Abstract
Primary and metastatic liver cancer is an increasingly common and difficult to control disease entity. Radiation offers a non-invasive treatment alternative for these patients who often have few options and a poor prognosis. However, the anatomy and aggressiveness of liver cancer poses significant challenges such as accurate localization at simulation and treatment, management of motion and appropriate selection of dose regimen. This article aims to review the options available and provide information for the practical implementation and/or improvement of liver cancer radiation programs within the context of stereotactic body radiotherapy and image-guided radiotherapy guidelines. Specific patient inclusion and exclusion criteria are presented given the significant toxicity found in certain sub-populations treated with radiation. Indeed, certain sub-populations, such as those with tumor thrombosis or those with larger lesions treated with transarterial chemoembolization, have been shown to have significant improvements in outcome with the addition of radiation and merit special consideration. Implementing a liver radiation program requires three primary challenges to be addressed: (1) immobilization and motion management; (2) localization; and (3) dose regimen and constraint selection. Strategies to deal with motion include simple internal target volume (ITV) expansions, non-gated ITV reduction strategies, breath hold methods, and surrogate marker methods to enable gating or tracking. Localization of the tumor and organs-at-risk are addressed using contrast infusion techniques to take advantage of different normal liver and cancer vascular anatomy, imaging modalities, and margin management. Finally, a dose response has been demonstrated and dose regimens appear to be converging. A more uniform approach to treatment in terms of technique, dose selection and patient selection will allow us to study liver radiation in larger and, hopefully, multicenter randomized studies.
Keywords: Hepatocellular carcinoma, Liver metastases, 4DCT, Image-guided radiotherapy, Stereotactic body radiation therapy
Core tip: Primary and metastatic liver cancer patients are a growing population seen in cancer centers. This population often has few options and a poor prognosis. Radiation offers a safe non-invasive treatment option, but those implementing a liver radiotherapy program must address specific challenges not always seen in other disease sites. A growing and large number of papers have investigated a wide range of strategies. Our objective is to consolidate this literature to provide a concise review of options to allow a pragmatic selection of management strategies.
INTRODUCTION
Liver cancer is a major area of investigation as it is increasingly common and remains one of the deadliest diseases where clinicians have few options. According to Surveillance, Epidemiology, and End Results (SEER) statistics, the estimated numbers of cases of liver cancer (including intrahepatic bile duct cancers) will be 35660 in 2015 representing the second largest annual increase in incidence amongst all cancers in the United States[1]. Liver remains the most frequent site of metastatic disease for patients with colorectal cancer. Approximately 50%-60% of patients with colorectal cancer (CRC) will develop liver metastases and one third will die from liver failure from progressive disease[2]. In patients with only limited liver metastases, aggressive local treatment with surgical extirpation could result in 5-year overall survival rates of 25%-40%[2]. Likewise, the mainstay of treatment for primary liver cancer is surgical resection or liver transplantation. Unfortunately, only 15%-25% of patients are eligible for curative resection or transplant at the time of diagnosis.
Traditionally, radiotherapy has not been routinely given to patients with liver tumors primarily due to the relatively low liver tolerance to radiation. With the advent of advanced radiation technology, it is now possible to deliver potentially curative radiation doses to liver tumors safely. Investigators from Sweden and Japan pioneered the use of stereotactic body radiotherapy (SBRT), a spin-off of intracranial stereotactic radiosurgery (SRS) for extracranial targets[3]. SBRT has also been applied for the treatment of liver tumors and the early results are promising. Following these results, advanced technologies such as protons have also been used to deliver radiotherapy to liver tumors with good results[4,5].
Despite the availability of advanced radiotherapy technologies and evidence of efficacy, the use of radiotherapy for liver has not become standard[6]. This may be due to the fact that there are several difficult challenges for radiotherapy of liver lesions and a myriad of approaches to deal with these challenges. Clinicians must select an appropriate patient population, a safe and effective dose regimen, image guidance methods for tumor localization, methods to deal with respiratory motion, and methods to avoid radiation-induced complications. This overview will provide a practical review of the challenges and options for the treatment of primary and secondary liver tumors. This will assist the practical selection and implementation of options for a high-quality program that follows the guidelines on SBRT[7].
APPROPRIATE PATIENT SELECTION AND ACHIEVABLE CLINICAL OUTCOMES
Hepatocellular carcinoma
There have been significant advances in the options available for hepatocellular carcinoma beyond surgery with level 1 evidence of an overall survival benefit for sorafenib, radiofrequency ablation and transarterial chemoembolization (TACE)[2]. Patient selection for sorafenib is limited to patients with hepatocellular carcinoma (HCC), that earlier treatments options are not suitable, or patients who have progressed on other treatments. This tends to be patients with extensive disease within or outside the liver, including patients with portal vein invasion based on two randomized controlled trials[8,9]. TACE has been shown in two randomized controlled trials and one metaanalysis to improve survival at two years[10-12]. Subsequent metaanalysis has added to the controversy indicating no improvement[13]. However, there are no prospective randomized studies to inform clinical practice beyond radiofrequency ablation, sorafenib, TACE and surgery. This makes selection of appropriate patients subject to interpretation of the evidence. For radiotherapy there is no prospective randomized trial, and we must rely on interpretation of multiple studies reporting case series data with variable patient inclusion, treatment and length of follow-up (Table 1). However, the literature suggests that one subgroup can be identified: Unresectable, locally advanced disease without extrahepatic metastasis, Child-Pugh class A or B, and occupying less than 2/3 of the liver. Several guidelines already include radiation for this subgroup[14,15]. This is based on a growing body of level II evidence (retrospective and prospective case series data); this data indicates a 1-year overall survival of 48%-100%, a 1-year local control rate of 64%-100%, and a grade 3 or greater toxicity of 0%-36% (Table 1). Yet, the role of radiation for hepatocellular carcinoma can span early curative presentations to palliative treatment also based on retrospective data[16]. For tumors near luminal structures, conventional radiotherapy may be recommended over SBRT depending on the dose selection method and planning constraints applied. For the subgroup of recurrences or incomplete responses post chemotherapy or chemoembolizations, the data also suggest that radiation is a strong option. In the landmark trial by Shim, the 2-year overall survival for those receiving radiation was 37% compared to 14% for those who did not receive radiation[17]. This trial also highlighted the importance of tumor size in predicting success of TACE and the possible value of adding for certain patients. For tumors greater than 8 and 10 cm, no patients lived beyond 2-years with TACE. However, if external beam radiation was added, the survival was 50% and 17% for the 8 and 10 cm groups, respectively. Combination therapy, particularly in those with larger lesions where TACE is indicated, the addition of radiation may play a significant role.
Table 1.
Summary of hepatocellular carcinoma radiotherapy studies
| Ref. | No. of patients | Percent of Child-Pugh B patients | Median tumor diameter (range), cm | Dose (range)/No. of fractions | Median follow-up interval, mo | 1-yr OS | 2-yr LC | Toxicity |
| Scorsetti et al[68], 2015 | 43 | 36% | ≤ 6 cm | 48-75 Gy/3 (51%) and 36-60 Gy/6 (49%) | 8 | 77.9% | 64.4% | ≥ gr3: 0 |
| Yamashita et al[69], 2015 | 79 | 11% | 2.7 cm | 48 Gy (40-60)/4-10 | 21 | 53% at 2 yr | 40% | gr3-4: 4.6% gr2: 2.3% |
| Huertas et al[70], 2015 | 77 | 14.3% | 2.4 cm | 45 Gy/3 | 12 | 81.8 | 99% | 14.9% |
| Zhong et al[71], 2014 | 72 | 26% | 13.1 cm | 35.6 Gy/12 | 18 | 56% | NR | gr1-2: 5.6% liver |
| gr1-2: 9.8% | ||||||||
| gastrointestinal | ||||||||
| Lo et al[72], 2014 | 53 | NR | 4.3 cm | 40 Gy/4-5 | 13.1 | 70.1% | RILD 9.4% | |
| Van de Voorde et al[73], 2014 | 5 | NR | NR | 93.6 Gy (62.5-150 )/3-10 | 21 | 85.4% | NR | 0 |
| Sanuki et al[74], 2014 | 63 | 16% | 2.6 cm | 35-40 Gy/5 | 31.1 | 100% | 95% | gr3: |
| early: 16% | ||||||||
| late: 21% | ||||||||
| gr4-5: 0% | ||||||||
| Park et al[75], 2013 | 26 | 27% | 2.8 cm | 40-50 Gy; 4-5 Gy per fraction | 20.2 | 88.5% | 87.6% | gr3: 4% |
| gr4-5: 0% | ||||||||
| Bujold et al[30], 2013 | 102 | 0% | 9.9 cm | 24-54 Gy (36)/6 | 31.4 | 75% | 74% | gr3: 21% gr4: 2.9% gr5: 6.9% |
| Yoon et al[76], 2013 | 93 | 26% | 2 cm | 45 Gy (30-60)/3-4 | 25.6 | 86.0% | 94.8%1 (2 yr) | gr3: 4.3% |
| gr4: 1.0% | ||||||||
| gr5: 1.0% | ||||||||
| Jang et al[65], 2013 | 108 | 10% | 3.0 cm | 51 Gy (33-60 )/3 | 30 | 83%1 | 87% | gr3: 6.5% |
| gr4: 1.9% | ||||||||
| gr5: 0% | ||||||||
| Jung et al[77], 2013 | 92 | 26% | Vol: 8.6 cc | 45 Gy (30-60 )/3-4 | 25.7 | 86.9% | 92.1% (3 yr) | gr ≥ 3: 7% |
| Bibault et al[78], 2013 | 75 | 11% | 3.7 cm | 40-45 Gy/3 | 10 | 78.5% | 89.8% | gr3: 4% |
| gr4: 1.3% | ||||||||
| gr5: 0% | ||||||||
| Honda et al[79], 2013 | 30 | 23% | 16 cm | 48 Gy/4 | 12.3 | 100% | 95%1 | gr3: 10% |
| gr4-5: 0% | ||||||||
| Yuan et al[80], 2013 | 22 | 45% | 4.3 cm | 45 Gy (39-54)/3-8 | 53.4 | 73% | 92.9% | gr2: 31.8% |
| Sanuki et al[81], 2013 | 185 | 15% | CP-A: 27 cm | CP-A: 40 Gy/5 | 24 | 95% | 93% (2 yr) | gr5: 1.1% |
| CP-B: 24 cm | CP-B: 35 Gy/5 | |||||||
| Xi et al[18], 2013 | 41 | 0% | Mean GTV vol: 65.4 cc (SD: 47.9) | 30-48 Gy (36) | 10 | 50.3% | NR | gr3: 2.4% |
| gr4-5: 0% | ||||||||
| Huang et al[82], 2012 | 36 | NR | 1.1-12.3 cm | 37 Gy (25-48)/4-5 | 14 | 64% at 2 yr | 98% | gr2: 3% |
| Kang et al[83], 2012 | 47 | 13% | 2.9 cm | 42-60 Gy/3 | 17 | 83%1 | 94.6% | gr3: 6.4% |
| gr4: 4.3% | ||||||||
| gr5: 0% | ||||||||
| Ibarra et al[84], 2012 | 21 | NR | GTV vol: 334.2 cc | 30 Gy (18-50 )/1-10 | 12.9 | 87% | 57%1 (2 yr) | gr3: 4.8% |
| gr4: 4.8% | ||||||||
| gr5: 0% | ||||||||
| Price et al[85], 2012 | 26 | 46% | Median GTV vol: 33.9 cc | 42 Gy (24-48 )/3-5 | 13 | 77% | NR | NR |
| Andolino et al[86], 2011 | 60 | 40% | 3.1 cm | CP-A: 30-48 Gy/3 | 27 | 82%1 | 90% (2 yr) | gr3: 35% |
| CP-B: 24-48 Gy/5 | gr4: 1.7% | |||||||
| gr5: 0% | ||||||||
| Chan et al[87], 2011 | 11 | 25% | 3 cm | 45 Gy/10 | 24 | 62% | NR | gr ≥ 3 22% |
| Louis et al[88], 2010 | 25 | 12% | 4.5 cm | 45 Gy/3 | 12.7 | 79% | 95% | gr3: |
| early 8% | ||||||||
| late 4% | ||||||||
| Kwon et al[89], 2010 | 42 | 10% | Vol: 15.4 cc | 30-39 Gy/3 | 28.7 | 92.9% | 67.5% | gr3: 0% |
| gr4: 2% | ||||||||
| gr5: 0% | ||||||||
| Cárdenes et al[66], 2010 | 17 | 65% | ≤ 6 cm | CP-A: 48 Gy/3 CP-B: 42 Gy/3 then 40/5 | 24 | 75% | 100% | gr3: 13 instances |
| gr4: 11.8% | ||||||||
| gr5: 0% | ||||||||
| Son et al[90], 2010 | 47 | 8% | 18.3 cm | 36 Gy (30-39)/3 | NR | NR | NR | gr2: 33% |
| Goyal et al[91], 2010 | 6 | NR | 9.3 cm | 34 (24-45 Gy)/1-3 | 10 | 83% | 100% at 9 mo | 0% |
| Seo et al[92], 2010 | 38 | 11% | Vol: 40.5 cc | 33-57 Gy/3-4 | 15 | 68.4% | 66.4% (local PFS) | gr3: 3% |
| gr4-5: 0% | ||||||||
| Choi et al[21], 2008 | 22 | 14% | Vol: 23.5 cc | 36 Gy (30-39 )/3 | 11.5 | 88.1% | NR | gr3: 4.5% |
| gr4-5: 0% | ||||||||
| Tse et al[61], 2008 | 31 | 0% | 173 cc | 36 Gy (24-54 )/6 | 17.6 | 48% | NR | gr3: 29% gr4-5: 0% |
| Méndez Romero et al[93], 2006 | 8 | 25% | 3.2 cm | < 4 cm: 37.5 Gy/3 ≥ 4 cm: 25 Gy/5 or 30 Gy/3 | 12.9 | 75% | 75% (22 mo) | gr5: 12.5% RILD |
Estimated from survival curve. CP-A: Child-Pugh class A; CP-B: Child-Pugh class B; gr: Grade; GTV: Gross tumor volume; LC: Local control; OS: Overall survival; NR: Not reported; PFS: Progression-free survival; RILD: Radiation induced liver disease; vol: Volume.
Lastly, another group with a very poor outcome are those with portal vein thrombosis. Radiotherapy may be particularly useful for tumor thrombosis where current median survivals remain at 2-4 mo without radiation. To date there have been twelve retrospective[18-29] and 1 prospective case[30] series demonstrating a median survival improvement of two to five times historical cohorts. The larger studies used older radiotherapy techniques, including the largest from Yoon et al[22]. Despite a relatively low median dose of 40 Gy in 2-5 fractions, the study achieved an impressive 43% one year overall survival and an acceptable 10% grade 3 or greater toxicity rate[22]. Randomized trials to address the value of radiation for patients with thrombosis are warranted given the possible survival benefit.
Liver metastases
There is growing interest in radiation for oligometastatic disease and palliation. Høyer et al[31] reviewed five retrospective and seven prospective trials to determine which patients should be considered for liver SBRT. This review of the literature by a subcommittee of the American Society of Radiation Oncology (ASTRO) including members from the European Society for Therapeutic Radiology and Oncology (ESTRO), the Canadian Association of Radiation Oncology (CARO) and the Trans-Tasman Radiation Oncology Group (TROG), concluded that the ideal radiotherapy candidate would have an ECOG 0-1, possess adequate hepatic function, have no extrahepatic disease, and have an uninvolved liver volume of 700 mL or greater. This would result in local control rates ranging from 56%-100% at 2 years. Table 2 summarizes prospective trials of SBRT for liver metastases. For a large proportion, extra-hepatic progression develops after local treatment. Though no threshold dose has been found, this group recommended liver metastases receive 48 Gy in three fractions based on the available evidence.
Table 2.
Prospective metastatic liver stereotactic body radiotherapy studies
| Ref. | No. of patients | Dose (Gy/fraction) | Median follow-up (mo) | 2-yr local control (%) |
| Herfarth et al[94], 2001 | 37 | 14-26 Gy/1 | 5.7 | 812 |
| Hoyer et al[95], 2006 | 44 | 45 Gy/3 | 51.6 | 79 |
| Kavanagh et al[96], 2006 | 36 | 60 Gy/3 | 19 | 93 |
| Ambrosino et al[97], 2009 | 27 | Median 36 (25-60) Gy/3 | 13 | 74 crude2 |
| Rusthoven et al[62], 2009 | 47 | 36-60, 60 Gy/3 | 16 | 92 |
| Lee et al[98], 2009 | 68 | Median 41.8 Gy/6 | 10.8 | 712 |
| Méndez Romero et al[93], 2006 | 171 | 30-37.5 Gy/2 | 86 | |
| Stintzing et al[99], 2010 | 361 | 24 Gy/l | 21.3 | 872 |
| Goodman et al[100], 2010 | 261 | 18-30 Gy/l | 17 | 772 |
| Rule et al[63], 2011 | 27 | 30 Gy/5 | 20 | 56 |
| 50 Gy/5 | 89 | |||
| 60 Gy/5 | 100 | |||
| Janoray et al[101], 2014 | 56 | 45 Gy/3-60 Gy/3 | 12.5 | 642 |
Included hepatocellular patients;
12-18 mo local control percentage.
TECHNICAL IMPLEMENTATION CHALLENGES
Immobilization and motion management
Surveys demonstrate that there is no universal standard for liver SBRT[32], but there are recommendations from large SBRT groups. This includes the CARO Scope of Practice guidelines that were published to ensure safe practice in the major SBRT sites[31,33]. In conventional treatments, a larger margin for internal target volume (ITV) may be acceptable as multiple fractions averaged the dose errors caused by inaccurate organ localization, motion or set up error. SBRT relies on the delivery of accurate high doses to the target and errors in localization could result in increased toxicity, geometric tumor miss and cannot be easily “corrected” in later fractions. Therefore, the use of techniques or devices to localize the radiation to the tumor, minimize margins and optimize on-treatment quality assurance is critical. Furthermore, with the use of IMRT, improved motion management results in fewer unplanned hot and cold spots due to the interplay of the motion of anatomical structures and MLC leaf motion[34].
The primary motion with liver SBRT is respiratory motion which can be controlled with fixed immobilization, breath hold and/or tracking. For immobilization, vacuum-bag systems or fixed body immobilizers are used where arms are kept up and out of field. A simple margin expansion to account for ITV is then applied based on a 4DCT scan, fluoroscopy and/or slow CT scanning to capture the full range of motion. These are categorized as ITV methods or motion encompassing methods. An additional margin for set-up motion is added for planning target volume (PTV) with recommendations ranging between 2 and 5 mm[35]. These methods in isolation necessitate larger treated volumes, greater normal tissue inclusion and a lower chance for dose escalation. Shallow breathing may be sufficient in many patients especially if patients are compliant and can maintain regular breathing motions. The American Association of Physicists in Medicine (AAPM) Task Group 76 emphasizes that some method of respiratory assessment be applied and a step-wise algorithm be applied to determine the amount of respiratory management required[36]. However, in a large proportion of patients, additional motion management techniques are necessary to achieve greater dose escalation and safety. The AAPM suggests a cut-off of 5 mm after which respiratory management is recommended. The options can be categorized into three types: (1) non-gated ITV reduction strategies; (2) active or passive breath hold techniques; and/or (3) surrogate markers. These are applied uniformly based on institutional practice or after a trial assessment of patients who are then assigned to one or more additional motion control methods.
Non-gated ITV reduction strategies: Abdominal compression was one of the earliest motion management strategies and was first used in Karolinska Hospital for lung and liver lesions in the 1990’s[37]. A compression plate was applied to the abdomen to reduce abdominal motion caused by respiration. Early data, primarily from lung cancer patients, has shown accuracy and reproducibility with median reductions of 7 mm[36,38]. Recent papers using fiducial markers to track motions have provided direct data on reproducibility and extent of motion reduction in liver patients using abdominal compression. Essentially, a motion minimization method, Wunderink and Méndez Romero[39] demonstrated reduced median excursion by 62% and essentially all residual excursions were reduced to less than 5 mm. Reproducibility was excellent between planning and treatment. Predating much of the 4D respiratory strategies, the appeal of this method includes better localization; this is due to more projection data from the entire breath cycle being available leading to better image quality than only a portion of the 4DCT data set. Another advantage of abdominal compression is the minimal technology requirements compared to more complicated strategies such as gating.
However, the magnitude of improvement may be smaller than initially reported. Updated data from Eccles reported in 2011 that the decrease in motion averaged 2.3 mm and 0.6 mm in the CC and AP direction; 28% saw an increase in motion with abdominal compression so this option does come with caveats[40]. Motion of other important structures such as the kidney do not appear to be improved with the use of this type of device[41]. Furthermore, not all patients require or can tolerate abdominal compression. Patients with abdominal aortic aneurysm also may not be suitable for abdominal compression. Therefore, other motion correction methods have been tested such as using the mean respiratory position for planning[42]. This strategy determines the diaphragm’s mean cranio-caudal position in the respiratory cycle or selects a mid-ventilation CT data set. Velec was able to show that this simple method resulted in a 34% lower irradiated volume due to the significantly smaller PTV compared to standard full-motion ITV-based and dose probability PTVs. However, this group demonstrated that rigid motion correction still results in an 8% and 7% change in dose accumulation for the tumor and normal tissues, respectively[43]. These changes were found in a majority of patients and suggest the need for some additional form of respiratory control and further investigation of adaptive SBRT to deal with organ deformation.
Breath hold methods: The second category of motion management techniques are the breath hold methods. The simplest application is deep inspiration breath hold (DIBH). Initially pioneered at the Memorial Sloan-Kettering Cancer Center[44], DIBH has shown reproducibility within a margin of 2.2 ± 2.0 mm[45]. Voluntary DIBH can reduce internal motion from 12.9 to 2.8 mm[46]. Additional margins for set-up error (typically 2-5 mm) and assessment of intra- and inter-fraction motion is required[26]. The addition of assisted or active breath hold, such as Active Breathing Control (ABC), reduces variability further. ABC was commercialized by Elekta, Inc. and uses a spirometer that monitors the phase of breathing. Usually after two preparatory breaths, a valve is closed at expiration thereby “holding the patient’s breath”. Issues with this strategy include concerns regarding reproducibility, cost of non-reusable components, time required, maintenance and patient tolerance. Reproducibility assessments have demonstrated good intrafraction absolute offsets of 3 mm or less. However, interfraction errors > 3 mm are found in 46% of cases further emphasizing the need for image guidance[47]. Alternatively, shallow breathing and voluntary breath hold can be monitored using the spirometry system; planning margins, treatment activation, and reproducibility of set up can be determined using the same equipment. This is useful as not all patients can tolerate active breath hold. While it has its own limitations, voluntary breath hold has many advantages compared to gating including no marker/tumor motion lag issues, about half the treatment time required, less specialized equipment, less training and software, plus more efficient simulation[29].
Surrogate markers to enable gating or tracking: The third variation in motion management is the use of surrogate markers to enable gating. Depending on the surrogate, this overlaps with tumor localization strategies. Surrogates are used to assess the degree of motion which leads to individualization of motion management. Compared to breath hold techniques alone, this method is better tolerated and may represent a more accurate anatomical picture than deep inspiration or expiration. External respiratory surrogates include the Varian 3D infrared marker (Real-time Positioning Management or RPM) system, the Siemens respiratory strain gauge belt, and 3D laser surface respiratory assessment systems (C-RAD Sentinel). Internal surrogates include the diaphragm/lung interface, radiotransmitters (Calypso), and radio-opaque markers (including surgical clips, stents, lipiodol or anatomical calcifications). Internal markers provide the best surrogates, but usually are invasive to insert, risk complications and have a higher relative cost in time and money. Various gating options can by chosen based on the information from these surrogates. Respiratory gating can be grouped into three major categories: First, phase gating consists of treating the patient during a particular phase such as end-expiration. The advantage is that this portion of the breathing cycle is often the most stable with the least motion. Second, amplitude gating selects a certain portion of the respiratory cycle defined by a percentage of the amplitude of each cycle. As phase gating may result in binning errors due to breath to breath variations in slope, length of cycles and amplitudes, detractors suggest better sorting with fixed amplitude gating[48]. Third, gating may be based on the surrogate marker at breath hold. In addition to motion assessment, these markers may be used during treatment for synchronizing treatment delivery. For example, simply gating the treatment beam when the surrogate indicates the tumor is in a certain position, or synchronizing the aperture via dynamic multileaf collimator (DMLC), or moving beam to the location of the lesion (Cyberknife) are valuable strategies[49]. A method to select an appropriate clinical target volume (CTV) to PTV margin has been developed by Keall and Vedam based on three challenges: (1) selection of amplitude vs phase gating; (2) accounting for phase shifts between markers and the lesion; and (3) the management of intrafraction motion vs increased delivery time[50]. Typical GTV to PTV margins are 5 mm axially and 10 mm craniocaudally[35]. Periodic monitoring during treatment is still necessary to confirm reproducibility of the motion compared to planning. Patient training plus visual or verbal prompting may allow better reproducibility and margins[50].
Localization
At simulation, IV contrast is considered standard particularly for hepatocellular carcinoma. However, this does introduce fusion errors as the contrast infusion must be captured over several respiratory cycles. Various protocols are in place such as the MD Anderson standardized protocol[51] or those that individualize[52] binning by visualizing contrast in specific vessels. The later method accounts for patient differences in the time contrast reaches and leaves the lesion, anatomical location of the tumor relative to the start of the scan, body weight, time-density curves and cirrhosis (Figure 1). Figure 1 demonstrates the time-density intravenous contrast enhancement called Dynamic Contrast Enhanced CT (DCECT). Images are binned by location in respiratory cycle and when the contrast density within a vessel (such as the aorta or portal vein) signifies the arterial, portal venous, and delayed phase. Images are specific to each patient and individualized contrast enhanced images (Figure 2) offer the possibility of improved delineation without additional equipment or technology, but methods to eliminate motion during the long acquisitions are required[53,54].
Figure 1.
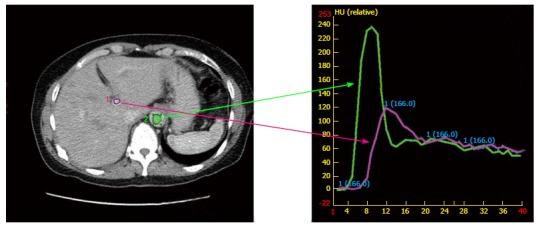
Image of a time-density intravenous contrast enhancement called Dynamic Contrast Enhanced computerized tomography. Images are binned by location in respiratory cycle and when the contrast density within a vessel (such as the aorta and portal vein) signifies the non-contrast, arterial and wash-out phase. Time is measured in seconds and density is measured in Hounsefield units (HU).
Figure 2.
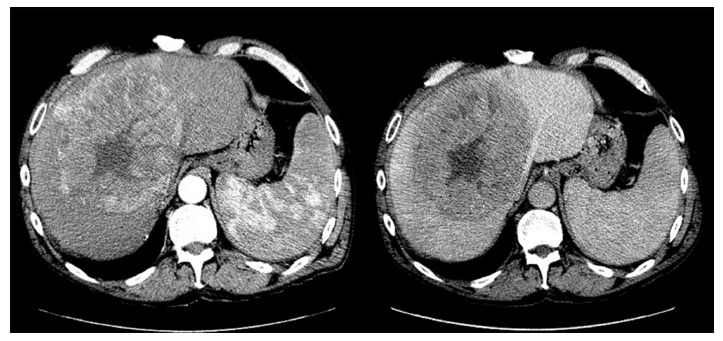
Arterial and portal-venous phase images.
MRI and PET are becoming a standard part of management for liver lesions due to improved sensitivity and specificity[55]. MRI is particularly useful for small tumors, cirrhotic patients or those who are unable to tolerate IV contrast. MRI may play a greater role as experience with 4DCT, gated MRI and cine MRI accumulates[56,57]. Functional imaging assessments are useful for follow-up, and to determine the necessity to add additional treatments post radiation[58]. However, both MRI and PET have long acquisition times and require strategies to account for motion. Strategies such as multiple breath holds, parallel imaging for rapid acquisition and respiratory correlated PET are being investigated. Even if accurate localization is possible with the elimination of respiratory motion, strategies to register the MRI and/or PET to the CT image are then required. Additional margins will be required after deformable or rigid image registration in the range of 2.2 to 21.3 mm[59]. Therefore, the value of more accurate localization must be balanced against the additional margin, time, and cost.
Oral contrast is useful to localize luminal structures, which often represent the most critical organs at risk. The contrast is assigned a CT number for tissue equivalence prior to planning. Lastly, calcifications, vessels and other anatomical landmarks can be extremely useful. If possible, contouring these structures provides information to the therapists; communication with therapists to indicate which critical structures to localize, priorize and/or avoid is a practical and valuable routine to incorporate.
At treatment, localization of the tumor in the liver is sometimes not possible in contrast to other sites such as lung cancer where the tumor location is often very clear. Internal and/or external surrogate markers or structures may be used as described earlier. At treatment the consistency of correlation with respiratory motion or breath hold ability at time of simulation must be verified. A commonly used structure is the diaphragm. Vedam has shown a strong linear correlation between the diaphragm and the external marker; a superior-inferior CTV-PTV margin of 0.8 cm provided sufficient coverage over multiple sessions with or without training[60]. Static images are acceptable, but real-time or near-real-time options exist. Some systems have the ability to acquire images in fluoroscopy or cine-mode and new systems now enable almost real-time dose accumulation to enable adaptive treatment. However, with fewer fractions used in SBRT, the opportunity to correct dosing errors is limited and localization prior to and during each treatment remains the primary goal. Non-radiographic dependent internal tumor markers such as Calypso can track motion during treatment to provide a more accurate assessment of tumor motion. This real-time tracking has significant advantages over other motion control strategies including the ability to adjust beam delivery via synchronized aperture tracking methods or by directly following the lesion motion with the radiation beam.
Dose selection and constraints
An optimal dose for primary and secondary liver cancer has not been identified. Essentially there are two types of research approaches in the literature for dose finding: Radiobiologically-guided dose escalation and step-wise dose escalation. The first approach, such as the pioneering work of Tse et al[61], uses radiobiological calculation of risk to provide individualized dose recommendations. The second relies on maximally tolerated dose (MTD) techniques used successfully in drug trials. In many cases, the dose has been determined by normal tissue constraints. Furthermore, patient tolerance of radiation may vary due to underlying hepatic insufficiency, and previous or concurrent treatments (resections, chemotherapy). Despite the varied approaches and dose regimens, a convergence of dose recommendations may be occurring (Table 1, summary of studies for HCC; and Table 2, summary of SBRT studies of metastatic cancer). For hepatic metastases, work published by Rusthoven demonstrated that 60 Gy in 3 fractions resulted in a local control rate of 92% at 2 years[62]. Similarly, Timmerman and Rule suggest that a 60 Gy in 5 fraction regimen is appropriate, particularly for tumors adjacent to critical structures[63]. Based on three dose escalation cohorts, the actuarial 24 mo local control was 100%. The authors state that a maximum tolerated dose was not reached; MTD was defined as the dose below which the dose limiting toxicity rate was ≥ 33%. Both groups used a critical volume model with at least 700 mL of normal liver receiving less than 15 Gy and 21 Gy for the 3 and 5 fraction regimen, respectively. However, in both studies tumors were highly selected with a median tumor size of less than 3 cm and few patients had centrally located lesions. Therefore, the excellent results may not be generalizable to a wider population especially those with larger lesions. However, for patients who can meet the trial constraints, the 100% local control rate is a strong argument that the optimal dose for hepatic metastases is 60 Gy in three or five fractions.
For primary liver cancer, a dose response relationship has been found[64], but outcomes and regimens remain somewhat more varied than with metastatic disease (Table 1). HCC patient population is very heterogeneous with important parameters such as size of lesion, liver dysfunction, previous treatments received, presence of vascular invasion and number of lesions all influencing outcome. This heterogeneity increases the difficulty in generalizing data. Modeling suggests that a 90% probability of 6-mo control could be achieved with 84 Gy in 2 Gy equivalent doses[55]; much higher than the 53 Gy in 2 Gy equivalent required for metastatic disease. Review of trials reporting 2-year outcomes of greater than 90%, suggests that a dose of 45 Gy in 3-4 fractions or 35-40 Gy in 5 fractions need to be achieved (Table 1). A critical dose threshold likely exists for both local control and overall survival. In one of the larger SBRT studies, Jang et al[65] demonstrated that above 54 Gy in 3 fractions, local control and survival was 100% and 71% at 2-years, respectively. However, if less than 45 Gy was achieved, the local control and overall survival dropped to 64% and 30%, respectively.
Unlike patients with liver metastases, a significant proportion of patients with HCC have underlying cirrhosis and/or other insufficiency. This factor has been a consistent parameter influencing dose selection, patient selection and outcome. Most commonly measured using the Child-Pugh score, groups have consistently found this issue to influence treatment and prognosis. Cárdenes et al[66]. from Indiana University conducted a phase I dose escalation trial of SBRT for HCC, where 17 Child-Pugh classes A or B patients with 25 tumors were included. The initial dose level was 36 Gy in 3 fractions and there was a 2-Gy per fraction increment. Patients received a maximum of two treatments per week. The protocol required 700 cc of normal liver would receive < 15 Gy. They were able to escalate the dose to 48 Gy in 3 fractions for Child-Pugh class A patients without causing dose-limiting toxicities. However, two Child-Pugh class B patients developed grade 3 liver toxicities when the dose was escalated to 42 Gy in 3 fractions. This observation has led these investigators to change the regimen for Child-Pugh class B patients, from 40 Gy in 3 fractions to 40 Gy in 5 fractions. This was considered the MTD and no further dose escalations are recommended. The most important factor associated with grade 3 or higher liver toxicities was a Child- Pugh score of ≥ 8. Based on their experience, the group has recommended that the dose to one-third of the uninvolved liver should be restricted to ≤ 10 Gy (3.3 Gy/fraction) and ≥ 500 cc of uninvolved liver should receive < 7 Gy (2.3 Gy/fraction) for Child-Pugh class A patients; for Child-Pugh class B patients, the dose to one-third of the uninvolved liver is restricted to ≤ 18 Gy, (3.6 Gy/fraction) and ≥ 500 cc of uninvolved liver should receive < 12 Gy (2.4 Gy/fraction)[66]. A summary of suggested constraints based on recent randomized clinical trials with accepted fraction regimens is summarized in Table 3.
Table 3.
Summary of dose constraints by number of fractions
| Organ at risk | 3 fraction (RAS trial[102]) | 5 fraction (RTOG 1112[103]) | QUANTEC (1.8-2 Gy per fraction)[104] | Toxicity |
| Liver excluding CTV | 700 mL < 15 Gy | V 10 < 70% | D mean < 30 Gy | Radiation induced liver dysfunction |
| Esophagus | D 1 mL < 21 Gy | D 0.5 mL < 32 Gy | V 35 < 50% | Esophagitis |
| Stomach | D 1 mL < 21 Gy | D 0.5 mL < 30 Gy | D 100 < 35 Gy | Ulceration |
| Kidney | D 35% < 15 Gy | D mean < 10 Gy | D mean < 28 Gy (1.8-2 Gy per fraction) | Renal dysfunction |
| Bowel and duodenum | D 1 mL < 21 Gy | D 0.5 mL < 30 Gy | D 45 < 195 cc | Enteritis/fistula |
| Spinal cord | Dmax < 18 Gy | D 0.5 mL < 25 Gy | Dmax = 45 | Myelopathy |
| Heart | D 1 mL, 30 Gy | D 30 mL < 30 Gy | V 25 < 10% | Pericarditis |
CTV: Clinical target volume.
FUTURE TRENDS
Patients with primary or secondary liver cancer are growing in incidence and have a rising mortality rate[1]. Current management with RFA, TACE, sorafenib and surgery often are not possible or result in moderate improvements[67]. Therefore, patients and their physicians must seek alternatives or combination of treatments. In addition to external beam radiation, work in the use of radionuclides, radiosensitizers (such as inhibitors of autophagy), epigenetic agents, liquid biopsies to better select patients, and immune modulation are exciting avenues of investigation. As for external beam radiotherapy, our review suggests that radiotherapy can be implemented safely and with high local control rates. In the future, we will continue to refine our technique and patient selection, but appropriate multidisciplinary randomized trials need to be completed before radiation can become a standard of care.
CONCLUSION
Radiation plays an important role in the treatment of primary and metastatic liver cancer. Control rates can be high and toxicity is minimal in well-selected patients. Indeed it may play a primary role in subgroups such as large tumors and those with thrombosis. The wide ranging outcomes, differing techniques and varied dosing strategies make specific treatment recommendations difficult, but the literature is converging on a short list of important components of a high quality liver radiation program. This article aims to provide a practical review of options to provide the best care possible in this evolving field.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Canada
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Conflict-of-interest statement: Lo SS has received research funding from Elekta AB through the International Oligometastasis Consortium; he has also received travel expenses and honorarium from Varian Medical Systems and travel expenses from Accuray Inc.; Lock MI has received fees as a consultant or research funding from AstraZeneca Limited, Accuray Incorporated, 3M Canada, Varian Medical Systems and Abbvie Corporation. No other potential conflicts of interest are declared. No financial support.
Peer-review started: August 29, 2016
First decision: November 21, 2016
Article in press: April 20, 2017
P- Reviewer: Cao GW, Cerwenka HR, Furka A, Tarazov PG S- Editor: Kong JX L- Editor: A E- Editor: Li D
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Lo SS, Moffatt-Bruce SD, Dawson LA, Schwarz RE, Teh BS, Mayr NA, Lu JJ, Grecula JC, Olencki TE, Timmerman RD. The role of local therapy in the management of lung and liver oligometastases. Nat Rev Clin Oncol. 2011;8:405–416. doi: 10.1038/nrclinonc.2011.75. [DOI] [PubMed] [Google Scholar]
- 3.Lo SS, Fakiris AJ, Chang EL, Mayr NA, Wang JZ, Papiez L, Teh BS, McGarry RC, Cardenes HR, Timmerman RD. Stereotactic body radiation therapy: a novel treatment modality. Nat Rev Clin Oncol. 2010;7:44–54. doi: 10.1038/nrclinonc.2009.188. [DOI] [PubMed] [Google Scholar]
- 4.Kim TH, Park JW, Kim YJ, Kim BH, Woo SM, Moon SH, Kim SS, Koh YH, Lee WJ, Park SJ, et al. Phase I dose-escalation study of proton beam therapy for inoperable hepatocellular carcinoma. Cancer Res Treat. 2015;47:34–45. doi: 10.4143/crt.2013.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hong TS, DeLaney TF, Mamon HJ, Willett CG, Yeap BY, Niemierko A, Wolfgang JA, Lu HM, Adams J, Weyman EA, et al. A prospective feasibility study of respiratory-gated proton beam therapy for liver tumors. Pract Radiat Oncol. 2010;4:316–322. doi: 10.1016/j.prro.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Song P, Tobe RG, Inagaki Y, Kokudo N, Hasegawa K, Sugawara Y, Tang W. The management of hepatocellular carcinoma around the world: a comparison of guidelines from 2001 to 2011. Liver Int. 2012;32:1053–1063. doi: 10.1111/j.1478-3231.2012.02792.x. [DOI] [PubMed] [Google Scholar]
- 7.Jaffray DA, Langen KM, Mageras G, Dawson LA, Yan D, Edd RA, Mundt AJ, Fraass B. Safety considerations for IGRT: Executive summary. Pract Radiat Oncol. 2013;3:167–170. doi: 10.1016/j.prro.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 9.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 10.Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology. 2003;37:429–442. doi: 10.1053/jhep.2003.50047. [DOI] [PubMed] [Google Scholar]
- 11.Llovet JM, Real MI, Montaña X, Planas R, Coll S, Aponte J, Ayuso C, Sala M, Muchart J, Solà R, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734–1739. doi: 10.1016/S0140-6736(02)08649-X. [DOI] [PubMed] [Google Scholar]
- 12.Lo CM, Ngan H, Tso WK, Liu CL, Lam CM, Poon RT, Fan ST, Wong J. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35:1164–1171. doi: 10.1053/jhep.2002.33156. [DOI] [PubMed] [Google Scholar]
- 13.Oliveri RS, Wetterslev J, Gluud C. Transarterial (chemo)embolisation for unresectable hepatocellular carcinoma. Cochrane Database Syst Rev. 2011;(3):CD004787. doi: 10.1002/14651858.CD004787.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park JW. [Practice guideline for diagnosis and treatment of hepatocellular carcinoma] Korean J Hepatol. 2004;10:88–98. [PubMed] [Google Scholar]
- 15.Korean Liver Cancer Study Group and National Cancer Center, Korea. [Practice guidelines for management of hepatocellular carcinoma 2009] Korean J Hepatol. 2009;15:391–423. doi: 10.3350/kjhep.2009.15.3.391. [DOI] [PubMed] [Google Scholar]
- 16.Wo JY, Dawson LA, Zhu AX, Hong TS. An emerging role for radiation therapy in the treatment of hepatocellular carcinoma and intrahepatic cholangiocarcinoma. Surg Oncol Clin N Am. 2014;23:353–368. doi: 10.1016/j.soc.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 17.Shim SJ, Seong J, Han KH, Chon CY, Suh CO, Lee JT. Local radiotherapy as a complement to incomplete transcatheter arterial chemoembolization in locally advanced hepatocellular carcinoma. Liver Int. 2005;25:1189–1196. doi: 10.1111/j.1478-3231.2005.01170.x. [DOI] [PubMed] [Google Scholar]
- 18.Xi M, Zhang L, Zhao L, Li QQ, Guo SP, Feng ZZ, Deng XW, Huang XY, Liu MZ. Effectiveness of stereotactic body radiotherapy for hepatocellular carcinoma with portal vein and/or inferior vena cava tumor thrombosis. PLoS One. 2013;8:e63864. doi: 10.1371/journal.pone.0063864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin CS, Jen YM, Chiu SY, Hwang JM, Chao HL, Lin HY, Shum WY. Treatment of portal vein tumor thrombosis of hepatoma patients with either stereotactic radiotherapy or three-dimensional conformal radiotherapy. Jpn J Clin Oncol. 2006;36:212–217. doi: 10.1093/jjco/hyl006. [DOI] [PubMed] [Google Scholar]
- 20.Sugahara S, Nakayama H, Fukuda K, Mizumoto M, Tokita M, Abei M, Shoda J, Matsuzaki Y, Thono E, Tsuboi K, et al. Proton-beam therapy for hepatocellular carcinoma associated with portal vein tumor thrombosis. Strahlenther Onkol. 2009;185:782–788. doi: 10.1007/s00066-009-2020-x. [DOI] [PubMed] [Google Scholar]
- 21.Choi BO, Choi IB, Jang HS, Kang YN, Jang JS, Bae SH, Yoon SK, Chai GY, Kang KM. Stereotactic body radiation therapy with or without transarterial chemoembolization for patients with primary hepatocellular carcinoma: preliminary analysis. BMC Cancer. 2008;8:351. doi: 10.1186/1471-2407-8-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoon SM, Lim YS, Won HJ, Kim JH, Kim KM, Lee HC, Chung YH, Lee YS, Lee SG, Park JH, et al. Radiotherapy plus transarterial chemoembolization for hepatocellular carcinoma invading the portal vein: long-term patient outcomes. Int J Radiat Oncol Biol Phys. 2012;82:2004–2011. doi: 10.1016/j.ijrobp.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 23.Chuma M, Taguchi H, Yamamoto Y, Shimizu S, Nakanishi M, Ogawa K, Sho T, Horimoto H, Kobayashi T, Nakai M, et al. Efficacy of therapy for advanced hepatocellular carcinoma: intra-arterial 5-fluorouracil and subcutaneous interferon with image-guided radiation. J Gastroenterol Hepatol. 2011;26:1123–1132. doi: 10.1111/j.1440-1746.2011.06745.x. [DOI] [PubMed] [Google Scholar]
- 24.Hata M, Tokuuye K, Sugahara S, Kagei K, Igaki H, Hashimoto T, Ohara K, Matsuzaki Y, Tanaka N, Akine Y. Proton beam therapy for hepatocellular carcinoma with portal vein tumor thrombus. Cancer. 2005;104:794–801. doi: 10.1002/cncr.21237. [DOI] [PubMed] [Google Scholar]
- 25.Shirai S, Sato M, Suwa K, Kishi K, Shimono C, Kawai N, Tanihata H, Minamiguchi H, Nakai M. Single photon emission computed tomography-based three-dimensional conformal radiotherapy for hepatocellular carcinoma with portal vein tumor thrombus. Int J Radiat Oncol Biol Phys. 2009;73:824–831. doi: 10.1016/j.ijrobp.2008.04.055. [DOI] [PubMed] [Google Scholar]
- 26.Huang YJ, Hsu HC, Wang CY, Wang CJ, Chen HC, Huang EY, Fang FM, Lu SN. The treatment responses in cases of radiation therapy to portal vein thrombosis in advanced hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2009;73:1155–1163. doi: 10.1016/j.ijrobp.2008.06.1486. [DOI] [PubMed] [Google Scholar]
- 27.Nakagawa K, Yamashita H, Shiraishi K, Nakamura N, Tago M, Igaki H, Hosoi Y, Shiina S, Omata M, Makuuchi M, et al. Radiation therapy for portal venous invasion by hepatocellular carcinoma. World J Gastroenterol. 2005;11:7237–7241. doi: 10.3748/wjg.v11.i46.7237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim DY, Park W, Lim DH, Lee JH, Yoo BC, Paik SW, Kho KC, Kim TH, Ahn YC, Huh SJ. Three-dimensional conformal radiotherapy for portal vein thrombosis of hepatocellular carcinoma. Cancer. 2005;103:2419–2426. doi: 10.1002/cncr.21043. [DOI] [PubMed] [Google Scholar]
- 29.Zeng ZC, Fan J, Tang ZY, Zhou J, Qin LX, Wang JH, Sun HC, Wang BL, Zhang JY, Jiang GL, et al. A comparison of treatment combinations with and without radiotherapy for hepatocellular carcinoma with portal vein and/or inferior vena cava tumor thrombus. Int J Radiat Oncol Biol Phys. 2005;61:432–443. doi: 10.1016/j.ijrobp.2004.05.025. [DOI] [PubMed] [Google Scholar]
- 30.Bujold A, Massey CA, Kim JJ, Brierley J, Cho C, Wong RK, Dinniwell RE, Kassam Z, Ringash J, Cummings B, et al. Sequential phase I and II trials of stereotactic body radiotherapy for locally advanced hepatocellular carcinoma. J Clin Oncol. 2013;31:1631–1639. doi: 10.1200/JCO.2012.44.1659. [DOI] [PubMed] [Google Scholar]
- 31.Høyer M, Swaminath A, Bydder S, Lock M, Méndez Romero A, Kavanagh B, Goodman KA, Okunieff P, Dawson LA. Radiotherapy for liver metastases: a review of evidence. Int J Radiat Oncol Biol Phys. 2012;82:1047–1057. doi: 10.1016/j.ijrobp.2011.07.020. [DOI] [PubMed] [Google Scholar]
- 32.Lock MI, Hoyer M, Bydder SA, Okunieff P, Hahn CA, Vichare A, Dawson LA. An international survey on liver metastases radiotherapy. Acta Oncol. 2012;51:568–574. doi: 10.3109/0284186X.2012.681700. [DOI] [PubMed] [Google Scholar]
- 33.Sahgal A, Roberge D, Schellenberg D, Purdie TG, Swaminath A, Pantarotto J, Filion E, Gabos Z, Butler J, Letourneau D, et al. The Canadian Association of Radiation Oncology scope of practice guidelines for lung, liver and spine stereotactic body radiotherapy. Clin Oncol (R Coll Radiol) 2012;24:629–639. doi: 10.1016/j.clon.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 34.Yu CX, Jaffray DA, Wong JW. The effects of intra-fraction organ motion on the delivery of dynamic intensity modulation. Phys Med Biol. 1998;43:91–104. doi: 10.1088/0031-9155/43/1/006. [DOI] [PubMed] [Google Scholar]
- 35.Cárdenes HR, Lasley F. Primary liver cancer. In: Lo SS, Teh BS, Lu JJ, Schefter TE (Eds.). Stereotactic body radiation therapy. Berlin: Springer-Verlag, 2012: 163-182 In: Lo SS, Teh BS, Lu JJ, Schefter TE (Eds, editors. [Google Scholar]
- 36.Keall PJ, Mageras GS, Balter JM, Emery RS, Forster KM, Jiang SB, Kapatoes JM, Low DA, Murphy MJ, Murray BR, et al. The management of respiratory motion in radiation oncology report of AAPM Task Group 76. Med Phys. 2006;33:3874–3900. doi: 10.1118/1.2349696. [DOI] [PubMed] [Google Scholar]
- 37.Lax I, Blomgren H, Näslund I, Svanström R. Stereotactic radiotherapy of malignancies in the abdomen. Methodological aspects. Acta Oncol. 1994;33:677–683. doi: 10.3109/02841869409121782. [DOI] [PubMed] [Google Scholar]
- 38.Herfarth KK, Debus J, Lohr F, Bahner ML, Fritz P, Höss A, Schlegel W, Wannenmacher MF. Extracranial stereotactic radiation therapy: set-up accuracy of patients treated for liver metastases. Int J Radiat Oncol Biol Phys. 2000;46:329–335. doi: 10.1016/s0360-3016(99)00413-7. [DOI] [PubMed] [Google Scholar]
- 39.Wunderink W, Méndez Romero A, de Kruijf W, de Boer H, Levendag P, Heijmen B. Reduction of respiratory liver tumor motion by abdominal compression in stereotactic body frame, analyzed by tracking fiducial markers implanted in liver. Int J Radiat Oncol Biol Phys. 2008;71:907–915. doi: 10.1016/j.ijrobp.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 40.Eccles CL, Patel R, Simeonov AK, Lockwood G, Haider M, Dawson LA. Comparison of liver tumor motion with and without abdominal compression using cine-magnetic resonance imaging. Int J Radiat Oncol Biol Phys. 2011;79:602–608. doi: 10.1016/j.ijrobp.2010.04.028. [DOI] [PubMed] [Google Scholar]
- 41.Heinzerling JH, Anderson JF, Papiez L, Boike T, Chien S, Zhang G, Abdulrahman R, Timmerman R. Four-dimensional computed tomography scan analysis of tumor and organ motion at varying levels of abdominal compression during stereotactic treatment of lung and liver. Int J Radiat Oncol Biol Phys. 2008;70:1571–1578. doi: 10.1016/j.ijrobp.2007.12.023. [DOI] [PubMed] [Google Scholar]
- 42.Velec M, Moseley JL, Brock KK. Simplified strategies to determine the mean respiratory position for liver radiation therapy planning. Pract Radiat Oncol. 2014;4:160–166. doi: 10.1016/j.prro.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Velec M, Moseley JL, Eccles CL, Craig T, Sharpe MB, Dawson LA, Brock KK. Effect of breathing motion on radiotherapy dose accumulation in the abdomen using deformable registration. Int J Radiat Oncol Biol Phys. 2011;80:265–272. doi: 10.1016/j.ijrobp.2010.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mageras GS, Yorke E. Deep inspiration breath hold and respiratory gating strategies for reducing organ motion in radiation treatment. Semin Radiat Oncol. 2004;14:65–75. doi: 10.1053/j.semradonc.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 45.Brock KK. Imaging and image-guided radiation therapy in liver cancer. Semin Radiat Oncol. 2011;21:247–255. doi: 10.1016/j.semradonc.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barnes EA, Murray BR, Robinson DM, Underwood LJ, Hanson J, Roa WH. Dosimetric evaluation of lung tumor immobilization using breath hold at deep inspiration. Int J Radiat Oncol Biol Phys. 2001;50:1091–1098. doi: 10.1016/s0360-3016(01)01592-9. [DOI] [PubMed] [Google Scholar]
- 47.Eccles C, Brock KK, Bissonnette JP, Hawkins M, Dawson LA. Reproducibility of liver position using active breathing coordinator for liver cancer radiotherapy. Int J Radiat Oncol Biol Phys. 2006;64:751–759. doi: 10.1016/j.ijrobp.2005.05.066. [DOI] [PubMed] [Google Scholar]
- 48.Wink N, Panknin C, Solberg TD. Phase versus amplitude sorting of 4D-CT data. J Appl Clin Med Phys. 2006;7:77–85. doi: 10.1120/jacmp.v7i1.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Keall P. 4-dimensional computed tomography imaging and treatment planning. Semin Radiat Oncol. 2004;14:81–90. doi: 10.1053/j.semradonc.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 50.Vedam SS, Keall PJ, Kini VR, Mohan R. Determining parameters for respiration-gated radiotherapy. Med Phys. 2001;28:2139–2146. doi: 10.1118/1.1406524. [DOI] [PubMed] [Google Scholar]
- 51.Beddar AS, Briere TM, Balter P, Pan T, Tolani N, Ng C, Szklaruk J, Krishnan S. 4D-CT imaging with synchronized intravenous contrast injection to improve delineation of liver tumors for treatment planning. Radiother Oncol. 2008;87:445–448. doi: 10.1016/j.radonc.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 52.Ichikawa T, Erturk SM, Araki T. Multiphasic contrast-enhanced multidetector-row CT of liver: contrast-enhancement theory and practical scan protocol with a combination of fixed injection duration and patients' body-weight-tailored dose of contrast material. Eur J Radiol. 2006;58:165–176. doi: 10.1016/j.ejrad.2005.11.037. [DOI] [PubMed] [Google Scholar]
- 53.Jensen NK, Mulder D, Lock M, Fisher B, Zener R, Beech B, Kozak R, Chen J, Lee TY, Wong E. Dynamic contrast enhanced CT aiding gross tumor volume delineation of liver tumors: an interobserver variability study. Radiother Oncol. 2014;111:153–157. doi: 10.1016/j.radonc.2014.01.026. [DOI] [PubMed] [Google Scholar]
- 54.Jensen NK, Stewart E, Lock M, Fisher B, Kozak R, Chen J, Lee TY, Wong E. Assessment of contrast enhanced respiration managed cone-beam CT for image guided radiotherapy of intrahepatic tumors. Med Phys. 2014;41:051905. doi: 10.1118/1.4870385. [DOI] [PubMed] [Google Scholar]
- 55.Hussain SM, Semelka RC. Hepatic imaging: comparison of modalities. Radiol Clin North Am. 2005;43:929–947, ix. doi: 10.1016/j.rcl.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 56.Yu JI, Kim JS, Park HC, Lim DH, Han YY, Lim HC, Paik SW. Evaluation of anatomical landmark position differences between respiration-gated MRI and four-dimensional CT for radiation therapy in patients with hepatocellular carcinoma. Br J Radiol. 2013;86:20120221. doi: 10.1259/bjr.20120221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu Y, Yin FF, Chen NK, Chu ML, Cai J. Four dimensional magnetic resonance imaging with retrospective k-space reordering: a feasibility study. Med Phys. 2015;42:534–541. doi: 10.1118/1.4905044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Huang WY, Kao CH, Huang WS, Chen CM, Chang LP, Lee MS, Chao HL, Chiu CH, Lo CH, Jen YM. 18F-FDG PET and combined 18F-FDG-contrast CT parameters as predictors of tumor control for hepatocellular carcinoma after stereotactic ablative radiotherapy. J Nucl Med. 2013;54:1710–1716. doi: 10.2967/jnumed.112.119370. [DOI] [PubMed] [Google Scholar]
- 59.Voroney JP, Brock KK, Eccles C, Haider M, Dawson LA. Prospective comparison of computed tomography and magnetic resonance imaging for liver cancer delineation using deformable image registration. Int J Radiat Oncol Biol Phys. 2006;66:780–791. doi: 10.1016/j.ijrobp.2006.05.035. [DOI] [PubMed] [Google Scholar]
- 60.Vedam SS, Kini VR, Keall PJ, Ramakrishnan V, Mostafavi H, Mohan R. Quantifying the predictability of diaphragm motion during respiration with a noninvasive external marker. Med Phys. 2003;30:505–513. doi: 10.1118/1.1558675. [DOI] [PubMed] [Google Scholar]
- 61.Tse RV, Hawkins M, Lockwood G, Kim JJ, Cummings B, Knox J, Sherman M, Dawson LA. Phase I study of individualized stereotactic body radiotherapy for hepatocellular carcinoma and intrahepatic cholangiocarcinoma. J Clin Oncol. 2008;26:657–664. doi: 10.1200/JCO.2007.14.3529. [DOI] [PubMed] [Google Scholar]
- 62.Rusthoven KE, Kavanagh BD, Cardenes H, Stieber VW, Burri SH, Feigenberg SJ, Chidel MA, Pugh TJ, Franklin W, Kane M, et al. Multi-institutional phase I/II trial of stereotactic body radiation therapy for liver metastases. J Clin Oncol. 2009;27:1572–1578. doi: 10.1200/JCO.2008.19.6329. [DOI] [PubMed] [Google Scholar]
- 63.Rule W, Timmerman R, Tong L, Abdulrahman R, Meyer J, Boike T, Schwarz RE, Weatherall P, Chinsoo Cho L. Phase I dose-escalation study of stereotactic body radiotherapy in patients with hepatic metastases. Ann Surg Oncol. 2011;18:1081–1087. doi: 10.1245/s10434-010-1405-5. [DOI] [PubMed] [Google Scholar]
- 64.Lausch A, Sinclair K, Lock M, Fisher B, Jensen N, Gaede S, Chen J, Wong E. Determination and comparison of radiotherapy dose responses for hepatocellular carcinoma and metastatic colorectal liver tumours. Br J Radiol. 2013;86:20130147. doi: 10.1259/bjr.20130147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jang WI, Kim MS, Bae SH, Cho CK, Yoo HJ, Seo YS, Kang JK, Kim SY, Lee DH, Han CJ, et al. High-dose stereotactic body radiotherapy correlates increased local control and overall survival in patients with inoperable hepatocellular carcinoma. Radiat Oncol. 2013;8:250. doi: 10.1186/1748-717X-8-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cárdenes HR, Price TR, Perkins SM, Maluccio M, Kwo P, Breen TE, Henderson MA, Schefter TE, Tudor K, Deluca J, et al. Phase I feasibility trial of stereotactic body radiation therapy for primary hepatocellular carcinoma. Clin Transl Oncol. 2010;12:218–225. doi: 10.1007/s12094-010-0492-x. [DOI] [PubMed] [Google Scholar]
- 67.Ohri N, Dawson LA, Krishnan S, Seong J, Cheng JC, Sarin SK, Kinkhabwala M, Ahmed MM, Vikram B, Coleman CN, et al. Radiotherapy for Hepatocellular Carcinoma: New Indications and Directions for Future Study. J Natl Cancer Inst. 2016;108:pii: djw133. doi: 10.1093/jnci/djw133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Scorsetti M, Comito T, Cozzi L, Clerici E, Tozzi A, Franzese C, Navarria P, Fogliata A, Tomatis S, D'Agostino G, et al. The challenge of inoperable hepatocellular carcinoma (HCC): results of a single-institutional experience on stereotactic body radiation therapy (SBRT) J Cancer Res Clin Oncol. 2015;141:1301–1309. doi: 10.1007/s00432-015-1929-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yamashita H, Onishi H, Murakami N, Matsumoto Y, Matsuo Y, Nomiya T, Nakagawa K. Survival outcomes after stereotactic body radiotherapy for 79 Japanese patients with hepatocellular carcinoma. J Radiat Res. 2015;56:561–567. doi: 10.1093/jrr/rru130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Huertas A, Baumann AS, Saunier-Kubs F, Salleron J, Oldrini G, Croisé-Laurent V, Barraud H, Ayav A, Bronowicki JP, Peiffert D. Stereotactic body radiation therapy as an ablative treatment for inoperable hepatocellular carcinoma. Radiother Oncol. 2015;115:211–216. doi: 10.1016/j.radonc.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 71.Zhong NB, Lv GM, Chen ZH. Stereotactic body radiotherapy combined with transarterial chemoembolization for huge (≥10 cm) hepatocellular carcinomas: A clinical study. Mol Clin Oncol. 2014;2:839–844. doi: 10.3892/mco.2014.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lo CH, Huang WY, Lee MS, Lin KT, Lin TP, Chang PY, Fan CY, Jen YM. Stereotactic ablative radiotherapy for unresectable hepatocellular carcinoma patients who failed or were unsuitable for transarterial chemoembolization. Eur J Gastroenterol Hepatol. 2014;26:345–352. doi: 10.1097/MEG.0000000000000032. [DOI] [PubMed] [Google Scholar]
- 73.Van De Voorde L, Vanneste B, Houben R, Damen P, van den Bogaard J, Lammering G, Dejong K, de Vos-Geelen J, Buijsen J, Öllers M, et al. Image-guided stereotactic ablative radiotherapy for the liver: a safe and effective treatment. Eur J Surg Oncol. 2015;41:249–256. doi: 10.1016/j.ejso.2014.10.053. [DOI] [PubMed] [Google Scholar]
- 74.Takeda A, Sanuki N, Eriguchi T, Kobayashi T, Iwabutchi S, Matsunaga K, Mizuno T, Yashiro K, Nisimura S, Kunieda E. Stereotactic ablative body radiotherapy for previously untreated solitary hepatocellular carcinoma. J Gastroenterol Hepatol. 2014;29:372–379. doi: 10.1111/jgh.12350. [DOI] [PubMed] [Google Scholar]
- 75.Park JH, Yoon SM, Lim YS, Kim SY, Shim JH, Kim KM, Lee HC, Cho B, Park G, Kim JH. Two-week schedule of hypofractionated radiotherapy as a local salvage treatment for small hepatocellular carcinoma. J Gastroenterol Hepatol. 2013;28:1638–1642. doi: 10.1111/jgh.12249. [DOI] [PubMed] [Google Scholar]
- 76.Yoon SM, Lim YS, Park MJ, Kim SY, Cho B, Shim JH, Kim KM, Lee HC, Chung YH, Lee YS, et al. Stereotactic body radiation therapy as an alternative treatment for small hepatocellular carcinoma. PLoS One. 2013;8:e79854. doi: 10.1371/journal.pone.0079854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jung J, Yoon SM, Kim SY, Cho B, Park JH, Kim SS, Song SY, Lee SW, Ahn SD, Choi EK, et al. Radiation-induced liver disease after stereotactic body radiotherapy for small hepatocellular carcinoma: clinical and dose-volumetric parameters. Radiat Oncol. 2013;8:249. doi: 10.1186/1748-717X-8-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bibault JE, Dewas S, Vautravers-Dewas C, Hollebecque A, Jarraya H, Lacornerie T, Lartigau E, Mirabel X. Stereotactic body radiation therapy for hepatocellular carcinoma: prognostic factors of local control, overall survival, and toxicity. PLoS One. 2013;8:e77472. doi: 10.1371/journal.pone.0077472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Honda Y, Kimura T, Aikata H, Kobayashi T, Fukuhara T, Masaki K, Nakahara T, Naeshiro N, Ono A, Miyaki D, et al. Stereotactic body radiation therapy combined with transcatheter arterial chemoembolization for small hepatocellular carcinoma. J Gastroenterol Hepatol. 2013;28:530–536. doi: 10.1111/jgh.12087. [DOI] [PubMed] [Google Scholar]
- 80.Yuan Z, Tian L, Wang P, Song Y, Dong Y, Zhuang H. Comparative research on the efficacy of CyberKnife® and surgical excision for Stage I hepatocellular carcinoma. Onco Targets Ther. 2013;6:1527–1532. doi: 10.2147/OTT.S51452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sanuki N, Takeda A, Mizuno T, Oku Y, Eriguchi T, Iwabuchi S, Kunieda E. Tumor response on CT following hypofractionated stereotactic ablative body radiotherapy for small hypervascular hepatocellular carcinoma with cirrhosis. AJR Am J Roentgenol. 2013;201:W812–W820. doi: 10.2214/AJR.12.10169. [DOI] [PubMed] [Google Scholar]
- 82.Huang WY, Jen YM, Lee MS, Chang LP, Chen CM, Ko KH, Lin KT, Lin JC, Chao HL, Lin CS, et al. Stereotactic body radiation therapy in recurrent hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2012;84:355–361. doi: 10.1016/j.ijrobp.2011.11.058. [DOI] [PubMed] [Google Scholar]
- 83.Kang JK, Kim MS, Cho CK, Yang KM, Yoo HJ, Kim JH, Bae SH, Jung DH, Kim KB, Lee DH, et al. Stereotactic body radiation therapy for inoperable hepatocellular carcinoma as a local salvage treatment after incomplete transarterial chemoembolization. Cancer. 2012;118:5424–5431. doi: 10.1002/cncr.27533. [DOI] [PubMed] [Google Scholar]
- 84.Ibarra RA, Rojas D, Snyder L, Yao M, Fabien J, Milano M, Katz A, Goodman K, Stephans K, El-Gazzaz G, et al. Multicenter results of stereotactic body radiotherapy (SBRT) for non-resectable primary liver tumors. Acta Oncol. 2012;51:575–583. doi: 10.3109/0284186X.2011.652736. [DOI] [PubMed] [Google Scholar]
- 85.Price TR, Perkins SM, Sandrasegaran K, Henderson MA, Maluccio MA, Zook JE, Tector AJ, Vianna RM, Johnstone PA, Cardenes HR. Evaluation of response after stereotactic body radiotherapy for hepatocellular carcinoma. Cancer. 2012;118:3191–3198. doi: 10.1002/cncr.26404. [DOI] [PubMed] [Google Scholar]
- 86.Andolino DL, Johnson CS, Maluccio M, Kwo P, Tector AJ, Zook J, Johnstone PA, Cardenes HR. Stereotactic body radiotherapy for primary hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2011;81:e447–e453. doi: 10.1016/j.ijrobp.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 87.Chan LC, Chiu SK, Chan SL. Stereotactic radiotherapy for hepatocellular carcinoma: report of a local single-centre experience. Hong Kong Med J. 2011;17:112–118. [PubMed] [Google Scholar]
- 88.Louis C, Dewas S, Mirabel X, Lacornerie T, Adenis A, Bonodeau F, Lartigau E. Stereotactic radiotherapy of hepatocellular carcinoma: preliminary results. Technol Cancer Res Treat. 2010;9:479–487. doi: 10.1177/153303461000900506. [DOI] [PubMed] [Google Scholar]
- 89.Kwon JH, Bae SH, Kim JY, Choi BO, Jang HS, Jang JW, Choi JY, Yoon SK, Chung KW. Long-term effect of stereotactic body radiation therapy for primary hepatocellular carcinoma ineligible for local ablation therapy or surgical resection. Stereotactic radiotherapy for liver cancer. BMC Cancer. 2010;10:475. doi: 10.1186/1471-2407-10-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Son SH, Choi BO, Ryu MR, Kang YN, Jang JS, Bae SH, Yoon SK, Choi IB, Kang KM, Jang HS. Stereotactic body radiotherapy for patients with unresectable primary hepatocellular carcinoma: dose-volumetric parameters predicting the hepatic complication. Int J Radiat Oncol Biol Phys. 2010;78:1073–1080. doi: 10.1016/j.ijrobp.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 91.Goyal K, Einstein D, Yao M, Kunos C, Barton F, Singh D, Siegel C, Stulberg J, Sanabria J. Cyberknife stereotactic body radiation therapy for nonresectable tumors of the liver: preliminary results. HPB Surg. 2010;2010:Epub 2010 Jun 28. doi: 10.1155/2010/309780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Seo YS, Kim MS, Yoo SY, Cho CK, Choi CW, Kim JH, Han CJ, Park SC, Lee BH, Kim YH, et al. Preliminary result of stereotactic body radiotherapy as a local salvage treatment for inoperable hepatocellular carcinoma. J Surg Oncol. 2010;102:209–214. doi: 10.1002/jso.21593. [DOI] [PubMed] [Google Scholar]
- 93.Méndez Romero A, Wunderink W, Hussain SM, De Pooter JA, Heijmen BJ, Nowak PC, Nuyttens JJ, Brandwijk RP, Verhoef C, Ijzermans JN, et al. Stereotactic body radiation therapy for primary and metastatic liver tumors: A single institution phase i-ii study. Acta Oncol. 2006;45:831–837. doi: 10.1080/02841860600897934. [DOI] [PubMed] [Google Scholar]
- 94.Herfarth KK, Debus J, Lohr F, Bahner ML, Rhein B, Fritz P, Höss A, Schlegel W, Wannenmacher MF. Stereotactic single-dose radiation therapy of liver tumors: results of a phase I/II trial. J Clin Oncol. 2001;19:164–170. doi: 10.1200/JCO.2001.19.1.164. [DOI] [PubMed] [Google Scholar]
- 95.Hoyer M, Roed H, Traberg Hansen A, Ohlhuis L, Petersen J, Nellemann H, Kiil Berthelsen A, Grau C, Aage Engelholm S, Von der Maase H. Phase II study on stereotactic body radiotherapy of colorectal metastases. Acta Oncol. 2006;45:823–830. doi: 10.1080/02841860600904854. [DOI] [PubMed] [Google Scholar]
- 96.Kavanagh BD, Schefter TE, Cardenes HR, Stieber VW, Raben D, Timmerman RD, McCarter MD, Burri S, Nedzi LA, Sawyer TE, et al. Interim analysis of a prospective phase I/II trial of SBRT for liver metastases. Acta Oncol. 2006;45:848–855. doi: 10.1080/02841860600904870. [DOI] [PubMed] [Google Scholar]
- 97.Ambrosino G, Polistina F, Costantin G, Francescon P, Guglielmi R, Zanco P, Casamassima F, Febbraro A, Gerunda G, Lumachi F. Image-guided robotic stereotactic radiosurgery for unresectable liver metastases: preliminary results. Anticancer Res. 2009;29:3381–3384. [PubMed] [Google Scholar]
- 98.Lee MT, Kim JJ, Dinniwell R, Brierley J, Lockwood G, Wong R, Cummings B, Ringash J, Tse RV, Knox JJ, et al. Phase I study of individualized stereotactic body radiotherapy of liver metastases. J Clin Oncol. 2009;27:1585–1591. doi: 10.1200/JCO.2008.20.0600. [DOI] [PubMed] [Google Scholar]
- 99.Stintzing S, Hoffmann RT, Heinemann V, Kufeld M, Rentsch M, Muacevic A. Radiosurgery of liver tumors: value of robotic radiosurgical device to treat liver tumors. Ann Surg Oncol. 2010;17:2877–2883. doi: 10.1245/s10434-010-1187-9. [DOI] [PubMed] [Google Scholar]
- 100.Goodman KA, Wiegner EA, Maturen KE, Zhang Z, Mo Q, Yang G, Gibbs IC, Fisher GA, Koong AC. Dose-escalation study of single-fraction stereotactic body radiotherapy for liver malignancies. Int J Radiat Oncol Biol Phys. 2010;78:486–493. doi: 10.1016/j.ijrobp.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 101.Janoray G, Chapet S, Ruffier-Loubière A, Bernadou G, Pointreau Y, Calais G. Robotic stereotactic body radiation therapy for tumors of the liver: radiation-induced liver disease, incidence and predictive factors. Cancer Radiother. 2014;18:191–197. doi: 10.1016/j.canrad.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 102.University of Aarhus. Radiofrequency Ablation Versus Stereotactic Radiotherapy in Colorectal Liver Metastases (RAS01). In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US) Available from: http://clinicaltrials.gov/ct2/show/NCT01233544 NLM Identifier: NCT01233544.
- 103.University Health Network, Toronto. Sorafenib-RT Treatment for Liver Metastasis (SLIM). In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US) Available from: http://clinicaltrials.gov/ct2/show/NCT00892424 NLM Identifier: NCT00892424.
- 104.Bentzen SM, Constine LS, Deasy JO, Eisbruch A, Jackson A, Marks LB, Ten Haken RK, Yorke ED. Quantitative Analyses of Normal Tissue Effects in the Clinic (QUANTEC): an introduction to the scientific issues. Int J Radiat Oncol Biol Phys. 2010;76:S3–S9. doi: 10.1016/j.ijrobp.2009.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]


