Abstract
Background
Sarcocephalus latifolius is used as a traditional medicine for curing many diseases in Sudan. The main objective of the current study was to determine the antioxidant activity and acetylcholinesterase inhibition (AChEI) of S. latifolius, and to estimate its total phenolic and flavonoid contents.
Methods
Antioxidant activity of the tested plant extracts was carried out by determining their ability to scavenge the 2,2-diphenyl-1-picryl hydrazyl (DPPH) free radical. On the other hand, AChE inhibitory activity was determined spectrophotometrically using the Ellman’s colorimetric method. The levels of total phenols and flavonoids were determined quantitatively using spectrophotometric methods. MTT assay was consumed to assess the cytotoxic effect of the most active fractions. These fractions were subjected to phytochemical analysis using GC-MS techniques to determine thier chemical composition.
Results
Hexane and chloroform fractions exhibited the highest antioxidant activity with IC50 values of (0.098 ± 0.08 and 0.099 ± 0.029 mg/ml) respectively. Standard propyl gallate had the lowest IC50 value of 0.0414 ± 0.11 mg/ml. The ethanolic crude extract showed low AChEI activity with 40.2 ± 0.10%. High concentrations of phenolic and flavonoid contents were observed. GCMS revealed the presence of well-known antioxidants compounds e.g. Vitamin E and caffeic acid.
Conclusion
The ethanolic extract of bark of S. latifolius showed potent antioxidant effects and low AChEI activity, high phenolic and flavonoid contents and presence of pharmacologically active compounds. These findings explain its wide usages in traditional medicine.
Keywords: S. latifolius, Antioxidant, Acetylcholinesterase, Phenolics, Flavonoids, Gcms
Background
Due to its high reactivity, Oxygen is capable of becoming part of potentially damaging molecules called reactive oxygen species (ROS) [1]. Humans have evolved a highly sophisticated antioxidant protective system, both endogenous and exogenous in origin [2]. Whenever the balance between ROS production and antioxidant effect is disturbed, ‘oxidative stress’ results leading to various pathological conditions [3]. Many researchers have focused on antioxidant activity of phenolic compounds especially flavonoids and a positive correlation was observed [4–6]. ROS are playing a dangerous role in the pathogenesis of various diseases, including neurodegenerative disorders, cancer and artherosclerosis [7, 8]. Oxidative processes are the pathogens associated with the central nervous system in Alzheimer’s disease (AD). The brain in particular is highly vulnerable to oxidative damage as it consumes about 20% of the body’s total oxygen, with a high content of polyunsaturated fatty acids and lower levels of endogenous antioxidants [9, 10]. The brain of patients suffering from AD is said to be under oxidative stress. [11, 12]. AD is the most common neurodegenerative disorder, characterized clinically by progressive memory deficits and impaired cognitive function [13]. It is a major public health concern due to the increasing number of sufferers, placing strains on caregivers as well as on financial resources [14]. A deficiency in levels of the neurotransmitter acetylcholine (ACh) has been observed in the brains of AD patients, and inhibition of acetylcholinesterase (AChE), the key enzyme which hydrolyses ACh, is a major treatment option for AD [15]. Traditionally plants have been shown to be good options in the search for AChE inhibitors. Recently, several plants have been identified as containing AChEI activity [7]. In this respect, medicinal plants provide a rich source of biologically active constituents with multiple activities. Sarcocephalus latifolius Sm. (family: Rubiaceae), locally known as “Karmadoda”, have many uses in traditional medicine including malaria, dysentery, fever, hypertension and health promotion (antioxidant) [16–18].
Methods
Plant materials
Bark samples of S. latifolius were collected from South Kordfan state in February 2015. Identified and authenticated by Prof. Hatel H. Alkamali, Faculty of Science and Technology, Omdurman Islamic University, and confirmed by plant taxonomists at the herbarium of Medicinal and Aromatic Plants Research Institute, National Center for Research. Khartoum, Sudan.
Extraction
The fresh samples were dried in shade for seven days, pulverized then used for extraction. Cold maceration methodology was carried out according to published method of Osama and Awdelkarim, 2015 [19].
Fractionation
The crude extract was fractionated using liquid- liquid extraction methodology, which were carried by dissolving the samples in dist. H2O then partitioned between n-hexane, chloroform, ethyl acetate and n-butanol respectively using separating funnel apparatus.
Qualitative phytochemical evaluation
Phytochemical screening was conducted to determine the presence of natural products in the fractions of selected plants using standard methods [20, 21].
Determination of total phenolic content
Total phenolic content was determined by Folin Ciocalteu method [22]. Calibration curve was constructed using gallic acid standards Fig. 1 and the total phenolic content was expressed as mg gallic acid equivalents (GAE)/g dry weight (DW).
Fig. 1.
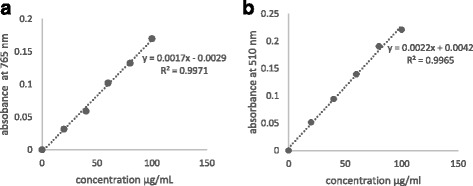
Standard curves of (a) Gallic acid and (b) Quercetin shows the absorbance against concentration
Determination of flavonoid content
The flavonoid content was measured by the aluminium chloride colorimetric assay [23]. Calibration curve was plotted using quercetin standards and flavonoid content was expressed as mg quercetin equivalents (QE)/g DW.
GC-MS analysis
GC-MS analysis was carried out using GCMS instrument (Model GCMS-QP2010 Ultra, Shimadzu Co., Japan) equipped with a capillary column Rtx-5 (0.25 휇m film × 0.25 mm i.d. × 30 m length). The instrument was operated in electron impact mode at ionization voltage (70 eV), injector temperature (250 ∘C), and detector temperature (280 ∘C). The carrier gas used was helium (99.9% purity) at a flow rate of 1.2 mL/min and about one 휇L of the sample was injected. The oven temperature was initially programmed at 110 °C (7 min) to 200 °C at 10 °C/min and from 200 to 280 °C at 5 °C/min withhold time 0 and 9 min respectively. The identification of compounds from the spectral data was based on the available mass spectral records (NIST and WILEY libraries).
Antioxidant activity: 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging assay
The test samples were prepared in DMSO as 10× stocks from each test concentration (between 0 and 100 μg/ml) and briefly sonicated when necessary in an ultrasonic water bath. Solvents fractions producing radical scavenging activities equal to or higher than 50% at 100 μg/mL in a preliminary screen were further tested and IC50 (concentration of the sample producing 50% scavenging of DPPH radicals) determined using EZ-Fit Enzyme Kinetic Program. propyl gallate was tested in the assay as positive control. The assay method used in the present study was based on Shimada et al., 1992 [24] method. The samples stock solutions (20 μL/well) were dispensed in triplicate onto 96-well plates. The assay was started with the addition of DPPH reagent (300 μM in ethanol, 180 μL/well). Appropriate blanks were prepared using the solvent only in addition to the same amount of DPPH reagent to get rid of any inherent solvent activity. Negative controls were also run in parallel to correct for any non-DPPH absorbance by coloured extracts at the test wavelength. The plate was immediately shaken for 30 s and incubated in the dark for 30 min at room temperature. The remaining DPPH was measured in the microplate reader at 517 nm. Percentage of radical scavenging activity (RSA) was calculated as following:
Where, At = Absorbance value of test compound; Ac = Absorbance value of control.
The multi-well plate AChE inhibition assay
The AChE inhibitory activity was tested using 96 well micro-plate assay based on Ellmam et al., 1961 [25] method with minor modifications. Each extract (10 μL of 5 mg/mL in ethanol) was dispensed in triplicate onto 96 well microplate and mixed with 190 μL of Ellman’s mixture containing 20 μL of enzyme, 140 μL to phosphate buffer, pH 8, containing 10 μL of 0.5 mM of 5, 5’- dithio-bis-(2-nitrobenzoic acid) (DTNB, Sigma-Aldrich, Germany) and 20 μL acetylthiocholine iodide (ATCI, Sigma-Aldrich, Germany). The control wells contained ethanol instead of the extract. The enzymatic activity was monitored at 412 nm every 30 s intervals for 3 min (linear reaction). The enzyme rate was calculated from the slope of the curve of absorbance vs time. As screening strategy, final concentration of 1000 μg/mL from each extract was examined and the average % inhibition was calculated relative to the enzyme rate at the control wells according to the following equation:
MTT (3-(4, 5-dimethylthazol-2-yl)-2, 5-diphenyl tetrazonium bromide) cytotoxicity assay
In the present investigation, Vero (normal, African green monkey kidney) cell line was used and cytotoxicity on these cells was assessed as described previously [26]. For each experiment, cultures were seeded from frozen stocks. Vero cells were maintained complete medium consisting of 10% fetal bovine serum and 90% minimal essential medium (MEM). The cells were incubated at 37 °C in a 5% CO2 atmosphere and were in the logarithmic phase of growth at the time of the neutral red (NR) and tetrazolium (MTT) assays. Cells were harvested and seeded into 96-well tissue culture plates at a density of 1 × 104 cells per well of aliquots of medium (200 μL). The cells were allowed to adhere to the wells for 24 h at 37 °C in a humid atmosphere optimized with 5% CO2 in air. The next day, the plant fractions were added at the desired final concentrations and incubated for 72 h. All experiments were performed at least four times. Phosphate-buffered saline (PBS) was used as a negative. After the 72 h exposure period, the toxic endpoints were determined at 570 nm. Viability was defined as the ratio (expressed as a percentage) of absorbance of treated cells to untreated cells that served as negative control.
Statistical analysis
All data were expressed as means ± SD for three experiments. P values <0.05 were considered statistically significant. Statistical analyses were performed using Excel software (Microsoft 2010).
Results and discussion
Extraction yield and phytochemical screening
The percentage yield of S. latifolius ethanolic extract was 47.33% of dry weight. The polar fractions (water and butanol) showed the highest percentage yield (Table 2), this could be attributed to the polar nature of the crude extract obtained with polar solvent (ethanol).
Table 2.
Yield percentages, Total phenolic and flavonoid contents of ethanolic extract and solvent fractions of S. latifolius
| Sample | Extraction yield (w/w% of dry weight) | Phenolic content (mg /g GAE) | Total flavonoid (mg/g QE) |
|---|---|---|---|
| Crude | 47.33 | 78.21 ± 2.4 | 91.36 ± 0.84 |
| Hexane | 6.18 | 98.78 ± 2.1 | 81.01 ± 0.012 |
| CHCl3 | 1.64 | 71.49 ± 0.5 | 118.29 ± 0.21 |
| EtOAc | 3.09 | 86.12 ± 0.7 | 80.23 ± 0.03 |
| BuOH | 19.09 | 56.20 ± 1.23 | 94.32 ± 0.71 |
| H2O | 70.91 | 83.20 ± 3.7 | 40.22 ± 0.28 |
The results of preliminary investigation on the phytochemicals present in different solvent fractions are presented in Table 1. Different phytoconstituents such as phenolics, flavonoids, tannins, alkaloids, saponins, quinones, steroids, and terpenoids were detected in the tested fractions. The phytochemicals investigated in the present study are known to be beneficial in industrial and medicinal sciences [27]. Also, this preliminary knowledge can be looked at as a decipher in the search of a new source of economically valued chemical compounds [28, 29].
Table 1.
Preliminary screening of secondary metabolites in the fractions of S. latifolius
| Family of compound | Type of test | interference | ||||
|---|---|---|---|---|---|---|
| n-hexane | CHCl3 | EtOAc | n-BuOH | H2O | ||
| Phenols | FeCl3 | +v | +v | +v | +v | +v |
| Tannins | FeCl3 | -v | +v | +v | +v | +v |
| Flavonoids | KOH | +v | +v | +v | +v | +v |
| Alkaline | +v | +v | +v | +v | +v | |
| Lead acetate | +v | +v | +v | +v | +v | |
| Quinones | H2SO4 | -v | +v | +v | +v | +v |
| Alkaloids | Dragendorff’s | +v | +v | +v | +v | +v |
| Wagner’s | +v | +v | +v | +v | +v | |
| Triterpenes | Salkowski | +v | +v | +v | +v | +v |
| Diterpenes | Copper acetate | -v | -v | -v | -v | -v |
| Steroids | Salkowski | +v | +v | +v | +v | +v |
| Saponins | Forth | -v | +v | +v | -v | +v |
+ve positive -ve negative
Total phenolic and flavonoid contents
Crude natural extracts and compounds purified from these extracts can serve as better herbal drug sources owing to their fewer side effects and nutritional value [30]. As presented in Table 2, the ethanolic crude extract of S. latifolius bark exhibited total phenolic content of 78.21 ± 2.4 mg GAE/g DW. Fig. 2 a similar study carried out using the methanolic leaf and root extracts of S. latifolius, has shown that the total phenolic content present was (0.016 ± 0.03 and 0.036 ± 0.05 mg GAE/g DW) respectively, which is very low compared to the present study [31]. This high variation could be due to many reasons including, the part of plant under study, which contains different chemical composition with different percentage, the method, solvent used for extraction, the origin of plant samples, and the environmental factors. The hexane fraction showed the highest phenolic content (98.78 ± 2.1 mg GAE/g DW), this result indicates the presence of high lipid soluble phenolic compounds such as vitamin E, whose existence was confirmed by GCMS analysis.
Fig. 2.

Comparison charts of (a) Total phenolic content. b Total flavonoid content of ethanolic extract and solvent fractions of S. latifolius
Successful determination of biologically active compounds from plant material is largely dependent on the type of solvent used in the extraction procedure. Higher concentrations of more bioactive flavonoid compounds were detected with 80% ethanol [32]. Therefore, ethanol was chosen for extraction. Plant phenolic compounds especially flavonoids are currently receiving greater interest due to their antioxidants potential [27, 33]. Aluminium chloride colorimetric assay yielded total flavonoid content of (91.36 ± 0.84 mg QE/g DW) for the crude extract. Highest flavonoid contents were observed in chloroform fraction (118.29 ± 0.21 mg QE/g DW), which indicate the high amount of less polar flavonoids (aglycones) such as isoflavones, flavanones, highly methoxylated flavones, and flavonols [34]. These results could give a clue interpreting the observed high bioactivities of this plant.
Antioxidant and AChE inhibitory activities
Recently, interest has increased in naturally occurring antioxidants that can be used to protect human beings from oxidative stress damages [35, 36]. In the current study, the ethanolic crude extract exhibited high antioxidant activity with 87 ± 0.03%. The order of the activity (IC50 mg/ml) was as follow: hexane (0.098 ± 0.08) > chloroform (0.099 ± 0.029) > butanol (0.104 ± 0.19) > ethyl acetate (0.148 ± 0.33) and eventually water fraction (2.015 ± 0.3), Table 3 and Figs. 3, and 4 illustrate these results. The activity of hexane and chloroform fractions (0.098 ± 0.08 and 0.099 ± 0.029 mg/ml) respectively, is comparable values to the standard antioxidant PG (0.0414 ± 0.11 mg/ml); they also showed high amount of phenolic and flavonoid contents, it’s possible that could be the reason of this high antioxidant potential.
Table 3.
Antioxidant activity (%RSA and IC50) of solvent fractions of S. latifolius
| Sample | % RSA | IC50 (mg/ml) |
|---|---|---|
| Hexane | 76 ± 0.02 | 0.098 ± 0.08 |
| CHCl3 | 86 ± 0.03 | 0.099 ± 0.029 |
| EtOAc | 84 ± 0.03 | 0.148 ± 0.33 |
| BuOH | 83 ± 0.02 | 0.104 ± 0.19 |
| H2O | 79 ± 0.10 | 2.015 ± 0.3 |
Fig. 3.
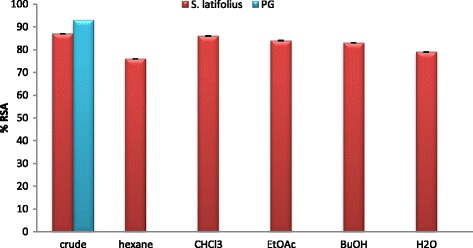
Percentage of RSA of S. latifolius ehanolic extract and solvent fractions
Fig. 4.
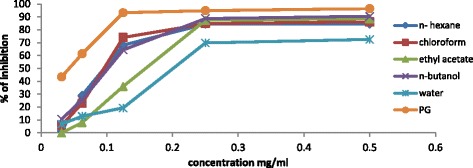
Comparison Chart of Antioxidant potentials of S. latifolius fractions with the decrease of concentration (IC50 evaluation)
Inhibition of AChE has been considered as a promising strategy for the treatment of neurological disorders such as Alzheimer’s disease, senile dementia, ataxia and myasthenia gravis, in which a deficit in cholinergic neurotransmission is involved [37, 38]. The side effects of anti-AChE drugs such as toxicity, tolerability, and loss of efficiency stimulates the researchers to screen alternative natural anti-AD drugs for medication switch [39]. Ethanolic extract displayed low AChE inhibitory activity with (40.2 ± 0.10). GCMS analysis of chloroform fraction indicates the presence of caffeic acid which has been reported to be a potent inhibitor of both AChE and BChE [40]. It is possible that the solvent used for extraction was not able to isolate the active ingredients with a proper amount.
GCMS analysis
Due to their superior antioxidant activities hexane and chloroform fractions were analyzed with GC-MS, to identify their chemical composition which may be responsible of the measured activities. The GC-MS analysis lead to the identification of a number of compounds. These compounds were identified through mass spectrometry attached with GC. Interpretation of mass spectrum was conducted using the database of National Institute Standard and Technology (NIST). The name, molecular weight and structure of the components of the test materials were ascertained, illustrated in Tables 4, and 5 and Figs. 5, and 6.
Table 4.
GC-MS spectral analysis of hexane fraction of S. latifolius bark
| Peak no. | R. Time | Area % | Compunde name | Molecular Formula | Mass |
|---|---|---|---|---|---|
| 1 | 13.736 | 1.68 | 5,5-Dimethyl-1,5-oxasilonan-9-one | C9H18O2Si | 186 |
| 2 | 15.467 | 5.67 | Quinic acid | C7 H12 O6 | 192 |
| 3 | 19.188 | 0.29 | Pentadecanoic acid, methyl ester | C16 H32 O2 | 256 |
| 4 | 19.425 | 0.67 | Ethyl (2E)-3-(4-hydroxy-3-methoxyphenyl)-2-propenoate | C12H14O4 | 222 |
| 5 | 19.805 | 6.89 | Pentadecanoic acid | C15H30O2 | 242 |
| 6 | 20.147 | 2.20 | Palmitic acid ethyl ester | C18 H36 O2 | 284 |
| 7 | 20.761 | 0.53 | 2-Cyclopropylideneadamantane | C13H18 | 174 |
| 8 | 22.455 | 16.49 | Oleic Acid | C18H34O2 | 282 |
| 9 | 22.694 | 3.23 | Methyl linoleate | C19 H34 O2 | 294 |
| 10 | 22.765 | 1.64 | Ethyl octadec-9-enoate | C20H38O2 | 310 |
| 11 | 22.814 | 0.48 | Linolenic acid methyl ester | C19 H32 O2 | 292 |
| 12 | 23.122 | 3.91 | Heptadecanoic acid, ethyl ester | C19 H38 O2 | 298 |
| 13 | 25.920 | 14.21 | Oelic acid amide | C18 H35 N O | 281 |
| 14 | 25.994 | 0.23 | Oelic acid amide | C18 H35 N O | 281 |
| 15 | 26.187 | 0.44 | Palmitic acid ethyl ester | C18 H36 O2 | 284 |
| 16 | 26.265 | 0.60 | Stearic acid amide | C18H37NO | 283 |
| 17 | 26.509 | 0.43 | 2-Pentyl-2-nonenal | C14H26O | 210 |
| 18 | 26.761 | 0.43 | Oxirane, hexadecyl- | C18H36O | 268 |
| 19 | 28.299 | 0.48 | Octadecanal | C18H36O | 268 |
| 20 | 28.665 | 9.23 | Phthalic acid, mono-(2-ethylhexyl) ester | C16H22O4 | 278 |
| 21 | 29.192 | 0.97 | Ethyl palmitate | C18 H36 O2 | 284 |
| 22 | 29.881 | 19.50 | Cinchol | C29 H50 O | 414 |
| 23 | 32.086 | 0.57 | Ethyl docosanoate | C24H48O2 | 368 |
| 24 | 32.642 | 1.64 | Spinacene | C30H50 | 410 |
| 25 | 33.176 | 0.59 | 3-[(Trimethylsilyl)oxy]lanosta-8,24-diene | C33H58OSi | 498 |
| 26 | 33.343 | 1.44 | Lupenyl acetate | C32H52O2 | 468 |
| 27 | 35.907 | 3.46 | Stigmast-4-en-3-one | C29H48O | 412 |
| 28 | 38.248 | 1.09 | Cholesteryl bromide | C27 H45 Br | 448 |
| 29 | 38.974 | 1.01 | Vitamin E | C29H50O2 | 430 |
Table 5.
GC-MS spectral analysis of chloroform fraction of S. latifolius bark
| Number | R. Time | Area % | Compound name | Molecular Formula | Mass |
|---|---|---|---|---|---|
| 1 | 13.735 | 2.97 | 5,5-Dimethyl-1-oxa-5-silacyclononanone-9 | C9H18O2Si | 186 |
| 2 | 14.635 | 0.29 | 9-Eicosene, (E)- | C20H40 | 280 |
| 3 | 15.989 | 1.99 | 2H–Pyran-2-one, 5-ethylidenetetrahydro-4-(2-hydroxyethyl)- | C9H14O3 | 170 |
| 4 | 17.029 | 0.85 | 4-((1E)-3-Hydroxy-1-propenyl)-2-methoxyphenol | C10H12O3 | 180 |
| 5 | 17.334 | 0.35 | 1-Heptadecene | C17H34 | 238 |
| 6 | 18.302 | 0.84 | p-Hydroxycinnamic acid, ethyl ester | C11H12O3 | 192 |
| 7 | 19.187 | 0.35 | 9-Octadecenoic acid, 12-(acetyloxy)-, methyl ester, [R-(Z)]- | C21H38O4 | 354 |
| 8 | 19.361 | 0.18 | 7,9-Di-tert-butyl-1-oxaspiro(4,5)deca-6,9-diene-2,8-dione | C17H24O3 | 276 |
| 9 | 19.422 | 0.15 | alpha.-D-Xylofuranose, 1,2-O-isopropylidene-5-(t-butyldimethylsilyl)- | C14H28O5Si | 304 |
| 10 | 19.800 | 5.89 | Pentadecanoic acid | C15 H30 O2 | 242 |
| 11 | 20.115 | 0.30 | 9-Tricosene, (Z)- | C23H46 | 322 |
| 12 | 20.360 | 7.84 | 2H-1-Benzopyran-2-one, 7-hydroxy-6-methoxy- | C10 H8 O4 | 192 |
| 13 | 20.761 | 0.62 | Bicylo[4.1.0]heptane, 7-bicyclo[4.1.0]hept-7-ylidene- | C14H20 | 188 |
| 14 | 21.139 | 0.22 | Trimethylsilyl 3-methoxy-4-(trimethylsilyloxy)cinnamate | C16H26O4Si2 | 338 |
| 15 | 21.769 | 0.31 | 9-Octadecenoic acid (Z)-, methyl ester | C19H36O2 | 296 |
| 16 | 21.996 | 25.46 | 2-Propenoic acid, 3-(3,4-dihydroxyphenyl)- | C9 H8 O4 | 180 |
| 17 | 22.450 | 9.78 | Oleic Acid | C18H34O2 | 282 |
| 18 | 22.762 | 3.28 | Octadecanoic acid | C18H36O2 | 284 |
| 19 | 23.104 | 1.19 | 1-Hexadecene | C16 H32 | 224 |
| 20 | 23.740 | 0.61 | 9,12-Octadecadienoic acid (Z,Z)- | C18H32O2 | 280 |
| 21 | 25.816 | 0.39 | O O′-BIPHENOL, 4,4’,6,6’-TETRA-T-BUTYL- | C28 H42 O2 | 410 |
| 22 | 25.909 | 1.08 | 9-Octadecenamide, (Z)- | C18 H35 N O | 281 |
| 23 | 26.155 | 0.93 | 1-Nonadecene | C19H38 | 266 |
| 24 | 28.301 | 0.19 | Oxirane, heptadecyl- | C19H38O | 282 |
| 25 | 28.662 | 1.17 | 1,2-Benzenedicarboxylic acid, mono(2-ethylhexyl) ester | C16H22O4 | 278 |
| 26 | 29.163 | 1.28 | 1-Nonadecene | C19H38 | 266 |
| 27 | 29.732 | 1.15 | gamma.-Sitosterol | C29H50O | 414 |
| 28 | 32.053 | 1.83 | 1-Triacontanol | C24H50O | 354 |
| 29 | 35.350 | 1.02 | 17-Pentatriacontene | C35H70 | 490 |
| 30 | 35.848 | 5.09 | Methyl commate C | C31 H50 O4 | 486 |
| 31 | 38.123 | 22.40 | Lup-20 (29)-en-3-ol, acetate, (3.beta.)- | C32H52O2 | 468 |
Fig. 5.
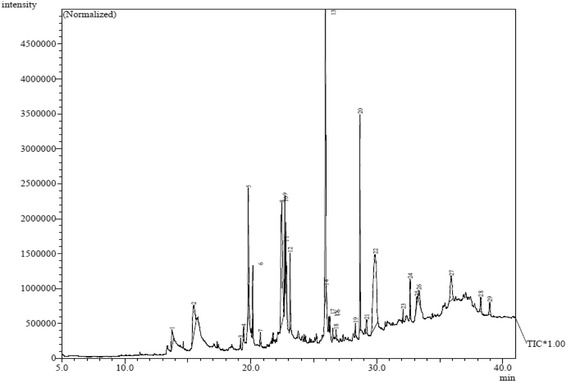
GC-MS chromatogram for hexane fraction of S. latifolius bark
Fig. 6.

GC-MS chromatogram for chloroform fraction of S. latifolius bark
GC-MS spectrum of the hexane and chloroform fractions revealed the presence of 29 and 31 compounds respectively. Two phenolic compounds (Ethyl (2E)-3-(4-hydroxy-3-methoxyphenyl)-2-propenoate and Vitamin E) were observed in hexane fraction. Fat soluble vitamin E is one of the most active natural antioxidants, it is the most effective chain-breaking antioxidant within the cell membrane where it protects membrane fatty acids from lipid peroxidation. The supplemental intakes of this powerful antioxidant have been documented to be useful against cancer [41]. Vitamin E also acts in the prevention of free radical formation.
chloroform fraction declared the presence of five phenolic substances (4-((1E)-3-Hydroxy-1-propenyl)-2-methoxyphenol; p-Hydroxycinnamic acid, ethyl ester; 2H-1-Benzopyran-2-one, 7-hydroxy-6-methoxy; Caffeic acid, O O’-biphenol, 4,4’,6,6’-tetra-T-butyl) these phenolic compounds are known antioxidants, therefore the antioxidant potentials of this plant could be justified. However, further studies on the isolation, characterization, and biological evaluation of these identified compounds are necessary to confirm their potential benefits.
Cytotoxicity study
MTT assay reveled that, all tested fractions have no toxic effects on Vero cells with IC50 more than 200 μg/ml, these results are shown in (Table 6).
Table 6.
The cytotoxic effect (expressed as % inhibition and IC50 values) of hexane and chloroform fractions of S. latifolius tested at 125, 250 and 500 μg/ml against Vero cells
| Code of extract | Concentration (μg/ml) | IC50 (μg/ml) | IC50 | ||
|---|---|---|---|---|---|
| Inhibition % ± SD | |||||
| 500 | 250 | 125 | |||
| Hexane | 55.3 ± 0.04 | 50.4 ± 0.02 | 40.9 ± 0.03 | 277.8 | >100 |
| Chloroform | 67.6 ± 0.08 | 55.0 ± 0.01 | 35.9 ± 0.05 | 224.9 | >100 |
| Control | 95.3 ± 0.00 | <30 | |||
IC50 < 30 μg/ml: High toxic. Control = triton was used as positive control at 0.2 μg/ml. the maximum concentration used was 500 μg/ml
The MTT assay is a test of metabolic competence based upon assessment of mitochondrial performance relying on the conversion of yellow MTT to the purple formazan derivative by mitochondrial succinate dehydrogenase in viable cells [42]. Increasing concentrations of the tested fractions did not affect mitochondrial respiration as measured by the MTT cytotoxicity assay. However, the results of this assay measuring cell integrity showed that these solvent fractions are not toxic over this concentration range tested.
Conclusion
S. latifolius is a Sudanese medicinal plants commonly used as herbal medicine for several purposes. In the present study, the selected plant was investigated in vitro for AChEI and antioxidant properties. In addition, the total phenolic and flavonoids contents were measured. GC-MS analysis of S. latifolius (hexane and chloroform) fractions reveled the presence of well known antioxidant compound such as Vitamin E and Caffeic acid. According to these findings it could be suggested that S. latifolius (hexane and chloroform) fractions might be potent and safe antioxidant materials in medicine or food industry.
Acknowledgements
We would like to express our deep gratitude to Prof. Hatel H. Alkamali, Dean of Faculty of Science and Technology, Omdurman Islamic University, and Prof. Asaad Khalid, Medical Biochemistry Research Department, Medicinal and Aromatic Plants Research Institute, National Center for Research, Khartoum, Sudan, for their advices.
Funding
These analyses were done with self-funding.
Availability of data and materials
All data and materials used in this research are available from the corresponding author on reasonable request.
Authors’ contributions
AO Conduct the extraction, fractionation, phytochemical screening, total phenolic and total flavonoid content as well as the GCMS analysis and wrote the first draft. SA Supervised all the experimental work and data interpretation and also corrects the first draft. AA Conduct the antioxidant and anti- AChE inhibitory activities and the cytotoxicity experiment. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Ethics approval and consent to participate
Not applicable.
Declaration
The experimental work described in this article was conducted in the Chemical Laboratories Complex, Department of Chemistry, Faculty of Sciences and Technology, Omdurman Islamic University, and Research Lab, Medical Biochemistry Department, Medicinal and Aromatic Plants Research Institute, National Center for Research. Khartoum, Sudan, from December 2014 to September 2015. These studies are the result of our own investigations, except where the work of others is acknowledged and have not been submitted in any other form to another journal.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- Abs
Absorbance
- Ac
Absorbance value of Control
- Ach
Acetylcholine
- AChE
Acetylcholinesterase
- AChEI
Acetylcholinesterase Inhibitory
- AD
Alzheimer’s Disease
- At
Absorbance value of Test compound
- BuOH
Butanol
- DMSO
Dimethyl sulfoxide
- DPPH
2,2-diphenyl-1-picryl hydrazyl
- DTNB
5,5’-Dithiobs (2-nitro benzoic acid)
- EI
Electron Ionization
- EtOAc
Ethyl Acetate
- FBS
Fetal Bovine Serum
- GAE
Gallic Acid Equivalent
- GC
Gas Chromatography
- GC-MS
Gas Chromatography- Mass Spectrometer
- MEM
Minimal Essential Medium
- MTT
Microculture tetrazolium
- NIST
National Institute of Standards and Technology
- PG
Propyl Gallate
- QE
Quercetin Equivalent
- RSA
Radical Scavenging Activity
Contributor Information
Alsiddig Osama, Email: alsiddigosama@gmail.com.
Sufyan Awadelkarim, Email: sufyan69@gmail.com.
Amna Ali, Email: amna9ali@yahoo.com.
References
- 1.Bandyopudya U. ROS: oxidative damage and pathogenesis. Curr Sci. 1999;77:658–666. [Google Scholar]
- 2.Percival M. Antioxidants. Clin Nutr Insights. 1998;31:01–04. [Google Scholar]
- 3.Chitra KP, Pillai KS. Antioxidants in health. Ind J Physiol Pharmacol. 2002;46(1):01–05. [PubMed] [Google Scholar]
- 4.Cai Y, Luo Q, Sun M, Corke H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. 2004;74:2175–2184. doi: 10.1016/j.lfs.2003.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Djeridane A, Yousfi M, Nadjemi B, Boutassouna D, Stocher P, Vidal N. Antioxidant activity of some Algerian medicinal plants extracts containing phenolic compounds. Food Chem. 2006;97:654–660. doi: 10.1016/j.foodchem.2005.04.028. [DOI] [Google Scholar]
- 6.Sroka Z, Cisowski W. Hydrogen peroxide scavenging, antioxidant and anti-radical activity of some phenolic acids. Food Chem Toxicol. 2002;41:753–758. doi: 10.1016/S0278-6915(02)00329-0. [DOI] [PubMed] [Google Scholar]
- 7.Winrow VR, Winyard PG, Morris CJ, Blake DR. Free radicals in inflammation: second messengers and mediators of tissue destruction. Brit Med Bull. 1993;49(3):506–522. doi: 10.1093/oxfordjournals.bmb.a072627. [DOI] [PubMed] [Google Scholar]
- 8.Confortia F, Sosa S, Marrelli M, Menichini F, Statti G, Uzunov D. In vivo anti-inflammatory and in vitro antioxidant activities of Mediterranean dietary plants. J Ethnopharmacol. 2007;116(1):144–151. doi: 10.1016/j.jep.2007.11.015. [DOI] [PubMed] [Google Scholar]
- 9.Halliwell B, Gutteridge JMC. Oxygen radicals and the nervous system. Trends Neuro Sc. 1985;8:22–26. doi: 10.1016/0166-2236(85)90010-4. [DOI] [Google Scholar]
- 10.Shulman RG, Rothman DL, Behar KL, Hyder F. Energetic basis of brain activity: implications for neuroimaging. Trends Neurosci. 2004;27:489–495. doi: 10.1016/j.tins.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 11.Emilien G, Beyreuther K, Master CL, Maloteaux JM. Prospects for pharmacological intervention in Alzheimer’s disease. Arch Neurol. 2000;57(4):454–459. doi: 10.1001/archneur.57.4.454. [DOI] [PubMed] [Google Scholar]
- 12.Tabet N. Acetylcholinesterase inhibitors for Alzheimer’s disease: anti-inflammatories in acetylcholine clothing. Age Ageing. 2006;35(4):336–338. doi: 10.1093/ageing/afl027. [DOI] [PubMed] [Google Scholar]
- 13.Mattson MP. Pathways towards and away from Alzheimer’s disease. Nature. 2004;430:631–639. doi: 10.1038/nature02621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Houghton PJ, Howes MJ. Natural products and derivatives affecting neurotransmission relevant to Alzheimer’s and Parkinson’s disease. Neurosignals. 2005;14:6–22. doi: 10.1159/000085382. [DOI] [PubMed] [Google Scholar]
- 15.Şenol FS, Orhan İ, Yilmaz G, Çiçek M, Şener B. Acetylcholinesterase, butyrylcholinesterase, and tyrosinase inhibition studies and antioxidant activities of 33 Scutellaria L. taxa from Turkey. Food Chem Toxicol. 2010;48:781–788. doi: 10.1016/j.fct.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 16.Amos S, Abba J, Chindo B, Edmond I, Binda L, Adzu B, Buhari S, Odutola AA, Wambebe C, Gamaniel K. Neuropharmacological effects of the aqueous extract of Nauclea latifolia root back in rats and mice. J Ethnopharmacol. 2005;97(1):53–57. doi: 10.1016/j.jep.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 17.Ngo Bum E, Taiwe GS, Motto FC, Ngoupaye GT, Nkantchoua GC, Pelanken MM, Rakotonirina SV, Rakotonirina A. Anticonvulsant, nxiolytic and sedative properties of the roots of Nauclea latifolia smith in mice. Epilepsy Behav. 2009;15(4):434–440. doi: 10.1016/j.yebeh.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 18.Abbah J, Amos S, Chindoc B, Ngazalc I, Vongtaue HO, Adzuc B, Faridad T, Odutolad AA, Wambebec C, Gamaniel KS. Pharmacological evidence favouring the use of Nauclea latifolia in malaria ethnopharmacy: effects against nociception, inflammation, and pyrexia in rats and mice. J Ethnopharmacol. 2010;127:85–90. doi: 10.1016/j.jep.2009.09.045. [DOI] [PubMed] [Google Scholar]
- 19.Osama A, Awdelkarim S. Phytochemical screening of Ficus sycomorus L. bark and Cleome gynandra L. aerial parts. J. Pharmacog. and. Phytochemistry. 2015;4(4):24–27. [Google Scholar]
- 20.Trease GE and Evans MD. A text book of Pharmacognosy. 13th ed. London: Bailliere Tindall; 1989.144–48.
- 21.Odebiyi O, Sofowora EA. Phytochemical screening of Nigerian medicinal plants. L Coydia. 1978;41:41–234. [PubMed] [Google Scholar]
- 22.Wolfe K, Wu X, Liu RH. Antioxidant activity of apple peels. J Agric Food Chem. 2003;51(3):609–614. doi: 10.1021/jf020782a. [DOI] [PubMed] [Google Scholar]
- 23.Kim DO, Chun OK, Kim YJ, Moon HY, Lee CY. Quantification of polyphenolics and their antioxidant capacity in fresh plums. J Agric Food Chem. 2003;516:509–6515. doi: 10.1021/jf0343074. [DOI] [PubMed] [Google Scholar]
- 24.Shimada K, Fujikawa K, Yahara K, Nakamura T. Antioxidative properties of xanthan on the antioxidation of soybean oil in cyclodextrin emulsion. J Agric Food Chem. 1992;40:945–948. doi: 10.1021/jf00018a005. [DOI] [Google Scholar]
- 25.Ellman G, Diane C, Valentino A, Robert M. Biochemical pharmacolog. Pergamon P press Ltd. 1961;7:88–95. [Google Scholar]
- 26.Patel S, Gheewala N, Suthar A, Saha A. In- vitro cytotoxicity activity of Solanum Nigerian extract aginst Hela cell line and Vero cell line. In J pharm Pharmac Sci. 2009;1(1):38–46. [Google Scholar]
- 27.Rauha JP, Remes S, Herinonen W, Hopia M, Kgjala T, Pitinlaja K, Vaorela H, Vaorela P. Antimicrobial effects of finished plant extract containing flavanoids and other phenolic compounds. Int J Food Microbiol. 2000;56:3–12. doi: 10.1016/S0168-1605(00)00218-X. [DOI] [PubMed] [Google Scholar]
- 28.Gezahegn Z, Akhtar MS, Woyessa D. And TarikuY. Antibacterial potential of Thevetia peruviana leaf extracts against food associated bacterial pathogens. J Coastal Life Med. 2015;3(2):150–157. [Google Scholar]
- 29.Mohanty SK. MalappaK, Godavarthi a, Subbanarasiman B and Maniyam a. Evaluation of antioxidant, in vitro cytotoxicity of micropropagated and naturally grown plants of Leptadenia reticulata (Retz.) Wight & Arn.-an endangered medicinal plant. Asian Pac J Trop Med. 2014;7(1):S267–S271. doi: 10.1016/S1995-7645(14)60244-3. [DOI] [PubMed] [Google Scholar]
- 30.Saetung A, Itharat A, Dechsukum C, Wattanapiro-msakul C, Keaprodub N, Ratansuwa P. Cytotoxic activity of Thai medicinal plants for cancer treatment. Sci Technol. 2005;27(2):469–478. [Google Scholar]
- 31.Dhalwal K, Shinde VM, Namdeo AG. Antioxidant profile and HPTLC densitometric analysis of umbelliferone and psoralen in Aegle marmelos. Pharm Biol. 2008;46:266–272. doi: 10.1080/13880200701741088. [DOI] [Google Scholar]
- 32.Bimakr M. Comparison of different extraction methods for the extraction of major bioactive flavonoid compounds from spearmint (Mentha spicata L.) leaves. Food Bioprod Process. 2010;89(1):1–6.
- 33.Oise IE, Adesina AB, Oluwasegun AA. Comparative phytochemical, cytotoxic and growth inhibitory effects of the leaf and root barks of Sarcocephalus Latifolius (J.E. Smith) E.A. Bruce (Rubiaceae) Inter J Biosci. 2014;4(4):162–169. [Google Scholar]
- 34.Waksmundzka-Hajnos M, Sherma J, Kowalska T. Thin Layer Chromatography in Phytochemistry. CRC Press is an imprint of the Taylor & Francis Group. 2008;99:334–36.
- 35.Aruoma OI, Halliwell B, Aeschbach R, Löligers J. Antioxidant and prooxidant properties of active rosemary constituents: Carnosol and carnosic acid. Xenobiotica. 1992;22:257–268. doi: 10.3109/00498259209046624. [DOI] [PubMed] [Google Scholar]
- 36.Scalbert A, Manach C, Morand C, Remesy C. Dietary polyphenols and the prevention of diseases. Crit Rev Food Sci Nutr. 2005;45:287–306. doi: 10.1080/1040869059096. [DOI] [PubMed] [Google Scholar]
- 37.López MD, Pascual-Villalobos MJ. Mode of inhibition of acetylcholinesterase by monoterpenoids and implications for pest control. Ind Crop Prod. 2010;31:284–288. doi: 10.1016/j.indcrop.2009.11.005. [DOI] [Google Scholar]
- 38.Mukherjee PK, Kumar V, Mal M, Houghton PJ. Acetylcholinesterase inhibitors from plants. Phytomedicine. 2007;14:289–300. doi: 10.1016/j.phymed.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 39.Gauthier S, Emre M, Farlow MR, Bullock R, Grossberg GT, Potkin SG. Strategies for continued successful treatment of Alzheimer’s disease: switching cholinesterase inhibitors. Curr Med Res Opin. 2003;19(8):707–714. doi: 10.1185/030079903125002450. [DOI] [PubMed] [Google Scholar]
- 40.Oboh G, Agunloye OM, Akinyemi AJ, Ademiluyi AO, Adefegha SA. Comparative study on the inhibitory effect of caffeic and chlorogenic acids on key enzymes linked to Alzheimer’s disease and some pro-oxidant induced oxidative stress in rats’ brain-in vitro. Neurochem Res. 2013;38(2):413–419. doi: 10.1007/s11064-012-0935-6. [DOI] [PubMed] [Google Scholar]
- 41.White E, Shannon JS, Patterson RE. Relationship between vitamin and calcium supplement use and colon cancer. Cancer Epidemiol Biomark Prev. 1997;6:769–774. [PubMed] [Google Scholar]
- 42.Mesaik MA, Zaheer Ul H, Murad S. Biological and molecular docking studies on coagulin-H: human IL-2 novel natural inhibitor. Mol Immunol. 2006;3(11):1855–1163. doi: 10.1016/j.molimm.2005.10.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data and materials used in this research are available from the corresponding author on reasonable request.


