] [PubMed] [Google Scholar]
- 5.Kokoglu E, et al. Alterations of serum lipids and lipoproteins in breast cancer. Cancer Lett. 1994;82(2):175–178. doi: 10.1016/0304-3835(94)90008-6. [DOI] [PubMed] [Google Scholar]
- 6.Ray G, Husain SA. Role of lipids, lipoproteins and vitamins in women with breast cancer. Clin Biochem. 2001;34(1):71–76. doi: 10.1016/S0009-9120(00)00200-9. [DOI] [PubMed] [Google Scholar]
- 7.Fay MP, et al. Effect of different types and amounts of fat on the development of mammary tumors in rodents: a review. Cancer Res. 1997;57(18):3979–3988. [PubMed] [Google Scholar]
- 8.Chajes V, et al. Omega-3 and omega-6 polyunsaturated fatty acid intakes and the risk of breast cancer in Mexican women: impact of obesity status. Cancer Epidemiol Biomark Prev. 2012;21(2):319–326. doi: 10.1158/1055-9965.EPI-11-0896. [DOI] [PubMed] [Google Scholar]
- 9.Howe GR, et al. Dietary factors and risk of breast cancer: combined analysis of 12 case-control studies. J Natl Cancer Inst. 1990;82(7):561–569. doi: 10.1093/jnci/82.7.561. [DOI] [PubMed] [Google Scholar]
- 10.Azrad M, Turgeon C, Demark-Wahnefried W. Current evidence linking polyunsaturated fatty acids with cancer risk and progression. Front Oncol. 2013;3:224. doi: 10.3389/fonc.2013.00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pauwels EK, Kairemo K. Fatty acid facts, part II: role in the prevention of carcinogenesis, or, more fish on the dish? Drug News Perspect. 2008;21(9):504–510. doi: 10.1358/dnp.2008.21.9.1290819. [DOI] [PubMed] [Google Scholar]
- 12.Larsson SC, et al. Dietary long-chain n-3 fatty acids for the prevention of cancer: a review of potential mechanisms. Am J Clin Nutr. 2004;79(6):935–945. doi: 10.1093/ajcn/79.6.935. [DOI] [PubMed] [Google Scholar]
- 13.Boyd NF, et al. Dietary fat and breast cancer risk revisited: a meta-analysis of the published literature. Br J Cancer. 2003;89(9):1672–1685. doi: 10.1038/sj.bjc.6601314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.MacLean CH, et al. Effects of omega-3 fatty acids on cancer risk: a systematic review. JAMA. 2006;295(4):403–415. doi: 10.1001/jama.295.4.403. [DOI] [PubMed] [Google Scholar]
- 15.Rose DP, Connolly JM. Omega-3 fatty acids as cancer chemopreventive agents. Pharmacol Ther. 1999;83(3):217–244. doi: 10.1016/S0163-7258(99)00026-1. [DOI] [PubMed] [Google Scholar]
- 16.Knapp ML, al-Sheibani S, Riches PG. Alterations of serum lipids in breast cancer: effects of disease activity, treatment, and hormonal factors. Clin Chem. 1991;37(12):2093–2101. [PubMed] [Google Scholar]
- 17.Arain SQ, Talpur FN, Channa NA. A comparative study of serum lipid contents in pre and post IFN-alpha treated acute hepatitis C patients. Lipids Health Dis. 2015;14:117. doi: 10.1186/s12944-015-0119-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jurczyszyn A, Czepiel J, Gdula-Argasińska J, Paśko P, Czapkiewicz A, Librowski T, Perucki W, Butrym A, Castillo JJ, Skotnicki AB. Plasma fatty acid profile in multiple myeloma patients. Leuk Res. 2015;39(4):400–405. doi: 10.1016/j.leukres.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 19.Abdelsalam KE, Hassan IK, Sadig IA. The role of developing breast cancer in alteration of serum lipid profile. J Res Med Sci. 2012;17(6):562–565. [PMC free article] [PubMed] [Google Scholar]
- 20.Weylandt KH, Kang JX. Rethinking lipid mediators in physiology and pathophysiology. Lancet. 2005;366:618–620. doi: 10.1016/S0140-6736(05)67119-X. [DOI] [PubMed] [Google Scholar]
- 21.Kang JX, Weylandt KH. Modulation of inflammatory cytokines byomega-3 fatty acids. Subcell Biochem. 2008;49:133–143. doi: 10.1007/978-1-4020-8831-5_5. [DOI] [PubMed] [Google Scholar]
- 22.Gdula-Argasinska J, Garbacik A, Tyszka-Czochara M, Wozniakiewicz M, Pasko P, Czepiel J. Identification of lipid derivatives in Hep G2 cells. Acta Biochim Pol. 2013;60:811–815. [PubMed] [Google Scholar]
- 23.Hardy S, et al. Saturated fatty acid-induced apoptosis in MDA-MB-231 breast cancer cells. A role for cardiolipin. J Biol Chem. 2003;278(34):31861–31870. doi: 10.1074/jbc.M300190200. [DOI] [PubMed] [Google Scholar]
- 24.Hilvo M, et al. Novel theranostic opportunities offered by characterization of altered membrane lipid metabolism in breast cancer progression. Cancer Res. 2011;71(9):3236–3245. doi: 10.1158/0008-5472.CAN-10-3894. [DOI] [PubMed] [Google Scholar]
- 25.Folsom AR, et al. Relation between plasma phospholipid saturated fatty acids and hyperinsulinemia. Metabolism. 1996;45(2):223–228. doi: 10.1016/S0026-0495(96)90058-X. [DOI] [PubMed] [Google Scholar]
- 26.Bakker N, Van't Veer P, Zock PL. Adipose fatty acids and cancers of the breast, prostate and colon: an ecological study. EURAMIC study group. Int J Cancer. 1997;72(4):587–591. doi: 10.1002/(SICI)1097-0215(19970807)72:4<587::AID-IJC6>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 27.Do MH, et al. Intake of dietary fat and vitamin in relation to breast cancer risk in Korean women: a case-control study. J Korean Med Sci. 2003;18(4):534–540. doi: 10.3346/jkms.2003.18.4.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bartsch H, Nair J, Owen RW. Dietary polyunsaturated fatty acids and cancers of the breast and colorectum: emerging evidence for their role as risk modifiers. Carcinogenesis. 1999;20(12):2209–2218. doi: 10.1093/carcin/20.12.2209. [DOI] [PubMed] [Google Scholar]
- 29.Welsch CW. Relationship between dietary fat and experimental mammary tumorigenesis: a review and critique. Cancer Res. 1992;52(Suppl 7):2040s–2048s. [PubMed] [Google Scholar]
- 30.Velie E, et al. Dietary fat, fat subtypes, and breast cancer in postmenopausal women: a prospective cohort study. J Natl Cancer Inst. 2000;92(10):833–839. doi: 10.1093/jnci/92.10.833. [DOI] [PubMed] [Google Scholar]
- 31.Serini S, et al. Dietary polyunsaturated fatty acids as inducers of apoptosis: implications for cancer. Apoptosis. 2009;14(2):135–152. doi: 10.1007/s10495-008-0298-2. [DOI] [PubMed] [Google Scholar]
- 32.Sasaki S, Horacsek M, Kesteloot H. An ecological study of the relationship between dietary fat intake and breast cancer mortality. Prev Med. 1993;22(2):187–202. doi: 10.1006/pmed.1993.1016. [DOI] [PubMed] [Google Scholar]
- 33.Darby S, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011;378(9804):1707–1716. doi: 10.1016/S0140-6736(11)61629-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wirfalt E, et al. Postmenopausal breast cancer is associated with high intakes of omega6 fatty acids (Sweden) Cancer Causes Control. 2002;13(10):883–893. doi: 10.1023/A:1021922917489. [DOI] [PubMed] [Google Scholar]
- 35.De Stefani E, et al. Essential fatty acids and breast cancer: a case-control study in Uruguay. Int J Cancer. 1998;76(4):491–494. doi: 10.1002/(SICI)1097-0215(19980518)76:4<491::AID-IJC8>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 36.Saadatian-Elahi M, et al. Biomarkers of dietary fatty acid intake and the risk of breast cancer: a meta-analysis. Int J Cancer. 2004;111(4):584–591. doi: 10.1002/ijc.20284. [DOI] [PubMed] [Google Scholar]
- 37.Gerber M. Omega-3 fatty acids and cancers: a systematic update review of epidemiological studies. Br J Nutr. 2012;107(Suppl 2):S228–S239. doi: 10.1017/S0007114512001614. [DOI] [PubMed] [Google Scholar]
- 38.Bougnoux P, Maillard V, Chajes V. Omega-6/omega-3 polyunsaturated fatty acids ratio and breast cancer. World Rev Nutr Diet. 2005;94:158–165. doi: 10.1159/000088235. [DOI] [PubMed] [Google Scholar]
- 39.Rysman E, et al. De novo lipogenesis protects cancer cells from free radicals and chemotherapeutics by promoting membrane lipid saturation. Cancer Res. 2010;70(20):8117–8126. doi: 10.1158/0008-5472.CAN-09-3871. [DOI] [PubMed] [Google Scholar]
- 40.Qadir MI, Malik SA. Plasma lipid profile in gynecologic cancers. Eur J Gynaecol Oncol. 2008;29(2):158–161. [PubMed] [Google Scholar]
- 41.Mady EA. Association between estradiol, estrogen receptors, total lipids, triglycerides, and cholesterol in patients with benign and malignant breast tumors. J Steroid Biochem Mol Biol. 2000;75(4-5):323–328. doi: 10.1016/S0960-0760(00)00183-7. [DOI] [PubMed] [Google Scholar]
- 42.Hasija K, Bagga HK. Alterations of serum cholesterol and serum lipoprotein in breast cancer of women. Indian J Clin Biochem. 2005;20(1):61–66. doi: 10.1007/BF02893044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Elkhadrawy TM, Ahsan H, Neugut AI. Serum cholesterol and the risk of ductal carcinoma in situ: a case-control study. Eur J Cancer Prev. 1998;7(5):393–396. doi: 10.1097/00008469-199810000-00004. [DOI] [PubMed] [Google Scholar]
- 44.Kumar K, Sachdanandam P, Arivazhagan R. Studies on the changes in plasma lipids and lipoproteins in patients with benign and malignant breast cancer. Biochem Int. 1991;23(3):581–589. [PubMed] [Google Scholar]
- 45.Bhat SA, Mir MR, Majid S, Reshi AA, Husain I, Hassan T, Ahmad H. Serum lipid profile of breast cancer patients in Kashmir. J Invest Biochem. 2013;2(1):26–31. doi: 10.5455/jib.20121125075314. [DOI] [Google Scholar]
- 46.Borrelli R, del Sordo G, De Filippo E, Contaldo F, Parisi V, Beneduce G. High serum HDL-cholesterol in pre- and post-menopausal women with breast cancer in southern Italy. Adv Exp Med Biol. 1993;348:149–153. doi: 10.1007/978-1-4615-2942-2_17. [DOI] [PubMed] [Google Scholar]
- 47.Delimaris E, Faviou G, Antonakos E, Stathopoulou A, Zachari A. Dionyssiou-Asteriou oxidized LDL, serum breast or ovarian cancer. ClinBiochem. 2007;40:1129–1134. doi: 10.1016/j.clinbiochem.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 48.Franky Dhaval S, et al. Significance of alterations in plasma lipid profile levels in breast cancer. Integr Cancer Ther. 2008;7(1):33–41. doi: 10.1177/1534735407313883. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Serum free fatty acids in pre treated BC patients. (PDF 165 kb)
Serum total fatty acid concentrations in Pre treated BC patients. (PDF 166 kb)
Serum total fatty acid concentrations in controls. (PDF 158 kb)
Serum free fatty acid concentrations in controls. (PDF 158 kb)
Serum lipid profile of pre treated BC patients. (PDF 162 kb)
Serum lipid profile of post treated BC patients. (PDF 154 kb)
Serum lipid profile of controls. (PDF 232 kb)
GC-FID Chromatogram showing serum total fatty acid profile of pre-treated BC patients. (PDF 233 kb)
Serum free fatty acids in post-treated BC patients. (PDF 159 kb)
Serum total fatty acids in post treated BC patients. (PDF 402 kb)
Data Availability Statement
Data sheet of serum lipid profile and fatty acid composition of control and Breast cancer is attached as Additional file 1 and representative GC chromatograms are attached as Additional file 2.


